Abstract
Background
Probiotics may offer a safe intervention in acute infectious diarrhoea to reduce the duration and severity of the illness.
Objectives
To assess the effects of probiotics in proven or presumed acute infectious diarrhoea.
Search methods
We searched the Cochrane Infectious Diseases Group's trials register (July 2010), the Cochrane Controlled Trials Register (The Cochrane Library Issue 2, 2010), MEDLINE (1966 to July 2010), EMBASE (1988 to July 2010), and reference lists from studies and reviews. We also contacted organizations and individuals working in the field, and pharmaceutical companies manufacturing probiotic agents.
Selection criteria
Randomized and quasi‐randomized controlled trials comparing a specified probiotic agent with a placebo or no probiotic in people with acute diarrhoea that is proven or presumed to be caused by an infectious agent.
Data collection and analysis
Two reviewers independently assessed the methodological quality of the trial and extracted data. Primary outcomes were the mean duration of diarrhoea, stool frequency on day 2 after intervention and ongoing diarrhoea on day 4. A random‐effects model was used.
Main results
Sixty‐three studies met the inclusion criteria with a total of 8014 participants. Of these, 56 trials recruited infants and young children. The trials varied in the definition used for acute diarrhoea and the end of the diarrhoeal illness, as well as in the risk of bias. The trials were undertaken in a wide range of different settings and also varied greatly in organisms tested, dosage, and participants' characteristics. No adverse events were attributed to the probiotic intervention.
Probiotics reduced the duration of diarrhoea, although the size of the effect varied considerably between studies.
The average of the effect was significant for mean duration of diarrhoea (mean difference 24.76 hours; 95% confidence interval 15.9 to 33.6 hours; n=4555, trials=35) diarrhoea lasting ≥4 days (risk ratio 0.41; 0.32 to 0.53; n=2853, trials=29) and stool frequency on day 2 (mean difference 0.80; 0.45 to 1.14; n=2751, trials=20).
The differences in effect size between studies was not explained by study quality, probiotic strain, the number of different strains, the viability of the organisms, dosage of organisms, the causes of diarrhoea, or the severity of the diarrhoea, or whether the studies were done in developed or developing countries.
Authors' conclusions
Used alongside rehydration therapy, probiotics appear to be safe and have clear beneficial effects in shortening the duration and reducing stool frequency in acute infectious diarrhoea. However, more research is needed to guide the use of particular probiotic regimens in specific patient groups.
22 March 2019
Update pending
Authors currently updating
The update is due to be published in 2019.
Plain language summary
Probiotics for treating acute infectious diarrhoea
Episodes of acute infectious diarrhoea remain a major disease burden throughout the world, especially in developing countries. They are due to infection by many different organisms. Most episodes are self‐limiting and usually investigations are not done to identify the infectious agent. The main risk to health is dehydration and management aims to improve and maintain hydration status. However, rehydration fluids do not reduce the stool volume or shorten the episode of diarrhoea. Probiotics are "friendly" bacteria that improve health and are not harmful in themselves. A number of randomized controlled trials have been done to see whether probiotics are beneficial in acute infectious diarrhoea. We have searched for as many of these trials as possible and collected together the data in a systematic way to try to discover whether or not probiotics are beneficial in acute diarrhoea. We identified 63 trials, which included a total of 8014 people ‐ mainly infants and children. Probiotics were not associated with any adverse effects. Nearly all studies reported a shortened duration of diarrhoea and reduced stool frequency in people who received probiotics compared to the controls. Overall, probiotics reduced the duration of diarrhoea by around 25 hours, the risk of diarrhoea lasting four or more days by 59% and resulted in about one fewer diarrhoeal stool on day 2 after the intervention. However, there was very marked variability in the study findings and so these estimates are approximate. We concluded that these results were very encouraging but more research is needed to identify exactly which probiotics should be used for which groups of people, and also to assess the cost effectiveness of this treatment.
Background
Definition
Diarrhoea is defined by the World Health Organization (WHO) as three or more loose or watery stools (taking the shape of the container) in a 24‐hour period. Diarrhoea is acute if the illness started less than 14 days previously, and persistent if the episode has lasted 14 days or more (Anonymous 1988). Normal infants who are exclusively breast fed may pass loose, "pasty" stools frequently. In this group the definition is usually based on what the mother considers to be diarrhoea (WHO 1990). Infectious diarrhoea is an episode of diarrhoea that is caused by an infectious agent.
Incidence and disease burden
Infectious diarrhoea occurs much more commonly in developing countries than industrialized countries (Guerrant 1990). Attack rates in developing countries are typically six to 12 episodes per child per year, compared with two in the USA (Savarino 1993). In a systematic analysis of population health data available for 2001, diarrhoeal diseases accounted for 1.78 million deaths (3.7% of total deaths) in low‐ and middle‐income countries (Lopez 2006). Most of these deaths occur in children under five years of age. Although 50% or more children with diarrhoea receive oral rehydration therapy and continued feeding in only six of 60 priority countries, and only seven countries include zinc in diarrhoeal management (Bryce 2006), diarrhoeal deaths have reduced in this age group. However, diarrhoea still accounted for about 1.6 million deaths in 2001 (15% of all deaths in the under fives; Lopez 2006). In industrialized countries deaths from infectious diarrhoea occur mainly in the elderly (Savarino 1993).
Causes
More than 20 viruses, bacteria and parasites are associated with acute diarrhoea (Gadewar 2005). Worldwide, rotavirus is the most common cause of severe diarrhoea and diarrhoea mortality in children (Cunliffe 1998). Other important viral pathogens are astrovirus, human caliciviruses (norovirus and sapovirus) and enteric adenoviruses. Important bacterial pathogens are diarrheogenic Escherichia coli, Salmonella, Shigella, Yersinia, Campylobacter, and Vibrio cholerae. The main parasitic causes of diarrhoea are Cryptosporidium and Giardia (reviewed by O'Ryan 2005). An aetiological study of young children attending hospitals in China, India, Mexico, Myanmar, and Pakistan showed that rotavirus, enterotoxigenic E. coli and Shigella spp. were the most commonly isolated pathogens (Huilan 1991). Acute diarrhoea is frequent among travellers, in whom enterotoxigenic E. coli is particularly common (Black 1986). In practice, most episodes of acute diarrhoea that are assumed to be caused by an infectious agent are treated without the causative agent being identified. The major causes of acute infectious diarrhoea differ according to local factors, such as availability of clean water and sanitation. In contrast with acute infectious diarrhoea, infection is likely to be only one of several factors that contribute to the pathogenesis of persistent diarrhoea (Walker‐Smith 1993).
Treatment
The aim of treatment is to prevent or reverse dehydration, shorten the duration of the illness (important for preventing progression to persistent diarrhoea, which is associated with adverse outcomes such as malnutrition), and to reduce the period that a person is infectious. Treatment options available are oral rehydration solution, antibiotics, and gut motility‐suppressing agents such as loperamide, codeine, and probiotics. This review considers the use of probiotics only.
Probiotics
Probiotics have been defined as microbial cell preparations or components of microbial cells that have a beneficial effect on the health and well‐being of the host (Salminen 1999). Although organisms used in clinical trials may not have a proven health benefit for the indication being investigated, we have used the term "probiotic" in this review for simplicity. Fermenting foods to enhance their taste and nutritional value is an ancient and widespread practice. Well‐known probiotics are the lactic acid bacteria and the yeast Saccharomyces (Naidu 1999). The taxonomy of the lactic acid bacteria relied traditionally on their phenotypic characteristics. Modern molecular techniques have shown these to be unreliable, and polyphasic taxonomy using both phenotypical and molecular techniques is now recommended (Klein 1998). Even closely related probiotic strains can have different clinical effects, and the Food and Agricultural Organization (FAO) of the United Nations and WHO expert consultation committee have emphasized that the beneficial effects observed in one strain cannot be assumed to occur in other strains (FAO/WHO 2001). This implies that the reliable identification of organisms at the strain level is necessary for clinical studies.
The rationale for using probiotics in infectious diarrhoea is that they act against enteric pathogens by competing for available nutrients and binding sites, making the gut contents acid, producing a variety of chemicals, and increasing specific and non‐specific immune responses (Gismondo 1999; Goldin 1998; Vanderhoof 1998). No serious adverse effects of probiotics have been suggested in well people, but rarely, infections have been reported in people with impaired immune systems or indwelling catheters (Hata 1988; Piarroux 1999; Salminen 1998; Saxelin 1996; Sussman 1986).
Six systematic reviews of probiotics in acute diarrhoea have been published. Szajewska 2001 included only published, randomized, placebo‐controlled double‐blind studies of acute diarrhoea lasting three or more days in infants and children. A score was used to assess the methodological quality of these trials. The effects of all probiotics and individual strains were analysed. The risk of diarrhoea lasting three or more days was reduced by 0.40 in the probiotic compared with the placebo group (95% confidence interval (CI) 0.28 to 0.57, random‐effects model, eight trials including 731 children), and probiotics reduced the duration of diarrhoea by 18.2 hours (95% CI 9.5 to 26.9 hours, random‐effects model, eight trials including 773 children). The statistically significant heterogeneity in this result was resolved when one study, which employed a mixture of three probiotic organisms, was excluded. Lactobacillus GG was thought to be particularly effective in rotavirus diarrhoea.
A meta‐analysis undertaken by Van Niel 2002 was restricted to adequately randomized and blinded studies of several strains of lactobacilli in children. Children who had received recent antibiotics were excluded from the study. Probiotics reduced the duration of diarrhoea by 0.7 days (95% CI 0.3 to 1.2 days, seven studies including 675 children) and diarrhoea frequency on day 2 by 1.6 (95% CI 0.7 to 2.6, three studies including 122 children). The heterogeneity of results among the studies prevented an analysis of the effects of individual strains of lactobacilli.
Three meta‐analyses have focused on randomized controlled trials of specific probiotics in acute infectious diarrhoea in children. Szajewska 2007a analysed trials of Lactobacillus casei strain GG where a > 80% follow up was achieved. Trial results published as letters to the editor, abstracts, and proceedings from scientific meetings were not included. L.casei GG reduced the duration of diarrhoea by 1.1 days (95% CI 0.3 to 1.9, seven trials, 876 infants) and was particularly effective in rotavirus diarrhoea (duration reduced by 2.1 days, 95% CI 0.6 to 3.6). However, the authors urged caution in the interpretation of the results in view of methodological limitations in the trials and the heterogeneity of the results in the studies. Chmielewska 2008 identified two trials of Lactobacillus reuteri strain ATTCC 55730. This probiotic reduced the duration of diarrhoea by 22 hours (95% CI 6 to 38, 106 participants). In an update of a previous review (Szajewska 2007b), Szajewska 2009 pooled data from seven randomized controlled trials of Saccharomyces boulardii in 944 otherwise healthy children with acute gastroenteritis. The duration of diarrhoea was reduced by 1.08 days (95% CI 0.53 to 1.64) in children who received the yeast compared with the placebo although there was marked heterogeneity in results among the studies.
A recent review concluded that the beneficial effects of probiotics in acute infectious diarrhoea were dependent on the strain of bacteria and the dose (a greater effect with doses >1010‐1011 colony‐forming units (CFU)/day). They were significant in watery diarrhoea and viral gastroenteritis but absent in invasive bacterial diarrhoea, and were greater when probiotics were administered early in the illness and were more evident in developed countries (Wolvers 2010).
Our review aims to assess the evidence base to inform the use of probiotics in acute infectious diarrhoea. To maximize use of available data, we included participants of all ages, unpublished studies, and non‐blinded (open) studies. We assessed the relevant methodological aspects of trials individually (Juni 1999). These were the generation of allocation sequence, allocation concealment, blinding, and loss to follow up. To maximize the relevance of our findings for clinical practice we included studies in which participants with infectious diarrhoea had received antibiotics prior to recruitment.
For primary outcomes, we chose the duration of diarrhoea and diarrhoea lasting ≥ 4 days, as these are directly relevant to the development of persistent diarrhoea, and stool frequency on day 2 after intervention as a marker of diarrhoea severity.
This review is a substantial update of the original version, first published in 2003 (Allen 2003).
Objectives
To assess the effects of probiotics in proven or presumed acute infectious diarrhoea.
Methods
Criteria for considering studies for this review
Types of studies
Randomized and quasi‐randomized controlled trials reporting the effect of probiotic(s) on acute infectious diarrhoea. Studies of probiotics in acute diarrhoea that reported other outcomes (eg their effect on rotavirus shedding in stools) but no diarrhoea outcomes were not included.
Types of participants
Adults and children with acute diarrhoea (duration < 14 days) that was proven or presumed to be caused by an infectious agent.
Excluded: studies of diarrhoea known or thought to have other causes (eg antibiotic‐associated diarrhoea and studies of persistent diarrhoea).
Types of interventions
Intervention
Specific, identified probiotic.
Excluded: yogurt or other fermented foods in which specific probiotic organisms were not identified.
Control
Placebo or no probiotic.
Intervention and control arm to be otherwise treated identically in relation to other treatments and drugs.
Types of outcome measures
Primary
Duration of diarrhoea Diarrhoea lasting ≥ 4 days Stool frequency on day 2 after intervention
Secondary
Diarrhoea lasting ≥ 3 days
Stool frequency on day 3 after intervention
Search methods for identification of studies
We have attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress). Searches of all databases was done on 1 July 2010.
We searched the Cochrane Infectious Diseases Group's trials register using the search terms: diarrhea/; diarr$(tw); diarhea(tw); probiotic(tw); Lactobacill$(tw); Lactococc$(tw); Bifidobacter$(tw); Enterococc$(tw); Streptococc$(tw); Saccharomyces(tw). Full details of the Cochrane Infectious Diseases Group's methods and the journals handsearched are published in The Cochrane Library in the section on 'Collaborative Review Groups'.
We searched the Cochrane Controlled Trials Register published on The Cochrane Library (Issue 2, 2010) using the search terms: diarrhea/; diarr$(tw); diarhea(tw); probiotic(tw); Lactobacill$(tw); Lactococc$(tw); Bifidobacter$(tw); Enterococc$(tw); Streptococc$(tw); Saccharomyces(tw).
We searched MEDLINE (1966 to 2010) and EMBASE (1988 to 2010 using the search strategy defined by The Cochrane Collaboration (Clarke 2003) and following search terms: diarrhea/; diarr$(tw); diarhea(tw); probiotic(tw); Lactobacill$(tw); Lactococc$(tw); Bifidobacter$(tw); Enterococc$(tw); Streptococc$(tw); Saccharomyces(tw).
The detailed search strategy is shown in Appendix 1.
In preparation for the original review (Allen 2003), we contacted organizations and individuals working in the field, and the following pharmaceutical companies that manufacture probiotic agents to help identify additional published trials and unpublished data: Biogaia Biologicals, Lund, Sweden; Nestle Foundation, Lausanne, Switzerland; Probiotics International Ltd, Somerset, UK; Ross Products Division of Abbott Laboratories, Ohio, USA, and Yakult, London, UK. We have not re‐contacted individuals or companies for this update.
We also drew on existing reviews of this topic and checked the citations of all trials identified by the above methods.
Data collection and analysis
Study selection
SA and LD independently reviewed the titles of articles and, where available, abstracts generated by the search to identify potentially relevant studies. All articles that could meet the inclusion criteria as identified by either of the reviewers were selected and the full article reviewed. Eligibility was assessed independently by SA and LD using a form based on the information presented in the article. We planned to contact trial authors if eligibility was unclear. Discrepancies among reviewers' eligibility assessments were resolved by discussion. Trial reports were scrutinized to ensure that multiple publications from the same trial were included only once. Excluded studies and the reasons for their exclusion were listed.
Assessment of methodological quality
Two reviewers (EM, GG), blinded to the origin of the articles, independently assessed the methodological quality of identified studies using generation of allocation sequence, allocation concealment, blinding, and loss to follow up, and we recorded this information on a standard form.
We considered the generation of allocation sequence to be adequate if the study authors stated that they used a method resulting in unpredictable sequences (such as a random number table or list or computer‐generated random numbers), unclear if a trial was stated to be randomized but no further information was provided and inadequate where allocation could be related to prognosis and therefore introduced selection bias (for example, the date of birth or date of admission to hospital).
We considered allocation concealment to be adequate if the assignment to arms of the study could not be predicted by the investigators or participants (for example, central randomization or numbered, identical drug containers), unclear if the method used to achieve concealment was not described or inadequate if they used a method such as alternation where the allocation of participants could be predicted.
We considered blinding to be adequate when studies were double blind (when an identical placebo was used and recruitment to intervention or control arms was not known by either the investigator or the participants), unclear if methods of blinding were not described adequately, and inadequate when blinding was not used or where the authors stated that unblinding had occurred.
We considered loss to follow up to be adequate when study endpoints were presented for 90% or more of the participants enrolled at the beginning, inadequate when follow up was less than this and unclear when either or both the number of participants recruited at the beginning of the study and the number of participants who completed the study were not clear.
LD resolved disagreements regarding the assessment of methodological quality.
Data extraction
SA, BO, SP, and SA independently extracted data using standard forms. Key data items were the aetiology and duration of diarrhoea, details of probiotic organism, participants' characteristics (nutritional and human immunodeficiency virus (HIV) status), location (countries classified according to mortality stratum; WHO 2001), and the outcome measures listed above. The number of participants recruited and the number for whom outcome data was reported were extracted and included in the Characteristics of included studies table.
For dichotomous outcomes, the number of participants experiencing the event, and the total number of participants in each intervention group was extracted. For continuous outcomes, arithmetic means, standard deviations (SD), and the numbers of participants in each intervention group was extracted. SDs were calculated from 95% CI and standard errors, where these were reported. The findings of trials that presented data that could not be included in pooled analyses (eg median and inter‐quartile range (IQR)), or reported outcomes other than the primary and secondary outcomes employed in this review were reported in the text.
Data analysis
We pooled data from studies that used comparable outcome measures. For the duration of diarrhoea and number of stools per day of intervention, we achieved a pooled estimate of treatment effect by calculating the weighted mean difference. For the number of participants with diarrhoea lasting 3 days or more, or 4 days or more after starting the intervention, we calculated a pooled estimate of the relative risk (RR) among probiotic and non‐probiotic groups.
We reported the proportion of participants for whom outcome data were available in a 'Risk of bias' table for each study. We performed analyses according to the intention‐to‐treat principle using an available case analysis approach.
Where there was significant heterogeneity (P < 0.1) in outcomes across studies assessed by the Chi2 test a random‐effects model was used; otherwise a fixed‐effect approach was taken.
We inspected the forest plots to detect non‐overlapping CIs, applied the Chi2 test and also implemented the I2 statistic (with a value of ≥ 50%) to assess heterogeneity in findings. Where there was significant statistical heterogeneity in primary outcomes for the probiotic versus no probiotic group comparisons, we conducted sensitivity analyses according to each of the four parameters of trial methodological quality (Characteristics of included studies).
We proceeded to pool data for meta‐analysis to provide a qualitative assessment of probiotic effect as a guide to clinical practice.
We expected that heterogeneity in results among studies would result from clinical diversity, including differences in probiotic(s) used, dose of organisms, types of participants, causes and severity of diarrhoea and the socioeconomic status of countries where the studies were undertaken (Wolvers 2010). Therefore, where there were results for a diarrhoea outcome available from three or more studies we conducted subgroup analyses according to the:
probiotic strain; single probiotic organisms versus combinations of two or more organisms, dose of live organisms (high dose [> 1010 CFU/day] versus lower dose [≤1010 CFU/day]); killed organisms;
age of participants;
identified diarrhoeal pathogens (rotavirus, bacterial diarrhoea);
severity of diarrhoea according to whether the participants were likely to have had mild diarrhoea and were, therefore, managed as outpatients;
mortality stratum for children and adults in the country or countries where the trial was undertaken (WHO 2001) to account for regional differences in major diarrhoeal pathogens and diarrhoea severity related to the availability of clean water and level of sanitation. To facilitate meta‐analysis, countries were divided into two groups according to whether either child or adult mortality, or both, was classified as high.
Finally, we inspected funnel plots for the primary outcomes to assess publication bias.
Results
Description of studies
Our search identified 120 potentially relevant studies. Of these, 63 met the inclusion criteria. Overall, 57 studies were excluded, including five that were preliminary or duplicate reports of other included studies (Characteristics of excluded studies). Eligibility regarding inclusion in this review was clear for all studies and clarification from trial authors was not required. We have not been able to locate the reports of two studies (Contreras 1983; Salgado) and one study is ongoing (Freedman 2010). None of the 56 included trials were cluster randomized.
Publication status
Of the 63 included studies, 23 were published in the 1980s‐1990s, 37 between 2000‐2009 and two in 2010; one study was unpublished.
Study location
According to country mortality strata for children/adults (WHO 2001), 41 trials were undertaken in countries where both child and adult mortality was classified as low or very low and 19 where either child or adult mortality was high. Two international studies recruited participants from countries crossing the mortality strata (Guandalini 2000; Jasinski 2002). Finally, the study by Ritchie 2010 was undertaken in Australia (very low child and low adult mortality) but recruited Aboriginal children who commonly had co‐morbidities such as pneumonia and malnutrition related to poverty and social disadvantage in the top end of the Northern Territory. Therefore, data from this study were not included in analysis according to country mortality strata.
A total of 47 studies were conducted in a single centre; 15 recruited participants from two to 150 centres. The number of recruitment centres was unclear in one study (D'Apuzzo 1982).
Participants
The 63 selected studies recruited a total of 8014 participants. There were 6489 infants and children (age < 18 years) and 352 adults. In three studies (1173 participants) the exact ages of participants was not clear: Bruno 1983 studied participants aged 14 years and above, participants in Wunderlich 1989 had a mean age of 33 years (age range not stated) and the age of the participants in Frigerio 1986 was not stated.
Forty‐four studies recruited inpatients, seven recruited outpatients and seven recruited both inpatients and outpatients. It was unclear in five studies whether the participants were inpatients or outpatients.
Although all studies recruited participants with acute diarrhoea, the criteria for acute diarrhoea varied considerably among studies (see Characteristics of included studies). Descriptions of stool consistency included watery, loose or liquid stools, or both, semi‐liquid, increased fluidity, pasty, mucousy or non‐formed in 46 studies but no description was stated in 17 studies. The minimum number of stools/day was specified in 36 studies; this ranged from ≥one to ≥ five stools with the most commonly used criteria being ≥ three (16 studies) and ≥four stools in 24 hours (13 studies). One study specified stool frequency as at least twice normal frequency, one as increased frequency and in one study stool consistency was taken into account. The minimum number of stools was not specified in 24 studies. The maximum duration of diarrhoea at recruitment was specified in 40 studies and varied between one and 14 days. The maximum diarrhoea duration was not specified in 23 studies.
Similarly, criteria used for the end of the diarrhoeal episode varied markedly among studies. The last liquid or watery stool (nine studies) and first normal stool (seven studies) were the most common. Twenty‐one studies used a variety of criteria based on stool frequency and consistency in a specified period (eg first formed stool if followed by two consecutive non‐watery stools or 12 hours without evacuation; Mao 2008). Four studies also included the resolution of associated symptoms (eg < two stools/day, formed, yellow/brown stools without mucus and no abdominal pains, vomiting, or fever for the whole day; D'Apuzzo 1982). Criteria were not stated in 17 studies.
Eighteen studies were either restricted to children with rotavirus diarrhoea or reported outcomes for a subgroup of children with rotavirus diarrhoea. Children with rotavirus diarrhoea were excluded in one study (Lievin Le‐Maol 2007). Ten studies stated that participants with bloody diarrhoea were included whereas these were excluded in 32 studies. It was unclear whether participants with bloody diarrhoea were included in 21 studies. No study specifically recruited or excluded travellers, and none identified any of the participants as suffering from travellers' diarrhoea.
No study specifically recruited participants known to have HIV infection and no study stated HIV positivity as an exclusion criterion, but many excluded participants with chronic illness or immunosuppression, or both.
Nutritional status was reported in 35 studies, all undertaken in children. Ten studies either recruited malnourished children only or included malnourished children; 20 studies excluded severe malnutrition; five studies recruited well‐nourished children only or excluded those with a chronic illness.
Twenty‐six studies excluded participants who had received antibiotic treatment before recruitment, eight included participants who had received antibiotic treatment before recruitment and this information was unclear in 29 studies.
The hydration status of the participants was reported in 35 studies; 22 studies included participants with severe dehydration whereas 10 studies recruited only children with mild or moderate dehydration.
Interventions
Many different probiotics were tested. Most studies tested live preparations of lactic acid bacteria and bifidobacteria. Several studies identified the probiotic organisms only by the species name without specific identification details such as a culture collection number. Few studies undertook analyses to confirm the identity or viability of the organism(s).
Forty‐six studies tested a single organism and 17 tested combinations of between two to eight organisms. The most common organisms evaluated were L. casei strain GG (13 studies), S. boulardii (10 studies) and Enterococcus lactic acid bacteria (LAB) SF68 (five studies). All other organisms and all combinations were tested in three or fewer studies. Canani 2007 allocated children to one of five different probiotic regimens and compared outcomes with a single control group. For the purposes of this review, we selected the L. casei GG group for inclusion because several other studies tested this probiotic and we wanted to maximize the data available for meta‐analysis. Grandi 2009 allocated children either to a single organism or a four‐organism group and compared outcomes with a single control group. No data extractable for meta‐analysis were reported in this study.
Forty‐seven studies tested live organisms, five studies tested a killed probiotic preparation (Billoo 2006; Boulloche 1994; Lievin Le‐Maol 2007; Simakachorn 2000; Khanna 2005), and one a pasteurized yogurt (Pashapour 2006). The viability of the organisms was unclear in 10 studies.
Three studies compared different dosages (number of organisms) of the same probiotic (Basu 2009, Mao 2008, Shornikova 1997b) with a single control group. We selected the higher probiotic dose group for inclusion in the review but have included results from the lower dose group in the text. Overall, 15 studies used a high dose of organisms (> 1010 CFU/day), 26 used a low dose (≤ 1010 CFU/day) and the dose was unclear in 22 studies.
As well as differences in dose or organisms, there was a wide variation in the treatment regimens according to timing of intervention, means of administration and duration of treatment. Probiotics were administered directly to the participants or mixed with a variety of fluids and foods. Although expressed breast milk was used to administer probiotics in some studies, some studies excluded exclusively breast‐fed infants to minimize the interruption of normal feeding.
Forty‐three studies used a placebo in the no probiotic control group; the remaining studies managed participants according to usual clinical practice.
Risk of bias in included studies
Methodological quality varied considerably (see Characteristics of included studies). Twenty‐three studies were considered adequate for generation of the allocation sequence, 15 for concealment of allocation, 35 for blinding and 45 for loss to follow up. Ten studies were adequate for all of the four methodological quality assessment parameters and five studies were inadequate for all four parameters.
Effects of interventions
Primary outcomes
The forest plots demonstrate that probiotics reduce the duration of diarrhoea. Values for duration of diarrhoea in the control arm varied widely, from 39.1 to 173.5 hours, and the difference between the intervention groups ranged from ‐79.2 to 7.0 hours (Analysis 1.1). Similar variability was evident in the other outcomes. Despite the high level of quantitative heterogeneity, the pattern was striking, and meta‐analysis shows an important effect which is statistically significant. Using a random effects approach, probiotics reduced the mean duration of diarrhoea (mean difference 24.76 hours; 95% confidence interval 15.9 to 33.6 hours; n=4555, trials=35; Analysis 1.1), diarrhoea lasting ≥4 days (risk ratio 0.41; 0.32 to 0.53; n=2853, trials=29; Analysis 1.2) and stool frequency on day 2 (mean difference 0.80; 0.45 to 1.14; n=2751, trials=20; Analysis 1.3). The differences in these analyses are an average across all studies with quantitative heterogeneity, demonstrating that probiotics have a substantive and significant effect, rather than being a precise estimate of the size of the effect.
1.1. Analysis.
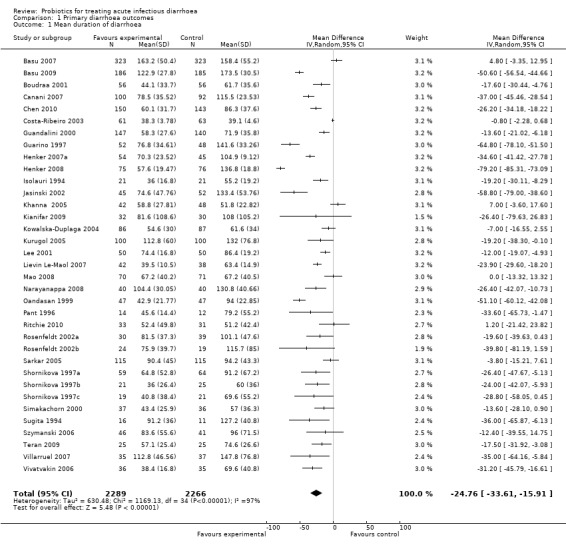
Comparison 1 Primary diarrhoea outcomes, Outcome 1 Mean duration of diarrhoea.
1.2. Analysis.
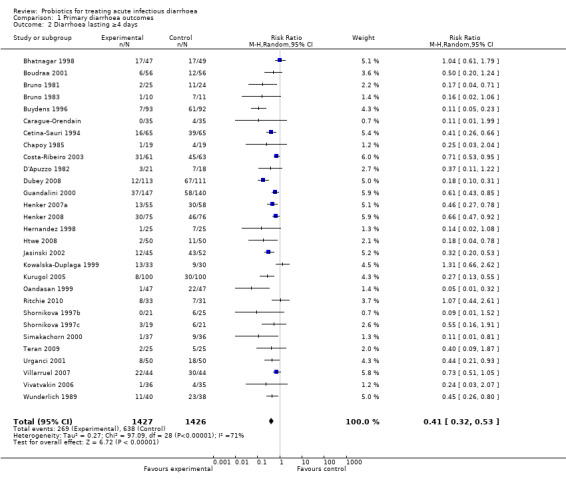
Comparison 1 Primary diarrhoea outcomes, Outcome 2 Diarrhoea lasting ≥4 days.
1.3. Analysis.
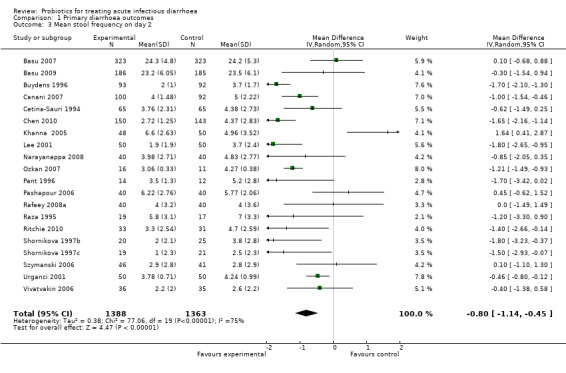
Comparison 1 Primary diarrhoea outcomes, Outcome 3 Mean stool frequency on day 2.
The funnel plots for the primary outcomes (Figure 1, Figure 2, Figure 3) did not indicate publication bias as the largest intervention effects were observed in studies with a large number of participants as well as smaller studies.
1.
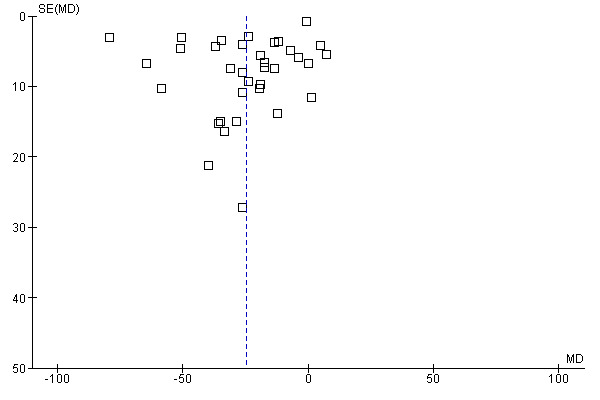
Funnel plot of comparison: 1 Primary diarrhoea outcomes, outcome: 1.1 Mean duration of diarrhoea.
2.

Funnel plot of comparison: 1 Primary diarrhoea outcomes, outcome: 1.2 Diarrhoea lasting ≥ 4 days.
3.
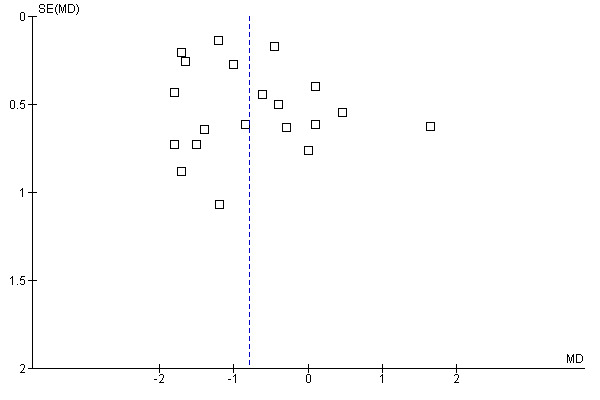
Funnel plot of comparison: 1 Primary diarrhoea outcomes, outcome: 1.3 Mean stool frequency on day 2.
Secondary outcomes
The findings for diarrhoea lasting ≥ 3days (Analysis 2.1) and stool frequency on day 3 after intervention (Analysis 2.2) were broadly similar to the primary outcomes and there was also marked statistical heterogeneity among studies.
2.1. Analysis.
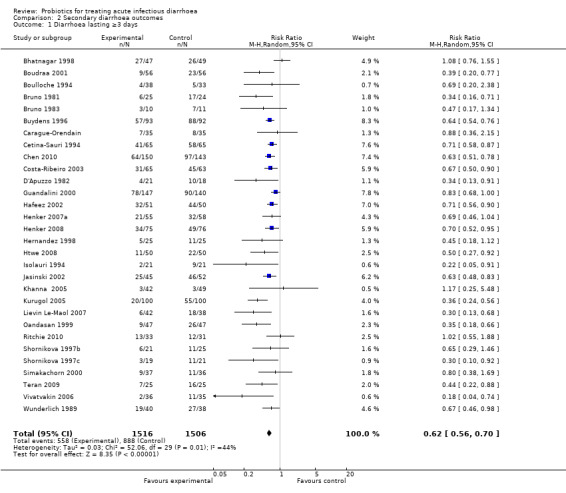
Comparison 2 Secondary diarrhoea outcomes, Outcome 1 Diarrhoea lasting ≥3 days.
2.2. Analysis.
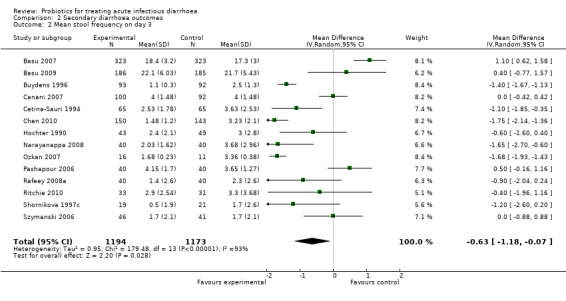
Comparison 2 Secondary diarrhoea outcomes, Outcome 2 Mean stool frequency on day 3.
Seven studies reported diarrhoea outcomes data that could not be included in analyses. Billoo 2006 evaluated S. boulardii in infants and children admitted with acute watery diarrhoea of mild to moderate severity in Pakistan. The mean duration of diarrhoea was reduced in the probiotic compared with the control group (n = 50, 86.4 hours versus n = 50, 115.2 hours, respectively; P = 0.001). Stool frequency on days 3 (P = 0.01) and 6 (P = 0.001) was also reduced in the probiotic group. Czerwionka 2009 evaluated Lactobacillus rhamnosus in children with acute diarrhoea in Poland. The total number of stools per child was statistically significantly lower in the probiotic group than the controls. Misra 2009 evaluated L. rhamnosus GG in children in India. The mean duration of diarrhoea was 70.6 hours in the probiotic and 78.0 hours in the control group (P = 0.20).
Grandi 2009 allocated young children admitted with acute rotavirus diarrhoea to receive either oral rehydration fluid (ORF) + S. boulardii, ORF + Lactobacillus acidophilus, L. rhamnosus, Bifidobacterium longum andS. boulardii, or ORF alone (control group). The median duration of diarrhoea was shorter in both of the probiotic groups compared with the controls but this was statistically significant only for S. boulardii (58 hours versus 84.5 hours, respectively; P = 0.04).
In a short abstract, Frigerio 1986 reported that the duration of diarrhoea was significantly reduced (P < 0.01) among 540 patients with an acute diarrhoeal disorder attending hospitals in Italy who received Enterococcus LAB SF 68 compared with 534 who received a placebo.
In a further study, Sepp 1995 evaluated adding L. casei GG to trimethoprim‐sulfamethoxazole, compared to trimethoprim‐sulfamethoxazole alone, in children with acute diarrhoea caused by shigellosis in Estonia. The duration of diarrhoea was similar in the probiotic (median 0.5 days) and the control group (1 day; not statistically significant). Also, the proportion of children with ongoing diarrhoea on day 5 was similar in the probiotic and control groups (6/13 (46.3%) versus 9/12 (75.0%); not statistically significant). However, a greater proportion was cured in the probiotic than the control group on day 10 (P < 0.05). Finally, in an open study, Táborská 1997 evaluated live L. acidophilus ND in infants and children admitted with acute gastroenteritis in the Czech Republic. The resolution of enteric symptoms during days 1 to 5 of the intervention was similar in the two groups.
Exploration of heterogeneity
Sensitivity analysis for primary outcomes
When analysis was restricted to trials assessed to be adequate for the four criteria of study quality (Characteristics of included studies), highly statistically significant between‐study heterogeneity persisted (forest plots not shown (Table 10). This suggests that differences in outcomes between studies were caused by factors other than differences in methodological quality.
1. Heterogeneity in sensitivity analysis of primary outcomes1.
| Sensitivity analysis | Outcome | Studies (no.) | χ2 | P value | I2 (%) |
| Generation of allocation sequence | Mean duration diarrhoea Diarrhoea ≥4 days Stool frequency day 2 |
16 13 9 |
1077.2 46.2 26.9 |
< 0.00001 < 0.00001 0.0007 |
99 74 70 |
| Concealment of allocation sequence | Mean duration diarrhoea Diarrhoea ≥4 days Stool frequency day 2 |
14 8 8 |
438.3 34.2 42.4 |
< 0.00001 < 0.0001 < 0.00001 |
97 8% 83 |
| Blinding | Mean duration diarrhoea Diarrhoea ≥4 days Stool frequency day 2 |
26 16 14 |
1070.9 64.8 48.8 |
< 0.00001 < 0.00001 < 0.00001 |
98 77 73 |
| Follow‐up | Mean duration diarrhoea Diarrhoea ≥4 days Stool frequency day 2 |
25 19 15 |
672.3 52.3 54.5 |
< 0.00001 < 0.0001 < 0.00001 |
96 66 74 |
1. Only trials considered adequate for quality assessment included; forest plots not shown
In addition to the methodological quality of studies as a potential source of heterogeneity in the primary outcomes, we explored other prespecified factors in subgroup analyses where outcomes were reported in three or more studies (probiotic strain: Analysis 3.1, Analysis 3.2, Analysis 3.3; single organism versus combinations: Analysis 4.1, Analysis 4.2, Analysis 4.3; live versus killed organisms: Analysis 5.1; dose or organisms: Analysis 6.1, Analysis 6.2, Analysis 6.3; children with rotavirus diarrhoea: Analysis 7.1, Analysis 7.2; severity of diarrhoea: Analysis 8.1 and, finally, mortality stratum for children and adults in the countries where the studies were undertaken: Analysis 9.1, Analysis 9.2, Analysis 9.3). With few exceptions, the magnitude of probiotic effect on diarrhoea outcomes was similar to that for all trials and marked heterogeneity in results persisted in the sub‐group analyses.
3.1. Analysis.
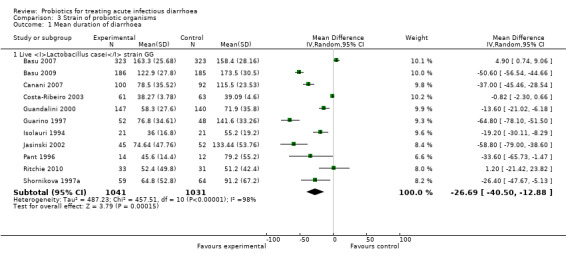
Comparison 3 Strain of probiotic organisms, Outcome 1 Mean duration of diarrhoea.
3.2. Analysis.

Comparison 3 Strain of probiotic organisms, Outcome 2 Diarrhoea lasting ≥4 days.
3.3. Analysis.
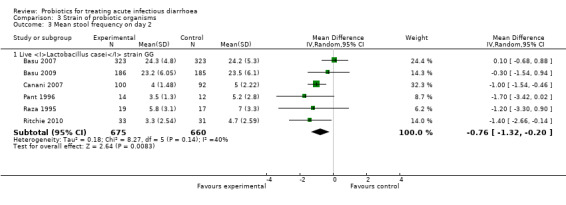
Comparison 3 Strain of probiotic organisms, Outcome 3 Mean stool frequency on day 2.
4.1. Analysis.

Comparison 4 Single organism versus combinations, Outcome 1 Mean duration of diarrhoea.
4.2. Analysis.
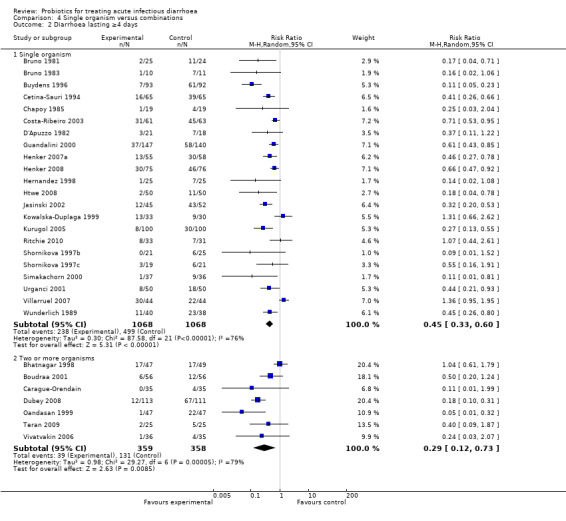
Comparison 4 Single organism versus combinations, Outcome 2 Diarrhoea lasting ≥4 days.
4.3. Analysis.
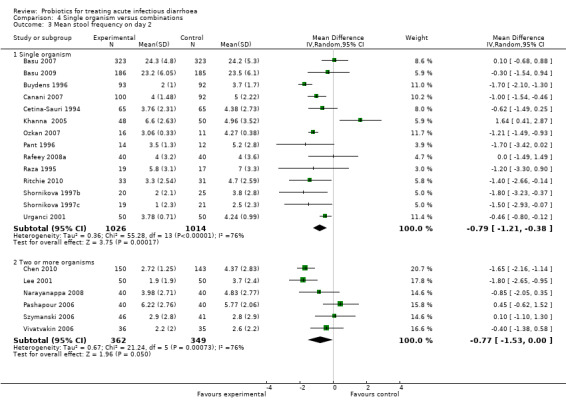
Comparison 4 Single organism versus combinations, Outcome 3 Mean stool frequency on day 2.
5.1. Analysis.

Comparison 5 Live versus killed organisms, Outcome 1 Mean duration of diarrhoea.
6.1. Analysis.
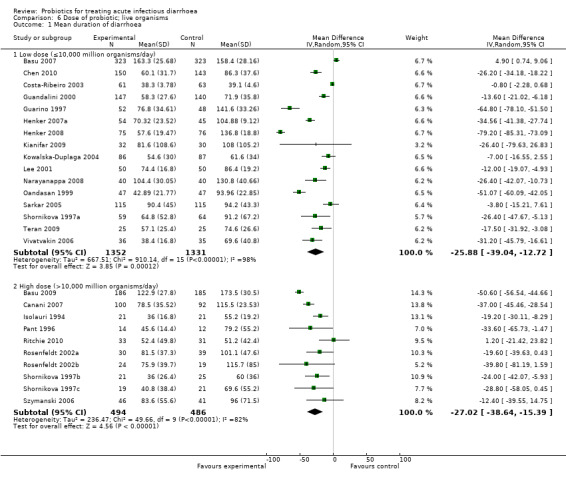
Comparison 6 Dose of probiotic; live organisms, Outcome 1 Mean duration of diarrhoea.
6.2. Analysis.
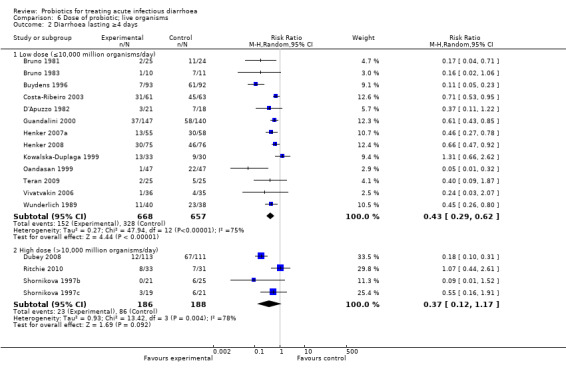
Comparison 6 Dose of probiotic; live organisms, Outcome 2 Diarrhoea lasting ≥4 days.
6.3. Analysis.
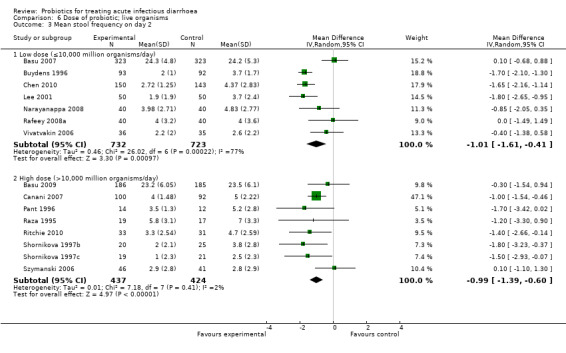
Comparison 6 Dose of probiotic; live organisms, Outcome 3 Mean stool frequency on day 2.
7.1. Analysis.
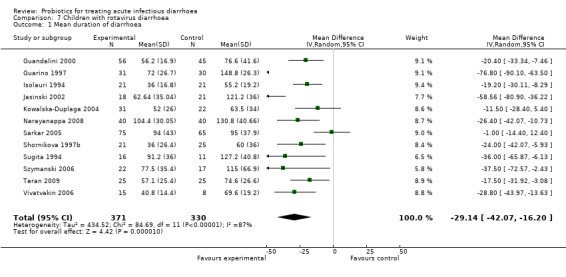
Comparison 7 Children with rotavirus diarrhoea, Outcome 1 Mean duration of diarrhoea.
7.2. Analysis.
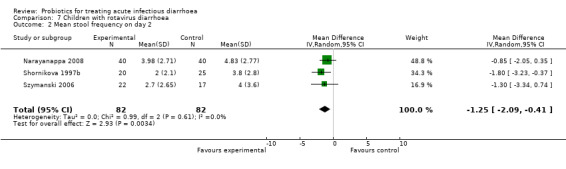
Comparison 7 Children with rotavirus diarrhoea, Outcome 2 Mean stool frequency on day 2.
8.1. Analysis.
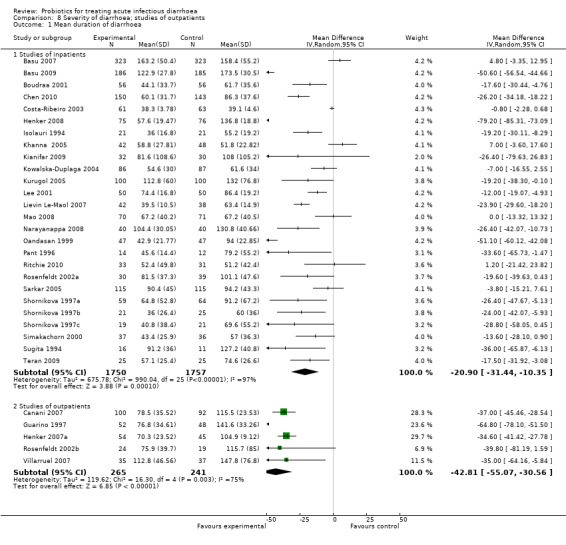
Comparison 8 Severity of diarrhoea; studies of outpatients, Outcome 1 Mean duration of diarrhoea.
9.1. Analysis.
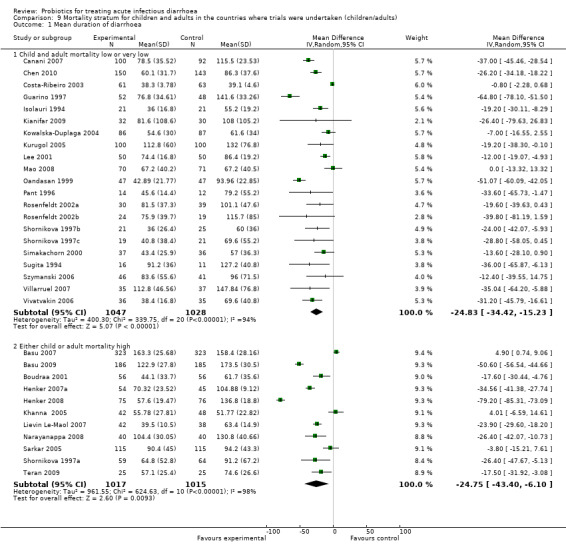
Comparison 9 Mortality stratum for children and adults in the countries where trials were undertaken (children/adults), Outcome 1 Mean duration of diarrhoea.
9.2. Analysis.
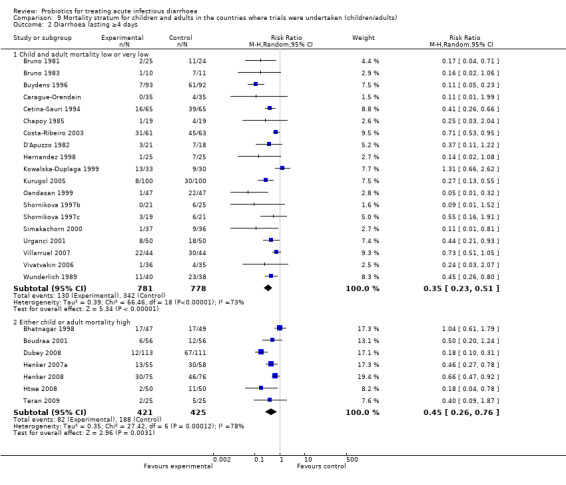
Comparison 9 Mortality stratum for children and adults in the countries where trials were undertaken (children/adults), Outcome 2 Diarrhoea lasting ≥4 days.
9.3. Analysis.
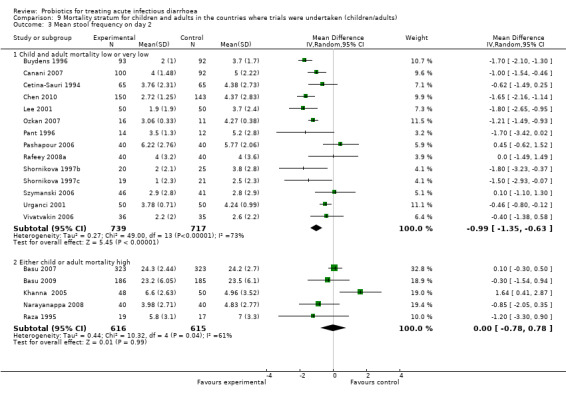
Comparison 9 Mortality stratum for children and adults in the countries where trials were undertaken (children/adults), Outcome 3 Mean stool frequency on day 2.
In three of the sub‐group analyses of trials that reported mean stool frequency on day 2, the magnitude of the effect in the intervention group was similar to that for all trials but there was greater consistency in the findings. This occurred in six trials (1335 participants) that assessed L. casei strain GG (Analysis 3.3), eight trials (861 participants) that used a high dose of live organisms (> 1010 organisms/day; Analysis 6.3) and three trials (164 participants) of children with rotavirus diarrhoea (Analysis 7.2). However, marked heterogeneity persisted in the corresponding sub‐group analyses that reported the other primary diarrhoea outcomes (Analysis 3.1 and Analysis 3.2; Analysis 6.1 and Analysis 6.2; Analysis 7.1 respectively). Therefore, the significance of the greater consistency in the sub‐group analyses reporting mean stool frequency on day 2 is unclear.
The sub‐group analysis according to diarrhoea severity suggested that probiotics resulted in a greater reduction in mean duration of diarrhoea in mild diarrhoea (studies of out‐patients) than in more severe diarrhoea (inpatients; Analysis 8.1). However, marked heterogeneity in findings persisted and, therefore, the significance of this finding is unclear.
Finally, probiotics appeared to be less effective in reducing mean stool frequency on day 2 in countries with high child and adult mortality rates compared with those with low or very low mortality rates (Analysis 9.3). However, marked heterogeneity persisted and probiotic effects were similar in both settings for the other diarrhoea outcomes (Analysis 9.1; Analysis 9.2).
On balance, we found no clear evidence that stratification according to the sub‐groups modified probiotic effect.
Several studies reported findings relevant to the subgroup analyses that could not be included in the analyses.
Probiotic organisms; strain, single organisms versus combinations and dose
Canani 2007 reported a statistically significantly reduced mean duration of diarrhoea for three different probiotics (live L. casei strain GG (Analysis 1.1), a combination of live Lactobacillus delbrueckii, L. acidophilus, Streptococcus thermophilus and Bacillus bifidum, and S. boulardii) compared with controls but there was no effect of live Enterococcus faecium SF68 or live Bacillus clausii strains O/C84, N/R84, T84, SIN84. These findings were generally supported by effectiveness in reducing stool frequency on d 2 and 3 reported in this study, except that the live L. casei strain GG did not reduce stool frequency on day 3 (Analysis 2.2) and S. boulardii did not reduce stool frequency on day 2.
Grandi 2009 allocated children with rotavirus diarrhoea to either an S. boulardii group or a group treated with a combination of four organisms (L. acidophilus, L. rhamnosus, B. longum andS. boulardii). The median duration of diarrhoea was shorter in both of the probiotic groups compared with the controls, but this was statistically significant only for S. boulardii (58 hours versus 84.5 hours, respectively; P = 0.04).
Three studies directly compared different doses of the same probiotic preparation in infants and children, most of whom had rotavirus diarrhoea. Mao 2008 evaluated two dose levels of a combination of Bifidobacterium lactis B12 and S. thermophilus TH4. Probiotics were administered in milk powder but the number of organisms administered in each group was not clear. The mean duration of diarrhoea and number of liquid stools/day were similar in the low dose and high dose groups.
Shornikova 1997b evaluated L. reuteri 107 CFU/day for up to 5 days. In 20 children in the low dose probiotic group, the mean (SD) duration of diarrhoea was 36.0 (26.4) hours, the mean stool frequency on day 2 was 2.0 (2.1), and diarrhoea lasting ≥ 4 days occurred in one (5.0%) child. These outcomes were not statistically significantly different from the control group. In contrast, both the mean duration of diarrhoea and the mean stool frequency on day 2 were statistically significantly improved in the high dose group (1010‐11 CFU/day; Analysis 1.1; Analysis 1.3).
Finally, on the basis of their previous study that did not show an effect of a low dose of L. rhamnosus GG on acute diarrhoea in a dose of 120 x 106 CFU/day (Basu 2007; Analysis 1.1; Analysis 1.3), these researchers evaluated two higher doses of this probiotic (2 x 1010 and 2 x 1012 CFU/day) in similar participants and a similar study setting (Basu 2009). In contrast to their earlier study, they reported that both higher doses had similar and statistically significant beneficial effects in acute diarrhoea (Analysis 1.1; Analysis 1.3).
Age of participants
It was not possible to assess the effects of probiotics in adults as < three studies reported the same diarrhoea outcomes. The primary analysis of mean duration of diarrhoea did not include studies undertaken in adults (Analysis 1.1). Removing studies of adults from the other primary analyses did not reduce heterogeneity (forest plots not shown). Overall, there was insufficient evidence regarding the efficacy of probiotics according to participants' age.
Children with rotavirus diarrhoea
In keeping with the findings for all children in their study, Simakachorn 2000 reported that fewer children with rotavirus diarrhoea in the probiotic than the control group had watery diarrhoea after 24 hours (3/19 versus 9/16; P = 0.012). Similarly, Boulloche 1994 reported that the resolution of diarrhoea in the probiotic group was similar for rotavirus positive and rotavirus negative participants.
Guandalini 2000 reported that mean stool frequency on day 3 of intervention was lower in the probiotic group (0.4, n = 56) than in the controls (2.0, n = 45; P < 0.05) and this was a greater reduction than that seen in all‐cause diarrhoea in this study. In contrast, Costa‐Ribeiro 2003 reported that there was no significant difference in the stool output or duration of diarrhoea between children allocated to probiotics versus placebo.
Bacterial diarrhoea
Only four trials reported outcomes for participants confirmed to have bacterial diarrhoea. Two studies assessed L. casei strain GG. Shornikova 1997a reported that the stool frequency was similar in the probiotic (n = 11) and placebo (n = 15) groups (P = 0.42). Guandalini 2000 reported that the mean (SD) duration of diarrhoea was similar in the probiotic and control groups (n = 35, 73.3 (29.3) versus n = 34, 72.0 (32.4) hours, respectively). The mean stool frequency on day 2 was also similar in the probiotic and control groups (5.0 and 5.5, respectively). Chen 2010 evaluated a combination of three organisms and reported that the mean (SD) duration of diarrhoea was not reduced significantly in children receiving probiotics (n = 27, 71.6 (32.8) hours) compared with controls (n = 30, 101.5 (46.8) hours; P = 0.082). In contrast, Htwe 2008 reported that in 21 children with pathogenic E. coli in stools, S. boulardii significantly improved stool consistency on d 3 (P = 0.004) and 4 (P = 0.025) compared with controls.
Adverse events
Of all 63 selected studies, 43 studies reported no adverse events and 20 gave no information on adverse events. Henker 2008 reported that one participant in the probiotic group had a mild hypersensitivity reaction that was assessed as being possibly related to the intervention. However, these authors commented that the probiotic was safe and well tolerated. With this exception, no authors reported an adverse effect that they considered to be attributable to the probiotic.
Many studies reported on vomiting. Boudraa 2001 reported a similar frequency of vomiting in the probiotic and control groups. Pant 1996 reported that 1/19 children in the control group vomited one dose of the medication, but no vomiting occurred in the 20 children in the probiotic group. Raza 1995 reported that the frequency of vomiting on the second day of intervention was statistically significantly less in children in the probiotic than the placebo group. Shornikova 1997c reported that fewer children in the probiotic than the placebo group vomited from the second day of treatment and this was statistically significant on day 2 and day 4. No child in the probiotic group vomited after the third day of treatment whereas vomiting persisted to the sixth day in 2/21 children in the placebo group. Kurugol 2005 reported that one child had meteorism but the group allocation was not stated.
Discussion
A striking finding of this review is that most trials reported that probiotics improved diarrhoea. A beneficial effect of probiotics was consistent across the different diarrhoea outcomes and was statistically significant in many trials.
With the exception of possible mild hypersensitivity to E. coli strain Nissle reported in one participant (Henker 2008), no authors reported adverse events that they attributed to probiotics. Vomiting is common in acute diarrhoea and was the most frequently reported adverse event. Vomiting occurred less frequently in the probiotic than the control groups and, therefore, would appear to be a symptom of the illness rather than an adverse effect of probiotics. The reasons for non‐compliance with protocol in some studies were not stated, but were unlikely to be related to the adverse events of probiotics since similar numbers of participants in the probiotic and control groups failed to comply. The causes of the withdrawal of participants from trials were related mostly to their primary illness rather than the interventions. Although this review supports the excellent safety record of probiotics, most of the studies recruited previously healthy people and more studies of susceptible individuals, for example, malnourished children and people with human immunodeficiency virus infection, are required to further evaluate safety.
The marked statistical heterogeneity between studies was expected given the marked clinical diversity in the definitions of diarrhoea and end of the diarrhoeal episode, the probiotic(s) tested, the treatment regimens, the diarrhoeal pathogens identified, the types of participants and the settings in which the trials were undertaken. Although these factors varied greatly among studies, individual studies used the same criteria and outcomes for both the probiotic and control groups. Although there was great variability in the methodological quality of the trials, there was no evidence that poor study design had led to an overestimate of the effects of probiotics.
Few studies reported outcomes for participants with bacterial diarrhoea and it was not possible to extract data for meta‐analysis from any of these studies. Many of the other studies that reported a beneficial effect of probiotics included a significant proportion of participants with bacterial diarrhoea or bloody stools, or both. Although this suggests that probiotics are efficacious, more research is needed to assess probiotics in bacterial diarrhoea.
The subgroup analyses did not explain between‐study statistical heterogeneity. Therefore, this review does not find important differences in probiotic effect according to probiotic strain, the number of different strains, the viability of the organisms, low versus high dose preparations, the causes or severity of diarrhoea or whether the studies were done in developed or developing countries. These findings are encouraging as effective interventions to prevent the progression from acute to persistent diarrhoea (> 14 days; closely associated with malnutrition in children in developing countries [Walker‐Smith 1993]), are a priority.
The persistence of statistical heterogeneity in subgroup analyses is perhaps not surprising given the marked clinical variability among studies. This was demonstrated clearly by the wide range of values for primary outcomes reported in participants allocated to the control groups. There is general consensus that effects of probiotics are strain‐specific and that results obtained with one probiotic cannot be extrapolated to other organisms, including closely related strains (Rijkers 2010). However, this review found that studies tested many different probiotics in many different settings yet nearly all reported beneficial outcomes. This suggests that a mechanism common to most probiotics, for example, colonization resistance, is effective against a wide range of gut pathogens. Probiotics are likely to have multiple mechanisms of action in the gut that may include effects on host immunity and gut mucosal barrier integrity as well as effects against diarrhoeal pathogens. Variations in several host and environmental factors that may determine the commensal gut flora may modify probiotic efficacy (Wolvers 2010). These include age, diet and eating practices, level of sanitation and exposure to antibiotics. It is likely that other factors, not considered in this review, underlie the marked among‐studies heterogeneity.
The marked clinical variability among studies complicates meta‐analysis and, therefore, weakens the evidence base to inform clinical practice. In particular, variability in the definition of diarrhoeal episodes results in misclassification and impairs the comparability of the findings from different studies (Baqui 1991). More large, well‐designed studies are needed of specific probiotic regimens in specific settings. In future research, the standardization of definitions of acute diarrhoea, treatment regimens, inclusion criteria and outcome measures are needed to facilitate comparison of results across studies. All studies should try to present data separately for important subgroups, for example, according to participant nutritional status and identified causes of diarrhoea, such as rotavirus or bacterial causes. Guidance on undertaking trials with probiotics, such as reliably identifying the agent used, testing the viability of organisms and confirming their quantity, is readily available (Rijkers 2010; Wolvers 2010). Since most episodes of acute diarrhoea are uncomplicated, self‐limiting, and require no specific treatment, cost‐effect analyses need to determine whether probiotics should be used in particular groups of people.
Authors' conclusions
Implications for practice.
Probiotics administered in addition to rehydration therapy resulted in clear reductions in the duration and severity of diarrhoea, and were not associated with adverse effects. This review supports the use of probiotics in acute, infectious diarrhoea. However, marked clinical variability between studies resulted in insufficient studies of specific probiotic regimens in defined groups of children or adults to inform the development of evidence‐based treatment guidelines.
Implications for research.
Although many different probiotics were effective in reducing diarrhoea, to better inform clinical practice studies of specific probiotic regimens in large numbers of participants with well‐defined diarrhoeal illness are needed. Trials need to use standardized definitions for acute diarrhoea and the resolution of the illness. They need to identify infectious causes of diarrhoea and present data separately for important participant subgroups, such as viral and bacterial causes of diarrhoea. All studies should include a reliable identification of the probiotic being tested, and confirm the viability and number of organisms for live probiotics. More research is needed to assess the role of probiotics in developing countries, especially in preventing the progression from acute to persistent diarrhoea and associated malnutrition.
Basic research is needed to identify generic and strain‐specific mechanisms underlying the apparent beneficial effects of probiotics in acute diarrhoea.
What's new
| Date | Event | Description |
|---|---|---|
| 9 November 2010 | Amended | Detailed search strategy added to appendices |
History
Protocol first published: Issue 2, 2001 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 11 August 2010 | New citation required but conclusions have not changed | Title changed ("acute" added) to emphasise that persistent diarrhoea is not considered. The authorship of the update has changed due to the untimely death of Dr Okoko. |
| 11 August 2010 | New search has been performed | The table showing clinical variability among studies has been removed and this information added to the Characteristics of included studies table. A table has been added to show the marked statistical heterogeneity in primary outcomes and subgroup analyses (Table 10). The following secondary outcomes have been removed as they were either uncommon or not reported: need for unscheduled intravenous (IV) rehydration after randomization; deaths; adverse events, such as vomiting; withdrawal from study. Details regarding adverse events and reasons for withdrawal are included in the "details of included studies" table. |
| 22 July 2008 | Amended | Converted to new review format. |
| 8 December 2007 | New citation required and conclusions have changed | Substantive amendment |
Notes
This review is a substantial update of the original version, first published in 2003 (Allen 2003).
Acknowledgements
Thanks to Dr Brown Okoko, Sam Parker and Stephanie Allen for help with data extraction.
The authors would like to dedicate this review to the memory of Dr Brown Okoko, an author on the previous version of this review, who died unexpectedly in 2008.
Appendices
Appendix 1. Detailed search strategy
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | Diarrhea* | DIARRHEA | DIARRHEA | INFECTIOUS DIARRHEA | Diarrhea$ |
| 2 | Diarrhoea* | Diarrhoea* | Diarrhoea* | Diarrhoea* | Diarrhoea$ |
| 3 | 1 or 2 | 1 or 2 | 1 or 2 | 1 or 2 | 1 or 2 |
| 4 | Probiotic* | Probiotic* | PROBIOTICS | PROBIOTIC AGENT | Probiotic$ |
| 5 | Lactobacill* | Lactobacill* | Lactobacill* | Lactobacill$ | Lactobacill$ |
| 6 | Lactococc* | Lactococc* | Lactococc* | Lactococc$ | Lactococc$ |
| 7 | Bifidobacter* | Bifidobacter* | Bifidobacter* | Bifidobacter$ | Bifidobacter$ |
| 8 | Enterococc* | Enterococc* | Enterococc* | Enterococc$ | Enterococc$ |
| 9 | Streptococc* | Streptococc* | Streptococc* | Streptococc$ | Streptococc$ |
| 10 | saccharomyces | saccharomyces | saccharomyces | saccharomyces | saccharomyces |
| 11 | 4 or 5 or 6 or 7 or 8 or 9 or 10 | 4 or 5 or 6 or 7 or 8 or 9 or 10 | 4 or 5 or 6 or 7 or 8 or 9 or 10 | 4 or 5 or 6 or 7 or 8 or 9 or 10 | 4 or 5 or 6 or 7 or 8 or 9 or 10 |
| 12 | 3 and 11 | 3 and 11 | 3 and 11 | 3 and 11 | 3 and 11 |
| 13 | Limit 12 to Humans | Limit 12 to Human |
Footnotes
a Cochrane Infectious Diseases Group Specialized Register
b Search terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins 2008); Upper case: MeSH or EMTREE heading; Lower case: free text term
Data and analyses
Comparison 1. Primary diarrhoea outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean duration of diarrhoea | 35 | 4555 | Mean Difference (IV, Random, 95% CI) | ‐24.76 [‐33.61, ‐15.91] |
| 2 Diarrhoea lasting ≥4 days | 29 | 2853 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.32, 0.53] |
| 3 Mean stool frequency on day 2 | 20 | 2751 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.14, ‐0.45] |
Comparison 2. Secondary diarrhoea outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diarrhoea lasting ≥3 days | 30 | 3022 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.56, 0.70] |
| 2 Mean stool frequency on day 3 | 14 | 2367 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.18, ‐0.07] |
Comparison 3. Strain of probiotic organisms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean duration of diarrhoea | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Live Lactobacillus casei strain GG | 11 | 2072 | Mean Difference (IV, Random, 95% CI) | ‐26.69 [‐40.50, ‐12.88] |
| 2 Diarrhoea lasting ≥4 days | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Live Lactobacillus casei strain GG | 4 | 572 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.40, 0.87] |
| 2.2 LIve Enterococcus LAB SF68 | 4 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.08, 0.52] |
| 2.3 Saccharomyces boulardii | 6 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.21, 0.65] |
| 3 Mean stool frequency on day 2 | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Live Lactobacillus casei strain GG | 6 | 1335 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.32, ‐0.20] |
Comparison 4. Single organism versus combinations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean duration of diarrhoea | 35 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Single organism | 22 | 3196 | Mean Difference (IV, Random, 95% CI) | ‐23.95 [‐35.57, ‐12.32] |
| 1.2 Two or more organisms | 13 | 1375 | Mean Difference (IV, Random, 95% CI) | ‐21.23 [‐30.38, ‐12.09] |
| 2 Diarrhoea lasting ≥4 days | 29 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Single organism | 22 | 2136 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.33, 0.60] |
| 2.2 Two or more organisms | 7 | 717 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.73] |
| 3 Mean stool frequency on day 2 | 20 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Single organism | 14 | 2040 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.21, ‐0.38] |
| 3.2 Two or more organisms | 6 | 711 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.53, 0.00] |
Comparison 5. Live versus killed organisms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean duration of diarrhoea | 32 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Live organisms | 29 | 3990 | Mean Difference (IV, Random, 95% CI) | ‐26.55 [‐36.95, ‐16.16] |
| 1.2 Killed organisms | 3 | 243 | Mean Difference (IV, Random, 95% CI) | ‐10.39 [‐30.75, 9.97] |
Comparison 6. Dose of probiotic; live organisms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean duration of diarrhoea | 26 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Low dose (≤10,000 million organisms/day) | 16 | 2683 | Mean Difference (IV, Random, 95% CI) | ‐25.88 [‐39.04, ‐12.72] |
| 1.2 High dose (>10,000 million organisms/day) | 10 | 980 | Mean Difference (IV, Random, 95% CI) | ‐27.02 [‐38.64, ‐15.39] |
| 2 Diarrhoea lasting ≥4 days | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Low dose (≤10,000 million organisms/day) | 13 | 1325 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.29, 0.62] |
| 2.2 High dose (>10,000 million organisms/day) | 4 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.12, 1.17] |
| 3 Mean stool frequency on day 2 | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Low dose (≤10,000 million organisms/day) | 7 | 1455 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.61, ‐0.41] |
| 3.2 High dose (>10,000 million organisms/day) | 8 | 861 | Mean Difference (IV, Random, 95% CI) | ‐0.99 [‐1.39, ‐0.60] |
Comparison 7. Children with rotavirus diarrhoea.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean duration of diarrhoea | 12 | 701 | Mean Difference (IV, Random, 95% CI) | ‐29.14 [‐42.07, ‐16.20] |
| 2 Mean stool frequency on day 2 | 3 | 164 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐2.09, ‐0.41] |
Comparison 8. Severity of diarrhoea; studies of outpatients.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean duration of diarrhoea | 31 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Studies of inpatients | 26 | 3507 | Mean Difference (IV, Random, 95% CI) | ‐20.90 [‐31.44, ‐10.35] |
| 1.2 Studies of outpatients | 5 | 506 | Mean Difference (IV, Random, 95% CI) | ‐42.81 [‐55.07, ‐30.56] |
Comparison 9. Mortality stratum for children and adults in the countries where trials were undertaken (children/adults).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean duration of diarrhoea | 32 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Child and adult mortality low or very low | 21 | 2075 | Mean Difference (IV, Random, 95% CI) | ‐24.83 [‐34.42, ‐15.23] |
| 1.2 Either child or adult mortality high | 11 | 2032 | Mean Difference (IV, Random, 95% CI) | ‐24.75 [‐43.40, ‐6.10] |
| 2 Diarrhoea lasting ≥4 days | 26 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Child and adult mortality low or very low | 19 | 1559 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.23, 0.51] |
| 2.2 Either child or adult mortality high | 7 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.26, 0.76] |
| 3 Mean stool frequency on day 2 | 19 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Child and adult mortality low or very low | 14 | 1456 | Mean Difference (IV, Random, 95% CI) | ‐0.99 [‐1.35, ‐0.63] |
| 3.2 Either child or adult mortality high | 5 | 1231 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.78, 0.78] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Basu 2007.
| Methods | Randomized controlled trial; 1 centre Duration: 1 year (January ‐December 2003) |
|
| Participants | Inclusion criteria: inpatients; infants and children with ≥ 3 watery stools/day without visible blood or mucus (duration not stated); < 10 white blood cells/high power field and no red cells, mucus flakes and bacteria on stool microscopy; negative hanging drop preparation; negative bacterial stool culture. Exclusion criteria: systemic illness other than diarrhoea on admission; systemic complication of diarrhoea during hospital stay; failure to give informed consent. Number completing study: 323/330 (97.9%) in the probiotic group (3 participants had electrolyte imbalance, 2 had septicaemia, 2 withdrew consent); 323/332 (97.3%) in control group (3 participants had electrolyte imbalance, 2 had septicaemia, 2 withdrew consent, 1 was discharged, 1 died). |
|
| Interventions |
Dehydration was corrected using oral rehydration fluid (ORF) following WHO guidelines |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: India (high child and adult mortality) Cause of diarrhoea: bacterial diarrhoea excluded. Rotavirus identified in 241 (74.6%) probiotic and 249 (77.1%) control group. Nutritional status: most participants malnourished: probiotic group; 198/323 moderately malnourished, 31/323 severely malnourished; control group; 185/323 moderately malnourished, 33/323 severely malnourished. Hydration status: all participants dehydrated: probiotic group: 48 mild, 173 moderate, 102 severe dehydration; control group: 51 mild, 168 moderate, 104 severe dehydration. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | computer randomization |
| Allocation concealment? | Low risk | concealed in envelopes |
| Blinding? All outcomes | Low risk | double blind |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow‐up ≥90% in both groups |
Basu 2009.
| Methods | Randomized controlled trial; 1 centre Duration: 1 year (period not stated) |
|
| Participants | Inclusion criteria: inpatients; infants and children with ≥3 watery stools/day, without macroscopic blood or mucus, white cells < 10 high power field and absent red blood cells, mucus flakes and bacteria on stool microscopy, negative hanging drop preparation and negative bacterial stool culture. Exclusion criteria: symptoms of illness other than diarrhoea; development of any systemic complication of diarrhoea during hospitalization; failure to give informed consent. Number completing the study: probiotic group: 186/196 (94.9%; withdrawals: 5 electrolyte imbalance, 3 septicaemia, 2 withdrew consent); placebo group: 185/196 (94.4%; withdrawals: 4 electrolyte imbalance, 3 septicaemia, 2 withdrew consent; 1 discharged on request; 1 died). |
|
| Interventions |
Interventions started after initial rehydration and stabilization. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: India (high child and adult mortality) Cause of diarrhoea: bacterial diarrhoea excluded. Rotavirus identified in 106 (57.0%) probiotic and 102 (55.1%) control group. Nutritional status: severe malnutrition in 17 (9.1%) probiotic and 12 (6.5%) control group; mild/moderate malnutrition in 102 (54.8%) probiotic and 100 (54.1%) control group. Hydration status: severe dehydration in 35 (18.8%) probiotic and 39 (21.1%) control group; mild/moderate dehydration in 121 (65.1%) probiotic and 122 (66.0%) control group. Source of funding not stated but no authors had a financial arrangement regarding this study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random numbers |
| Allocation concealment? | Low risk | Opaque, sealed envelopes |
| Blinding? All outcomes | Low risk | Interventions prepared by pharmacy; packets of similar appearance |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Bhatnagar 1998.
| Methods | Randomized controlled trial; 2 centres. Duration: 16 months |
|
| Participants | Inclusion criteria: inpatients; malnourished boys (weight for height < 80% NCHS median) with diarrhoea (≥ 5 liquid stools in preceding 24 hours) for ≤ 96 hours. Nearly all children were dehydrated (48/49 milk group and 43/47 yogurt group). Exclusion criteria: females; severe non‐gastrointestinal illness; gross blood in the stools; exclusive breast‐feeding. Number completing study: 47/49 (95.9%) in probiotic group (2 withdrawn because cholera in stool cultures); 49/53 (92.5%) in control group (2 withdrawn because cholera in stool cultures and 2 left against medical advice). |
|
| Interventions |
Given after 8 hours initial observation. All participants received rehydration fluids (IV if stool > 4 g/kg/hour), IV cephalosporin and gentamicin, and fed with rice lentil oil gruel. |
|
| Outcomes |
No comment regarding adverse events. |
|
| Notes | Study location: India (high child and adult mortality). Cause of diarrhoea: excluded if gross bloody stools. Nutritional status: all malnourished boys (weight for height < 80% NCHS median); mean weight for length and length for age (% NHCS median) similar in both groups. Hydration status: Nearly all children were dehydrated: 43/47 (91.5%) probiotic and 48/49 (98.0%) control group. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | randomisation list |
| Allocation concealment? | Unclear risk | not stated |
| Blinding? All outcomes | Unclear risk | probably open study |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow‐up ≥ 90% in both groups |
Billoo 2006.
| Methods | Randomized trial; probably open study; 1 centre Duration: not stated |
|
| Participants | Inclusion criteria: inpatients; infants and children with acute watery diarrhoea of mild to moderate severity Exclusion criteria: Severe intercurrent illness; severe diarrhoea and dehydration requiring admission and IV rehydration; temperature > 38.5°C; anti‐diarrhoeals or antibiotics in last 24 hours; severe malnutrition Number completing study: 50/50 (100%) in probiotic group; 50/50 (100%) in control group. |
|
| Interventions |
Timing of interventions not stated. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: Pakistan (high child and adult mortality) Cause of diarrhoea: Rotavirus identified in 8 (16.0%) probiotic and 10 (20.0%) control group. Bacterial diarrhoea identified in 13 (26.0%) probiotic and 6 (12.0%) control group. Nutritional status: severe malnutrition excluded; no further data presented Hydration status: severe dehydration excluded; no further data presented Source of funding: supported by Laboratoires Biocedex (France); Hilton Pharma (Pvt.) Ltd. Pakistan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomized controlled trial but methods not described |
| Allocation concealment? | Unclear risk | Methods not described |
| Blinding? All outcomes | High risk | No placebo; probably open study |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥90% in both groups |
Boudraa 2001.
| Methods | Randomized controlled trial; 1 centre Duration: not stated |
|
| Participants | Inclusion criteria: inpatients; well‐nourished children aged 3‐24 months with watery diarrhoea < 5 day duration and > 3 watery stools in previous 24 hours. All children were dehydrated, including some with severe dehydration. Exclusion criteria: exclusive breast feeding, history of allergy to cow's milk, severe malnutrition (weight or height < 70% or oedema) Number completing study: 49/56 (87.5%) in probiotic group (3 with urinary tract infection and 1 with bronchopneumonia withdrawn, others withdrawn by parents) and 48/56 (85.7%) in non‐probiotic group (2 with urinary tract infection, 1 with amebiasis withdrawn and 1 failed to attend for follow up, others withdrawn by parents). Reasons for withdrawal by parents not stated. Diarrhoea outcomes reported for all randomized children. |
|
| Interventions |
180 mL/kg/day of either fermented or non‐fermented infant formula given after initial oral rehydration. All infants also received other foods. |
|
| Outcomes |
Frequency of vomiting similar in both groups. No other comment regarding adverse events. |
|
| Notes | Study location: Algeria (high child and adult mortality). Cause of diarrhoea: rotavirus identified in 25/56 (44.6%) probiotic and 26/56 (46.4%) in control group. No bacterial pathogens isolated. Nutritional status: all well‐nourished Hydration status: all dehydrated; severe dehydration in 5 (8.9%) in the probiotic and 4 (7.1%) in the control group. Reduced duration of diarrhoea in the probiotic compared with non‐probiotic group observed only in children with reducing substances in stools. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | High risk | Stated as double blind but mothers able to distinguish fermented from non‐fermented infant formula |
| Incomplete outcome data addressed? All outcomes | High risk | Follow up < 90% in both groups |
Boulloche 1994.
| Methods | Randomized controlled trial; 1 centre. Duration: 3 years |
|
| Participants | Inclusion criteria: inpatients; young children with acute diarrhoea (definition not stated; 3/4 had diarrhoea < 3 days); weight loss of at least 5%. Exclusion criteria: any treatment that could have affected diarrhoea during hospitalization. Number completing study: 38/38 (100%) in probiotic group and 33/33 (100%) in control group. |
|
| Interventions |
Timing of start of administration not stated. All young infants were given Pregestimil, and older children were given an anti‐diarrhoeal diet. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: France (very low child and adult mortality). Cause of diarrhoea: 18% all participants had positive stool cultures and 49% positive virology tests (no further details given). Nutritional status: no data presented. Hydration status: all dehydrated with weight loss of at least 5%. Results presented for oral rehydration group only and all children. Resolution of diarrhoea in killed L. acidophilus group similar for rotavirus positive and negative participants. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number table stratified in groups of 18 |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Unclear risk | Not described |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Bruno 1981.
| Methods | Randomized controlled trial; 1 centre. Duration: not stated |
|
| Participants | Inclusion criteria: inpatients; adults with acute enteritis (diarrhoea, fever, vomiting, nausea, abdominal pain with or without toxicity; duration not stated). Exclusion criteria: typhoid cases. Number completing study: stool cultures available after randomization; participants with Salmonella typhi withdrawn (number not stated); for non‐typhoid participants, results presented for 25/25 (100%) in probiotic group and 24/24 (100%) in control group. |
|
| Interventions |
Timing of start of administration not stated. |
|
| Outcomes |
Resolution of diarrhoea defined as 2 or less formed stools/day and no abdominal pain or fever. No adverse events attributed to probiotic. |
|
| Notes | Study location: Italy (very low child and adult mortality). Cause of diarrhoea: non‐typhoid. Bacterial stool culture (probiotic group/placebo group): Salmonella 4/3; enteropathogenic E. coli 18/20; other enteropathogen 1/3. Nutritional status: no data presented. Hydration status: no data presented. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Bruno 1983.
| Methods | Randomized controlled trial; 1 centre. Duration: not stated |
|
| Participants | Inclusion criteria: inpatients; adults with acute febrile enteritis (duration of diarrhoea not stated). Exclusion criteria: typhoid cases. Number completing study: 10/10 (100%) in the probiotic group and 11/11 (100%) in the control group. |
|
| Interventions |
Intervention started after initial treatment with chloramphenicol (all participants) and after stool culture results available. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: Italy (very low child and adult mortality). Cause of diarrhoea: non‐typhoid. Nutritional status: no data presented. Hydration status: no data presented. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization list |
| Allocation concealment? | High risk | Not described |
| Blinding? All outcomes | High risk | Not described |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Buydens 1996.
| Methods | Randomized controlled trial; 2 centres. Duration: not stated |
|
| Participants | Inclusion criteria: inpatients and outpatients; adults with acute diarrhoea (>= 3 watery or loose stools in last 24 hours). Exclusion criteria: diarrhoea > 3 days; blood in faeces; faecal leukocytes; temperature > 39 °C; friable and haemorrhagic mucosa in rectosigmoid; history of chronic diarrhoea; polyps; colon cancer; Crohn's disease; ulcerative colitis; malabsorption; use of antidiarrhoeals or antibiotics in past 7 days; severe diarrhoea (dehydration with weight loss >10%); associated major diseases. Number completing study: 93/105 (88.6%) in probiotic group (4 violated protocol, 5 did not comply with study medications, 3 lost to follow up) and 92/106 (86.8%) in control group (5 violated protocol, 7 did not comply with study medications, 2 lost to follow up). |
|
| Interventions |
Started on day of presentation. |
|
| Outcomes |
Diarrhoea resolved when stool frequency < 3/day and semisolid or solid and no associated symptoms. No adverse events attributed to probiotic. |
|
| Notes | Study location: Belgium (very low child and adult mortality). Cause of diarrhoea: bloody diarrhoea excluded. Bacterial diarrhoea identified in 12 (11.4%) in the probiotic and 16 (15.1%) in the control group. Nutritional status: no data presented Hydration status: > 10% dehydration excluded; no further data presented. Highly significant reduction in duration of diarrhoea in the probiotic group confirmed by an intention‐to‐treat analysis, which included the excluded participants as non‐recovered on day 7 (but no data shown). Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization by central computer |
| Allocation concealment? | Low risk | Randomization by central computer |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | High risk | < 90% follow‐up in probiotic and placebo groups |
Canani 2007.
| Methods | Randomized controlled trial; 6 centres Duration: 12 months, October 1999 to September 2000 |
|
| Participants | Inclusion criteria: outpatients; infants and children aged 3 to 36 months with >2 loose or liquid stools/day for <48 hours. Exclusion criteria; malnutrition, severe dehydration; coexisting acute systemic illness (meningitis, sepsis, pneumonia), immunodeficiency; underlying severe chronic disease; cystic fibrosis; food allergy or other chronic GI diseases; use of probiotics in the previous 3 weeks; antibiotics or any other antidiarrhoeal medication in the previous 3 weeks; poor compliance (< 4 doses of the study medication administered). Number completing study: 95/100 in the probiotic group (2 did not receive the allocated intervention, 1 faster remission, 1 worsening symptoms, 1 poor compliance); 88/92 in the control group (1 did not receive the allocated intervention, 1 worsening symptoms, 1 contracted pneumonia, 1 had coeliac disease). |
|
| Interventions |
Intervention started within 48 hours of admission. ORF given for 3‐6 hours after admission, lactose‐containing formula milk or cow's milk according to age. |
|
| Outcomes |
1 patient with poor compliance in the probiotic group; 31 and 34 participants had vomiting in the probiotic and placebo groups, respectively. No adverse events attributed to probiotic. |
|
| Notes | Study location: Italy (very low child and adult mortality). Cause of diarrhoea: stool culture in only few participants; no data presented. Nutritional status: malnutrition excluded Hydration status: severe dehydration excluded; no other data presented. Source of funding: none Single blind trial. Parents instructed to buy probiotic preparation. This study also allocated children to 4 other probiotic groups: 1) S. boulardii It 5 × 109 live organisms daily (Codex) for 5 days; 2) Bacillus clausii O/C84, N/R84, T84, SIN84 (Enterogermina) 109 CFU bd for 5 days; 3) a combination of L. delbrueckii var bulgaricus LMG‐P17550 109 CFU daily, L. acidophilus LMG‐P 17549 109 CFU daily, S. thermophilus LMG‐P 17503 109 CFU daily, B. bifidum LMG‐P 17500 5 × 108 CFU daily (Lactogermina) for 5 d; 4) Enterococcus faecium SF 68 (Bioflorin) 7.5×107 CFU daily for 5 days and compared each of the probiotic groups with the single control group. Mean duration of diarrhoea and mean stool frequency on day 2 and 3 were significantly shorter than in the control group for intervention groups 1 and 3. These outcomes were similar to the control group for the other probiotic groups. To avoid a unit‐of‐analysis error as a result of the multiple comparisons between the intervention groups and the single control group, we elected to include data for the L. GG group only in this review. We selected L. GG because this was the probiotic most frequently evaluated in acute infectious diarrhoea and we wished to maximize the body of evidence. We rejected the alternative approach of pooling the data from all of the different probiotic intervention groups into a single group because this would not be helpful in selecting a specific probiotic intervention for use in clinical practice. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomization list allocation in blocks of 6 |
| Allocation concealment? | Low risk | Concealed until treatment assigned |
| Blinding? All outcomes | Low risk | Blinded third‐party blind assessor |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Carague‐Orendain.
| Methods | Randomized controlled trial; 1 centre Duration: not stated |
|
| Participants | Inclusion criteria: inpatients and outpatients; children with non‐bloody diarrhoea (not defined) of less than 5 days duration. Exclusion criteria: antimicrobials in the last 72 hours; concomitant illness; severe malnutrition; antidiarrhoeal drugs; immunocompromised. Participants completing study: 35/35 (100%) in probiotic group and 35/35 (100%) in control group. |
|
| Interventions |
No details of when interventions started. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Unpublished data. Study location: Philippines (low child and adult mortality). Cause of diarrhoea: bloody diarrhoea excluded. Nutritional status: severe malnutrition excluded; no other data presented. Hydration status: overall, 42 children had some dehydration (none severe) and 28 had no dehydration Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Unclear risk | Unclear whether placebo identical to probiotic |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Cetina‐Sauri 1994.
| Methods | Randomized controlled trial; 1 centre Duration: 11 months, 1April 1988 to 15 March 1989 |
|
| Participants | Inclusion criteria: unclear whether inpatients or outpatients, or both; children aged 3 months to 3 years with acute (duration not stated) non‐bloody diarrhoea; no dehydration; no concomitant illness; no antibiotics or drugs affecting gut motility. Number completing study: unclear how many participants randomized; participants who deteriorated, developed concomitant illness, and needed other drugs, or who wished to withdraw were excluded from the analysis (details not given). |
|
| Interventions |
No details of when interventions started. |
|
| Outcomes |
Cure defined as < 4 stools in 24 hours and absence of liquid stools. No adverse events attributed to probiotic. |
|
| Notes | Study location: Mexico (low child and adult mortality). Cause of diarrhoea: bloody diarrhoea excluded Nutritional status: all well nourished. Hydration status: dehydration excluded. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random table |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Unclear risk | Unclear whether placebo was identical to the probiotic |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Unclear how many participants were randomized at beginning of study |
Chapoy 1985.
| Methods | Intervention study; 1 centre Duration: not stated |
|
| Participants | Inclusion criteria: inpatients; infants and children with sudden, recent onset of watery diarrhoea (not defined) of variable importance with or without fever and vomiting. Exclusion criteria: dehydration >10% needing IV rehydration; bloody or purulent stools; fever >39°C; associated pathology. Number completing study: 19/19 (100%) probiotic group and 19/19 (100%) control group. |
|
| Interventions |
When the probiotic was administered was not stated. |
|
| Outcomes | Mean number of stools, mean stool weight and carmine red transit time on days 1 and 4. Stool consistency on day 4. Stool frequency on day 4 was lower in the probiotic than the control group (n = 19; mean 2.1 [SD 0.9] versus n = 19; 3.4 [1.9] respectively). The reduction in stool frequency from baseline was statistically significantly greater in the probiotic than control group (P < 0.01). No adverse events attributed to probiotic. |
|
| Notes | Location: France (very low child and adult mortality) Cause of diarrhoea: bloody or purulent stools excluded; pathogenic bacteria isolated from 9 children in the probiotic and 6 in the control group. Nutritional status: no data presented. Hydration status: dehydration > 10% needing IV rehydration excluded; Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Infants allocated alternately to the two groups as enrolled in trial |
| Allocation concealment? | High risk | Alternate allocation |
| Blinding? All outcomes | High risk | No placebo; open study |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Chen 2010.
| Methods | Randomized controlled trial; 1 centre Duration: 22 months; February 2006 to November 2007 |
|
| Participants | Inclusion criteria: inpatients; children aged 3 months to 6 years with acute diarrhoea defined as 3 or more loose or liquid stools per day of less than 72 hours duration. Exclusion criteria: immunodeficiency, severe abdominal distension with risk of bowel perforation, severe infection or sepsis, history with gastrointestinal tract surgery, probiotics use in the preceding 1 week. Number completing study: 304 children enrolled and 293 were included in the analysis (150 in the probiotic and 143 in the control group). Overall, 7 children discontinued medication and 4 were lost to follow up; group allocation unclear. |
|
| Interventions |
When interventions started not stated. |
|
| Outcomes |
Duration of diarrhoea also reported for children with rotavirus diarrhoea and those with bacterial diarrhoea No adverse events attributed to probiotic. |
|
| Notes | Study location: Taiwan (low child and adult mortality). Cause of diarrhoea: 47 (31.3%) of children in probiotic and 44 (30.8%) in control group had rotavirus in stools. Norovirus and adenovirus also identified. 27 (18.0%) children in probiotic and 30 (20.0%) in the control group had bacteria in stools (either Salmonella enterica or Campylobacter jejuni). Nutritional status: no data presented Hydration status: no data presented Source of funding: The study was supported in part by a grant from Chang Gung Memorial Hospital research project grant XMRPG440021, Northern Taiwan. First author was contacted and asked to clarify
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Costa‐Ribeiro 2003.
| Methods | Randomized controlled trial; 1 centre Duration: not stated |
|
| Participants | Inclusion criteria: inpatients; boys, age 1 to 24 months with acute diarrhoea (3 or more watery or loose stools per 24 hours during at least one 24 hour period in the 72 hours before admission) with moderate dehydration or severe dehydration after correction by rapid IV fluids. Exclusion criteria: systemic infections requiring antibiotics, severe malnutrition (weight for age < 65% of NCHS standards), bloody diarrhoea. Number completing study: 61/61 (100%) in the probiotic group and 63/63 (100%) in the control group. |
|
| Interventions |
Interventions started after correction of severe dehydration if required |
|
| Outcomes |
No comment regarding adverse events. |
|
| Notes | Study location: Brazil (low child and adult mortality). Cause of diarrhoea: bloody diarrhoea excluded; 52% of children in the probiotic and 48% in the control group had rotavirus in stools; no data shown for outcomes in rotavirus diarrhoea although stated as "no significant difference" between groups. Nutritional status: severe malnutrition excluded; median WHZ score ‐1.13 (IQR −1.63 to −0.43) in control and ‐1.22 (−1.87 to −0.62) in probiotic group. Hydration status: all dehydrated; moderate or severe dehydration in 92% in the probiotic and 94% in the control group. Source of funding: the study was supported in part by a grant from Pronex/CNPq (661086/1998‐4), Brazil. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization code |
| Allocation concealment? | High risk | Sequential administration |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Czerwionka 2009.
| Methods | Randomized controlled trial; 1 centre Duration: not stated |
|
| Participants | Inclusion criteria: inpatients; infants and children with acute infectious diarrhoea who had failed oral rehydration. Exclusion criteria: bloody stools; coexisting disease that might influence the course of diarrhoea. Number completing study: 50/50 (100%) in the probiotic group and 50/50 (100%) in the control group. |
|
| Interventions |
Interventions started after rapid IV rehydration |
|
| Outcomes |
No specific comment regarding adverse events. |
|
| Notes | Study location: Poland (low child, low adult mortality). Cause of diarrhoea: bloody diarrhoea excluded; 28/50 in the probiotic and 30/50 in the control group had rotavirus diarrhoea. Nutritional status: no data presented Hydration status: no data presented Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not stated |
| Allocation concealment? | Unclear risk | Not stated |
| Blinding? All outcomes | Unclear risk | Not stated |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
D'Apuzzo 1982.
| Methods | Randomized controlled trial; unclear whether single or multi‐centre. Duration: not stated |
|
| Participants | Inclusion criteria: unclear whether inpatients or outpatients, or both; children with acute enteritis (duration and definition not given). Exclusion criteria: none stated. Number completing study: 21/21 (100%) in the probiotic group and 18/18 (100%) in the control group. |
|
| Interventions |
When interventions started not stated. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: Switzerland (very low child and adult mortality). Cause of diarrhoea: 7 participants in each group had positive stool cultures for bacteria. Nutritional status: no data presented Hydration status: no data presented S. faecium 68 also appeared to promote recovery from abdominal pains, fever, and vomiting. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Unclear risk | Unclear whether placebo identical to probiotic |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Dubey 2008.
| Methods | Randomized controlled trial; 1 centre Duration: February 2005 to February 2007 |
|
| Participants | Inclusion criteria: inpatients; infants and children with watery diarrhoea (defined as watery stools) <72 hours duration due to rotavirus infection, parental consent. Exclusion criteria: systemic infection, chronic disease, body weight <60% NCHS standard, vomiting, need for antibiotics. Number completing study: 113/113 (100%) in the probiotic group and 111/111 (100%) in the control group. Six children did not complete the study; no group allocation or reasons given. |
|
| Interventions |
When interventions started not stated. |
|
| Outcomes | Number stools/day; duration diarrhoea; IV fluid requirement; ORF requirement. No adverse effects attributed to probiotic. |
|
| Notes | Study location: India (high child and high adult mortality) Cause of diarrhoea: all rotavirus Nutritional status: severe malnutrition excluded; statement that "malnutrition status similar in two groups" Hydration status: dehydration status similar in two groups at baseline but no data presented. Source of funding: supported by grant from VSL |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | identical sachets |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Frigerio 1986.
| Methods | Randomized controlled trial; 150 hospitals Duration not stated |
|
| Participants | Inclusion criteria: acute diarrhoeal disorder; diarrhoea defined as ≥ 3 not formed stools/day; duration not stated Exclusion criteria: not stated Number participants recruited at baseline not reported. 534 patients in the placebo group and 540 in the probiotic group completed the study. |
|
| Interventions |
When interventions started not stated. |
|
| Outcomes | Duration of diarrhoea (only statistical analysis reported; no raw data) No adverse effects attributed to probiotic. |
|
| Notes | Study location: Italy (very low child and adult mortality) Cause of diarrhoea: no data presented Nutritional status: no data presented Hydration status: no data presented Source of funding: not stated Probiotic also evaluated in antibiotic‐associated diarrhoea |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random allocation; no details reported |
| Allocation concealment? | Unclear risk | No details reported |
| Blinding? All outcomes | Unclear risk | No details regarding placebo reported. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Number participants recruited not reported |
Grandi 2009.
| Methods | Randomized controlled trial; 1 centre Duration not stated |
|
| Participants | Inclusion criteria: inpatients; children with acute rotavirus diarrhoea Exclusion criteria: not stated Number completing study: overall, 64/70 (91.4%) completed study. Number in each intervention group not stated. |
|
| Interventions |
When interventions started not stated. |
|
| Outcomes |
No comment regarding adverse events. |
|
| Notes | Study location: Chile (low child and adult mortality) Cause of diarrhoea: all rotavirus Nutritional status: no data presented Hydration status: no data presented Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Unclear risk | Not described |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Number children in each intervention group not stated |
Guandalini 2000.
| Methods | Randomized controlled trial; multi‐centre Duration: 1 year, 1996 |
|
| Participants | Inclusion criteria: inpatients and outpatients; infants and children with > 4 liquid or semi‐liquid stools/day for 1 to 5 days. Exclusion criteria: previous probiotic usage; underlying chronic untreated small bowel disease; inflammatory bowel disease; any underlying chronic disease or immunosuppressive disease or treatment. Number completing study: 287 forms (269 participants) of total of 323 forms (88.9%) received at the coordinating centre were analysed (36 incomplete data or not compliant with protocol); unclear whether withdrawals occurred at participating centres. |
|
| Interventions |
Interventions added to ORF and started at recruitment. |
|
| Outcomes |
Some outcomes also reported for rotavirus, bacterial, and no organism‐isolated subgroups. No comment regarding adverse events. |
|
| Notes | Study locations: Poland (low child and adult mortality), Egypt (high child and high adult mortality), Croatia, Italy, Slovenia, The Netherlands, Greece, Israel, United Kingdom, Portugal (all very low child and very low adult mortality). Cause of diarrhoea: rotavirus (56 probiotic/45 placebo); bacteria (35/34); parasites (7/6); no pathogen (45/54). 10 (6.8) probiotic and 15 (10.7) control group had bloody diarrhoea. Nutritional status: no data presented. Hydration status: severe dehydration in 1 (0.7) probiotic and 1 (0.7) control group; mild/moderate dehydration in 107 (72.7%) probiotic and 96 (68.2%) control group. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Low risk | Code broken at end of study |
| Blinding? All outcomes | Unclear risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Unclear whether withdrawals occurred at participating centres; also 36/323 (11.2%) participant data forms received at the co‐ordinating centre were not analysed as incomplete and/or not compliant with protocol. |
Guarino 1997.
| Methods | Randomized controlled trial; 1 centre Duration: 3 months, November 1995 to January 1996 |
|
| Participants | Inclusion criteria: consecutive outpatients attending 3 family physicians; infants and children with ≥ 3 watery stools/day of < 48 hours duration. Exclusion criteria: antibiotic treatment in preceding 3 weeks, breastfeeding, and weight:height ratio < 5th percentile. Number completing study: 52/52 (100%) in probiotic group and 48/48 (100%) in control group. |
|
| Interventions |
Interventions started after 6 hours of ORF. |
|
| Outcomes |
Results for rotavirus subgroup also presented. No comment regarding adverse events. |
|
| Notes | Study location: Italy (very low child and adult mortality). Cause of diarrhoea: Rotavirus identified in 30 (57.7%) probiotic and 31 (64.6%) control group. Nutritional status: weight:height ratio < 5th percentile excluded. Hydration status: all had mild to moderate dehydration. The study author clarified that Figure 1 in the published article reports the mean and standard error for the duration of diarrhoea; SDs derived from graph. We also extracted data from Canani 1997 (abstract), which also reports standard errors. Probiotic also reduced prevalence of rotavirus in stools on day 6. Source of funding: Ministero della Sanità, AIDS Project (9205.30) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number table |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | High risk | Open study |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Hafeez 2002.
| Methods | Randomized controlled trial ‐ randomization according to odd and even participant numbers; three centres Duration: 2 months |
|
| Participants | Inclusion criteria: outpatients; children aged 6 months to 5 years with acute watery diarrhoea of mild or moderate severity (not defined), suitable for ambulatory treatment. Exclusion criteria: anti‐diarrhoeals or antibiotics before admission, grade III malnutrition, bloody diarrhoea, needed IV rehydration, diarrhoea for >14 days. Number completing study: 51/54 (94%) probiotic group and 50/54 (93%) control group. |
|
| Interventions |
Unclear whether researchers and participants able to distinguish between interventions. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: Pakistan (high child and adult mortality). Cause of diarrhoea: bloody diarrhoea excluded; stool analysis not done. Nutritional status: grade III malnutrition excluded Hydration status: participants who needed IV rehydration excluded.. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Alternate allocation |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Unclear risk | Probably open study; no placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Henker 2007a.
| Methods | Randomized controlled trial; 11 centres Duration: 3 months, February to April 2005 |
|
| Participants | Inclusion criteria: outpatients; infants and toddlers < 4 years with > 3 watery or loose and non‐bloody stools /day for ≤ 3 days. Exclusion criteria: > 5% dehydration; intake of E. coli Nissle 1917 in last 3 months; intake of food supplements or drugs which contain living microorganisms or their metabolic products or components within 7 days prior to enrolment or during the trial; other antidiarrhoeal drugs; breast‐feeding, premature birth; severe or chronic disease of the bowel or severe concomitant diseases. Antibiotics stated as exclusion criteria but some children included. Number completing study: 54/55 (98.2%) probiotic group and 45/58 (93.8%) control group. Reason for withdrawals in both groups stated as intervention no longer suitable or required other treatment. |
|
| Interventions |
|
|
| Outcomes |
Diarrhea resolution: reduction in stool frequency to < 3 watery or loose stools in 24 hours over a period of at least 2 consecutive days. Adverse effects: 1 had rhinitis and 1 had abdominal cramps in the probiotic group. 2 had acute otitis media in the placebo group. 1 participant with poor compliance in the placebo group. No adverse events attributed to probiotic. |
|
| Notes | Study location: Ukraine, Russia (low child, high adult mortality) Cause of diarrhoea: bloody diarrhoea excluded; 16/55 (29.1%) probiotic and 19/58 (32.8%) control group had viral diarrhoea. Bacterial pathogens isolated from 9/55 (16.4%) probiotic and 4/58 (6.8%) control group. Nutritional status: most children well nourished. Hydration status: > 5% dehydration excluded; 0/55 probiotic and 1/58 control children had mild dehydration. Better outcomes in probiotic than placebo for abdominal pain (28/30 vs. 24/33) and abdominal cramps (17/18 vs. 21/26). Parents reported slightly better tolerance of probiotic than placebo, although investigators noted no difference. Authors supplied data regarding SDs for diarrhoea duration. Source of funding: ARDEYPHARM provided verum and placebo medications and reimbursed study‐related expenses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomly permuted blocks of 4 |
| Allocation concealment? | Low risk | Sequence concealed from parents and researchers |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Henker 2008.
| Methods | Randomized controlled trial; 11 centres Duration: 3 months, February to April 2005 |
|
| Participants | Inclusion criteria: inpatients; > 3 loose or watery stools without blood / 24 hours for > 4 days and < 14 days; moderate dehydration (5‐10% loss of body weight). Exclusion criteria: other severe organic or infectious disease; participation in another trial; intake of trial preparation in the past 3 months; intake of probiotic preparations within the past 7 days; antibiotics or antidiarrhoeals; severe dehydration (>10% weight loss); weight <5th percentile; growth faltering; breast‐feeding; preterm birth. Number completing study: 72/75 (96.0%) probiotic group (trial intervention no longer suitable/different treatment needed ‐ 2; personal reasons ‐ 1); 59/76 (77.6%) control group (trial intervention no longer suitable/different treatment needed ‐ 11; personal reasons ‐ 5f; intolerable adverse event ‐ 1). |
|
| Interventions |
|
|
| Outcomes |
1 participant in the probiotic group had a mild hypersensitivity reaction which was assessed as possibly related to the intervention. In the control group, 1 participant had vomiting, 1 abdominal pain, 1 dermatitis and 1 withdrawn because of influenza. Authors commented that the probiotic was safe and well tolerated. |
|
| Notes | Study location: Ukraine, Russia (low child, high adult mortality) Cause of diarrhoea: bloody diarrhoea excluded; 12 (16.0) probiotic and 15 (21.1) control group had viral diarrhoea. Bacterial pathogens isolated from 15 (20.0) probiotic and 19 (25.0) control group. Nutritional status: weight < 5th percentile and growth faltering excluded; 2 (2.7) probiotic and 3 (3.9) controls had mild/moderate malnutrition. Hydration status: all had moderate dehydration (5‐10% loss of body weight). Fewer children with dehydration at the end of the study in the probiotic than the placebo group. General state of health improved to a greater extent in the probiotic than the placebo group. Significantly fewer children with diarrhoea > 21 days in the probiotic than the placebo group. At the end of the study the rates of mucus in stool, abdominal cramps, and abdominal pain were all lower in the probiotic group. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomly permuted blocks of 4 |
| Allocation concealment? | Low risk | Study personnel and participants blinded to treatment assignment for the duration of the study |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | High risk | Follow up < 90% in placebo group |
Hernandez 1998.
| Methods | Randomized controlled trial; 1 centre Duration: not stated |
|
| Participants | Inclusion criteria: inpatients; uncomplicated acute diarrhoea (not defined) and mild dehydration. Exclusion criteria: fever; malnutrition; bloody stools. Number completing study: 25/25 (100%) probiotic group; 25/25 (100%) control group. |
|
| Interventions |
|
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: Mexico (low child and adult mortality) Cause of diarrhoea: bloody diarrhoea excluded Nutritional status: malnutrition (not defined) excluded. Hydration status: all had mild dehydration. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | High risk | Not described |
| Blinding? All outcomes | Unclear risk | Not described |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Hochter 1990.
| Methods | Randomized controlled trial; multi‐centre Duration: not stated |
|
| Participants | Inclusion criteria: outpatients attending general practitioners, gastroenterologists, and internal physicians; adults with acute diarrhoea (> 3 liquid stools in last 24 hours; in great majority duration 2 days or less; 1 participant in the placebo group had diarrhoea for >10 days). Exclusion criteria: chronic diarrhoea; blood in stools; drug‐induced diarrhoea; antimicrobial treatment; inflammatory bowel disease. Number completing study: 92/107 (86.0%) randomized participants completed study (1 took additional drugs, 14 < 3 liquid stools at presentation). 3 participants dropped out (2 probiotic, 1 placebo) because intervention not effective; results included in analysis. |
|
| Interventions |
Interventions started at presentation. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: Germany (very low child and adult mortality). Cause of diarrhoea: Stool analyses in first 50 participants only: 2 had rotavirus and 3 Salmonella Nutritional status: all well nourished. Hydration status: no data presented. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | identical placebo |
| Incomplete outcome data addressed? All outcomes | High risk | < 90% in probiotic and placebo groups |
Htwe 2008.
| Methods | Randomized controlled trial ‐ participants alternately assigned to the probiotic or control group on hospital admission; 1 centre Duration: not stated |
|
| Participants | Inclusion criteria: inpatients; infants and children aged 3 months to 10 years; acute watery diarrhoea of duration < 7 days. Exclusion criteria: fever > 38°C; severely dehydrated; macroscopic blood in the stools; intake of antifungals; existing severe malnutrition. Number completing the study: 50 (100%) probiotic group, 50 (100%) control group. |
|
| Interventions |
Interventions started on admission. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: Myanmar (high child and high adult mortality) Cause of diarrhoea: bloody diarrhoea excluded Nutritional status: severe malnutrition excluded, no other data presented Hydration status: severe dehydration excluded, no other data presented SDs for the duration of diarrhoea were not reported. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Alternate allocation |
| Allocation concealment? | High risk | Alternate allocation |
| Blinding? All outcomes | High risk | Probably open study; no placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Isolauri 1994.
| Methods | Randomized controlled trial; 1 centre Duration: not stated |
|
| Participants | Inclusion criteria: inpatients; infants and children with > 3 watery stools/day for < 7 days and stools positive for rotavirus. Average dehydration about5% in both groups. Exclusion criteria: not stated. Number completing study: 21/21 (100%) in probiotic group and 21/21 (100%) in control group. |
|
| Interventions |
Interventions started after 6 hours ORF. |
|
| Outcomes |
No comment regarding adverse events. |
|
| Notes | Study location: Finland (very low child and adult mortality). Cause of diarrhoea: all rotavirus diarrhoea. Nutritional status: all well nourished Hydration status: mean dehydration about 5% in both groups. Source of funding: Academy of Finland and the Foundation for Nutrition Research (Finland). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not defined |
| Allocation concealment? | Unclear risk | Not defined |
| Blinding? All outcomes | High risk | Open study; no placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Jasinski 2002.
| Methods | Randomized controlled trial; 12 centres Duration: not stated |
|
| Participants | Inclusion criteria: inpatients and outpatients; age 1month to 3 years; acute diarrhoea (3 or more liquid stools in 12 hours or single liquid or semi‐solid stool with mucus or blood, or both, for 5 days or less). Exclusion criteria: antibiotics or probiotics in last 5 days; chronic diseases of small or large intestine (eg coeliac, cow milk protein allergy, inflammatory bowel disease), immunosuppression, phenylketonuria Number completing study: 45/45 (100%) probiotic and 52/52 (100%) placebo |
|
| Interventions |
Start time for administration unclear. |
|
| Outcomes |
No comment regarding adverse events. |
|
| Notes | Study location: Europe, Egypt, Africa, and single site (Montevideo) in S. America (variable child and adult mortality) Cause of diarrhoea: bacterial pathogens: probiotic group 29 (64.4%) and placebo group 37 (71.2%); rotavirus: probiotic group 18 (40.0%) and placebo group 21 (40.4%); parasites: probiotic group 2 (4.4%) and placebo group 4 (7.7%); no pathogens identified: probiotic group 11 (24.4%) and placebo group 14 (26.9%). Nutritional status: 15 (33.3%) in the probiotic and 20 (38.5%) in the control group had at least some malnutrition. Hydration status: mild/moderate dehydration in 15 (33.3%) probiotic and 17 (32.7%) control group. Severer dehydration in 0 in the probiotic and 2 (3.8%) control group. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Alternate allocation |
| Allocation concealment? | High risk | Alternate allocation |
| Blinding? All outcomes | Unclear risk | Not described |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Khanna 2005.
| Methods | Randomized controlled trial; 1 centre Duration: 19 months, April 2001 to September 2002 |
|
| Participants | Inclusion criteria: inpatients; aged 6 months to 12 years with acute diarrhoea (not defined) Exclusion criteria: systemic infection; encephalopathy; convulsions; use of pharmaceutical probiotics Number completing study: 1/49 (2.0%) in the probiotic group and 3/53 (5.7%) controls left before the completion of the study. |
|
| Interventions |
Interventions started on admission. All children received ORF, feeding and IV fluids if needed |
|
| Outcomes |
No comment regarding adverse events. |
|
| Notes | Study location: India (high child and adult mortality) Cause of diarrhoea: overall, 14/22 (63.6%) children tested were rotavirus positive and 8/98 (8.2%) has a positive culture for cholera. Nutritional status: most children were stunted and some had wasting. Hydration status: 19 in (39.6%) in the probiotic and 15 (30.0%) in the control group had severe dehydration. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as "simple randomisation done by a non‐departmental colleague" |
| Allocation concealment? | Low risk | Investigators blinded to group allocation |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow‐up > 90% in both groups |
Kianifar 2009.
| Methods | Randomized controlled trial; 1 centre Duration: 18 months, April 2006 to September 2007 |
|
| Participants | Inclusion criteria: inpatients; infants and children aged 6 to 36 months with acute non‐bloody, non‐bacterial diarrhoea (not defined) of less than 2 days' duration and moderate dehydration Exclusion criteria: severe dehydration, antibiotic consumption, severe vomiting, convulsion, inflammatory cells in stool samples Number completing study: 32/34 (94.1%) probiotic and 30/34 (88.2%) placebo; participants excluded because of poor compliance. |
|
| Interventions |
Start time for administration not stated. All children received IV fluid therapy, oral rehydration solution, and mother’s milk in breast‐feeding infants, or complementary food according to the patient's age. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: Iran (low child and adult mortality) Cause of diarrhoea: non‐bloody, non‐bacterial diarrhoea (not defined) Nutritional status: not stated. Hydration status: all had moderate dehydration; severe dehydration excluded. Source of funding: grant from the Vice Chancellery for Research, Mashad University of Medical Sciences. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random number table sequence |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | placebo sachets matched for size, shape, and volume of contents; physicians, nurses and parents were blinded to the treatment protocol. |
| Incomplete outcome data addressed? All outcomes | High risk | Follow up < 90% in placebo group |
Kowalska‐Duplaga 1999.
| Methods | Randomized controlled trial; 1 centre Duration: not stated |
|
| Participants | Inclusion criteria: unclear whether inpatients or outpatients, or both; age < 24 months with acute rotavirus diarrhoea (> 3 loose or watery stools/24 hours lasting < 48 hours prior to inclusion). Exclusion criteria: not stated. Number completing study: 33/33 (100%) in probiotic group and 30/30 (100%) in control group. |
|
| Interventions |
Timing of administration not stated. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: Poland (low child and adult mortality). Cause of diarrhoea: all rotavirus diarrhoea. Nutritional status: no data presented. Hydration status: dehydration status similar in both group; no other data presented. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Kowalska‐Duplaga 2004.
| Methods | Randomized controlled trial; 3 centres Duration: not stated |
|
| Participants | Inclusion criteria: inpatients; infants and children with 3 or more loose stools within 24h period of < 72 hours duration Exclusion criteria: history of acute diarrhoea within 14 days preceding the inclusion in the study; antibiotic treatment; received probiotic up to 7 days before the participation in the study; exclusively breast fed; chronic alimentary disease; diagnosis of malabsorption; lack of parental consent; lack of diarrhoea. Number completing study: 86/87 (98.9%) probiotic group and 87/89 (97.8%) placebo group. |
|
| Interventions |
Interventions administered from recruitment. |
|
| Outcomes |
Mean duration of diarrhoea reported for children with rotavirus diarrhoea. No adverse effects attributed to probiotic. |
|
| Notes | Study location: Poland (low child and adult mortality) Cause of diarrhoea: rotavirus identified in 31 (37.3%) probiotic and 22 (26.8%) placebo group. Bacterial pathogens identified in 6 (7.2%) probiotic and 14 (17%) placebo group. Nutritional status: no data presented. Hydration status: no data presented. Source of funding: interventions provided by Allergon, Sweden |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Allocated according to order of presentation |
| Allocation concealment? | High risk | Allocated according to order of presentation |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Kurugol 2005.
| Methods | Randomized controlled trial; 1 centre Duration: not stated |
|
| Participants | Inclusion criteria: inpatients; aged 3 months to 7 years with acute diarrhoea (liquid, mucous, or bloody stools passed at least twice as frequently as usual for ≥ 24 hours and < 7 days) Exclusion criteria: chronic disease; malnutrition; use of antibiotics, antidiarrhoeal or other drugs influencing gut motility Number completing study: probiotic group 100/115 (87.0%; 10 required antibiotics, 5 non‐compliant); control group 100/117 (85.5%; 13 required antibiotics, 4 non‐compliant) |
|
| Interventions |
Interventions administered from admission. All children received ORF, normal food for age and IV fluids as required |
|
| Outcomes |
1 child had meteorism (group allocation unclear). No adverse events attributed to probiotic. |
|
| Notes | Study location: Turkey (low child and adult mortality). Cause of diarrhoea: 39 (39.0%) children in probiotic group and 44 (44.0%) controls had rotavirus diarrhoea. Overall, bacterial pathogens were isolated in 9 and parasites in 11 children. Nutritional status: malnutrition excluded; no other data presented. Hydration status: severe or moderate dehydration in 3 (3.0%) probiotic and 5 (5.0%) control group; mild/moderate dehydration in 17 (17.0%) probiotic and 24 (24.0%) control group. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | High risk | Follow up < 90% in both groups |
Lee 2001.
| Methods | Randomized controlled trial, non‐blinded; 1 centre Duration: 6 months, October 1999 to March 2000 |
|
| Participants | Inclusion criteria: inpatients; consecutive admissions aged 6‐60 months; diarrhoea < 5 days and > 3 watery stools in last 24 hours. Average dehydration about 5% in both groups. Exclusion criteria: bloody stools, antidiarrhoeal or antiperistaltic drugs; children receiving lactose‐free, protein hydrolysated formula for malabsorptive disorder; compromised immune system. Number completing study: 50/50 (100%) probiotic and 50/50 (100%) control group. |
|
| Interventions |
All children had IV fluids because of vomiting. Interventions administered after initial fluid therapy. |
|
| Outcomes |
No comment regarding adverse effects. |
|
| Notes | Study location: Taiwan (low child and adult mortality). Cause of diarrhoea: bloody diarrhoea excluded. Nutritional status: no data presented. Hydration status: % average dehydration 4.3 (SD 1.5) in probiotic and 4.0 (1.4) in control group. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Unclear risk | Probably open study; no placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Lievin Le‐Maol 2007.
| Methods | Randomized controlled trial; 1 centre Duration: not stated |
|
| Participants | Inclusion criteria: inpatients; infants and children aged ≤24 months; > 4 liquid stools/24 hours of < 72 hours duration. Exclusion criteria: rotavirus diarrhoea Number completing study: 42/42 (100%) probiotic and 38/38 (100%) control group. |
|
| Interventions |
All sachets diluted in ORF. Every admitted child was given at least 100 mL/kg of ORF. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: Ecuador (high child and high adult mortality) Cause of diarrhoea: rotavirus diarrhoea excluded; bloody diarrhoea included. Nutritional status: no data presented Hydration status: severe dehydration in 0 probiotic and 1 (2.6%) control group; mild/moderate dehydration in 4 (10.5%) probiotic and 7 (23.3%) control group. Source of funding: Laboratoire du Lacte´ol (Houdan, France) provided strain LB and batches of lyophilized, heat‐killed LB bacteria plus their culture medium to Dr Servin and Lactéol Fort sachets and placebo sachets to Dr Sarrazin‐Davila. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Sequential allocation |
| Allocation concealment? | High risk | Sequential allocation |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Mao 2008.
| Methods | Randomized controlled trial; 1 centre Duration: not stated |
|
| Participants | Inclusion criteria: inpatients; infants and children with severe acute diarrhoea (defined as 1 watery or mucous stool or 3 or more loose stools daily for > 24 hours). Exclusion criteria: moderate or severe malnutrition; total or partial breast feeding; diarrhoea > 48 hours; need for antibiotic treatment; allergy to cow's milk; gastrointestinal or other chronic pathologies. 12/212 (5.7%; 3 study groups) withdrawn after recruitment as they did not match the age criteria. Number completing study: 70/70 (100%) probiotic and 71/71 (100%) control group. |
|
| Interventions |
Interventions administered after oral or parenteral rehydration. |
|
| Outcomes |
No specific comment regarding adverse effects. |
|
| Notes | Study location: China; low child and adult mortality Cause of diarrhoea: rotavirus diarrhoea occurred in 87% and bacterial diarrhoea in 13% in both groups. Nutritional status: moderate or severe malnutrition excluded. Hydration status: no data presented. Source of funding:not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Unclear risk | Reported as double blind but methods of blinding not described |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Misra 2009.
| Methods | Randomized controlled trial; 1 centre Duration: not stated |
|
| Participants | Inclusion criteria: inpatients; infants and children with diarrhoea (> 3 stools per day, watery or taking the shape of the container); duration not stated. Exclusion criteria: parents refused consent, children living outside the municipal area, bloody diarrhoea, severe dehydration, shock, inability to take and retain oral feeds, suspected systemic infection. Number completing study: 105/111 (94.6%) probiotic and 105/118 (89.0%) control group; children withdrawn as they did not complete allocated treatment. |
|
| Interventions |
Start of interventions not stated |
|
| Outcomes |
No comment regarding adverse effects |
|
| Notes | Study location: India; high child and adult mortality Cause of diarrhoea: rotavirus identified in 29/105 (27.6%) probiotic and 25/105 (23.8%) in control group. Bloody diarrhoea excluded but 30/105 (28.6%) in probiotic and 30/105 (28.6%) in control group had white blood cells in stools and, overall, 10 children had bacterial diarrhoea. Nutritional status: no data presented. Hydration status: severe dehydration excluded; mild/moderate dehydration in 18 (17.1%) probiotic and 23 (21.9%) control group. Source of funding: partly by the International Development Fund of the John Nuveen Centre for International Affairs, University of Illinois, Chicago, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomization |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | Identical capsules |
| Incomplete outcome data addressed? All outcomes | High risk | Follow up < 90% in placebo group |
Narayanappa 2008.
| Methods | Randomized controlled trial; 1 centre Duration: not stated |
|
| Participants | Inclusion criteria: inpatients; infants and children with acute rotavirus diarrhoea (stool frequency and consistency not stated) of duration of ≤ 3 days. Exclusion criteria: infectious diarrhoea other than rotaviral diarrhoea; serum sodium > 155 mmol/L or <130 mmol/L; history of malabsorption, respiratory or systemic infections Number completing study: 40/40 (100%) probiotic and 40/40 (100%) control group. |
|
| Interventions |
When interventions started not stated |
|
| Outcomes |
No adverse effects attributed to the probiotic. |
|
| Notes | Study location: India; high child and adult mortality Cause of diarrhoea: all rotavirus diarrhoea. Nutritional status: no data presented. Hydration status: no data presented. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Unclear risk | Reported as double blind but no details given |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Oandasan 1999.
| Methods | Randomized controlled trial; 1 centre Duration: 1 year, 16 January 1998 to 15 January 1999 |
|
| Participants | Inclusion criteria: inpatients; infants and children with non‐bloody diarrhoea (characteristics not stated) for < 5 days. Exclusion criteria: antibiotics in last 72 hours; antidiarrhoeal drugs; other illness; severe malnutrition; compromised immune system, severe electrolyte disturbance and dehydration. Number completing study: 47/47 (100%) in probiotic group and 47/47 (100%) in placebo group. |
|
| Interventions |
When interventions started not stated. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: Philippines (low child and adult mortality). Cause of diarrhoea: bloody diarrhoea excluded. Nutritional status: severe malnutrition excluded; no other data presented. Hydration status: dehydration excluded. Unpublished data. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number table |
| Allocation concealment? | Low risk | Randomization by independent person |
| Blinding? All outcomes | Low risk | Administration of interventions by independent person |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Ozkan 2007.
| Methods | Randomized controlled trial; 1centre Duration: 6 months, October 2004 to March 2005 |
|
| Participants | Inclusion criteria: inpatients and outpatients; previously healthy children; aged 6 months to 10 years; acute diarrhoea (not defined). Exclusion criteria: severe systemic infection or sepsis; chronic disease; previous antibiotics; anti‐diarrhoeal drugs; primary/secondary immune deficiency. Number completing study: 16/16 (100%) for the probiotic group. 11/11(100%) for the control group. |
|
| Interventions |
Start of intervention unclear. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: Turkey (low child, low adult mortality) Cause of diarrhoea: 1 (6.3%) child in probiotic and 0 in control group had bacterial diarrhoea. Nutritional status: mild/moderate malnutrition in 2 (12.5%) in the probiotic and 1 (9.1%) in the control group. Hydration status: severe dehydration in 1 (6.3%) in the probiotic and 0 in the control group; mild/moderate dehydration in 3 (18.8%) in the probiotic and 2 (18.2%) in the control group. Source of funding: Sanofi‐Aventis (Paris, France) provided laboratory reagents and a commercial preparation of S. boulardii |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Pant 1996.
| Methods | Randomized controlled trial; 1 centre Duration: 6 weeks, July to mid‐August 1993. |
|
| Participants | Inclusion criteria: inpatients; infants and children with > 3 watery stools in last 24 hours and diarrhoea for < 14 days. . Mean (SD) weight for age z score ‐1.15 (0.95) in the probiotic group and ‐1.8 (1.4) in the placebo group. Exclusion criteria: exclusive breast‐feeding; septicaemia. Number completing study: 20/20 (100%) in probiotic group and 19/19 (100%) in placebo group. However, data extractable for subset with watery diarrhoea only: 14/20 (70%) in probiotic group and 12/19 (63.2%) in placebo group. No data for children with bloody stools presented. |
|
| Interventions |
Interventions started after 6 hours ORF. |
|
| Outcomes |
Vomiting occurred in 1 child in the placebo group. No adverse events attributed to probiotic. |
|
| Notes | Study location: Thailand (low child and adult mortality). Cause of diarrhoea: bloody stools in 6 children in probiotic and 7 in placebo group. All negative for parasites and cryptosporidium; 2 rotavirus and 1 astrovirus patients in the probiotic group and 5 rotavirus patients in the control group Nutritional status: no data presented Hydration status: severe dehydration in 2 (10%) in the probiotic and 4 (21%) in the control group; mild/moderate dehydration in all remaining children. Source of funding: Scientific Hospital Supplies, UK, provided the probiotic |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | follow up ≥ 90% in both groups |
Pashapour 2006.
| Methods | Randomized controlled trial; 1 centre Duration: 4 months, September to December 2002 |
|
| Participants | Inclusion criteria: inpatients; aged 6 to 24 months, breast fed with increased frequency, fluidity and volume of faeces of duration less than 4 days and moderate dehydration. Exclusion criteria: mucoid or bloody stools; oral feeding contra‐indicated or intolerance; pneumonia; septicaemia; malnutrition; severe dehydration; stool culture positive for bacteria; recent intake of yogurt; poor compliance with yogurt intervention Number completing study: 3/43 (7.0%) withdrew from probiotic and 3/43 (7.0%) from control group all due to poor compliance with management |
|
| Interventions |
Interventions administered from admission to discharge. All infants received ORF, IV fluids, complementary feeds |
|
| Outcomes |
No comment regarding adverse effects. |
|
| Notes | Study location: Pakistan (high child and adult mortality). Cause of diarrhoea: no data presented Nutritional status: malnutrition excluded. Hydration status: all had moderate dehydration. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | High risk | No placebo; probably open study |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Rafeey 2008a.
| Methods | Randomized controlled trial; 1 centre Duration: 12 months; May 2005 to May 2006 |
|
| Participants | Inclusion criteria: inpatients; infants and children with 3 or more watery stools/day for less than 48 hours and clinical dehydration. Exclusion criteria: bloody stools; hypovolaemic shock; acute systemic illness; antibiotic or anti‐diarrhoeal medication. 18/178 children withdrawn mainly because of parent non‐compliance; likely to have been withdrawn before recruitment. Number completing study: 40/40 (100%) in the probiotic group and 40/40 (100%) in the placebo group. |
|
| Interventions | Children randomized to one of 4 groups: A, yogurt fermented with L. acidophilus, B, L. acidophilus supplement, C, conventional yogurt and D, placebo. Groups B and D selected for review.
Start of administration not stated. |
|
| Outcomes |
No adverse effects attributed to probiotic. |
|
| Notes | Study location: Iran; low child and adult mortality Cause of diarrhoea: bloody diarrhoea excluded; no bacteria or parasites identified in stool samples. Nutritional status: severe malnutrition excluded. Hydration status: severe dehydration in 1/40 (2.5%) probiotic and 2/40 (5%); all the rest had mild/moderate dehydration. Source of funding: supported by a grant from Tabriz Medical University |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Restricted randomization using random permuted blocks |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Unclear risk | Not described |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up ≥ 90% in both groups |
Raza 1995.
| Methods | Randomized controlled trial; 1 centre Duration: 2 months, July and August 1993 |
|
| Participants | Inclusion criteria: inpatients; undernourished infants and children with > 3 watery stools in last 24 hours for < 14 days duration and at least moderate dehydration. Exclusion criteria: severe malnutrition; septicaemia. Number completing study: 36/40 participants; 4 withdrawals (2 diagnosed with cholera, 1 developed pneumonia, 1 refused anything by mouth). Results presented for 19/21 (90.5%) in the probiotic group and 17/19 (89.5%) in the placebo group. |
|
| Interventions |
Interventions started after 4 to 6 hours ORF. |
|
| Outcomes |
Outcomes for watery (non‐bloody) diarrhoea also presented: mean (SD) stool frequency day 2 for probiotic (n = 16) versus placebo (n = 16) was 4.4 (2.0) versus 6.6 (4.2), P = < 0.05, and persistent diarrhoea at 48 hours in 5 (31%) versus 12 (75%) patients, P = < 0.01. Definition of persistent diarrhoea not stated. Less vomiting in the probiotic group; myoclonic jerks occurred in one child in each group. No adverse events attributed to probiotic. |
|
| Notes | Study location: Pakistan (high child and adult mortality). Cause of diarrhoea: bloody diarrhoea included. Nutritional status: all had mild/moderate malnutrition; severe malnutrition excluded. Hydration status: severe dehydration in 14 (66.7) probiotic and 7 (37) control group; all the rest had moderate dehydration. Duration of diarrhoea not measured (many children discharged before stool character had changed). Source of funding: Scientific Hospital Supplies, UK, provided the probiotic |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not defined |
| Allocation concealment? | Unclear risk | Not defined |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Approximately 90% follow up in both groups |
Ritchie 2010.
| Methods | Randomized controlled trial; 1 centre Duration: 21 months, June 2002 to March 2004. |
|
| Participants | Inclusion criteria: inpatients; Aboriginal children aged 4 months to 2 years with acute diarrhoea defined as ≥ 3 loose stools during 24 hours before presentation and duration < 7 days and able to tolerate ORF. Exclusion criteria: oxygen required during the study period; chronic cardiac, renal or respiratory disease; previous gastrointestinal surgery; proven sucrose intolerance; suspected on known immunodeficiency; received probiotic before enrolment; younger than 4 months. Number completing study: 201 assessed for eligibility; 103 refused participation and 28 failed to consent. Probiotic arm: 4 discharged before intervention, 1 parental withdrawal, 33 completed study. Control arm: 1 parental withdrawal, 31 completed study. |
|
| Interventions |
Interventions administered within 24 hours of admission. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: Australia (very low child and low adult mortality). However, this study recruited Aboriginal children who commonly had co‐morbidities such as pneumonia and malnutrition related to poverty and social disadvantage in the top end of the Northern Territory. Therefore, data not included in analysis according to country mortality strata. Cause of diarrhoea: bacterial pathogens identified in 4 (12%) probiotic and 4 (13%) in the control group; rotavirus identified in 11 (33%) in the probiotic and 6 (19%) in the control group; parasites identified in 2 (6%) probiotic and 2 (6%) control group. Nutritional status: mild/moderate malnutrition common amongst participants; no other data presented. Hydration status: severe dehydration in 0 probiotic and 1 (3.2%) in the control group; all the rest had mild/moderate dehydration. Source of funding: Project supported by Australian National Health and Medical Research Council |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated block randomization |
| Allocation concealment? | Low risk | Randomization by independent research institute; allocation concealed until recruitment, data collection and analyses were completed |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | High risk | Follow up < 90% in probiotic group |
Rosenfeldt 2002a.
| Methods | Randomized controlled trial; 2 centres Duration: 6 months, December 1998 to May 1999 |
|
| Participants | Inclusion: inpatients; children aged 6 to 36 months with 2 or more consecutive loose stools in 24 hours and a duration no more than 7 days. Exclusion criteria: underlying chronic disease or antibiotics prescribed during the study period. Number completing study: 86 children enrolled, of whom 69 (80.2%) completed the study; exclusions were made after randomization because antibiotics were prescribed (3 in the control group and 2 in the probiotic group), rapid recovery before intervention started (3 in the control group and one in the probiotic group), non‐compliance with the protocol (4 in the control group and 4 in the probiotic group). |
|
| Interventions |
Interventions started as soon as possible after randomization and did not await rehydration. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: Denmark (very low child and adult mortality). Cause of diarrhoea: overall, rotavirus was the only pathogen in 40 (58%) children; 6 children had rotavirus and a bacterial pathogen was identified; in addition, Campylobacter jejuni was isolated in 3 children and Salmonella typhimurium in 1 child. Nutritional status: no data presented. Hydration status: no severe dehydration; mild/moderate dehydration in 5 (16.7%) in the probiotic and 17 (43.6%) in the control group. The probiotics appeared to reduce significantly the duration of diarrhoea in children treated within 60 hours of the onset of diarrhoea. Hospital stay was shorter in the probiotic group than the controls (mean 1.6 (SD 1.0) versus 2.7 (SD 2.0) respectively; P = 0.02). The probiotics also appeared to reduce significantly the number of children excreting rotavirus in stools on day 5. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | High risk | Follow up < 90% in both groups |
Rosenfeldt 2002b.
| Methods | Randomized controlled trial; 19 day‐care centres Duration: 6 months, December 1998 to May 1999 |
|
| Participants | Inclusion criteria: outpatients; children aged 6 to 36 months with 2 or more consecutive loose stools in 24 hours as assessed by parents and with a duration no more than 7 days. Exclusion criteria: underlying chronic disease; antibiotics prescribed during study period. Number completing study: 50 children enrolled, of whom 43 (86%) participants completed the study. Exclusions were because of hospitalization with excessive vomiting and moderate dehydration (2 in the placebo group and 3 in the probiotic group), 1 because antibiotics were prescribed (placebo group), 1 non‐compliant with protocol (placebo group). |
|
| Interventions |
Interventions started as soon as possible after randomization. |
|
| Outcomes |
One participant in the probiotic group complained of constipation (no stools passed from day 3 for 10 days). No adverse events attributed to probiotic. |
|
| Notes | Study location: Denmark (very low child and adult mortality). Cause of diarrhoea: overall, rotavirus was the only pathogen in 25 children, 2 had rotavirus and a bacterial pathogen identified, 2 had an infection with C. jejuni and Salmonella typhimurium. Nutritional status: no data presented. Hydration status: mild/moderate dehydration in 3 patients (12.5%) in the probiotic and 4 (13.8%) in the control group; no severe dehydration. The probiotics appeared to reduce significantly the duration of diarrhoea in children treated within 60 hours of the onset of diarrhoea. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | High risk | Follow up < 90% in both groups |
Sarkar 2005.
| Methods | Randomized controlled trial; 1 centre Duration: 23 months, February 2001 to December 2002 |
|
| Participants | Inclusion criteria: inpatients; boys aged 4 to 24 months of age; acute watery diarrhoea (> 4 liquid stools during 24
hours) of < 48 hours duration. Exclusion criteria: severe malnutrition (< 65% weight for age by the standard of the National Centre for Health Statistics (NCHS)); systemic infection requiring antimicrobial therapy; bloody diarrhoea; spot sample of stool revealed V. cholerae by dark‐field microscopy; antibiotic treatment in the preceding 2 weeks Number completing study: 112/115 (97.4%) in the probiotic group (3 withdrawn by parents) and 115/115 (100.0%) in the control group. |
|
| Interventions |
Interventions started after enrolment. All children received ORF and continued feeding, including breast milk if breast fed. |
|
| Outcomes |
No comment regarding adverse outcomes. |
|
| Notes | Study location: Bangladesh (high child and adult mortality). Cause of diarrhoea: bloody diarrhoea excluded. Rotavirus detected in 78 (69.6%) in the probiotic and 73 (63.5%) in the placebo group; V. cholera detected in 14 (12.2%) in the probiotic and 16 (13.9%) in the placebo group. Nutritional status: severe malnutrition (weight < 65% weight for age of NCHS standard) excluded no further data presented. Hydration status: mild/moderate dehydration in 54 (47.0%) in the probiotic and 65 (56.5%) in the control group. Source of funding: Nestle Research provided L. paracasei. Research supported by the Swedish Agency for Research in Developing Countries , the Karolinska Institute (Stockholm, Sweden), and Nestlé Research Centre (Lausanne, Switzerland). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomly permutated blocks |
| Allocation concealment? | Low risk | Randomization code generated by an independent statistician |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up >90% in both groups |
Sepp 1995.
| Methods | Randomized trial; 1 centre Duration: 1 year; November 1992 to October 1993 |
|
| Participants | Inclusion criteria: inpatients; infants and children with ≥2 loose stools for 1 to 3 days or haemorrhagic colitis, fever ≥ 38.0oC,or second stage dehydration, or both. All had shigellosis. Exclusion criteria: not stated Number completing study: 13/13 (100%) children in the probiotic group and 12/12 (100%) in the control group. |
|
| Interventions |
When interventions started was not stated. |
|
| Outcomes |
No comment regarding adverse effects |
|
| Notes | Study location: Estonia (low child, high adult mortality) Cause of diarrhoea: all shigellosis. 9 (69.2%) in the probiotic and 4 (33.3%) in the controls had bloody diarrhoea. Nutritional status: no data presented. Hydration status: no data presented. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | High risk | No placebo; probably open study |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up > 90% in both groups |
Shornikova 1997a.
| Methods | Randomized controlled trial; 1 centre Duration: 1 year, 1 April 1994 to 31 May 1995 |
|
| Participants | Inclusion criteria: inpatients; infants and children with ≥ 1 watery stool in the last 24 hours and diarrhoea for < 5 days. Exclusion criteria: not stated. Number completing study: 123/214 (57%) eligible children admitted during the study period enrolled; no reasons given for those not recruited. A total of 59/59 (100%) children allocated to the probiotic group and 64/64 (100%) in the placebo group completed the trial. |
|
| Interventions |
Interventions started with oral rehydration solution. All participants with positive stool cultures received antibiotics. Effect of isotonic versus hypotonic oral rehydration solution also assessed. |
|
| Outcomes |
No comment regarding adverse events. |
|
| Notes | Study location: Russia (low child and high adult mortality). Cause of diarrhoea: rotavirus identified in 13 (22.0%) in the probiotic and 21 (32.8%) in the control group. Bacterial diarrhoea identified in 11 (18.7%) in the probiotic and 15 (23.4%) in the control group. Nutritional status: no data presented. Hydration status: mean dehydration ˜4% in both groups. Among children with rotavirus diarrhoea, the probiotic (n = 13) reduced the number of watery stools compared with the placebo (n = 21; P = 0.02, but no data given). No beneficial effect of the probiotic was seen in those with bacterial diarrhoea (probiotic (n = 11) and placebo (n = 115), P = 0.42). Stool samples tested for rotavirus (Rotazyme, Dakopotts AS, Denmark) and cultured for Salmonella and Shigella. Source of funding: Leiras, Turku, Finland and Valio, Helsinki, Finland |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization schedule |
| Allocation concealment? | Low risk | Randomization numbers sequentially assigned to participants as enrolled |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up > 90% in both groups |
Shornikova 1997b.
| Methods | Randomized controlled trial; 2 centres Duration: 6 months, 22 January to 15 July 1996 |
|
| Participants | Inclusion criteria: inpatients; infants and children with ≥ 3 watery stools in last 24 hours, diarrhoea for < 7 days; stools positive for rotavirus antigen (IDEIA Rotavirus, UK). Mean dehydration about 4% in both groups. Exclusion criteria: 20 participants who received exclusively or mainly IV fluids were excluded. 86/97 (89%) enrolled participants were positive for rotavirus. Number completing study: 21/21 (100%) in the probiotics and 25/25 (100%) in the placebo group. (20 allocated to a low dose probiotic group). |
|
| Interventions | Participants randomized to one of 3 groups: 20 in the probiotic small dose (107 CFU/day) group, 21 in the probiotic large dose group, 25 in the placebo group. Data from the large dose group were used in this review.
Interventions started with ORF |
|
| Outcomes |
No comment regarding adverse events. |
|
| Notes | Study location: Finland (very low child and adult mortality). Cause of diarrhoea: all rotavirus. Nutritional status: no data presented. Hydration status: mean dehydration about4% in both groups. Data from high dose probiotic group used for continuous outcomes. Duration of diarrhoea before admission greater in probiotic group (4.2 (SD 1.4) days) than in the placebo group (2.9 (SD 1.2) days). Number with persistent diarrhoea on day 3 derived from graph. Source of funding: BioGaia Biologicals AB |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | High risk | Participants receiving IV fluids excluded |
Shornikova 1997c.
| Methods | Randomized controlled trial; 1 centre Duration: 5 months, 29 January to 3 July , 1995 |
|
| Participants | Inclusion criteria: inpatients; infants and children with ≥ 3 watery stools in last 24 hours; diarrhoea for < 7 days; ingested bovine dairy products. Exclusion criteria: immunosuppressive therapy or immune deficiency; allergy to bovine milk; serious underlying disorder; undergoing an investigational product during the preceding month. Number completing study: 41 participants initially enrolled; 19/19 (100%) in the probiotic group and 21/22 (95.5%) in the placebo group (1 participant in the placebo group removed because the probiotic agent (L. reuteri) was detected in stool; the probiotic was administered to his sibling). |
|
| Interventions |
Interventions started at recruitment. |
|
| Outcomes |
Less vomiting in the probiotic group. No adverse events attributed to probiotic. |
|
| Notes | Study location: Finland (very low child and adult mortality). Cause of diarrhoea: rotavirus identified in 18 (86%) in the probiotic group and 12 (63%) in the control group. Nutritional status: no data presented. Hydration status: mean dehydration at baseline greater in the probiotic (3.9% (SD 1.3)) than the control group (3.0 (SD 1.2)). Source of funding: BioGaia Biologicals, Inc., Raleigh,NC, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomiation schedule |
| Allocation concealment? | Low risk | Randomization numbers sequentially assigned to participants as enrolled |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up > 90% in both groups |
Simakachorn 2000.
| Methods | Randomized controlled trial; 1 centre Duration: 1 year, September 1995 to August 1996 |
|
| Participants | Inclusion criteria: inpatients; infants and children with acute, watery diarrhoea (stool frequency not stated) for ≤ 5 days. Exclusion criteria: mucous bloody stools or major systemic illness. Number completing study: 37/37 (100%) in the probiotic group and 36/36 (100%) in the placebo group. |
|
| Interventions |
Interventions mixed with 5 mL water and started with ORF. |
|
| Outcomes |
No comment regarding adverse events. |
|
| Notes | Study location: Thailand (low child and adult mortality). Cause of diarrhoea: bloody diarrhoea excluded. Nutritional status: severe malnutrition in 1 (2.7%) probiotic participant and 1 (2.8%) in the control group; mild/moderate malnutrition in 8 (21.6%) in the probiotic and 12 (33.3%) in the control group. Hydration status: no severe dehydration; all had mild/moderate dehydration. 40 children (17 probiotic and 23 placebo) had received antibiotics before admission. The effect of the probiotic in shortening the duration of diarrhoea more marked in children who had not received antibiotics before admission. Source of funding: Merck Ltd., Bangkok, Thailand. National Collection of Pasteur Institute provided the probiotic. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization code |
| Allocation concealment? | Low risk | Numerically coded packages |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow‐up >90% in both groups |
Sugita 1994.
| Methods | Quasi‐randomized controlled trial; 1 centre Duration: not stated |
|
| Participants | Inclusion criteria: inpatients; infants and children with acute rotavirus diarrhoea (stool characteristics described for each participant; stool frequency x 1‐10/day; duration not stated); none had bloody stools. Exclusion criteria: none stated. Number completing study: 16/17 (94.1%) in the probiotic group and 11/15 (73.3%) in the control group. |
|
| Interventions |
Not stated when interventions started. All participants received lactase (1.5 g/day in 3 doses) and albumin tannate (0.1/kg/day in 3 doses). |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: Japan (very low child and adult mortality). Cause of diarrhoea: all rotavirus diarrhoea. Nutritional status: no data presented. Hydration status: no data presented. Results for time to first formed stool given for 16/17 (94.1%) participants in the probiotic group and 11/15 (73.3%) in the control group. Reasons for missing data not stated. Rotavirus antigen persisted in the stools of 1/9 (11.1%) children in the probiotic group and 2/8 (25%) in the control group. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Allocation in order of hospitalization |
| Allocation concealment? | High risk | Allocation in order of hospitalization |
| Blinding? All outcomes | High risk | Open study |
| Incomplete outcome data addressed? All outcomes | High risk | Overall < 90% follow up in placebo group |
Szymanski 2006.
| Methods | Randomized controlled trial; 1 centre Duration: 10 months, September 2003 to June 2004 |
|
| Participants | Inclusion criteria: inpatients and outpatients; aged 2 months to 6 years with acute diarrhoea (3 or more stools/day looser than normal that may contain blood or mucous for > 1 and < 5 days). Exclusion criteria: organic gut disease; underlying chronic illness; immunosuppression, exclusive breast‐feeding Number completing study: 46/49 (93.9%) in probiotic group; 41/44 (93.2) controls. Withdrawals stated to be due to non‐compliance or incomplete data. |
|
| Interventions |
Onset of intervention delayed >72 hours in many participants. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: Poland (low child and adult mortality) Cause of diarrhoea: bloody diarrhoea included. Overall, 39/87 (45%) had rotavirus (22 probiotic and 17 control group), 5/87 (6%) had adenovirus, 9/87 (10%) had a bacterial pathogen and many children had norovirus infection. Nutritional status: no data presented. Hydration status: no severe dehydration. Mild/moderate dehydration in 34 (73.9%) in the probiotic and 31 (75.6%) in the control group. Source of funding: Wellcome Travel Award |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated block randomization |
| Allocation concealment? | Low risk | Sequential assignment of randomization numbers |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up > 90% in both groups |
Teran 2009.
| Methods | Randomized, single blind controlled trial; 1 centre Duration: 7 months; August 2007 to February 2008 |
|
| Participants | Inclusion criteria: inpatients; infants and children with a history of acute watery diarrhoea (defined as ≥3 stools of liquid consistency/day < 72 hours duration) positive for rotavirus and with moderate to severe dehydration. Exclusion criteria: severe malnutrition; systemic infections requiring antibiotic therapy; severe chronic disease; identification of a second pathogen in the stool; ingestion of antibiotics; probiotics or nitazoxanide 3 weeks before admission; recurrence of diarrhoea after discharge. Patients in whom a cause of diarrhoea other than rotavirus were withdrawn (probiotic group: 3 with adenovirus, 2 with E. histolytica; control group: 3 with E. histolytica, 2 with Giardia, 1 with S. flexneri). Number completing study: 25/25 (100%) probiotic group; 25/25 (100%) control group. |
|
| Interventions | Participants were allocated to one of three groups: a nitazoxanide, a probiotic and a control group that received rehydration solutions only. Data from the probiotic group and controls used for this review.
Time when interventions started not described. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: Bolivia (high child and high adult mortality) Cause of diarrhoea: all rotavirus diarrhoea. Nutritional status: severe malnutrition excluded; mild/moderate malnutrition in 5 (20%) in the probiotic and 15 (60%) in the control group. Hydration status: all had moderate to severe dehydration; no further data presented. Source of funding: the research was not sponsored by any pharmaceutical company |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computerised admissions list |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | High risk | Single blind; only parents/caretakers not aware of group allocation. No placebo. |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up > 90% in both groups |
Táborská 1997.
| Methods | Randomized trial; 1 centre Duration: 1994‐1995 |
|
| Participants | Inclusion criteria: inpatients; infants and children Exclusion criteria: nosocomial rotaviral infection Number completing the study: 50/50 (100%) probiotic group; 50/50 (100%) control group. |
|
| Interventions |
Time when interventions started: |
|
| Outcomes |
No adverse events attributed to the probiotic. |
|
| Notes | Study location: Czech Reublic (very low child and adult mortality) Cause of diarrhoea: nosocomial rotavirus diarrhoea excluded; 16 (32.0%) probiotic and 21 (42.0%) control group had viral diarrhoea. A total of 22 (44.0%) in the probiotic and 24 (48.0%) in the control group had bacterial diarrhoea. Nutritional status: no data presented. Hydration status: severe dehydration in 3 (6.0%) probiotic and 2 (4.0%) in the control group; all the rest had mild/moderate dehydration. No data presented that could be extracted for meta‐analysis. Source of funding not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | High risk | No placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up > 90% in both groups |
Urganci 2001.
| Methods | Randomized controlled trial; 1 centre Duration: 1 year, June 200 to May 2001 |
|
| Participants | Inclusion criteria: consecutive inpatients aged 2 to 29 months with acute, non‐bacterial diarrhoea (definition not stated) lasting >48 hours able to receive oral medication. Exclusion criteria: concomitant illness, antimicrobial, antidiarrhoeal or other drugs affecting gut motility, severe electrolyte disturbance or dehydration. Number completing study: 50 cases reported in both arms; number withdrawn because of the deterioration in diarrhoea, concomitant disease requiring other drugs unclear. |
|
| Interventions |
Time of starting interventions and duration of administration not stated. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: Turkey (low child and adult mortality) Cause of diarrhoea: non‐bacterial diarrhoea Nutritional status: no data presented. Hydration status: none dehydrated. Lactose intolerance identified in 8% in the probiotic and 26% in the placebo group. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Unclear risk | Unclear if placebo identical or not |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Number of children withdrawn not stated |
Villarruel 2007.
| Methods | Randomized controlled trial; 1 centre Duration: 1 year |
|
| Participants | Inclusion criteria: outpatients; infants and children aged 3 months to 2 years (urban population, middle social class); acute, mild to moderate diarrhoea. Exclusion criteria: use of probiotic in the preceding 7 days; chronic intestinal disease; short bowel syndrome; malabsorption; ≥ grade 2 malnutrition; severe disease (including dehydration requiring hospitalization at the time of the consultation); known immunodeficiency; immunosuppressant treatment (oral or IV corticoids in the preceding 7 days); oral nystatin; oral or parenteral imidazoles; other systemic antifungal agents; macrolides; drugs that alter intestinal motility (antispasmodics, cisapride, antiemetics and antidiarrhoeal drugs) in the preceding 7 days. Number completing study: 6/50 (12.0%) excluded from the probiotic group and 6/50 (12.0%) from the control group for lack of compliance with protocol medication. |
|
| Interventions |
ORF and antibiotics given when indicated. |
|
| Outcomes |
No comment regarding adverse events. |
|
| Notes | Study location: Argentina (low child and adult mortality) Cause of diarrhoea: none had bloody diarrhoea; no other data presented. Nutritional status: ≥ grade 2 malnutrition excluded. Hydration status: dehydration requiring hospitalisation excluded; all had dehydration <7%. Stool frequency significantly lower in probiotic than placebo group on days 4 and 7. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random computer‐generated into blocks of 20 |
| Allocation concealment? | Low risk | Paediatricians recruiting patients received batches of coded containers |
| Blinding? All outcomes | Low risk | Identical placebo |
| Incomplete outcome data addressed? All outcomes | High risk | Followup < 90% in both groups |
Vivatvakin 2006.
| Methods | Randomized open study; 1 centre Duration: March 2003 to January 2004; 11 months |
|
| Participants | Inclusion criteria: inpatients and outpatients; infants and children with watery diarrhoea (not defined) for < 5 days. Exclusion criteria: immunocompromised; suspected dysentery; diagnosed with persistent or chronic diarrhoea; chronic cardiac, pulmonary or haematological illness; undergoing antibiotic treatment in the last 2 weeks; severe dehydration or shock. 4/75 withdrawn (1 febrile seizure, 1 urinary tract infection, 2 with pneumonia). Two patients were withdrawn from each group. Number completing study: 36/38 (94.7%) in the probiotic group; 35/37 (94.6%) in the control group. |
|
| Interventions |
Timing of administration not stated |
|
| Outcomes |
Duration of diarrhoea reported for rotavirus diarrhoea. No adverse events attributed to probiotic. |
|
| Notes | Location: Thailand; low child and adult mortality Cause of diarrhoea: suspected dysentery excluded; overall, 34% had rotavirus and 12.1% Salmonella in stools. Nutritional status: no data presented. Hydration status: severe dehydration excluded; mild/moderate dehydration in 25 (69.4%) probiotic and 23 (65.7%) control group. Source of funding: AIS donation fund, Thai Red Cross Society |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | High risk | Open study; no placebo |
| Incomplete outcome data addressed? All outcomes | Low risk | Follow up > 90% in both groups |
Wunderlich 1989.
| Methods | Randomized controlled trial; 10 centres Duration: not stated |
|
| Participants | Inclusion criteria: adults with acute diarrhoea (characteristics and duration not stated). Exclusion criteria: not stated. 3 participants from each group withdrawn on day 4 or later (causes for dropouts stated to be unrelated to medication); 4 participants assigned to the probiotic group and 5 assigned to the placebo group did not complete the study (reasons not stated). Number completing study (for persisting diarrhoea outcomes): 40/47 (85.1%) in the probiotic group and 38/46 (82.6%) in the placebo group. |
|
| Interventions |
Not stated when interventions started. |
|
| Outcomes |
No adverse events attributed to probiotic. |
|
| Notes | Study location: Switzerland and Lichtenstein (very low child and adult mortality). Cause of diarrhoea: no data presented. Nutritional status: no data presented. Hydration status: no data presented. Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Low risk | identical placebo |
| Incomplete outcome data addressed? All outcomes | High risk | Follow up <90% in both groups |
CFU: colony‐forming units IV: intravenous NCHS: National Centre for Health Statistics ORF: oral rehydration fluid RCT: randomized controlled trial SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agarwal 2001 | No non‐probiotic group. Participants included in Agarwal 2002 study |
| Agarwal 2002 | No non‐probiotic group |
| Alexander 1971 | Not a randomized controlled trial; no non‐probiotic group |
| Alvisi 1982 | Intervention groups not treated equally; antibiotics given to the non‐probiotic group |
| Barone 2000 | No non‐probiotic group |
| Beck 1961 | Not a randomized controlled trial |
| Bellomo 1979 | Cause of diarrhoea unclear. Additional treatment given to children with persisting diarrhoea |
| Bellomo 1980 | No non‐probiotic group. Study included children with diarrhoea secondary to antibiotic treatment or associated with respiratory infection |
| Bellomo 1982 | Cause of diarrhoea unclear |
| Bin Li Xie 1995 | Intervention groups not treated equally; antibacterials given to the non‐probiotic group |
| Brewster 2004 | Secondary publication to Ritchie 2010 |
| Camarri 1981 | Intervention groups not treated equally; antibiotics given to the non‐probiotic group |
| Cetina Sauri 1990 | Secondary publication to Cetina‐Sauri 1994 |
| Chandra 2002 | Prevention of rotavirus diarrhoea study |
| Chicoine 1973 | Unclear if acute diarrhoea |
| Costa‐Ribeiro 2000a | Unclear whether a randomized controlled trial |
| Costa‐Ribeiro 2000b | Prevention of diarrhoea study |
| Cui 2004 | No non‐probiotic group |
| de dios Pozo‐O 1978 | Assessment of probiotic in the prevention of traveller's diarrhoea |
| Eren 2010 | No non‐probiotic group |
| Fang 2009 | Study of effect of probiotic on rotavirus shedding in stools; no diarrhoea outcomes reported |
| Fourrier 1968 | No non‐probiotic group |
| Girola 1995 | Children with gastroenteritis and antibiotic‐associated diarrhoea studied together |
| Gracheva 1996 | No non‐probiotic group |
| Henker 2007b | Secondary reference to Henker 2007a |
| Heydarian 2010 | No non‐probiotic group |
| Isolauri 1991 | No non‐probiotic group |
| Kaila 1992 | No non‐probiotic group |
| Kaila 1995 | No non‐probiotic group |
| Korviakova 2000 | Not a randomized controlled trial; probiotic versus antibiotic |
| Le Leyur 2010 | Intervention group received an adapted lactose‐free formula fortified with S. boulardii and control group received a standard formula; difference in diarrhoea outcomes between groups cannot be attributed to the probiotic |
| Lei 2006 | Probiotic used was not specified |
| Lin 2009 | Prevention study |
| Magreiter 2006 | No non‐probiotic control group |
| Majamaa 1995 | No non‐probiotic group |
| Mazo 2006 | Prevention study |
| Michielutti 1995 | Not a randomized controlled trial |
| Mitra 1990 | No non‐probiotic group |
| Moraes 2001 | No non‐probiotic group |
| Niv 1963 | Not a randomized controlled trial; some children with diarrhoea thought to be caused by antibiotic treatment included |
| Ortlieb 1974 | Participants with acute diarrhoea and antibiotic‐associated diarrhoea combined |
| Pearce 1974 | Intervention groups not treated equally; calcium carbonate given as the placebo and may have reduced diarrhoea in the non‐probiotic group |
| Pedone 1999 | Prevention of diarrhoea study |
| Pedone 2000 | Prevention of diarrhoea study |
| Pene 1966 | No non‐probiotic group; participants with diarrhoea of various causes (infectious, post‐antibiotics) grouped together |
| Rafeey 2008b | Secondary publication to Rafeey 2008a |
| Rautanen 1998 | No data presented for placebo group |
| Saint‐Marc 1991 | Not a randomized controlled trial; no non‐probiotic group |
| Salazar‐Lindo 2004 | Mean duration of diarrhoea reported from responders only; children with ongoing diarrhoea excluded from analysis |
| Salazar‐Lindo 2007 | Active placebo |
| Satoh 1984 | Not a randomized controlled trial; no non‐probiotic group |
| Savas‐Erdeve 2009 | Study of amoebiasis‐associated diarrhoea and not acute infectious diarrhoea |
| Schrezenmeir 2004 | Antibiotic‐associated diarrhoea included in the study |
| Singh 1987 | No probiotic specified |
| Sudarmo 2003 | Other than the probiotic, unclear whether two intervention groups were treated the same. Probiotic group received high‐lactose formula containing B. bifidum. Unclear whether control group received high‐lactose or normal formula |
| Szymanski 2005 | Preliminary publication of Szymanski 2006 |
| Tojo 1987 | Unclear whether diarrhoea acute and whether a randomized controlled trial |
Characteristics of studies awaiting assessment [ordered by study ID]
Contreras 1983.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | No details of study available |
Salgado.
| Methods | |
| Participants | |
| Interventions | Heat‐killed L. acidophilus, Lacteol strain |
| Outcomes | |
| Notes | No other details available |
Differences between protocol and review
The following secondary outcomes have been removed as they were either uncommon or not reported: need for unscheduled intravenous (IV) rehydration after randomization; deaths; adverse events, such as vomiting; withdrawal from study.
Contributions of authors
Stephen Allen and Leonila Dans identified articles for inclusion in the review. Leonila Dans, Elizabeth Martinez, and Germana Gregorio assessed study quality, and Leonila Dans settled any disagreements. Stephen Allen extracted data. Stephen Allen took the main responsibility for analysis and writing the review. All reviewers contributed to the final version.
Sources of support
Internal sources
Swansea School of Medicine, UK.
External sources
Cochrane Infectious Disease Group, Liverpool School of Tropical Medicine, UK.
Declarations of interest
Stephen Allen is participating in ongoing research studies of lactobacilli and bifidobacteria provided by Cultech Ltd, UK, in the prevention of atopic disorders in infants and antibiotic‐associated diarrhoea in older people. In previous research, Scientific Hospital Supplies, UK, and Valio Ltd, Finland, have provided L. casei strain GG and also supported his attendance at a training workshop. Elizabeth Martinez is a Medical Manager for United Laboratories Inc., Philippines.
Unchanged
References
References to studies included in this review
Basu 2007 {published data only}
- Basu S, Chatterjee M, Ganguly S, Chandra P.K. Efficacy of Lactobacillus rhamnosus GG in acute watery diarrhoea of Indian children: a randomised controlled trial. Journal of Paediatrics and Child Health 2007;43(12):837‐42. [DOI] [PubMed] [Google Scholar]
Basu 2009 {published data only}
- Basu S, Paul DK, Ganguly S, Chatterjee M, Chandra PK. Efficacy of high‐dose Lactobacillus rhamnosus GG in controlling acute watery diarrhea in Indian children: a randomized controlled trial. Journal of Clinical Gastroenterology 2009;43(3):208‐13. [DOI] [PubMed] [Google Scholar]
Bhatnagar 1998 {published data only}
- Bhatnagar S, Singh KD, Sazawal S, Saxena SK, Bhan MK. Efficacy of milk versus yogurt offered as part of a mixed diet in acute noncholera diarrhea among malnourished children. Journal of Pediatrics 1998;132(6):999‐1004. [DOI] [PubMed] [Google Scholar]
Billoo 2006 {published data only}
- Billoo AG, Memon MA, Khaskheli SA, Murtaza G, Iqbal K, Shekhani MS, et al. Role of a probiotic (Saccharomyces boulardii) in management and prevention of diarrhoea. World Journal of Gastroenterology 2006;12(28):4557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boudraa 2001 {published data only}
- Boudraa G, Benbouabdellah M, Hachelaf W, Boisset M, Desjeux JF, Touhami M. Effect of feeding yogurt versus milk in children with acute diarrhea and carbohydrate malabsorption. Journal of Pediatric Gastroenterology and Nutrition 2001;33(3):307‐13. [DOI] [PubMed] [Google Scholar]
Boulloche 1994 {published data only}
- Boulloche J, Mouterde O, Mallet E. Management of acute diarrhoea in infants and young children. Controlled study of the anti‐diarrheal efficacy of killed L. acidophilus (LB strain) versus a placebo and a reference drug (loperamide). Annales de Pediatrie 1994;41(7):457‐63. [Google Scholar]
Bruno 1981 {published data only}
- Bruno F, Frigerio G. A new therapeutic alternative for the treatment of enteritis ‐‐ controlled double‐blind tests with the strain SF 68 [Eine neuartige möglichkeit zur behandlung der enteritis ‐ kontrollierte doppel‐blindversuche mit dem Stamm SF 68]. Schweizerische Rundschau fur Medizin Praxis 1981;70(39):1717‐20. [PubMed] [Google Scholar]
Bruno 1983 {published data only}
- Bruno F, Nastasi A, Bruno M. Double‐blind controlled study of the effect of the lactogenic enterococcus SF68 strain on various enterocolitis associated manifestations and on salmonella infections [Studio controllato dopio‐cieco sull'effecto dell'enterococco lattoproduttore ceppo SF 68 su manifestazione associate a forme enterocolitiche variee a salmonellosi]. La Clinica Terapeutica 1983;105(3):203‐7. [PubMed] [Google Scholar]
Buydens 1996 {published data only}
- Buydens P, Debeuckelaere S. Efficacy of SF68 in the treatment of acute diarrhea. A placebo‐controlled trial. Scandanavian Journal of Gastroenterology 1996;31(9):887‐91. [DOI] [PubMed] [Google Scholar]
Canani 2007 {published data only}
- Canani RB, Cirillo P, Terrin G, Cesarano L, Spagnuolo MI, Vincenzo A, et al. Probiotics for treatment of acute diarrhea in children: randomised clinical trial of five different preparations. BMJ 2007;335(7615):340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carague‐Orendain {unpublished data only}
- Carrague‐Orendain A. Randomized, double blind placebo‐controlled trial on the efficacy and safety of lactobacillus (Infloran Berna capsules) in the treatment of acute non‐bloody diarrhoea in children two to five years of age.
Cetina‐Sauri 1994 {published data only}
- Cetina‐Sauri G, Sierra Basto G. Evaluation of Saccharomyces boulardii in children with acute diarrhea [Evaluation therapeutique de Saccharomyces boulardii chez des enfants souffrant de diarrhee aigue]. Annales de Pediatrie 1994;41(6):397‐400. [Google Scholar]
Chapoy 1985 {published data only}
- Chapoy P. Treatment of acute infantile diarrhea: controlled trial of Saccharomyces boulardii [Traitement des diarrhées aiguës infantiles]. Annales de Pediatrie 1985;32(6):561‐3. [PubMed] [Google Scholar]
Chen 2010 {published data only}
- Chen CC, Kong MS, Lai MW, Chao HC, Chang KW, Chen SY, et al. Probiotics have clinical, microbiologic, and immunologic efficacy in acute infectious diarrhea. The Pediatric Infectious Diseases Journal 2010;29(2):135‐8. [DOI] [PubMed] [Google Scholar]
Costa‐Ribeiro 2003 {published data only}
- Costa‐Ribeiro H, Ribeiro TC, Mattos AP, Valois SS, Neri DA, Almeida P, et al. Limitations of probiotic therapy in acute, severe dehydrating diarrhea. Journal of Pediatric Gastroentology and Nutrition 2003;36(1):112‐5. [DOI] [PubMed] [Google Scholar]
Czerwionka 2009 {published data only}
- Czerwionka‐Szaflarska M, Murawska S, Swincow G. Evaluation of influence of oral treatment with probiotic and/or oral rehydration solution on course of acute diarrhoea in children. Przeglad Gastroenterologiczny 2009;4(3):166‐72. [Google Scholar]
D'Apuzzo 1982 {published data only}
- D'Apuzzo V, Salzberg R. The treatment of acute diarrhoea in paediatrics using Streptococcus faecium: results of a double blind trial [Die Behandlung der akuten Diarrho in der Padiatrie mit Streptococcus faecium: Resultae einer doppleblindstudie]. Therapeutische Umschau 1982;39(12):1033‐5. [PubMed] [Google Scholar]
Dubey 2008 {published data only}
- Dubey AP, Rajeshwari K, Chakravarty A, Famularo G. Use of VSL#3 in the treatment of rotavirus diarrhea in children: preliminary results. Journal of Clinincal Gastroenterology 2008;42 Suppl 3 Pt 1:S126‐9. [DOI] [PubMed] [Google Scholar]
Frigerio 1986 {published data only}
- Frigerio G. A lactic acid producer enterococcus in the prevention of antibiotic‐associated diarrhoea and in the treatment of acute diarrhoeal disorders: a double‐blind multicentre placebo‐controlled clinical trial (Abstract). Digestive Diseases and Sciences 1986;31 Suppl:496. [Google Scholar]
Grandi 2009 {published data only}
- Grandi G, Medina M, Soria R, Teran C, Araya M. Probiotics in the management of acute rotavirus diarrhea in Bolivian children: a randomized, double‐blind, controlled trial of two different preparations. Pediatric Research. 2010; Vol. 67:447 (abstract 10). [DOI] [PMC free article] [PubMed]
Guandalini 2000 {published data only}
- Guandalini S, Pensabene L, Zikri MA, Dias JA, Casali LB, Hoekstra H, et al. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhoea: a multicenter European trial. Journal of Pediatric Gastroenterology and Nutrition 2000;30(1):54‐60. [DOI] [PubMed] [Google Scholar]
Guarino 1997 {published data only}
- Canani RB, Albano F, Spagnuolo MI, Benedetto L, Stabile A, Guarino A. Effect of oral administration of Lactobacillus GG on the duration of diarrhea and on rotavirus excretion in ambulatory children (Abstract). Journal of Pediatric Gastroenterology and Nutrition 1997;24(4):469. [Google Scholar]
- Guarino A, Canani RB, Spagnuolo MI, Albano F, Benedetto L. Oral bacterial therapy reduces the duration of symptoms and viral excretion in children with mild diarrhoea. Journal of Pediatric Gastroenterology and Nutrition 1997;25(5):516‐9. [DOI] [PubMed] [Google Scholar]
Hafeez 2002 {published data only}
- Hafeez A, Tariq P, Ali S, Kundi ZU, Khan A, Hassan M. The efficacy of Saccharomyces boulardii in the treatment of acute watery diarrhoea in children: a multicentre randomized controlled trial. Journal of College of Physicians and Surgeons Pakistan 2002;12(7):432‐4. [Google Scholar]
Henker 2007a {published data only}
- Henker J, Laass M, Blokhin BM, Bolbot YK, Maydannik VG, Elze M, et al. The probioticE. coli strain Nissle 1917 (EcN) stops acute diarrhoea in infants and toddlers. European Journal of Paediatrics 2007;166(4):311‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henker 2008 {published data only}
- Henker J, Laass MW, Blokhin BM, Maydannik VG, Bolbot YK, Elze M, et al. Probiotic E.Coli Nissle 1917 versus placebo for treating diarrhea of greater than 4 days duration in infants and toddlers. The Pediatric Infectious Disease Journal 2008;27(6):494‐99. [DOI] [PubMed] [Google Scholar]
Hernandez 1998 {published data only}
- Hernandez CL, Pineda EE, Jimenez MIR, Lucena MS. Clinical therapeutic affect of Saccharomyces boulardii on children with acute diarrhea. Revista de Enfermedades Infecciosas en Pediatria March 1998;11(43):87‐9. [Google Scholar]
Hochter 1990 {published data only}
- Höchter W, Chase D, Hagenhoff G. Saccharomyces boulardii in the treatment of acute adult diarrhoea. [Saccharomyces boulardii bei acuter Erwachsenendiarrhoea]. Münchener medizinische Wochenschrift 1990;132(12):188‐92. [Google Scholar]
Htwe 2008 {published data only}
- Htwe K, Yee K.S, Tin M, Vandenplas Y. Effect of Saccharomyces boulardii in the treatment of acute watery diarrhea in Myanmar children: a randomized control study. American Journal of Tropical Medicine and Hygiene 2008;78(2):214‐16. [PubMed] [Google Scholar]
Isolauri 1994 {published data only}
- Isolauri E, Kaila M, Mykkanen H, Ling WH, Salminen S. Oral bacteriotherapy for viral gastroenteritis. Digestive Diseases and Sciences 1994;39(12):2595‐600. [DOI] [PubMed] [Google Scholar]
Jasinski 2002 {published data only}
- Jasinski C, Tanzi MN, Schelotto F, Varela G, Zanetta E, Acuna AM, et al. Efficacy of Lactobacillus GG in oral rehydration solution [Efectop del Lactobacillus casei administrado en el suero de rehidratacion oral, en el tratamiento de la enfermedad diarreica aguda]. Pediatrika 2002;22(7):231‐43. [Google Scholar]
Khanna 2005 {published data only}
- Khanna V, Seema A, Ashraf M, Abida M. Efficacy of tyndalized lactobacillus acidophilus in acute diarrhoea. Indian Journal of Pediatrics 2005;72(11):935‐8. [DOI] [PubMed] [Google Scholar]
Kianifar 2009 {published data only}
- Kianifar HR, Farid R, Ahanchian H, Jabbari F, Moghiman T, Sistanian A. Probiotics in the treatment of acute diarrhea in young children. Iranian Journal of Medical Sciences 2009;34(3):204‐7. [Google Scholar]
Kowalska‐Duplaga 1999 {published data only}
- Kowalska‐Duplaga K, Strus M, Heczko P, Krobicka B, Kurowska‐Baran D, Mrukowicz JZ. Lactobif, a marketed probiotic product containing Bifidobacterium ruminantium, was not effective in the treatment of acute rotavirus diarrhoea in infants. Gut 1999;44:17‐25. [Google Scholar]
Kowalska‐Duplaga 2004 {published data only}
- Kowalska‐Duplaga K, Krzysztof F, Szajewska H, Janiak R. Efficacy of Trilac® in the treatment of acute diarrhoea in infants and young children ‐ a multicentre, randomised, double blind placebo‐controlled study. Pediatria Wspolczesna, Gastroenterologia, Hepatologia i Zywienie Dziecka 2004;6(3):295‐9. [Google Scholar]
Kurugol 2005 {published data only}
- Kurugol Z, Koturoglu G. Effects of Saccharomyces boulardii in children with acute diarrhoea. Acta Paediatrica 2005;94(1):44‐7. [DOI] [PubMed] [Google Scholar]
Lee 2001 {published data only}
- Lee M‐C, Lin L‐H, Hung K‐L, Wu H‐Y. Oral bacterial therapy promotes recovery from acute diarrhoea in children. Acta Paediatrica Taiwan 2001;42(5):301‐5. [PubMed] [Google Scholar]
Lievin Le‐Maol 2007 {published data only}
- Lievin‐Le Maol V, Sarrazin‐Davilla L.E, Servin A.L. An experimental study and a randomized, double‐blind, placebo‐controlled clinical trial to evaluate the antisecretory activity of Lactobacillus acidophilus strain LB against non‐rotavirus diarrhea. Pediatrics 2007;120(4):795‐803. [DOI] [PubMed] [Google Scholar]
Mao 2008 {published data only}
- Mao M, Yu T, Xiong Y, Wang Z, Liu H, Gotteland M, et al. Effect of a lactose‐free milk formula supplemented with bifidobacteria and streptococci on the recovery from acute diarrhoea. Asia Pacific Journal of Clinical Nutrition 2008;17(1):30‐4. [PubMed] [Google Scholar]
Misra 2009 {published data only}
- Misra S, Sabui TK, Pal NK. A randomized controlled trial to evaluate the efficacy of Lactobacillus GG in infantile diarrhea. The Journal of Pediatrics 2009;155(1):129‐32. [DOI] [PubMed] [Google Scholar]
Narayanappa 2008 {published data only}
- Narayanappa D. Randomized double blinded controlled trial to evaluate the efficacy and safety of Bifilac in patients with acute viral diarrhea. Indian Journal of Pediatrics 2008;75(7):709‐13. [DOI] [PubMed] [Google Scholar]
Oandasan 1999 {unpublished data only}
- Oandasan M, Gatcheco F, Kapahmgan S. Randomized, double blind placebo‐controlled clinical trial on the efficacy and safety of Infloran berna capsules in the treatment of acute non‐bloody diarrhea in infants.
Ozkan 2007 {published data only}
- Ozkan TB, Sahin E, Erdemir G, Budak F. Effect of Saccharomyces boulardii in children with acute gastroenteritis and its relationship to the immune response. The Journal of International Medical Research 2007;35(2):201‐12. [DOI] [PubMed] [Google Scholar]
Pant 1996 {published data only}
- Pant AR, Graham SM, Allen SJ, Harikul S, Sabchareon A, Cuevas L, et al. Lactobacillus GG and acute diarrhoea in young children in the tropics. Journal of Tropical Pediatrics 1996;42(3):162‐5. [DOI] [PubMed] [Google Scholar]
Pashapour 2006 {published and unpublished data}
- Pashapour N, Iou SG. Evaluation of yogurt effect on acute diarrhea in 6‐24‐month‐old hospitalized infants. Turkish Journal of Pediatrics 2006;48(2):115‐18. [PubMed] [Google Scholar]
Rafeey 2008a {published data only}
- Rafeey M, Ostadrahimi A, Boniadi M, Ghorashi Z, Alizadeh MM, Hadafey V. Lactobacillus acidophilus yogurt and supplement in children with acute diarrhea: a clinical trial. Research Journal of Medical Sciences 2008;2(1):13‐18. [Google Scholar]
Raza 1995 {published data only}
- Raza S, Graham SM, Allen SJ, Sultana S, Cuevas L, Hart CA. Lactobacillus GG promotes recovery from acute nonbloody diarrhoea in Pakistan. The Pediatric Infectious Disease Journal 1995;14(2):107‐11. [DOI] [PubMed] [Google Scholar]
Ritchie 2010 {published data only}
- Ritchie BK, Brewster DR, Tran CD, Davidson GP, McNeil Y, Butler RN. Efficacy of Lactobacillus GG in Aboriginal children with acute diarrhoeal disease: a randomised clinical trial. Journal of Pediatric Gastroenterology and Nutrition 2010;50(6):619‐24. [DOI] [PubMed] [Google Scholar]
Rosenfeldt 2002a {published data only}
- Rosenfeldt V, Michaelsen KF, Jakobsen M, Larsen CN, Moller PL, Pedersen P. Effect of probiotic Lactobacillus strains in young children hospitalized with acute diarrhea. The Pediatric Infectious Disease Journal 2002;21(5):411‐6. [DOI] [PubMed] [Google Scholar]
Rosenfeldt 2002b {published data only}
- Rosenfeldt V, Michaelsen KF, Jakobsen M, Larsen CN, Moller PL, Tvede M, et al. Effect of probiotic Lactobacillus strains on acute diarrhea in a cohort of nonhospitalized children attending day‐care centers. The Pediatric Infectious Disease Journal 2002;21(5):417‐9. [DOI] [PubMed] [Google Scholar]
Sarkar 2005 {published data only}
- Sarker SA, Sultana S, Fuchs GJ, Alam NH, Azim T, Brűssow H, et al. Lactobacillus paracasei strain ST11 has no effect on rotavirus but ameliorates the outcome of nonrotavirus diarrhea in children from Bangladesh. Pediatrics 2005;116(2):e221‐8. [DOI] [PubMed] [Google Scholar]
Sepp 1995 {published data only}
- Sepp E, Tamm E, Torm S, Lutsar I, Mikelsaar M, Salminen S. Impact of a Lactobacillus probiotic on the faecal microflora in children with shigellosis. Microecology and Therapy 1995;23(1):74‐80. [Google Scholar]
Shornikova 1997a {published data only}
- Shornikova AV, Isolauri E, Burkanova L, Lukovnikova S, Vesikari T. A trial in the Karelian Republic of oral rehydration and Lactobacillus GG for treatment of acute diarrhoea. Acta Paediatrica 1997;86(5):460‐5. [DOI] [PubMed] [Google Scholar]
Shornikova 1997b {published data only}
- Shornikova AV, Casas IA, Mykkanen H, Salo E, Vesikari T. Bacteriotherapy with Lactobacillus reuteri in rotavirus gastroenteritis. The Pediatric Infectious Disease Journal 1997;16(12):1103‐7. [DOI] [PubMed] [Google Scholar]
Shornikova 1997c {published data only}
- Shornikova AV, Casas IA, Isolauri E, Mykkanen H, Vesikari T. Lactobacillus reuteri as a therapeutic agent in acute diarrhea in young children. Journal of Pediatric Gastroenterology and Nutrition 1997;24(4):399‐404. [DOI] [PubMed] [Google Scholar]
Simakachorn 2000 {published data only}
- Simakachorn N, Pichaipat V, Rithipornpaisarn P, Kongkaew C, Tongpradit P, Varavithya W. Clinical evaluation of the addition of lyophilized, heat‐killed Lactobacillus acidophilus LB to oral rehydration therapy in the treatment of acute diarrhoea in children. Journal of Pediatric Gastroenterology and Nutrition 2000;30(1):68‐72. [DOI] [PubMed] [Google Scholar]
- Simakachorn N, Pichaipat V, Rithipornpaisarn P, Kongkaew C, Tongpradit P, Varavithya W. Erratum. Journal of Pediatric Gastroenterology 2000;30(2):228. [DOI] [PubMed] [Google Scholar]
Sugita 1994 {published data only}
- Sugita T, Togawa M. Efficacy of Lactobacillus preparation bioloactis powder in children with rotavirus enteritis. Japan Journal of Pediatrics 1994;47:2755‐62. [Google Scholar]
Szymanski 2006 {published data only}
- Szymanski H, Pejcz J, Jawien M, Chmielarczyk A, Strus M, Heczko PB. Treatment of acute infectious diarrhoea in infants and children with a mixture of three Lactobacillus rhamnosus strains ‐‐ a randomized, double‐blind, placebo‐controlled trial. Alimentary Pharmacology and Therapeutics 2006;23(2):247‐53. [DOI] [PubMed] [Google Scholar]
Teran 2009 {published data only}
- Teran CG, Teran‐Escalera CN, Villarroel P. Nitazoxanide vs. probiotics for the treatment of acute rotavirus diarrhea in children: a randomized, single‐blind, controlled trial in Bolivian children. International Journal of Infectiouos Diseases 2009;13(4):518—23. [DOI] [PubMed] [Google Scholar]
Táborská 1997 {published data only}
- Táborská J, Pazdiora P. Smecta and Lactobacillus acidophilus ND in the treatment of acute diarrhoea in children [Smecta a Lactobacillus acidophilus ND v lécbe akutních detských prujmu]. Ceskoslovenská pediatrie 1997;52(1):29‐33. [Google Scholar]
Urganci 2001 {published data only}
- Urganci N, Polat T, Uysalol M, Cetinkaya F. Evaluation of the efficacy of Saccharomyces boulardii in children with acute diarrhoea. Archives of Gastroenterohepatology 2001;20(3‐4):81‐3. [Google Scholar]
Villarruel 2007 {published data only}
- Villarruel G, Rubio DM, Lopez F, Cintioni J, Gurevech R, Romero G, et al. Saccharomyces boulardii in acute childhood diarrhea: a randomised placebo controlled study. Acta Paediatrica 2007;96(4):538‐41. [DOI] [PubMed] [Google Scholar]
Vivatvakin 2006 {published data only}
- Vivatvakin B, Kowitdamrong E. Randomized control trial of live Lactobacillus acidophilus plus Bifidobacterium infantis in treatment of infantile acute watery diarrhea. Journal of the Medical Association of Thailand 2006;89:Suppl 3:S126‐33. [PubMed] [Google Scholar]
Wunderlich 1989 {published data only}
- Wunderlich PF, Braun L, Fumagalli I, D'Apuzzo V, Heim F, Karly M, et al. Double‐blind report of the efficacy of lactic acid‐producing Enterococcus SF68 in the prevention of antibiotic‐associated diarrhoea and in the treatment of acute diarrhoea. The Journal of International Medical Research 1989;17(4):333‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agarwal 2001 {published data only}
- Agarwal KN, Bhasin SK, Faridi MMA, Mathur M, Gupta S. Lactobacillus casei in the control of acute diarrhoea ‐ a pilot study. Indian Pediatrics 2001;38(8):905‐10. [PubMed] [Google Scholar]
Agarwal 2002 {published data only}
- Agarwal KN, Bhasin SK. Feasibility studies to control acute diarrhoea in children by feeding fermented milk preparations Actimel and Indian Dahi. European Journal of Clinical Nutrition 2002;56(Suppl. 4):S56‐9. [DOI] [PubMed] [Google Scholar]
Alexander 1971 {published data only}
- Alexander JG. Lactobacillus casei tablets in the treatment of intestinal infection. The Journal of the Royal College of General Practitioners 1971;21(111):623‐4. [PMC free article] [PubMed] [Google Scholar]
Alvisi 1982 {published data only}
- Alvisi V, Tralli M, Loponte A, Pavani F, Massari M. Double‐blind study of treatment with SF 68 or with antibiotics in acute enteritis in adults [Studio in doppio cieco sul trattamento con SF68 o con antibiotici nelle enteritis acute dell'adulto]. La Clinica Terapeutica 1982;101(6):581‐6. [PubMed] [Google Scholar]
Barone 2000 {published data only}
- Barone C, Pettinato R, Avola E, Alberti A, Greco D, Failla P, et al. Comparison of three probiotics in the treatment of acute diarrhea in mentally retarded children. Minerva Pediatrica 2000;52(3):161‐5. [PubMed] [Google Scholar]
Beck 1961 {published data only}
- Beck C, Necheles H. Beneficial effects of administration of Lactobacillus acidophilus in diarrheal and other intestinal disorders. The American Journal of Gastroenterology 1961;35:522‐30. [PubMed] [Google Scholar]
Bellomo 1979 {published data only}
- Bellomo G, Finocchiaro C, Frigerio G. A new approach for the treatment of enteritides in paediatrics [Une novelle approche pour le traitement des entérites en pédiatrie]. Médecine et Hygiène 1979;37:3781‐4. [Google Scholar]
Bellomo 1980 {published data only}
- Bellomo G, Mangiagle A, Nicastro L, Frigeria G. A controlled double‐blind study of SF68 strain as a new biological preparation for the treatment of diarrhoea in pediatrics. Current Therapeutic Research: Clinical and Experimental 1980;28(6):927‐35. [Google Scholar]
Bellomo 1982 {published data only}
- Bellomo G, Finocchiaro C, Frigerio G, Mangiagli A, Nicastro L. Controlled study ofEnterococcus LAB strain SF68 in acute enteritis in children with concomitant respiratory infection [Studio controllato sull'enterococco L.A.B. ceppo SF 68 nelle enteriti acute del bambino concomitanti ad infezioni delle vie respiratorie]. Clinica Pediatrica 1982;64:219‐27. [Google Scholar]
Bin Li Xie 1995 {published data only}
- Bin Li Xie. Controlled clinical trial of Lacteol Fort Sachet versus furazolidone or berberine in treatment of acute diarrhoea in children [Étude contrôlée du Lactéol Fort sachets versus furazolidone ou berbérine dans le traitement des diarrhées aiguës de l'enfant]. Annales de Pediatrie 1995;42(6):396‐401. [Google Scholar]
Brewster 2004 {published data only}
- Brewster DR, Ritchie B, McNeil Y, Davidson G, Butler R. Efficacy of probiotic therapy in Aboriginal children with acute diarrheal disease. Poster presentation 2004.
Camarri 1981 {published data only}
- Camarri E, Belvisi A, Guidoni G, Marini G, Frigerio G. A double‐blind comparison of two different treatments for acute enteritis in adults. Chemotherapy 1981;27(6):466‐70. [DOI] [PubMed] [Google Scholar]
Cetina Sauri 1990 {published data only}
- Cetina‐Sauri G, Basto GS. Therapeutic evaluation of children with acute diarrhea. Tribuna Medica 1990;81(3):141‐4. [Google Scholar]
Chandra 2002 {published data only}
- Chandra RK. Effect of Lactobacillus on the incidence and severity of acute rotavirus diarrhoea in infants. A prospective placebo‐controlled double‐blind study. Nutrition Research 2002;22(1):65‐9. [Google Scholar]
Chicoine 1973 {published data only}
- Chicoine L, Joncas JH. Use of lactic enzymes in non‐bacterial gastroenteritis [Emploi des ferments lactiques dans la gastro‐entérite non bactérienne]. L'Union Médicale du Canada 1973;102(5):1114‐5. [PubMed] [Google Scholar]
Costa‐Ribeiro 2000a {published data only}
- Costa‐Ribeiro H, Ribeiro TCM, Mattos AP, Almeida PS, Valois SS, Vanderhoof JA. Use of Lactobacillus GG in the treatment of severe, acute diarrhoea in adverse environmental conditions.. Journal of Pediatric Gastroenterology and Nutrition 2000;31 Suppl 2:251‐2. [Google Scholar]
Costa‐Ribeiro 2000b {published data only}
- Costa‐Ribeiro H, Ribeiro TCM, Mattos AP, Lins EV, Neri DA, Valois SS, Vanderhoof JA. Prophylactic administration of Lactobacillus GG to children in a daycare center. Journal of Pediatric Gastroenterology and Nutrition 2000;31 Suppl 2:252. [Google Scholar]
Cui 2004 {published data only}
- Cui Y‐L, Wan F‐C, Tang D‐L, Wu S‐H. Efficacy of Bacillus coagulans tablets in the treatment of acute and chronic diarrhoea. International Journal of Immunotherapy 2004;20(1):17‐22. [Google Scholar]
de dios Pozo‐O 1978 {published data only}
- dios Pozo‐Olano J, Warram JH Jr, Gomez RG, Cavazos MG. Effect of a lactobacilli preparation on traveler's diarrhoea. A randomised, double blind clinical trial. Gastroenterology 1978;74(5 Pt 1):829‐30. [PubMed] [Google Scholar]
Eren 2010 {published data only}
- Eren M, Dinleyici EC, Vandenplas Y. Clinical efficacy comparison of Saccharomyces boulardii and yogurt fluid in acute non‐bloody diarrhea in children: a randomized, controlled, open label study. American Journal of Tropical Hygiene 2010;82(3):488‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fang 2009 {published data only}
- Fang S B, Lee H‐C, Hu J‐J, Hou S‐Y, Liu H‐L, Fang H‐W. Dose‐dependent effect of Lactobacillus rhamnosus on quantitative reduction of faecal rotavirus shedding in children. Journal of Tropical Pediatrics 2009;55(5):297‐301. [DOI] [PubMed] [Google Scholar]
Fourrier 1968 {published data only}
- Fourrier A, Lequien P. The treatment of infantile gastroenteritis by the use solely of a combination of colibacillus and lactobacillus. Apropos of 56 cases. Annales de Pediatrie 1968;15:491‐5. [PubMed] [Google Scholar]
Girola 1995 {published data only}
- Girola M, Ventura P. Efficacy of probiotic preparation with living, freeze‐dried lactic acid bacteria and yeast on child diarrhoea [Efficacia di un prodotto probiotico a base di fermenti lattici e lievito vitali liofilizzati nel trattamento della diarrea del bambino]. Archivio di Medicina Interna 1995;47(2‐3):61‐72. [Google Scholar]
Gracheva 1996 {published data only}
- Gracheva NM, Gavrilov AF, Solov'eva AI, Smirnov VV, Sorokulova IB, Reznik SR, et al. The efficacy of the new bacterial preparation biosporin in treating acute intestinal infections. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1996;1(1):75‐7. [PubMed] [Google Scholar]
Henker 2007b {published data only}
- Henker J, Blokhin BM, Bolbot YK, Maydannik VG. Acute diarrhoea in infants and small children. Successful adjuvant therapy with the probiotic Mutaflor [Akute diarrhő bei säuglingen und kleinkindern. Erfolgreiche adjuvante therapie mit dem probiotikum Mutaflor]. Pädiat. Prax 2007;71:605‐10. [Google Scholar]
Heydarian 2010 {published data only}
- Heydarian F, Kianifar HR, Ahanchian H, Khakshure A, Seyedi J, Moshirian D. A comparison between traditional yogurt and probiotic yogurt in non‐inflammatory acute gastroenteritis. Saudi Med J 2010;31(3):280‐3. [PubMed] [Google Scholar]
Isolauri 1991 {published data only}
- Isolauri E, Juntunen M, Rautanen T, Sillanaukee P, Koivula T. A human Lactobacillus strain (Lactobacillus casei GG) promotes recovery from acute diarrhea in children. Pediatrics 1991;88(1):90‐7. [PubMed] [Google Scholar]
Kaila 1992 {published data only}
- Kaila M, Isolauri E, Soppi E, Virtanen E, Laine S, Arvilommi H. Enhancement of the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus strain. Pediatric Research 1992;32(2):141‐4. [DOI] [PubMed] [Google Scholar]
Kaila 1995 {published data only}
- Kaila M, Isolauri E, Saxelin M, Arvilommi H, Vesikari T. Viable versus inactivated Lactobacillus strain GG in acute rotavirus diarrhoea. Archives of Disease in Childhood 1995;72(1):51‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Korviakova 2000 {published data only}
- Korviakova EP. Use of loading doses of Bifidumbacterin forte for the treatment of patients with acute enteric infections. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 2000;6:58‐61. [PubMed] [Google Scholar]
Le Leyur 2010 {published data only}
- Luyer B, Makhoul G, Duhamel JF. A multicentric study of a lactose free formula supplemented with Saccharomyces boulardii in children with acute diarrhea. Archives de Pédiatrie 2010;17(5):459‐65. [DOI] [PubMed] [Google Scholar]
Lei 2006 {published data only}
- Lei V, Friis H, Michaelsen KF. Spontaneously fermented millet product as a natural probiotic treatment for diarrhea in young children: an intervention study Northern Ghana. International Journal of Food Microbiology 2006;110(3):246‐53. [DOI] [PubMed] [Google Scholar]
Lin 2009 {published data only}
- Lin JS, Chiu YH, Lin NT, Chu CH, Huang KC, Liao KW, et al. Different effects of probiotic species/strains on infections in preschool children: a double‐blind, randomized, controlled study. Vaccine 2009;27(7):1073‐9. [DOI] [PubMed] [Google Scholar]
Magreiter 2006 {published data only}
- Margreiter M, Ludl K, Phleps W, Kaehler ST. Therapeutic value of a Lactobacillus gasseri and Bifdobacterium longum fixed bacterium combination in acute diarrhea: a randomized, double‐blind, controlled clinical trial. International Journal of Clinical Pharmacology and Therapeutics May 2006;44(5):207‐15. [DOI] [PubMed] [Google Scholar]
Majamaa 1995 {published data only}
- Majamaa H, Isolauri E, Saxelin M, Vesikari T. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. Journal of Pediatric Gastroenterology and Nutrition 1995;20(3):333‐8. [DOI] [PubMed] [Google Scholar]
Mazo 2006 {published data only}
- Mazo SA, Arias SA. Efficacy and safety of milk fermented by lactobacillus (kumis) in nutritional recovery of undernourished children and control of their diarrhoea episodes. Revista Facultad Nacional de Salud Pública 2006;24(2):83‐97. [Google Scholar]
Michielutti 1995 {published data only}
- Michielutti F, Bertini M, Presciuttini B, Andreotti G. Clinical assessment of a new oral bacterial treatment for children with acute diarrhea [Valutazione clinica di un nuovo batterioterapico orale in pazienti di eta pediatrica con diarrea acuta]. Minerva Medica 1996;87(11):545‐50. [PubMed] [Google Scholar]
Mitra 1990 {published data only}
- Mitra AK, Rabbini GH. A double‐blind, controlled trial of bioflorin (Streptococcus faecium SF68) in adults with acute diarrhea due to Vibrio chlolerae and entertoxigenic Escherichia coli. Gastroenterology 1990;99(4):1149‐52. [DOI] [PubMed] [Google Scholar]
Moraes 2001 {published data only}
- Moraes E, Chinzon D, Coelho LG, Fernandes TF, Haddad MT, et al. A multicentric, randomised, investigator‐blind, parallel group study to assess the efficacy, safety and tolerability of Racecadotril versus Saccharomyces boulardii in the treatment of acute diarrhoea in adults [Estudo multicêntrico de grupos paralelos, randomizado, cego para o investigador, para avaliar a eficácia, segurança e tolerabilidade do racecadotril versus Saccharomyces boulardii no tratamento da diarréia aguda em adultos]. Revista Brasileira de Medicina 2001;58(1‐2):65‐74. [Google Scholar]
Niv 1963 {published data only}
- Niv M, Levy W, Greenstein NM. Yogurt in the treatment of infantile diarrhea. Clinical Pediatrics 1963;2(7):407‐11. [Google Scholar]
Ortlieb 1974 {published data only}
- Ortlieb R. Randomized comparative testing of a new drug in intestinal disorders in a child. Therapie der Gegenwart 1974;113(1):76‐8. [PubMed] [Google Scholar]
Pearce 1974 {published data only}
- Pearce JL, Hamilton JR. Controlled trial of orally administered lactobacilli in acute infantile diarrhea. The Journal of Pediatrics 1974;84(2):261‐2. [DOI] [PubMed] [Google Scholar]
Pedone 1999 {published data only}
- Pedone CA, Bernabeu AO, Postaire ER, Bouley CF, Reinert P. The effect of supplementation with milk fermented by Lactobacillus casei (strain DN‐114 001) on acute diarrhoea in children attending day care centres. International Journal of Clinical Practice 1999;53(3):179‐84. [PubMed] [Google Scholar]
Pedone 2000 {published data only}
- Pedone CA, Arnaud CC, Postaire ER, Bouley CF, Reinert P. Multicentric study of the effect of milk fermented by Lactobacillus casei on the incidence of diarrhoea. International Journal of Clinical Practice 2000;54(9):568‐71. [PubMed] [Google Scholar]
Pene 1966 {published data only}
- Pene P, Linhard J, Bernou JC. The colibacillus‐lactobacillus combination in the treatment of diarrhea in adults, children and infants [L'association colibacilles‐lactobacilles dans le traitement des diarrhées de l'adulte, de l'enfant et du nourrisson]. La Semaine Des Hopitaux 1966;42(4):241‐4. [PubMed] [Google Scholar]
Rafeey 2008b {published data only}
- Rafeey M, Ghojazadeh M, Hadari V. Probiotics in children with acute diarrhea. WCPGHAN 3: World Congress of Pediatric Gastroenterology, Hepatology and Nutrition 2008:129‐35. [Google Scholar]
Rautanen 1998 {published data only}
- Rautanen T, Isolauri E, Salo E, Vesikari T. Management of acute diarrhoea with low osmolarity oral rehydration solutions and Lactobacillus strain GG. Archives of Disease in Childhood 1998;79(2):157‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saint‐Marc 1991 {published data only}
- Saint‐Marc T, Rossello‐Prats L, Touraine JL. Efficacy of Saccharomyces boulardii in the treatment of diarrhea in AIDS [Efficacite de Saccharomyces boulardii dans le traitement des diarrhees du SIDA]. Annales de Médecine Interne 1991;142(1):64‐5. [PubMed] [Google Scholar]
Salazar‐Lindo 2004 {published data only}
- Salazar‐Lindo E, Miranda‐Langschwager P, Campos‐Sanchez M, Chea‐Woo E, Bradley Sack R. Lactobacillus casei strain GG in the treatment of infants with acute watery diarrhoea: a randomised, double‐blind, placebo controlled clinical trial. BMC Pediatrics 2004; Vol. 4, issue 18. [http://www.biomedcentral.com/1471‐2431/4/18] [DOI] [PMC free article] [PubMed]
Salazar‐Lindo 2007 {published data only}
- Salazar‐Lindo E, Figueroa‐Quintanilla D, Caciano MI, Reto‐Valiente V, Chauviere G, Colin P, et al. Effectiveness and safety of Lactobacillus LB in the treatment of mild acute darrhea in children. Journal of Paediatric Gastroenterology and Nutrition 2006;44(5):571‐6. [DOI] [PubMed] [Google Scholar]
Satoh 1984 {published data only}
- Satoh Y, Iwata S, Iwata Y, Yamashita N, Oikawa T, Osano M, et al. Effect of Bifidobacterium breve administration on clinical course and intestinal flora in children with acute diarrhea [Abstract]. Nippon Shonikagakkai Zasshi 1984;88:2178‐9. [Google Scholar]
Savas‐Erdeve 2009 {published data only}
- Savas‐Erdeve S, Gökay S, Dallar Y. Efficacy and safety of Saccharomyces boulardii in amebiasis‐associated diarrhea in children. The Turkish Journal of Pediatrics 2009;51(3):220‐4. [PubMed] [Google Scholar]
Schrezenmeir 2004 {published data only}
- Schrezenmeir J, Heller K, McCue M, Llamas C, Lam W, Burrow H, et al. Benefits of oral supplementation with and without synbiotics in young children. Clinical Paediatrics 2004;43:239‐49. [DOI] [PubMed] [Google Scholar]
Singh 1987 {published data only}
- Singh T. Yoghurt feeding during acute diarrhea. Indian Pediatrics 1987;24(6):530. [PubMed] [Google Scholar]
Sudarmo 2003 {published data only}
- Sudarmo SM, Ranuh RG, Rochim A, Soeparto P. Management of infant diarrhea with high‐lactose probiotic‐containing formula. Southeast Asian Journal of Tropical Medicine and Public Health 2003;34(4):845‐8. [PubMed] [Google Scholar]
Szymanski 2005 {published data only}
- Szymanski H, Pejcz J, Jawien M, Kucharska A, Strus M, Heczko PB. Treatment of acute infectious diarrhoea in children with a mixture of three new Lactobacillus rhamnosus strains (573L/1,573L/2, 573L/3). A randomized, double‐blind, placebo‐controlled trial. Journal of Pediatric Gastroenterology and Nutrition 2005;40(5):652. [Google Scholar]
Tojo 1987 {published data only}
- Tojo M, Oikawa T, Morikawa Y, Yamashita N, Iwata S, Satoh Y, et al. The effects of Bifidobacterium breve administration on campylobacter enteritis. Acta Paediatrica Japonica 1987;29(1):160‐7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Contreras 1983 {published data only}
- Contreras G. Compend Invest Clin Latinoam 1983;3:114‐6. [Google Scholar]
Salgado {published data only}
- Salgado AJ, Garcia Jara JA. Use of heat‐killedLactobacillus acidophilus, Lacteol strain in the treatment of acute diarrhoea. Open trial. Revista del Hospital de la Muyer 2. [Google Scholar]
References to ongoing studies
Freedman 2010 {published data only}
- Ongoing study Starting date of trial not provided. Contact author for more information.
Additional references
Allen 2003
- Allen SJ, Okoko B, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating infectious diarrhoea. Cochrane Database of Systematic Reviews 2003, Issue 4. [Art. No.: CD003048. DOI: 10.1002/14651858.CD003048.pub2] [DOI] [PubMed] [Google Scholar]
Anonymous 1988
- Anonymous. Persistent diarrhoea in children in developing countries: memorandum from a WHO meeting. Bulletin of the World Health Organization 1988;66(6):709‐17. [PMC free article] [PubMed] [Google Scholar]
Baqui 1991
- Baqui AH, Black RE, Yunus M, Hoque AR, Chowdhury HR, Sack RB. Methodological issues in diarrhoeal diseases epidemiology: definition of diarrhoeal episodes. International Journal of Epidemiology 1991;20(4):1057‐63. [DOI] [PubMed] [Google Scholar]
Black 1986
- Black RE. Pathogens that cause traveler's diarrhea in Latin America and Africa. Reviews of Infectious Diseases 1986;8 Suppl 2:131‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bryce 2006
- Bryce J, Terreri N, Victora CG, Mason E, Daelmans B, Bhutta ZA, et al. Countdown to 2015: tracking intervention coverage for child survival. Lancet 2006;368(9541):1067‐76. [DOI] [PubMed] [Google Scholar]
Chmielewska 2008
- Chmielewska A, Ruszczyński M, Szajewska H. Lactobacillus reuteri strain ATCC 55730 for the treatment of acute infectious diarrhoea in children: a meta‐analysis of randomized controlled trials. Metaanalysis 2008;10:32‐6. [Google Scholar]
Clarke 2003
- Clarke M, Oxman A, editors. Optimal search strategy. Cochrane Reviewers' Handbook 4.2 [updated March 2003]; Appendix 5c. In: The Cochrane Library. The Cochrane Collaboration. Chichester, UK: John Wiley & Sons, Ltd.; 2003, Issue 3.
Cunliffe 1998
- Cunliffe NA, Kilgore PE, Bresee JS, Steele AD, Luo N, Hart CA, et al. Epidemiology of rotavirus diarrhoea in Africa: a review to assess the need for rotavirus immunization. Bulletin of the World Health Organization 1998;76(5):525‐37. [PMC free article] [PubMed] [Google Scholar]
FAO/WHO 2001
- Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO). Joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk and live lactic acid bacteria. ftp://ftp.fao.es/esn/food/probio_report_en.pdf 2001.
Gadewar 2005
- Gadewar S, Fasano A. Current concepts in the evaluation, diagnosis and management of acute infectious diarrhea. Current Opinion in Pharmacology 2005;5(6):559‐65. [DOI] [PubMed] [Google Scholar]
Gismondo 1999
- Gismondo MR, Drago L, Lombardi A. Review of probiotics available to modify gastrointestinal flora. International Journal of Antimicrobial Agents 1999;12(4):287‐92. [DOI] [PubMed] [Google Scholar]
Goldin 1998
- Goldin BR. Health benefits of probiotics. The British Journal of Nutrition 1998;80 Suppl(4):203‐7. [PubMed] [Google Scholar]
Guerrant 1990
- Guerrant RL, Hughes JM, Lima NL, Crane J. Diarrhea in developed and developing countries: magnitude, special settings, and etiologies. Reviews of Infectious Diseases 1990;12 Suppl 1:41‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hata 1988
- Hata D, Yoshida A, Ohkubo H, Mochizuki Y, Hosoki Y, Tanaka R, et al. Meningitis caused by Bifidobacterium in an infant. The Pediatric Infectious Disease Journal 1988;7(9):669‐71. [DOI] [PubMed] [Google Scholar]
Huilan 1991
- Huilan S, Zhen LG, Mathan MM, Mathew MM, Olarte J, Espejo R, et al. Etiology of acute diarrhoea among children in developing countries: a multicentre study in five countries. Bulletin of the World Health Organization 1991;69(5):549‐55. [PMC free article] [PubMed] [Google Scholar]
Juni 1999
- Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta‐analysis. JAMA: the Journal of the American Medical Association 1999;282(11):1054‐60. [DOI] [PubMed] [Google Scholar]
Klein 1998
- Klein G, Pack A, Bonaparte C, Reuter G. Taxonomy and physiology of the probiotic lactic acid bacteria. International Journal of Food Microbiology 1998;41(2):103‐25. [DOI] [PubMed] [Google Scholar]
Lopez 2006
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367(9524):1747‐57. [DOI] [PubMed] [Google Scholar]
Naidu 1999
- Naidu AS, Bidlack WR, Clemens RA. Probiotic spectra of lactic acid bacteria (LAB). Critical Reviews in Food Science and Nutrition 1999;39(1):13‐126. [DOI] [PubMed] [Google Scholar]
O'Ryan 2005
- O'Ryan M, Prado V, Pickering LK. A millennium update on pediatric diarrhoeal illness in the developing world. Seminars in Pediatric Infectious Diseases 2005;16(2):125‐36. [DOI] [PubMed] [Google Scholar]
Piarroux 1999
- Piarroux R, Millon L, Bardonnet K, Vagner O, Koenig H. Are live saccharomyces yeasts harmful to patients?. Lancet 1999;353(9167):1851‐2. [DOI] [PubMed] [Google Scholar]
Rijkers 2010
- Rijkers GT, Bengmark S, Enck P, Haller D, Herz U, Kaolliomaki M, et al. Guidance for substantiating the evidence for beneficial effects of probiotics: current status and recommendations for future research. Journal of Nutrition 2010;140(3):671S‐6S. [DOI] [PubMed] [Google Scholar]
Salminen 1998
- Salminen S, Wright A, Morelli L, Marteau P, Brassart D, Vos WM, et al. Demonstration of safety of probiotics ‐‐ a review. International Journal of Food Microbiology 1998;44(1‐2):93‐106. [DOI] [PubMed] [Google Scholar]
Salminen 1999
- Salminen S, Ouwehand A, Benno Y, Lee YK. Probiotics: how should they be defined?. Trends in Food Science & Technology 1999;10:107‐10. [Google Scholar]
Savarino 1993
- Savarino SJ, Bourgeois AL. Diarrhoeal disease: current concepts and future challenges. Epidemiology of diarrhoeal diseases in developed countries. Transactions of the Royal Society of Tropical Medicine and Hygiene 1993;87 Suppl 3:7‐11. [DOI] [PubMed] [Google Scholar]
Saxelin 1996
- Saxelin M, Chuang NH, Chassy B, Rautelin H, Makela PH, Salminen S, et al. Lactobacilli and bacteremia in southern Finland, 1989‐1992. Clinical Infectious Diseases 1996;22(3):564‐6. [DOI] [PubMed] [Google Scholar]
Sussman 1986
- Sussman J, Baron E, Goldberg S, Kaplan M, Pizzarello R. Clinical manifestations and therapy of Lactobacillus endocarditis: report of a case and review of the literature. Reviews of Infectious Diseases 1986;8(5):771‐6. [DOI] [PubMed] [Google Scholar]
Szajewska 2001
- Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhoea in infants and children; a systematic review of published randomized, double‐blind, placebo‐controlled trials. Journal of Pediatric Gastroenterology and Nutrition 2001;33 Suppl 2:17‐25. [DOI] [PubMed] [Google Scholar]
Szajewska 2007a
- Szajewska H, Skorka A, Ruszczynski M, Gieruszczak‐Bialek D. Meta‐analysis: Lactobacillus GG for treating acute diarrhoea in children. Alimentary Pharmacology & Therapeutics 2007;25(8):871–81. [DOI] [PubMed] [Google Scholar]
Szajewska 2007b
- Szajewska H, Skórka A, Dylag M. Meta‐analysis: Saccharomyces boulardii for treating acute diarrhoea in children. Alimentary Pharmacology & Therapeutics 2007;25:257‐64. [DOI] [PubMed] [Google Scholar]
Szajewska 2009
- Szajewska H, Skórka A. Saccharomyces boulardii for treating acute gastroenteritis in children: updated meta‐analysis of randomized controlled trials. Alimentary Pharmacology & Therapeutics 2009;30:960‐1. [DOI] [PubMed] [Google Scholar]
Van Niel 2002
- Niel CW, Feudtner C, Garrison MM, Christakis DA. Lactobacillus therapy for acute infectious diarrhoea in children: a meta‐analysis. Pediatrics 2002;109(4):678‐84. [DOI] [PubMed] [Google Scholar]
Vanderhoof 1998
- Vanderhoof JA, Young RJ. Use of probiotics in childhood gastrointestinal disorders. Journal of Pediatric Gastroenterology and Nutrition 1998;27(3):323‐32. [DOI] [PubMed] [Google Scholar]
Walker‐Smith 1993
- Walker‐Smith JA. Diarrhoeal disease: current concepts and future challenges. Malnutrition and infection. Transactions of the Royal Society of Tropical Medicine 1993;87 Suppl 3:13‐5. [DOI] [PubMed] [Google Scholar]
WHO 1990
- Division of Diarrhoeal and Acute Respiratory Disease Control. The treatment of diarrhoea. A manual for physicians and other senior health workers. Geneva: World Health Organization, 1990:20‐1. [Google Scholar]
WHO 2001
- World Health Organization. The world health report : 2001: mental health : new understanding, new hope. Geneva: World Health Organization, 2001. [Google Scholar]
Wolvers 2010
- Wolvers D, Antoine JM, Myllyluoma E, Schrezenmeir J, Szajewska H, Rijkers GT. Guidance for substantiating the evidence for beneficial effects of probiotics: prevention and management of infections by probiotics. Journal of Nutrition 2010;140(3):698S‐712S. [DOI] [PubMed] [Google Scholar]


