Abstract
Marked gender differences in the expression of sulfotransferases (Sults) are known to exist in several species including rats, mice and hamsters. However, the mechanism for this gender difference is not known. Therefore, in the present study, it was determined whether sex and/or growth hormone (GH) are responsible for the gender difference in the expression of Sults using gonadectomized (GNX), hypophysectomized (HX) and GH-releasing hormone receptor-deficient little (lit/lit) mouse models.
Sult1a1 and Papss2 in liver and kidney, and Sult1d1 in liver are female-predominant in mice because of suppressive effects of both androgens and male-pattern GH secretion. Sult2a1/a2 is the most markedly female-predominant Sult in mouse liver due to suppressive effects of androgens and male-pattern GH secretion, as well as stimulatory effects by estrogens and female-pattern GH secretion. Sult3a1 is female-predominant in mouse liver due to suppressive effects of androgens as well as stimulatory effects of estrogens and female-pattern GH secretion. Sult1c1 expression is male-predominant in mouse liver and kidney because of stimulatory effects of androgens in males. Sult4a1 expression is female-predominant in mouse brain due to stimulatory effects of estrogens.
In conclusion, gender-divergent Sults are mostly female-predominant and Sult1c1 is the only male-dominant Sult. The gender differences in expression of various mouse Sults are influenced by various mechanisms involving sex and/or GHs.
Keywords: Sulfotransferase, Sult, PAPS, Papss, gender, mRNA, bDNA
INTRODUCTION
Sulfonation is the transfer of a sulfonate group (SO3−) from the activated sulfate compound, 3′-phosphoadenosine 5′-phosphosulphate (PAPS), to an oxygen, nitrogen, or sulfur atom of a substrate to form sulfates, sulfamates, or thiosulfates, respectively. The resulting sulfo-conjugated metabolites are usually pharmacologically inactive and more water soluble, and therefore are readily eliminated in bile and urine (Glatt, 2000). However, sulfonation can also bioactivate molecules and result in the formation of reactive metabolites that covalently bind with macromolecules (Glatt et al., 1998).
Sulfonation is catalyzed by a super-family of enzymes called sulfotransferases (Sults). There are two classes of Sults: cytosolic Sults, which catalyze the sulfonation of various small endogenous and exogenous compounds, and Golgi-associated Sults, which catalyze the sulfonation of macromolecules, such as proteins and carbohydrates. Therefore, cytosolic Sults represent the class relevant for the phase II metabolic conjugation of xeno- and endobiotics. The synthesis of PAPS from inorganic sulfates is catalyzed by two PAPS synthases isoforms (Papss1 and Papss2) (Matsui and Homma, 1994).
Sults are divided into five families and several subfamilies based on the similarity of their amino acid sequences. The various Sult isoforms have broad and overlapping substrate specificity. However, in general, phenols, thyroid hormones, hydroxylamines, eicosanoids and estrogens are prototypical substrates for Sult1a, 1b, 1c, 1d and 1e subfamilies, respectively. The hydroxysteroid Sults, Sult2a and Sult2b, have a broad substrate specificity including androgens, estrogens, dehydroepiandrosterone (DHEA), bile acids and cholesterol. The Sult3 family catalyzes the sulfonation of heterocyclic amines, whereas the substrate specificity of the Sult4 and -5 enzymes have not been well-characterized. Papss has two isoforms (Papss1 and Papss2), which differ in their tissue distribution and catalytic activity (Strott, 2002).
Gender differences in sulfonation were reported more than 50 years ago, when it was demonstrated that the sulfonation of glucocorticoids by male rats was only 20% of that in females (Roy, 1956). Gender differences in the sulfonation of many substrates have been reported (DeBaun et al., 1970; Matsui and Watanabe, 1982; Borthwick et al., 1993). Furthermore, the mRNA expression of various Sult isoforms was shown to be gender-related in rats (Liu and Klaassen, 1996a, 1996b; Klaassen et al., 1998).
Sexual dimorphism of drug metabolizing enzymes and transporters are considered major factors that cause gender differences in the pharmacokinetic, pharmacologic and toxicologic profiles of many chemicals (Meibohm et al., 2002). Hundreds of hepatic proteins including enzymes, transporters and nuclear receptors have sexual-dimorphic expression (Hines and McCarver, 2002; McCarver and Hines, 2002; Ahluwalia et al., 2004). The sexual dimorphism of many proteins is mediated via hormones, namely sex hormones and growth hormone (GH) secreted by the gonads and pituitary gland, respectively (Roy and Chatterjee, 1983). In male rats and mice, GH secretion is characterized by high-amplitude pulses with GH levels being undetectable between pulses. However, in female rats and mice, GH is secreted in more frequent pulses of lower amplitude, and therefore a continuous baseline of GH levels in blood remains detectable (Edén, 1979; MacLeod et al., 1991).
Gonadectomized (GNX), hypophysectomized (HX) and mice with spontaneous mutation in the GH-releasing hormone receptor (lit/lit) represent animal models often used to study the regulation of gene expression by sex hormones and GH. In GNX-animals, the gonads, which are the primary organs for sex-hormone production, are surgically removed. In HX-animals, the pituitary gland, which produces GH, is surgically removed. However, the pituitary gland also regulates the production of sex hormones by the gonads via the action of lutenizing hormone. Therefore, the lit/lit model, which lacks a functional receptor to stimulate GH secretion, is a more specific model that distinguishes the role of GH in the regulation of gene expression (Beamer and Eicher, 1976; Kasukawa et al., 2003).
We recently reported gender differences in the expression of Sult1a1, Sult1c1, Sult1d1, Sult2a1, Sult3a1, Sult4a1 and Papss2 in mice (Alnouti and Klaassen, 2006). However, the mechanisms underlying these gender differences in mice are largely unknown. Therefore, in the present study, we studied the hormonal regulation of these sexually dimorphic enzymes in mice. The sex-hormone effect was studied by hormone replacement in GNX and HX mice, whereas the GH effect was studied by simulating the gender-specific pattern of GH secretion in HX and lit/lit mice. hormone effect was studied by hormone replacement in GNX and HX mice, whereas the GH effect was studied by simulating the gender-specific pattern of GH secretion in HX and lit/lit mice.
MATERIALS AND METHODS
Materials
Pellets for subcutaneous release of the hormones used in this study, 5α-dihydroxytestosterone (DHT) (5 mg; 21-day release) and 17β-estradiol (E2) (0.5 mg; 21-day release), were formulated by Innovative Research of America (Sarasota, FL) to deliver approximately physiological levels of these hormones (700–1500 ng/ml for E2 and 6–9 ng/ml for DHT) (Cai et al., 2003).
Animals
Eight-week-old male and female C57BL/6 mice were purchased from Charles River Laboratories Inc. (Wilmington, MA). Animals were housed in a temperature-, light- and humidity-controlled environment. Mice were fed Laboratory Rodent Chow W (Harlan Teklad, Madison, WI) ad libitum. Tissues were removed from five mice of each gender, frozen in liquid nitrogen and stored at −80ºC until mRNA isolation.
Gonadectomy and sex-hormone replacement
Mice were castrated or ovariectomized at 37 days of age by Charles River Laboratories. At 54 days of age, DHT (5 mg) or 17β-estradiol (0.5 mg) in 21-day-release pellets (Innovative Research of America) were subcutaneously implanted interscapularly in intact and GNX male and female mice under isofluorane anesthesia. The mice were separated into seven treatment groups, with five mice/gender/treatment: (1) intact animals + placebo, (2) castration + DHT, (3) castration + 17β-estradiol (E2), (4) castration + placebo, (5) ovariectomy + DHT (6) ovariectomy + E2 and (7) ovariectomy + placebo. Tissues were removed at 64 days of age from GNX and age-matched intact control mice.
GH Replacement of lit/lit Mice
Breeding pairs of GH-releasing hormone receptor-mutant homozygous C57BL/six mice (lit/lit) were purchased from Jackson Laboratory (Bar Harbor, ME). Mice (n = 6/group) were treated for 10 days with GH in male pattern (twice daily, i.p. injection, dose of 2.5 mg of GH/day/kg), female pattern (continuous infusion via 21-day-release 1-mg GH pellet implanted subcutaneously), and placebo (Innovative Research of America). Tissues were removed after treatments for total RNA isolation.
Hormone replacement of HX mice
Mice were HX at 38 days of age by Charles River Laboratories. HX mice received 5% glucose (w/v) ad libitum. HX mice that gained weight before the start of the study were excluded under the assumption that surgery was incomplete. At 45 days of age, the mice (n = 5/gender/treatment) were treated for 10 days with placebo, 21-day-release pellets (containing 5 mg of DHT or 0.5 mg of E2), GH in male pattern, or GH in female pattern. Placebo-treated, age-matched mice were used as controls. Tissues were removed at 64 days of age for total RNA isolation.
Total RNA isolation
Total RNA was isolated using RNA-Bee reagent (Tel-Test Inc., Friendswood, TX) according to the manufacturer’s protocol. Total RNA concentrations were determined spectrophotometrically at 260 nm. One μg/μl solutions were prepared from the stock RNA solution by dilution with diethyl pyrocarbonate-treated deionized water. Integrity of RNA samples was evaluated visually using agarose gel electrophoresis. Samples were visualized under ultraviolet light by ethidium bromide fluorescence.
bDNA signal amplification Analysis
mRNA was quantified using the branched DNA (bDNA) assay (Quantigene® bDNA signal amplification kit; Panomics Inc., Fremont, CA) and expressed in relative light unit(s) as described previously (Alnouti and Klaassen, 2006, 2008).
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Statistical differences between genders in naive control mice were determined by a two-tailed Student’s t-test (P < 0.05). Statistical differences between untreated, placebo and hormone treatment groups were determined by a one-way analysis of variance followed by Duncan’s multiple range post hoc test.
RESULTS
Regulation of mouse Sults and Papss by sex hormones
Female dominant
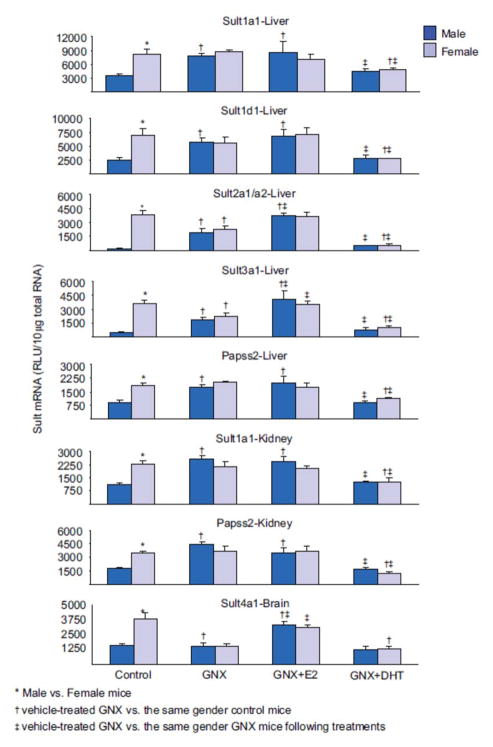
The expression of Sult1a1 and Papss2 mRNA in liver and kidney, and Sult1d1 mRNA in liver were 2–3-fold higher in female than male mice (Figure 1). However, Sult1d1 was expressed equally in kidneys of male and female mice (data not shown). The effects of GNX and sex-hormone replacement on the mRNA expression of Sult1a1, Papss2 and Sult1d1 in liver, and Sult1a1 and Papss2 in kidney were similar. The gender differences in mRNA expression of these Sult isoforms were abolished in GNX mice (Figure 1). After gonadectomy, mRNA expression in males increased to similar levels to that in control females, whereas mRNA expression was not altered in females. Estrogen replacement had no effect on the mRNA expression of these Sult isoforms, whereas androgen replacement suppressed mRNA expression in both male and female GNX mice, to levels similar to that in control male mice.
Figure 1.
Effects of gonadectomy and sex-hormone replacements on the mRNA expression of female-predominant Sults in mice. Total liver, kidney or brain RNA were isolated and analyzed by bDNA for each Sult mRNA content. The data are presented as mean RLU ± SEM (n = 5). GNX (vehicle administered to gonadectomized mice), GNX + DHT (5α-dihydroxytestosterone administered to GNX mice), and GNX + E2 (17β-estradiol administered to GNX mice). Asterisk (*) represents statistically significant differences (P < 0.05) between male and female mice; single dagger (†) represents statistically significant differences (P < 0.05) between control mice and the same gender, vehicle-treated GNX mice; and double dagger (‡) represents statistically significant differences (P < 0.05) between vehicle-treated GNX mice and the same gender, GNX mice administered DHT or E2.
Hepatic Sult2a1/a2 and Sult3a1 mRNA expressions were 100- and 8-fold higher in female than male mice, respectively. Gonadectomy abolished the gender difference in hepatic Sult2a1/a2 and Sult3a1 by increasing their expression in males and decreasing it in females (Figure 1). Estrogen replacement in GNX male and female mice increased hepatic Sult2a1/a2 and Sult3a1 expression to levels similar to that in control female mice, whereas androgen replacement suppressed their expression to levels similar to that in control male mice.
Sult4a1 expression in brains of female mice was ~2.5-fold higher than in male mice (Figure 1). Sult4a1 mRNA in brains of male mice were not affected by gonadectomy, whereas mRNA expression in females decreased to levels similar to that in male control mice (Figure 1). As a result, the gender difference in Sult4a1 mRNA expression in brain was abolished in GNX mice. Androgen replacement had no effect on Sult4a1 expression in either gender, whereas estrogen replacement increased the mRNA expression in the brains of both male and female GNX mice to levels similar to that in control female mice.
Male-dominant Sults
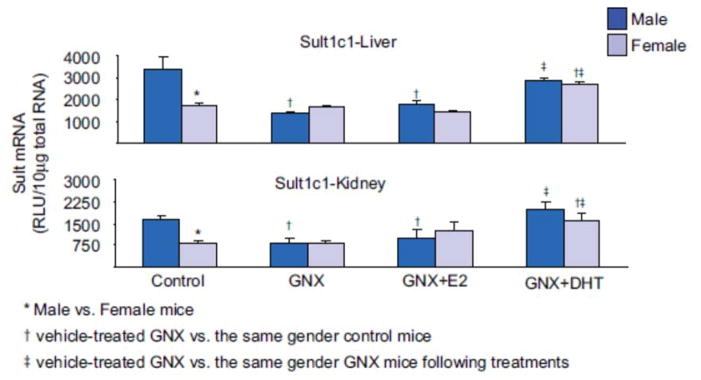
Sult1c1 mRNA expression in liver and kidney of male mice was approximately twice that in female mice (Figure 2). GNX decreased the mRNA expression of Sult1c1 in male livers and kidneys to levels similar to that in control females, whereas mRNA expression in female mice was not affected by gonadectomy (Figure 2). As a result, the gender differences in both hepatic and renal Sult1c1 mRNA expression were abolished by gonadectomy. Hepatic and renal Sult1c1 expression was not affected by estrogen replacement in either gender. However, androgen replacement increased the Sult1c1 mRNA expression in both livers and kidneys of male and female mice to levels similar to that in control male mice.
Figure 2.
Effects of gonadectomy and sex-hormone replacements on the mRNA expression of male-predominant Sult1c1 in mice. Total liver or kidney RNA were isolated and analyzed by bDNA for each Sult mRNA content. The data Sare presented as mean RLU ± SEM (n = 5). GNX (vehicle administered to gonadectomized mice), GNX + DHT (5α-dihydroxytestosterone administered to GNX mice), and GNX + E2 (17β-estradiol administered to GNX mice). Asterisk (*) represents statistically significant differences (P < 0.05) between male and female mice; single dagger (†) represents statistically significant differences (P < 0.05) between control mice and the same gender, vehicle-treated GNX mice; and double dagger (‡) represents statistically significant differences (P < 0.05) between vehicle-treated GNX mice and the same gender, GNX mice administered DHT or E2.
Regulation of Sults and Papss in HX mice
Female dominant
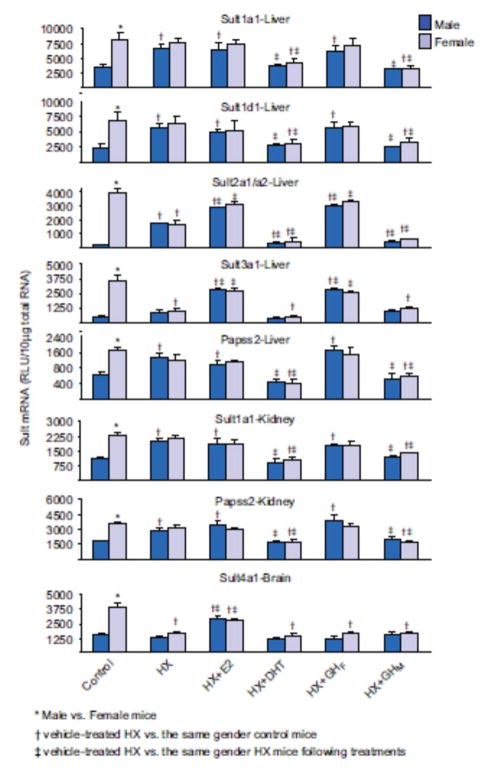
In male HX mice, mRNA expressions of Sult1a1 and Papss2 in liver and kidney, and expression of Sult1d1 in liver were increased to levels similar to that in control females, whereas mRNA expression in females was not affected by HX (Figure 3). Estrogen replacement had no effect on the hepatic or renal expression of Sult1a1 and Papss2, or the hepatic expression of Sult1d1 in either gender. In contrast, androgen replacement decreased the expression of hepatic and renal expression of Sult1a1 and Papss2, as well as hepatic expression of Sult1d1 in both male and female HX mice to that in control male mice. Similarly, female-pattern GH replacement did not affect the expression of Sult1a1 and PAPSs in livers and kidneys, and Sult1d1 in livers from HX mice, whereas male-pattern GH replacement decreased expression of the same isoforms in both male and female HX mice to levels similar to that in control male mice.
Figure 3.
Effects of hypophysectomy and hormone replacements on the mRNA expression of female-predominant Sults in mice. Total liver, kidney, or brain RNA were isolated and analyzed by bDNA for each Sult mRNA content. The data are presented as mean RLU ± SEM (n = 5). HX (placebo administered to hypophysectomized mice), HX + GHM (rat GH twice daily administered by i.p. injection to HX mice mimicking male-pattern GH secretion), HX + GHF (continuous infusion to HX mice via s.c. implanted 21-day-release 1-mg rat GH pellet mimicking female-pattern GH secretion), HX + DHT (5α-dihydroxytestosterone administered to HX mice), and HX + E2 (17β-estradiol administered to HX mice). Asterisk (*) represents statistically significant differences (P < 0.05) between male and female mice; single dagger (†) represents statistically significant differences (P < 0.05) between control mice and the same gender, vehicle-treated HX mice; and double dagger (‡) represents statistically significant differences (P < 0.05) between vehicle-treated HX mice and the same gender, HX mice following hormone replacement treatments.
Hepatic expression of Sult2a1/a2 after hypophysectomy increased in males and decreased in females, resulting in similar levels in both genders (Figure 3). Estrogen replacement in HX mice increased hepatic expression of Sult2a1/a2 to levels similar to that in control female mice, whereas androgen replacement lowered the hepatic expression to values similar to that in male control mice. Similarly, male-pattern GH replacement in HX mice lowered hepatic expression of Sult2a1/a2 in both male and female HX mice to levels similar to that in control male mice, whereas female-pattern GH replacement increased hepatic expression in both male and female HX mice to levels similar to that in control female mice.
After hypophysectomy, hepatic expression of Sult3a1 decreased in females to values similar to that in control male mice, whereas expression in males remained unchanged (Figure 3). Estrogen replacement increased the hepatic expression of Sult3a1 in male and female HX mice to levels similar to that in control female mice, whereas androgen replacement tended to further repress Sult3a1 (the androgen effect was not statistically significant) (Figure 3). Female-pattern GH replacement increased expression of Sult3a1 in the livers of both genders to levels similar to that in control female mice, whereas male-pattern GH replacement had no effect on the hepatic expression in either male or female mice.
Hypophysectomy decreased expression of Sult4a1 in brains of female mice to levels similar to that in control male mice, whereas expression in brains of HX males was not changed (Figure 3). Estrogen replacement increased Sult4a1 expression in both male and female brains of HX mice, but did not fully restore expression to that in control female mice. Androgen replacement had no effect on Sult4a1 expression in brains of either male or female mice. Both female- and male-pattern GH replacement had no effect on the hepatic expression in either male or female mice.
Male dominant
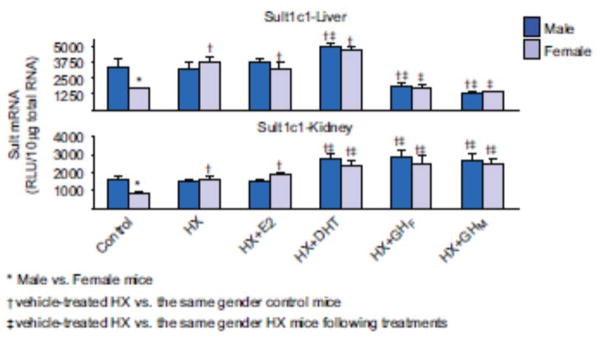
HX increased Sult1c1 mRNA in both liver and kidney in female mice but it remained unchanged in males (Figure 4). Estrogen replacement did not alter the hepatic or renal expression of Sult1c1, whereas androgen replacement increased the hepatic and renal expression of Sult1c1 in both male and female HX mice. Female-pattern GH replacement decreased hepatic expression, but increased renal expression of Sult1c1 in both male and female mice. In contrast, male-pattern GH replacement decreased hepatic expression, but increased renal expression of Sult1c1 in both genders.
Figure 4.
Effects of hypophysectomy and hormone replacements on the mRNA expression of male-predominant Sult1c1 in mice. Total liver or kidney brain RNA were isolated and analyzed by bDNA for each Sult mRNA content. The data are presented as mean RLU ± SEM (n = 5). HX (placebo administered to hypophysectomized mice), HX + GHM (rat GH twice daily administered by i.p. injection to HX mice mimicking male-pattern GH secretion), HX + GHF (continuous infusion to HX mice via s.c. implanted 21-day-release 1-mg rat GH pellet mimicking female-pattern GH secretion), HX + DHT (5α-dihydroxytestosterone administered to HX mice), and HX + E2 (17β-estradiol administered to HX mice). Asterisk (*) represents statistically significant differences (P < 0.05) between male and female mice; single dagger (†) represents statistically significant differences (P < 0.05) between control mice and the same gender, vehicle-treated HX mice; and double dagger (‡) represents statistically significant differences (P < 0.05) between vehicle-treated HX mice and the same gender, HX mice following hormone replacement treatments.
Regulation of mouse Sults and Papss by GH in lit/lit mice
Female dominant
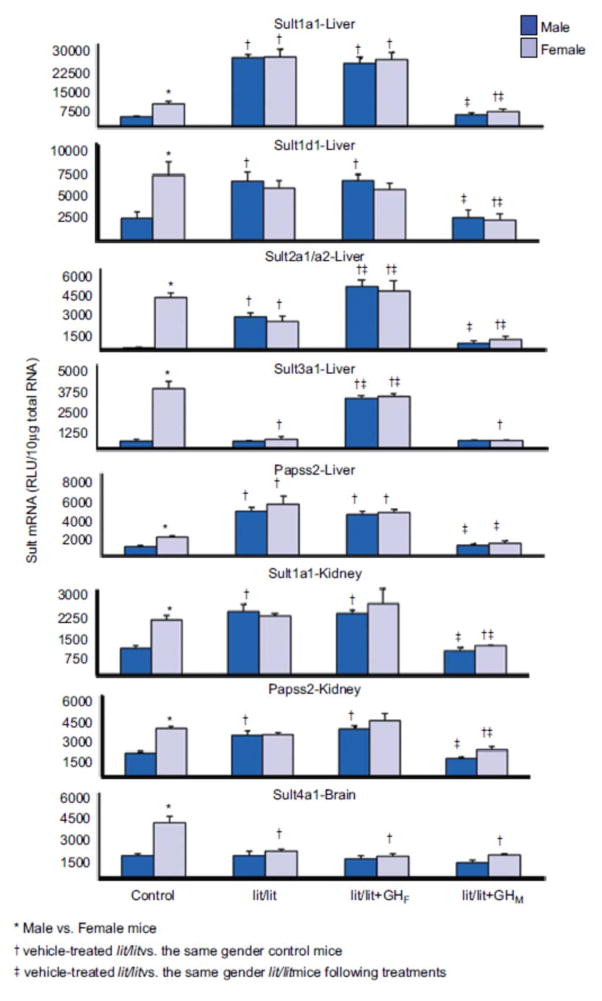
Hepatic expression of Sult1a1 and Papss2 was higher in both male and female lit/lit than control mice (Figure 5). Renal expression of Sult1a1 and Papss2, as well as hepatic expression of Sult1d1 in lit/lit male mice was higher than that in control male but similar to that in control female mice, whereas their expression in female lit/lit mice was similar to that in control female mice (Figure 5). Male-pattern GH replacement decreased the hepatic and renal expression of Sult1a1 and Paps2 as well as renal expression of Sult1d1 in both genders to that in control male mice, whereas female-pattern GH replacement did not alter expression in either gender.
Figure 5.
Effects of GH secretion pattern on the mRNA expression of female-predominant Sults in mice. Total liver, kidney, or brain RNA were isolated and analyzed bybDNA for each Sult mRNA content. The data are presented as mean RLU ± SEM (n = 5). lit/lit, placebo administered to lit/lit mice; lit/lit + GHM, rat GH twice daily administered by i.p. injection to lit/lit mice mimicking male-pattern GH secretion; lit/lit + GHF, continuous infusion to lit/lit mice via s.c. implanted 21-day-release 1-mg rat GH pellet mimicking female-pattern GHsecretion. Asterisk (*) represents statistically significant differences (P < 0.05) between male and female mice; single dagger (†) represents statistically significant differences (P < 0.05) between control mice and the same gender, vehicle-treated lit/lit mice; and double dagger (‡) represents statistically significant differences (P < 0.05) between vehicle-treated lit/lit mice and the same gender lit/lit mice following GH replacement treatments.
The gender difference in hepatic expression of Sult2a1/a2 was abolished in lit/lit mice due to a decrease in mRNA expression in females and an increase in mRNA expression in males (Figure 5). Male-pattern GH replacement lowered hepatic expression of Sult2a1/a2 in both male and female lit/lit mice to levels similar to that in control male mice, whereas female-pattern GH replacement increased hepatic expression in both male and female HX mice to levels similar to that in control female mice.
In female lit/lit mice, the hepatic expression of Sult3a1 was lower than that in control female and similar to that in control male mice, whereas expression in male lit/lit mice was similar to that in control males (Figure 5). Male-pattern GH replacement had no effect on the hepatic expression of Sult3a1 in either gender, whereas female-pattern GH replacement markedly increased expression in both male and female lit/lit mice to levels similar to that in control female mice.
Sult4a1 expression in brain was lower in female lit/lit mice than that in female control mice and similar to that in male control mice, whereas expression in the male lit/lit mice was similar to that in control male mice (Figure 5). Both male- and female-pattern GH replacement had no effect on the expression of Sult4a1 in the brains of lit/lit female and male mice.
Male dominant
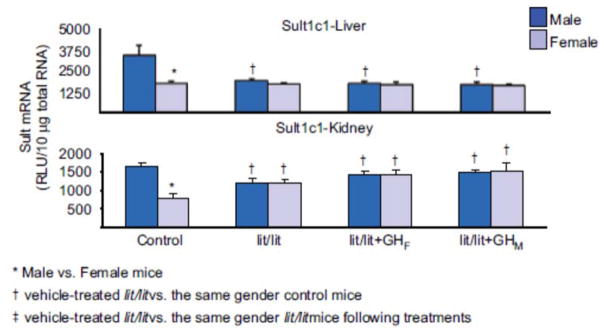
Hepatic and renal expression of Sult1c1 in male lit/lit mice was lower than that in control mice, whereas expression in female lit/lit males was similar to that in control mice (Figure 6). In lit/lit mice, male-pattern GH replacement had no effect on hepatic and renal expression of Sult1c1 in either gender.
Figure 6.
Effects of GH secretion pattern on the mRNA expression of male-predominant Sult1c1 in mice. Total liver, or kidney RNA were isolated and analyzed by bDNA for each Sult mRNA content. The data are presented as mean RLU ± SEM (n = 5). lit/lit, placebo administered to lit/lit mice; lit/lit + GHM, rat GH twice daily administered by i.p. injection to lit/lit mice mimicking male-pattern GH secretion; lit/lit + GHF, continuous infusion to lit/lit mice via s.c. implanted 21-day-release 1-mg rat GH pellet mimicking female-pattern GH secretion. Asterisk (*) represents statistically significant differences (P < 0.05) between male and female mice; single dagger (†) represents statistically significant differences (P < 0.05) between control mice and the same gender, vehicle-treated lit/lit mice; and double dagger (‡) represents statistically significant differences (P < 0.05) between vehicle-treated lit/lit mice and the same gender lit/lit mice following GH replacement treatments.
DISCUSSION
Female dominant
Sult1a1 and Papss2 mRNA expression in kidneys and livers from female mice are 2–3-fold higher than that in male mice. The gender differences in Sult1a1 and PAPSs2 expression do not appear until puberty (~4 weeks of age) (Alnouti and Klaassen, 2006). In humans, there were no gender differences in platelet SULT activity toward 4-nitrophenol, which is attributed to various SULT1 members, including SULT1A1 (Marazziti et al., 1998). In contrast to humans and similar to mice, Sult1a1 expression and 4-nitrophenolsulfonation are male-predominant in several rat tissues such as liver and kidney (Singer et al., 1982; Ozawa et al., 1993; Liu and Klaassen, 1996b; Klaassen et al., 1998). This difference among rodents and between rodents and humans may be because 4-nitrophenol is not a selective substrate for Sult1a1 or may result from species differences in the hormonal regulation of Sult1a1 expression.
In the current report, the female predominance of Sult1a1 and Papss2 expression in livers and kidney, as well as Sult1d1 in livers was not observed in GNX or HX mice due to the increased expression of these genes in the male GNX (Figure 1) and HX mice (Figure 3). Androgen administration to the GNX and HX mice decreases the expression of these genes to that observed in control male mice. Replacement of the male-GH secretion pattern to male and female HX mice decreased expression of these genes to their background levels in control males. The gender differences of these genes were also not observed in the lit/lit mice (Figure 5). Similar to HX mice, male-pattern GH replacement restored expression of these genes in male and female lit/lit mice to their background levels in control males. Therefore, Sult1a1 and Papss2 in liver and kidney, and Sult1d1 in liver are female-predominant in mice because of suppressive effects of androgens and male GH secretion pattern.
The hepatic expression of Sult2a1/a2 is more than a 100-fold higher in female than male mice. The gender difference in Sult2a1/a2 was previously reported in mice (Wu et al., 2001; Alnouti and Klaassen, 2006) and in rats (Chatterjee et al., 1987; Runge-Morris and Wilusz, 1991; Labrie et al., 1994; Liu and Klaassen, 1996a; Dunn and Klaassen, 1998). Furthermore, Sult2a1/a2 enzyme activities toward hydroxysteroids, such as bile acids, cortisol, and DHEA, are also female-predominant in rats (Roy, 1956; Carlstedt-Duke and Gustafsson, 1973; Singer et al., 1976; Hammerman et al., 1978; Chen et al., 1982; Balistreri et al., 1984; Kane et al., 1984; Yamazoe et al., 1987), hamsters (Barnes et al., 1979; Borthwick et al., 1995a), and mice (Borthwick et al., 1995a). In contrast, no gender differences in DHEA activity were detected in humans (Aksoy et al., 1993). Several hormones have been suggested to contribute to the female-predominant expression and enzymatic activity of hepatic Sult2a1 in rats, including sex hormones and GH (Torday et al., 1971; Carlstedt-Duke and Gustafsson, 1973; Hammerman et al., 1978; Kane et al., 1984; Yamazoe et al., 1987; Labrie et al., 1994; Borthwick et al., 1995b; Liu and Klaassen, 1996a). Therefore, the sexual dimorphism of Sult2a1 appear to be conserved across several rodent species but does not exist in humans. In contrast, the mechanism of the gender differences in Sult3a1 has not been reported previously.
In this current report, Sult2a1/a2 and Sult3a1 in mouse liver are regulated in a similar manner by sex hormones. The gender differences in the hepatic expression of Sult2a1/a2 and Sult3a1 disappeared in GNX and HX mice, due to an increase in male and decrease in female expression (Figures 1 and 3). Hepatic expression of both of these Sult isoforms is increased in GNX and HX female mice to their background levels in control female mice by estrogen replacement, and decreased in GNX and HX male mice to their background levels in control male mice by androgen replacement.
Hepatic expression of Sult2a1/a2 is lower in male- and higher in female-HX and -lit/lit mice than control mice, and thus the gender difference in Sult2a1/a2 hepatic expression was abolished in both animal models (Figures 3 and 5). In contrast, the gender difference in hepatic Sult3a1 disappeared in HX and lit/lit mice due to a decrease in female expression, whereas male expression remained unchanged (Figures 3 and 5). Female-pattern GH replacement to both male and female HX and lit/lit mice increased Sult2a1/a2 expression to its background levels in control males, whereas male-pattern GH replacement decreased Sult2a1/a2 expression to its background levels in control males. In contrast, only female-pattern GH restored hepatic Sult3a1 expression to its background in control female mice. Therefore, Sult2a1/a2 is female-predominant in mouse liver due to suppressive effects of androgens and male GH secretion pattern, as well as stimulatory effects of estrogens and female-GH secretion pattern. In contrast, Sult3a1 is female-predominant due to suppressive effects of androgens and stimulatory effects of both estrogens and female-GH secretion pattern.
Sult4a1 expression in brain is higher in female than male mice. This gender difference is abolished after GNX due to the decrease in Sult4a1 expression in female GNX mice (Figure 1). Estrogen replacement restored Sult4a1 expression in both male and female GNX mice to back-ground levels in control females. The gender differences in Sult4a1 expression also did not exist in brains from HX and lit/lit mice (Figures 3 and 5). Estrogen replacement increased Sult4a1 expression in brains of HX mice. However, neither female- nor male-pattern GH replacement altered the expression of Sult4a1 in these mice. Therefore, the female-predominant expression of Sult4a1 in mice is due to stimulatory effects of estrogens.
Male dominant
The hepatic expression of Sult1c1 is male-predominant in rats (Gong et al., 1991; Nagata et al., 1993; Liu and Klaassen, 1996b; Klaassen et al., 1998) and mice (Alnouti and Klaassen, 2006). Furthermore, male rats are more susceptible to the carcinogenicity of N-hydroxy-2-acetylaminofluorene, which is activated to reactive intermediates by Sult1c1 (DeBaun et al., 1970; Yamazoe et al., 1987; Nagata et al., 1993). The male-predominance of Sult1c1 mRNA expression and enzyme activity in rats were attributed to stimulatory effects by androgens and male-pattern of GH secretion in rats (DeBaun et al., 1970; Yamazoe et al., 1987; Nagata et al., 1993; Liu and Klaassen, 1996b). Therefore, the sexual dimorphism of Sult1c1 appear to be conserved among mice and rats, and there are no data available from humans to compare with data obtained from rodents.
The male-predominant hepatic and renal expression of Sult1c1 did not exist in GNX mice, due to a decreased expression in male mice after gonadectomy (Figure 2). Androgen replacement to GNX mice increased hepatic and renal expressions of Sult1c1. Similarly, androgen replacement induced hepatic and renal expression of Sult1c1 in male and female HX mice (Figure 4). In contrast, estrogen replacement did not have any effect on hepatic or renal Sult1c1 expression in GNX or HX mice. The male-predominant hepatic and renal expression of Sult1c1 was also abolished in HX and lit/lit mice, due to an increase in expression in females (Figure 6). In HX mice, hepatic expression of Sult1c1 was suppressed by both female- and male-pattern GH replacement, whereas renal expression was induced by both female- and male-pattern GH replacement. In contrast, GH replacement had no effect on renal or hepatic Sult1c1 expression in lit/lit mice. Therefore, GH is not likely involved in the regulation of Sult1c1 expression. Overall, our data supports the conclusion that the male-predominant Sult1c1 expression is due to stimulatory effects of androgens.
In summary, the role of sex hormones and gender-divergent GH secretion pattern in the regulation of sex dimorphic Sults and Papss were studied in mice. We used GNX, HX and lit/lit mice models. The gender differences in expression of various mouse Sults are influenced by various mechanisms involving sex and/or GHs as summarized in Table 1. Sult1a1 and Papss2 in liver and kidney, and Sult1d1 in liver are female-predominant in mice because of suppressive effects by androgens and male-GH secreting pattern. Sult2a1/a2 is markedly female-predominant in mouse liver due to suppressive effects of androgens and male-GH secreting pattern, as well as stimulatory effects by estrogens and female-GH secreting pattern. Sult3a1 is female-predominant in mouse liver due to suppressive effects by androgens and stimulatory effects of estrogens and female-GH secreting pattern. Sult1c1 expression is male-predominant in mouse liver and kidney because of stimulatory effects of androgens. Sult4a1 expression is female-predominant in mouse brain due to stimulatory effects of estrogens. Major differences in the sexual dimorphic expression of Sults exist across species, and very scarce data are available from humans. Our data are restricted to mRNA expression because of the lack of specific antibodies and selective probe substrates for most of the mouse Sult isozymes. However, previous functional data produced by relatively selective substrates such as DHEA and N-hydroxy-2-acetylaminofluorene, are in a very good agreement with our mRNA data.
Table 1.
Summary of the overall effects of DHT (dehydroepiandrosterone), MP-GH (male-pattern growth hormone), E2 (estrogen) and FP-GH (female-pattern growth hormone) on the mRNA expression of the gender-divergent Sults in mouse liver (Sult1a1, 1c1, 1d1, 2a1/a2, 3a1 and Papss2), kidney (Sult1a1, 1c1 and Papss2), and brain (Sult4a1).
| DHT | MP-GH | E2 | FP-GH | |
|---|---|---|---|---|
| Female-predominant | ||||
| Sult1a1 | ||||
| Sult1d1 | ↓ | ↓ | — | — |
| Papss2 | ||||
| Sult2a1/a2 | ↓ | ↓ | ↑ | ↑ |
| Sult3a1 | ↓ | — | ↑ | ↑ |
| Sult4a1 | — | — | ↑ | — |
| Male-predominant | ||||
| Sult1c1 | ↑ | — | — | — |
Acknowledgments
The authors would like to thank David Buckly, Peizhen Song, Xiaohong Lei, and Drs. Chuan Chen and Hong Lu for technical assistance.
Footnotes
DECLARATION OF INTEREST
This work was supported by NIH grants ES-09649, ES-09716, ES013714 and COBRE grant P20-RR-021940.
References
- Ahluwalia A, Clodfelter KH, Waxman DJ. Sexual dimorphism f rat liver gene expression: regulatory role of growth hormone revealed by deoxyribonucleic Acid microarray analysis. Mol Endocrinol. 2004;18:747–760. doi: 10.1210/me.2003-0138. [DOI] [PubMed] [Google Scholar]
- Aksoy IA, Sochorová V, Weinshilboum RM. Human liver dehydroepiandrosterone sulfotransferase: nature and extent of individual variation. Clin Pharmacol Ther. 1993;54:498–506. doi: 10.1038/clpt.1993.181. [DOI] [PubMed] [Google Scholar]
- Alnouti Y, Klaassen CD. Tissue distribution and ontogeny of sulfotransferase enzymes in mice. Toxicol Sci. 2006;93:242–255. doi: 10.1093/toxsci/kfl050. [DOI] [PubMed] [Google Scholar]
- Alnouti Y, Klaassen CD. Regulation of sulfotransferase enzymes by prototypical microsomal enzyme inducers in mice. J Pharmacol Exp Ther. 2008;324:612–621. doi: 10.1124/jpet.107.129650. [DOI] [PubMed] [Google Scholar]
- Balistreri WF, Zimmer L, Suchy FJ, Bove KE. Bile salt sulfotransferase: alterations during maturation and non-inducibility during substrate ingestion. J Lipid Res. 1984;25:228–235. [PubMed] [Google Scholar]
- Barnes S, Burhol PG, Zander R, Haggstrom G, Settine RL, Hirschowitz BI. Enzymatic sulfation of glycochenodeoxycholic acid by tissue fractions from adult hamsters. J Lipid Res. 1979;20:952–959. [PubMed] [Google Scholar]
- Beamer WH, Eicher EM. Stimulation of growth in the little mouse. J Endocrinol. 1976;71:37–45. doi: 10.1677/joe.0.0710037. [DOI] [PubMed] [Google Scholar]
- Borthwick EB, Burchell A, Coughtrie MW. Purification and immunochemical characterization of a male-specific rat liver oestrogen sulphotransferase. Biochem J. 1993;289(Pt 3):719–725. doi: 10.1042/bj2890719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthwick EB, Burchell A, Coughtrie MW. Differential expression of hepatic oestrogen, phenol and dehydroepiandrosterone sulphotransferases in genetically obese diabetic (ob/ob) male and female mice. J Endocrinol. 1995a;144:31–37. doi: 10.1677/joe.0.1440031. [DOI] [PubMed] [Google Scholar]
- Borthwick EB, Voice MW, Burchell A, Coughtrie MW. Effects of hypophysectomy and thyroxine on the expression of hepatic oestrogen, hydroxysteroid and phenol sulphotransferases. Biochem Pharmacol. 1995b;49:1381–1386. doi: 10.1016/0006-2952(95)00055-5. [DOI] [PubMed] [Google Scholar]
- Cai Y, Dai T, Ao Y, Konishi T, Chuang KH, Lue Y, Chang C, Wan YJ. Cytochrome P450 genes are differentially expressed in female and male hepatocyte retinoid X receptor alpha-deficient mice. Endocrinology. 2003;144:2311–2318. doi: 10.1210/en.2002-0129. [DOI] [PubMed] [Google Scholar]
- Carlstedt-Duke J, Gustafsson JA. Sexual differences in hepatic sulphurylation of deoxcorticosterone in rats. Eur J Biochem. 1973;36:172–177. doi: 10.1111/j.1432-1033.1973.tb02898.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee B, Majumdar D, Ozbilen O, Murty CV, Roy AK. Molecular cloning and characterization of cDNA for androgen-repressible rat liver protein, SMP-2. J Biol Chem. 1987;262:822–825. [PubMed] [Google Scholar]
- Chen LJ, Kane B, 3rd, Bujanover Y, Thaler MM. Development and regulation of bile salt sulfotransferase in rat liver. Biochim Biophys Acta. 1982;713:358–364. doi: 10.1016/0005-2760(82)90254-5. [DOI] [PubMed] [Google Scholar]
- DeBaun JR, Miller EC, Miller JA. N-hydroxy-2-acetylaminofluorene sulfotransferase: its probable role in carcinogenesis and in protein-(methion-S-yl) binding in rat liver. Cancer Res. 1970;30:577–595. [PubMed] [Google Scholar]
- Dunn RT, 2nd, Klaassen CD. Tissue-specific expression of rat sulfotransferase messenger RNAs. Drug Metab Dispos. 1998;26:598–604. [PubMed] [Google Scholar]
- Edén S. Age- and sex-related differences in episodic growth hormone secretion in the rat. Endocrinology. 1979;105:555–560. doi: 10.1210/endo-105-2-555. [DOI] [PubMed] [Google Scholar]
- Glatt H. Sulfotransferases in the bioactivation of xenobiotics. Chem Biol Interact. 2000;129:141–170. doi: 10.1016/s0009-2797(00)00202-7. [DOI] [PubMed] [Google Scholar]
- Glatt H, Bartsch I, Christoph S, Coughtrie MW, Falany CN, Hagen M, Landsiedel R, Pabel U, Phillips DH, Seidel A, Yamazoe Y. Sulfotransferase-mediated activation of mutagens studied using heterologous expression systems. Chem Biol Interact. 1998;109:195–219. doi: 10.1016/s0009-2797(97)00133-6. [DOI] [PubMed] [Google Scholar]
- Gong DW, Ozawa S, Yamazoe Y, Kato R. Purification of hepatic N-hydroxyarylamine sulfotransferases and their regulation by growth hormone and thyroid hormone in rats. J Biochem. 1991;110:226–231. doi: 10.1093/oxfordjournals.jbchem.a123561. [DOI] [PubMed] [Google Scholar]
- Hammerman KJ, Chen LJ, Fernandez-Corugedo A, Earnest DL. Sex differences in hepatic sulfation of taurolithocholate in the rat. Gastroenterology. 1978;75:1021–1025. [PubMed] [Google Scholar]
- Hines RN, McCarver DG. The ontogeny of human drug-metabolizing enzymes: phase I oxidative enzymes. J Pharmacol Exp Ther. 2002;300:355–360. doi: 10.1124/jpet.300.2.355. [DOI] [PubMed] [Google Scholar]
- Kane RE, 3rd, Chen LJ, Thaler MM. Regulation of bile salt sulfotransferase isoenzymes by gonadal hormones. Hepatology. 1984;4:1195–1199. doi: 10.1002/hep.1840040616. [DOI] [PubMed] [Google Scholar]
- Kasukawa Y, Baylink DJ, Guo R, Mohan S. Evidence that sensitivity to growth hormone (GH) is growth period and tissue type dependent: studies in GH-deficient lit/lit mice. Endocrinology. 2003;144:3950–3957. doi: 10.1210/en.2002-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen CD, Liu L, Dunn RT., 2nd Regulation of sulfotransferase mRNA expression in male and female rats of various ages. Chem Biol Interact. 1998;109:299–313. doi: 10.1016/s0009-2797(97)00141-5. [DOI] [PubMed] [Google Scholar]
- Labrie Y, Couët J, Simard J, Labrie F. Multihormonal regulation of dehydroepiandrosterone sulfotransferase messenger ribonucleic acid levels in adult rat liver. Endocrinology. 1994;134:1693–1699. doi: 10.1210/endo.134.4.8137732. [DOI] [PubMed] [Google Scholar]
- Liu L, Klaassen CD. Ontogeny and hormonal basis of female-dominant rat hepatic sulfotransferases. J Pharmacol Exp Ther. 1996a;279:386–391. [PubMed] [Google Scholar]
- Liu L, Klaassen CD. Ontogeny and hormonal basis of male dominant rat hepatic sulfotransferases. Mol Pharmacol. 1996b;50:565–572. [PubMed] [Google Scholar]
- MacLeod JN, Pampori NA, Shapiro BH. Sex differences in the ultradian pattern of plasma growth hormone concentrations in mice. J Endocrinol. 1991;131:395–399. doi: 10.1677/joe.0.1310395. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Palego L, Rossi A, Cassano GB. Gender-related seasonality of human platelet phenolsulfotransferase activity. Neuropsychobiology. 1998;38:1–5. doi: 10.1159/000026509. [DOI] [PubMed] [Google Scholar]
- Matsui M, Homma H. Biochemistry and molecular biology of drug-metabolizing sulfotransferase. Int J Biochem. 1994;26:1237–1247. doi: 10.1016/0020-711x(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Matsui M, Watanabe HK. Developmental alteration of hepatic UDP-glucuronosyltransferase and sulphotransferase towards androsterone and 4-nitrophenol in Wistar rats. Biochem J. 1982;204:441–447. doi: 10.1042/bj2040441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarver DG, Hines RN. The ontogeny of human drug-metabolizing enzymes: phase II conjugation enzymes and regulatory mechanisms. J Pharmacol Exp Ther. 2002;300:361–366. doi: 10.1124/jpet.300.2.361. [DOI] [PubMed] [Google Scholar]
- Meibohm B, Beierle I, Derendorf H. How important are gender differences in pharmacokinetics? Clin Pharmacokinet. 2002;41:329–342. doi: 10.2165/00003088-200241050-00002. [DOI] [PubMed] [Google Scholar]
- Nagata K, Ozawa S, Miyata M, Shimada M, Gong DW, Yamazoe Y, Kato R. Isolation and expression of a cDNA encoding a male-specific rat sulfotransferase that catalyzes activation of N-hydroxy-2-acetylaminofluorene. J Biol Chem. 1993;268:24720–24725. [PubMed] [Google Scholar]
- Ozawa S, Nagata K, Gong DW, Yamazoe Y, Kato R. Expression and functional characterization of a rat sulfotransferase (ST1A1) cDNA for sulfations of phenolic substrates in COS-1 cells. Jpn J Pharmacol. 1993;61:153–156. doi: 10.1254/jjp.61.153. [DOI] [PubMed] [Google Scholar]
- Roy AB. The enzymic synthesis of steroid sulphates. Biochem J. 1956;63:294–300. doi: 10.1042/bj0630294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Chatterjee B. Sexual dimorphism in the liver. Annu Rev Physiol. 1983;45:37–50. doi: 10.1146/annurev.ph.45.030183.000345. [DOI] [PubMed] [Google Scholar]
- Runge-Morris M, Wilusz J. Age and gender-related gene expression of hydroxysteroid sulfotransferase-a in rat liver. Biochem Biophys Res Commun. 1991;175:1051–1056. doi: 10.1016/0006-291x(91)91671-x. [DOI] [PubMed] [Google Scholar]
- Singer SS, Federspiel MJ, Green J, Lewis WG, Martin V, Witt KR, Tappel J. Enzymatic sulfation of steroids. XV. Studies differentiating between rat liver androgen, estrogen, bile acid, glucocorticoid and phenol sulfotransferases. Biochim Biophys Acta. 1982;700:110–117. doi: 10.1016/0167-4838(82)90298-9. [DOI] [PubMed] [Google Scholar]
- Singer SS, Giera D, Johnson J, Sylvester S. Enzymatic sulfation of steroids: I. The enzymatic basis for the sex difference in cortisol sulfation by rat liver preparations. Endocrinology. 1976;98:963–974. doi: 10.1210/endo-98-4-963. [DOI] [PubMed] [Google Scholar]
- Strott CA. Sulfonation and molecular action. Endocr Rev. 2002;23:703–732. doi: 10.1210/er.2001-0040. [DOI] [PubMed] [Google Scholar]
- Torday JS, Klein GP, Giroud CJ. Influence of gonads on the sulfurylation of 11-deoxycorticosterone and corticosterone by rat liver cytosol. Can J Biochem. 1971;49:437–440. doi: 10.1139/o71-064. [DOI] [PubMed] [Google Scholar]
- Wu W, Kocarek TA, Runge-Morris M. Sex-dependent regulation by dexamethasone of murine hydroxysteroid sulfotransferase gene expression. Toxicol Lett. 2001;119:235–246. doi: 10.1016/s0378-4274(01)00263-6. [DOI] [PubMed] [Google Scholar]
- Yamazoe Y, Manabe S, Murayama N, Kato R. Regulation of hepatic sulfotransferase catalyzing the activation of N-hydroxyarylamide and N-hydroxyarylamine by growth hormone. Mol Pharmacol. 1987;32:536–541. [PubMed] [Google Scholar]