Abstract
The protective effect of antibodies (Abs) is generally attributed to neutralization or complement activation. Using Legionella pneumophila and Mycobacterium bovis bacillus Calmette–Guérin as a model, we discovered an additional mechanism of Ab-mediated protection effective against intracellular pathogens that normally evade lysosomal fusion. We show that Fc receptor (FcR) engagement by Abs, which can be temporally and spatially separated from bacterial infection, renders the host cell nonpermissive for bacterial replication and targets the pathogens to lysosomes. This process is strictly dependent on kinases involved in FcR signaling but not on host cell protein synthesis or protease activation. Based on these findings, we propose a mechanism whereby Abs and FcR engagement subverts the strategies by which intracellular bacterial pathogens evade lysosomal degradation.
Keywords: immunoglobulins, opsonization, macrophage, intracellular trafficking, intracellular bacterial replication
Infections with intracellular bacterial pathogens cause a number of severe respiratory diseases, such as tuberculosis, or intestinal tract-associated medical conditions, such as listeriosis or salmonellosis. These intracellular bacteria survive and replicate inside phagocytes by effectively avoiding the host cell's antimicrobial processes. To achieve this common goal, they have evolved multiple strategies to interfere with phagosomal maturation. Mycobacterium tuberculosis, Legionella pneumophila (Lpn), and Listeria monocytogenes, for example, paralyze the innate defense mechanisms of their host cells by arresting or reprogramming phagosomal maturation or by escaping from the maturing phagosome, allowing them to replicate and cause disease (reviewed in ref. 1). M. tuberculosis survives in macrophages (MΦ) by arresting phagosomal maturation at an early stage through inhibition of cytosolic Ca2+ flux and PI(3)P hydrolysis, whose depletion from early phagosomes results in impaired maturation to the late phagolysosomal stage (2). Similarly, Lpn arrests phagosome maturation at an early stage by active modulation of the Legionella-containing vacuole (LCV), thereby avoiding contact with the lysosomal pathway (3).
We have used Lpn, the etiological agent of Legionnaires’ disease, as a model to study the protective mechanism of antibodies (Abs) in infections with intracellular bacterial pathogens that are specialized to interfere with phagolysosomal maturation (4). Inhalation of contaminated aerosols allows Lpn to gain access to the lung, where they infect alveolar MΦ, replicate inside these cells, and cause disease. The interaction between Lpn and MΦ is governed by the bacterial Icm/Dot type IV secretion system (T4SS), through which the bacteria secrete effector proteins into the host cell cytoplasm, resulting in the formation of a replication-permissive LCV (5–7).
Although primary infection of Lpn is largely controlled by innate immune mechanisms, particularly, natural killer cell-derived IFNγ (8, 9), early reports also documented adaptive cell-mediated and humoral immune responses upon Lpn infection (10). We have previously analyzed the Lpn-specific humoral response in detail and documented that Lpn-specific Abs can mediate protection against Lpn challenge (11).
In this study, we dissect the mechanisms by which Abs mediate protection against intracellular bacterial pathogens that have evolved strategies to evade phagolysosomal fusion. Using the vacuolar bacterial pathogen Lpn, we show that Abs protect by altering intracellular trafficking of this pathogenic bacterium into lysosomal compartments. This lysosomal targeting is independent of Fc receptor (FcR)-mediated phagocytosis but strictly dependent on FcR signaling, and it is operational in vitro and in vivo.
Results
Lpn-Specific Abs Constitute the Dominant Protective Mechanism in Vivo.
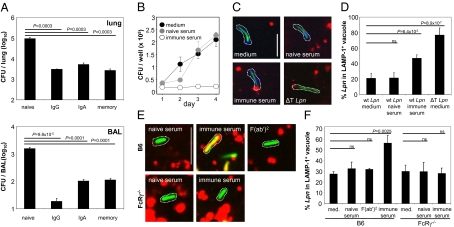
To address whether Abs are the major component mediating protection on secondary Lpn infection in vivo, wild-type (wt) B6 and B cell-deficient JHT mice, intranasally (i.n.) immunized with Lpn, were challenged through the same route, and bacterial titers were determined 2 d later. Preimmunized (memory) wt mice showed a marked reduction (100-fold) in colony-forming units compared with the naive controls, whereas only a minor reduction could be observed in JHT memory mice (Fig. S1), indicating that Ab-mediated protection is the dominant protective mechanism in secondary Lpn infection. To determine which Ab isotype confers this protection, we challenged naive mice with Lpn opsonized with purified polyclonal IgG or IgA from the bronchio-alveolar lavage (BAL) of Lpn immune mice, and we found that both isotypes were equally protective and led to a reduction in bacterial titers comparable with memory mice (Fig. 1A).
Fig. 1.
Antibodies protect from Lpn infection and inhibit Lpn growth by redirection into lysosomes in vitro. (A) B6 mice were vaccinated with Lpn (memory) or left naive. Colony-forming units (CFU) were determined in the lung and BAL 2 d after i.n. challenge with IgG- or IgA-opsonized Lpn (IgG and IgA) or untreated Lpn (naive; n = 3). (B–D) RAW MΦ were infected with Lpn pretreated with medium, naive serum, or Lpn-specific immune serum, and (B) intracellular growth of Lpn was measured. (C and D) LCVs were isolated from infected MΦ and stained for the T4SS substrate SidC (blue) and the lysosomal associated membrane protein 1 (LAMP-1) (red), and the percentage of Lpn (green) localizing in LAMP-1+ vacuoles was quantified. T4SS-deficient Lpn (ΔT), which are unable to evade lysosomal fusion, were used as positive controls for the localization in LAMP-1+ vacuoles. Dotted lines visualize LCVs. (Scale bar: 2 μm.) At least 40 LCVs were analyzed in each experimental condition. SDs from three independent experiments are shown. (E and F) Bone marrow-derived MΦ from B6 or FcRγ−/− mice were infected with Lpn pretreated with medium, naive serum, Lpn-specific immune serum, or Lpn-specific F(ab′)2. LCVs were isolated from infected MΦ and stained for the lysosomal marker LAMP-1 (red), and the percentage of Lpn (green) localized in LAMP-1+ vacuoles was quantified. Dotted lines visualize LCVs. (Scale bar: 2 μm.) At least 40 LCVs were analyzed in each experimental condition, and SDs from three independent experiments are shown. Means ± SD are indicated. P values were calculated by Student t test (two-tailed, unpaired). ns, not significant.
Complement Is Not Involved in Protection Against Lpn.
To investigate the mechanisms by which Lpn-specific Abs confer protection against this intracellular bacterium, we addressed the role of complement in this process, because Ab-induced complement activation can result in lysis of Gram-negative bacteria. Although Lpn was reported to be resistant to complement-mediated lysis in vitro (12), the role of complement in the protection from Lpn infection in vivo has not been investigated. We addressed this by either depleting memory mice from complement or infecting C3−/− mice with Ab-opsonized Lpn, and we show that complement does not play a role in protection from Lpn infection in vivo (Fig. S2). Furthermore, because IgA is unable to activate complement, our finding that Lpn-specific IgA is protective (Fig. 1A) further supports the conclusion that complement activation is dispensable for Ab-mediated protection against Lpn infection in vivo.
Opsonized Lpn Fails to Replicate in Mammalian Host Cells.
We next addressed the question if opsonization with Abs has an effect on the ability of Lpn to replicate within their host cells. To test this in vitro, we infected MΦ with Lpn that had been pretreated with immune serum, naive serum, or medium and monitored bacterial growth. Interestingly, Lpn pretreated with immune serum were no longer able to replicate intracellularly (Fig. 1B).
Because Lpn depend on their T4SS to evade lysosomal fusion and generate a replication-permissive LCV, it is conceivable that Lpn-specific Abs block the T4SS leading to Lpn degradation in the lysosome, as seen with T4SS-deficient Lpn ΔT. To address this, we isolated LCVs from MΦ infected with Lpn, which had been pretreated with immune serum, and from controls and stained the vacuoles for the lysosomal associated membrane protein 1 (LAMP-1) as well as the T4SS substrate SidC (13) (Fig. 1C). Opsonized Lpn showed significantly increased lysosomal localization (LAMP-1+) compared with control infections (47% vs. 21%) (Fig. 1 C and D). Further characterization of the compartment into which the opsonized Lpn are targeted revealed that it is positive for the late endosomal marker Rab7 (Fig. S3A) and associates with the acidic organelle label LysoTracker (Fig. S3B). The lysosomal localization of opsonized Lpn was not caused by an impaired function of the T4SS, because they were still able to secrete comparable amounts of the effector protein SidC (Fig. S4). Furthermore, persistence and replication of Lpn in the amoeba Dictyostelium discoideum, which requires a functional T4SS, were not affected by opsonization (Fig. S5), confirming that the T4SS of opsonized Lpn remained fully functional. Finally, lysosomal targeting of opsonized Lpn was also observed with human macrophages, indicating that the Ab-mediated lysosomal targeting of Lpn is conserved across species (Fig. S6).
FcR Engagement Targets Opsonized Lpn to Lysosomal Compartments.
The fact that opsonized Lpn are able to replicate in D. discoideum but not in MΦ indicates that a host cell component specific for mammalian cells is critical for growth restriction of opsonized Lpn. Potential candidates are FcRs, because they are absent in D. discoideum. Therefore, we hypothesized that FcR engagement is involved in redirecting Lpn to lysosomal compartments, resulting in restriction of intracellular growth. To address this, we infected bone marrow-derived MΦ from wt or FcRγ−/− mice, which lack all activating FcγRs, with Lpn that had been pretreated with immune or naive serum and stained LCVs for LAMP-1 (Fig. 1E). Although opsonized Lpn preferentially localized to lysosomes in wt MΦ, trafficking of opsonized Lpn was not affected in FcRγ−/− MΦ, and the bacteria were preferentially found in LAMP-1–negative vacuoles (Fig. 1 E and F). In agreement with a role of FcRs for altered trafficking of opsonized Lpn, bacteria opsonized with F(ab′)2 fragments derived from immune serum were able to evade phagolysosomal fusion in wt MΦ (Fig. 1 E and F). These results show that the host FcRs and the Fc moiety of the Ab are essential to redirect Lpn to lysosomes, where they are no longer able to replicate.
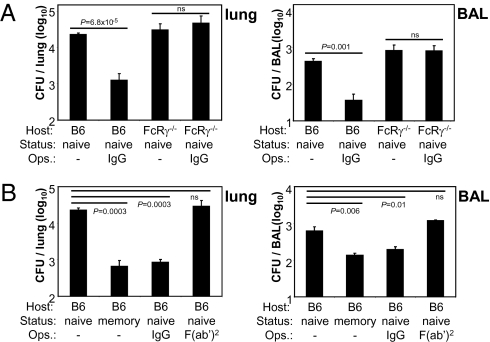
To verify the importance of FcR in the protective mechanism against Lpn infection in vivo, we infected FcRγ−/− mice with IgG-opsonized Lpn. In accordance with our in vitro results, IgG opsonization had no protective effect in these animals (Fig. 2A). Furthermore, opsonization of Lpn with F(ab′)2 fragments did not result in decreased bacterial titers compared with naive unopsonized controls (Fig. 2B). Thus, in agreement with our in vitro results, both the FcR and the Fc moiety of the Ab are essential to mediate in vivo protection against Lpn infection.
Fig. 2.
Host FcR and the Fc portion of Abs are essential for protection from Lpn in vivo. B6 mice were vaccinated with Lpn (memory) or were left naive. (A) B6 or FcRγ−/− mice were challenged i.n. with IgG- or (B) F(ab′)2-opsonized Lpn (IgG, F(ab′)2) or untreated Lpn (naive, memory), and CFUs were determined in the lung and BAL 2 d later. Means ± SD are indicated (n = 3). P values were calculated by Student t test (two-tailed, unpaired). ns, not significant.
FcR Triggering Is Sufficient to Target Lpn to Lysosomal Compartments.
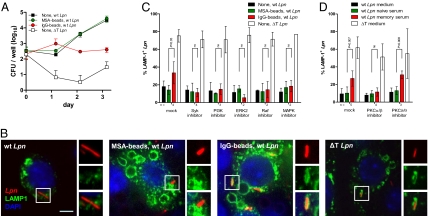
To address whether the process of FcR-dependent phagocytosis itself or alternatively, downstream signaling events triggered by FcR engagement would be responsible for lysosomal targeting of Lpn, we pretreated MΦ with IgG-coupled latex beads (IgG-beads) or mouse serum albumin-coupled beads (MSA-beads) as a control (Fig. 3A). In this setup, only MΦ that were in contact with beads decorated with IgG received an activating signal through their FcRs. The MΦ were then washed and infected with wt Lpn, and intracellular replication was quantified. Although MΦ that were treated with control beads readily supported Lpn growth, MΦ that were pretreated with IgG-beads were no longer permissive for Lpn replication, despite unaltered bacterial uptake (Fig. 3A). Comparable results were obtained when opsonized replication-deficient Lpn ΔT were used to stimulate FcRs before infection with untreated wt Lpn (Fig. S7). In line with our previous findings, pretreatment with IgG-beads led to significantly increased Lpn localization in LAMP-1+ compartments (35% vs. 16% in controls) (Fig. 3 B and C). These results show that FcR engagement, even if it is temporally and spatially separated from the uptake of the bacteria, renders MΦ nonpermissive for Lpn by targeting the bacteria to lysosomes.
Fig. 3.
FcR activation and downstream signaling are essential for directing Lpn into lysosomes in vitro. (A) RAW MΦ were incubated with IgG- (red ●) or MSA-beads (green ○) or were left untreated (squares). Cells were then infected with wt (■, red ●, and green ○) or ΔT Lpn (□), and intracellular growth was measured. (B and C) RAW MΦ expressing LAMP-1–GFP were pretreated with FcR signaling inhibitors or left untreated, and they were subsequently incubated with IgG- or MSA-beads or media. The cells were then infected with DsRed-expressing wt or ΔT Lpn, and the percentage of bacteria localizing in LAMP-1+ vacuoles was quantified. (Scale bar: B, 5 μm.) (C) One hundred LCVs were analyzed in each experimental condition, and SDs from two to four independent experiments are shown. (D) RAW MΦ expressing LAMP-1–GFP were pretreated with PKC inhibitors or left untreated and then were infected with DsRed-expressing wt or ΔT Lpn pretreated with medium, naive serum, or Lpn-specific immune serum; the percentage of bacteria localizing in LAMP-1+ vacuoles was quantified. One hundred LCVs were analyzed in each experimental condition, and SDs from three to five independent experiments are shown. P values were calculated by Student t test (two-tailed, unpaired). ns, not significant.
To analyze whether downstream FcR signaling events were required for redirecting Lpn into lysosomes, we pharmacologically inhibited kinases involved in FcR signaling and analyzed the lysosomal targeting of Lpn (Fig. 3 C and D). We targeted kinases that are involved in proximal FcR signaling (phosphoinositide 3-kinases and Syk) as well as kinases involved in signaling further downstream of FcRs (ERK2, Raf, p38 MAPK, and PKCα/β). Targeting of wt Lpn into LAMP-1+ compartments on FcR stimulation was completely abolished in the presence of any tested inhibitor relevant for FcR signaling, whereas the lysosomal targeting of replication-incompetent Lpn ΔT was not affected. In contrast to kinases involved in FcR signaling, blocking of PKCδ/θ, which is not implicated in FcR signaling, had no effect on Lpn localization (Fig. 3D). FcR-dependent signaling, involving all major downstream protein kinases in a nonredundant manner, is, therefore, essential for redirecting Lpn into lysosomes.
Although the short time period in which the opsonized bacteria are targeted to lysosomes suggests that involvement of de novo host cell protein synthesis in the lysosomal targeting is unlikely, we nevertheless tested if inhibition of protein synthesis affected the intracellular localization of the bacteria. As expected, the preferential localization of opsonized wt Lpn in LAMP-1+ compartments was not altered in the presence of the inhibitor (Fig. S8), confirming that de novo host cell protein synthesis is not required for FcR-dependent lysosomal targeting of intracellular bacteria.
Host cell proteases, such as the MΦ elastase, can exhibit bactericidal activity against Gram-positive and Gram-negative bacteria (14). To address whether FcR signaling ultimately induces activation and mobilization of host proteases to the LCV, we pretreated the host cells with cell-permeable protease inhibitors before infecting them with opsonized Lpn. These targeted elastase-like serine proteases, matrix metalloproteinases, trypsin-like proteases, cysteine proteases, aspartic proteases, serine proteases, peptidyl arginine aldehyde proteases, or the proteasome, respectively. The lysosomal targeting of opsonized wt Lpn in MΦ was affected neither by any of the inhibitors used nor by a combination thereof (Fig. S8). Thus, neither host cell proteases nor host cell protein synthesis is involved in lysosomal targeting of Lpn on FcR activation.
FcR Triggering Is Sufficient to Target Mycobacterium bovis bacillus Calmette–Guérin into Lysosomal Compartments.
Because evasion of phagolysosomal fusion represents a pivotal step in establishing infection for many intracellular pathogens, we next tested whether the lysosomal targeting on FcR stimulation was restricted to Lpn or whether other intracellular bacteria were similarly affected. To this end, we infected MΦ that had been pretreated with IgG- or MSA- beads with Mycobacterium bovis bacillus Calmette–Guérin (BCG) and analyzed its intracellular localization. In line with our findings for Lpn, FcR stimulation readily targeted the bacteria into LAMP-1+ compartments (62% vs. 35% in controls) (Fig. S9), indicating that this pathway is more generally used to protect mammalian cells from infections with intracellular vacuolar bacteria.
Lpn-Specific Abs Target Lpn to Lysosomal Compartments in Vivo.
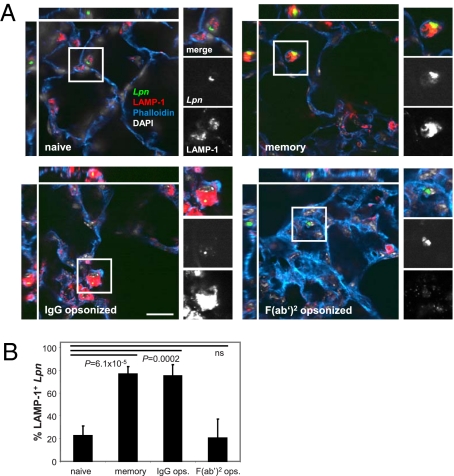
To confirm that this mechanism was also effective in vivo, we infected naive and memory mice with wt Lpn and analyzed their intracellular localization in the lung. In line with our in vitro observations, untreated bacteria did not colocalize with lysosomal markers within alveolar MΦ in naive mice (Fig. 4). In contrast, in memory mice and mice infected with opsonized Lpn, the majority of the bacteria colocalized with LAMP-1. Furthermore, F(ab′)2-opsonized Lpn did not colocalize with the lysosomal marker, confirming the central role of FcRs in lysosomal targeting of the bacteria in vivo.
Fig. 4.
Abs redirect Lpn to lysosomes in vivo. B6 mice were vaccinated with Lpn (memory) or were left naive. The mice were challenged i.n. with IgG- or F(ab′)2-opsonized (IgG, F(ab′)2) or untreated Lpn GFP (naive, memory), and the localization of the bacteria was analyzed 16–20 h after infection. Lungs were stained for the lysosomal marker LAMP-1 (red), actin (blue), and nuclei (white), and the percentage of Lpn (green) localizing in LAMP-1+ compartments was quantified. (Scale bar: A, 20 μm.) (B) n = 20 each; means ± SD are depicted. P values were calculated by Student t test (two-tailed, unpaired). ns, not significant.
Discussion
Antibodies mediate protection against infections by many different intracellular pathogens, and the mechanisms by which they protect the organism from secondary challenges are as manifold as the strategies used by the pathogens to evade destruction by the host. Nevertheless, the protective function of Abs can be attributed to several general mechanisms, including (i) neutralization, which is an important protective mechanism against viral infections (15) and extra- and intracellular bacterial toxins (16, 17), (ii) antibody-dependent cellular cytotoxicity (ADCC), which plays a role in responses against multicellular parasites as well as in eliminating infected host cells (18–20), and (iii) complement activation, which can result in lysis of Gram-negative bacteria (21). With the results presented in this study, we add lysosomal targeting to this list, a mechanism in which Abs engage FcRs and the subsequent downstream signaling renders the host cell nonpermissive for intracellular replication of the pathogen, resulting in its lysosomal degradation.
We show that, in the presence of specific Abs, the Lpn-mediated subversion of host cell trafficking no longer takes place, because Lpn are targeted to lysosomes and can no longer replicate intracellularly. Thus, opsonized Lpn are targeted into degradative pathways, indicating that specific Abs can effectively oppose the downstream events initiated by the T4SS. The opsonization of Lpn with specific Abs does not interfere with the function of the T4SS itself, because opsonized bacteria are still able to secrete effector proteins and replicate in D. discoideum—a process that is dependent on a functional T4SS. The lysosomal targeting of Lpn is, however, dependent on functional engagement of activating FcRs on the surface of MΦ. Lpn opsonization with F(ab′)2 fragments has no protective effect against intracellular multiplication within MΦ in vitro or in in vivo protection experiments, clearly showing the importance of the Fc moiety of the Abs for the altered intracellular trafficking into lysosomal compartments. The importance of FcR triggering for this protective effect is shown in vitro by the finding that opsonized Lpn are not targeted into lysosomal compartments in FcRγ−/− MΦ, which lack activating FcRs. More importantly, Lpn-specific Abs do not mediate protection in FcRγ−/− mice in vivo. Furthermore, the lysosomal targeting of Lpn is not merely a consequence of Ab-mediated phagocytosis, because the signal delivered through the FcR can be temporally and spatially separated from the infection. In addition, FcR-dependent lysosomal targeting is not restricted to Lpn infection but is also effective in BCG infection of MΦ.
The fact that MΦ, which had received an FcR trigger before infection, effectively targeted nonopsonized bacteria to lysosomal compartments indicates that FcR cross-linking induces a signaling cascade that counteracts the modulation of host cell trafficking by Lpn or BCG effectors and redirects these vacuolar pathogens to lysosomes. Indeed, pharmacological inhibition of protein kinases involved in FcR signaling abolished the Ab-induced lysosomal targeting of Lpn. Accordingly, all major FcR downstream signaling pathways are involved in the altered bacterial trafficking in the host cell in a nonredundant manner. In contrast, lysosomal targeting is dependent on neither de novo host cell protein synthesis nor protease activation resulting from FcR activation.
FcR engagement by inert particles was shown to accelerate phagolysosome fusion and maturation (22), and in case of opsonized bacteria, it has been shown to lead to increased bacterial antigen presentation (23, 24). Our results indicate another function of FcR signaling, namely to counteract the subversive strategies used by Lpn and BCG within their host cells, even if the Abs are not directly bound to the pathogen.
It is conceivable that this Ab-mediated intracellular re-routing is also relevant in infections by other prokaryotic and eukaryotic intracellular pathogens, such as Toxoplasma gondii (25, 26), Encephalitozoon cuniculi (27), Rickettsia conorii (28), and Salmonella enterica serovar Typhimurium (23, 24, 29). Like Lpn and BCG, Salmonella also actively induces a replication-permissive vacuole within host cells (30). The presence of Salmonella-specific Abs leads to enhanced antigen presentation by DCs and thereby, augments T-cell responses in an FcγRIII-dependent manner (23, 24). It is, therefore, very likely that Ab-coated Salmonella are targeted to lysosomes by the same mechanism described in this study and that this not only modulates T-cell responses but also has a direct protective effect. This is supported by the fact that passive immunization protects mice from lethal challenge with Salmonella within a time frame that would not allow the generation of effective T-cell responses (31). We therefore propose that specific Abs mediate protection against Salmonella by targeting the bacteria into lysosomes, where they are degraded.
Results from studies with the intracellular parasite T. gondii suggest that protection through FcR-dependent lysosomal targeting is not restricted to intracellular bacteria but is also effective against eukaryotic intracellular pathogens. Toxoplasma builds up a modified phagocytic vacuole in which the parasite resides and replicates (26, 32). In contrast to live Toxoplasma, dead or opsonized parasites are primarily located in lysosomes (25, 26); after Toxoplasma is located in the lysosomal compartment, MΦ are able to kill the parasites, and replication can no longer take place (33). Studies using μMT mice showed that Abs also play a crucial role in mediating resistance to Toxoplasma in vivo. It is, therefore, likely that, as in Lpn infection, Abs are able to activate MΦ through FcRs and convert them into a nonpermissive state for Toxoplasma replication (34). However, Ab-mediated enhanced lysosomal fusion of bacteria that normally evade phagolysosomal fusion might not always lead to control of intracellular bacterial replication, because it seems controversial in case of M. tuberculosis/BCG (35–37). These differences in Ab-mediated growth restriction might well relate to differential susceptibility to lysosomal degradation of the respective bacteria (36).
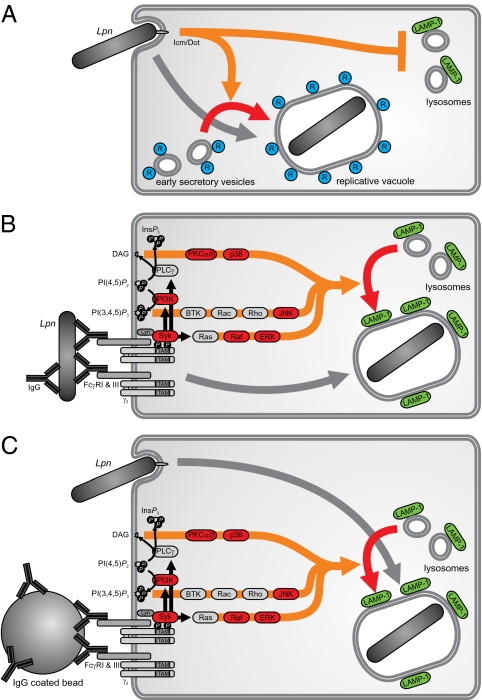
In conclusion, we propose a mechanism of Ab-mediated host cell protection against intracellular pathogens, which is effective against vacuolar pathogens that evade phagolysosomal fusion (Fig. 5A). In the presence of specific Abs, FcRs are engaged, resulting in a signaling cascade that activates the host cell and thereby renders the bacterial subversion of the phagolysosomal pathway ineffective (Fig. 5B). Moreover, FcR engagement and bacterial uptake can be temporally and spatially separated in this process (Fig. 5C). Through this pathway, the host cell becomes nonpermissive for intracellular replication, and the pathogens are targeted to the lysosome for degradation.
Fig. 5.
The relevance of the FcR trigger and its downstream signaling for the intracellular fate of Lpn in host cells. Schematic view of Lpn taken up by a MΦ. (A) Using their T4SS, the bacteria actively avoid fusion with lysosomes but acquire vesicles trafficking between the endoplasmic reticulum (ER) and Golgi, and finally, they replicate in a cellular compartment studded with ribosomes. (B) Opsonized bacteria activate FcRs and are subsequently targeted to lysosomal compartments. (C) Temporal and spatial separation of the FcR activation and FcR-independent uptake of Lpn into the MΦ also result in lysosomal targeting of the bacteria.
Materials and Methods
Bacteria, Mice, and Immunizations.
Lpn strains used in this study were JR32 (wt Philadelphia-1) (38), GS3011 (icmT deletion mutant lacking a functional Icm/Dot T4SS; ΔT) (39), thymidine-auxotroph derivatives of Lpn ΔT, and Corby (wt or flaA deletion mutant). Where indicated, Lpn constitutively expressed GFP from plasmid pMMB207-Km14-GFPc (8) or DsRed from plasmid pSW001 (40). Before infections, Lpn was grown for 3 d at 37 °C on charcoal yeast-extract agar (CYE) plates. Liquid cultures were inoculated in ACES-buffered yeast extract medium at an OD600 of 0.1 and grown for 21 h at 37 °C. To maintain plasmids, chloramphenicol (5 μg/mL) was added.
C57BL/6 (B6), B6.129P2-Igh-Jtm1Cgn (JHT) (41), C3−/− (42), and Fcer1gtm1Rav (FcRγ−/−) (43) mice were bred at the Eidgenössiche Technische Hochschule Zurich or Biosupport (Zurich-Schlieren, Switzerland) and used at 7–16 wk of age (sex- and age-matched within the experiments). All animal experiments were performed in accordance with institutional policies and have been reviewed by the cantonal veterinary office.
I.n. immunizations of mice were performed on days −64 and −32 before challenge with 5 × 106 CFU Lpn Corby as described (11). For i.n. challenge experiments, Lpn were opsonized by incubation for 1 h at room temperature with neat BAL from secondarily infected mice containing Lpn-specific IgG and IgA, purified Lpn-specific IgG or IgA from BAL, serum of secondarily infected mice, Lpn-specific F(ab′)2, or PBS as control. Mice were challenged i.n. with 5,000 CFU Lpn flaA or Lpn flaA-GFP.
Intracellular Growth of Lpn in Macrophages and Immunofluorescence Microscopy.
Bone marrow-derived MΦ were differentiated by culturing in medium supplemented with 20% L929 cell supernatant for 1 wk. RAW 264.7 MΦ and RAW MΦ expressing LAMP-1–GFP (44) or monomeric red fluorescent protein 1 (mRFP1)-Rab7 (45) were grown in Roswell Park Memorial Institute (RPMI) medium with 10% heat-inactivated FCS, which does not support extracellular Lpn growth. MΦ were seeded at 5 × 104 cells/well in 96-well plates (growth curves) or at 2.5 × 105 cells/well on sterile cover glasses in 24-well plates (microscopy) 1 d before the experiment. On day 0, Lpn from liquid cultures were opsonized with serum from naive or Lpn-infected mice, Lpn-specific F(ab′)2, or medium for 1 h at room temperature. For intracellular replication assays, MΦ were infected at a multiplicity of infection (MOI) of 0.1 (immune serum-opsonized Lpn) or 1 (all others). Infection was synchronized by centrifugation, and the infected MΦ were incubated at 37 °C. Where indicated, the MΦ were pretreated 1 h before infection with opsonized thymidine-auxotroph Lpn ΔT or latex beads coated with IgG or MSA. At the time points indicated, MΦ were lysed and the lysate was combined with supernatant from the same well and plated on CYE plates. Colonies were enumerated after 3 d of incubation at 37 °C.
For immunofluorescence microscopy, Lpn expressing GFP or DsRed was used. MΦ were infected at an MOI of 10–100, and the infection was synchronized by centrifugation. After 1 h incubation at 37 °C, the cells were washed with PBS, and vacuoles were isolated and stained as described previously (46) or were fixed as intact cells. Where indicated, the MΦ were pretreated 2 h before infection with the PI3K inhibitor LY294002 (Calbiochem), a Syk inhibitor, an ERK2 inhibitor, a Raf inhibitor, or a p38 MAPK inhibitor (Novartis Pharma AG) and 1 h before infection with IgG- or MSA- beads. In separate experiments, MΦ were pretreated with the PKC inhibitors Rottlerin and Gö6976, the host protein synthesis inhibitor cycloheximide, or selected host cell-permeable protease inhibitors, all at concentrations recommended by the manufacturers, or with DMSO as control. The MΦ were then infected with wt or ΔT Lpn pretreated with medium, naive serum, or Lpn-specific immune serum.
For the preparation of lungs for microscopy, lungs were perfused with PBS and fixed by infusion of formalin, PBS, 20% sucrose, and finally, optimum cutting temperature (O.C.T.) compound (Sakura) into the lung lumen. The organs were embedded in O.C.T. compound and snap-frozen; 60 μm cryosections were air-dried, permeabilized using 0.1% Triton X-100 (Sigma-Aldrich) in PBS, blocked (10% goat serum in PBS), and then stained and mounted with Vectashield (Vector Laboratories). Detailed information on antibodies, fluorescent dyes, and sample analysis is in SI Materials and Methods.
Determination of Bacterial Titers.
Organs were excised and homogenized using a TissueLyser (Qiagen), and serial dilutions were plated on CYE plates. Bacteria in the BAL were extracted with PBS and plated directly. Colonies were enumerated after 3 d of incubation at 37 °C.
Supplementary Material
Acknowledgments
We thank P. Wolint for technical assistance, M. Kopf (ETH Zurich, Zurich), N. Harris (EPFL Lausanne, Lausanne, Switzerland), and A. Verschoor (University of Zurich, Zurich) for the kind provision of various mouse strains, E. C. Dell'Angelica (University of California, Los Angeles) for the LAMP-1–GFP plasmid, A. Heer, B. Marsland, Ch. Dumelin, J. Scheuermann (all ETH Zurich, Zurich), and the members of the H.H. and A.O. group for discussions, and V. Kuchroo (Harvard Medical School, Boston) for critically reading the manuscript. This work was supported by the Roche Research Fund for Biology, the Bonizzi-Theler Stiftung, the GEBERT-RÜF-STIFTUNG, the Swiss National Science Foundation, the European Union, the Vontobel Foundation, and Union Bank of Switzerland AG on behalf of a client.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013827107/-/DCSupplemental.
References
- 1.Flannagan RS, Cosío G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009;7:355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- 2.Vergne I, et al. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2005;102:4033–4038. doi: 10.1073/pnas.0409716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clemens DL, Lee BY, Horwitz MA. Deviant expression of Rab5 on phagosomes containing the intracellular pathogens Mycobacterium tuberculosis and Legionella pneumophila is associated with altered phagosomal fate. Infect Immun. 2000;68:2671–2684. doi: 10.1128/iai.68.5.2671-2684.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDade JE, et al. Legionnaires’ disease: Isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1977;297:1197–1203. doi: 10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- 5.Vogel JP, Andrews HL, Wong SK, Isberg RR. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 6.Isberg RR, O'Connor TJ, Heidtman M. The Legionella pneumophila replication vacuole: Making a cosy niche inside host cells. Nat Rev Microbiol. 2009;7:13–24. doi: 10.1038/nrmicro1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber SS, Ragaz C, Hilbi H. Pathogen trafficking pathways and host phosphoinositide metabolism. Mol Microbiol. 2009;71:1341–1352. doi: 10.1111/j.1365-2958.2009.06608.x. [DOI] [PubMed] [Google Scholar]
- 8.Spörri R, Joller N, Albers U, Hilbi H, Oxenius A. MyD88-dependent IFN-γ production by NK cells is key for control of Legionella pneumophila infection. J Immunol. 2006;176:6162–6171. doi: 10.4049/jimmunol.176.10.6162. [DOI] [PubMed] [Google Scholar]
- 9.Brieland JK, et al. Immunomodulatory role of endogenous interleukin-18 in gamma interferon-mediated resolution of replicative Legionella pneumophila lung infection. Infect Immun. 2000;68:6567–6573. doi: 10.1128/iai.68.12.6567-6573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horwitz MA. Cell-mediated immunity in Legionnaires’ disease. J Clin Invest. 1983;71:1686–1697. doi: 10.1172/JCI110923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joller N, Spörri R, Hilbi H, Oxenius A. Induction and protective role of antibodies in Legionella pneumophila infection. Eur J Immunol. 2007;37:3414–3423. doi: 10.1002/eji.200737591. [DOI] [PubMed] [Google Scholar]
- 12.Horwitz MA, Silverstein SC. Interaction of the Legionnaires’ disease bacterium (Legionella pneumophila) with human phagocytes. I. L. pneumophila resists killing by polymorphonuclear leukocytes, antibody, and complement. J Exp Med. 1981;153:386–397. doi: 10.1084/jem.153.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo ZQ, Isberg RR. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc Natl Acad Sci USA. 2004;101:841–846. doi: 10.1073/pnas.0304916101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houghton AM, Hartzell WO, Robbins CS, Gomis-Rüth FX, Shapiro SD. Macrophage elastase kills bacteria within murine macrophages. Nature. 2009;460:637–641. doi: 10.1038/nature08181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dörner T, Radbruch A. Antibodies and B cell memory in viral immunity. Immunity. 2007;27:384–392. doi: 10.1016/j.immuni.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Keller MA, Stiehm ER. Passive immunity in prevention and treatment of infectious diseases. Clin Microbiol Rev. 2000;13:602–614. doi: 10.1128/cmr.13.4.602-614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edelson BT, Unanue ER. Intracellular antibody neutralizes Listeria growth. Immunity. 2001;14:503–512. doi: 10.1016/s1074-7613(01)00139-x. [DOI] [PubMed] [Google Scholar]
- 18.Lachmann PJ, Davies A. Complement and immunity to viruses. Immunol Rev. 1997;159:69–77. doi: 10.1111/j.1600-065x.1997.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 19.Bouharoun-Tayoun H, Oeuvray C, Lunel F, Druilhe P. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med. 1995;182:409–418. doi: 10.1084/jem.182.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butterworth AE, Sturrock RF, Houba V, Rees PH. Antibody-dependent cell-mediated damage to schistosomula in vitro. Nature. 1974;252:503–505. doi: 10.1038/252503a0. [DOI] [PubMed] [Google Scholar]
- 21.Tomlinson S. Complement defense mechanisms. Curr Opin Immunol. 1993;5:83–89. doi: 10.1016/0952-7915(93)90085-7. [DOI] [PubMed] [Google Scholar]
- 22.Trivedi V, et al. Immunoglobulin G signaling activates lysosome/phagosome docking. Proc Natl Acad Sci USA. 2006;103:18226–18231. doi: 10.1073/pnas.0609182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrada AA, Contreras FJ, Tobar JA, Pacheco R, Kalergis AM. Immune complex-induced enhancement of bacterial antigen presentation requires Fcgamma receptor III expression on dendritic cells. Proc Natl Acad Sci USA. 2007;104:13402–13407. doi: 10.1073/pnas.0700999104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobar JA, González PA, Kalergis AM. Salmonella escape from antigen presentation can be overcome by targeting bacteria to Fc γ receptors on dendritic cells. J Immunol. 2004;173:4058–4065. doi: 10.4049/jimmunol.173.6.4058. [DOI] [PubMed] [Google Scholar]
- 25.Joiner KA, Fuhrman SA, Miettinen HM, Kasper LH, Mellman I. Toxoplasma gondii: Fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science. 1990;249:641–646. doi: 10.1126/science.2200126. [DOI] [PubMed] [Google Scholar]
- 26.Sibley LD, Weidner E, Krahenbuhl JL. Phagosome acidification blocked by intracellular Toxoplasma gondii. Nature. 1985;315:416–419. doi: 10.1038/315416a0. [DOI] [PubMed] [Google Scholar]
- 27.Niederkorn JY, Shadduck JA. Role of antibody and complement in the control of Encephalitozoon cuniculi infections by rabbit macrophages. Infect Immun. 1980;27:995–1002. doi: 10.1128/iai.27.3.995-1002.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng HM, Whitworth T, Popov V, Walker DH. Effect of antibody on the Rickettsia-host cell interaction. Infect Immun. 2004;72:3524–3530. doi: 10.1128/IAI.72.6.3524-3530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drecktrah D, Knodler LA, Ireland R, Steele-Mortimer O. The mechanism of Salmonella entry determines the vacuolar environment and intracellular gene expression. Traffic. 2006;7:39–51. doi: 10.1111/j.1600-0854.2005.00360.x. [DOI] [PubMed] [Google Scholar]
- 30.Schlumberger MC, Hardt WD. Salmonella type III secretion effectors: Pulling the host cell's strings. Curr Opin Microbiol. 2006;9:46–54. doi: 10.1016/j.mib.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Colwell DE, Michalek SM, Briles DE, Jirillo E, McGhee JR. Monoclonal antibodies to Salmonella lipopolysaccharide: Anti-O-polysaccharide antibodies protect C3H mice against challenge with virulent Salmonella typhimurium. J Immunol. 1984;133:950–957. [PubMed] [Google Scholar]
- 32.Black MW, Boothroyd JC. Lytic cycle of Toxoplasma gondii. Microbiol Mol Biol Rev. 2000;64:607–623. doi: 10.1128/mmbr.64.3.607-623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson SE, Jr, Bautista SC, Remington JS. Specific antibody-dependent killing of Toxoplasma gondii by normal macrophages. Clin Exp Immunol. 1976;26:375–380. [PMC free article] [PubMed] [Google Scholar]
- 34.Kang H, Remington JS, Suzuki Y. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-gamma, TNF-alpha, and inducible nitric oxide synthase. J Immunol. 2000;164:2629–2634. doi: 10.4049/jimmunol.164.5.2629. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong JA, Hart PD. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975;142:1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomes MS, et al. Survival of Mycobacterium avium and Mycobacterum tuberculosis in acidified vacuoles of murine macrophages. Infect Immun. 1999;67:3199–3206. doi: 10.1128/iai.67.7.3199-3206.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Vallière S, Abate G, Blazevic A, Heuertz RM, Hoft DF. Enhancement of innate and cell-mediated immunity by antimycobacterial antibodies. Infect Immun. 2005;73:6711–6720. doi: 10.1128/IAI.73.10.6711-6720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sadosky AB, Wiater LA, Shuman HA. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect Immun. 1993;61:5361–5373. doi: 10.1128/iai.61.12.5361-5373.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segal G, Purcell M, Shuman HA. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc Natl Acad Sci USA. 1998;95:1669–1674. doi: 10.1073/pnas.95.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mampel J, et al. Planktonic replication is essential for biofilm formation by Legionella pneumophila in a complex medium under static and dynamic flow conditions. Appl Environ Microbiol. 2006;72:2885–2895. doi: 10.1128/AEM.72.4.2885-2895.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu H, Zou YR, Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993;73:1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- 42.Wessels MR, et al. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci USA. 1995;92:11490–11494. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR γ chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 44.Falcón-Pérez JM, Nazarian R, Sabatti C, Dell'Angelica EC. Distribution and dynamics of Lamp1-containing endocytic organelles in fibroblasts deficient in BLOC-3. J Cell Sci. 2005;118:5243–5255. doi: 10.1242/jcs.02633. [DOI] [PubMed] [Google Scholar]
- 45.Vonderheit A, Helenius A. Rab7 associates with early endosomes to mediate sorting and transport of Semliki forest virus to late endosomes. PLoS Biol. 2005;3:e233. doi: 10.1371/journal.pbio.0030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber SS, Ragaz C, Reus K, Nyfeler Y, Hilbi H. Legionella pneumophila exploits PI(4)P to anchor secreted effector proteins to the replicative vacuole. PLoS Pathog. 2006;2:e46. doi: 10.1371/journal.ppat.0020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.