Abstract
Natriuretic peptides (NPs) are cyclic vasoactive peptide hormones with high therapeutic potential. Three distinct NPs (ANP, BNP, and CNP) can selectively activate natriuretic peptide receptors, NPR-A and NPR-B, raising the cyclic GMP (cGMP) levels. Insulin-degrading enzyme (IDE) was found to rapidly cleave ANP, but the functional consequences of such cleavages in the cellular environment and the molecular mechanism of recognition and cleavage remain unknown. Here, we show that reducing expression levels of IDE profoundly alters the response of NPR-A and NPR-B to the stimulation of ANP, BNP, and CNP in cultured cells. IDE rapidly cleaves ANP and CNP, thus inactivating their ability to raise intracellular cGMP. Conversely, reduced IDE expression enhances the stimulation of NPR-A and NPR-B by ANP and CNP, respectively. Instead of proteolytic inactivation, IDE cleavage can lead to hyperactivation of BNP toward NPR-A. Conversely, decreasing IDE expression reduces BNP-mediated signaling. Additionally, the cleavages of ANP and BNP by IDE render them active with NPR-B and a reduction of IDE expression diminishes the ability of ANP and BNP to stimulate NPR-B. Our kinetic and crystallographic analyses offer the molecular basis for the selective degradation of NPs and their variants by IDE. Furthermore, our studies reveal how IDE utilizes its catalytic chamber and exosite to engulf and bind up to two NPs leading to biased stochastic, non-sequential cleavages and the ability of IDE to switch its substrate selectivity. Thus, the evolutionarily conserved IDE may play a key role in modulating and reshaping the strength and duration of NP-mediated signaling.
Keywords: Cyclic GMP (cGMP), Enzyme Mechanisms, Mass Spectrometry (MS), Metalloprotease, Signal Transduction, X-ray Crystallography
Introduction
The natriuretic peptides (NPs),2 mainly atrial (ANP), B-type (BNP), and C-type natriuretic peptides (CNP), play key roles in many cardiovascular functions (1). Their diuretic, natriuretic, and vasodilatory properties have been developed as therapeutic strategies for human cardiovascular diseases (2). The actions of NPs are mediated through binding and signal transduction of three natriuretic peptide receptors (NPR). NPR-A and NPR-B have guanylyl cyclase activity that raises intracellular cGMP levels. Effects of these receptors are mediated by the preferential binding of ANP and BNP to NPR-A and by that of CNP to NPR-B. NPR-C is a non-guanylyl cyclase receptor. In addition to its role in the clearance of NPs, NPR-C also can transmit signals via heterotrimeric G protein, Gi (3, 4).
NPs have a short half-life and their circulation levels are tightly controlled (5, 6). In addition to the regulation of NPs by gene transcription, secretion, and NPR-C mediated clearance, NP maturation and breakdown by multiple proteases are also key in NP regulation. For example, active NPs are converted from pro-NPs by furin, corin, and likely, by other proteases (7, 8). Active NPs are postulated to be proteolytically inactivated by a membrane-bound metalloprotease, neprilysin (NEP) (9–12). However, growing evidence propose the role of other proteases in the clearance of NPs. Meprin A is shown to be involved in the initial N-terminal cleavage of BNP and meprin A and NEP are thought to work together in the clearance of BNP (13). In addition, insulin-degrading enzyme (IDE) and DPP-IV have been shown to cleave NPs in vitro (14–16) However, the functional consequence in the cleavage of NPs by these two proteases in the cellular setting remains unknown.
IDE is a ubiquitously expressed zinc-metalloprotease that is involved in the clearance of insulin and amyloid-β (Aβ), peptides implicated in the pathogenesis of diabetes and Alzheimer's disease, respectively (17–19). We have recently solved the structure of human IDE in complex with various substrates and elucidated the molecular basis for the recognition and selective degradation of substrates by IDE (20–22). Our structures reveal that IDE uses a sizable catalytic chamber to entrap, unfold, and degrade insulin, Aβ, and other substrates. IDE recognizes its substrates mainly based on their tertiary structures. The size, dipole moment, structure flexibility, and location of the N-terminal end of the substrates are key factors for the selectivity of IDE.
IDE was shown to degrade ANP and BNP (15, 16), however it was never established whether the cleavages of ANP and BNP by IDE have any functional consequences. Further, the cleavage rate of ANP by IDE was significantly higher than that of BNP, but the functional implications of this rate difference remains unknown. In addition, the molecular details that dictate the differences in NP selectivity by IDE have not been elucidated. In this report, we evaluate the biological impact of IDE on NP-mediated signaling. We also used key members of the NP family from various species, as well as truncation mutants of NPs, to investigate the mechanism of how human IDE selectively degrades certain NPs.
MATERIALS AND METHODS
Expression and Purification of Recombinant IDE
Wild-type and mutant human IDE proteins were expressed in Escherichia coli Rosetta (DE3) cells (at 25 °C and 18 h, IPTG induction) and purified by Ni-NTA, Source-Q, and Superdex S-200 columns as described previously (20, 21).
Competition Assays
IDE enzyme activities were assayed using substrate V (20, 22). For IC50 determinations, 85 μl of 0.35 μm substrate V was mixed with 10 μl of NPs at various concentrations. The reactions were initiated by addition of 5 μl of IDE protein (0.2 mg/ml), carried out at 37 °C for 15 min. The substrate V degradation was monitored by fluorescence intensity on a microplate reader as described previously (20, 22).
X-ray Diffraction Data Collection and Structural Determination of IDE·ANP and IDE·BNP
IDE-CF-E111Q was incubated with a 4-fold molar excess of ANP and BNP prior to each of 5 gel-filtration steps and crystallized as described (21, 22). Diffraction data were collected at the beamline 19-ID at the Structural Biology Center at Argonne National Laboratory. The data sets were processed using HKL2000. Structure determinations were performed by molecular replacement using IDE (PDB 3CWW) as a search model. Structure refinement and model building were performed with REFMAC, Phenix, and Coot. The final model for ANP-bound and BNP-bound IDE structures had Rwork/Rfree of 20.61/24.15% and 20.22/24.27%, respectively.
Proteolysis of NPs by IDE and MALDI-TOF MS Analysis
IDE was incubated at 37 °C for a maximum of 1 h with each NP in 50 mm Tris-HCl buffer, pH 7.0 containing 20 mm NaCl. Each reaction was subsequently quenched by the addition of a solution containing 0.5 mm EDTA and 0.1% TFA. The peptide digests were first analyzed by MALDI-TOF MS. The quenched reaction mixtures were mixed with an equal volume of 0.1% TFA and desalted through a C-18 ZipTip (Millipore) and analyzed using an Applied Biosystems Voyager mass spectrometer. The acquired spectra were analyzed using DataExplorer.
LC-ESI FT-ICR MS/MS Analysis of NP Fragments
The IDE-treated NPs (20 pmol) were separated by nanoLC on a reverse-phase C8 column with a linear gradient of 5–95% acetonitrile in 0.1% formic acid. FT-ICR MS analysis was performed on a linear quadrupole ion trap (LTQ) FT ICR hybrid mass spectrometer. Eluted NP fragments were electrosprayed at 2 kV. Peptide fragmentation was induced by both collision-induced dissociation (CID) and electron-capture dissociation (ECD) in the ion trap, and fragment ions were also analyzed in the ion trap. The mass spectrometer was set to switch between an FT-ICR MS full scan (200 m/z to 2,000 m/z) followed by successive FT-ICR MS single-ion monitoring scans and LTQ MS/MS scans of the three most abundant precursor ions in the FT-ICR MS full scan as determined by the Xcalibur software. Dynamic exclusion was enabled after a repeat count of three for a period of 90 s.
RNAi-mediated Inhibition of IDE
Human embryonic 293 cells stably expressing human NPR-A or NPR-B were maintained as previously described (23). RNA inhibition was accomplished with the use of recombinant lentiviruses. A set of five plasmids (1 CONTROL Non-Targeting and 4 IDE-specific) was purchased from Open Biosystem (RMM4534-NM-031156) and lentiviruses were produced by co-transfecting shRNA plasmids with pHR8.2ΔR packaging plasmid and pCMV VSVG envelope plasmid into HEK293T cells. HEK-293 stable lines containing the IDE-specific shRNA were obtained by lentiviral infection and selection with 5 μg/ml puromycin. IDE knockdown levels were examined by Western blotting with a polyclonal anti-IDE antibody raised against human IDE and affinity purified using immobilized IDE column and 293 cells with the most knocked down IDE levels were chosen in our studies.
Bioassay
Whole cell stimulations were performed as described (23). Cellular cGMP levels were measured using enzyme immunoassays (cGMP EIA System, GE Healthcare). Data are presented as means ± S.E., performed in duplicate.
RESULTS
Effect of Decreased IDE Levels on the NP-stimulated cGMP Accumulation in HEK293 Cells
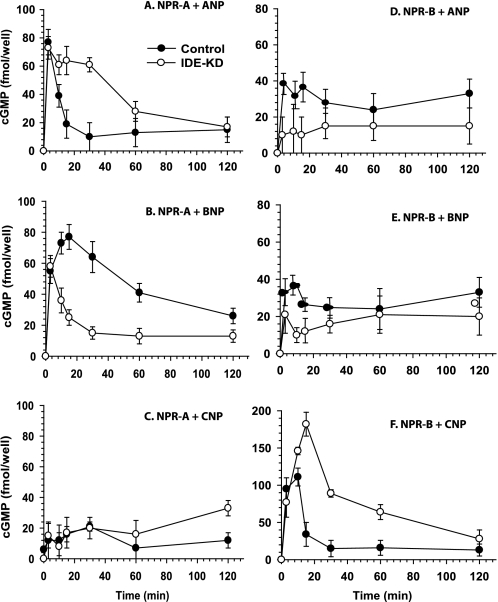
If IDE participates in the function of NPs, the reduction of IDE expression would result in an altered NP-mediated cGMP accumulation in cultured cells. To test this, we knocked down IDE through the expression of short-hairpin RNA against IDE in human embryonic kidney 293 cells that stably express human NPR-A or NPR-B and achieved >95% reduction of IDE levels (supplemental Fig. S1). We then compared the time course of cGMP accumulation of these cells with the cells that express control shRNA upon stimulation by ANP, BNP, or CNP (Fig. 1). In control NPR-A cells, ANP elicited an immediate and substantial increase in intracellular cGMP content, reaching a maximum within 3 min and declining rapidly by 30 min to a steady level over the next 120 min. The early phase of response for HEK293-NPR-A cells that have low levels of IDE is similar to that with the normal lDE levels. However, the decline in cGMP accumulation was delayed significantly by the knockdown of IDE expression (Fig. 1A). Interestingly, the opposite effect was observed for BNP stimulation. For control cells, the cGMP accumulation reached a maximum in 15 min with a continual decline over the next 120 min. However, in IDE-knockdown cells, the decline of cGMP level was rapid and robust after the 3 min maximum (Fig. 1B). Consistent with the notion that CNP activates NPR-A poorly, we observed a weak response by CNP stimulation in both control and IDE-knockdown cells (Fig. 1C). Next, we investigated the NPR-B cells. As expected, CNP potently stimulated cGMP accumulation of HEK 293 cells stably expressing NPR-B, while ANP and BNP did not (Fig. 1, D--F). Interestingly, the knockdown of IDE profoundly enhanced the cGMP accumulation of NPR-B-overexpressing HEK293 cells (Fig. 1F). Our findings support the notion that IDE modulates NP-mediated signaling. IDE appears to be a negative regulator of the ANP- mediated NPR-A signaling and CNP-mediated NPR-B signaling, while IDE serves as a positive regulator of BNP-mediated NPR-A signaling. Further, our observation that IDE only affected the decline phase of NP-stimulated response is consistent with the role for IDE in the time-dependent cleavage of NPs, either by the accessibility and/or cleavage rate of NPs by IDE.
FIGURE 1.
Differential responses to NPs upon IDE knockdown in human NPR-A- and NPR-B-expressing 293 cells. Intracellular cGMP content was determined in 293-neo cells stably expressing human NPR-A (A–C) or NPR-B (D–F) lentivirally infected with control (●) or IDE siRNA (○) at the indicated times of treatment with 100 nm ANP, BNP, and CNP. Data are means ± S.E. for three independent experiments with two replications per condition.
Effect of IDE Degradation on the Ability of NPs to Activate Their Receptors
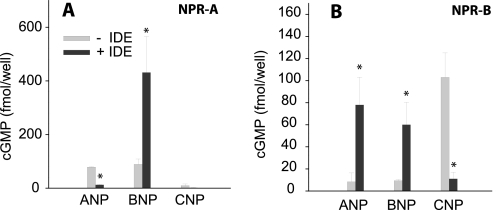
The bioactivity of IDE-digested NPs was evaluated by measuring their ability to raise intracellular cGMP levels in HEK293-NPR-A and HEK 293-NPR-B cells. As expected, cells expressing NPR-A were stimulated by ANP and BNP but had little response to CNP (Fig. 2A). The incubation with IDE caused ANP to lose more than 90% ability to raise intracellular cGMP levels. In contrast, the stimulation of NPR-A receptor by BNP was enhanced ∼4-fold. These data suggest that IDE cleavage may produce highly active BNP-derived products.
FIGURE 2.
IDE treatment changes the ability of NPs to stimulate cGMP signaling. 100 nm of each peptide was incubated with 2 nm human recombinant IDE for 5 min in a total volume of 0.1 ml. Proteolysis was terminated by addition of 0.1 ml of 0.1 m HCl, and bioactivity was evaluated by measuring the ability of neutralized extracts to elevate cGMP concentration in 293-neo cells stably expressing human NPR-A (A) or NPR-B (B). Data are means ± S.E. for three independent experiments with two replications per condition. Statistical significance reflects comparison with the activity of the peptide with no IDE. *, p < 0.05.
As expected, NPR-B-expressing cells were only sensitive to CNP (Fig. 2B). We found that CNP lost >90% capacity to raise intracellular cGMP levels after incubation with IDE (Fig. 2B). This is consistent with the data that show that reduced IDE levels lead to an increased response of NPR-B to CNP. We also found that the incubation with IDE increased the signal induced by ANP and BNP, toward NPR-B by ∼10- and ∼7-fold, respectively. These data indicate that the cleavage of ANP and BNP by IDE could generate CNP-like products that serve as agonists of NPR-B. Consistent with this notion, the IDE knockdown cells appeared to reduce the already weak response of NPR-B elicited by ANP and BNP (Fig. 1, D and E). Together, our data show that IDE could modulate the intracellular cGMP accumulation elicited by NPs in both positive and negative manners and the response is dependent upon the given NP and the cognate receptor.
Mass Spectrometry and Model of the Degradation of Human ANP by Human IDE
To understand the intricate regulation of NP-mediated signaling, we used mass spectrometry to analyze the cleavage sites of NPs by IDE and the time-dependent production of IDE-degraded NP fragments. The reports on the degradation of NPs by IDE used NPs from different species (rat ANP, BNP, and CNP) and rat IDE (15, 16). Because these sequences are substantially different from human NPs in some cases, we chose to use human IDE and NPs.
For mass spectrometry (MS) analysis of IDE-digested NP fragments, we used Fourier transform ion cyclotron resonance (FTICR) MS because of its high accuracy and resolution of precursor and product ion spectra. IDE-degraded NPs were applied to an HPLC C8 column to separate cleavage products and thus ensure maximal sampling of low abundance NP fragments. After incubation for various times at 37 °C, the IDE-degraded NPs were analyzed by LC-electrospray ionization (ESI)-FT ICR-MS in conjunction with collision-induced (CID) and electron-capture (ECD) dissociation MS/MS (Fig. 3, supplemental Tables S1–S7). All proteolytic peptide fragments were then identified based on the MS and MS/MS data having accuracies of <5 ppm (supplemental Tables S1–S7).
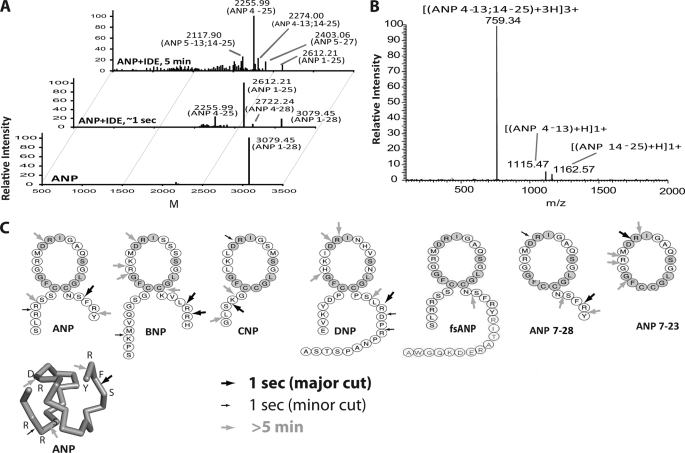
FIGURE 3.
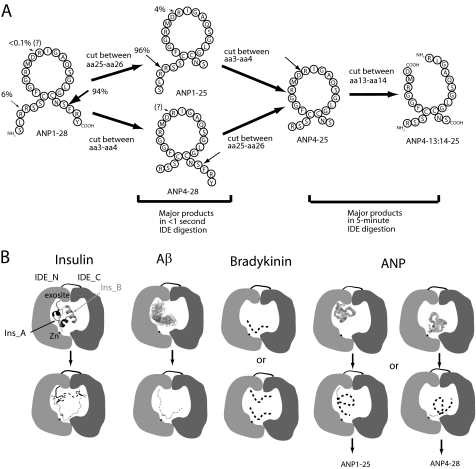
Mass spectrometry analyses of NP degradation by human IDE. A, representative ESI-FTICR-MS spectrum of ANP before (lower panel) and after IDE digestion (upper two panels). ANP and IDE were mixed in a 50:1 molar ratio. After 1-s and 5-min incubations, EDTA (80 mm) and trifluoroacetic acid (0.03%) were added to stop the reactions. B, representative ESI-ECD mass of 2274 Da. C, primary sequence and IDE cleavage sites of seven natriuretic peptides. Initial cleavage sites and secondary cutting sites are shown as big and small arrows, respectively. The gray shaded amino acids indicate residues conserved in all NPs. Similarly, the cleavage sites are also shown on the tertiary structure of ANP (PDB:1ANP).
To establish our methodology, we first analyzed the interaction of human ANP by human IDE. We found that ANP can be rapidly degraded by IDE (Fig. 3A), resulting in a number of major ANP fragments (supplemental Table S1). Three major ANP fragments were obtained (1–25, 4–25, and 4–28 by the order of descending intensity) after a 1 s incubation with IDE (Fig. 3A, middle panel). The predominant products (ANP 1–25 and 4–25) have a C-terminal cleavage between Ser-25 and Phe-26, suggesting that IDE preferentially cuts at this site. However, IDE also cuts once at the N terminus of ANP between the two arginines at positions 3 and 4, generating the ANP4–28 fragment.
The 5-min incubation of ANP with IDE led to a near complete breakdown of ANP and the formation of complex ANP fragments (Fig. 3A top). The longer incubation shifted the dominant fragment from ANP1–25 to ANP4–25. In addition, we found three new major fragments, one is ANP5–27 and the other two fragments are ANP4–25 and ANP 5–25 with an increased 18-Da mass. These fragments could have resulted from the hydrolysis at any position within the 17-amino acid-long ring of ANP4–25 and ANP5–25.
To unambiguously identify the hydrolysis position within the ring, we applied ECD-MS2 (22). We found that ANP4–13 and ANP14–25 product ions could be derived from ANP4–25 precursor ion as well as that of ANP5–13 and ANP14–25 product ions from ANP5–25 (Fig. 3B and supplemental Table S1). Thus, our data unequivocally identifies that the cleavage site inside the loop of ANP4–25 and ANP5–25 occurs between Asp-13 and Arg-14 (Fig. 3B and supplemental Table S1). Given the vital role of the intact ring for ANP function, this cleavage between Asp-13 and Arg-14 would undoubtedly inactivate ANP. This is consistent with our cell-based studies that IDE could serve as a negative regulator of ANP-mediated NPR-A signaling.
We also analyzed the kinetics of the degradation of ANP by IDE (Fig. 4A). First, we used the ability of ANP to compete and inhibit the cleavage of fluorogenic bradykinin-mimetic substrate, substrate V, by IDE to assess the affinity of ANP to IDE. The observed IC50 value is 40 nm, which is about 5-fold lower than that of insulin (∼200 nm). We also used MS data to assess the rate of ANP cleavage by IDE and found the kcat to be ∼10 s−1, which is comparable with the rate of IDE degradation of insulin (2 s−1). Thus, the binding affinity and rate of degradation of ANP by IDE is comparable to those of insulin, a physiologically relevant IDE substrate (22).
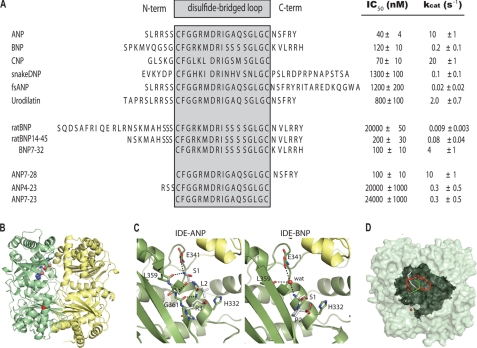
FIGURE 4.
Kinetics of hydrolysis of NPs by IDE and structural analysis of ANP- and BNP-bound IDE. A, sequence alignment and kinetic parameters of NPs with the upper part containing naturally occurring NPs, and the middle and lower parts containing variants of BNP and ANP, respectively. The region within the loop is enclosed within the gray shaded box. Unless otherwise noted, all NPs are of human origin. B, global view of the structure of ANP-bound IDE-CF-E111Q monomer. IDE-N and IDE-C are colored green and yellow, respectively. ANP is in a sphere representation with atoms colored red for oxygen, blue for nitrogen, and white for carbon. C, detailed interaction of the N terminus of ANP (left) and BNP (right) with the exosite of IDE. D, model for the interaction of IDE and ANP (ANP is based on a solution structure from PDB:1ANP). The IDE monomer is shown as a light green surface representation and the catalytic chamber is shown in dark green (red sphere for catalytic zinc). The backbone of ANP is shown as a ribbon with the N and C termini depicted in gray and green, respectively, and the disulfide-linked ring shown in red.
The presence of ANP4–28 fragment as an initial cleavage product of ANP by IDE negated the previously proposed model that IDE sequentially cuts ANP, first at the C terminus of ANP and then at its N terminus (16). Instead, we propose that the degradation of ANP by IDE is a biased stochastic process with a preferential cleavage at the C terminus over the cut at the N terminus (Fig. 5A). This discrepancy is probably due to the failure to observe the low abundant ANP4–28 fragment in the previous study (16). Thus, the cleavage of ANP by IDE reveals a novel mechanism for substrate hydrolysis, involving a biased stochastic, and not sequential, mode.
FIGURE 5.
Model for the cleavage of ANP by IDE. A, biased stochastic mode for the cleavage of ANP by IDE. ANP cleavage by IDE has revealed a novel mechanism for substrate hydrolysis by IDE, involving a biased stochastic mode, as opposed to the previously proposed sequential mode. B, four modes of substrate cleavage by IDE exemplified by the cleavages of insulin, Aβ, bradykinin, and ANP. See “Results” for details about these four modes.
Based on the accumulated structural and biochemical evidence, IDE-mediated substrate cleavage is achieved by at least three different mechanisms (Fig. 5B). Two of those mechanisms require the anchoring of the substrate at the exosite, a conserved site distal to the catalytic cleft of IDE. The binding of this exosite to the N-terminal end of substrate allows IDE to processively cut insulin at both A and B chains to generate two halves (22) or to stochastically cut at the middle portion of other substrates, as is the case with Aβ (20, 22). The third mechanism occurs with short peptide substrates like bradykinin and it is exosite-independent (21). For ANP, we find that the first cleavage site by IDE is random with preference given to the C-terminal cleavage. The preference for the C-terminal end of ANP is expected since the anchoring of the N-terminal of ANP to the exosite of IDE would allow the C-terminal end to reach the catalytic cleft (Fig. 5B). However, the previous modes of substrate degradation by IDE, as described above, do not readily explain an initial cleavage at the N-terminal end of ANP. Given that the exosite of IDE is about 30 Å away from the catalytic site, this precludes the involvement of the IDE exosite in the binding of the N-terminal end of ANP for the cleavage of the same ANP molecule (Fig. 5B).
The probabilistic initial cleavage of the N-terminal end of ANP by IDE is likely exosite-independent (similar to the cleavage of short peptide substrates-see Fig. 5B). Alternatively, IDE may entrap more than one NP molecule within the catalytic chamber. In such a case, one ANP could be anchored to the exosite, confining the space within the catalytic chamber, while the other is oriented in such a way that the N-terminal end of ANP can enter into the catalytic cleft for its subsequent cleavage. These interesting results prompted us to characterize the degradation by IDE of other NPs and NP mutants (alone or in combination), and to use x-ray crystallography to better understand how IDE degrades NP in such a biased stochastic manner.
Cleavages of Other NPs by IDE
We performed MS and kinetic analyses for the degradation of human BNP by human IDE (Figs. 3C and 4A, and supplemental Table S2–S3). BNP potently prevents the degradation of substrate V by IDE with IC50 values of 120 nm, suggesting that BNP binds IDE with the high affinity. However, the rate of BNP degradation by IDE is about 50–100-fold slower than that of ANP (Fig. 4A). The cleavage pattern of BNP by IDE also follows a biased stochastic pattern already observed with ANP. Interestingly, the cleavages of BNP by IDE occur mostly at the C terminus, which convert BNP to ANP-like fragments. The slower cleavage of BNP by IDE would likely allow these ANP-like fragments to activate NPR-A signaling in a manner analogous to ANP.
CNP potently inhibits the cleavage of substrate V by IDE (70 nm) and it can be rapidly degraded by IDE (20 s−1). Because CNP does not contain a C-terminal tail that would promote a biased preferential cleavage in the C-terminal tail, we instead found that IDE preferentially cuts at two sites, one at its N-terminal end (Ser-3/Lys-4) and one inside the ring (Asp-12/Arg-13) (Fig. 3C). Such cleavages, particularly the cut at the ring, would inactivate CNP. This supports the notion that the reduction of IDE expression increases CNP-mediated NPR-B signaling as described above.
We then examined whether IDE can degrade two new members of NPs, dendroaspis natriuretic peptide (DNP), and urodilatin. DNP, originally isolated from green mamba snake, is markedly natriuretic and diuretic (24). DNP is poorly bound and degraded by IDE (Fig. 4A and supplemental Table S4). However, similar to ANP, BNP, and CNP, IDE degraded DNP by a biased stochastic process with a strong bias for cutting the C-term of DNP (Fig. 3C). Urodilatin is a new member of the NPs mainly located in kidneys (25). We found that while urodilatin has an IC50 value that is 20-fold greater than ANP, IDE effectively degrades urodilatin with a rate of 2 s−1.
Determination of Factors That Influence the Degradation of Various NPs by IDE
We also utilized NPs from other species and truncated forms of NPs to assess the factors crucial for the selectivity of NPs by IDE. To address the contribution of various regions of ANP for its recognition by IDE, we also examined the degradation of ANP from various species and ANP variants by IDE (supplemental Fig. S2 and Fig. 4A). Human IDE binds and degrades human and rat ANP equally well, with only one mutation at residue 12. However, human IDE has a lower affinity and kcat for chicken and frog ANP. We then measured the ability of IDE to degrade three ANP variants: ANP7–28, ANP4–23, and ANP7–23 (Fig. 4A and supplemental Tables S6 and S7). We found that the removal of the entire N-terminal end of ANP did not alter the recognition and degradation of ANP by IDE. However, the combined deletion at both N and C termini rendered ANP to be poor IDE substrates, as indicated by the low kcat and high IC50 values for ANP4–23 and ANP7–23.
We also performed a similar analysis with BNP from various species and BNP variants. Mammalian BNPs have the high sequence diversity. Most noticeable is the 12-amino acid N-terminal extension in rodent BNPs. We found that such extension greatly impedes binding and degradation of BNP by IDE (Fig. 4A and supplemental Fig. S2). The rat BNP variant lacking such an extension has 300-fold higher binding affinity and a 9-fold increased rate of degradation by IDE. Thus, our data indicate that the N terminus of NPs is crucial in the recognition and degradation by IDE. Interestingly, the removal of the N-terminal end resulted in a significant increase in degradation of BNP by IDE.
Taken together, these results reveal several key findings about the interaction of IDE with NPs. First, the IDE-mediated degradation of the various NPs suggest that IDE has no clear preference for P1-P1′ residues (Fig. 3C and supplemental Tables S1–S7). We also note that if the NPs have both N- and C-terminal tails, the first cleavage occurs at either termini, but not within the disulfide-linked loop (ANP, BNP, DNP). In contrast, if the NPs lack either terminal end, the first cleavage event can occur within the loop (Fig. 3C, CNP and ANP 7–28). Furthermore, the size of the terminal tails of NPs is important determinants of NP selectivity by IDE. NPs of all chain lengths likely slip into the catalytic chamber of IDE, whereby the correct orientation of the NP within the cavity is dependent on the N- and C-terminal lengths and the volumes defined by differently sized amino acids. The cleavage site of the optimally sized NPs can be adjusted properly to the catalytic site of IDE by amino acid interactions. Longer N- and C-terminal extensions cause spatial clashes or unfavorable turns, which impede the correct orientation of the NP within the chamber, leading to the inability of IDE to hydrolyze the target sequence. Conversely, very short NPs do not have the necessary interactions with IDE needed for optimal degradability.
Crystal Structure of ANP-bound and BNP-bound IDE
To understand the molecular detail for the interaction of NPs with IDE, we crystallized the complex of IDE with ANP and BNP, which represent a NP substrate with good and poor degradability, respectively (supplemental Table S8). Despite significant optimization efforts, ANP- and BNP-bound IDE crystals only diffracted at 3.0 Å and 3.1 Å, respectively. As described above, our biochemical analyses show that the initial cleavages of ANP and BNP can occur at either N- or C-terminal end of ANP and BNP. Thus, the low resolution of ANP- or BNP-bound IDE crystals may be due to the conformational heterogeneity of ANP and BNP within the catalytic chamber of IDE that results in the biased stochastic cleavage patterns.
The overall fold of these complexes shows IDE in the closed conformation similar to the previous structures of substrate-bound IDE (Fig. 4B). Our ANP-bound IDE structure has an electron density that can fit well with the first three residues of ANP (Ser-1, Leu-2, Arg-3) adjacent to the exosite of IDE (also known as the distal binding site) (Fig. 4C and supplemental Fig. S3A, left). This region of ANP forms hydrogen bonds with the main chain carbonyl of IDE residues, Gly-361 and Leu-359, and the side chain carboxyl of Glu-341. This is a typical interaction for the N terminus of IDE substrate with the exosite of IDE (20–22, 26). However, the electron density at the exosite of BNP-bound IDE can best fit with only two N-terminal residues of BNP (S1, P2). It appears that the presence of proline at residue 2 of BNP shifts the N-terminal residues of BNP away from the typical IDE-binding interaction partners (Fig. 4D and supplemental Fig. S3A, right). Thus, the interactions of BNP with the exosite of IDE are primarily mediated by a water molecule followed by the N-terminal Ser-1 residue of BNP. This weaker interaction between BNP and IDE likely impedes the proper adjustment of BNP to the active site, leading to the poor degradability of BNP by IDE.
In the active site, the electron densities were discontinuous for the IDE-ANP and BNP complexes. This could be explained by a highly flexible C-terminal portion of ANP that can adopt more than one conformation. Alternatively, it may also suggest that IDE binds two ANP molecules within the chamber: one which uses the exosite anchoring. Indeed, we performed docking studies based on our crystal structures and developed a 3D model of the interaction of ANP and IDE (Fig. 4E and supplemental Fig. S3, B–D). According to this model, the catalytic chamber of IDE is spacious enough to fit two NP molecules. We thus seek whether the capture of two NPs by the catalytic chamber of IDE indeed occurs.
IDE Efficiently Degrades fsANP in the Presence of ANP or CNP but Not Alone
A frameshift mutation of ANP (fsANP) rendering a 40 amino acid aberrant form of ANP, has been associated with familial atrial fibrillation in heterozygous patients (27). In such patients, fsANP is accumulated in higher levels compared with ANP, which may be due to its resistance to proteolysis, as demonstrated using kidney membrane preparations (23, 27). Interestingly, we found that fsANP is a substrate of IDE (Figs. 3C and 4A and supplemental Table S5). However, as compared with ANP, we found that the IDE IC50 value for fsANP is ∼30-fold higher and is degraded poorly. The lower apparent affinity and lower rate of degradation is similar to those of DNP, which also has a longer C-terminal tail. Consistent with the poor degradation of fsANP by IDE, we also found that IDE treatment did not alter the ability of fsANP to raise the intracellular cGMP in NPR-A expressing 293 cells (supplemental Fig. S4).
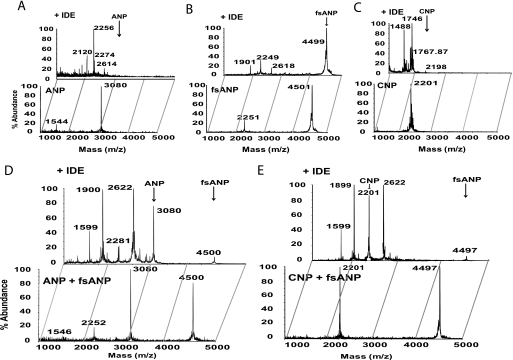
Because fsANP is present along with the normal ANP in heterozygous patients, we sought to compare the IDE-mediated cleavage pattern of an equimolar mixture of ANP and fsANP with those of each peptide alone (Fig. 6). Our MALDI-TOF MS spectra showed that IDE degraded ANP and CNP rapidly and much faster than fsANP (Fig. 6, A–C). However, the IDE digestion pattern changed dramatically for the 1:1 mixture of ANP and fsANP, with the degradation being markedly faster for fsANP compared with ANP (Fig. 6D). Indeed, this pattern was evident for ANP to fsANP ratios when the level of ANP is reduced to 1:50 of ANP to fsANP (see supplemental Fig. S5). In this case, ANP is at a molar ratio equal to IDE, suggesting that this switch in selectivity can occur even when only one molecule of ANP is bound to IDE. Because it can be argued that the undigested peak for ANP is a product of fsANP degradation, we also incubated IDE with an equimolar mixture of CNP and fsANP. This also resulted in the rapid cleavage of fsANP, while CNP was mainly undigested (Fig. 6E). However, fsANP in the presence of BNP, also a poor substrate of IDE, remained mostly uncleaved upon incubation with IDE (supplemental Fig. S6). This indicates that the insensitivity of IDE toward fsANP can be reverted by the inclusion of ANP or CNP, but not BNP.
FIGURE 6.
IDE-mediated degradation of fsANP in the absence and presence of ANP and CNP. MALDI-TOF mass spectra of (A) ANP, (B) fsANP, (C) CNP, and a 1:1 molar ratio of (D) ANP + fsANP and (E) CNP + fsANP. Each spectrum depicts the peptide(s) alone (bottom panel) or after incubation with IDE (top panel).
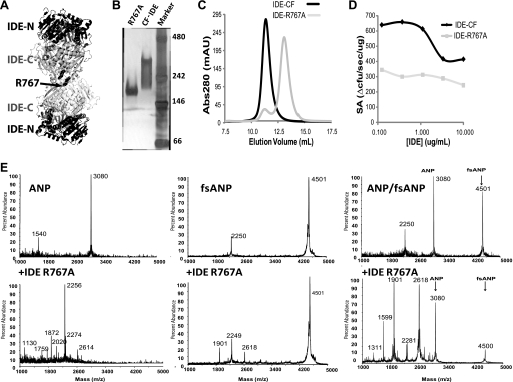
The switch in NP substrate selectivity may be the result of IDE allosterism. IDE is shown to require oligomerization for its allosteric regulation (28, 29). Based on the IDE dimer structure, Arg-767 can form a network of hydrogen bonds as well as a salt bridge at the IDE dimer interface; thus the mutation of Arg-767 to alanine should disrupt the oligomerization of IDE and its allosteric properties (Fig. 7A). Indeed, IDE-R767A is mostly monomeric as judged by the migration of this mutant in the native-PAGE and in size exclusion chromatography (Fig. 7, B and C). As expected, this mutant does not exhibit concentration-dependent alteration of specific activity as compared with wild-type IDE (Fig. 7D). Interestingly, upon incubation with a 1:1 mixture of ANP:fsANP, IDE-R767A retained its ability to switch its substrate selectivity toward fsANP degradation similar to wild-type IDE (Fig. 7E). This suggests that allosterism, driven by oligomerization, is not the mechanism that drives this switch in fsANP selectivity by IDE (Fig. 7E).
FIGURE 7.
Monomeric IDE mutant R767A retains its ability to switch NP substrate selectivity. A, cartoon diagram depicting IDE dimer arrangement revealed in IDE crystal structures. Arg-767 located at the dimer interface is highlighted. Native-PAGE analysis (B) and size exclusion chromatography (C) of IDE-R767A are shown. D, enzymatic activity of IDE-R767A using bradykinin mimetic substrate V. E, IDE-R767A mediated degradation of ANP, fsANP, and a 1:1 mixture of both NPs. The MALDI-TOF spectra depict the peptides alone (left panels) or after incubation with IDE-R767A (right panels).
DISCUSSION
Our studies indicate that IDE is likely involved in the catabolism of NPs. Our observations supporting this statement include the affinity of ANP, BNP, and CNP being in the same range as insulin, the main substrate of IDE. In addition, the cleavage rate of ANP and CNP, as compared with BNP correlates well with the observed half-life in circulation of ∼2 min for ANP and CNP and ∼20 min for BNP (5, 6). Furthermore, one of the main cleavage sites for ANP by IDE is at the C-terminal tripeptide, which correlates with reports describing a soluble proteolytic activity, having similar properties as IDE, involved in the degradation of ANP by the removal of the C-terminal tripeptide (14, 30, 31). In addition, IDE has a much higher affinity for NPs (nm) than NEP (μm) (9), also a chamber-containing protease that catabolizes NPs. Other observations supporting the role of IDE in NP metabolism include our cell-based studies, where the treatment of ANP and CNP with IDE greatly reduces the ability of these two peptides to activate their cognate receptors, NPR-A and NPR-B, respectively. Additionally, the RNAi knockdown of IDE in the cultured cells prolongs their ability to raise intracellular cGMP levels.
Our studies also offer an exciting possibility that IDE is not limited to the inactivation and clearance of NPs, but could also be involved in the enhancement of their function. The cleavage of ANP and BNP by IDE appears to generate potent activators of NPR-B since the treatment of ANP and BNP with IDE greatly enhances their ability to stimulate NPR-B in HEK293 cells. Conversely, the reduction of IDE levels further decreases the weak response of NPR-B elicited by ANP and BNP. This suggests that a fraction of the IDE-cleaved products of ANP and BNP cleavage may resemble CNP leading to the activation of NPR-B (Fig. 3). It is worth highlighting that the incubation of BNP with IDE not only resulted in the activation of NPR-B in HEK-293 cells, but also generated a highly potent cGMP signal for NPR-A in HEK-293 cells. Therefore, although IDE does not rapidly cleave BNP, it is likely that some of the BNP cleavage products are highly potent thus activating NPR-A before they are further cleaved and ultimately inactivated by IDE (Fig. 3). This is consistent with the observation that the truncated BNP can be more potent than the matured BNP (32). It is important to note that BNP from various species has a high sequence length variation (supplemental Fig. S2): rodent BNP is substantially longer than human BNP. We found that mouse BNP is an extremely poor substrate for IDE. Thus, the IDE-mediated regulation of BNP signaling appears to be a species-specific event.
This study also subjoins further structural features that better predict the degradability of peptides by IDE. They refer, for example, to the relationship between the size of the substrate and its ability to adjust within the catalytic chamber of the IDE molecule and an “adjustment site” at the exosite likely formed by Glu-341, Gly-360, Gly-361, and Leu-359 of IDE, which interacts with the NP substrate, fixes it, and modulates the catalytic attack by IDE. These structural features give rise to the possible development of more potent synthetic natriuretic peptides with reduced degradability by endogenous proteases. Finally, the systematic study of IDE-NP interaction should be useful for similar strategies with other pathophysiologically important IDE substrates, considering that IDE is mainly involved in the catabolism of several peptide hormones with vital roles in, for example, cardiovascular diseases, Alzheimer disease, and diabetes.
An unexpected finding derived from this study is the ability of IDE to switch its substrate selectivity. We showed that fsANP, as compared with ANP, is degraded slowly by IDE, but with a 1:1 stoichiometric amount of ANP and fsANP, the selectivity of IDE swapped to efficiently digesting fsANP, while ANP is mostly resistant to proteolysis. We postulate that this change in recognition could be due to ANP or its products acting as allosteric regulators of IDE. It is also possible that both peptides bind within the catalytic chamber as proposed above and that the binding of ANP restricts the space within the chamber, allowing fsANP to bind in an orientation that favors its digestion by IDE. Alternatively, the presence of the reaction products from ANP could also change the allosteric interaction or the chamber properties thus allowing the preferential cleavage of fsANP. This interesting phenomenon warrants future investigation.
In conclusion, our results point to the possibility that the physiological role of IDE may not be strictly limited to the inactivation of its peptide substrates, but may further enhance their potency in certain circumstances. We propose that the rapid cleavage rate of ANP and CNP by IDE could dampen the signaling of ANP and CNP and contribute to their rapid turnover. Conversely, the slower cleavage of BNP by IDE allows IDE to modulate and fine-tune BNP-mediated signaling. However, these hypotheses require further investigation. In addition, it would be interesting to dissect which of the NP products generated by IDE confer the changes in the signaling activities that we observed, as it may provide a basis for the rational design of new therapeutic NPs.
Supplementary Material
Acknowledgments
We thank the staff of APS SBC for help in data collection. The use of the Advanced Photon Source was supported by the U.S. Dept. of Energy, Office of Basic Energy Sciences, under Contract No. W-31-109-ENG-38. The use of the proteomics service facility was supported by Chicago Biomedical Consortium.
This work was supported, in whole or in part, by National Institutes of Health Grants GM81539 (to W.-J. T.), F32 GM87093 (to L. A. R.), 5T32HL07237-33 (to T. F.), and R21HL093402-01 (to L. R. P.).
The atomic coordinates and structure factors (codes 3N56 and 3N57) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1–S8 and Figs. S1–S6.
- NP
- natriuretic peptide
- IDE
- insulin-degrading enzyme
- ANP
- atrial natriuretic peptide
- BNP
- B-type natriuretic peptide
- CNP
- C-type natriuretic peptide
- DNP
- dendroaspis natriuretic peptide
- fsANP
- frameshift mutant atrial natriuretic peptide
- NPR-A
- natriuretic peptide receptor-A
- NPR-B
- natriuretic peptide receptor B
- HEK
- human embryonic kidney
- Aβ
- amyloid-β; DPP-IV-dipeptidyl peptidase-4
- FT-ICR MS
- Fourier transform ion cyclotron resonance mass spectrometry.
REFERENCES
- 1. Potter L. R., Yoder A. R., Flora D. R., Antos L. K., Dickey D. M. (2009) Handb. Exp. Pharmacol. 191, 341–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rubattu S., Sciarretta S., Valenti V., Stanzione R., Volpe M. (2008) Am. J. Hypertens. 21, 733–741 [DOI] [PubMed] [Google Scholar]
- 3. Nussenzveig D. R., Lewicki J. A., Maack T. (1990) J. Biol. Chem. 265, 20952–20958 [PubMed] [Google Scholar]
- 4. Matsukawa N., Grzesik W. J., Takahashi N., Pandey K. N., Pang S., Yamauchi M., Smithies O. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 7403–7408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Potter L. R., Abbey-Hosch S., Dickey D. M. (2006) Endocr. Rev. 27, 47–72 [DOI] [PubMed] [Google Scholar]
- 6. Potter L. R., Yoder A. R., Flora D. R., Antos L. K., Dickey D. M. (2009) Handb. Exp. Pharmacol. 191, 341–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chinkers M., Garbers D. L., Chang M. S., Lowe D. G., Chin H. M., Goeddel D. V., Schulz S. (1989) Nature 338, 78–83 [DOI] [PubMed] [Google Scholar]
- 8. Leissring M. A., Farris W., Chang A. Y., Walsh D. M., Wu X., Sun X., Frosch M. P., Selkoe D. J. (2003) Neuron 40, 1087–1093 [DOI] [PubMed] [Google Scholar]
- 9. Kenny A. J., Bourne A., Ingram J. (1993) Biochem. J. 291, 83–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vanneste Y., Pauwels S., Lambotte L., Michel A., Dimaline R., Deschodt-Lanckman M. (1990) Biochem. J. 269, 801–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seymour A. A., Norman J. A., Asaad M. M., Fennell S. A., Abboa-Offei B., Little D. K., Kratunis V. J., Delaney N. G., Hunt J. T., Di Donato G. (1991) J. Pharmacol. Exp. Ther. 256, 1002–1009 [PubMed] [Google Scholar]
- 12. Webb R. L., Yasay G. D., Jr., McMartin C., McNeal R. B., Jr., Zimmerman M. B. (1989) J. Cardiovasc. Pharmacol. 14, 285–293 [DOI] [PubMed] [Google Scholar]
- 13. Pankow K., Wang Y., Gembardt F., Krause E., Sun X., Krause G., Schultheiss H. P., Siems W. E., Walther T. (2007) Circ. Res. 101, 875–882 [DOI] [PubMed] [Google Scholar]
- 14. Toll L., Brandt S. R., Olsen C. M., Judd A. K., Almquist R. G. (1991) Biochem. Biophys. Res. Commun. 175, 886–893 [DOI] [PubMed] [Google Scholar]
- 15. Müller D., Baumeister H., Buck F., Richter D. (1991) Eur. J. Biochem. 202, 285–292 [DOI] [PubMed] [Google Scholar]
- 16. Müller D., Schulze C., Baumeister H., Buck F., Richter D. (1992) Biochemistry 31, 11138–11143 [DOI] [PubMed] [Google Scholar]
- 17. Malito E., Hulse R. E., Tang W. J. (2008) Cell Mol. Life Sci. 65, 2574–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hersh L. B. (2006) Cell Mol. Life Sci. 63, 2432–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duckworth W. C., Hamel F. G., Liepnieks J., Peavy D., Frank B., Rabkin R. (1989) Am. J. Physiol. 256, E208–E214 [DOI] [PubMed] [Google Scholar]
- 20. Shen Y., Joachimiak A., Rosner M. R., Tang W. J. (2006) Nature 443, 870–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Malito E., Ralat L. A., Manolopoulou M., Tsay J. L., Wadlington N. L., Tang W. J. (2008) Biochemistry 47, 12822–12834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manolopoulou M., Guo Q., Malito E., Schilling A. B., Tang W. J. (2009) J. Biol. Chem. 284, 14177–14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dickey D. M., Yoder A. R., Potter L. R. (2009) J. Biol. Chem. 284, 19196–19202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schweitz H., Vigne P., Moinier D., Frelin C., Lazdunski M. (1992) J. Biol. Chem. 267, 13928–13932 [PubMed] [Google Scholar]
- 25. Saxenhofer H., Raselli A., Weidmann P., Forssmann W. G., Bub A., Ferrari P., Shaw S. G. (1990) Am. J. Physiol. 259, F832–F838 [DOI] [PubMed] [Google Scholar]
- 26. Guo Q., Manolopoulou M., Bian Y., Schilling A. B., Tang W. J. (2010) J. Mol. Biol. 395, 430–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hodgson-Zingman D. M., Karst M. L., Zingman L. V., Heublein D. M., Darbar D., Herron K. J., Ballew J. D., de Andrade M., Burnett J. C., Jr., Olson T. M. (2008) N. Engl. J. Med. 359, 158–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Song E. S., Juliano M. A., Juliano L., Hersh L. B. (2003) J. Biol. Chem. 278, 49789–49794 [DOI] [PubMed] [Google Scholar]
- 29. Song E. S., Rodgers D. W., Hersh L. B. (2010) PLoS One 5, e9719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson G. R., Arik L., Foster C. J. (1989) J. Biol. Chem. 264, 11637–11642 [PubMed] [Google Scholar]
- 31. Johnson G. R., Foster C. J. (1990) Biochem. Biophys. Res. Commun. 167, 110–116 [DOI] [PubMed] [Google Scholar]
- 32. Shimekake Y., Kawabata T., Nakamura M., Nagata K. (1992) FEBS Lett. 309, 185–189 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.