Abstract
Sustained spermatogenesis in adult males relies on the activity of spermatogonial stem cells (SSCs). In general, tissue-specific stem cell populations such as SSCs are influenced by contributions of support cells that form niche microenvironments. Previous studies have provided indirect evidence that several somatic cell populations and the interstitial vasculature influence SSC functions, but an individual orchestrator of niches has not been described. In this study, functional transplantation of SSCs, in combination with experimental alteration of Sertoli cell content by polythiouracil (PTU)-induced transient hypothyroidism, was used to explore the relationship of Sertoli cells with SSCs in testes of adult mice. Transplantation of SSCs from PTU-treated donor mice into seminiferous tubules of normal recipient mice revealed a greater than 3-fold increase in SSCs compared to those from testes of non-PTU-treated donors. In addition, use of PTU-treated mice as recipients for transplantation of SSCs from normal donors revealed a greater than 3-fold increase of accessible niches compared to those of testes of non-PTU treated recipient mice with normal numbers of Sertoli cells. Importantly, the area of seminiferous tubules bordered by interstitial tissue and percentage of seminiferous tubules associated with blood vessels was found to be no different in testes of PTU-treated mice compared to controls, indicating that neither the vasculature nor interstitial support cell populations influenced the alteration of niche number. Collectively, these results provide direct evidence that Sertoli cells are the key somatic cell population dictating the number of SSCs and niches in mammalian testes.
Keywords: niche, Sertoli cells, spermatogenesis, spermatogonial stem cell
Sertoli cell number was found to dictate the number of spermatogonial stem cells (SSCs) and accessible niches in testes of adult mice.
INTRODUCTION
During steady-state spermatogenesis, millions of spermatozoa are produced daily within testes to ensure continual male fertility [1]. This process relies on the self-renewal and differentiation of a tissue-specific stem cell population referred to as spermatogonial stem cells (SSCs) in mammals [2, 3]. Similar to other stem cell populations, SSC activities are supported within a niche microenvironment. In general, stem cell niches are formed by contributions of support cells that provide a milieu of growth factors and specialized microarchitecture to promote stem cell self-renewal and survival. For most stem cell populations, niches are orchestrated by one or more main support cell populations. For example, in the nervous system, neural stem cells (NSCs) receive cues from endothelial cells lining blood vessels [4], whereas, in the bone marrow system, osteoblasts and bone marrow stromal cells are the major support cells for hematopoietic stem cell (HSC) functions [5, 6]. Currently, the main orchestrating niche support cell populations are undefined for SSCs.
The somatic support cell system in mammalian testes consists primarily of Sertoli, Leydig, and myoid cells. Previous studies have provided indirect evidence that these cell populations contribute in varying capacities to the function of SSCs. Studies by Tadokoro et al. [7] indicate that Sertoli cells express the cytokine glial cell line-derived neurotrophic factor (GDNF) under control of follicle-stimulating hormone. GDNF is an essential regulator of SSC self-renewal and survival in vitro [8] and is required for maintenance of the undifferentiated spermatogonial population in vivo [9]. Another cytokine, colony-stimulating factor 1 (CSF-1), enhances GDNF-induced self-renewal of mouse SSCs in vitro and is expressed by Leydig and myoid cells in vivo [10]. In addition to these somatic cell populations, several studies have indicated that the interstitial space between seminiferous tubules, including the vascular network, is associated with development of the undifferentiated spermatogonial population that includes SSCs [11–13]. Though these previous studies indicate that contributions from multiple somatic support cell populations and the vasculature influence SSC functions and are thereby contributors of the niche, direct evidence of a main orchestrating niche cell population in mammalian testes has not been uncovered. Because of their intimate association with developing germ cells and their role in the formation of testicular architecture, Sertoli cells have been regarded as the main contributor to germ cell development and the maintenance of spermatogenesis. In fact, Sertoli cell number is highly correlated to daily sperm production in the testes of several mammalian species [14–16].
Transient induction of hypothyroidism by treatment with polythiouracil (PTU) during neonatal development in rodents extends the period of Sertoli cell proliferation, resulting in an increase of Sertoli cell numbers at puberty [17–19]. With mice, PTU treatment was shown to increase Sertoli cell content by 30% compared to normal mice not treated with PTU [19]. Further studies by Holsberger et al. [20–22] determined that the cyclin-dependent kinase inhibitor p27(Kip1) is activated in neonatal Sertoli cells by stimulation from triiodothyronine (T3) to regulate the period of postnatal proliferation. Thus, PTU-induced hypothyroidism delays the timing of T3 output during neonatal development to prolong the period of Sertoli cell mitosis. Consequently, the total germ cell content, including spermatogonial population and production of spermatozoa, is increased in testes of PTU-treated adult mice. This alteration of the seminiferous epithelium is associated with increases in testis size and seminiferous tubule diameter [17–19]. In addition, Leydig cell number is increased within testes from PTU-treated mice; however, testosterone production is similar to normal mice, indicating that overall Leydig cell function may not be altered in testes of PTU-treated animals [19]. Recently, studies by Auharek and de França [23] revealed that overall vascularization of the interstitial space between seminiferous tubules is increased in testes of PTU-treated adult mice. These observations demonstrate that Sertoli cell content influences the capacity for spermatogenesis within seminiferous tubules. However, to our knowledge, the impact of increasing Sertoli cell numbers on SSCs or niches within mouse testes has not been explored.
The objective of this study was to identify unequivocally the main support cell population that dictates formation of stem cell niches within mammalian testes, and we hypothesized that Sertoli cells serve in this capacity. To test this, we devised an experimental strategy that would determine directly whether Sertoli cells dictate niche numbers within mouse testes. We reasoned that if Sertoli cells orchestrate formation of SSC niches, an increase in Sertoli cell number would correspond to an increase in both the number of SSCs and the number of accessible niches for colonization by SSCs. To alter Sertoli cell numbers in adult mouse testes, we induced hypothyroidism during neonatal development in mouse pups via treatment with PTU. This method was used in conjunction with the germ cell transplantation technique [24, 25] to assay directly for changes in SSC and niche numbers within testes of adult mice that contain increased Sertoli cell content. Upon transplantation into recipient seminiferous tubules, donor SSCs migrate to open niches and reestablish spermatogenesis, and counting colonies provides a faithful determination of SSC number in the injected cell suspension from donor testes and niche accessibility within the recipient testis [24–27].
MATERIALS AND METHODS
Animals
Mice used as donors for SSC transplantation analyses were LacZ expressing B6;129s-Gt(ROSA)26Sor/J (designated Rosa) and were purchased from The Jackson Laboratory (Bar Harbor, ME). Recipient mice for microinjection of donor SSCs into seminiferous tubules were 129SvCP (129) × C57BL/6 (The Jackson Laboratory) that had been treated with busulfan (60 mg/kg) at least 6 wk prior to eliminate endogenous germ cells. All animal procedures were approved by the Institutional Animal Care and Use Committee at Penn State University.
PTU Treatment
To induce transient hypothyroidism during postnatal development, 6-Propyl-2-Thiouracil (PTU; 50 mg/L; Sigma Inc., St. Louis, MO) was added to the mothers' water immediately after parturition. Approximately 5 g/L of cherry Kool-Aid was added to the water to enhance palatability and ensure constant intake by the mother. Fresh PTU was supplied to mothers every 3–4 days until the pups were weaned at 25 days old. Weaned males were then maintained without PTU supplementation.
Isolation of THY1+ Germ Cells
Testes of adult (2- to 4-mo-old) donor mice were collected following euthanasia and incubated with collagenase (1 mg/ml in Hanks balanced salt solution [HBSS]) to separate seminiferous tubules, followed by washing five times in HBSS to eliminate interstitial cells. Single cell suspensions of seminiferous tubules were generated by incubation with trypsin-ethylenediaminetetraacetic acid, and THY1+ germ cells were isolated by magnetic-activated cell sorting (MACS) as described previously [25, 28, 29] and washed several times in mouse serum-free medium (mSFM) [29].
SSC Transplantation and Analysis of Recipient Testes
To assay for numbers of SSCs and stem cell niches in testes of PTU-treated and control mice, the germ cell transplantation technique was performed as described previously [19]. Briefly, to assay for total SSC numbers, THY1+ germ cells isolated from PTU-treated and control donor Rosa mice were suspended in mSFM at a concentration of 1 × 106 cells per milliliter, and 7 μl of donor cell suspension was microinjected into seminiferous tubules of busulfan-treated recipient mice. To assay for niche number in PTU-treated and control mice, THY1+ germ cells isolated from normal adult Rosa donor males were suspended in mSFM at a concentration of 1 × 106 cells per ml, and 7 μl of donor cell suspension was microinjected into the seminiferous tubules of PTU-treated and control recipient mice that had also been treated with busulfan at least 6 wk prior. Analysis of recipient testes for colonies of donor-derived spermatogenesis, a measure of stem cell content in the microinjected donor cell suspension and niche number in recipient testes, was conducted 2 mo after transplantation by incubation with X-gal (5-bromo-4-chloro-3-indolyly-β-D-galactosidase) to stain donor cells blue. The number of blue-stained colonies in each recipient testis was determined by counting with the aid of a dissecting microscope (Olympus, Center Valley, PA), and digital images were captured with an Olympus SZX stereo microscope equipped with a digital imaging system (DP71 Camera; Olympus). Determination of niche number in PTU-treated and control recipient testes was made based on colonies of donor-derived spermatogenesis per 1 × 105 THY1+ cells injected. Because similar numbers of THY1+ germ cells were injected into recipient testes to avoid effects on colonization efficiency due to unequal administration of overall cell numbers, determining differences in the SSC content of testes from PTU-treated and control donors involved multiplying donor-derived colonies of spermatogenesis by the number of THY1+ germ cells isolated.
Immunohistochemistry for GATA4 and Determination of Sertoli Cell Numbers
Testes from PTU-treated and control mice were fixed by incubating in Bouin solution overnight, followed by washing in 70% ethanol. Tissues were then embedded in paraffin, and 5-μm-thick cross sections were adhered to glass slides. Sections were deparaffinized, rehydrated, and nonspecific antibody binding was blocked by incubating with 10% normal goat serum. Sertoli cell nuclei were then labeled by incubating sections overnight at 4°C with goat anti-mouse GATA4 polyclonal antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA). The next day, sections were washed in PBS and incubated for 1 h at room temperature with biotinylated rabbit anti-goat secondary antibody (Santa Cruz Biotechnology). Sections were then washed in PBS and stained with a DAB substrate kit (Vector Lab Inc., Burlingame, CA), followed by counterstaining with hematoxylin. Cross sections were visualized microscopically at 10× magnification, and Sertoli cell numbers were quantified by counting GATA4+ nuclei in 10 round seminiferous tubules per cross section. Analyses were conducted in triplicate, thus 30 round seminiferous tubules were analyzed for PTU- and control-treated animals.
Evaluation of Blood Vessel and Interstitial Tissue Association with Seminiferous Tubules
Testes were excised and small holes were made in tunica albuginae, followed by incubating in Bouin solution overnight according to methods described by Russell et al. [30]. Fixed testes were then washed in 70% ethanol and subsequently embedded in paraffin. Cross sections were cut at 7-μm thickness and adhered to glass slides. Following deparaffinization and rehydration, cross sections were stained with hematoxylin and eosin, and samples were visualized microscopically at 10× and 20× magnification, and digital images of cross sections were captured using a DP71 camera and CellSense imaging software (Olympus Inc.). Composite images representing entire cross sections were compiled using CellSense imaging software and evaluated for the number of blood vessels within the interstitial space. Two cross sections of each testis from PTU-treated and control mice were analyzed. The number of seminiferous tubules bordered by blood vessels was counted for each cross section and divided by the total number of seminiferous tubules within the cross section to determine the percentage of seminiferous tubules associated with blood vessels. The average length of seminiferous tubule basement membrane bordered by blood vessels or interstitial tissue within each cross section was determined using NIH Image J (http://imagej.nih.gov/ij/). All tubules associated with blood vessels were analyzed, but only 10 round tubules of each cross section were analyzed for association with interstitial tissue. Lengths of seminiferous tubule basement membrane were analyzed as arbitrary values.
Statistics
All data are presented as average (AVG) ± SEM for three independent replicate experiments. Differences between means were determined using the t-test function of SPSS statistical software (version 17; Chicago, IL).
RESULTS
Increased Numbers of SSCs in Testes of PTU-Treated Mice Containing Greater Sertoli Cell Content
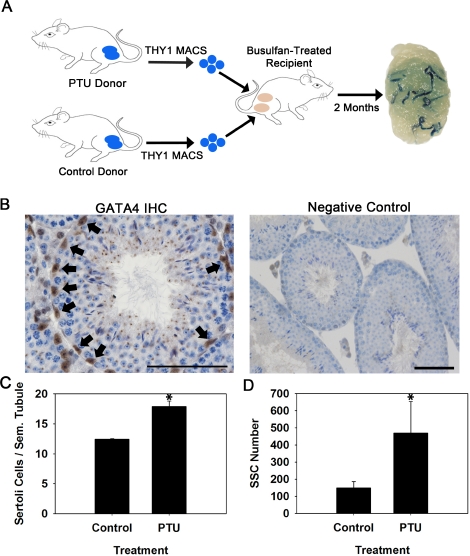
First, we devised an experimental strategy utilizing PTU-treated donors and transplantation analyses to examine whether an increase in the number of Sertoli cells corresponds to an increase in the number of SSCs within testes of adult mice (Fig. 1A). For these experiments, Rosa mice that express a LacZ marker transgene in all germ cell types were used as donors. Immunohistochemistry staining for expression of GATA4, a marker of Sertoli cells, in seminiferous tubules was used to determine differences in Sertoli cell numbers (Fig. 1B). Treatment with PTU during the postnatal period of 0–25 days postpartum (dpp) resulted in a significant (P = 0.01) increase of testis weight by 32% (Table 1), seminiferous tubule diameter by 7% (Table 1), and Sertoli cell number by 44% (Fig. 1C) compared to non-PTU-treated controls when the mice were 3–4 mo old (n = 3 different mice for each treatment). This alteration in Sertoli cell content is similar to the 30% increase measured in previous studies with PTU-treated mice [16]. The THY1+ cell fraction, which contains nearly all (>90%) of the SSCs in adult mouse testes [28, 29], was then isolated from 3- to 4-mo-old PTU-treated and non-PTU-treated control Rosa donors and transplanted separately into the testes of wild-type 129XC57 F1 recipient mice to assay for SSC numbers. As expected, the total number of cells recovered after enzymatic digestion of donor seminiferous tubules was greater by ∼59% for PTU-Rosa donors compared to control-Rosa donors (Table 1). Similarly, the number of THY1+ cells isolated by MACS from PTU-Rosa donor testes was greater by ∼36% compared to control-Rosa donors (Table 1). Colonies of donor spermatogenesis were observed in testes of recipient mice 2 mo after transplantation regardless of whether they received THY1+ cells from PTU-treated or control Rosa donors. The number of colonies generated by microinjected cells from PTU-Rosa donors (32.5 ± 7.9 colonies per 105 THY1+ cells injected; mean ± SEM for three different donors and seven recipient testes) was significantly (P = 0.05) greater by 2.2-fold compared to control-Rosa donors (14.8 ± 2.6 colonies per 105 THY1+ cells injected; mean ± SEM for three different donors and seven recipient testes). Because the number of donor THY1+ cells injected into each recipient testis was the same to eliminate differences in homing efficiency due to unequal cell concentrations, evaluation of total SSC numbers in donor testes required consideration of the number of isolated THY1+ cells from each donor. When this normalization was conducted, testes of PTU-Rosa donor mice were found to contain 3.1-fold more SSCs compared to control-Rosa donors (Fig. 1D). Together, results of these experiments indicate that the number of Sertoli cells positively influences the total number of SSCs within adult mouse testes. However, whether this increase was due to expansion of the number of niches or altered balance of SSC self-renewal and differentiation remained undefined.
FIG. 1.
Evaluation of the number of SSCs in testes of mice with experimentally induced increase of Sertoli cell numbers. A) Experimental strategy using PTU treatment during the neonatal period to increase Sertoli cell numbers at puberty in Rosa donor mice and transplantation analyses to assay for SSC numbers. Each recipient mouse received MACS-isolated THY1+ germ cells from a PTU-treated donor in one testis and MACS-isolated THY1+ germ cells from a control donor in the other testis. The numbers of blue colonies of spermatogenesis are a measure of SSC content in the microinjected donor cell suspension. B) Immunohistochemistry staining for GATA4+ nuclei (arrows) in cross sections of testis from a PTU-treated Rosa mouse. Within seminiferous tubules, GATA4 expression is localized specifically to Sertoli cell nuclei. Bar = 50 μm. C) Numbers of GATA4+ nuclei within cross sections of seminiferous tubules from PTU-treated and control Rosa mice. Data are mean ± SEM for three testes from different mice; *P = 0.01. D) Numbers of SSCs in testes of PTU-treated and control Rosa donor mice. SSC number is derived from quantification of colonies within recipient testes arising from 1 × 105 THY1+ donor germ cells microinjected and normalized to the number of THY1+ cells isolated by MACS. Data are mean ± SEM for three independent experiments using different donors for each treatment and 10–12 recipient testes for each replicate experiment; *P = 0.05.
TABLE 1.
Characteristics of testes from PTU-treated and control donor Rosa mice used as donors for SSC transplantation.
Association of Seminiferous Tubules with the Vasculature and Interstitial Tissue Is Not Altered in Testes of PTU-Treated Mice Containing Greater Numbers of Sertoli Cells
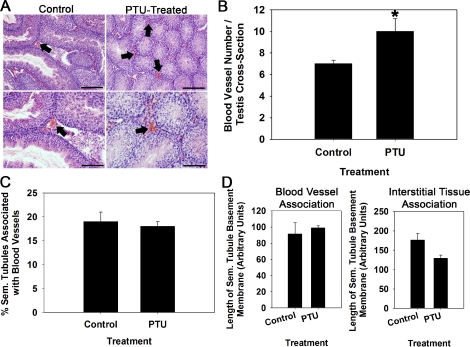
In addition to Sertoli cells, previous studies have implicated contributions from the vascular network and interstitial tissue between seminiferous tubules as regulators of SSC function in mouse testes [11–13]. Therefore, we investigated the association of seminiferous tubules with both blood vessels and interstitial tissue in testes with increased Sertoli cell numbers from PTU treatment. Staining of cross sections from testes of PTU-treated and control 129XC57 mice with hematoxylin and eosin allowed for identification of blood vessels within the interstitial space (Fig. 2A). Quantification of these revealed a significant (P = 0.01) increase of ∼30% (n = 3 different mice examined for each treatment) more blood vessels in testes of PTU-treated males compared to nontreated controls (Fig. 2B). This altered vascularization of the interstitial space could have been a major contributor to the increase of SSC numbers within testes of PTU-treated mice. Thus, we reasoned that if the vasculature was a key contributor of the SSC niche, the percentage of seminiferous tubules and length of seminiferous tubule basement membrane in close association with blood vessels would be elevated in testes of PTU-treated mice that contain increased numbers of SSCs. Examination of cross sections from PTU-treated and control mice revealed no differences (P = 0.13) in the percentage of seminiferous tubules that are associated with blood vessels (Fig. 2C). Similarly, the length of seminiferous tubule basement membrane associated with blood vessels or interstitial tissue was found to be no different (P > 0.05) in testes of PTU-treated and control mice (Fig. 2D). Collectively, these findings indicate that increased SSC content in testes of PTU-treated mice is a result of increased Sertoli cell content and not altered association of seminiferous tubule area with the vasculature or interstitial tissue.
FIG. 2.
Effects of increased Sertoli cell numbers on vascularization of the interstitial space within testes of adult mice. A) Cross sections of testes from PTU-treated mice and non PTU-treated control mice stained with hematoxylin and eosin to identify blood vessels within the interstitial space (arrows). Bottom images are 10× magnification of top images. Bars = 100 μm for top images and 50 μm for bottom images. B) Numbers of blood vessels in cross sections of testes from PTU-treated and non-PTU-treated control mice. Data are mean ± SEM for three different mice in each treatment; *P = 0.01. C) Percentage of seminiferous tubules in close association with blood vessels in testes of PTU-treated and non-PTU-treated control mice. Data are mean ± SEM for three different mice in each treatment. D) Length of seminiferous tubule basement membrane bordered by blood vessels or interstitial tissue in testes of PTU-treated and non-PTU-treated control mice. Data are mean ± SEM for three different mice in each treatment.
Increased Numbers of SSC Niches in Testes of PTU-Treated Mice Containing Greater Numbers of Sertoli Cells
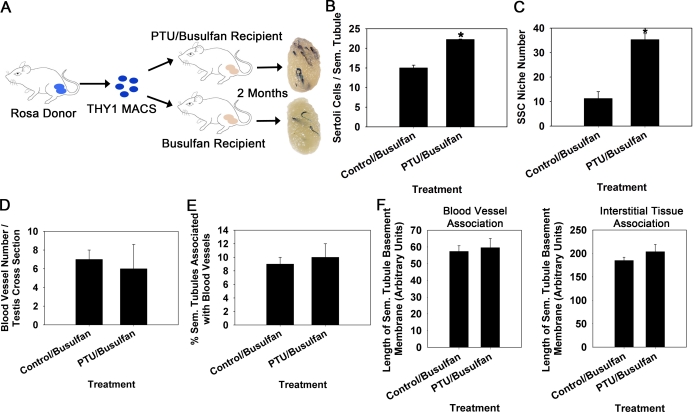
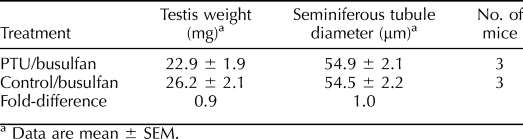
Next, we aimed to determine whether an increase in Sertoli cell numbers corresponds to an increase in niche accessibility within adult mouse testes. We devised an experimental strategy in which Sertoli cell numbers were artificially increased in hybrid 129XC57 F1 recipient mice by treatment with PTU from 0–25 dpp (Fig. 3A). At 8 wk of age, PTU-treated and nontreated control 129XC57 F1 mice were administered the chemotoxic drug busulfan to deplete endogenous spermatogenesis. These mice were then used as recipients 6 wk after busulfan treatment for transplantation with THY1+ germ cells from normal Rosa donor males. Prior to conducting transplantation analyses, we confirmed that testes from PTU-treated males contained a greater number of Sertoli cells compared to control males even after treatment with busulfan. Based on quantification of the number of GATA4+ nuclei within cross sections of seminiferous tubules, we found that the number of Sertoli cells was significantly (P = 0.05) greater by ∼48% (n = 3 different mice evaluated for each treatment) in testes of PTU/busulfan-treated males compared to busulfan-treated control males that did not receive PTU (Fig. 3B). In contrast to previous studies showing increased size of seminiferous tubules and testis weight in PTU-treated mice not treated with busulfan [14–16] (Fig. 1), the diameter of seminiferous tubules and weight of testes from PTU/busulfan-treated mice was not different compared to busulfan-treated control mice (Table 2). After transplantation, colonies of donor-derived spermatogenesis were observed in both PTU/busulfan-treated and control/busulfan recipient testes 2 mo later. Quantification of these colonies revealed that testes of PTU-treated recipients (35.3 ± 2.4 colonies per 105 THY1+ cells injected; mean ± SEM for 3 different donors and 10–12 recipient testes) contained 3.1-fold more niches that were colonized by donor SSCs with reestablished spermatogenesis compared to testes of non-PTU-treated controls (11.3 ± 2.7 colonies per 105 THY1+ cells injected; mean ± SEM for 3 different donors and 10–12 recipient testes; Fig. 3C). These values were in agreement with the 3.1-fold more SSCs found in PTU-treated donor testes from previous analyses (Fig. 1D). Previous studies suggest that SSC niches in mouse testes are regions of seminiferous tubules associated with the vasculature and interstitial tissue [11–13]. Thus, we examined whether these associations were altered in testes of PTU/busulfan-treated recipient mice that contain greater numbers of SSC niches compared to normal busulfan-treated recipient mice. Surprisingly, the number of blood vessels within the interstitial space was not different (P = 0.82) in cross sections of testes from PTU/busuflan-treated mice compared to control busulfan-treated mice (Fig. 3D). As expected, the percentage of seminiferous tubules associated with blood vessels was also found to be no different (P = 0.35) between PTU/busulfan-treated mice and control busulfan-treated mice (Fig. 3E). Similarly, the length of seminiferous tubule basement membrane associated with either the interstitial tissue or blood vessels was found to be no different (P > 0.05) in testes of PTU/busulfan-treated mice compared to control busuflan-treated mice (Fig. 3F). Overall, these results indicate that the number of Sertoli cells dictates the number of accessible SSC niches within testes of adult mice, and this interaction is not through alteration of the area of seminiferous tubules associated with the vasculature or interstitial tissue.
FIG. 3.
Evaluation of SSC niche numbers in testes of mice with experimentally induced increase of Sertoli cell numbers. A) Experimental strategy using germ cell transplantation to assay for numbers of niches in recipient mice with normal numbers of Sertoli cells and recipient mice treated with PTU during postnatal development to increase Sertoli cell content. MACS-isolated THY1+ germ cells from normal Rosa donors were used as a source of SSCs for microinjection into PTU and non-PTU-treated recipient testes. B) Numbers of GATA4+ nuclei within cross sections of seminiferous tubules from PTU/busulfan-treated and control busulfan-treated 129XC57 mice. Data are mean ± SEM for three different replicate samples of each treatment; *P = 0.05. C) Numbers of donor-derived colonies of spermatogenesis, a reflection of colonized SSC niches, in testes of PTU/busulfan-treated and control busulfan-treated recipient mice 2 mo after transplantation. Data are mean ± SEM for three independent transplantation experiments and 10–12 recipient testes for each treatment; *P = 0.01. D) Number of blood vessels within the interstitial space in cross sections of testes from PTU/busulfan-treated and control busulfan-treated mice. Data are mean ± SEM for three different mice in each treatment. E) Percentage of seminiferous tubules in close association with blood vessels in testes of PTU/busulfan-treated and busulfan-treated control mice. Data are mean ± SEM for three different mice of each treatment. F) Length of seminiferous tubule basement membrane bordered by blood vessels or interstitial tissue in testes of PTU/busulfan-treated and busulfan-treated control mice. Data are mean ± SEM for three different mice in each treatment.
TABLE 2.
Characteristics of testes from PTU-treated and control 129XC57 mice representing those prepared as recipients for SSC transplantation by treatment with busulfan.
DISCUSSION
The seminiferous epithelium is divided into basal and adluminal compartments, of which the spermatogonial population including SSCs is located within the basal compartment below the blood-testis-barrier formed by Sertoli cell tight junctions [30]. This anatomical localization allows for exposure of SSCs to factors secreted not only by Sertoli cells but also those coming from interstitial cell populations, including Leydig cells and factors carried in the vasculature. Thus, several somatic cell populations have been implicated as contributors to the SSC niche, yet a single cell type that orchestrates niche formation in mammalian testes has not been identified. Sertoli cells have been regarded as the key contributor of the SSC niche, a belief born from circumstantial evidence that these cells express GDNF, and this factor is required for SSC self-renewal. However, direct evidence that these cells dictate niche formation and accessibility to SSCs has not been reported. Using the PTU-induced hypothyroidism model to artificially manipulate Sertoli cell numbers in testes of adult mice, we show that increased Sertoli cell number is correlated to increases of both the number of SSCs and niches accessible for SSC colonization following transplantation. Though total seminiferous tubular volume is increased in testes of PTU-treated mice, and this would be expected to increase SSC numbers accordingly, the volume is not altered in testes of PTU/busulfan-treated mice compared to control busulfan-treated mice not exposed to PTU. Importantly, use of these animals as recipients for transplantation revealed a greater than 3-fold increase in accessible niches for colonization by donor SSCs. Thus, these observations indicate that Sertoli cell numbers dictate stem cell niches formation in mammalian testes, similar to the notion of a single niche support cell population that governs activities of other tissue-specific stem cell populations such as NSCs [4] and HSCs [5, 6].
Though the current study indicates that Sertoli cells are the key drivers of SSC niche formation and function, the mechanisms by which they orchestrate this feat are still poorly defined. One possibility is formation of local gradients of Sertoli cell-secreted factors such as GDNF. In this respect, Sertoli cell density could affect the number of SSCs. Based on the number of Sertoli cell nuclei per seminiferous tubule and in relation to tubule diameter, the density of Sertoli cells in testes of adult PTU-treated mice (8.6 Sertoli cells per 100 μm) was 1.4-fold greater compared to that of control mice (6.2 Sertoli cells per 100 μm). Thus, it is likely that concentration of secreted niche factors that influence SSC activities was greater in testes of PTU-treated mice, resulting in enhanced numbers of SSCs. Another potential mechanism by which Sertoli cells orchestrate the SSC niche is influencing output of soluble factors from interstitial cell populations, including Leydig cells. Previous studies showed that Leydig cell numbers are increased in testes of PTU-treated rodents [19]. Also, our recent studies revealed that CSF1 enhances GDNF-induced self-renewal of mouse SSCs, and this factor is expressed by Leydig cells [10]. Thus, it is likely that concentrations of secreted factors from Leydig cells such as CSF1 are also elevated in testes of PTU-treated mice, and these could contribute to activity of the SSC population. Intriguingly, cross-talk between Sertoli and Leydig cells could be a major mechanism by which stem cell niche formation and function in mammalian testes is regulated. Similar to PTU-treated donor mice, testes of PTU/busulfan-treated mice used as recipients for SSC transplantation also contained a greater density of Sertoli cells compared to busulfan-treated mice not exposed to PTU. Use of these animals as recipients revealed greater than 3-fold more accessible niches for colonization by transplanted donor SSCs. However, individual donor-derived colonies were randomly distributed throughout recipient testes; thus, alteration in the total number of colonies generated was likely a result of increased numbers of individual niches and not from elevated concentrations of specific factors within a given niche. Collectively, results of the current study indicate that Sertoli cells dictate the number of SSC niches within mouse testes resulting in greater SSC numbers, which is likely influenced by concentrations of soluble niche factors secreted by both Sertoli and Leydig cells.
Previous studies suggest an important role of interstitial cell populations in formation of SSC niches [11–13]. In particular, associative observations from studies of Yoshida et al. [13] suggest that vascular patterning of the interstitial space dictates niche formation within mouse testes, yet direct evidence that manipulation of the vasculature effects SSC function has not been reported. In the current study, we found that total vascular development of the interstitial space is increased in accordance with increased Sertoli cell numbers. This finding suggests that Sertoli cells may regulate vascularization of the interstitial space, but a direct connection has not been made. However, the percentage of seminiferous tubules and area of seminiferous tubule basement membrane associated with the vasculature was found to be unaltered in testes of PTU-treated mice compared to controls. Likewise, the area of basement membrane associated with interstitial tissue was found to be no different in PTU-treated mice. These findings indicate that the increased number of SSCs in testes of PTU-treated mice result from alteration of Sertoli cell numbers. In support of this conclusion, the percentage of seminiferous tubules and area of basement membrane associated with either the interstitial tissue or blood vessels was found to be no different in testes of PTU/busulfan-treated mice compared to non-PTU-treated controls also receiving busulfan. Importantly, the number of niches accessible for colonization by transplanted SSCs was increased by greater than 3-fold in the PTU/busulfan-treated mice, and the number of Sertoli cells was also increased by 48%. Collectively, these findings provide direct evidence that manipulation of the Sertoli cell population alters both the number of SSCs and niches within testes of adult mice. Moreover, these results indicate that in the PTU model the vasculature and interstitial tissue does not dictate the number of SSC niches in mouse testes, rather Sertoli cells are the main orchestrating support cell population.
Overall, results of this study show that Sertoli cells dictate formation of SSC niches within mouse testes likely through secretion of specific growth factors and through inducing output of secreted factors from Leydig cells and possibly other interstitial cell populations that influence SSC activities. Future studies will be aimed at identifying the mechanism by which Sertoli cells coordinate the contributions by other support cell populations. Decline in tissue homeostasis and regenerative capacity are hallmarks of aging and degenerative diseases, which may be due to impaired activity of tissue-specific stem cells. Previous studies indicate that failure of niches to support stem cell activities leads to aging-related decline of tissue function in liver and skeletal muscle [31, 32]. Similarly, studies by Ryu et al. [33] showed that declining fertility in aged males is a result of impaired niche function. Thus, defining the main support cell populations and their contributions to the stem cell niche is essential for diagnosing underlying causes of degenerative diseases, including male infertility.
Acknowledgments
We thank E. Cloninger for help with preparing figures and Dr. O. Ocon-Grove for assistance with data collection.
Footnotes
Supported by grants HD061665 and HD058137 awarded to J.M.O. from the National Institutes of Health.
REFERENCES
- Sharpe R. Regulation of spermatogenesis. Knobil E, Neill JD. (Eds.), The Physiology of Reproduction, vol. 1, 2nd ed. New York: Raven Press; 1994: 1363 1434 [Google Scholar]
- de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl 2000; 21: 776 798 [PubMed] [Google Scholar]
- Oatley JM, Brinster RL. Regulation of spermatogonial stem cell self-renewal in mammals. Ann Rev Cell Dev Biol 2008; 24: 263 286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 2004; 304: 1338 1340 [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003; 425: 836 841 [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003; 425: 841 846 [DOI] [PubMed] [Google Scholar]
- Tadokoro Y, Yomogida K, Ohta H, Tohda A, Nishimune Y. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech Dev 2002; 113: 29 39 [DOI] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A 2004; 101: 16489 16494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvönen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 2000; 287: 1489 1493 [DOI] [PubMed] [Google Scholar]
- Oatley JM, Oatley MJ, Avarbock MR, Tobias JW, Brinster RL. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development 2009; 136: 1191 1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarini-Garcia H, Hornick JR, Griswold MD, Russell LD. Distribution of type A spermatogonia in the mouse is not random. Biol Reprod 2001; 65: 1179 1185 [DOI] [PubMed] [Google Scholar]
- Chiarini-Garcia H, Raymer AM, Russell LD. Non-random distribution of spermatogonia in rats: evidence of niches in the seminiferous tubules. Reproduction 2003; 126: 669 680 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science 2007; 317: 1722 1726 [DOI] [PubMed] [Google Scholar]
- Berndtson WE, Igboeli G, Pickett BW. Relationship of absolute numbers of Sertoli cells to testicular size and spermatogenesis in young beef bulls. J Anim Sci 1987; 64: 241 246 [DOI] [PubMed] [Google Scholar]
- Berndtson WE, Thompson TL. Changing relationships between testis size, Sertoli cell number and spermatogenesis in Sprague-Dawley rats. J Androl 1990; 11: 429 435 [PubMed] [Google Scholar]
- Thompson TL, Berndtson WE. Testicular weight, Sertoli cell number, daily sperm production, and sperm output of sexually mature rabbits after neonatal or prepubertal hemicastration. Biol Reprod 1993; 48: 952 957 [DOI] [PubMed] [Google Scholar]
- Cooke PS, Hess RA, Porcelli J, Meisami E. Increased sperm production in adult rats after transient neonatal hypothyroidism. Endocrinology 1991; 129: 244 248 [DOI] [PubMed] [Google Scholar]
- Hess RA, Cooke PS, Bunick D, Kirby JD. Adult testicular enlargement induced by neonatal hypothyroidism is accompanied by increased Sertoli and germ cell numbers. Endocrinology 1993; 132: 2607 2613 [DOI] [PubMed] [Google Scholar]
- Joyce KL, Porcelli J, Cooke PS. Neonatal goitrogen treatment increases adult testis size and sperm production in the mouse. J Androl 1993; 14: 448 455 [PubMed] [Google Scholar]
- Holsberger DR, Jirawatnotai S, Kiyokawa H, Cooke PS. Thyroid hormone regulates the cell cycle inhibitor p27Kip1 in postnatal murine Sertoli cells. Endocrinology 2003; 144: 3732 3738 [DOI] [PubMed] [Google Scholar]
- Holsberger DR, Kiesewetter SE, Cooke PS. Regulation of neonatal Sertoli cell development by thyroid hormone receptor alpha1. Biol Reprod 2005; 73: 396 403 [DOI] [PubMed] [Google Scholar]
- Holsberger DR, Buchold GM, Leal MC, Kiesewetter SE, O'Brien DA, Hess RA, França LR, Kiyokawa H, Cooke PS. Cell-cycle inhibitors p27Kip1 and p21Cip1 regulate murine Sertoli cell proliferation. Biol Reprod 2005; 72: 1429 1436 [DOI] [PubMed] [Google Scholar]
- Auharek SA, de França LR. Postnatal testis development, Sertoli cell proliferation and number of different spermatogonial types in C57BL/6J mice made transiently hypo- and hyperthyroidic during the neonatal period. J Anat 2010; 216: 577 588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A 1994; 91: 11298 11302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Brinster RL. Spermatogonial stem cells. Methods Enzymol 2006; 419: 259 282 [DOI] [PubMed] [Google Scholar]
- Ogawa T, Aréchaga JM, Avarbock MR, Brinster RL. Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol 1997; 41: 111 122 [PubMed] [Google Scholar]
- Dobrinski I, Ogawa T, Avarbock MR, Brinster RL. Computer-assisted image analysis to assess colonization of recipient seminiferous tubules by spermatogonial stem cells from transgenic donor mice. Mol Reprod Dev 1999; 53: 142 148 [DOI] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci U S A 2003; 100: 6487 6492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL. Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biol Reprod 2004; 71: 722 731 [DOI] [PubMed] [Google Scholar]
- Russell LD, Ettlin R, Sinha Hikim A, Clegg E. Histological and Histopathological Evaluation of the Testis. Clearwater, FL: Cache River Press; 1990. [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005; 433: 760 764 [DOI] [PubMed] [Google Scholar]
- Carlson ME, Conboy IM. Loss of stem cell regenerative capacity within aged niches. Aging Cell 2007; 6: 371 382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu BY, Orwig KE, Oatley JM, Avarbock MR, Brinster RL. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells 2006; 24: 1505 1511 [DOI] [PMC free article] [PubMed] [Google Scholar]