Abstract
RecJ-like proteins belonging to the DHH family have been proposed to function as oligoribonucleases and 3′-phosphoadenosine 5′-phosphate (pAp) phosphatases in bacteria and archaea, which do not have Orn (oligoribonuclease) and CysQ (pAp phosphatase) homologs. In this study, we analyzed the biochemical and physiological characterization of the RecJ-like protein TTHA0118 from Thermus thermophilus HB8. TTHA0118 had high enzymatic activity as an oligodeoxyribonucleotide- and oligoribonucleotide-specific exonuclease and as pAp phosphatase. The polarity of degradation was 5′ to 3′, in contrast to previous reports about Bacillus subtilis NrnA, a RecJ-like protein. TTHA0118 preferentially hydrolyzed short oligodeoxyribonucleotides and oligoribonucleotides, whereas the RecJ exonuclease from T. thermophilus HB8 showed no such length dependence on oligodeoxyribonucleotide substrates. An insertion mutation of the ttha0118 gene led to growth reduction in minimum essential medium. Added 5′-mononucleotides, nucleosides, and cysteine increased growth of the ttha0118 mutant in minimum essential medium. The RecJ-like protein Mpn140 from Mycoplasma pneumoniae M129, which cannot synthesize nucleic acid precursors de novo, showed similar biochemical features to TTHA0118. Furthermore, B. subtilis NrnA also hydrolyzed oligo(deoxy)ribonucleotides in a 5′-3′ direction. These results suggested that these RecJ-like proteins act in recycling short oligonucleotides to mononucleotides and in controlling pAp concentrations in vivo.
Keywords: DNase, Enzyme Mechanisms, Phosphatase, Ribonuclease, RNA Metabolism, RNA Turnover, RecJ, Exonuclease, pAp Phosphatase
Introduction
Nucleases are essential for nucleic acid metabolism and cell viability. DNA repair and recombination require a variety of specific deoxyribonucleases (1, 2). RNA metabolism also requires various ribonucleases (RNases)2 for a broad range of processes (3, 4). In mRNA turnover, mRNA decay is an important determinant of gene expression and is mediated by several RNases with endonuclease and exonuclease activity. Nucleases belonging to different superfamilies have been identified with endonuclease and exonuclease activities (5–8). In addition, genome sequences have revealed numerous ORFs annotated as putative nucleases.
One of the phosphoesterase families is the DHH superfamily (9), which includes phosphodiesterase and exopolyphosphatase (10–12). RecJ is a DNA exonuclease in the DHH protein superfamily, with DHH motifs I-IV and an associated DHHA1 motif (9), as described in the Pfam database (13). The RecJ protein is a Mg2+- or Mn2+-dependent single-stranded DNA (ssDNA)-specific 5′-3′ exonuclease/deoxyribophosphodiesterase and plays a role in homologous recombination, mismatch repair, and base excision repair (14–19). Although the ORFs annotated as RecJ-related proteins also have DHH and DHHA1 motifs, each RecJ-related protein has to be investigated for nuclease activity similar to RecJ protein. According to the SYSTERS database (20), proteins with DHH and DHHA1 motifs can be further divided into three main families. Representatives of each family are RecJ (N_155469 in SYSTERS), putative poly(A) polymerase (O_142140), and exopolyphosphate-related protein (N_142143, S_59202, and O_155470). We have designated this third family as RecJ-like proteins in this work. ORFs encoding RecJ-like proteins have been found in many bacterial and archaeal genomes.
Recent research has elucidated the activity and function of RecJ-like proteins. NrnA (YtqI), a RecJ-like protein from Bacillus subtilis, hydrolyzes short single-stranded RNA (ssRNA) in vitro, and the gene complements an Escherichia coli orn mutant (21). E. coli Orn (oligoribonuclease) degrades 5-mer or shorter ssDNA and ssRNA to mononucleotides in a 3′-5′ direction (22). Since exonucleases that hydrolyze mRNA cannot hydrolyze ssRNAs shorter than 2–5-mers (23–26), E. coli Orn is essential for the final step of mRNA degradation and cellular nucleotide recycling (27–29). However, Orn homologs are only present in the β and γ subdivisions of Proteobacteria and Actinobacteria, whereas RecJ-like proteins homologous to NrnA are absent in the majority of these Proteobacteria subdivisions. In contrast, these RecJ-like proteins are present in Firmicutes, Bacteroides, Chlorobi, the δ and ϵ subdivisions of Proteobacteria, Actinobacteria, Crenarchaeota, and Euryarchaeota, whereas Orn homologs are absent in the majority of these bacteria and archaea. This phylogenetic distribution suggests a negative correlation, perhaps due to non-orthologous gene displacements (21). Therefore, RecJ-like proteins may act as functional analogues of Orn.
NrnA also hydrolyzes 3′-phosphoadenosine 5′-phosphate (pAp) in vitro, and the gene can complement an E. coli cysQ (pAp phosphatase) mutant (21). CysQ hydrolyzes pAp generated from phosphoadenosine 5′-phosphosulfate (30–32). Control of a cellular pAp concentration is essential because high intracellular concentrations of pAp are toxic (33, 34). Interestingly, some negative correlation can also be observed in the phylogenetic distribution of CysQ and RecJ-like proteins (21). Therefore, RecJ-like proteins may also function as CysQ analogs.
However, unlike Orn and CysQ, the biochemical and physiological properties of RecJ-like proteins have not been investigated in detail. In particular, there are little data on the exonuclease and pAp phosphatase activities (e.g. substrate specificity, polarity, cleavage pattern, kinetic constants, and metal ion dependence) of RecJ-like proteins. One important point is the polarity of exonuclease degradation by NrnA, which was reported to be 3′ to 5′ (21). However, RecJ exonuclease has 5′ to 3′ polarity (14, 15, 18, 19). In addition, the detailed phenotype of the RecJ-like gene disruptant has not been reported until now.
We selected Thermus thermophilus HB8 as a model organism for studying RecJ-like proteins. T. thermophilus HB8 proteins are heat stable and useful for physiochemical studies. In addition, systems for genetic manipulation of T. thermophilus HB8 have been established (35, 36). T. thermophilus HB8 contains three DHH superfamily proteins: RecJ (TTHA1167, ttRecJ), a putative poly(A) polymerase (TTHA0831), and a RecJ-like protein (TTHA0118). Our group determined the crystal structures of the core domain (residues 40–463) of ttRecJ, designated cd-ttRecJ (37), and the intact ttRecJ (38). The intact ttRecJ structure has four domains which form a molecule with an O-like structure. The hole containing DHH and DHHA1 motifs and metal (Mn2+, Mg2+) ion(s) between the three domains is very suitable (11 Å) for binding ssDNA, but not double-stranded DNA (dsDNA). An oligonucleotide/oligosaccharide-binding (OB)-fold domain (39) also contributes specificity to ssDNA. The crystal structures of BF3670 and SH1221, RecJ-like proteins from Bacteroides fragilis and Staphylococcus hemeolyticus, have also been resolved (PDB ID: 3DMA and 3DEV), respectively. Nevertheless, studies including more detailed biochemical characterization are needed to understand the in vivo functions of RecJ-like proteins.
In this study, we purified and characterized the enzymatic activity of TTHA0118 from T. thermophilus HB8. TTHA0118 had higher nuclease activities for short ssDNA and ssRNA substrates than for long ones. A ttha0118 disruptant (Δttha0118) had reduced growth in minimum essential medium compared with the wild-type strain. Addition of 5′-mononucleotides, nucleosides, or cysteine to the minimum essential medium increased Δttha0118 growth. These results suggested that TTHA0118 acts in recycling short-length oligonucleotides to mononucleotides in vivo.
EXPERIMENTAL PROCEDURES
Materials
Enzymes that act on DNA, including restriction enzymes and LA Taq polymerase, were obtained from Takara Bio Inc. KOD-Plus polymerase and KOD polymerase were obtained from Toyobo. Polypeptone and yeast extract for cultivating E. coli were obtained from Difco. Yeast extract for rich medium (TT) for cultivating T. thermophilus HB8 was obtained from Nihon Pharmaceutical Co. DNA oligomers were synthesized by BEX Co. or Gene Design Inc. [γ-32P]ATP was obtained from ICN. All other reagents used were of the highest commercially available grade. TTHA0118, Mpn140, B. subtilis NrnA, ttRecJ, and cd-ttRecJ were prepared as described in supplemental experimental procedures. The concentrations of purified proteins were calculated as described by Kuramitsu et al. (40).
Size Exclusion Chromatography
The protein (50 μm) being studied was applied onto a Superdex 200 HR 10/30 column and eluted with buffer III with a flow rate of 0.5 ml/min by the ÄKTA Explorer System. The apparent molecular weight was estimated by comparing the protein's retention time with those of molecular weight markers (Sigma).
Spectroscopic Analysis
Circular dichroism (CD) spectra in the far-UV region (200–250 nm) were obtained at 25 °C with a Jasco J-720W spectropolarimeter, using 1.25 μm ttRecJ, 2 μm cd-ttRecJ, 5 μm TTHA0118, 5 μm Mpn140, or 5 μm NrnA in 50 mm potassium phosphate and 100 mm KCl (pH 7.5). Thermostability was investigated by recording the molar ellipticity at 222 nm from 25–95 °C under the same conditions as above.
Assay Using Radioisotope for Exonuclease Activity
The sequences of oligodeoxyribonucleotides used as substrates are: 3f, 5′-ATG-3′; 6f, 5′-ATGACA-3′; 11f, 5′-ATGACAACTAA-3′; 21f, 5′-ATGACAACTAAAGCAACACCC-3′; 33f, 5′-ATGACAAAAGCAACACCCAAAACAACTCCC-3′; and 27r, 5′-GGGAGTTGTTTTGGGTGTTGCTTTAGT-3′. The sequences of oligoribonucleotides used as substrates are: 3fR, 5′-AUG-3′; 6fR, 5′-AUGACA-3′; 11fR, 5′-AUGACAACUAA-3′; and 21fR, 5′-AUGACAACUAAAGCAACACCC-3′. These ssDNAs and ssRNAs were radiolabeled at the 5′-end with [γ-32P]ATP using polynucleotide kinase. Each reaction mixture (10 μl) contained 50 mm HEPES; 100 mm KCl; 5 mm MnCl2 or MgCl2; 10 nm 5′-32P-labeled ssDNA, ssRNA, or 5′-end 6-mer overhang dsDNA; various concentrations of unlabeled ssDNA, ssRNA, or 5′-end 6-mer overhang dsDNA; and various concentrations of TTHA0118, Mpn140, NrnA, ttRecJ, or cd-ttRecJ (pH 7.5). The mixtures were incubated at 37 °C and, at each time point, the reaction was quenched by adding 1 μl of 100 mm EDTA and 11 μl phenol/chloroform. The samples were centrifuged, and an equal volume of sample buffer (5 mm EDTA, 80% deionized formamide, 10 mm NaOH, 0.1% bromphenol blue, and 0.1% xylene cyanol) was added to each supernatant. The samples were denatured at 95 °C for 3 min, loaded onto a 25% (w/v) acrylamide gel containing 8 m urea and 1× TBE buffer, and then electrophoresed in 1× TBE buffer (41). After electrophoresis, each gel was dried and placed in contact with an imaging plate. Bands were visualized and analyzed using a BAS2500 image analyzer (Fuji Photo Film).
The percentage of degraded DNA or RNA was plotted for each incubation period. For each assay, the initial rate of reaction was calculated from the linear region of the plot. The initial rate was plotted against the concentration of the substrate. The data were fitted to the Michaelis-Menten equation, and the kinetic constant was calculated by the Igor Pro 3.14 software (WaveMetrics).
Exonuclease activity in the presence of various divalent cations was measured at 37 °C using the following mixture: 50 mm HEPES, 100 mm KCl, 5 mm divalent cation (MgCl2, MnCl2, ZnCl2, CoCl2, NiCl2, or CaCl2), 10 nm 5′-32P-labeled ssDNA, 5 μm cold ssDNA, and 25 nm enzyme (pH 7.5).
Assay Using Fourier Transform Ion Cyclotron Resonance (FT-ICR) Mass Spectrometer for Exonuclease Activity
To determine the polarity and products of exonuclease activity accurately, a reaction mixture (20 μl) containing 50 mm HEPES, 100 mm KCl, 5 mm MnCl2, 10 μm 5′-end phosphorylated 11-mer ssDNA (5′-end phosphorylated 11f), and 1 μm TTHA0118, 1 μm Mpn140, or 0.1 μm NrnA (pH 7.5) was incubated at 37 °C for 1, 3, and 6 h. The reaction was stopped by heat treatment at 95 °C for 3 min and then kept on ice. Each reaction product was extracted by using C18 ZipTip (Millipore Corp.) with ion-pairing agent, dibutylammonium format (DBAF), pH 8.0 to eliminate any contaminants and salts. First, C18 ZipTip was equilibrated with 4 mm DBAF after activation by using 100% acetonitrile. The reactant was introduced into the equilibrated C18 ZipTip several times and then washed with pure water. The reaction products that bound to C18 resin with DBAF were eluted with 50% acetonitrile. Finally, 1.0 m of piperidine and imidazole were mixed with each eluent to a final 10 mm concentration, respectively.
The resulting solutions were introduced into a solariX, FT-ICR mass spectrometer shielded with 9.4 T superconducting magnets (Bruker Daltonics Inc.) by electrospray ionization under negative mode with 2 μl/min flow rate, 4000 V capillary voltage and −500 voltage spray shield. For data acquisition at solariX FT-ICR MS, a free induction decay was set to 1 m size and mass detection range was set from 150 to 3,500 m/z. One hundred spectra for each reaction product were combined to obtain average spectra. Mass analyses of all samples were carried out under the same condition. The obtained mass results were charge deconvoluted by DataAnalysis software (version 4.0, Bruker Daltonics Inc.) for quantitative and comparative analysis of the enzyme products.
Assay for pAp Phosphatase Activity
To determine the pAp phosphatase steady-state kinetic parameters, hydrolytic activity was analyzed by measuring the production of inorganic orthophosphate using a colorimetric assay (42). The reaction mixture (100 μl), which contained 50 mm Tris-HCl, 100 mm KCl, 5 mm MnCl2 or MgCl2, 5–1,000 μm pAp, and 0.2 nm TTHA0118 or Mpn140 (pH 7.5), was incubated at 37 °C.
The reaction products were identified by applying the following mixture (80 μl) to CAPCELL PAK C18 column (Shiseido) as previously described (45). The reaction mixture (100 μl), which contained 50 mm Tris-HCl, 100 mm KCl, 5 mm MnCl2, 1 mm pAp, and 1 μm TTHA0118 (pH 7.5), was incubated at 37 °C for 15 min. The reaction was stopped by adding 20 μl of 500 mm EDTA, and the protein was removed by ultrafiltration using a membrane filter (cutoff MW, 5,000).
Assay for cAMP and cGMP Phosphodiesterases
To determine the phosphodiesterase steady-state kinetic parameters, the following reaction mixture (80 μl) was applied to a CAPCELL PAK C18 column as previously described (42). The reaction mixture (100 μl), which contained 50 mm Tris-HCl, 100 mm KCl, 5 mm MnCl2, 0.1–2 mm cAMP or cGMP, and 1 μm TTHA0118 (pH 7.5), was incubated at 37 °C. The reaction was stopped by adding 20 μl of 500 mm EDTA, and the protein was removed by ultrafiltration using a membrane filter (cutoff MW, 5,000).
Insertion Mutant of ttha0118 and Growth Analysis
An insertion mutant of T. thermophilus HB8 ttha0118 (Δttha0118) was constructed by homologous recombination using a thermostable kanamycin-resistant marker (36). Wild-type and Δttha0118 strains were grown at 70 °C in rich TT medium, on TT plates, or in minimum essential (CS) medium. TT medium contained: 0.4% tryptone, 0.2% yeast extract, 0.1% NaCl, 0.4 mm MgCl2, and 0.4 mm CaCl2 (pH 7.3, adjusted with NaOH). The TT plate contained 1.5% gelan gum in TT medium. CS medium contained: 2% sucrose, 2% sodium glutamate, 0.055% K2HPO4, 0.018% KH2PO4, 0.2% NaCl, 0.05% (NH4)2SO4, 0.0125% MgCl2·6H2O, 0.0025% CaCl2·2H2O, 0.001% FeSO4·7H2O, 0.00012% NaMoO3·2H2O, 0.00001% VOSO4·xH2O, 0.00005% MnCl2·4H2O, 0.000006% ZnSO4·7H2O, 0.0000015% CuSO4·5H2O, 0.00008% CaCl2·6H2O, 0.000002% NiCl2·2H2O, 0.001% biotin, and 0.01% thiamine (pH 7.3, adjusted with NaOH). For some studies, a 5′-mononucleotide mixture containing AMP, GMP, CMP, and UMP; a nucleoside mixture containing adenosine, guanosine, cytidine, and uridine; or cysteine was added to the CS medium (final concentration, 0.1 mm).
For growth studies, cells were grown in TT medium and used to inoculate TT and CS media with 4 × 104 cells/ml. Samples were taken after 21 h of incubation at 70 °C, diluted, and counted. For growth on TT plates, bacterial samples from preincubation TT cultures (3 × 107 cells/ml) were diluted to 5 × 102 colonies/plate.
RESULTS
Preparation of TTHA0118, Mpn140, NrnA, ttRecJ, and cd-ttRecJ
To investigate molecular functions of RecJ-like proteins, we selected TTHA0118 from T. thermophilus HB8 as a main target. We also selected Mpn140, a RecJ-like protein from M. pneumoniae M129, to further examine the contribution of a RecJ-like protein to cellular nucleotide recycling because Mycoplasma species lack enzymes for de novo biosynthesis of nucleic acid precursors (43–45). In addition, we targeted B. subtilis NrnA to reexamine the polarity of its exonuclease activity (21). Furthermore, we used ttRecJ (38) and cd-ttRecJ (37) from T. thermophilus HB8 as a control to highlight differences between RecJ and RecJ-like proteins.
The TTHA0118, Mpn140, NrnA, ttRecJ, and cd-ttRecJ proteins used in this study are composed of 324, 324, 329, 666, and 426 amino acids, respectively, and each has a calculated molecular mass of 35.1, 37.2, 36.9, 72.9, and 46.1 kDa and a theoretical pI of 5.2, 9.2, 5.1, 5.9, and 5.5, respectively. All these sequences share DHH motifs I-IV and the DHHA1 motif, but the C-terminal regions of TTHA0118, Mpn140 and NrnA are shorter than those of RecJ proteins (Fig. 1). These five proteins were overexpressed and purified at least 95% pure (supplemental Fig. S1). By size exclusion chromatography, the apparent molecular masses of TTHA0118, Mpn140, NrnA, ttRecJ, and cd-ttRecJ were estimated to be 550 kDa, 58 kDa, 150 kDa, 98 kDa, and 60 kDa, respectively. These results suggest that TTHA0118 exists in an oligomeric form, Mpn140 in a monomeric or dimeric form, NrnA in a tetrameric form, ttRecJ in a monomeric form, and cd-ttRecJ in a monomeric form in solution. Far-UV (200–250 nm) CD spectra showed that all five proteins had an α/β or α+β fold (data not shown). TTHA0118, Mpn140, NrnA, ttRecJ, and cd-ttRecJ were stable up to 85 °C, 55 °C, 55 °C, 80 °C, and 65 °C at pH 7.5, respectively (data not shown). These results suggested that the purified proteins were properly folded.
FIGURE 1.
Amino acid sequences and schematic representation of RecJ-like and RecJ proteins. A, amino acid sequences of TTHA0118, Mpn140, NrnA, NrnB, SMU.1297, BF3670, ttRecJ, E. coli RecJ (ecRecJ), and B. subtilis RecJ (bsRecJ) are aligned according to Aravind and Koonin (9). The numbers of amino acids not shown in the alignments are in parentheses. Motifs with variant residues are numbered. U indicates a bulky hydrophobic residue, and O indicates a small residue. TTHA0118 (T. thermophilus HB8, YP_143384); Mpn140 (M. pneumoniae M129, NP_109828); NrnA (B. subtilis M168, NP_390803); NrnB (B. subtilis M168, NP_389702); SMU.1297 (S. mutans UA159, NP_721668); BF3670 (B. fragilis NCTC 9343, YP_213263); ttRecJ (T. thermophilus HB8, YP_144433); ecRecJ (E. coli ATCC 8739, YP_001723816); and bsRecJ (B. subtilis M168, NP_390640). B, schematic representation of TTHA0118, ttRecJ, and cd-ttRecJ. DHH and DHHA1 indicate DHH motifs I-IV and DHHA1 motif, respectively. OB indicates OB-fold.
Exonuclease Activity of TTHA0118
Exonuclease activity was analyzed with 5′-end 32P-labeled ssDNA, ssRNA, and dsDNA with a 5′-overhang. Gel electrophoresis analysis of the reaction of TTHA0118 with labeled 11-mer ssDNA (11f) in the presence of MnCl2 showed only two radioactive bands (Fig. 2, lanes 3 and 4). The upper and lower band corresponded to undegraded substrate ssDNA and the mononucleotides (1-mer) excised from the 5′-end, respectively. This result was similar to that for ttRecJ (Fig. 2, lanes 9 and 10) and cd-ttRecJ (Fig. 2, lanes 11 and 12) in the presence of MgCl2. Because RecJ is known to be a 5′-3′ exonuclease (14, 15, 18, 19), these results indicated that TTHA0118 had 5′-3′ exonuclease activity for ssDNA.
FIGURE 2.
Exonuclease activity of TTHA0118, Mpn140, NrnA, ttRecJ, and cd-ttRecJ. An aliquot of 10 nm 5′-32P-labeled 11-mer ssDNA (11f) was reacted with 0.1 or 1 μm TTHA0118 (tt0118), Mpn140, NrnA, ttRecJ, or cd-ttRecJ for 1 h at 37 °C. The reaction mixtures contained 50 mm HEPES, 100 mm KCl, 5 mm MnCl2 (for TTHA0118, Mpn140, and NrnA reactions) or 5 mm MgCl2 (for ttRecJ and cd-ttRecJ reactions) (pH 7.5). Lane 1, 5 mm MnCl2; lane 2, 5 mm MgCl2; lane 3, 0.1 μm TTHA0118; lane 4, 1 μm TTHA0118; lane 5, 0.1 μm Mpn140; lane 6, 1 μm Mpn140; lane 7, 0.1 μm NrnA; lane 8, 1 μm NrnA; lane 9, 0.1 μm ttRecJ; lane 10, 1 μm ttRecJ; lane 11, 0.1 μm cd-ttRecJ; lane 12, 1 μm cd-ttRecJ. The 11-mer arrow indicates the position of 5′-end labeled substrate 11-mer ssDNA, and the 1-mer arrows indicate the position of released 5′-end-labeled mononucleotides in control reactions with buffer containing Mn2+ or Mg2+ (lanes 1 and 2). Authentic 5′-end-labeled AMP was used as a marker for the position of mononucleotides.
The polarity and products of TTHA0118 exonuclease activity with a 5′-end phosphorylated 11-mer ssDNA were analyzed using FT-ICR mass spectrometer accurately (Fig. 3A). A deviation between the theoretical and measured molecular mass was estimated under 5 ppm (Fig. 3A, inset). TTHA0118 hydrolyzed the substrate one by one in the 5′-3′ polarity, yielding 3′-OH and 5′-phosphate ends, because x-ions were observed (supplemental Fig. S2) (46). The products which are shorter than x5 (x1–x5) in each reaction were not detected. We suppose that each enzyme rapidly hydrolyzed such short nucleotides under this condition. TTHA0118 also hydrolyzed the ssDNA region of dsDNA with a 6-mer overhang at the 5′-end (33f-27r), and ssRNAs producing the same cleavage pattern as for ssDNAs; i.e. release of 5′-labeled mononucleotide (1-mer) (data not shown). These results indicated that TTHA0118 hydrolyzed ssDNA and ssRNA in a 5′-3′ direction. The polarity of TTHA0118 hydrolysis was different from that of NrnA in the previous study (21) (see “Discussion” below). TTHA0118 hydrolyzed both ssDNA and ssRNA (Table 1). However, ttRecJ and cd-ttRecJ had no activity against ssRNA, even with a 100-fold molar enzyme excess, in contrast to their activity against ssDNA (data not shown).
FIGURE 3.
FT-ICR mass spectra of the products of TTHA0118, Mpn140, and NrnA exonuclease reaction. A–C, mass analysis results of the products generated by TTHA0118, Mpn140, and NrnA, respectively. The substrate (S), 5′-end phosphorylated 11-mer ssDNA, was incubated at 37 °C with each enzyme for 1 h (b, f, and j), 3 h (c, g, and k), 6 h (d, h, and l), or incubated for 6 h without enzyme (a, e, and i) as a negative control. The relative intensities of b, c and d were four times magnified. The isotopic mass distribution of 5′-end phosphorylated 11-mer ssDNA (black line) and its simulated pattern (red dotted line) are shown in the inset of panel a in A. Peak labels show x-ions by the letter x and nucleotide length by numbers written as subscripts (see supplemental Fig. S2).
TABLE 1.
Kinetic constants for 5′-3′ exonuclease and pAp phosphatase activities of TTHA0118, Mpn140, NrnA, ttRecJ, and cd-ttRecJ
| Protein | Substrate | Kma | kcata | kcat/Km |
|---|---|---|---|---|
| μm | s−1 | m−1s−1 | ||
| TTHA0118b | 3f | 65 ± 8.7 | 200 ± 33 | 3,100,000 |
| 6f | 120 ± 17 | 6.3 ± 1.2 | 53,000 | |
| 11f | 68 ± 11 | 0.025 ± 0.0041 | 370 | |
| 21f | 78 ± 17 | 0.0047 ± 0.0011 | 60 | |
| 33f-27rg | 120 ± 29 | 0.0052 ± 0.0020 | 43 | |
| 3fR | 280 ± 36 | 280 ± 18 | 1,000,000 | |
| 6fR | 270 ± 26 | 6.2 ± 0.82 | 23,000 | |
| 11fR | 450 ± 63 | 2.5 ± 0.33 | 5,600 | |
| 21fR | 470 ± 52 | 0.097 ± 0.0094 | 210 | |
| pAph | 18 ± 5.7 | 95 ± 4.1 | 5,300,000 | |
| Mpn140c | 6f (Mg2+) | 0.75 ± 0.12 | 0.45 ± 0.12 | 600,000 |
| 6f (Mn2+) | 1.5 ± 0.33 | 0.012 ± 0.0033 | 8,000 | |
| 11f (Mn2+) | 3.5 ± 0.41 | 0.0028 ± 0.00041 | 800 | |
| 6fR (Mg2+) | 0.32 ± 0.15 | 0.033 ± 0.0065 | 100,000 | |
| 6fR (Mn2+) | 0.96 ± 0.049 | 0.010 ± 0.0016 | 10,000 | |
| 11fR (Mn2+) | 2.9 ± 0.49 | 0.0038 ± 0.00057 | 1,300 | |
| pAph | 32 ± 3.3 | 0.75 ± 0.041 | 23,000 | |
| NrnAd | 3f | 280 ± 29 | 490 ± 39 | 1,800,000 |
| ttRecJe | 3f | N.D.i | N.D.i | N.D.i |
| 6f | 0.17 ± 0.033 | 0.020 ± 0.0041 | 120,000 | |
| 11f | 0.084 ± 0.021 | 0.012 ± 0.0024 | 140,000 | |
| 21f | 0.037 ± 0.0054 | 0.0047 ± 0.00025 | 130,000 | |
| cd-ttRecJf | 3f | 33 ± 9.4 | 0.0011 ± 0.00018 | 33 |
| 6f | 42 ± 10 | 0.021 ± 0.0086 | 500 | |
| 11f | 23 ± 5.7 | 0.0071 ± 0.0014 | 310 | |
| 21f | 20 ± 2.8 | 0.0073 ± 0.00071 | 370 |
a Assays were performed at pH 7.5 as described under “Experimental Procedures.” In these assays, the enzyme was mixed with various concentrations of substrate. Three independent experiments were performed. Km and kcat were determined by nonlinear regression analysis of the assay data.
b Reactions were done in the presence of 5 mm MnCl2, 10–1,500 μm substrate, and 0.25–250 nm enzyme.
c Reactions were done in the presence of 5 mm metal (MgCl2 or MnCl2) shown in the parentheses, 0.1–10 μm substrate, and 5–25 nm enzyme.
d Reaction was done in the presence of 5 mm MnCl2, 20–900 μm substrate, and 0.25 nm enzyme.
e Reactions were done in the presence of 5 mm MnCl2, 0.01–0.5 μm substrate, and 2.5 nm enzyme.
f Reactions were done in the presence of 5 mm MnCl2, 1–100 μm substrate, and 25 nm enzyme.
g 33f-27r denotes dsDNA with 5′-end 6-mer overhang, in which 33f was radiolabeled at the 5′-end with [γ-32P]ATP.
h Reactions were done in the presence of 5 mm MnCl2.
i N.D. denotes that the activity was not detected, even with a 100-fold molar enzyme excess, in contrast to the activity for 6f, 11f, and 21f.
Interestingly, TTHA0118 hydrolyzed short ssDNA and ssRNA oligomers more efficiently than long oligomers (Table 1). The kcat/Km values for 3-, 6-, 11-, and 21-mer ssDNAs were 3,100,000, 53,000, 370, and 60 m−1 s−1, respectively (Table 1). Similarly, the kcat/Km values for 3-, 6-, 11-, and 21-mer ssRNAs were 1,000,000, 23,000, 5,600, and 210 m−1 s−1, respectively (Table 1). The Km values for the substrates studied were all within a relatively narrow range (65–470 μm). However, kcat values increased as ssDNA substrate length decreased, with kcat increasing by ∼5 orders of magnitude as ssDNA length decreased from 21-mer to 3-mer. There was not a significant difference in kcat values between hydrolysis of ssDNA and ssRNA substrates. Hydrolysis of the 5′-ssDNA overhang of dsDNA (33f-27r) was much less efficient than that of ssDNA; the kcat value for dsDNA with a 6-mer overhang was 0.0052 s−1 compared with 6.3 s−1 for 6-mer ssDNA.
To highlight the preference for short-length oligonucleotides, we examined the dependence of ttRecJ and cd-ttRecJ on substrate length. However, unlike TTHA0118, ttRecJ and cd-ttRecJ enzymatic activities did not show such a dependence on substrate length (Table 1). Also, while ttRecJ binding affinity for ssDNA was much higher than that of TTHA0118, cd-ttRecJ binding affinity for ssDNA was relatively similar to that of TTHA0118. These results indicated that TTHA0118 and ttRecJ (and TTHA0118 and cd-ttRecJ) had a significant difference in substrate recognition although both belong to the DHH protein superfamily.
We made TTHA0118 D114A mutant, in which Asp in DHH motif was replaced by Ala. The Km and kcat values of 5′-3′ exonuclease activity for 6 mer ssDNA of D114A mutant were 420 μm and 0.1 s−1, respectively, showing lower substrate affinity and catalytic activity than that of the wild-type protein (120 μm and 6.3 s−1, respectively). This indicated that the observed nuclease activity was ascribed to TTHA0118.
To determine any preference of TTHA0118 exonuclease activity for phosphorylation status of 5′-end in substrate, the assay using 10 nm 5′-32P-labeled 11f and large excess of 11f (5′-end hydroxylated form) or 5′-end phosphorylated 11f was performed. The apparent rate constant (kapp) values of these two samples were the same, suggesting that a 5′-phosphate is not required for exonuclease activity of TTHA0118 similar to RecJ (18).
The presence of a divalent cation was essential for both TTHA0118 and ttRecJ exonuclease activity. Among the divalent cations examined (each at 5 mm) in exonuclease assays using 6-mer ssDNA as substrate, Co2+ was the most effective cation and Mn2+ was second. The kapp values in the presence of Mn2+, Zn2+, Mg2+, Ca2+, and Ni2+ were ∼51%, 16%, 15%, 11%, and 8%, respectively, of that of Co2+. No activity was observed without a metal ion. However, for ttRecJ, of the divalent cations tested (each at 5 mm) in exonuclease assays using 21-mer ssDNA as substrate, Mg2+ was most effective. The presence of Mn2+, Co2+, and Zn2+ resulted in only ∼65%, 59%, and 2%, respectively, of the exonuclease activity observed with Mg2+. No activity was observed with Ca2+ and without metal ions.
Exonuclease Activity of Mpn140
We then characterized Mpn140, another RecJ-like protein, from M. pneumoniae. Because Mycoplasma species lack de novo biosynthetic pathway of nucleotides (42), we thought that the study of its RecJ-like protein might be useful in understanding physiological roles of RecJ-like proteins. Mpn140 hydrolyzed ssDNAs and ssRNAs in a 5′-3′ direction similar to TTHA0118 and ttRecJ (Fig. 2, lanes 5 and 6; Fig. 3B; and Table 1). Both kcat and Km values of Mpn140 were much lower than those of TTHA0118. However, the kcat and kcat/Km values of Mpn140 also showed a significant dependence on substrate length, although only 6-mer and 11-mer substrates were examined (Table 1). This result suggested that substrate preference for short oligonucleotides is a feature common to RecJ-like proteins. It should be noted that Mpn140 exhibited exonuclease activity even in the absence of added metal ion (data not shown). However, some metal ions may have bound to Mpn140 during purification.
Exonuclease Activity of NrnA
Furthermore, we reexamined the polarity of exonuclease activity of Bacillus subtilis NrnA, another RecJ-like protein, because its nuclease polarity was reported to be 3′ to 5′ (21). In this study, NrnA hydrolyzed ssDNA and ssRNA in a 5′-3′ direction similar to TTHA0118 and Mpn140 (Fig. 2, lanes 7 and 8; 3C). Both kcat and Km values for 3-mer ssDNA were roughly similar to those of TTHA0118 (Table 1). The result of FT-ICR mass spectrometry analysis also demonstrated that the polarity of NrnA exonuclease activity was 5′ to 3′ (Fig. 3C). This result was different from the data in the previous study (21) (see “Discussion” below).
pAp Phosphatase Activity of TTHA0118 and Mpn140
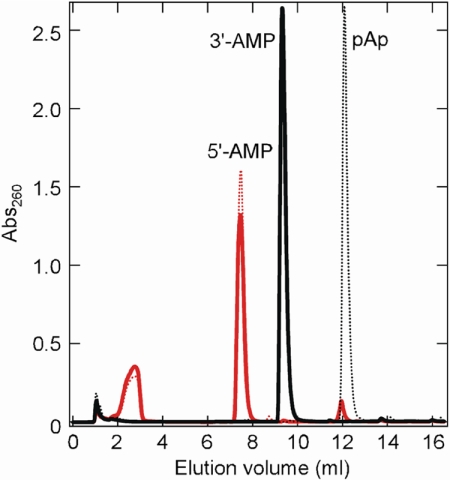
TTHA0118 had phosphatase activity for pAp (Table 1), similar to NrnA (21). HPLC analysis of the TTHA0118 reaction product with pAp showed that the main peak eluted at 7.5 ml, corresponding to 5′-AMP (Fig. 4), indicating that the 3′-phosphate was cleaved off by TTHA0118. Thus, the pAp phosphatase reaction was assumed to be: pAp + H2O → 5′-AMP + inorganic phosphate. This cleavage pattern was basically the same as that of the exonuclease reaction (supplemental Fig. S2). TTHA0118 had high pAp phosphatase activity with a kcat/Km value of 5,300,000 m−1 s−1, comparable to exonuclease activity for 3-mer ssDNA and ssRNA (Table 1). Therefore, the smallest nucleotide TTHA0118 can hydrolyze is pAp.
FIGURE 4.
HPLC profile of the products of TTHA0118 phosphatase activity on a pAp substrate. Elution profile of the product of pAp hydrolysis by TTHA0118 (red solid line), a pAp control reaction mixture (black dotted line), a 5′-AMP (red dotted line) control reaction mixture, and a 3′-AMP control reaction mixture (black solid line). The enzyme reaction mixture was prepared as described in “Experimental Procedures.” The control reaction mixture did not contain TTHA0118.
TTHA0118 also hydrolyzed cAMP and cyclic GMP (cGMP), forming 5′-AMP and 5′-GMP, respectively (data not shown). Such cAMP phosphodiesterase activity has been reported for human Prune belonging to the DHH protein superfamily (10). However, the hydrolase activity of TTHA0118 was very low: the Km, kcat, and kcat/Km values for cAMP and cGMP were 1.4 and 2.4 mm, 0.0035 and 0.017 s−1, and 2.5 and 7.1 m−1 s−1, respectively. Thus, the phosphatase activity of TTHA0118 on 3′-phosphorylated mononucleotides may be specific for nucleoside 5′, 3′-diphosphate. Similarly, Mpn140 had a high kcat/Km value (23,000 m−1 s−1) for pAp phosphatase activity similar to TTHA0118 (Table 1).
Growth of the Δttha0118 Strain in Minimum Essential Medium
To investigate the functions of TTHA0118 in T. thermophilus HB8 cells, we disrupted the chromosomal ttha0118 gene by insertion of a kanamycin resistance gene to produce a Δttha0118 strain. TT medium (rich medium) and CS medium (minimum essential medium) were inoculated with 4 × 104 cells/ml and total bacterial counts were assayed after 21 h of incubation (Fig. 5). Both T. thermophilus HB8 wild-type and Δttha0118 strains showed almost the same cell density (∼6 × 109 cells/ml) at late-log-phase growth in TT medium. However, colonies of the Δttha0118 strain were significantly (about 4-fold) smaller than those of the wild-type strain (data not shown), suggesting that Δttha0118 cells grew more slowly than wild-type cells on TT plates. Furthermore, the Δttha0118 strain had significantly less growth in CS medium (7.3 × 108 cells/ml) than in TT medium (5.9 × 109 cells/ml) (Fig. 5). The growth of the Δttha0118 strain was ∼2-fold slower in CS medium than in TT medium during log phase (data not shown). Because growth of the wild-type strain was similar in TT and CS media, the Δttha0118 strain may be auxotrophic for some components in CS minimum essential medium.
FIGURE 5.
Phenotype of the Δttha0118 strain. Cell density after 21 h cultivation of wild-type (gray columns) and Δttha0118 (white columns) strains in liquid TT or CS medium in the absence or presence of 0.1 mm 5′-mononucleotides (5′-NMP), nucleosides (Nuc), or cysteine (Cys). Each cell density is the arithmetic mean ± standard deviation for four independent experiments.
To investigate the relationship between auxotrophic phenotype of the Δttha0118 strain and enzymatic activities in vitro, we added a 5′-mononucleotide mixture (i.e. the products of TTHA0118 exonuclease activity) to CS medium and studied growth of the Δttha0118 strain in this medium. We also examined the effect of addition of a nucleoside mixture to CS medium on growth of the Δttha0118 strain, because nucleosides are electrically neutral and thought be more membrane-permeable than 5′-mononucleotides. Addition of either the 5′-mononucleotide mixture or the nucleoside mixture to CS medium increased the Δttha0118 cell density after 21 h cultivation about 5-fold (3.2 × 109 or 3.9 × 109 cells/ml, respectively) than in CS medium alone (Fig. 5). These results suggested that TTHA0118 is involved in recycling nucleotides in vivo, probably via its exonuclease activity. Addition of cysteine to CS medium also increased the Δttha0118 cell density after 21 h cultivation about 3-fold (2.3 × 109 cells/ml) than in CS medium alone (Fig. 5). This result suggested that the pAp phosphatase activity of TTHA0118 affected cysteine assimilation in vivo.
DISCUSSION
In this study, we investigated the molecular and physiological functions of TTHA0118, a RecJ-like protein, and characterized some biochemical properties of Mpn140 and NrnA, other RecJ-like proteins, and ttRecJ and its truncated form cd-ttRecJ. These five proteins had 5′-3′ exonuclease activities, indicating that enzymes in the DHH protein superfamily may have the same cleavage polarity (Fig. 2). The FT-ICR mass spectra of reaction products of these three RecJ-like proteins showed that these proteins have 5′-3′ exonuclease activities definitely (Fig. 3). However, this result is in contrast to previous work about NrnA, which degraded short ssRNA in a 3′-5′ direction (21). In addition, the apparent rate constant for NrnA cleavage of 3-mer ssRNA was reported to be 1.5 pmol/μg/min (21), which was equivalent to ∼0.001 s−1 based on the theoretical molecular weight of NrnA. This value is five orders of magnitude lower than that for NrnA cleavage of 3-mer ssDNA and that for TTHA0118 cleavage for 3-mer ssRNA in this study (Table 1). Different experimental conditions might cause this difference; for example, the substrates used in the previous study were 5′-Cy5-labeled oligoribonucleotides, whereas the substrates in this study were 5′-phosphorylated. Although it was reported that E. coli RecJ had very weak 3′-5′ exonuclease activity (14), the 5′-3′ exonuclease activity of NrnA might be severely affected by such a sterically-bulky modification at the 5′-terminus of its substrates. It should be mentioned here that NrnB (YngD) (family S_59202, Fig. 1), another RecJ-like protein from B. subtilis, was also reported to hydrolyze short ssRNA oligomer in a 3′-5′ direction (47).
An important finding of this study was that TTHA0118 and Mpn140 preferentially hydrolyzed short ssDNA and ssRNA oligomers. ttRecJ and cd-ttRecJ did not show a dependence on substrate length. Although a preference for short oligomers has been shown qualitatively for NrnA in the previously study (21), this report was about its 3′-5′ exonuclease activity. Thus, this study is the first report showing that 5′-3′ exonuclease of RecJ-like proteins has a preference for short oligomer. In the study reported here, the substrate preference of TTHA0118 has been shown quantitatively. The kcat/Km value of TTHA0118 for a 3-mer substrate was approximately two orders of magnitude greater than for a 6-mer substrate, but was comparable to that for pAp, the smallest nucleotide (Table 1). These results imply that a 3-mer is the optimal oligomer length for TTHA0118 cleavage. This study further showed that TTHA0118 substrate preference was determined by the catalytic step, not the substrate-binding step, as shown by the kcat values (Table 1). The major contribution of kcat into the substrate preference was also reported for the exopolyphosphatase activity of human Prune, which hydrolyzes short-chain polyphosphate (11) as well as cAMP (10).
The difference in substrate length dependence between RecJ and RecJ-like proteins may be due to their different tertiary structures. RecJ-like proteins are shorter than RecJ proteins (Fig. 1). Although cd-ttRecJ has a similar size to RecJ-like proteins (Fig. 1B), its enzymatic activity showed no dependence on substrate length (Table 1). Therefore, the tertiary structure of cd-ttRecJ (37) may be limited in helping understand the mechanism underlying substrate length preference. Recently, the crystal structures of RecJ-like proteins BF3670 from B. fragilis and SH1221 from S. hemeolyticus have been resolved (PDB ID: 3DMA and 3DEV), but have not yet been published. When the BF3670 monomer crystal structure was compared with that of cd-ttRecJ, the root mean square deviation was 4.1 Å, indicating that these proteins are structurally similar to each other. The residues in the DHH and DHHA1 motifs are highly conserved in both proteins, suggesting a common catalytic mechanism. In addition to these motifs, cd-ttRecJ contains highly conserved residues (Arg-110, Asn-273, Arg-277, Asn-307, Arg-310, Gln-311, Arg-350, and Arg-370) whose side chains have been suggested to interact with the nucleic acid backbone (Fig. 6A) (37, 38). However, only one residue in BF3670 (Arg291 in BF3670) is conserved in RecJ-like proteins (Fig. 6B), based on comparison of BF3670 with cd-ttRecJ structures and multiple alignment analysis. The BF3670 Arg-291 corresponds to Arg-370 in cd-ttRecJ. In addition, Arg-105 and Asn-184 in BF3670, which corresponds to His-142 and Asn-229, respectively, in cd-ttRecJ, may interact with nucleic acids (Fig. 6B). This suggests that RecJ-like proteins have a smaller number of residues for interaction with nucleic acids than RecJ proteins. Therefore, the length dependence of RecJ and RecJ-like exonucleases may be attributed to differences in their interactions with the backbone of their nucleic acid substrates. Many interactions may be necessary for ttRecJ and cd-ttRecJ to adopt a catalytically effective conformation regardless of substrate length. RecJ-like proteins may adopt such a conformation only on binding to short oligonucleotides due to a lack of tight interactions with long substrates. Conversely, a long substrate may hamper the adoption or stabilization of a catalytically effective RecJ-like protein conformation.
FIGURE 6.
Structural comparison between RecJ and RecJ-like protein. The active site is surrounded by a dashed line. A, crystal structure of cd-ttRecJ (PDB ID: 1I6R). Residues thought to be important for ssDNA binding are shown in stick form (37, 38). His-394 is a residue in DHHA1 motif. B, crystal structure of BF3670 (PDB ID: 3DMA). Residues thought to be important for the substrate binding are shown in stick form. His-311 is a residue in DHHA1 motif. C, crystal structure of ttRecJ (PDB ID: 2ZXP). The OB-fold-containing domain is shown in orange. Residues thought to be important for ssDNA binding in this domain are shown in stick form (38).
It should be noted that cd-ttRecJ showed lower affinity for substrates than ttRecJ. This can be explained by the fact that cd-ttRecJ lacks the domain containing a novel OB-fold (39), that is in the C-terminal region of ttRecJ (Figs. 1B and 6C) (38). Lack of the OB-fold-containing domain might also explain the low substrate affinity of TTHA0118, but not the high affinity of Mpn140. Mpn140 might have an appropriate active site with high affinity for oligonucleotides, compared with that of TTHA0118. The relatively low sequence identity (29%) between TTHA0118 and Mpn140 might account for the difference in their active site conformation. This difference may also be related to the different metal ion dependences of these two proteins.
Another important finding of this study was that TTHA0118, Mpn140, and NrnA had not only oligoribonuclease activity but also oligodeoxyribonuclease activity, as do E. coli Orn (22) and human Sfn (small fragment nuclease) (29). The kcat/Km values of Sfn for 5-mer ssDNA and 5-mer ssRNA were 17,000 and 41,000 m−1 s−1, respectively (the kcat values were 0.025 s−1 and 0.065 s−1, and the Km values were 1.5 μm and 1.6 μm, respectively) (29). These values are similar to those of TTHA0118 and Mpn140 for 6-mer oligonucleotides (Table 1). While it has not been determined whether NrnA has oligodeoxyribonuclease activity in the previously study (21), our results suggest that RecJ-like proteins hydrolyze both short ssRNAs and ssDNAs. Therefore, our data strongly support the suggestion that RecJ-like proteins are functional analogs of the Orn/Sfn protein family.
With regard to TTHA0118 functions, growth of Δttha0118 cells in minimum essential medium was less than that of wild-type cells, indicating that ttha0118 was not an essential gene in T. thermophilus HB8. This is in contrast to the observation that inactivation of the E. coli orn gene stopped cell growth, indicating that orn is an essential E. coli gene (48). This difference suggests there should be another enzyme with oligo(deoxy)ribonuclease activity in T. thermophilus HB8. We also found that addition of 5′-mononucleotides, nucleosides, or cysteine to minimum essential medium improved growth of the Δttha0118 strain (Fig. 5). This is the first report of the effect of nucleotides and nucleosides on growth of cells with a RecJ-like gene mutant. Cysteine (final concentration, 0.1 mm) shortened the doubling time of a B. subtilis nrnA mutant in MOPS minimal medium containing all amino acids except for cysteine, but nucleotides and nucleosides were not examined (21). Our results support the suggestion that TTHA0118 hydrolyzes short nucleic acids to mononucleotides to provide precursors for nucleotide and/or nucleic acid metabolism in vivo (Fig. 7). Based on this model, depletion of the mononucleotide pool may have affected growth of the Δttha0118 strain.
FIGURE 7.
Overview of RecJ-like protein functions. RecJ-like protein has exonuclease activity for short ssDNA and ssRNA oligomers to produce mononucleotides, and/or phosphatase activity for pAp to control pAp concentration.
However, possible accumulation of pAp might be partly responsible for the observed changes in Δttha0118 cells, because TTHA0118 also has pAp phosphatase activity. A reduction in cysteine or sulfate assimilation via pAp might also be considered, because addition of cysteine restored the growth of Δttha0118 cells in minimum essential medium (Fig. 5). Because pAp acts as a competitive inhibitor of several enzymes (31, 49, 50), high concentrations of pAp are toxic for cells. RecJ-like proteins may degrade pAp to control the concentration in bacteria and archaea that do not have CysQ (30–34) (Fig. 7).
A possible role of RecJ-like proteins in oligo(deoxy)ribonucleotide degradation was further suggested by studies of Mpn140. The amino acid sequence of Mpn140, from M. pneumoniae M129, showed a 29% identity match with TTHA0118 (Fig. 1). M. pneumoniae is a common agent of respiratory infections in humans (51, 52). No Mycoplasma gene or gene product involved in de novo biosynthesis of nucleic acid precursors, including purines and pyrimidines, has been identified thus far (40–42). Mycoplasma species are thought to be completely dependent on their host for supplying these nucleic acid precursors. Genomic sequence analyses of a number of Mycoplasma species have identified novel enzymes associated with salvage pathways for biosynthesis of nucleic acids from nucleotides (41, 53, 54). Mycoplasma nucleases are thought to play a metabolic role in the production of nucleotide substrates from host or microbial nucleic acids released through natural and induced cell death. Mpn140 is part of a polycistronic transcriptional unit that includes with Mpn141, the cellular major adhesin protein, and Mpn142, a cytoadherence-related B/C protein (52). Our study showed Mpn140 has oligo(deoxy)ribonuclease and pAp phosphatase activities. Therefore, Mpn140 may hydrolyze short ssDNA and ssRNA oligomers in the human respiratory tract to produce precursors for M. pneumoniae nucleic acids.
Data on two other RecJ-like proteins have recently been reported. SMU.1297 (family N_142143, Fig. 1A) from Streptococcus mutans, a facultative anaerobe, hydrolyzed pAp, but not short ssRNA, and complemented an E. coli cysQ mutant (55). A smu.1297 mutant was sensitive to superoxide stress, suggesting that SMU.1297 was involved in superoxide stress tolerance. However, this phenotype may be unique to S. mutans because the S. mutans lifestyle is very different from that of other bacteria. E. coli cysQ and B. subtilis nrnA mutant did not show a superoxide stress-sensitive phenotype (55).
NrnB was reported to hydrolyze short ssRNA and ssDNA oligomers but not pAp (47). NrnB has low sequence identity with NrnA, and NrnB homologs are found in a smaller number of bacterial genomes than those of NrnA. NrnB was able to complement an E. coli orn mutant but not an E. coli cysQ mutant (47). Considering these substrate specificities, NrnA and NrnB might be different RecJ-like protein subtypes. The fact that many bacteria that do not carry orn and cysQ have a NrnA homolog, but not a NrnB homolog, supports the suggestion that the NrnA homolog may be physiologically significant (47).
In conclusion, RecJ-like proteins may function to produce mononucleotides and/or to control the cellular pAp concentration (Fig. 7). Considering these functions and the physiological distribution of RecJ-like proteins, these proteins may have functions similar to Orn and CysQ.
Supplementary Material
Acknowledgments
We thank Hirohumi Ohmori for DNA sequencing. We thank Dr. Naoto Ohtani (Keio University) for providing us with the genome of M. pneumoniae M129. We thank Dr. Yasuhiro Takahashi (Saitama University) for providing us with the genomic DNA of B. subtilis M168.
This work was supported in part by Grant-in-Aid for Scientific Research 20570131 (to R. M.) and 19770083 (to N. N.) from the Ministry of Education, Science, Sports and Culture of Japan.

The on-line version of this article (available at http://www.jbc.org) contains supplemental experimental procedures and Figs. S1 and S2.
- RNase
- ribonuclease
- cd-ttRecJ
- core domain of ttRecJ
- dsDNA
- double-stranded DNA
- OB-fold
- oligonucleotide/oligosaccharide-binding-fold
- pAp
- 3′-phosphoadenosine 5′-phosphate
- ssDNA
- single-stranded DNA
- ssRNA
- single-stranded RNA
- ttRecJ
- T. thermophilus RecJ.
REFERENCES
- 1. Nishino T., Morikawa K. (2002) Oncogene 21, 9022–9032 [DOI] [PubMed] [Google Scholar]
- 2. Marti T. M., Fleck O. (2004) Cell Mol. Life Sci. 61, 336–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deutscher M. P. (2006) Nucleic Acids Res. 34, 659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Condon C. (2007) Curr. Opin. Microbiol. 10, 271–278 [DOI] [PubMed] [Google Scholar]
- 5. Aravind L., Makarova K. S., Koonin E. V. (2000) Nucleic Acids Res. 28, 3417–3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sapranauskas R., Sasnauskas G., Lagunavicius A., Vilkaitis G., Lubys A., Siksnys V. (2000) J. Biol. Chem. 275, 30878–30885 [DOI] [PubMed] [Google Scholar]
- 7. Bujnicki J. M., Radlinska M., Rychlewski L. (2001) Trends Biochem. Sci. 26, 9–11 [DOI] [PubMed] [Google Scholar]
- 8. Zuo Y., Deutscher M. P. (2001) Nucleic Acids Res. 29, 1017–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aravind L., Koonin E. V. (1998) Trends Biochem. Sci. 23, 17–19 [DOI] [PubMed] [Google Scholar]
- 10. D'Angelo A., Garzia L., André A., Carotenuto P., Aglio V., Guardiola O., Arrigoni G., Cossu A., Palmieri G., Aravind L., Zollo M. (2004) Cancer Cell 5, 137–149 [DOI] [PubMed] [Google Scholar]
- 11. Tammenkoski M., Koivula K., Cusanelli E., Zollo M., Steegborn C., Baykov A. A., Lahti R. (2008) Biochemistry 47, 9707–9713 [DOI] [PubMed] [Google Scholar]
- 12. Wurst H., Kornberg A. (1994) J. Biol. Chem. 269, 10996–11001 [PubMed] [Google Scholar]
- 13. Finn R. D., Mistry J., Tate J., Coggill P., Heger A., Pollington J. E., Gavin O. L., Gunasekaran P., Ceric G., Forslund K., Holm L., Sonnhammer E. L., Eddy S. R., Bateman A. (2010) Nucleic Acids Res. 38, D211–D222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lovett S. T., Kolodner R. D. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 2627–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cooper D. L., Lahue R. S., Modrich P. (1993) J. Biol. Chem. 268, 11823–11829 [PubMed] [Google Scholar]
- 16. Dianov G., Sedgwick B., Daly G., Olsson M., Lovett S., Lindahl T. (1994) Nucleic Acids Res. 22, 993–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burdett V., Baitinger C., Viswanathan M., Lovett S. T., Modrich P. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 6765–6770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han E. S., Cooper D. L., Persky N. S., Sutera V. A., Jr., Whitaker R. D., Montello M. L., Lovett S. T. (2006) Nucleic Acids Res. 34, 1084–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sharma R., Rao D. N. (2009) J. Mol. Biol. 385, 1375–1396 [DOI] [PubMed] [Google Scholar]
- 20. Krause A., Nicodème P., Bornberg-Bauer E., Rehmsmeier M., Vingron M. (1999) Bioinformatics 15, 262–263 [DOI] [PubMed] [Google Scholar]
- 21. Mechold U., Fang G., Ngo S., Ogryzko V., Danchin A. (2007) Nucleic Acids Res. 35, 4552–4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mechold U., Ogryzko V., Ngo S., Danchin A. (2006) Nucleic Acids Res. 34, 2364–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheng Z. F., Deutscher M. P. (2002) J. Biol. Chem. 277, 21624–21629 [DOI] [PubMed] [Google Scholar]
- 24. Amblar M., Barbas A., Fialho A. M., Arraiano C. M. (2006) J. Mol. Biol. 360, 921–933 [DOI] [PubMed] [Google Scholar]
- 25. Vincent H. A., Deutscher M. P. (2006) J. Biol. Chem. 281, 29769–29775 [DOI] [PubMed] [Google Scholar]
- 26. Amblar M., Barbas A., Gomez-Puertas P., Arraiano C. M. (2007) RNA 13, 317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Datta A. K., Niyogi K. (1975) J. Biol. Chem. 250, 7313–7319 [PubMed] [Google Scholar]
- 28. Zhang X., Zhu L., Deutscher M. P. (1998) J. Bacteriol. 180, 2779–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nguyen L. H., Erzberger J. P., Root J., Wilson D. M., 3rd (2000) J. Biol. Chem. 275, 25900–25906 [DOI] [PubMed] [Google Scholar]
- 30. Neuwald A. F., Krishnan B. R., Brikun I., Kulakauskas S., Suziedelis K., Tomcsanyi T., Leyh T. S., Berg D. E. (1992) J. Bacteriol. 174, 415–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klaassen C. D., Boles J. W. (1997) FASEB J. 11, 404–418 [DOI] [PubMed] [Google Scholar]
- 32. Hatzios S. K., Iavarone A. T., Bertozzi C. R. (2008) Biochemistry 47, 5823–5831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murguía J. R., Bellés J. M., Serrano R. (1995) Science 267, 232–234 [DOI] [PubMed] [Google Scholar]
- 34. Peng Z., Verma D. P. (1995) J. Biol. Chem. 270, 29105–29110 [DOI] [PubMed] [Google Scholar]
- 35. Yokoyama S., Hirota H., Kigawa T., Yabuki T., Shirouzu M., Terada T., Ito Y., Matsuo Y., Kuroda Y., Nishimura Y., Kyogoku Y., Miki K., Masui R., Kuramitsu S. (2000) Nat. Struct. Biol. 7, 943–945 [DOI] [PubMed] [Google Scholar]
- 36. Hashimoto Y., Yano T., Kuramitsu S., Kagamiyama H. (2001) FEBS Lett. 506, 231–234 [DOI] [PubMed] [Google Scholar]
- 37. Yamagata A., Kakuta Y., Masui R., Fukuyama K. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 5908–5912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wakamatsu T., Kitamura Y., Kotera Y., Nakagawa N., Kuramitsu S., Masui R. (2010) J. Biol. Chem. 285, 9762–9769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Theobald D. L., Mitton-Fry R. M., Wuttke D. S. (2003) Annu. Rev. Biophys. Biomol. Struct. 32, 115–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kuramitsu S., Hiromi K., Hayashi H., Morino Y., Kagamiyama H. (1990) Biochemistry 29, 5469–5476 [DOI] [PubMed] [Google Scholar]
- 41. Yamagata A., Masui R., Kakuta Y., Kuramitsu S., Fukuyama K. (2001) Nucleic Acids Res. 29, 4617–4624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wakamatsu T., Nakagawa N., Kuramitsu S., Masui R. (2008) J. Bacteriol. 190, 1108–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Razin S. (1978) Microbiol. Rev. 42, 414–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Himmelreich R., Hilbert H., Plagens H., Pirkl E., Li B. C., Herrmann R. (1996) Nucleic Acids Res. 24, 4420–4449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pollack J. D., Williams M. V., McElhaney R. N. (1997) Crit. Rev. Microbiol. 23, 269–354 [DOI] [PubMed] [Google Scholar]
- 46. McLuckey S. A., Van Berkel G. J., Glish G. L. (1992) J. Am. Soc. Mass Spectrom. 3, 60–70 [DOI] [PubMed] [Google Scholar]
- 47. Fang M., Zeisberg W. M., Condon C., Ogryzko V., Danchin A., Mechold U. (2009) Nucleic Acids Res. 37, 5114–5125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ghosh S., Deutscher M. P. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 4372–4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dichtl B., Stevens A., Tollervey D. (1997) EMBO J. 6, 7184–7195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schneider B., Xu Y. W., Janin J., Véron M., Deville-Bonne D. (1998) J. Biol. Chem. 273, 28773–28778 [DOI] [PubMed] [Google Scholar]
- 51. Waites K. B., Talkington D. F. (2004) Clin. Microbiol. Rev. 17, 697–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Waldo R. H., 3rd, Krause D. C. (2006) J. Bacteriol. 188, 569–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang L., Westberg J., Bölske G., Eriksson S. (2001) Mol. Microbiol. 42, 1065–1073 [DOI] [PubMed] [Google Scholar]
- 54. Westberg J., Persson A., Holmberg A., Goesmann A., Lundeberg J., Johansson K. E., Pettersson B., Uhlén M. (2004) Genome Res. 14, 221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang J., Biswas I. (2009) J. Bacteriol. 191, 4330–4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.