Abstract
Objective
Epithelial ovarian carcinoma (OvCa) is rarely detected early, and it is also difficult to determine whether an adnexal mass is benign or malignant. Previously, we noted differences in methylation patterns of cell-free plasma DNA (cfpDNA) in women without disease compared to patients with OvCa. In this work we investigated whether methylation patterns of cfpDNA can differentiate between benign and malignant tumors.
Methods
Methylation patterns in cfpDNA were determined in three cohorts (30 samples each) using a microarray-based assay (MethDet 56). Principal component analysis, supervised clustering, linear discrimination analysis, and 25 rounds of 5-fold cross validation were used to determine informative genes and assess the sensitivity and specificity of differentiating between OvCa vs. healthy control (HC), benign ovarian disease (mostly serous cystadenoma, BOD) vs. HC, and OvCa vs. BOD samples.
Results
Differential methylation of three promoters (RASSF1A, CALCA, and EP300) differentiated between OvCa vs. HC with a sensitivity of 90.0% and a specificity of 86.7%. Three different promoters (BRCA1, CALCA, and CDKN1C) were informative for differentiating between BOD vs. HC, with a sensitivity of 90.0% and a specificity of 76.7%. Finally, two promoters (RASSF1A and PGR-PROX) were informative for differentiating between OvCa vs. BOD, with a sensitivity of 80.0% and a specificity of 73.3%.
Conclusions
This proof-of-principle data show that differential methylation of promoters in cfpDNA may be a useful biomarker to differentiate between certain benign and malignant ovarian tumors.
Keywords: cell-free plasma DNA, methylation, ovarian, benign, malignant
Introduction
Ovarian cancer (OvCa) has the highest mortality rate of any gynecologic malignancy [1]. The five-year survival rate is very low, which is likely a reflection of the advanced stage at the time of initial diagnosis for most patients [2, 3]. However, women with early stage disease have an improved survival [4], indicating that early detection improves the outcome of this disease. Early symptoms of OvCa are rare, and when present they are nonspecific and similar to other diseases, including benign gynecological conditions [5, 6]. Present imaging techniques often are unable to distinguish benign and malignant ovarian tumors [7, 8].
Cancer antigen 125 (CA-125) has used as a biomarker; unfortunately, it lacks the sensitivity or specificity required for a screening test [9, 10]. CA-125 often is elevated in patients with benign ovarian disease, increasing the false discovery rate for this marker [10-12] and emphasizing the need for the development of new and improved screening biomarkers for detection of ovarian cancer [12, 13].
The high false-positive rates of the CA-125 screening test often lead to unnecessary medical interventions. Recent estimates indicate that a screening test should be at least 75% sensitive and 99.6% specific to produce a positive predictive value of 10% [14]. Imaging studies (e.g., transvaginal sonography) have been used to confirm CA-125 results; however, imaging is inadequate to classify an early adnexal mass as benign or malignant. Development of a new blood-based test to differentiate between benign and malignant adnexal masses may be important to improve screening.
A new approach (termed MethDet) has been developed for analysis of methylation patterns of DNA from tissues [15] or peripheral blood (cell-free plasma DNA; cfpDNA) [16, 17]. MethDet has shown great promise for diagnosing cancer in both tissue and plasma and a significant degree of accuracy in different types of cancers, including ovarian cancer [16]. Instead of concentrating on one or two genes, the MethDet technique tests methylation at multiple genes in each clinical sample to identify differentially methylated genes that are specific for an individual disease. This technique has been successful in differential identification of chronic inflammation and neoplastic disease [18], suggesting that methylation patterns in cfpDNA can provide differential diagnosis of diverse diseases occurring in the same organ.
The purpose of our study was to determine the differences in cfpDNA methylation in patients with OvCa and benign ovarian disease (BOD), and to assess whether differential methylation of cfpDNA could be used to differentiate between these diseases. Our results strongly suggested that methylation of cfpDNA may be able to differentiate benign and malignant tumors.
Materials and methods
Patient enrollment
This study was approved by the institutional review boards of the Rush University Medical Center and the M. D. Anderson Cancer Center (MDACC). Each individual signed an informed consent prior to enrollment. All patients and controls were Caucasian females, and each cohort contained specimens from 30 individuals. Diagnosis of ovarian serous carcinoma or benign disease (serous cystadenoma [n=25], serous cystadenofibroma [n=3], mucinous cystadenoma [n=1], and simple cyst [n=1]) was determined by pathological evaluation at the MDACC. All plasma specimens were obtained pre-operatively prior to any treatment.
Sample collection and DNA isolation
Whole blood was obtained by venipuncture, collected in Vacutainer tubes containing EDTA, and stored at 4°C for a maximum of 2 hr. Each tube was centrifuged twice (2600g) for 10 minutes at 4°C to separate the plasma from cellular elements. The plasma was then aliquoted and stored at -80°C. Similarly prepared plasma samples from healthy individuals were obtained from Analytical Biological Services (Wilmington, DE, USA) and used as controls. DNA was isolated and quantified as previously described [18, 19] using DNAzol BD and proteinase K.
Microarray-mediated methylation assay
The assay was performed as previously described [18]. Briefly, each DNA sample was split into two equal aliquots, and one of them was digested with Hin6I (Fermentas, Glen Burnie, MD, USA) while the other was mock digested. Both samples were amplified via a multiplexed, nested polymerase chain reaction (PCR). 5-aminoallyl dUTP (Biotium Inc., Hayward, CA, USA) was added for the second PCR; products of the Hin6I-digested DNA were labeled with Cy3, while products of the mock-digested DNA were labeled with Cy5. Both labeled products were mixed and hybridized to custom printed DNA microarrays (Microarrays Inc., Huntsville, AL, USA). The slides were then washed and scanned using a GenePix 4000B Microarray Scanner (Molecular Devices, Union City, CA, USA), and the data were analyzed using the GenePix Pro 6.0 software.
Data processing for statistical analysis
The microarray was composed of three identical subarrays with 64 (8 × 8) spots printed on each one. DNA probes for gene promoters occupied 56 spots, 5 spots had controls for nonspecific binding, and 3 empty spots were used to assess and subtract background. If the hybridization signal for a spot was less than 2.5 times the average of the five control spots, the spot was removed from analysis (Filter 1). The second filter removed any gene promoter that had less than two informative spots (NA) among the three subarrays. Finally, the methylation ratio of Cy5/Cy3 was calculated. For each cohort, genes classified as NA in greater than 25% of the samples were removed from analysis (Filter 3).
Statistical analysis
Specific methylation patterns in cfpDNA were determined using the MethDet 56 test in three different cohorts: OvCa, BOD, and healthy control (HC). Each cohort contained samples from 30 individuals, providing 85% power to detect an effect size of 0.8 in methylation ratio (difference divided by standard deviation) between any two groups. A Student`s t test was performed on the log-transformed methylation ratio for each gene, and those with a p value of < 0.01 were selected for further analysis. Unsupervised principal component analysis and hierarchical clustering in addition to supervised linear discrimination analysis coupled with cross validation were used to determine informative genes and the sensitivity and specificity of differentiation between OvCa and BOD.
Alternatively, data were processed using a fixed cutoff approach as previously described [16] by dichotomizing results into methylated and unmethylated, applying Fisher's exact test to rank differentially methylated genes, and selection of the most informative combination by naive Bayes algorithm with k-fold cross-validation.
Results
Patients
There were no significant differences in age between patients with ovarian cancer, benign disease (mostly serous cystadenoma), or healthy controls (Table 1; p > 0.90 by ANOVA). The clinicopathological data for patients with serous carcinoma are shown in Table 2. Eighteen (60%) patients had stage III and 12 (40%) had stage IV cancer. Their cytoreduction status was given as either optimal (< 1 cm of residual tumor; 24 patients or 80%) or suboptimal (> 1 cm of residual tumor; 6 patients or 20%). Five OvCa patients (16.6%) had CA-125 < 30 U/ml and four BOD patients (13.8%) had CA-125 > 30 U/ml. Duration of follow up ranged from 1 to 13.5 years with an average of 3.0 years. At the time of analysis, 10 (33%) patients were deceased, 18 (60%) patients had active disease, and two (6.7%) patients were alive without disease at the time of the study. There was no correlation between age, cancer stage, age of death, or cytoreduction status as determined by Spearman's rank correlation coefficient with p < 0.05 used as the cutoff.
Table 1.
Age of donors in each cohort
| Benign Disease | Ovarian Cancer | Healthy Controls | ||
|---|---|---|---|---|
| Age | Average | 61.2 | 61.7 | 60.7 |
| Std Dev | 11.3 | 10.1 | 11.1 | |
| Range | 45-84 | 41-85 | 45-81 | |
| Median | 62.5 | 61.5 | 61 | |
Table 2.
Clinicopathological data.
| A) Patients with Benign Ovarian Disease | |||
|---|---|---|---|
| Patient ID | Age | Diagnosis | CA-125* (U/ml) |
| BOD1 | 69 | serous cystadenoma | 13.1 |
| BOD2 | 59 | serous cystadenoma | 7.4 |
| BOD3 | 45 | serous cystadenoma | 29.1 |
| BOD4 | 75 | serous cystadenoma | 34.7 |
| BOD5 | 67 | serous cystadenoma | < 7.0 |
| BOD6 | 61 | serous cystadenofibroma | 16.2 |
| BOD7 | 79 | serous cystadenoma | 13.1 |
| BOD8 | 62 | mucinous cystadenoma | 7.4 |
| BOD9 | 46 | serous cystadenoma | 12 |
| BOD10 | 59 | serous cystadenoma | < 7 |
| BOD11 | 70 | serous cystadenoma | < 7 |
| BOD12 | 53 | serous cystadenoma | 18.2 |
| BOD13 | 55 | serous cystadenoma | 12.9 |
| BOD14 | 46 | serous cystadenoma | 13.4 |
| BOD15 | 60 | serous cystadenoma | 35.3 |
| BOD16 | 84 | serous cystadenoma | 7 |
| BOD17 | 36 | serous cystadenoma | 21.8 |
| BOD18 | 67 | serous cystadenofibroma | 31.6 |
| BOD19 | 61 | serous cystadenoma | 8.9 |
| BOD20 | 67 | serous cystadenoma | 24.4 |
| BOD21 | 46 | serous cystadenoma | 16.3 |
| BOD22 | 47 | serous cystadenofibroma | 17.3 |
| BOD23 | 69 | serous cystadenoma | 11.6 |
| BOD24 | 68 | serous cystadenoma | 7 |
| BOD25 | 66 | serous cystadenoma | ND* |
| BOD26 | 72 | serous cystadenoma | 10.1 |
| BOD27 | 66 | serous cystadenoma | 1828.9 |
| BOD28 | 72 | benign simple cyst | 10.4 |
| BOD29 | 63 | serous cystadenoma | 10.9 |
| BOD30 | 49 | serous cystadenoma | 14.4 |
| B) Patients with Ovarian Serous Carcinoma. ND – not determined | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient ID | Age | Menopausal Agea | pTNM | # nodes | Stageb | Optimal or Suboptimal | Follow-up (years) | Statusd | CA-125* (U/ml) |
| OvCal | 70 | 54 | TN0M1 | 0 | IV | Sub-O | 2 | DOD | 725.7 |
| OvCa2 | 59 | surgical | T3cN0M0 | 0 | IIIc | O | 2.75 | AWOD | 66.1 |
| OvCa3 | 41 | surgical | T3N1M0 | 1 | III* | O | 11.75 | DOD | 20.7 |
| OvCa4 | 47 | surgical | T3aN1M0 | 3 | IIIa | O | 13.5 | AWD | 501.3 |
| OvCa5 | 60 | NA | T3cN0M0 | 0 | IIIc | O | 2.5 | AWD | 51.5 |
| OvCa6 | 47 | surgical | T3cN0M0 | 0 | IIIc* | O | 6.5 | AWD | 952.2 |
| OvCa7 | 55 | NA | TN0M1 | 0 | IV | O | 1 | DOD | 543.6 |
| OvCa8 | 85 | NA | T3N0M0 | 0 | III | O | 1 | DOD | 15 |
| OvCa9 | 61 | NA | TN0M1 | 0 | IV | Sub-O | 2 | AWD | 391.7 |
| OvCa10 | 74 | NA | TN0M1 | 0 | IV | O | 2.5 | DOD | 32.9 |
| OvCa11 | 74 | NA | TN0M1 | 0 | IV | O | 1.75 | DOD | 839 |
| OvCa12 | 76 | 31-surgical | T3N1M0 | 1 | III | Sub-O | 2.75 | AWD | 17.4 |
| OvCa13 | 57 | 53 | T3cN1M0 | 1 | IIIc | O | 3.75 | AWD | 23335.5 |
| OvCa14 | 57 | 50 | T3cN1M0 | 5 | IIIc | O | 2.5 | AWD | 810.3 |
| OvCa15 | 62 | NA | T3aN0M0 | 0 | IIIa | O | 5.75 | DOD | 180.6 |
| OvCa16 | 62 | surgical | TN0M1 | 0 | IV | O | 1.25 | AWD | 1965.9 |
| OvCa17 | 72 | NA | TN1M1 | 25 | IV | O | 2.5 | AWD | 1.3 |
| OvCa18 | 67 | 60 | T3cN0M0 | 0 | IIIc | Sub-O | 2.5 | AWD | 150.9 |
| OvCa19 | 62 | 50 | T3N0M0 | 0 | III? | O | 2.5 | AWD | 173.1 |
| OvCa20 | 50 | surgical | T3cN0M0 | 0 | IIIc | Sub-O | 2 | DOD | 5.6 |
| OvCa21 | 59 | 37-surgical | T3N1M0 | 10 | III | O | 1.5 | DOD | 392.6 |
| OvCa22 | 66 | NA | TN1M1 | 1 | IV | O | 2.25 | AWD | 10120.4 |
| OvCa23 | 49 | surgical | T3cN1M0 | 9 | IIIC | O | 0.5 | AWOD | 843.1 |
| OvCa24 | 50 | NA | T3cN0M0 | 0 | IIIc | O | 1.5 | AWD | 150.9 |
| OvCa25 | 71 | 27-surgical | TN1M1 | 3 | IV | O | 1 | AWD | 557.4 |
| OvCa26 | 69 | surgical | T3cN1M0 | 3 | IIIc | O | 1.25 | AWD | 173.1 |
| OvCa27 | 68 | 50 | T3cN0M0 | 0 | IIIc | O | 2.25 | AWD | 139 |
| OvCa28 | 69 | 48 | TN1M1 | 7 | IV | O | 1.75 | DOD | 392.6 |
| OvCa29 | 59 | surgical | TN1M1 | 1 | IV | O | 4 | AWD | 10120.4 |
| OvCa30 | 54 | 51 | TN0M1 | 0 | IV | Sub-O | 1 | AWD | 843.1 |
Measured by chemiluminescent enzyme immuno assay
Age at menopause or hysterectomy
stages marked (*) were low-grade
coptimal (O): diameter of residual tumor < 1 cm; suboptimal (Sub-O): diameter of residual tumor > 1 cm
DOD: death of disease, AWD: alive with disease, AWOD: alive without disease.
Analysis of serous carcinoma and healthy controls
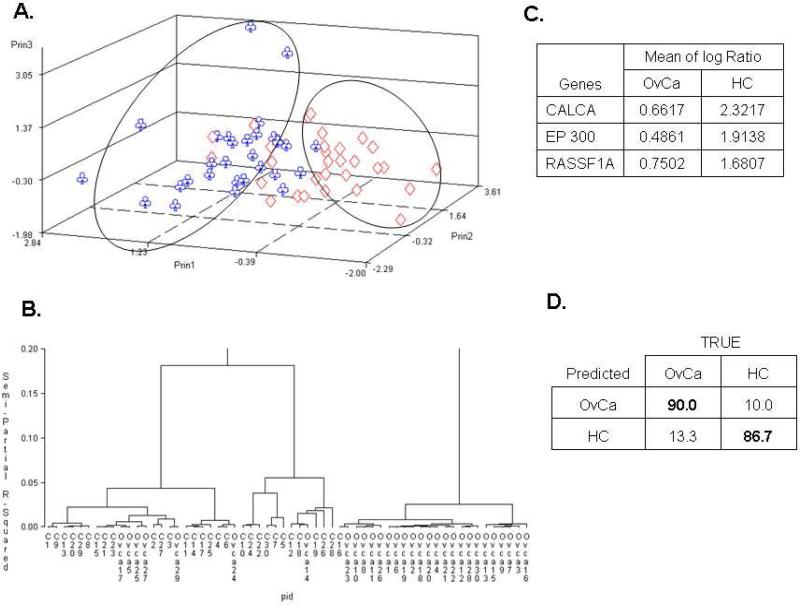
Data filtering was done as described in Methods. From the 5490 initial spots for the HC cohort, Filter 1 removed 757 observations, Filter 2 eliminated 61 additional observations (leaving 4672 for further analysis), and Filter 3 removed 310 observations, yielding 4362 observations for 53 genes. For the OvCa cohort, filtering removed 1033 observations, leaving 4457 observations and 53 genes for further analysis. Twenty-one genes were selected by the t-test (p < 0.01) for principal component analysis (PCA), which clearly identified two distinct groups with limited crossover between them (Figure 1A). The supervised hierarchical clustering analysis also segregated these two cohorts (Figure 1B). Three genes (CALCA, EP300, and RASSF1A) were determined to be differentially methylated (Figure 1C) by linear discrimination analysis and cross validation. When these genes were used in combination, a sensitivity of 90.0% (95% CI: 80.0-100%) and a specificity of 86.7% (CI: 66.7-96.7%) with positive predictive value (PPV) being 87.1% and negative predictive value (NPV) being 89.7% for cancer detection was determined (Figure 1D), similar to previously reported results [16].
Figure 1. Comparisons between ovarian serous carcinoma and healthy controls.
A) Unsupervised principal component analysis; B) hierarchical clustering; C) Supervised linear discrimination analysis coupled with cross validation were used to determine informative genes; D) Sensitivity (Bold upper left) and specificity (Bold lower right).
Analysis of benign disease and healthy controls
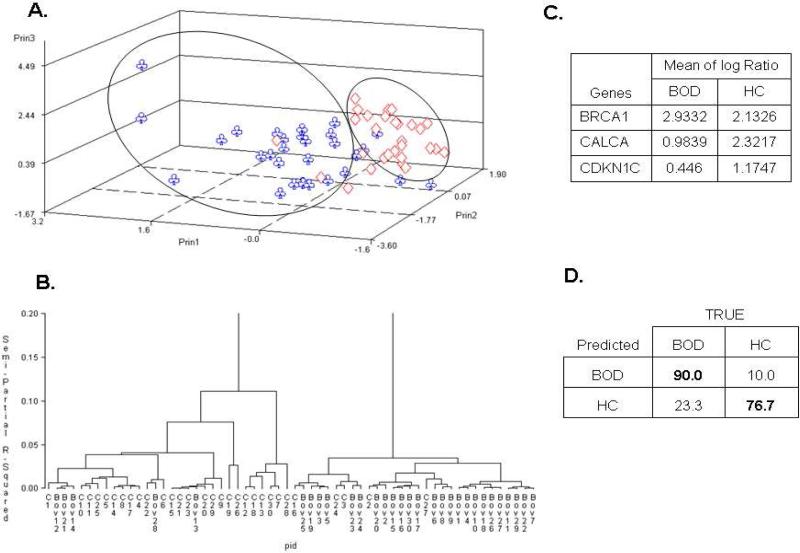
When data filters were applied, 4309 observations for 52 genes were left for the analysis of BOD vs. HC using the Student's t-test. Ten genes were selected as differentially methylated between these two groups (p < 0.01). PCA analysis showed distinct differences (Figure 2A), although the crossover was stronger than in the OvCa vs. HC comparison. Hierarchical clustering clearly segregated BOD and HC (Figure 2B), and three genes (BRCA1, CALCA, and CDKN1C) were selected as being differentially methylated (Figure 2C). The combination of these genes yielded a sensitivity of 90.0% (CI: 76.7 - 100%) and a specificity of 76.7% (CI: 66.7 - 96.7%) with PPV=79.4% and NPV=88.5% for discrimination between BOD and HC (Figure 2D), establishing for the first time the possibility of molecular detection of BOD.
Figure 2. Comparisons between benign ovarian disease and healthy controls.
A) Unsupervised principal component analysis; B) hierarchical clustering; C) Supervised linear discrimination analysis coupled with cross validation were used to determine informative genes; D) Sensitivity (Bold upper left) and specificity (Bold lower right).
Analysis of serous carcinoma and benign disease
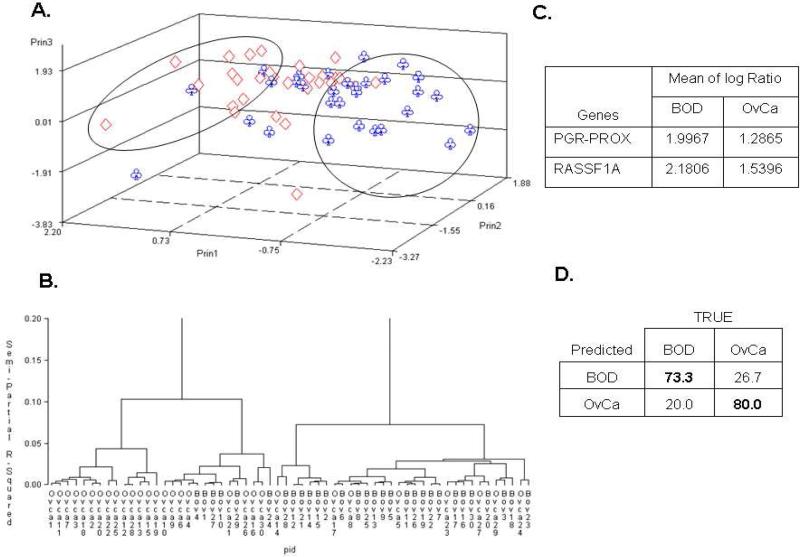
Thirteen genes were determined to be differentially methylated between the OvCa and BOD cohorts based on the Student's t-test (p < 0.01). PCA and hierarchical clustering showed greater crossover between the two groups (Figures 3A and B) than in the previous comparisons. Two genes (PGR-PROX and RASSF1A) were determined to be differentially methylated (Figure 3C), and their combination yielded a sensitivity of 73.3% (CI: 56.7-90.0%) and a specificity of 80.0% (CI: 66.7-93.3%) (Figure 3D) for OvCa identification. This observation demonstrated that methylation of cfpDNA could discriminate between benign and malignant disease, with PPV=78.6% and NPV=75.0%.
Figure 3. Comparisons between benign ovarian disease and ovarian serous carcinoma.
A) Unsupervised principal component analysis; B) hierarchical clustering; C) Supervised linear discrimination analysis coupled with cross validation were used to determine informative genes; D) Sensitivity (Bold upper left) and specificity (Bold lower right).
Analysis using a fixed cutoff approach
To confirm the validity of our results, the data were analyzed by a second statistical algorithm, which identified genes as either methylated or unmethylated using a fixed value for cutoff [16]. This approach had been previously developed for analysis of methylation data [15] and has generated verifiable results. This approach identified a large group of informative genes (Table 3A) that could be separated into five groups. Genes in groups 1, 3, and 5 were selected as informative for more than one comparison. For example, genes from group 1 were informative for differentiation of OvCa samples from both BOD and HC, while genes from group 5 were informative for differentiation of HC from patients with either benign or malignant ovarian disease. Genes in groups 2 and 4 were unique for a specific comparison.
Table 3.
Comparisons using the fixed cut off approach Analysis of the three cohorts using the fixed cutoff approach and comparison to the continuous approach. A: Differences between the informative genes; no value means that the gene was not selected as informative. (*) denotes genes identified previously by the same method [16]. B, C and D show 2×2 contingency tables with sensitivity (Bold upper left) and specificity (Bold lower right) indicated for each comparison. E: Sensitivity and specificity using the continuous and fixed cutoff data analysis with confidence interval in parenthesis. Note that the continuous analysis produces nearly the same or greater specificities and sensitivities, while picking fewer genes for each composite. F: Positive predictive value (PPV) and negative predictive value (NPV) for each comparison.

|
The number of informative genes for differentiation of BOD, OvCa, and HC was higher for the fixed cutoff approach (6 for BOD vs. HC, 10 for BOD vs. OvCa, and 13 for OvCa vs. BOD) compared to the analysis described above, suggesting a reduction of the informative value imposed by binning into methylated and unmethylated groups. Despite this loss of information, the sensitivity and specificity of disease differentiation were very close for both statistical algorithms (Table 3E), creating an interesting choice between a more intricate statistical analysis of identification of fewer genes (continuous) or a less complex test for identification of more genes (fixed cutoff). Positive predictive value (PPV) and negative predictive value (NPV) were calculated for all comparisons (Table 3F).
Discussion
The main finding from this study is the possibility of differential diagnosis of OvCa and benign ovarian tumors (mostly serous cystadenoma) by blood analysis. This result supports the previously reported differential methylation of cfpDNA from cancer patients and patients with chronic inflammatory disease [18], suggesting that methylation reflects the nature of the disease and may be useful for differential diagnosis. Applied to ovarian cancer, these findings indicated that methylation-based screening biomarkers will likely have a manageable rate of false-positive results for at least some of benign ovarian tumors, supporting further development of this approach for biomarker discovery.
Despite the low prevalence of OvCa, the disease has a poor outcome, which is often due to late detection. Imaging-based detection of advanced OvCa as an adnexal mass is most frequent, although it is difficult to determine whether an early adnexal mass is benign or malignant by imaging alone [13, 20, 21]. As a result, the clinical task of early diagnosis of OvCa can be described as having two components: differential diagnosis of malignant and benign disease in addition to detection of OvCa at the earliest possible stage. The low prevalence of this disease requires population-wide screening, which might be appropriate if an inexpensive and accurate test for differential diagnosis between BOD and OvCa is available.
Our data suggests that abnormal methylation of cfpDNA may be a useful test. Three different patterns observed between pairwise comparisons of OvCa, HC, and certain BOD (Figs. 1–3 and Table 3) can be used for disease identification.
Two algorithms were used for the data analysis. One applied binning of results for individual genes into one of two categories (methylated or unmethylated), ranked differentially methylated genes using the Fisher's exact test, and took advantage of a naive Bayes classification algorithm to select the most informative combination [16]. The other algorithm included unsupervised principal component analysis, hierarchical clustering, and supervised linear discrimination analysis as described in Figueroa et al. [22]. Significantly, the sensitivity and specificity were similar for both approaches (Fig. 4), which shows that the overall informative value of the results was not appreciably affected by binning. The alternative stepwise selection approach produced fewer informative differentially-methylated genes without sacrificing accuracy, which may simplify future clinical applications.
One of these genes, RASSF1A, was shown to be exclusively methylated in the OvCa cohort (Fig. 1 and 2), confirming previously published data from our own and other laboratories [16, 23-27]. Methylation of this promoter is a common feature of different malignancies, reflecting the gene's role as a negative regulator of cell proliferation. Methylation of the proximal promoter of PGR (PGR-PROX) has been previously observed in ovarian cancer tissues [16], so its methylation in cfpDNA from patients with OvCa (but not BOD) confirms its informative value. Finally, methylation of the EP300 promoter has been observed previously in OvCa tissues [16], suggesting disruptions in chromatin remodeling [28, 29]. Of note, a recent report indicates a role of EP300 in regulation of DNA methyltransferase 1 (DNMT1) expression, potentially through a p21-dependent mechanism [30]. Taken together, our results are well supported by previously published data from our own and other laboratories.
Hypermethylation of CALCA in both OvCa and BOD samples has not yet been described. It is interesting to note that a T-to-C transition at position -624 upstream of the translation start site significantly increases the risk of ovarian cancer [31]. It is tempting to speculate that this transition correlates with increased methylation of the CALCA promoter and/or its first exon. An earlier observation of loss of heterozygosity (LOH) on chromosome 11p (which contains the CALCA gene) in OvCa tumor tissues [32] also supports this hypothesis.
Increased methylation of BRCA1 in BOD (mostly serous cystadenoma) compared to HC may be indicative of a higher risk for both benign and malignant tumors in patients with silenced BRCA1. This hypothesis correlates with a well-established association between hereditary ovarian cancer and BRCA1 mutations [23, 33-35], suggesting that silencing of BRCA1 can be achieved by either a genetic or epigenetic route [36]. Hypermethylation of CDKN1C in the BOD vs. HC group has not yet been described. It is notable that the CDKN1C gene is located in the same 11p chromosome as CALCA, and LOH in this region is associated with OvCa [37], while decreased expression of CDKN1C has been described as a negative prognostic factor for ovarian tumor progression [38].
Significantly, our results support previous studies of abnormal DNA methylation in cfpDNA from patients with OvCa [23, 26], when RASSF1A and BRCA1 were found to be important for disease detection. While abnormal methylation of the HMLH1 promoter has been shown to correlate with poor survival after chemotherapy, in agreement with our results this promoter is uninformative in treatment-naïve OvCa [39].
In this proof-of-principle study we showed that all three cohorts–OvCa, BOD, and HC–have distinct patterns of methylation in their respective cfpDNA. These results suggested that the differences are sufficient to differentiate OvCa from certain BOD and that a differential diagnosis of benign and malignant ovarian tumors may be possible. An inexpensive diagnostic test for OvCa will promote more efficient screening and facilitate earlier detection of this disease in the future.
Acknowledgements
This work was supported in part by grants from Marsha Rivkin Foundation, R21RR024420, and DOD W81XWH0710505 to VVL. Portions of this work were supported by The University of Texas M. D. Anderson Cancer Center Specialized Program of Research Excellence (SPORE) in Ovarian Cancer (P50 CA083639), the Silicon Valley Foundation, and the Betty Ann Asche Murray Distinguished Professorship to AKS.
Grant information: Supported in part by Marsha Rivkin Foundation, R21RR024420, and DOD W81XWH0710505 (VVL); University of Texas M. D. Anderson Cancer Center SPORE in Ovarian Cancer (P50 CA083639); the Silicon Valley Foundation; and the Betty Ann Asche Murray Distinguished Professorship (AKS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: VVL is a consultant and member of the Board of Directors of CDI Biosciences, Inc and CEO of US Biomarkers, Inc.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Barnholtz-Sloan JS, Schwartz AG, Qureshi F, Jacques S, Malone J, Munkarah AR. Ovarian cancer: changes in patterns at diagnosis and relative survival over the last three decades. Am J Obstet Gynecol. 2003;189:1120–7. doi: 10.1067/s0002-9378(03)00579-9. [DOI] [PubMed] [Google Scholar]
- 3.Cannistra SA. Cancer of the ovary. N Engl J Med. 1993;329:1550–9. doi: 10.1056/NEJM199311183292108. [DOI] [PubMed] [Google Scholar]
- 4.Schlaerth AC, Chi DS, Poynor EA, Barakat RR, Brown CL. Long-term survival after fertility-sparing surgery for epithelial ovarian cancer. Int J Gynecol Cancer. 2009;19:1199–204. doi: 10.1111/IGC.0b013e31819d82c3. [DOI] [PubMed] [Google Scholar]
- 5.Attanucci CA, Ball HG, Zweizig SL, Chen AH. Differences in symptoms between patients with benign and malignant ovarian neoplasms. Am J Obstet Gynecol. 2004;190:1435–7. doi: 10.1016/j.ajog.2004.01.077. [DOI] [PubMed] [Google Scholar]
- 6.Menon U. Ovarian cancer: challenges of early detection. Nat Clin Pract Oncol. 2007;4:498–9. doi: 10.1038/ncponc0906. [DOI] [PubMed] [Google Scholar]
- 7.Twickler DM, Moschos E. Ultrasound and assessment of ovarian cancer risk. AJR Am J Roentgenol. 2010;194:322–9. doi: 10.2214/AJR.09.3562. [DOI] [PubMed] [Google Scholar]
- 8.Bell RJ, Healy DL, Robertson DM, Jobling T, Oehler MK, Edwards A, et al. Ovarian status in healthy postmenopausal women: follow-up 12 months after transvaginal ultrasound. Menopause. 2009;16:1149–55. doi: 10.1097/gme.0b013e3181aa1c93. [DOI] [PubMed] [Google Scholar]
- 9.Badgwell D, Bast RC., Jr. Early detection of ovarian cancer. Dis Markers. 2007;23:397–410. doi: 10.1155/2007/309382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Memarzadeh S, Lee SB, Berek JS, Farias-Eisner R. CA125 levels are a weak predictor of optimal cytoreductive surgery in patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2003;13:120–4. doi: 10.1046/j.1525-1438.2003.13019.x. [DOI] [PubMed] [Google Scholar]
- 11.Sjovall K, Nilsson B, Einhorn N. The significance of serum CA 125 elevation in malignant and nonmalignant diseases. Gynecol Oncol. 2002;85:175–8. doi: 10.1006/gyno.2002.6603. [DOI] [PubMed] [Google Scholar]
- 12.Arits AH, Stoot JE, Botterweck AA, Roumen FJ, Voogd AC. Preoperative serum CA125 levels do not predict suboptimal cytoreductive surgery in epithelial ovarian cancer. Int J Gynecol Cancer. 2008;18:621–8. doi: 10.1111/j.1525-1438.2007.01064.x. [DOI] [PubMed] [Google Scholar]
- 13.Gallup DG, Talledo E. Management of the adnexal mass in the 1990s. South Med J. 1997;90:972–81. doi: 10.1097/00007611-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Bast RC, Jr., Badgwell D, Lu Z, Marquez R, Rosen D, Liu J, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005;15(Suppl 3):274–81. doi: 10.1111/j.1525-1438.2005.00441.x. [DOI] [PubMed] [Google Scholar]
- 15.Melnikov AA, Scholtens DM, Wiley EL, Khan SA, Levenson VV. Array-based multiplex analysis of DNA methylation in breast cancer tissues. J Mol Diagn. 2008;10:93–101. doi: 10.2353/jmoldx.2008.070077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melnikov A, Scholtens D, Godwin A, Levenson V. Differential methylation profile of ovarian cancer in tissues and plasma. J Mol Diagn. 2009;11:60–5. doi: 10.2353/jmoldx.2009.080072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barton CA, Hacker NF, Clark SJ, O'Brien PM. DNA methylation changes in ovarian cancer: implications for early diagnosis, prognosis and treatment. Gynecol Oncol. 2008;109:129–39. doi: 10.1016/j.ygyno.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Liggett T, Melnikov A, Yi QL, Replogle C, Brand R, Kaul K, et al. Differential methylation of cell-free circulating DNA among patients with pancreatic cancer versus chronic pancreatitis. Cancer. 2010;116:1674–80. doi: 10.1002/cncr.24893. [DOI] [PubMed] [Google Scholar]
- 19.Liggett T, Melnikov A, Tilwalli S, Yi Q, Chen H, Replogle C, et al. Methylation patterns of cell-free plasma DNA in relapsing-remitting multiple sclerosis. J Neurol Sci. 2010;290:16–21. doi: 10.1016/j.jns.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuang H, Pourdehnad M, Lambright ES, Yamamoto AJ, Lanuti M, Li P, et al. Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med. 2001;42:1412–7. [PubMed] [Google Scholar]
- 21.Kyriazi S, Kaye SB, deSouza NM. Imaging ovarian cancer and peritoneal metastases--current and emerging techniques. Nat Rev Clin Oncol. 2010;7:381–93. doi: 10.1038/nrclinonc.2010.47. [DOI] [PubMed] [Google Scholar]
- 22.Figueroa ME, Skrabanek L, Li Y, Jiemjit A, Fandy TE, Paietta E, et al. MDS and secondary AML display unique patterns and abundance of aberrant DNA methylation. Blood. 2009;114:3448–58. doi: 10.1182/blood-2009-01-200519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibanez de Caceres I, Battagli C, Esteller M, Herman JG, Dulaimi E, Edelson MI, et al. Tumor cell-specific BRCA1 and RASSF1A hypermethylation in serum, plasma, and peritoneal fluid from ovarian cancer patients. Cancer Res. 2004;64:6476–81. doi: 10.1158/0008-5472.CAN-04-1529. [DOI] [PubMed] [Google Scholar]
- 24.Agathanggelou A, Honorio S, Macartney DP, Martinez A, Dallol A, Rader J, et al. Methylation associated inactivation of RASSF1A from region 3p21.3 in lung, breast and ovarian tumours. Oncogene. 2001;20:1509–18. doi: 10.1038/sj.onc.1204175. [DOI] [PubMed] [Google Scholar]
- 25.Ma L, Guo Q, Ma Y, Liu FR, Shen XY. Clinicopathological implications of inactivation of RASSF1A in serous epithelial ovarian cancers. Eur J Gynaecol Oncol. 2009;30:370–4. [PubMed] [Google Scholar]
- 26.Teodoridis JM, Hall J, Marsh S, Kannall HD, Smyth C, Curto J, et al. CpG island methylation of DNA damage response genes in advanced ovarian cancer. Cancer Res. 2005;65:8961–7. doi: 10.1158/0008-5472.CAN-05-1187. [DOI] [PubMed] [Google Scholar]
- 27.Yoon JH, Dammann R, Pfeifer GP. Hypermethylation of the CpG island of the RASSF1A gene in ovarian and renal cell carcinomas. Int J Cancer. 2001;94:212–7. doi: 10.1002/ijc.1466. [DOI] [PubMed] [Google Scholar]
- 28.Lang SE, Hearing P. The adenovirus E1A oncoprotein recruits the cellular TRRAP/GCN5 histone acetyltransferase complex. Oncogene. 2003;22:2836–41. doi: 10.1038/sj.onc.1206376. [DOI] [PubMed] [Google Scholar]
- 29.Wallberg AE, Yamamura S, Malik S, Spiegelman BM, Roeder RG. Coordination of p300-mediated chromatin remodeling and TRAP/mediator function through coactivator PGC-1alpha. Mol Cell. 2003;12:1137–49. doi: 10.1016/s1097-2765(03)00391-5. [DOI] [PubMed] [Google Scholar]
- 30.Tan HH, Porter AG. p21(WAF1) negatively regulates DNMT1 expression in mammalian cells. Biochem Biophys Res Commun. 2009;382:171–6. doi: 10.1016/j.bbrc.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Goodman MT, Ferrell R, McDuffie K, Thompson PJ, Wilkens LR, Bushley AW, et al. Calcitonin gene polymorphism CALCA-624 (T/C) and ovarian cancer. Environ Mol Mutagen. 2005;46:53–8. doi: 10.1002/em.20134. [DOI] [PubMed] [Google Scholar]
- 32.Kiechle-Schwarz M, Bauknecht T, Wienker T, Walz L, Pfleiderer A. Loss of constitutional heterozygosity on chromosome 11p in human ovarian cancer. Positive correlation with grade of differentiation. Cancer. 1993;72:2423–32. doi: 10.1002/1097-0142(19931015)72:8<2423::aid-cncr2820720821>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 33.Friedenson B. BRCA1 and BRCA2 pathways and the risk of cancers other than breast or ovarian. MedGenMed. 2005;7:60. [PMC free article] [PubMed] [Google Scholar]
- 34.Nicoletto MO, Donach M, De Nicolo A, Artioli G, Banna G, Monfardini S. BRCA-1 and BRCA-2 mutations as prognostic factors in clinical practice and genetic counselling. Cancer Treat Rev. 2001;27:295–304. doi: 10.1053/ctrv.2001.0233. [DOI] [PubMed] [Google Scholar]
- 35.Wilcox CB, Baysal BE, Gallion HH, Strange MA, DeLoia JA. High-resolution methylation analysis of the BRCA1 promoter in ovarian tumors. Cancer Genet Cytogenet. 2005;159:114–22. doi: 10.1016/j.cancergencyto.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 36.Baldwin RL, Nemeth E, Tran H, Shvartsman H, Cass I, Narod S, et al. BRCA1 promoter region hypermethylation in ovarian carcinoma: a population-based study. Cancer Res. 2000;60:5329–33. [PubMed] [Google Scholar]
- 37.Hu RJ, Lee MP, Connors TD, Johnson LA, Burn TC, Su K, et al. A 2.5-Mb transcript map of a tumor-suppressing subchromosomal transferable fragment from 11p15.5, and isolation and sequence analysis of three novel genes. Genomics. 1997;46:9–17. doi: 10.1006/geno.1997.4981. [DOI] [PubMed] [Google Scholar]
- 38.Sui L, Dong Y, Ohno M, Watanabe Y, Sugimoto K, Tokuda M. Expression of p57kip2 and its clinical relevance in epithelial ovarian tumors. Anticancer Res. 2002;22:3191–6. [PubMed] [Google Scholar]
- 39.Gifford G, Paul J, Vasey PA, Kaye SB, Brown R. The acquisition of hMLH1 methylation in plasma DNA after chemotherapy predicts poor survival for ovarian cancer patients. Clin Cancer Res. 2004;10:4420–6. doi: 10.1158/1078-0432.CCR-03-0732. [DOI] [PubMed] [Google Scholar]