Abstract
Typhoid fever is a systemic, persistent infection caused by host-specific strains of Salmonella. Although the use of antibiotics has reduced the complications associated with primary infection, recurrent infection remains an important cause of ongoing human morbidity and mortality. Herein, we investigated the impacts of antibiotic eradication of primary infection on protection against secondary recurrent infection. Using a murine model of persistent Salmonella infection, we demonstrate protection against recurrent infection is sustained despite early eradication of primary infection. In this model, protection is not mediated by CD4+ or CD8+ T cells because depletion of these cells either alone or in combination prior to rechallenge does not abrogate protection. Instead, infection followed by antibiotic-mediated clearance primes robust levels of Salmonella-specific antibody that can adoptively transfer protection to naïve mice. Thus, eradication of persistent Salmonella infection primes antibody-mediated protective immunity to recurrent infection.
Keywords: SALMONELLA, ANTIBIOTIC TREATMENT, RECURRENT INFECTION
1. Introduction
Typhoid fever is a persistent, systemic infection caused by host-adapted strains of Gram-negative bacteria within the Salmonella genus. The development and use of antimicrobials with bactericidal activity against Salmonella has transformed this once debilitating and often fatal infection into a readily treatable condition. Unfortunately even with antibiotic therapy, recurrent disease occurs in 5 to 15% of individuals [1–4]. Molecular genotyping and phenotyping of S. enterica serotype Typhi (S. typhi) isolates from individuals with primary and recurrent infection suggest recurrence may be caused by re-activation of latent or secondary infection [2, 5, 6]. However, for individuals living in endemic areas where re-exposure is essentially unavoidable, protection from recurrent infection is paramount, while the significance of distinguishing Salmonella isolates associated with re-activation or secondary infection appears less relevant.
Regardless of the specific etiology, the clinical symptoms of recurrent compared with primary infection are less severe and of shorter duration [7]. Similarly, reduced rates of clinical typhoid fever and infection relapse have been reported for human volunteers previously recovered from typhoid compared with naïve individuals after challenge with virulent Salmonella [8], and reduced attack rates occur for individuals with prior Salmonella infection during an outbreak among military personnel exposed to infected food handlers [9]. These epidemiological features of human typhoid suggest naturally-acquired Salmonella infection confers some protection against secondary infection.
Protection from recurrent disease triggered by primary infection is also reproduced in animal models of Salmonella infection. For example, natural recovery from experimental typhoid fever protects chimpanzees from fever, bacteremia, and systemic inflammation after secondary challenge with virulent Salmonella [10]. For mouse typhoid caused by S. enterica serotype Typhimurium (S. typhimurium) infection, primary infection with live attenuated Salmonella mutants confers a high level of protection against secondary challenge with virulent Salmonella [11, 12]. Thus, animal models of typhoid infection allow the potential impact of antibiotic treatment in priming protective immunity to be more precisely characterized. In this regard, a recent study reported sharply reduced protection against recurrent infection after early eradication of primary infection with virulent Salmonella compared with that primed by an attenuated Salmonella mutant that causes more sustained infection [13]. These findings suggest antimicrobial therapy, while beneficial for curtailing the sequelae of primary infection, may also blunt the priming of protective immunity conferred by natural infection. However, the inherent susceptibility C57BL/6 mice lacking the resistant allele of Nramp1 to virulent S. typhimurium used in this study required the eradication of primary infection within two days. Therefore, the effects of antibiotic-mediated clearance of primary infection during the later and persistent phase of this infection remain undefined. In this study, mice containing the resistant allele of Nramp1 that develop persistent infection with virulent Salmonella were used to investigate the impacts of primary infection eradication on protection against secondary Salmonella infection.
2. Materials and methods
2.1. Mice
C57BL/6 and 129SvJ mice were purchased from the National Cancer Institute. B6.129 F1 mice generated by intercrossing C57BL/6 females with 129SvJ males as a model for persistent infection with virulent Salmonella has been described [14–16]. All mice were generated and maintained in specific pathogen-free facilities and used between 6–8 weeks of age. These experiments were conducted under University of Minnesota IACUC approved protocols.
2.2. Bacteria, infections, and antibiotic treatment
The virulent S. enterica serotype Typhimurium (S. typhimurium) strain, SL1344, has been described [12, 17, 18]. For infections, S. typhimurium was grown to log phase in brain heart infusion (BHI) media at 37 °C, washed and diluted with saline and injected intravenously through the lateral tail vein [16]. The number of recoverable Salmonella CFUs was quantified by plating serial dilutions of organ homogenates onto BHI agar plates. Where indicated, enrofloxacin was added to the drinking water (2 mg/ml) beginning five or twenty days post-infection. Mice were withdrawn from antibiotics for at least five days prior to secondary infection. For re-challenge, 1 × 104 or 1 × 106 CFUs of SL1344 was injected intravenously. Heat-killed Salmonella was prepared by resuspending SL1344 in sterile saline and incubating at 75°C for 60 minutes, and plating to confirm the absence of live bacteria as described [19].
2.3. Reagents for cell staining, antibody ELISA, and cell depletion
Antibodies and other reagents for flow cytometry and ELISA were purchased from BD Biosciences (San Jose, CA) or eBioscience (San Diego, CA). For ELISA, flat bottom 96-well plates were coated with 1.25 × 107 CFUs heat-killed SL1344 diluted in 0.1 M NaHCO3 and incubated overnight at 4°C. Wells were then blocked with 1% albumin, assayed with serial dilutions of serum from Salmonella infected mice followed by biotinylated anti-mouse isotype specific antibodies, and developed with streptavidin conjugated to peroxidase and O-phenylenediamene substrate. For CD4+ and CD8+ T cell depletion, 500 μg of purified anti-mouse CD4 (clone GK1.5) and/or anti-mouse CD8 (clone 2.43) antibody (BioXCell) were inoculated intraperitoneally one day prior to Salmonella infection. For transfer, serum was collected from donor mice, and transferred intravenously into naïve recipient mice (350 to 400 μl/mouse) one day prior to Salmonella infection.
2.4. Statistics
The difference in number of recoverable bacterial CFUs and survival were evaluated using the Student’s t and log-rank tests, respectively (GraphPad, Prism Software) with P ≤ 0.05 taken as statistically significant.
3. Results
3.1. Protective immunity despite early eradication of primary Salmonella infection
The reduced severity, shorter duration, and lowered attack rates of recurrent compared with primary Salmonella infection in humans suggest natural infection primes some protective effects against secondary infection [8, 9]. To address how eradication of primary infection impacts these protective effects, we compared the susceptibility against secondary recurrent infection with virulent Salmonella for mice treated with antibiotics after primary infection or control mice without primary infection. We initially enumerated the relative susceptibility for S. typhimurium-infected mice (104 CFUs) treated with enrofloxacin beginning day 20 after primary infection. Enrofloxacin is a fluoroquinolone antibiotic, and this dose has been shown to eradicate systemic Salmonella infection within five to seven days after administration in the drinking water [13, 20]. Using this approach, highly significant (≥ 50–100-fold) reductions in recoverable Salmonella were found after secondary challenge (104 CFUs) for mice eradicated of primary infection compared with control mice without primary Salmonella infection (Fig. 1A). This reduction in number of recoverable Salmonella CFUs was not due to residual enrofloxacin because both groups of mice were treated and withdrawn from this antibiotic in parallel. Thus, protection against recurrent Salmonella infection is sustained despite eradication of primary infection.
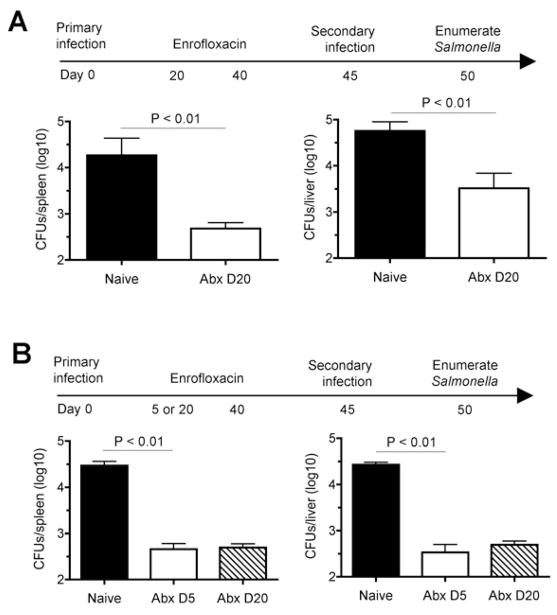
Fig. 1.
Protection against secondary Salmonella infection despite early eradication of primary infection. A. Number of recoverable Salmonella CFUs in the spleen and liver day 5 post-secondary challenge (104 CFUs) in B6.129F1 mice treated with antibiotics beginning day 20 after primary infection (104 CFUs) or control mice treated with antibiotics in parallel without primary infection. B. Number of recoverable Salmonella CFUs in the spleen and liver day 5 post- secondary challenge (104 CFUs) in B6.129F1 mice treated with antibiotics beginning either day 5 or day 20 after primary infection (104 CFUs), or control mice without primary infection. These results are representative of two independent experiments each containing 3–5 mice per group. Bar, one standard error.
To investigate if earlier eradication of primary Salmonella infection would impact protection against recurrent infection, we compared the effects of antibiotic initiation beginning day 5 compared with day 20 after primary infection. We found protection against secondary infection was sustained with eradication of primary infection beginning day 5, and these protective effects were indistinguishable compared with mice treated with antibiotics later (day 20) (Fig. 1B). Importantly, these protective effects could not be attributed to reduced efficiency of antibiotic eradication of virulent S. typhimurium that causes persistent infection in mice containing the resistant allele of Nramp1 because enrofloxacin readily eradicated primary infection within the first 5–7 days after treatment (Fig. 2).
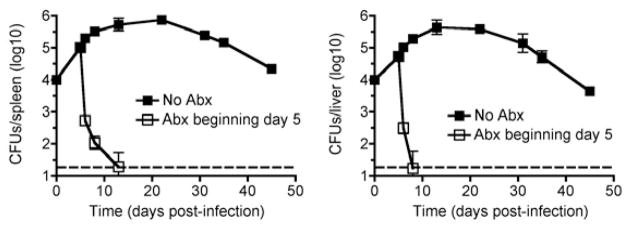
Fig. 2.
Enrofloxacin eradicates persistent S. typhimurium infection in B6.129F1 mice. Number of recoverable Salmonella CFUs in the spleen and liver at the indicated time points after infection for mice with (open squares) or without (filled squares) enrofloxacin (2mg/ml) supplementation in the drinking water beginning day 5 post-infection. These results are representative of two independent experiments each containing 3–5 mice per group per time point. Dotted line, limit of detection.
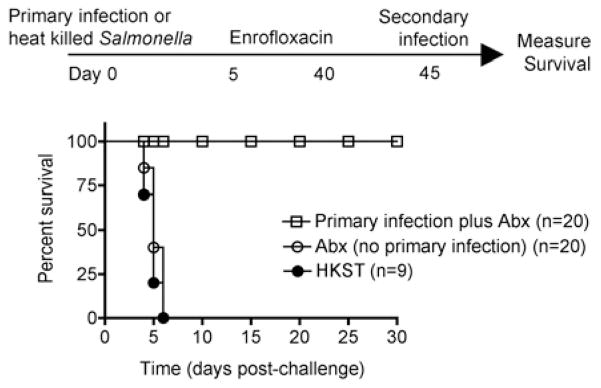
Additional experiments explored the magnitude of these protective effects by enumerating survival following secondary infection with a higher dose of virulent S. typhimurium (106 CFUs) that normally causes lethal infection even in mice containing the resistant allele of Nramp1. These experiments revealed that eradication of primary infection with antibiotics beginning day 5 was sufficient to protect mice from lethal Salmonella infection, while naïve control mice treated with antibiotics in parallel all succumbed within the first week after infection (Fig. 3). These protective effects of primary infection followed by eradication with antibiotics shortly thereafter were specific to live Salmonella infection because the same dose of heat-killed Salmonella used for primary infection (104 CFUs) provided no protective effects (Fig 3). These results demonstrate primary Salmonella infection even with antibiotic eradication primes protection against recurrent infection.
Fig. 3.
Protection against secondary challenge with virulent S. typhimurium despite early eradication of primary infection. Percent survival following secondary challenge with virulent S. typhimurium (106 CFUs) in mice treated with antibiotics beginning 5 days post-primary infection (104 CFUs) (open squares), mice treated with the same dose of heat-killed Salmonella (filled circles), or naïve mice given antibiotics in parallel (open circles). The number of mice in each group is indicated and combined from two to three independent experiments.
3.2. Neither CD4+ nor CD8+ T cells directly mediate protection against recurrent Salmonella infection
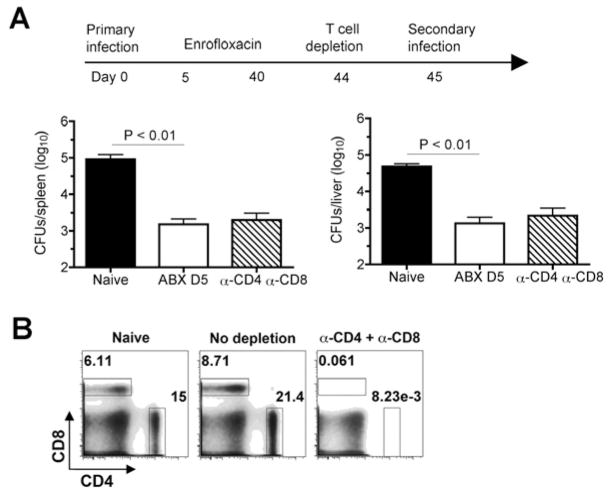
T cells are important mediators of host defense against Salmonella infection because mice with targeted defects in CD4+ and CD8+ T cells show defects in controlling both primary and secondary infection even with attenuated Salmonella mutant strains [21–26]. However, given the intricate cross-regulation between T cells and other immune cell subsets, the use of mice with targeted T cell defects cannot discriminate between whether these cells are required for priming protection by other immune mediators or if T cells directly provide protection. To overcome these limitations and determine the specific requirements for CD4+ and/or CD8+ T cells in protection against recurrent Salmonella infection after eradication of primary infection, we compared the susceptibility of antibiotic-treated mice following CD4+ and/or CD8+ T cell depletion immediately prior to secondary infection. We found CD4+ or CD8+ T cell depletion either alone or in combination had no significant effect on the level of protection primed by early eradication of primary Salmonella infection (Fig. 4A). Since each T cell subset was found to be depleted ≥ 99%, these results cannot be attributed to inefficient T cell depletion (Fig. 4B). Taken together, these results demonstrate CD4+ and CD8+ T cells are non-essential direct mediators of protection against recurrent Salmonella infection.
Fig. 4.
T cell depletion does not impact protection against secondary Salmonella infection conferred by antibiotic eradication of primary infection. A. Number of recoverable Salmonella CFUs in the spleen and liver day 5 post-challenge for mice with CD4+ and CD8+ T cells depleted beginning one day prior to secondary Salmonella challenge (104 CFUs). B. Representative FACS plots demonstrating the efficiency of in vivo CD4+ and CD8+ T cell depletions. The numbers in each plot indicate the percent cells in each gate. These results are combined from two independent experiments containing 7–10 mice per experimental group.
3.3. Protective Salmonella-specific antibodies are primed after early infection eradication
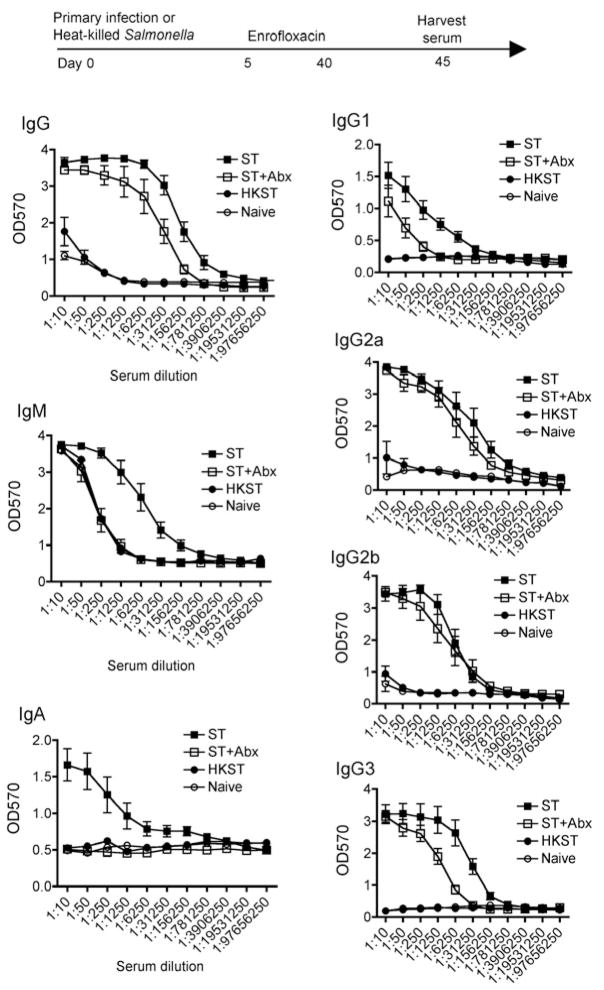
Given the sustained protection against recurrent infection even after CD4+ and CD8+ T cell depletion, and the importance of serum mediated protection especially in mice innately resistance to Salmonella infection [27], we also enumerated the impacts of early eradication of primary infection on the anti-Salmonella serological response. Remarkably, despite antibiotic treatment beginning day 5, total anti-Salmonella IgG in these mice was only modestly reduced compared with mice with persistent infection without antibiotic treatment 45 days after primary infection (Fig 5). Anti-Salmonella antibody was sustained for all IgG isotypes, and for IgG2a and IgG2b, the response between antibiotic treated and untreated controls was nearly indistinguishable (Fig 5). By contrast to IgG, anti-Salmonella IgM and IgA were each not sustained because the background levels in Salmonella infected mice after antibiotic eradication were significantly reduced compared with mice without antibiotic treatment and sustained infection. The anti-Salmonella serological IgG response despite early eradication of primary infection was specifically triggered by live bacteria because the same dose of heat-killed Salmonella (104 bacteria) did not prime a significant increase in antibody titer above the background levels found in naïve control mice (Fig 5).
Fig. 5.
Salmonella-specific IgG is sustained despite early eradication of primary Salmonella infection. Antibody titers of Salmonella-specific IgG, IgM, IgA, IgG1, IgG2a, IgG2b, and IgG3 in the serum of mice 45 days post-infection (104 CFUs) treated with enrofloxacin beginning day 5 (open squares) or without antibiotic treatment (filled squares), mice treated the same dose of heat-killed Salmonella (filled circles), or naïve mice (open circles). These results represent four independent experiments containing 4–6 mice per group.
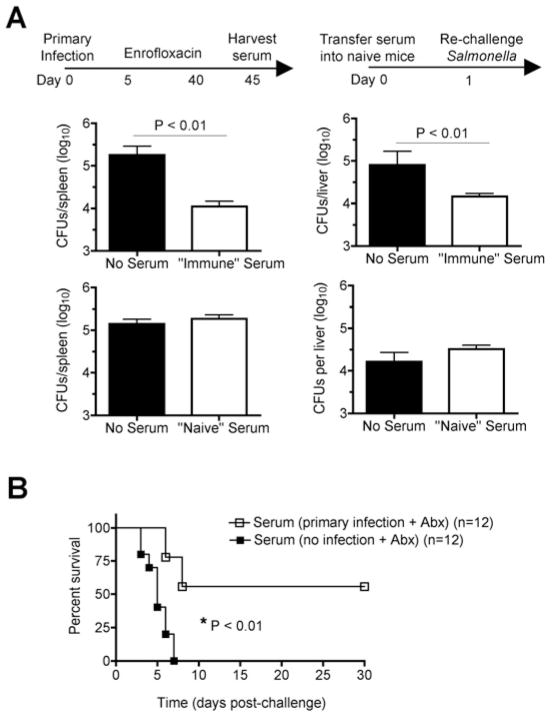
To determine if Salmonella-specific antibody primed after early antibiotic treatment of primary Salmonella infection provides protection against recurrent infection, the impact of adoptively transferred serum on infection susceptibility was enumerated. After challenge with a sub-lethal inocula of virulent Salmonella (104 CFUs), serum from Salmonella infected antibiotic-treated mice conferred significant reductions (≥ 10-fold) in recoverable Salmonella CFUs in the spleen and liver 5 days post-challenge, while serum from naïve mice did not significantly impact infection susceptibility (Fig. 6A). Similarly after challenge with a higher dose of virulent Salmonella (106 CFUs) normally lethal for naïve mice, protection was partially restored with serum from Salmonella infected antibiotic-treated mice while serum from naïve control mice failed to confer protection (Fig. 6B). Together, these results demonstrate Salmonella-specific antibody is primed despite early antibiotic-mediated resolution of primary infection, and that adoptive transfer of serum containing anti-Salmonella antibody confers protection against recurrent infection.
Fig. 6.
Adoptively transferred serum from mice eradicated of primary Salmonella infection confers protection to naïve recipients. A. Number of recoverable Salmonella CFUs in the spleen and liver day 5 after infection with virulent S. typhimurium (104 CFUs) for mice transferred serum from mice eradicated of primary Salmonella or no transfer control mice (top). Number of recoverable Salmonella CFUs in the spleen and liver day 5 post-challenge for mice transferred serum from naïve antibiotic-treated mice or no transfer control mice (bottom). B. Percent survival after infection with virulent S. typhimurium (106 CFUs) in mice transferred serum from mice eradicated of primary Salmonella (open squares) or control mice without primary infection (filled squares). These results are representative of two independent experiments containing 8 – 12 mice per group. Bar, one standard error.
4. Discussion
Given the widespread use of antimicrobials to eradicate and reduce the long-term complications associated with human typhoid, identifying how this therapy impacts of protection against recurrent infection is an important area for investigation. Although human epidemiological data demonstrating reduced attack rates of recurrent infection in individuals where primary typhoid was treated with antibiotics suggest protective immunity is generated [8, 9], these studies are limited by relatively small sample sizes, large heterogeneity in immune responses between individuals, and wide fluctuations in Salmonella inocula during natural human infection even during outbreak-type settings. To more definitively address this question, the impact of antibiotic-mediated clearance of primary Salmonella infection on protective immunity to recurrent infection was investigated using a murine model of persistent Salmonella infection where each of these parameters could be more precisely controlled. We demonstrate that protection is primed against recurrent Salmonella infection and sustained equally whether antibiotics are administered during the early (day 5) or later (day 20) phases of primary Salmonella infection. These results have important implications for treating and preventing recurrent Salmonella infection especially in typhoid endemic areas where re-infection is essentially unavoidable.
Furthermore, using immunological tools and experimental techniques that are more readily performed in rodent models of infection, the mediators of protective immunity primed by antibiotic-mediated eradication of primary Salmonella infection were identified. We demonstrate that protection against recurrent infection is largely mediated by Salmonella-specific antibody because resistance against secondary infection could be transferred with serum containing high titers of Salmonella-specific antibody. These findings are consistent with the demonstration that antibody plays a dominant role in protection against secondary infection in mice containing the resistant allele of Nramp1 that are inherently resistant to Salmonella [27, 28], the protection against human typhoid conferred by the Vi polysaccharide vaccine that primes a T cell-independent, Salmonella-specific serological response [29–33], and the significantly reduced levels of protection against secondary Salmonella infection in B cell (antibody)-deficient mice [12, 34, 35]. Importantly, since B cells are also potent antigen-presenting cells required for the optimal priming of Salmonella-specific Th1 CD4+ cells after infection with live-attenuated strains, the lack of protection against recurrent infection in B cell-deficient mice does not discriminate between the antibody-producing and antigen-presenting roles of these cells [34, 36]. To clarify these different roles, the ability of serum containing Salmonella antibody primed by early eradication of primary infection to transfer protection to naïve mice was demonstrated (Fig. 6). The more modest level of protection achieved with serum transfer compared to intact mice primed by primary Salmonella infection with antibiotic eradiation (Figs. 1, 3 and 6) is most likely due to diminished anti-Salmonella antibodies related to adoptive transfer. However, the potential importance of activated macrophages primed by live Salmonella that may also contribute to this differences in protection cannot be excluded [28, 37]. Nevertheless taken together, these results illustrate the importance of sustained Salmonella-specific antibody in protective immunity against recurrent infection.
In sharp contrast to their role in host defense against primary infection, neither CD4+ nor CD8+ T cells were essential mediators of protective immunity against recurrent infection because depletion of each cell type individually or in combination did not increase susceptibility to secondary Salmonella infection in antibiotic-treated mice [14–16]. Despite these observations, T cells clearly contribute to and play an important role in host defense against Salmonella infection because mice with targeted defects in CD4+ T cells are highly susceptible to and do not eradicate even attenuated strains of Salmonella, and similar defects in host defense against primary Salmonella infection are found with T cell depletion [13, 16, 21–26]. By extension, adoptively transferred T cells primed with attenuated Salmonella together with antibody confers protection in naïve mice, while T cell ablation eliminates protection against secondary infection in mice lacking the resistant allele of Nramp1 [38]. These findings in the mouse model of typhoid are consistent with the clinical observation that recovery from human typhoid and reductions in typhoid-associated complications such as gastrointestinal bleeding or perforation each correlates with the development of cell-mediated immunity [39–41]. Since T cells also play important and critical roles in the maturation and activation of antibody-producing cells, the increased susceptibility in T cell-deficient mice cannot discriminate between whether T cells provide protection indirectly through enhanced and sustained help to antibody-producing cells. Our experiments comparing differences in susceptibility to recurrent infection with T cell ablation immediately prior to secondary infection demonstrate that neither CD4+ nor CD8+ T cells are essential direct mediators of protection against recurrent Salmonella infection.
Protection against recurrent infection demonstrated by reductions in recoverable bacterial CFUs and increased survival despite early antibiotic eradication of primary infection we demonstrate here is consistent with the delayed time to death for antibiotic-treated mice after S. typhimurium infection in mice inherently susceptible to virulent Salmeonlla [13]. Although the level of protection primed by antibiotic eradication of primary infection was sharply reduced compared with that primed by an attenuated Salmonella mutant that causes more persistent primary infection [13], the level of protection we demonstrate using mice inherently more resistant to virulent Salmonella is sharply increased (100% survival in 20 mice) even after challenge with a significantly higher inoculum of virulent Salmonella that is normally lethal even in B6.129F1 mice containing a resistant allele of Nramp1 (Fig. 3). Taken together, these findings suggest vaccination strategies that prime a more robust and sustained serological response compared with primary infection will confer enhanced protection against Salmonella infection.
Acknowledgments
The authors thank Dr. Stephen McSorley for helpful discussions. This research was supported by National Institutes of Health grants R01AI087830 (NIAID), the Minnesota Vikings Children’s Fund, the Minnesota Medical Foundation, and University of Minnesota Grant-in-Aid.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhutta ZA, Khan IA, Shadmani M. Failure of short-course ceftriaxone chemotherapy for multidrug-resistant typhoid fever in children: a randomized controlled trial in Pakistan. Antimicrob Agents Chemother. 2000;44:450–452. doi: 10.1128/aac.44.2.450-452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gotuzzo E, Morris JG, Jr, Benavente L, Wood PK, Levine O, Black RE, Levine MM. Association between specific plasmids and relapse in typhoid fever. Journal of clinical microbiology. 1987;25:1779–1781. doi: 10.1128/jcm.25.9.1779-1781.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Islam A, Butler T, Kabir I, Alam NH. Treatment of typhoid fever with ceftriaxone for 5 days or chloramphenicol for 14 days: a randomized clinical trial. Antimicrob Agents Chemother. 1993;37:1572–1575. doi: 10.1128/aac.37.8.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yew FS, Chew SK, Goh KT, Monteiro EH, Lim YS. Typhoid fever in Singapore: a review of 370 cases. J Trop Med Hyg. 1991;94:352–357. [PubMed] [Google Scholar]
- 5.Islam A, Butler T, Ward LR. Reinfection with a different Vi-phage type of Salmonella typhi in an endemic area. The Journal of infectious diseases. 1987;155:155–156. doi: 10.1093/infdis/155.1.155. [DOI] [PubMed] [Google Scholar]
- 6.Wain J, Hien TT, Connerton P, Ali T, Parry CM, Chinh NT, Vinh H, Phuong CX, Ho VA, Diep TS, Farrar JJ, White NJ, Dougan G. Molecular typing of multiple-antibiotic-resistant Salmonella enterica serovar Typhi from Vietnam: application to acute and relapse cases of typhoid fever. Journal of clinical microbiology. 1999;37:2466–2472. doi: 10.1128/jcm.37.8.2466-2472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N Engl J Med. 2002;347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 8.Dupont HL, Hornick RB, Snyder MJ, Dawkins AT, Heiner GG, Woodward TE. Studies of immunity in typhoid fever. Protection induced by killed oral antigens or by primary infection. Bull World Health Organ. 1971;44:667–672. [PMC free article] [PubMed] [Google Scholar]
- 9.Marmion DE, Naylor GR, Stewart IO. Second attacks of typhoid fever. J Hyg (Lond) 1953;51:260–267. doi: 10.1017/s0022172400015680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaines S, Tully JG, Tigertt WD. Studies on infection and immunity in experimental typhoid fever. II. Susceptibility of recovered animals to re-exposure. J Exp Med. 1960;112:1023–1036. doi: 10.1084/jem.112.6.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoiseth SK, Stocker BA. Aromatic–dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 12.McSorley SJ, Jenkins MK. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar typhimurium. Infection and immunity. 2000;68:3344–3348. doi: 10.1128/iai.68.6.3344-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin A, Baraho-Hassan D, McSorley SJ. Successful treatment of bacterial infection hinders development of acquired immunity. J Immunol. 2009;183:1263–1270. doi: 10.4049/jimmunol.0900772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luu RA, Gurnani K, Dudani R, Kammara R, van Faassen H, Sirard JC, Krishnan L, Sad S. Delayed expansion and contraction of CD8+ T cell response during infection with virulent Salmonella typhimurium. J Immunol. 2006;177:1516–1525. doi: 10.4049/jimmunol.177.3.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mittrucker HW, Kohler A, Kaufmann SH. Characterization of the murine T-lymphocyte response to Salmonella enterica serovar Typhimurium infection. Infection and immunity. 2002;70:199–203. doi: 10.1128/IAI.70.1.199-203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johanns TM, Ertelt JM, Rowe JH, Way SS. Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection. PLoS pathogens. 2010;6 doi: 10.1371/journal.ppat.1001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McSorley SJ, Asch S, Costalonga M, Reinhardt RL, Jenkins MK. Tracking salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity. 2002;16:365–377. doi: 10.1016/s1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 18.Johanns TM, Ertelt JM, Lai JC, Rowe JH, Avant RA, Way SS. Naturally occurring altered peptide ligands control Salmonella-specific CD4+ T cell proliferation, IFN-gamma production, and protective potency. J Immunol. 2010;184:869–876. doi: 10.4049/jimmunol.0901804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasan A, Nanton M, Griffin A, McSorley SJ. Culling of activated CD4 T cells during typhoid is driven by Salmonella virulence genes. J Immunol. 2009;182:7838–7845. doi: 10.4049/jimmunol.0900382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunningham AF, Gaspal F, Serre K, Mohr E, Henderson IR, Scott-Tucker A, Kenny SM, Khan M, Toellner KM, Lane PJ, MacLennan IC. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J Immunol. 2007;178:6200–6207. doi: 10.4049/jimmunol.178.10.6200. [DOI] [PubMed] [Google Scholar]
- 21.Guilloteau L, Buzoni-Gatel D, Bernard F, Lantier I, Lantier F. Salmonella abortusovis infection in susceptible BALB/cby mice: importance of Lyt-2+ and L3T4+ T cells in acquired immunity and granuloma formation. Microb Pathog. 1993;14:45–55. doi: 10.1006/mpat.1993.1005. [DOI] [PubMed] [Google Scholar]
- 22.Hess J, Ladel C, Miko D, Kaufmann SH. Salmonella typhimurium aroA- infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J Immunol. 1996;156:3321–3326. [PubMed] [Google Scholar]
- 23.Lo WF, Ong H, Metcalf ES, Soloski MJ. T cell responses to Gram-negative intracellular bacterial pathogens: a role for CD8+ T cells in immunity to Salmonella infection and the involvement of MHC class Ib molecules. J Immunol. 1999;162:5398–5406. [PubMed] [Google Scholar]
- 24.Nauciel C. Role of CD4+ T cells and T-independent mechanisms in acquired resistance to Salmonella typhimurium infection. J Immunol. 1990;145:1265–1269. [PubMed] [Google Scholar]
- 25.Sinha K, Mastroeni P, Harrison J, de Hormaeche RD, Hormaeche CE. Salmonella typhimurium aroA, htrA, and aroD htrA mutants cause progressive infections in athymic (nu/nu) BALB/c mice. Infection and immunity. 1997;65:1566–1569. doi: 10.1128/iai.65.4.1566-1569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weintraub BC, Eckmann L, Okamoto S, Hense M, Hedrick SM, Fierer J. Role of alphabeta and gammadelta T cells in the host response to Salmonella infection as demonstrated in T-cell-receptor-deficient mice of defined Ity genotypes. Infection and immunity. 1997;65:2306–2312. doi: 10.1128/iai.65.6.2306-2312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenstein TK, Killar LM, Sultzer BM. Immunity to infection with Salmonella typhimurium: mouse-strain differences in vaccine- and serum-mediated protection. The Journal of infectious diseases. 1984;150:425–435. doi: 10.1093/infdis/150.3.425. [DOI] [PubMed] [Google Scholar]
- 28.Collins FM. Vaccines and cell-mediated immunity. Bacteriological reviews. 1974;38:371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acharya IL, Lowe CU, Thapa R, Gurubacharya VL, Shrestha MB, Cadoz M, Schulz D, Armand J, Bryla DA, Trollfors B, et al. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella typhi. A preliminary report. N Engl J Med. 1987;317:1101–1104. doi: 10.1056/NEJM198710293171801. [DOI] [PubMed] [Google Scholar]
- 30.Fraser A, Paul M, Goldberg E, Acosta CJ, Leibovici L. Typhoid fever vaccines: systematic review and meta-analysis of randomised controlled trials. Vaccine. 2007;25:7848–7857. doi: 10.1016/j.vaccine.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 31.Klugman KP, Gilbertson IT, Koornhof HJ, Robbins JB, Schneerson R, Schulz D, Cadoz M, Armand J. Protective activity of Vi capsular polysaccharide vaccine against typhoid fever. Lancet. 1987;2:1165–1169. doi: 10.1016/s0140-6736(87)91316-x. [DOI] [PubMed] [Google Scholar]
- 32.Levine MM, Tacket CO, Sztein MB. Host-Salmonella interaction: human trials. Microbes Infect. 2001;3:1271–1279. doi: 10.1016/s1286-4579(01)01487-3. [DOI] [PubMed] [Google Scholar]
- 33.Yang HH, Wu CG, Xie GZ, Gu QW, Wang BR, Wang LY, Wang HF, Ding ZS, Yang Y, Tan WS, Wang WY, Wang XC, Qin M, Wang JH, Tang HA, Jiang XM, Li YH, Wang ML, Zhang SL, Li GL. Efficacy trial of Vi polysaccharide vaccine against typhoid fever in south-western China. Bull World Health Organ. 2001;79:625–631. [PMC free article] [PubMed] [Google Scholar]
- 34.Mastroeni P, Simmons C, Fowler R, Hormaeche CE, Dougan G. Igh-6(−/−) (B-cell-deficient) mice fail to mount solid acquired resistance to oral challenge with virulent Salmonella enterica serovar typhimurium and show impaired Th1 T-cell responses to Salmonella antigens. Infection and immunity. 2000;68:46–53. doi: 10.1128/iai.68.1.46-53.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mittrucker HW, Raupach B, Kohler A, Kaufmann SH. Cutting edge: role of B lymphocytes in protective immunity against Salmonella typhimurium infection. J Immunol. 2000;164:1648–1652. doi: 10.4049/jimmunol.164.4.1648. [DOI] [PubMed] [Google Scholar]
- 36.Ugrinovic S, Menager N, Goh N, Mastroeni P. Characterization and development of T-Cell immune responses in B-cell-deficient (Igh-6(−/−)) mice with Salmonella enterica serovar Typhimurium infection. Infection and immunity. 2003;71:6808–6819. doi: 10.1128/IAI.71.12.6808-6819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Killar LM, Eisenstein TK. Immunity to Salmonella typhimurium infection in C3H/HeJ and C3H/HeNCrlBR mice: studies with an aromatic-dependent live S. typhimurium strain as a vaccine. Infection and immunity. 1985;47:605–612. doi: 10.1128/iai.47.3.605-612.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mastroeni P, Villarreal-Ramos B, Hormaeche CE. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infection and immunity. 1993;61:3981–3984. doi: 10.1128/iai.61.9.3981-3984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajagopalan P, Kumar R, Malaviya AN. A study of humoral and cell-mediated immune response following typhoid vaccination in human volunteers. Clin Exp Immunol. 1982;47:275–282. [PMC free article] [PubMed] [Google Scholar]
- 40.Rajagopalan P, Kumar R, Malaviya AN. Immunological studies in typhoid fever. II. Cell-mediated immune responses and lymphocyte subpopulations in patients with typhoid fever. Clin Exp Immunol. 1982;47:269–274. [PMC free article] [PubMed] [Google Scholar]
- 41.Sarma VN, Malaviya AN, Kumar R, Ghai OP, Bakhtary MM. Development of immune response during typhoid fever in man. Clin Exp Immunol. 1977;28:35–39. [PMC free article] [PubMed] [Google Scholar]