Abstract
Estrogen modulates pain perception but how it does so is not fully understood. The aim of this study was to determine if estradiol reduces nociceptive responses in part via hypothalamic-pituitary-adrenal (HPA) axis regulation of cyclooxygenases (COX)-1/COX-2 activity. The first study examined the effects of estradiol (20%) or vehicle with concurrent injection non-steroidal anti-inflammatory drugs (NSAIDs) on formalin-induced nociceptive responding (flinching) in ovariectomized (OVX) rats. The drugs were ibuprofen (COX-1 and COX-2 inhibitor), SC560 (COX-1 inhibitor), or NS398 (COX-2 inhibitor). In a second study, estradiol’s effects on formalin-induced nociception were tested in adrenalectomized (ADX), OVX, and ADX+OVX rats. Serum levels of prostaglandins (PG) PGE2 and corticosterone were measured. Estradiol significantly decreased nociceptive responses in OVX rats with effects during both the first and the second phase of the formalin test. The non-steroidal anti-inflammatory drugs (NSAIDs) did not alter nociception at the doses used here. Adrenalectomy neither altered flinching responses in female rats nor reversed estradiol-induced antinociceptive responses. Estradiol alone had no effect on corticosterone or prostaglandin levels after the formalin test, dissociating the effects of estrogen on behavior and these serum markers. Ibuprofen and NS398 significantly reduced PGE2 levels. CORT was not decreased by OVX surgery or by estradiol below that of ADX. Only IBU significantly increased corticosterone levels. Taken together, our results suggest that estradiol-induced antinociception in female rats is independent of COX activity and HPA axis activation.
Keywords: Nociception, formalin-test, NSAIDS, adrenalectomy, ovariectomy, estrogen, sex-differences, prostaglandins, corticosterone
INTRODUCTION
About 8 million women in the United States alone use estrogen-alone replacement therapy (source: www.NIH.gov). Nonetheless, previous reports of the efficacy of such therapy and of the effects of estrogen in general vary in their conclusions [see (Fillingim et al., 2009) for a comprehensive review]. However, in a variety of animal models, estradiol is consistently antinociceptive (Kuba et al., 2005a); (Bradshaw and Berkley, 2002; Kuba et al., 2006; Mannino et al., 2007; Tsao et al., 1999), acting through both alpha and beta estrogen receptors (Amandusson and Blomqvist, 2009; Gardell et al., 2008; Spooner et al., 2007). Estradiol also reduces the development of inflammation and tissue injury associated with spinal cord trauma (Cuzzocrea et al., 2008) and inflammation and tissue damage associated with paw edema and pleurisy (Cuzzocrea et al., 2000; Cuzzocrea et al., 2001). Yet the mechanisms underlying estradiol’s anti-inflammatory and antinociceptive effects are not well described.
Prostaglandins (PG) E2—through biosynthesis by COX 1 and COX 2 enzymes—are putative mediators of injury-induced nociception and inflammation (Malmberg and Yaksh, 1995a; Malmberg and Yaksh, 1995b; Scheuren et al., 1997; Vetter et al., 2001). For example, increased release of PGs follow closely the behavioral response during Phase 2 after formalin administration (Tegeder et al., 2001). Moreover, inhibition of COX activity with either celecoxib (a selective COX-2 inhibitor) or indomethacin and diclofenac (both non-selective COX inhibitors) attenuate formalin evoked flinching responses in male rats (Euchenhofer et al.; Malmberg and Yaksh, 1995a; Yaksh et al., 2001a; Yamamoto and Nozaki-Taguchi, 1996; Yamamoto and Nozaki-Taguchi, 2002). However, SC560, a specific COX-1 inhibitor, has been reported to reduce flinching behaviors, abolish formalin-evoked PGE2 release, or be ineffective in attenuating these responses, again done only in male subjects (Yaksh et al., 2001a) (Burian and Geisslinger, 2005; Tegeder et al., 2001; Whitehouse, 2005).
PGE2 and COX protein levels are also regulated by estradiol in the central nervous system (CNS) and in non-neuronal tissue (Amateau and McCarthy, 2002; Joldersma et al., 2001; Tamura et al., 2004), although this has not been studied in pain paradigms. It is feasible that estradiol attenuates formalin-induced flinching by reducing activation of COX-1 and/or COX-2, thereby dampening PGE2 synthesis. Estradiol also increases corticosterone (CORT) levels—a hormone known for its anti-inflammatory properties (Engblom et al., 2002; O'Banion et al., 1992; Samad et al., 2002). Thus, regulation of CORT levels is another possible mechanism by which exogenous administration of estradiol attenuates flinching responses after formalin. To this end, one goal of the present study was to describe the interactions of COX inhibition with estradiol on nociception. We used ovariectomized (OVX) female rats and hormone replacement to control cyclic endogenous changes in hormone level and to more closely mimic hormone replacement therapy (HRT) in postmenopausal women. An additional goal was to examine the role of the CORT in estradiol’s antinociceptive actions. Finally we compared the behavioral results to changes in serum prostaglandins and CORT to determine if the changes in prostanoids might mediate the behavioral responses. We had the following hypotheses: 1. Estradiol would reduce flinching in the formalin test, decrease serum PGE2, and increase CORT levels; 2. COX-2 inhibition by NS398 would reduce flinching and serum PGE2 levels; 3. SC560 would be less effective than NS398 in its effects on flinching and the reduction of serum PGE2. 4. Estradiol in conjunction with NS398 would not be additive in their effects on flinching suggesting that they may work through similar mechanisms; and 5. Adrenalectomy (ADX) would reduce flinching and attenuate estradiol’s antinociceptive effect.
METHODS AND MATERIALS
Animals
Eight-week-old OVX only, sham-OVX + ADX, sham-ADX + OVX, and ADX+OVX female Sprague-Dawley rats purchased from Taconic (Germantown, NY) were double-housed in 12-h light-dark cycle (lights on 8 a.m.) with food and water ad libitum. Animals were randomly assigned to experimental groups. At the time of nociceptive testing, rats weighed between 200 and 240 g. Animal care was in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication 85-23, Bethesda, MD) and approved by the Institutional Animal Care and Use Committee at Hunter College of The City University of New York.
Reagents
17-β-estradiol 3-benzoate, cholesterol, and dimethyl sulfoxide (DMSO) were purchased from Sigma Aldrich (St. Louis, MO). Ibuprofen (IBU), SC560, and NS398 were purchased from Cayman Chemical (Ann Arbor, MI). Formalin was purchased from Fluka BioChemika (Ronkonkoma, NY).
Estradiol replacement
Two weeks after ovariectomy or OVX-sham surgery (i.e., in 10-week-old rats), SILASTIC capsules (1 cm, 0.058 in. ID × 0.077 in. OD, Dow Corning) were inserted into the nape of the animal’s neck. Capsules contained either vehicle (100% cholesterol) or estradiol (20% 17-β-estradiol: 80% cholesterol). This dose was chosen because it produces serum levels of estrogen within the normal range of the rat the reproductive cycle and represents the maximally effective dose for attenuating formalin response without affecting basal activity (Mannino et al., 2005; Mannino et al., 2007). Moreover, this dose has been shown to produce persistent anti-hyperalgesic effects after formalin administration (Kuba et al., 2005b; Kuba et al., 2006; Mannino et al., 2005). Rats were tested 1 week after capsule implantation when estradiol serum levels approximate the peak levels during proestrus—40 to 60 pg/mL (Mannino et al., 2005; Mannino et al., 2007).
Hormones and drug pretreatment for each experimental design
For experiment one, which aimed to determine estradiol’s effects on flinching behavior and on serum PG’s and CORT levels, rats received estradiol or cholesterol administration as previously described (Kuba et al., 2005b; Mannino et al., 2005). For experiment two, which aimed to determine the effects of COX inhibitors on estradiol-induced antinociception, after one week of estradiol treatment rats were injected (i.p.) with either DMSO (vehicle), ibuprofen (40 or 100 mg/kg), SC560 (20 mg/kg), or NS398 (20 mg/kg). Immediately after the administration of inhibitors or DMSO, rats were placed in the testing chamber for a one-hour habituation period and then given formalin. Experiment 3 studied the role of adrenal hormones in estradiol’s reduction of formalin induced. ADX animals were maintained on water, supplemented with 0.9% sodium chloride and tested as described above. Furthermore, only in this experiment did OVX rats receive sham ADX surgeries for ADX.
Formalin assay
Formalin assay was carried out as previously described with minor modifications (Kuba et al., 2006; Mannino et al., 2007). This assay was used because it is a common assay in the literature (e.g. (Khakpay et al.; Multon et al., 2005; Spooner et al., 2007) and because it assesses both acute pain (first phase), inhibitory processes (interphase) and sensitization (second phase). Briefly, one week after hormone replacement, a soft metal band was placed on the right hind paw with the opening positioned at the plantar surface of the paw. To minimize the novelty of the testing environment and band, rats were placed inside the testing chamber for a total of one hour prior to the formalin injection. After the habituation period, 5%-formalin, in a volume of 50 µL, was injected into the plantar surface of the banded right hind paw. Rats were then placed in the chamber and behavioral data were collected at 1-min intervals for a total of 45 min. An automated flinch detecting system was used in the formalin nociceptive assay (Yaksh et al., 2001b). All parameters of the program were set to default values. Behavioral testing was conducted between 9:00 a.m. and 3:00 p.m.
Corticosterone and PGE2 measurements
Sixty minutes after formalin injection, rats were sacrificed by decapitation (following a 20 sec exposure to CO2), and trunk blood was collected and centrifuged (at 3,000 RPM for 30 min at 4 °C). Serum was collected and then stored at −80 °C for 30–60 days prior to assay. Serum levels of CORT were detected using Coat-A-Count radioimmunoassay kits (Diagnostic Products Corporation, Los Angeles, CA). Intra-assay coefficients of variation averaged 10.0% ± 1.0%. PGE2 serum levels were detected by using enzyme immunoassay kits from Cayman Chemical (Ann Arbor, MI). Results for these assays were determined via a log-logit analysis within Graph Pad Prism program (San Diego, CA).
Statistical analysis
For behavioral analysis, the minute-by-minute data for the first 45 minutes were collapsed into three-minute bins to reduce variability. These data were analyzed by a two factor ANOVA. The between factor was the experimental group and the within factor was bin. When the main effect of experimental groups was significant, subsequent analyses were used to determine which groups differed from each other [Least Significant Difference (LSD)]. Where there was a significant Bin by treatment interaction (only the EB data), tests of simple main effects were used to determine differences in individual bins. Data are presented as mean flinches/minute averaged over the entire session or in 3-minute bins. For the estradiol study, serum levels are expressed as pg/ml (PGE2) and ng/ml (CORT) and analyzed by a t-test. For the drug studies and the ADX studies the data were normalized to OVX-vehicle treated groups and are presented as mean % change ± SEM. For all analyses, significance was at the level of p <0.05.
RESULTS
For all experiments there was a significant bin effect, which is not discussed further. Specific bins X experimental conditions interactions are discussed in the relevant section below.
Effects of estradiol
Behavior
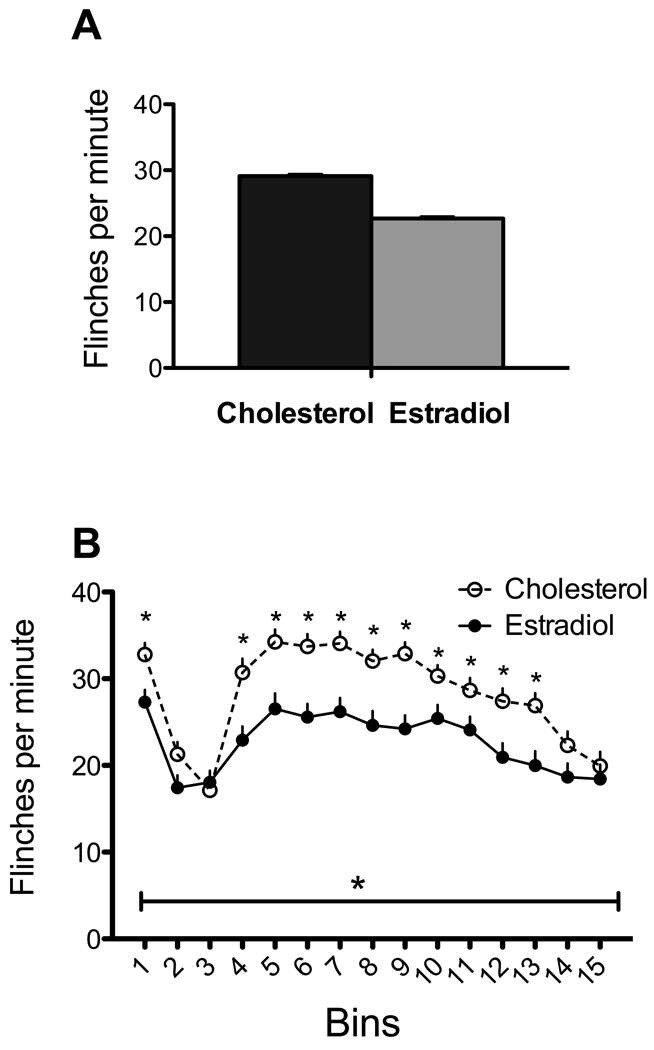
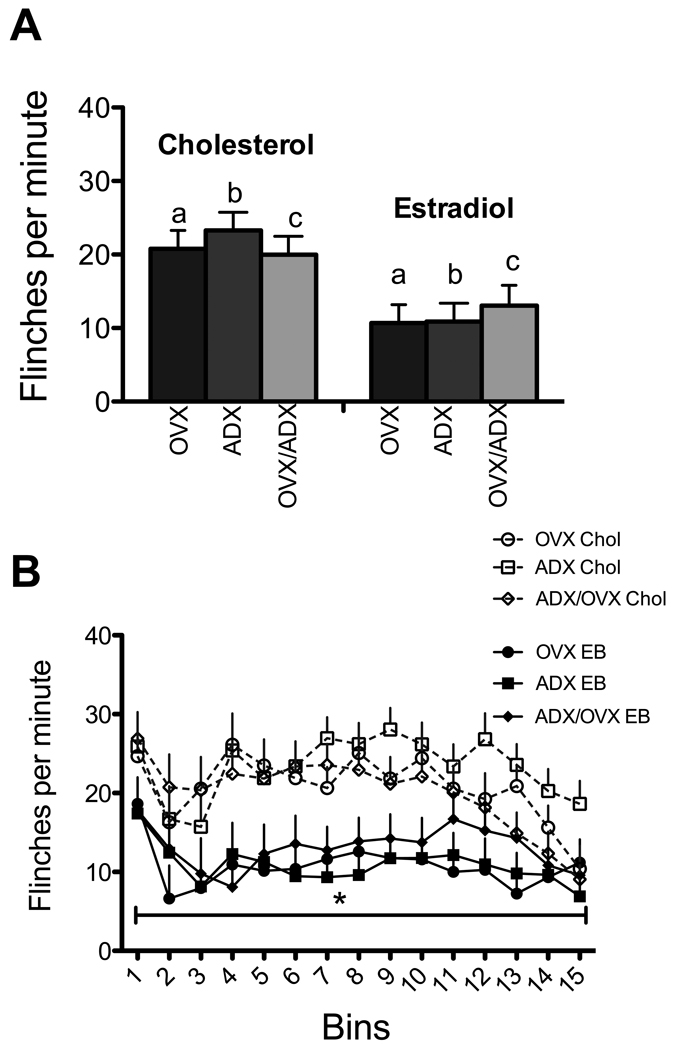
Estradiol significantly reduced flinching induced by formalin [main effect: F(1,89)=13.17, p<.001; Figure 1A]. There was also a significant bin X hormone interaction [F(14,1256)=3.26, p<.001; Figure 1B)]. Tests of simple main effects showed that responding in the first phase (bin 1; minute 1–3) and much of the second phase was reduced by estradiol. There was no effect in the interphase.
Figure 1. Effects of estradiol.
A. Estradiol significantly reduced flinching when totaled over the entire session (F(1,89)=13.17, p<.001). B. Effects of hormone treatment over bins. In addition to a significant bin effect (denoted by horizontal line with asterisk in all figures), there was a bin X hormone interaction (F(14,1256)=3.264, p<.001). Asterisks above the curves denote differences within the bin. N=41 and 50 for the estradiol (EB) and cholesterol (Chol) groups respectively.
PGE2 and corticosterone serum levels
Estradiol had no significant effect on serum prostaglandin [PGE2: F(1,35)=1.648, p=.207]. CORT serum levels were increased by estradiol but this effect failed to reach significance [F(1,57)=3.316, p=07; Table 1].
Table 1.
Effects of estradiol on prostaglandins and corticosterone
| PGE2 (pg/ml) | ||
| Cholesterol | Estradiol | |
| Mean | 288.3 | 205.1 |
| Std Error | 50.9 | 39.2 |
| Lower 95% CI | 184.4 | 122.3 |
| Upper 95% CI | 395.1 | 287.9 |
| PGD2 (pg/ml) | ||
| Cholesterol | Estradiol | |
| Mean | 4123.8 | 3853.6 |
| Std Error | 511.5 | 431.9 |
| Lower 95% CI | 3049.2 | 2942.2 |
| Upper 95% CI | 5198.3 | 4764.9 |
| Corticosterone (pg/ml) | ||
| Cholesterol | Estradiol | |
| Mean | 283.1 | 381.7 |
| Std Error | 32.7 | 44.0 |
| Lower 95% CI | 216.4 | 291.4 |
| Upper 95% CI | 349.8 | 471.9 |
Note: There were no significant effects of estradiol on either prostaglandin and only a trend for increasing corticosterone (p=0.074).
Effects of non-selective COX inhibition by ibuprofen
Behavior
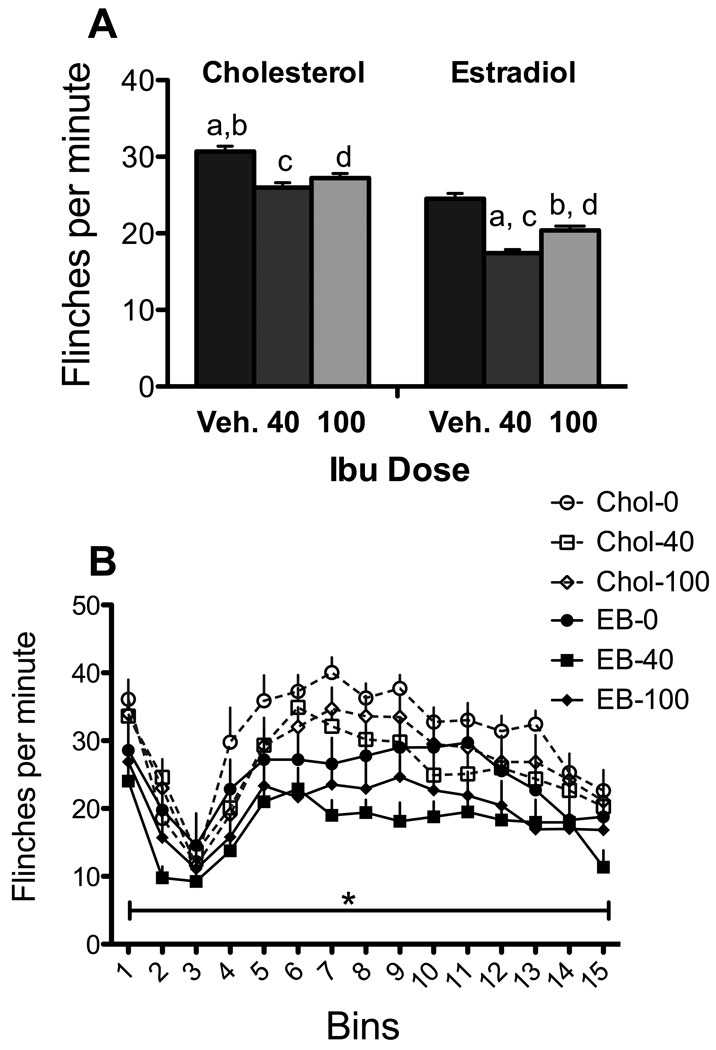
As shown in Figure 2A, there was a main effect of experimental condition on formalin-induced flinching [F (1, 54) =3.78; p=.005]. Subsequent comparisons showed that ibuprofen by itself did not significantly reduce flinching (although in estradiol treated animals, ibuprofen showed a trend towards reduce flinching at the 40 mg/kg dose compared to estradiol alone; p=.052). In contrast, estradiol was effective with and without IBU. Neither dose of ibuprofen alone was effective in cholesterol treated animals. When combined with estradiol both doses of ibuprofen were significantly different from cholesterol alone (p<.01), but not more so than estradiol alone. There were no significant differences between drug-treatment conditions or among interactions with bins (Figure 2B) although there was a significant overall hormone effect (p<.001).
Figure 2. Effects of ibuprofen and estradiol.
A. There was an overall effect of experimental condition. In this and subsequent figures, the same letter above pairs of bars identifies significant differences in the posthoc analyses. The difference between veh- estradiol and 40-estradiol showed a trend (p=.052). B. There was a significant effect of bins but no bin by experimental group interaction. N = 9–11 per group.
PGE2 and corticosterone serum levels
Ibuprofen reduced PGE2 in blood in both estradiol and cholesterol-treated females [Table 2; F(5,27)=5.744, p<.001]. Ibuprofen also increased serum CORT compared to controls with or without estradiol [100 mg/kg only; F(5,49)=3.72; =p=.006]. Thus although ibuprofen had little effect itself on behavior, it did alter PGE2 and CORT in predicted ways.
Table 2.
Effects of surgical treatments on prostaglandins and corticosterone
| Condition | PGE2 | PGD2 | CORT |
|---|---|---|---|
| OVX/ADX | 110.9 | *106.8 | 35.7 |
| OVX/ADX/EB | 108.0 | 115.0 | 52.7 |
| ADX | 96.2 | *^136.0 | 26.8 |
| ADX/EB | 118.3 | ^107.0 | 30.9 |
| VEH/IBU40 | 38.6 | 87.5 | 251.2 |
| VEH/IBU100 | 6.5 | 87.1 | 223.6 |
| EB/IBU40 | 11.9 | 102.5 | 194.4 |
| EB/IBU100 | 9.4 | 61.5 | 348.3 |
| VEH/NS398 | 15.4 | 116.6 | 138.9 |
| EB/NS398 | 17.0 | 142.2 | 134.7 |
| VEH/SC560 | 39.2 | 104.7 | *72.7 |
| EB/SC560 | 27.2 | 138.8 | *171.4 |
Note: Entries are percent of control (cholesterol and DMSO). Bolded entries are significantly different from the appropriate control. Entries that have common symbols within a treatment condition are different from each other. Estradiol treated controls are not included since overall estradiol had no effect on any of these measures.
Effect of COX-2 inhibition by NS398
Behavior
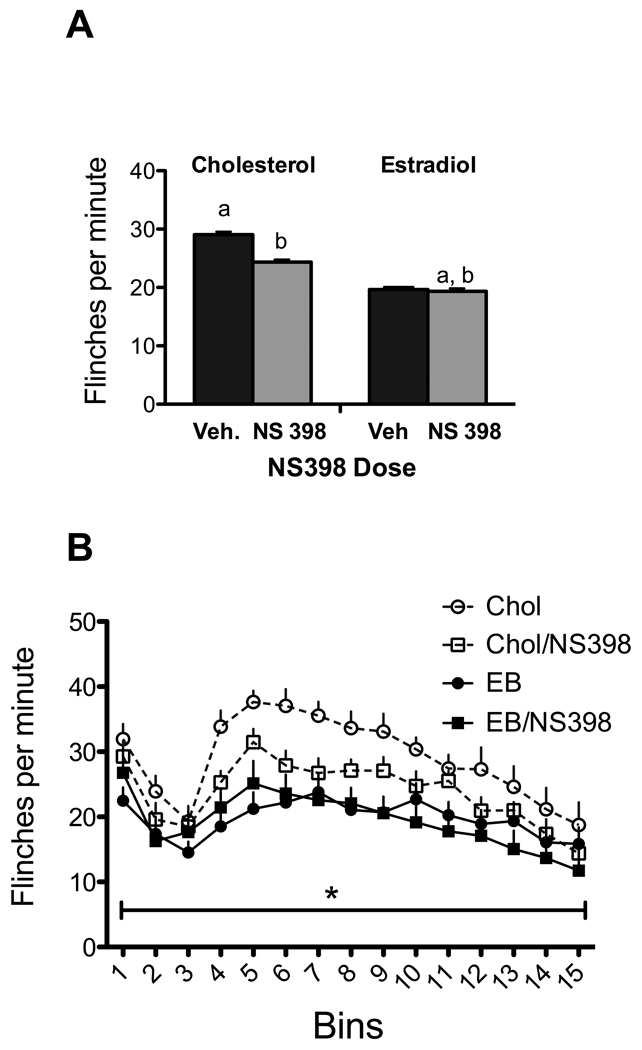
There was a main effect of experimental condition [Figure 3A; F (3, 53) =7.143, p<0.001]. The antinociceptive effects of estradiol were replicated (p<.005) but there were no interactions of drug with estradiol over individual bins. NS398 had only a trend towards an effect in control animals (p=.054) and none in estradiol treated animals. In addition, the interaction of experimental conditions with the three phases was a non-significant trend (p=.057), mostly due to the reduction of flinching by estradiol in the second phase (Figure 3B). However, when the effects of NS398 were compared to the three phases, there was no significant interaction between NS398 and phase or among NS398, phase and estradiol (all p’s >0.15).
Figure 3. Effects of NS-398 and estradiol.
A. There was an overall effect of experimental condition. The reduction in flinching induced by NS-398 in the cholesterol (Chol) treated animals was a trend (p=.054). B. There was a significant effect of bins and only a trend for a bin by experimental group interaction (p=.057). N= 13–15 per group.
PGE2 and corticosterone serum levels
NS398 with or without estradiol significantly lowered PGE2 serum levels compared to the control group [F(3,14)=7.032, p=.004; Table 2]. Posthoc analysis showed that NS398 combined with either estradiol or cholesterol elevated CORT levels [F(3,27)=6.838, p=.002).
Effect of COX-1 inhibition by SC560
Behavior
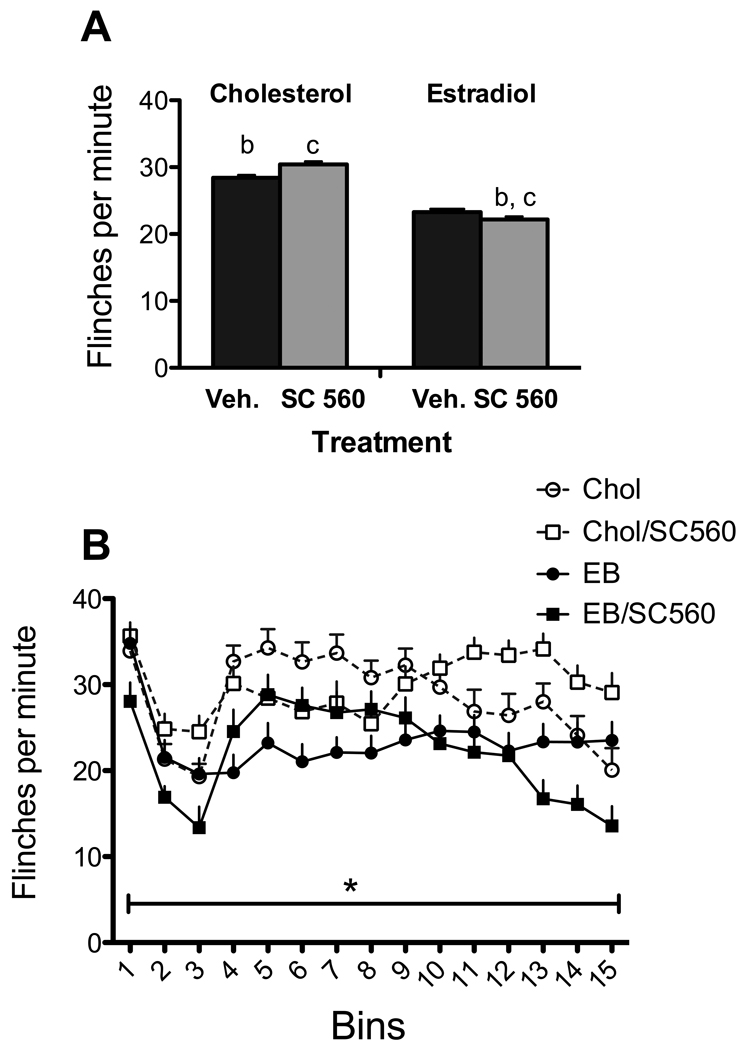
As shown in Figure 4a, there was a significant main effect of experimental condition [F (3, 64) =6.54, p=0.001]; there was no bin by treatment interaction [F(42,896)=1.037, p=.409]. EB reduced flinching over the 15-minute bins (p<.02). Follow-up analysis of the main effect of experimental conditioned confirmed that SC560 had no effect beyond that of estradiol in reducing flinching (Figure 4b).
Figure 4. Effects of SC-560 and estradiol.
A. There was a significant effect of experimental condition [F (1, 54) =3.78; p=.005]. B. There was a significant effect of bins but no bin by experimental group interaction. N= 17–18 per group.
PGE2-corticosterone serum levels
Analysis of PGE2 serum levels showed no significant effects of drug treatment [F(3,11)=1.543, p=.258 for PGE2]. The PGE2 data must be interpreted with caution since SC560 did reduce levels compared to controls but small numbers of subjects reduced power. SC560 + estradiol treatment significantly increased CORT serum levels, as compared with vehicle-vehicle treatment (p<0.05).
Role of adrenal hormones in the effects of estradiol
Behavior
There was a significant effect of experimental condition [Figure 5A; F (5,52) =4.912; p=0.001]; estradiol significantly reduced behavioral flinching in each of the three surgical groups although the analysis showed only a trend in the combined lesion groups (ovx/adx; p=.068). Adrenalectomy had no effect on behavior in either cholesterol or estradiol treated animals. However, there was a significant interaction between experimental condition and bins largely due to the effects of estradiol [F(70,728)=1.462, p=.011; Figure 5B].
Figure 5. Effects of adrenalectomy, ovariectomy and estradiol.
A. There was a significant effect of experimental condition [F (5,52) =4.912; p=0.001] but that was entirely due to effects of estradiol. Only the comparison between same surgical groups given cholesterol or estradiol are shown. In addition to the significant difference between OVX and the ADX groups with and without estradiol (“a” and “b”), the two OVX/ADX groups with and without estradiol, differed with a .068 probability (denoted by the letter “c”). B. There was a significant effect of bins and a significant bin by experimental group interaction (F(70,728)=1.462, p=.011). The major effect was of estradiol during the second phase. N= 10 except the ADX/OVX/EB group which had N=8.
PGE2-corticosterone serum levels
Neither surgery had an effect on PGE2 levels [Table 2; F(3,11)=1.543, p=.258]. Neither estradiol nor OVX altered CORT levels compared to those reduced by ADX.
DISCUSSION
The results of these experiments support prior findings that estradiol attenuates nociceptive responses to inflammatory stimuli in animal models (Ozaki-Okayama et al., 2004; Pardutz et al., 2002; Patrignani et al., 2005; Portanova et al., 1996; Taylor et al., 1998). Using a dose previously shown to be maximally effective in this paradigm [20%; (Kuba et al., 2005b)], estradiol reduced flinching in both the first and second phases but not the interphase. Because the interphase is usually considered the result of inhibitory processes (intraspinal, descending brain stem circuits or both) these findings suggest that estradiol dampens active pain signaling rather than enhancing inhibition. These effects may be peripheral or spinal in nature [see (Mogil, 2009) for review].
In contrast to studies using male rats (Yamamoto and Nozaki-Taguchi, 2002; Yashpal and Coderre, 1998), neither IBU, NS398 nor SC560 significantly decreased flinching responses in ovariectomized females. Moreover, when NS398 or SC560 was added to estradiol the reduction of flinching responses was no greater than that of estradiol alone. Only pairing IBU with estradiol showed a trend (.052) towards an additive effect of IBU at the lower dose (40 mg/kg). Sex differences associated with the efficacy of NSAIDs have not been well studied and the results of published work are mixed (Averbuch and Katzper, 2000; Hancock et al., 2009; Norrbrink Budh et al., 2003; Ousehal et al., 2009). In animal studies, the role of COX is controversial and COX inhibitors have been reported to be more effective in females or equally effective in female and male rats. [see (Fillingim et al., 2009) for a comprehensive review]. Moreover, mice lacking either the COX-1 or COX-2 gene differed from wild type mice in nociception and local inflammation; there were some sex differences but these differences were not universal (Chillingworth et al., 2006). Likewise, the effects of COX inhibition in clinical studies or experimental human work is mixed (Averbuch and Katzper, 2000; Compton et al., 2003). It is likely that there are multiple variables not yet understood that contribute to these inconsistencies.
However, there is consistency for the role of COX-2 in the mediation of inflammatory responses. Use of celecoxib, a selective COX-2 inhibitor revealed that formalin-induced secondary hyperalgesia was prevented by a local pre-injection, but not with a local post-injection. Furthermore, when celecoxib was administered spinally it inhibited formalin-induced secondary hyperalgesia with both pre- and post-injections (Veiga et al., 2004). Nantel et al., (1999) examined the regulation of COX-2 in a model where a carrageenan injection caused paw edema only or edema and mechanical hyperalgesia depending on dose. Carrageenan-induced mechanical hyperalgesia was associated with greater increases in COX-2 and PGE2 than was paw edema. Surprisingly, indomethacin administration blocked COX-2 induction in paw edema but not in hyperalgesia. The authors suggested that a positive feedback–loop regulating COX-2 expression in paw edema might be responsible (Nantel et al., 1999). After peripheral inflammation or axotomy carrageenan injection in the hind paw resulted in strong expression of COX-2 in non-neuronal cells in the grey and white matter along the leptomeninges and blood vessels (Ichitani et al., 1997). These findings suggest COX-2 expression in non-neuronal cells contributes to PG production in and around the spinal cord associated with peripheral inflammation. Moreover, pretreatment with dexamethasone and indomethacin anti-inflammatory drugs reduced edema in a dose dependent manner after subplantar carrageenan injection (Henriques et al., 1987). Here we found a non-significant trend towards analgesia with NS398 in cholesterol treated animals, which would be consistent with most studies.
Our data found no evidence for any effect of SC560 in female rats with or without estradiol. COX-1 inhibitors are inconsistent in their effects on pain and the role of COX-1 in nociceptive responses remains controversial. COX-1-inhibitors are effective in incision models (Zhu et al., 2005) but not bone cancer models (Saito et al., 2005); in an induced arthritic model, COX-1 inhibitors worked only with COX-2 inhibition (Martinez et al., 2002). Specifically, using the formalin model SC560 was found to be analgesic and to reduce formalin evoked PGE2 in some studies but not others (Burian and Geisslinger, 2005; Tegeder et al., 2001; Whitehouse, 2005). Recently selective COX-1 and COX-2 inhibitors were shown to reduce antinociceptive when administered before and during the development of inflammation but only the COX-2 inhibitor was able to reverse inflammation and hyperexcitability after it was established, suggesting multiple and alternate mechanisms in COX-2 compared to COX-1 antinociceptive effects (Telleria-Diaz et al., 2008).
Estradiol had no significant effects on serum levels of either PGE2 or CORT. This is in contrast to the findings of others who showed increases induced by estradiol. One possible explanation is that all drug controls including the cholesterol animals received DMSO as a vehicle. DMSO by itself elevates CORT levels (Ivanovic Matic et al., 2004) and thus the baseline was likely to be elevated. However, the surgical controls for the ADX/OVX experiments did not receive DMSO and analysis of that subset of data showed no significant effect of estradiol (p=.10), albeit with smaller numbers of subjects. In addition, the measurement of PGE2 and CORT were accessed 60 minute after formalin injection and cannot distinguish which phase in the test might have induced PGE2 or CORT production.
Thus our results suggest that activation of the HPA is not necessary for the analgesic effects of estradiol and estradiol’s effects are not mediated through prostaglandins in any simple manner. This conclusion is consistent with previous work showing that although formalin administration activates the HPA axis, CORT does not reduce nociception in the formalin test (Taylor et al., 1998). Here we observed significant differences in the effects of estradiol on flinching responses in all three surgical conditions (OVX, OVX + ADX and ADX). Therefore estradiol likely mediates its antinociceptive effect through non-HPA-dependent mechanisms, such as cytokine releases and/or nitric oxide (NO) activation. Indeed, estradiol reduced NO activation and cytokine release in vitro and in vivo [e.g., in spinal cord and brain tissues; (Cuzzocrea et al., 2008)]. In part this could occur by estradiol’s known regulation of microglial cell release of multiple inflammatory mediators including NO and PGE2 (Bruce-Keller et al., 2000; Vegeto et al., 2001).
Estradiol did not alter PGE2 serum levels. This is consistent with the lack of effect in vitro whereby estradiol did not alter PGE2 levels in neuronal cultures (Tenenbaum et al., 2007). In contrast to estradiol, NS398 and IBU reduced PGE2 serum levels compared to controls. However, neither drug significantly altered the flinching response, thus dissociating their behavioral and biochemical actions. The decrease in PG levels did not block or enhance estradiol’s antinociceptive effects in formalin responses further suggesting that estradiol’s effects may be independent of PGE2
Although our results confirm that estrogen reduces inflammatory nociceptive responses in females, women demonstrate significantly higher behavioral responses to chronic and inflammatory pain than do men (Fillingim et al., 2009). For example, numerous studies have reported that certain conditions such as migraine, temporomandibular disorders, neuropathic pain, spinal cord injuries and some forms of arthritis and fibromyalgia are significantly more prevalent in females than males. Similarly women display a greater use of opioids and NSAIDs (Norrbrink Budh et al., 2003). This paradox (estrogen being antinociceptive and anti-inflammatory but women having higher rates of chronic pain) may in part derive from interactions between estrogen and progesterone in women or the presence of testosterone in men. Indeed, we have demonstrated that progesterone reverses/reduces estrogen’s anti-inflammatory and antinociceptive effects (Kuba et al., 2006; Mannino et al., 2007). Thus, in intact women, because of the presence of both hormones, estrogen’s antinociceptive and anti-inflammatory effects may be masked by progesterone’s inhibition of estrogen’s effects. Alternatively, testosterone may serve a protective role in men, producing antinociceptive responses to inflammatory and chronic pain (Borzan and Fuchs, 2006; Fischer et al., 2008).
To conclude, we postulate that sex differences in response to analgesia (inflammatory pain) are, in part, independent of the influences of estradiol on inflammation-mediated responses. Furthermore, the fact that estradiol attenuates nociception and induces CORT levels suggests that during the different reproductive stages females may experience differential behavioral nociceptive responses, activation of inflammation-mediated mechanisms and efficacy of NSAIDs.
ACKNOWLEDGMENTS
We are grateful to Dr. Patricia Stephens for her editorial comments. This work was supported by RR-03037 (VQJ), NF39534 (VQJ), DA 12136 (VQJ), NS341073 (CEI), DA001457 (CEI), DA000198 (CEI) and K05 DA000325 (GAB).
REFERENCES
- Amandusson A, Blomqvist A. Estrogen receptor-alpha expression in nociceptive-responsive neurons in the medullary dorsal horn of the female rat. Eur J Pain. 2009;14(3):245–248. doi: 10.1016/j.ejpain.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J Neurosci. 2002;22(19):8586–8596. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbuch M, Katzper M. A search for sex differences in response to analgesia. Arch Intern Med. 2000;160(22):3424–3428. doi: 10.1001/archinte.160.22.3424. [DOI] [PubMed] [Google Scholar]
- Borzan J, Fuchs PN. Organizational and activational effects of testosterone on carrageenan-induced inflammatory pain and morphine analgesia. Neuroscience. 2006;143(3):885–893. doi: 10.1016/j.neuroscience.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Bradshaw HB, Berkley KJ. Estrogen replacement reverses ovariectomy-induced vaginal hyperalgesia in the rat. Maturitas. 2002;41:157–165. doi: 10.1016/s0378-5122(01)00261-4. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Antiinflammatory effects of estrogen on microglial activation. Endocrinology. 2000;141(10):3646–3656. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- Burian M, Geisslinger G. COX dependent mechanisms involved in the antinociceptive action of NSAIDS at central and peripheral sites. Pharmacology and therapeutics. 2005;107:139–154. doi: 10.1016/j.pharmthera.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Chillingworth NL, Morham SG, Donaldson LF. Sex differences in inflammation and inflammatory pain in cyclooxygenase-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R327–R334. doi: 10.1152/ajpregu.00901.2005. [DOI] [PubMed] [Google Scholar]
- Compton P, Charuvastra VC, Ling W. Effect of oral ketorolac and gender on human cold pressor pain tolerance. Clin Exp Pharmacol Physiol. 2003;30(10):759–763. doi: 10.1046/j.1440-1681.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Genovese T, Mazzon E, Esposito E, Di Paola R, Muia C, Crisafulli C, Peli A, Bramanti P, Chaudry IH. Effect of 17beta-estradiol on signal transduction pathways and secondary damage in experimental spinal cord trauma. Shock. 2008;29(3):362–371. doi: 10.1097/shk.0b013e31814545dc. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Mazzon E, Calabro G, Dugo L, De Sarro A, van De LF, Caputi AP. Inducible nitric oxide synthase-knockout mice exhibit resistance to pleurisy and lung injury caused by carrageenan. Am J Respir Crit Care Med. 2000;162(5):1859–1866. doi: 10.1164/ajrccm.162.5.9912125. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Mazzon E, Sautebin L, Serraino I, Dugo L, Calabro G, Caputi AP, Maggi A. The protective role of endogenous estrogens in carrageenan-induced lung injury in the rat. Mol Med. 2001;7(7):478–487. [PMC free article] [PubMed] [Google Scholar]
- Engblom D, Ek M, Saha SE-DA, Jakobsson Pj, Bomqvist A. Prostaglandins as inflammatory messengers across the blood brain barrier. J Mol Med. 2002;80:5–15. doi: 10.1007/s00109-001-0289-z. [DOI] [PubMed] [Google Scholar]
- Euchenhofer C, Maihofner C, Brune K, Tegeder I, Geisslinger G. Differential effect of selective cyclooxygenase-2 (COX-2) inhibitor NS 398 and diclofenac on formalin-induced nocicption in the rat. NeurosciLett. 1998;248:25–28. doi: 10.1016/s0304-3940(98)00325-5. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10(5):447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer L, Torres-Chavez KE, Clemente-Napimoga JT, Jorge D, Arsati F, de Arruda Veiga MC, Tambeli CH. The influence of sex and ovarian hormones on temporomandibular joint nociception in rats. J Pain. 2008;9(7):630–638. doi: 10.1016/j.jpain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Gardell LR, Hyldtoft L, Del Tredici AL, Andersen CB, Fairbairn LC, Lund BW, Gustafsson M, Brann MR, Olsson R, Piu F. Differential modulation of inflammatory pain by a selective estrogen receptor beta agonist. Eur J Pharmacol. 2008;592(1–3):158–159. doi: 10.1016/j.ejphar.2008.06.107. Epub 2008 Jul 2004. [DOI] [PubMed] [Google Scholar]
- Hancock MJ, Maher CG, Latimer J, McLachlan AJ, Day RO, Davies RA. Can predictors of response to NSAIDs be identified in patients with acute low back pain? Clin J Pain. 2009;25(8):659–665. doi: 10.1097/AJP.0b013e3181a7ee3a. [DOI] [PubMed] [Google Scholar]
- Henriques MG, Silva PM, Martins MA, Flores CA, Cunha FK, Assreuy-Filho J, Cordeiro RS. Mouse paw edema. A new model for inflammation. Braz J Med Biol Res. 1987;20(2):243–249. [PubMed] [Google Scholar]
- Ichitani Y, Shi T, Haeggstrom J, Samuelsson B, Hokfelt T. Increased levels of cyclooxygenase-2 mRNA in the rat spinal cord after peripheral inflammation: in situ hybridization study. NeuroReport. 1997;8(13):2949–2952. doi: 10.1097/00001756-199709080-00028. [DOI] [PubMed] [Google Scholar]
- Ivanovic Matic S, Dinic S, Mihailovic M, Grigorov I, Bogojevic DPG. Acute-phase protein expression in DMSO-intoxicated rats. Toxicol Lett. 2004;147(2):153–159. doi: 10.1016/j.toxlet.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Joldersma M, Klein-Nulend J, Oleksik AM, Heyligers IC, Burger EH. Estrogen enhances mechanical stress-induced prostaglandin production by bone cells from elderly women. Am J Physiol Endocrinol Metab. 2001;280(3):E436–E442. doi: 10.1152/ajpendo.2001.280.3.E436. [DOI] [PubMed] [Google Scholar]
- Khakpay R, Semnanian S, Javan M, Janahmadi M. The effect of intra-locus coeruleus injection of 17beta-estradiol on inflammatory pain modulation in male rat. Behav Brain Res. 2010:20. doi: 10.1016/j.bbr.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Kuba T, Jenab S, Quinones-Jenab V. Endogenous gonadal hormones mediate sex differences in formalin-induced behavioral responses through prostaglandin E2 release. 2005a Journal of Pain submitted. [PMC free article] [PubMed] [Google Scholar]
- Kuba T, Kemen LM, Quinones-Jenab V. Estradiol administration mediates the inflammatory response to formalin in female rats. Brain Res. 2005b;1047(1):119–122. doi: 10.1016/j.brainres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Kuba T, Wu HB, Nazarian A, Festa ED, Barr GA, Jenab S, Inturrisi CE, Quinones-Jenab V. Estradiol and progesterone differentially regulate formalin-induced nociception in ovariectomized female rats. Horm Behav. 2006;49(4):441–449. doi: 10.1016/j.yhbeh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Cyclooxygenase inhibition and the spinal release of prostaglandin e(2) and amino acids evoked by paw formalin injection: a microdialysis study in unanesthetized rats. Journal of Neuroscience. 1995a;15:2768–2776. doi: 10.1523/JNEUROSCI.15-04-02768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. The effect of morphine on formalin-evoked behaviour and spinal release of excitatory amino acids and prostaglandin e2 using microdialysis in conscious rats. British Journal of Pharmacology. 1995b;114:1069–1075. doi: 10.1111/j.1476-5381.1995.tb13315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannino CA, South SM, Inturrisi CE, Quinones-Jenab V. Pharmacokinetics and effects of 17beta-estradiol and progesterone implants in ovariectomized rats. J Pain. 2005;6(12):809–816. doi: 10.1016/j.jpain.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Mannino CA, South SM, Quinones-Jenab V, Inturrisi CE. Estradiol replacement in ovariectomized rats is antihyperalgesic in the formalin test. J Pain. 2007;8(4):334–342. doi: 10.1016/j.jpain.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Martinez RV, Reval M, Campos MD, Terron JA, Dominguez R, Lopez-Munoz FJ. Involvement of peripheral cyclooxygenase-1 and cyclooxygenase-2 in inflammatory pain. J Pharm Pharmacol. 2002;54(3):405–412. doi: 10.1211/0022357021778475. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10(4):283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Multon S, Pardutz A, Mosen J, Hua MT, Defays C, Honda S, Harada N, Bohotin C, Franzen R, Schoenen J. Lack of estrogen increases pain in the trigeminal formalin model: a behavioural and immunocytochemical study of transgenic ArKO mice. Pain. 2005;114(1–2):257–265. doi: 10.1016/j.pain.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Nantel F, Denis D, Gordon R, Northey A, Cirino M, Metters KM, Chan CC. Distribution and regulation of cyclooxygenase-2 in carrageenan-induced inflammation. Br J Pharmacol. 1999;128(4):853–859. doi: 10.1038/sj.bjp.0702866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrbrink Budh C, Lund I, Hultling C, Levi R, Werhagen L, Ertzgaard P, Lundeberg T. Gender related differences in pain in spinal cord injured individuals. Spinal Cord. 2003;41(2):122–128. doi: 10.1038/sj.sc.3101407. [DOI] [PubMed] [Google Scholar]
- O'Banion MR, Winn VD, Young DA. cDNA cloning and functional activity of glucorcorticoids regulated inflammatory cyclooxygenase. Proc Acad Sci USA. 1992;89(4888):4892. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousehal L, Lakhdar A, Elquars F. Comparison of the effect of paracetamol and ibuprofen on orthodontic pain. Int Orthod. 2009;7(2):193–206. doi: 10.1016/S1761-7227(09)74626-1. Epub 2009 Aug 2013. [DOI] [PubMed] [Google Scholar]
- Ozaki-Okayama Y, Matsumura K, Ibuki T, Ueda M, Yamazaki Y, Tanaka Y, Kobayashi S. Burn injury enhances brain prostaglandin E2 production through induction of cyclooxygenase-2 and microsomal prostaglandin E synthase in cerebral vascular endothelial cells in rats. Crit Care Med. 2004;32(3):795–800. doi: 10.1097/01.ccm.0000114576.60077.fc. [DOI] [PubMed] [Google Scholar]
- Pardutz A, Multon S, Malgrange B, Parducz A, Vescei L, Schoenen J. Effect of systemic nitroglycerin on CGRP and 5-HT afferents to rat caudal spinal trigeminal nucleus and its modulation by estrogen. Eur J Neurosci. 2002;15(11):1803–1809. doi: 10.1046/j.1460-9568.2002.02031.x. [DOI] [PubMed] [Google Scholar]
- Patrignani P, Tacconelli S, Sculli M, Capone M. New Insights into COX-2 biology and inhibition. Brain Research Reviews. 2005;48:352–359. doi: 10.1016/j.brainresrev.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Portanova JP, Zhang Y, Anderson GD, Hauser SD, Masferrer JL, Seibert K, Gregory SA, Isakson PC. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia and interleukin 6 production in vivo. J Exp Med. 1996;184:883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito O, Aoe T, Yamamoto T. Analgesic effects of nonsteroidal antiinflammatory drugs, acetaminophen, and morphine in a mouse model of bone cancer pain. J Anesth. 2005;19(3):218–224. doi: 10.1007/s00540-005-0323-3. [DOI] [PubMed] [Google Scholar]
- Samad TA, Sapirstein A, Woolf CJ. Prostanoids and pain: unraveling mechanisms and revealing therapeutic targets. Trends Molecular Medicine. 2002;8(8):390–396. doi: 10.1016/s1471-4914(02)02383-3. [DOI] [PubMed] [Google Scholar]
- Scheuren N, Neupert W, Ionac M, Neuhuber W, Brune K, Geisslinger G. Peripheral noxious stimulation releases spinal PGE2 during the first phase in the formalin assay of the rat. Life Sci. 1997;60:PL295–PL300. doi: 10.1016/s0024-3205(97)00155-0. [DOI] [PubMed] [Google Scholar]
- Spooner MF, Robichaud P, Carrier JC, Marchand S. Endogenous pain modulation during the formalin test in estrogen receptor beta knockout mice. Neuroscience. 2007;150(3):675–680. doi: 10.1016/j.neuroscience.2007.09.037. [DOI] [PubMed] [Google Scholar]
- Tamura M, Deb S, Sebastian S, Okamura K, Bulun SE. Estrogen up-regulates cyclooxygenase-2 via estrogen receptor in human uterine microvascular endothelial cells. Fertility and Sterility. 2004;81(5):1351–1356. doi: 10.1016/j.fertnstert.2003.09.076. [DOI] [PubMed] [Google Scholar]
- Taylor BK, Akana SF, Peterson MA, Dallman MF, Basbaum AI. Pituitary-adrenocortical responses to persistent noxious stimuli in the awake rats: Endogenous corticosterone does not reduce nociception in the formalin test. Endocrinology. 1998;139(5):2407–2413. doi: 10.1210/endo.139.5.5993. [DOI] [PubMed] [Google Scholar]
- Tegeder I, Niederberger E, Vetter G, Brautigam L, Geisslinger G. Effects of selective COX-1 and -2 inhibition on formalin-evoked nociceptive behaviour and prostaglandin E(2) release in the spinal cord. J Neurochem. 2001;79(4):777–786. doi: 10.1046/j.1471-4159.2001.00613.x. [DOI] [PubMed] [Google Scholar]
- Telleria-Diaz A, Ebersberger A, Vasquez E, Schache F, Kahlenbach J, Schaible HG. Different effects of spinally applied prostaglandin D2 on responses of dorsal horn neurons with knee input in normal rats and in rats with acute knee inflammation. Neuroscience. 2008;156(1):184–192. doi: 10.1016/j.neuroscience.2008.07.017. Epub 2008 Jul 2012. [DOI] [PubMed] [Google Scholar]
- Tenenbaum M, Azab AN, Kaplanski J. Effects of estrogen against LPS-induced inflammation and toxicity in primary glial and neuronal cultures. J Endotoxin R. 2007;13(3):158–166. doi: 10.1177/0968051907080428. [DOI] [PubMed] [Google Scholar]
- Tsao CM, Ho CM, Tasi SK, Lee TY. Effects of estrogen on autotomy in normal and ovariectomized rats. Pharmacology. 1999;59(3):142–148. doi: 10.1159/000028314. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Bonincontro C, Pollio G, Sala A, Viappiani S, Nardi F, Brusadelli A, Viviani B, Ciana P, Maggi A. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci. 2001;21(6):1809–1818. doi: 10.1523/JNEUROSCI.21-06-01809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga APC, Duarte IDG, Avila MN, da Motta PG, Tatsuo MAKF, Francischi JN. Prevention by celecoxib of secondary hyperalgesia induced by formalin in rats. Life Sci. 2004;75:2807–2817. doi: 10.1016/j.lfs.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Vetter G, Geisslinger G, Tegeder I. Release of glutamate, nitric oxide and prostaglandin E2 and metabolic activity in the spinal cord of rats following peripheral nociceptive stimulation. Pain. 2001;92(1–2):213–218. doi: 10.1016/s0304-3959(01)00258-5. [DOI] [PubMed] [Google Scholar]
- Whitehouse MW. Prostanoids as friends, not foes: further evidence from the interference by cyclooxygenase-inhibitory drugs when introducing tolerance to experimental arthritigens in rats. Inflammopharmacology. 2005;12(5–6):481–492. doi: 10.1163/156856005774382788. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Dirig DM, Conway CM, Svensson C, Luo ZD, Isakson PC. The acute antihyperalgesic action of nonsteroidal, anti-inflammatory drugs and release of spinal prostaglandin E2 is mediated by the inhibition of constitutive spinal cyclooxygenase-2 (COX-2) but not COX-1. J Neurosci. 2001a;21(16):5847–5853. doi: 10.1523/JNEUROSCI.21-16-05847.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Ozaki G, McCumber D, Rathbun M, Svensson C, Malkmus S, Yaksh MC. An automated flinch detecting system for use in the formalin nociceptive bioassay. J Appl Physiol. 2001b;90(6):2386–2402. doi: 10.1152/jappl.2001.90.6.2386. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N. Analysis of the effects of cyclooxygenase (COX)-1 and COX-2 in spinal nociceptive transmission using indomethacin, a non-selective COX inhibitor, and NS-398, a COX-2 selective inhibitor. Brain Res. 1996;739:104–110. doi: 10.1016/s0006-8993(96)00817-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N. The role of cyclooxygenase-1 and -2 in the rat formalin test. Anesth Analg. 2002;94(4):962–967. doi: 10.1097/00000539-200204000-00035. table of contents. [DOI] [PubMed] [Google Scholar]
- Yashpal K, Coderre TJ. Influence of formalin concentration on the antinociceptive effects of anti-inflammatory drugs in the formalin test in rats: separate mechanisms underlying the nociceptive effects of low- and high-concentration formalin. Euro J Pain. 1998;2:63–68. doi: 10.1016/s1090-3801(98)90047-7. [DOI] [PubMed] [Google Scholar]
- Zhu X, Conklin DR, Eisenach JC. Preoperative inhibition of cyclooxygenase-1 in the spinal cord reduces postoperative pain. Anesth Analg. 2005;100(5):1390–1393. doi: 10.1213/01.ANE.0000148127.53832.8E. [DOI] [PubMed] [Google Scholar]