Abstract
Nucleotide excision repair (NER) is the primary defense against the DNA damage implicit in skin cancer formation and is negatively affected by chronic exposure to UVB radiation. However, in-situ and in-vitro studies consistently yield equivocal results when addressing individual DNA repair capacity and melanoma susceptibility. The primary objective of this study was to determine if individual global NER capacity is a risk factor for melanoma formation in a prominent UVB inducible melanoma model, hybrid Xiphophorus fishes. After neonatal UVB irradiation, adult tumor-bearing and tumor-free fish were given a challenge UVB dose and (6-4) photoproduct repair was quantified in individual fish at 24 h using radioimmunoassay. Despite considerable inter-individual variation in repair capacity, ranging from 13% to 91%, we found no difference in mean NER capacity between fish with and without melanomas, thus detaching global NER from melanomagenesis. Furthermore, despite epidemiological data indicating that sex and age are important risk factors underlying melanoma susceptibility, we found no difference in mean NER rates among the sexes or as a function of age. We conclude with a discussion of the apparent paradox of how inter-individual variation in NER is not a risk factor given the clear evidence that DNA damage underlies melanoma susceptibility.
INTRODUCTION
Heredity can be a strong predisposing factor in human melanoma (1,2). Major heritable risk factors include a high frequency of normal and atypical melanocytic nevi, hair and skin type, and a familial history of melanoma. In addition to additive genetic factors, environmental exposure to solar ultraviolet radiation (UVR) is clearly important in determining individual melanoma susceptibility. However, the involvement of UVR is complicated and includes multiple aspects of exposure, including site, duration, frequency, and time of exposure (childhood or adulthood) (3). A further complication arises from the fact that different wavelengths of UVR (UVA, UVB) can result in different types of photoproducts in DNA and other cellular constituents. Both UVA (320–400 nm) and UVB (280–320 nm) result in the production of reactive oxygen species (ROS), which can have multiple deleterious effects including DNA damage. However, because the absorbance spectrum of DNA extends well into the UVB range, DNA directly absorbs UVB photon energy. A small portion of this absorbed energy is converted into covalent changes in DNA structure, the most prominent of which include the cyclobutane pyrimidine dimer (CPD) and (6-4) pyrimidine dimer [(6-4)PD]. Although the efficacy of UVA and UVB in initiating melanomas has been debated (3–6), there is little doubt that pyrimidine dimers are in some way involved. This is evidenced by the rare genetic disorder, Xeroderma pigmentosum (XP), which is characterized by an individual’s inability to repair bulky adducts in DNA induced primarily by UVB using nucleotide excision repair (NER). XP patients are 1000 times more likely to develop melanoma than individuals with normal DNA repair capacity (hereafter DRC) (7).
In order to increase our understanding of sunlight-induced DNA damage and its role in melanoma formation in the human population, it is critical to develop and test hypotheses that correlate an individual’s DRC with melanoma susceptibility using appropriate animal models. Since its inception in the late 1920s (8,9), the Xiphophorus melanoma model has proven to be a valuable and relevant animal model for human carcinogenesis (10). Melanoma development in Xiphophorus fishes is determined, at least in part, by the constitutive activation and overexpression of the Xiphophorus melanoma receptor tyrosine kinase (Xmrk), a fish paralog of the human epidermal growth factor receptor (EGFR) and the dysregulation of an as yet to be determined tumor suppressor gene, such as Cdkn2X (P16/INK4A homolog) (10). As with human melanoma formation (11,12), the activation of EGFR stimulates numerous downstream signaling cascades that result in altered cell cycle control and proliferation (13).
In order to avoid the consequences of sunlight induced DNA damage, including mortality, mutagenesis and carcinogenesis, organisms have evolved diverse and redundant DNA repair mechanisms that combine to reduce the amount of CPDs and (6-4)PDs in their genomes (for review 14). Fish utilize primarily two pathways to remove direct damage: (i) Photoenzymatic repair (PER) is a fairly simple, single enzyme reaction (photolyase + co-factors) that splits CPDs and (6-4)PDs in situ in the presence of visible/blue light (15); (ii) Nucleotide excision repair (NER) is a genetically complex and phenotypically diverse system directly and indirectly influenced by approximately 20–30 proteins, including those involved in DNA damage recognition, excision, re-synthesis and ligation as well as many genes that regulate NER and its accessibility to damaged DNA in chromatin (16).
Recently, we examined the wavelength dependence of UVR induced melanoma in a well-studied Xiphophorus hybrid model (Sp-couchianus backcross model). We found that neonatal exposure to UVB irradiation resulted in high frequencies of melanomas in adulthood (~ 12–14 month old animals). However, UVA irradiation resulted in adult melanoma frequencies that were not significantly different from the unirradiated control fish (5). Furthermore, previous work on this same Xiphophorus model found that neonatal UVB irradiation immediately followed by exposure to visible/blue light, which allows for rapid repair of DNA damage via PER, reduced the incidence of melanomas by 50% (17). This observation is not limited to the Sp-couchianus backcross model as other Xiphophorus hybrid models treated in this same fashion resulted in the reduction of melanomas to background (i.e., spontaneous) levels (18). Collectively, these results strongly suggest that the direct absorption of UVB wavelengths by DNA and the formation of CPDs and (6-4)PDs appear to be essential for melanoma induction in this model. Although UVB can result in ROS production, the fact that neonatal UVA exposure does not induce melanomas and efficient PER of direct damage decreases melanoma frequencies, suggests a decreased role of ROS in the etiology of Xiphophorus melanomas.
Previously, we found that F1 hybrids of the Sp-couchianus model have remarkably poor NER of UV-induced (6-4)PDs relative to the parental species of the X. maculatus and X. couchianus hybrid scheme (X. maculatus ~48 %, X. couchianus ~64 %, F1 hybrids ~18 % (6-4)PD removal 18 hrs post irradiation), whereas NER of UV-induced CPDs in the F1 hybrids was not different from the parental species (19). Most intriguing, was the finding that in the other two hybrid models examined, which are intractable to UVR induced melanomas, there was no difference between NER capacity of (6-4)PDs or CPDs for either of the parental species or their F1 hybrids. These observations led us to the current study and the hypothesis that deficient global NER of (6-4)PDs may underlie the significant increase in melanoma frequency of first generation backcross (BC1) hybrids in the Sp-couchianus model after neonatal UVB irradiation. The goals of this study were to (i) Determine if deficient global NER was a risk factor for individual melanoma susceptibility (ii) Determine if neonatal UVB irradiation resulted in irreversible damage to molecular elements of global NER that could explain increased melanomagenesis and (iii) Examine if gender or age differences might correspond to the inter-individual variation in global NER and melanoma susceptibility.
MATERIALS AND METHODS
Animals and breeding
Parental strains were originally obtained from the Xiphophorus Genetic Stock Center located at Texas State University in San Marcos, TX and have been maintained in our facility since 2000. The X. couchianus stock was collected in 1961 from the Huasteca canyon (Nuevo Leon, Mexico) and is currently in its 71st generation of full sibling inbreeding. As a result, individuals are believed to homozygous at most, if not all, loci. Progenitors of X. maculatus strain Jp 163 B were obtained from a single pregnant female collected in 1939 from the Rio Jamapa (Veracruz, Mexico) and as a result this stock is highly inbred. The mating scheme used in this study to generate Sp-couchianus BC1 individuals was F1 (X. couchianus x X. maculatus 163B) hybrid females bred to X. couchianus males. Therefore, the genetic background of BC1 individuals is primarily that of X. couchianus and not X. maculatus 163B (i.e., ~75% X. couchianus and 25% X. maculatus). Because a critical component for melanoma formation in Xiphophorus is the presence of melanocytes and the Xmrk oncogene (20), non-melanized BC1 individuals (i.e., fish that did not inherit the spotted side (Sp) pattern or Xmrk) were discarded in accordance with standard operating procedure for studies within the Xiphophorus melanoma model. The generation of BC1 animals involves breeding F1 hybrids, which are hemizygous for Xmrk, to X. couchianus animals that lack Xmrk, thus in accordance with Mendelian principles approximately fifty percent of the BC1 offspring are discarded because these fish lack melanocytes and Xmrk and, therefore, do not form melanomas (for review see 13).
Experimental design
We designed an irradiation apparatus in which un-anesthetized, free-swimming fish are exposed in a UV transparent (UVT) chamber to two Philips TL01 UVB lamps (peak λ = 313 nm) from both sides simultaneously (4 lamps total). This irradiation apparatus enhances exposure to the caudal, pectoral and dorsal fins in free-swimming fish (dorsal and pectoral fins collapse in anesthetized fish), which are the tissues used to quantify DNA damage. Irradiance measurements were made using a UVB-1 probe coupled to a Model IL 1400A radiometer (International Light Technologies, Peabody, MA, USA) at the distance measured to the center axis of the irradiation chamber. Negligible attenuation was observed by the UVT Plexiglas© and the small amount of water (~70 mL) in the UVT chamber.
Xiphophorus Sp-couchianus BC1 broods were randomly assigned to receive either neonatal UVB irradiation or not (control group). Neonate UVB irradiation protocol was identical to that of Mitchell and colleagues (5). In brief, UVB irradiation was administered on day 5 postpartum and thereafter for 5 consecutive days. During this treatment period, all animals were housed in the dark except during treatment. Fry were exposed for 8 min 45 sec at a fluence rate of 12.2 J/m2/sec at the same time of day on each of the 5 days. The individual dose was 6.4 kJ/m2. After the UVB irradiations were concluded, animals were communally housed by brood and monitored weekly for melanoma formation. At 13 to 14 months of age, we randomly chose a subset of tumor-bearing fish (TBF) and non-tumor-bearing fish (NTBF) from both the neonatal UVB treatment and control treatment to examine individual global NER. All adult fish were exposed to a challenge dose of 6.4 kJ/m2 using the same irradiation apparatus. A small section of the caudal fin was excised from anesthetized fish immediately after exposure (T0). The fish were returned to individual aquaria and allowed to recover for 24 h in the dark, in order to prevent PER, at which time they were sacrificed using excess anesthetic (i.e., 0.06% tricaine methanesulfonate) and the remaining caudal and dorsal fin tissues harvested, snap frozen and stored for subsequent radioimmunoassay (RIA). Care was taken to ensure that all fin tissues used in the RIA did not contain melanin, which increases the repeatability of RIA measurements and eliminates the possibility of melanoma formation in the fins of Sp-couchianus BC1 individuals.
RIA and photoproduct measurements
A standard DNA isolation kit (Qiagen Inc., Valencia, CA, USA) was used to purify DNA from fin tissue collected at T0 and T24 h. DNA concentrations were determined after heat denaturation with a ND-1000 Nanodrop® spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). RIA was used to measure (6-4)PDs in isolated DNA. Due to the small size of individual BC1 fish we were not able to assay both (6-4)PDs and CPDs photoproducts. This could have been accomplished with pooling individuals, however, a primary goal of this study was to establish individual global NER capacity and melanoma susceptibility therefore we opted not to do this. We chose to measure (6-4)PDs rather than CPDs because (i) the earlier NER study found that (6-4)PDs and not CPDs were poorly repaired in F1 hybrids (19) and (ii) the greater distortion of this photoproduct and rate of repair when compared to CPDs. Detailed methodology of the RIA, including the specificities of this RIA, is described in (21,22). Briefly, 2–5 μg of heat-denatured sample DNA were incubated with 5–10 pg of poly(dA):poly(dT) (labeled to >5 × 108 cpm/μg by nick translation with 32P-dTTP) in 10 mM Tris (pH 8.0), 1 mM EDTA, 150 mM NaCl, and 0.2% gelatin (Bovine Type A; Sigma-Aldrich, St. Louis, MO, USA). Antiserum was added at a dilution that yields optimal binding (40–60%) to labeled ligand. After 3 h incubation at 37°C the immune pellet was precipitated for 2 d at 4°C with goat anti-rabbit immunoglobulin and normal rabbit serum. The immune complex was centrifuged at 3700 rpm for 45 min at 10°C and the supernatant discarded. The pellet was dissolved in 100 μL NCS tissue solubilizer, mixed with 4 mL ScintiSafe (Thermo Fisher Scientific) containing 0.1% glacial acetic acid and quantified with a 1600 TR liquid scintillation analyzer (Packard Instruments, Downers Grove, IL, USA). Sample inhibitions were extrapolated through a standard (dose response) curve to determine the number of photoproducts in 106 bases and normalized to 0 h. Rates of photoproduct induction were previously quantified using HPLC/MS-MS (i.e., 0.6 P (6-4)PDs/mb/J/m2).
Statistics
Two-tailed unpaired t tests were used to determine if there was a difference in individual global NER of TBF and NTBF in the control and UVB irradiated backcross hybrids. Subsequently, we combined TBF and NTBF for each treatment group and ran another unpaired t test to determine if neonatal UVB irradiation influenced an individual’s ability to repair DNA using NER machinery. Examinations of age and gender differences in individual global NER were conducted on the UVB treatment group alone due to the lack of sufficient males in the control group. Within the UVB treatment group, potential differences in individual global NER as a result of age or gender were determined by unpaired t tests. All statistical analyses were performed using Graphpad PrismR Ver. 5.0a (Graphpad Software, La Jolla, CA, USA) and the statistical assumptions of unpaired t tests were met (e.g., homogeneity of variances).
RESULTS
Individual repair capacity was not a risk factor for melanoma
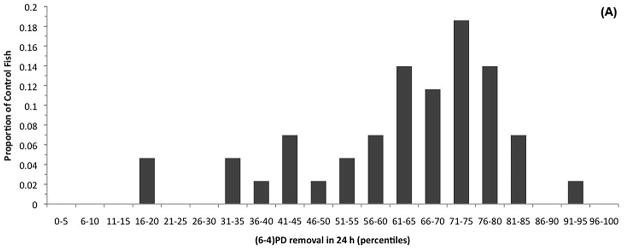
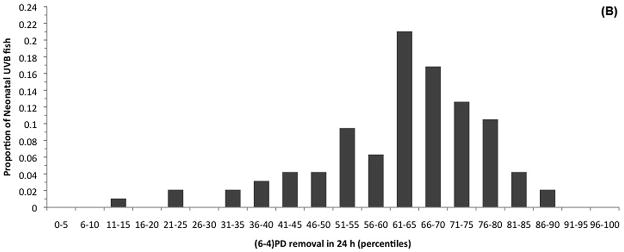
The primary goal of this study was to determine if global NER was correlated with melanoma susceptibility in the primary Xiphophorus UVR melanoma model, Sp-couchianus. Individual global NER was quantified in adult tumor-bearing fish (TBF) and non-tumor-bearing fish (NTBF) that were exposed to UVB between 5 and 10 days of age or not exposed to UVB (control). Global NER capacity was expressed as a percentage of the (6-4)PDs removed in 24 h after a single challenge dose of UVB was administered to adult animals. We found that ~62 % of the (6-4)PDs were repaired after 24 h and that there was no significant difference in global NER between TBF and NTBF in either the animals treated with UVB as neonates or the control animals (Table 1). Given the lack of differences in global NER capacity, we calculated the proportion of fish with DRC values within 5% increments to help assess the distribution of global NER among the control fish and among those fish exposed to UVB as neonates (Figure 1). It is noteworthy that there is a broad range of global NER capacity and that this type of distribution was characteristic of all subsets of the entire dataset (Table 1, Table 2). The fact that animals with 13.2 % global NER and 88.5% global NER both developed melanomas suggests that UVB induced melanomagenesis in Xiphophorus is not related to adult global NER.
Table 1.
(6-4)PD excision capacities of tumor bearing and non-tumor bearing Xiphophorus backcross hybrids (BC1)
| Treatment | Fish | % (6-4)PD excision
|
N | t statistic, df | Probability | |||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Median | Average ± SEM | |||||
| Control
|
||||||||
| NTBF | 18.8 | 84.9 | 67.3 | 63.8 ± 3.2 | 27 | 0.358, 41 | P = 0.722 | |
| TBF | 20.2 | 91.3 | 66.3 | 60.9 ± 4.7 | 16 | |||
| UVB
|
||||||||
| NTBF | 20.8 | 85.1 | 63.4 | 61.4 ± 2.4 | 39 | 0.548, 93 | P = 0.585 | |
| TBF | 13.2 | 88.5 | 62.9 | 63.1 ± 1.9 | 56 | |||
NTBF: Non-tumor bearing fish; TBF: Tumor bearing fish
Figure 1.
(6-4)PD excision capacity percentiles for the control (A) and neonatal UVB irradiated BC1 fish (B). There was considerable inter-individual variation in (6-4)PD removal in a 24 hour period, however, the degree of variation was not significantly different between the control and neonatal UVB irradiated groups [F(1,136) = 1.445, P = 0.144). It is noteworthy that a portion of the melanomas in the UVB irradiated group (B) are likely spontaneous melanomas (i.e., not induced via UVB irradiation), which would contribute to the similarity in the variances of the two data sets.
Table 2.
(6-4)PD excision capacities of non-irradiated and UVB irradiated Xiphophorus backcross hybrids (BC1)
| Treatment | % (6-4)PD excision
|
N | t statistic, df | Probability | |||
|---|---|---|---|---|---|---|---|
| Min | Max | Median | Average ± SEM | ||||
| Control | 18.8 | 91.3 | 67.3 | 62.7 ± 2.6 | 43 | 0.101, 136 | P = 0.92 |
| UVB | 13.2 | 88.5 | 63.0 | 62.4 ± 1.5 | 95 | ||
DNA repair capacity was not affected by early life exposure to UVB
We recently documented that neonatal UVB irradiation of BC1 Xiphophorus Sp-couchianus hybrids significantly increased the melanoma frequency when compared with the melanoma frequency in non-irradiated animals or in animals exposed to UVA as neonates (5). Because repeated exposure to UVB can have negative effects on cellular function, including DNA repair (23,24), we were interested to know if this increase in adult melanomagenesis of animals exposed to repeated doses of UVB as neonates was related to deficient DRC, which could exacerbate the effects of UVB irradiation. We found that adult global NER capacity was not affected by neonatal UVB exposure as animals in both the control and UVB treatment groups repaired about 62% of the (6-4)PDs after 24 h (Table 2). Therefore, repeated neonatal UVB irradiation does not significantly affect the functions, coding sequences or expression of the proteins involved in the NER pathway.
Individual repair capacity is not correlated with gender or age
In the U.S., the rates of cutaneous melanoma vary dramatically with age and gender. According to National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program, melanoma incidence is greater in females than males during 20 to 40 years of age, however, after 45 years of age this is reversed with melanomas being more frequent in men than women thereafter (25). This epidemiological data indicates that sex and age are important risk factors underlying melanoma susceptibility. In skin cancers, more generally, a number of studies have indicated that there is an inverse relationship between age and DRC (for discussion see 26). Despite this, the leading animal models of melanoma, Xiphophorus hybrids and HGF/SF transgenic mice, have not investigated DRC with respect to sex or age. We found that male and female Sp-couchianus BC1 hybrids did not differ in their global NER capacity (Table 3). In the UVB irradiated dataset, we noted that TBF were significantly younger than NTBF (days; mean ± SE; TBF: 406 ± 8.7, NTBF: 441 ± 8.3). However, despite this significant difference in their ages, TBF and NTBF were not different in terms of their ability to repair (6-4)PDs (Table 1). Furthermore, although it is difficult to measure NER in young fish due to their small size, our preliminary results of pooled 10 day old Sp-couchianus BC1 animals suggest that they are not different from adults in the rate of (6-4)PD repair after receiving an equivalent UVB dose (i.e., 58.5 % (6-4)PDs removal at 18 h post irradiation). Therefore, in addition to sex, it is unlikely that there are appreciable differences in global NER capacity as a function of age in Xiphophorus.
Table 3.
Male and female (6-4)PD excision capacities in UVB irradiated Xiphophorus backcross hybrids (BC1)
| Sex | % (6-4)PD excision
|
N | t statistic, df | Probability | |||
|---|---|---|---|---|---|---|---|
| Min | Max | Median | Average ± SEM | ||||
| Male | 30.8 | 88.5 | 61.9 | 62.6 ± 3.3 | 19 | 0.060, 127 | P = 0.952 |
| Female | 18.8 | 91.3 | 65.1 | 62.4 ± 1.4 | 110 | ||
DISCUSSION
Ultraviolet radiation is the primary environmental risk factor for skin carcinogenesis, with UVB exposure being particularly relevant in the etiology of cutaneous melanoma formation (3,5). Given the direct absorption of UVB by DNA and the clear importance of genomic integrity in avoiding melanomas (XP patients), it is surprising that DNA repair studies examining the removal of sunlight induced DNA damage and melanoma susceptibility are conflicting (for reviews see 27,28). Here we used Xiphophorus Sp-couchianus BC1 hybrids, a model that is susceptible to UVB induced melanomagenesis, and found that individual DRC does not correlate with melanoma susceptibility. Specifically, adult animals with and without cutaneous melanomas did not differ in their removal of UVB induced (6-4)PDs using global NER. Furthermore, global NER capacity was not affected by intermittent daily acute neonatal exposures that approached a lethal threshold because (6-4)PD repair was not significantly different between UVB-irradiated and unirradiated fish. Finally, despite the rapid rise of melanomas in older men compared to younger men and women in the United States (29,30), we found individual global NER was not significantly different between the sexes or as a function of age in this melanoma model. Collectively, this work corroborates a recent comprehensive in-vitro NER study that found similar excision rates of UV induced photoproducts in human melanocytes and several melanoma cell lines (31).
The impetus for this study was derived from our earlier observation of drastically reduced NER repair of (6-4)PDs in Sp-couchianus F1 adult hybrids after UVB irradiation (F1 hybrids ~18 % (6-4)PD removal 18 hrs post irradiation; 19). In interpreting these results it was suggested that the combination of individual NER components from two different species (X. maculatus and X. couchianus) is detrimental to the function of the multi-component, sequential pathway that characterizes NER. From these results it was speculated that further hybridization and the generation of BC1 fish would lead to even greater decreases in DRC and potentially account for the increased melanoma susceptibility of UVB irradiated BC1 fish. However, the results of the current study found that the global NER capacity of Sp-couchianus BC1 fish is similar to both parental species (X. maculatus shows ~48 % and X. couchianus ~64 % (6-4)PD removal 18 hrs post irradiation; (19). Although counterintuitive at first, the backcross hybridization process, in which the F1 fish is selectively bred back to one of the parental species, actually results in ‘less’ of a hybrid animal. In the case of the Sp-couchianus model, the predominant genetic makeup of BC1 fish is X. couchianus because this is the parental species mated to the F1 individuals. Therefore, the BC1 global NER rates in this study suggest that the greater proportion of X. couchianus repair components in BC1 as compared to F1 may account for the restoration of global NER efficiency with respect to (6-4)PD removal. It is noteworthy that we do not investigate transcription coupled repair (TCR), which could be involved in melanomagenesis (32). Although, TCR and global NER are very similar with the exception of damage recognition (33), future studies addressing TCR and melanomagenesis in appropriate animal models are warranted.
We believe that the current ideology of distinct pathways to melanoma formation may underlie much of the controversy involving individual DRC and melanoma susceptibility over the last decade. The “divergence pathway model” suggests that the site of a tumor and the nature of the UV exposure associated with that tumor are paramount in differentiating the etiologies of melanoma (34). Cutaneous melanomas on chronically sun exposed sites (head and neck melanomas) are characterized by a distinct set of mutations, including UV signature mutations (i.e., C→T and CC→TT transition mutations), whereas cutaneous melanomas on intermittently sun exposed sites (truncal melanomas), lack UV signature mutations but do have some underlying germline susceptibility (e.g., BRAF mutations) (35). The role of UV exposure in truncal melanomas is less transparent, however, it is generally accepted that early life acute erythemal sun exposures are important (3). Case-control DNA repair studies find that basal cell carcinoma and squamous cell carcinoma patients, two skins cancers associated with chronic UV exposure and displaying high levels of UVB signature mutations, show reduced DRC after treatment with UVB (36) and 4-NQO, a UVR mimetic chemical (37). However, these studies found that DRC in cutaneous melanoma patients was not different from control subjects. Remarkably, in silico searches reveal that very few DNA repair studies of cutaneous melanoma patients consider the site of tumor formation, which if there are DRC disparities between chronically exposed and intermittently exposed sites, could account for the equivocal results of individual DRC and melanoma susceptibility in humans. In the only study we could find that examined DRC with respect to site of tumor, Wei and colleagues (38) found that patients with melanomas on chronically exposed skin did have significantly lower DRC than patients with tumors on unexposed skin (truncal melanomas). This study highlights the importance of documenting tumor location in attempting to correlate individual DRC as a risk factor for melanomagenesis and is representative of modern DNA repair studies given our current knowledge of the distinct pathways to melanomas.
We believe that melanomas induced in Xiphophorus BC1 hybrids after intermittent neonatal UVB exposure are analogous to truncal melanomas rather than head and neck melanomas that arise after repeated UVR exposures. There are several lines of evidence to support this assertion. First, animals in this model do have germline susceptibility, a characteristic of truncal melanomas, as they are BC1 individuals that are genetically predisposed for melanoma formation. Second, experimental animals under the conventional UVB irradiation protocol are only subjected to UVB during early development (5 to 10 days of age) and never experience UVR again. Finally, melanomas induced after this neonatal UVB irradiation lack UV signature mutations in Tp53 (39). Thus, it seems probable that UVB induced melanomas in Xiphophorus fishes correspond to truncal melanomas in humans, particularly given the observation that melanomas do not arise in this system after low-grade chronic UVB irradiation (unpublished data). The distinct etiologies of chronic and intermittent melanomas likely explain why we did not find a correlation between adult DRC and melanoma susceptibility in Xiphophorus. In rodents, it is clear that chronic UVB irradiation is correlated with reduced DRC in the dermis and epidermis and that this decreased DRC persists for at least 60 days post UVB treatment (23,24). Given this, it is surprising that melanoma models, including rodents, are not susceptible to melanoma formation after chronic UVB irradiation. HGF/SF mice, like Xiphophorus, develop melanoma after neonate exposure to UVB but not after exposure to chronic UVB (40). Taken collectively, studies of UVB irradiation in these models suggest that melanoma induction is not related to chronic UV exposures and concomitant accumulation and persistence of DNA mutations.
A recent study conducted on the Sp-couchianus model provides further evidence that accumulated DNA damage does not underlie melanoma susceptibility after UVB irradiation in Xiphophorus fishes. Mitchell and colleagues (41) exposed Sp-couchianus hybrids to the 5 day neonatal UVB irradiation used in the current study and found that there was no residual DNA damage ((6-4)PDs and CPDs) remaining 10 days after the final day of UVB exposure. Even more striking was the lack of accumulated DNA damage during the five consecutive days of UVB treatment. The amount of DNA damage present after the fifth day of irradiation was similar to or even less than the amount of DNA damage induced after the first day of irradiation, suggesting a photoadaptive mechanism is present in Xiphophorus fishes (41). This work clearly demonstrates that Xiphophorus Sp-couchianus hybrids, despite being susceptible to UVB induced melanomagenesis, do not accumulate or sustain DNA damage as a result of their neonatal UVB exposure. Furthermore, because the current study found no correlation between adult global NER and individual melanoma susceptibility, the carcinogenic effects of neonatal UVB irradiation are not the result of ineffective NER mechanisms nor the subsequent accumulation of DNA damage.
Although the fish used in this study were highly inbred, we observed substantial inter-individual variation in global NER efficiency in both the UVB and control groups (Table 1, Figure 1). This observation parallels epidemiological studies that consistently find significant inter-individual variation in DRC, including NER, in the human population (42–44). Such variation has prompted many investigations attempting to identify specific NER genotypes (alleles) that correspond to an individual’s NER efficiency and ultimately one’s risk of developing cancer. However, to date there are very few cases of an association between certain NER genotypes and increased risk for cutaneous melanoma (28). A recent meta-analysis of 20 original reports that examined melanoma risk and single nucleotide polymorphisms (SNPs) of 16 different genes associated with DNA repair found that only one SNP (XPD/ERCC2 SNP rs13181) might represent a low-penetrance melanoma susceptibility gene (45). Within Xiphophorus, we believe it is unlikely that NER genotypes are responsible for the inter-individual variation observed due to the high degree of inbreeding in both parental stocks of the Sp-couchianus melanoma model.
All lines of research, from animal and human subjects, indicate that melanoma is a very complex cancer that arises due to both genetic and environmental factors. The nature of UV exposure clearly influences the characteristics (e.g., mutation spectra) of the melanomas that develop. In Xiphophorus fishes, early life intermittent UVB exposure results in the induction of melanomas in adult animals that is not related to individual global NER efficiency. This finding is in contrast to other skin cancers that are associated with chronic exposures and consistent UV signature mutation spectra. The generality of intermittent UVB exposures being associated with melanoma induction despite a correlation with NER capacity warrants further investigation in other animal models.
Acknowledgments
This work was supported by NCI grant CA11367, NIEHS Center grant ES07784 and NCI training grant CA009480.
References
- 1.Chin L, Merlino G, DePinho RA. Malignant melanoma: modern black plague and genetic black box. Genes Dev. 1998;12:3467–81. doi: 10.1101/gad.12.22.3467. [DOI] [PubMed] [Google Scholar]
- 2.Koh HK. Cutaneous melanoma. N Engl J Med. 1991;325:171–82. doi: 10.1056/NEJM199107183250306. [DOI] [PubMed] [Google Scholar]
- 3.Walker G. Cutaneous melanoma: how does ultraviolet light contribute to melanocyte transformation? Future Oncol. 2008;4:841–56. doi: 10.2217/14796694.4.6.841. [DOI] [PubMed] [Google Scholar]
- 4.De Fabo EC, Noonan FP, Fears T, Merlino G. Ultraviolet B but not ultraviolet A radiation initiates melanoma. Cancer Res. 2004;64:6372–6. doi: 10.1158/0008-5472.CAN-04-1454. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell DL, Fernandez AA, Nairn RS, Garcia R, Paniker L, Trono D, Thames HD, Gimenez-Conti I. Ultraviolet A does not induce melanomas in a Xiphophorus hybrid fish model. Proc Natl Acad Sci U S A. 2010;107:9329–34. doi: 10.1073/pnas.1000324107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood SR, Berwick M, Ley RD, Walter RB, Setlow RB, Timmins GS. UV causation of melanoma in Xiphophorus is dominated by melanin photosensitized oxidant production. Proc Natl Acad Sci U S A. 2006;103:4111–5. doi: 10.1073/pnas.0511248103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraemer KH, Levy DD, Parris CN, Gozukara EM, Moriwaki S, Adelberg S, Seidman MM. Xeroderma pigmentosum and related disorders: examining the linkage between defective DNA repair and cancer. J Invest Dermatol. 1994;103:96S–101S. doi: 10.1111/1523-1747.ep12399329. [DOI] [PubMed] [Google Scholar]
- 8.Gordon M. The genetics of viviparous top-minnow Platypoecilus: the inheritance of two kinds of melanophores. Genetics. 1927;12:253–283. doi: 10.1093/genetics/12.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosswig C. Über bastarde der Teleostier Platyopoecilus und Xiphophorus. Z Indukt Abstamm Vererbungsl. 1928;47:150–158. [Google Scholar]
- 10.Patton EE, Mitchell DL, Nairn RS. Genetic and environmental melanoma models in fish. Pigment Cell Melanoma Res. 2010;23:314–37. doi: 10.1111/j.1755-148X.2010.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haluska F, Pemberton T, Ibrahim N, Kalinsky K. The RTK/RAS/BRAF/PI3K pathways in melanoma: biology, small molecule inhibitors, and potential applications. Semin Oncol. 2007;34:546–54. doi: 10.1053/j.seminoncol.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Mattei S, Colombo MP, Melani C, Silvani A, Parmiani G, Herlyn M. Expression of cytokine/growth factors and their receptors in human melanoma and melanocytes. Int J Cancer. 1994;56:853–7. doi: 10.1002/ijc.2910560617. [DOI] [PubMed] [Google Scholar]
- 13.Meierjohann S, Schartl M. From Mendelian to molecular genetics: the Xiphophorus melanoma model. Trends Genet. 2006;22:654–61. doi: 10.1016/j.tig.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Cleaver JE, Mitchell DL. Ultraviolet radiation carcinogenesis. In: Hong WK Jr, RCB, Hait WN, Kufe DW, Pollock RE, Weichselbaum RR, Holland JFEF III, editors. Cancer Medicine. McGraw-Hill; New York: 2010. pp. 262–269. [Google Scholar]
- 15.Sancar A. DNA excision repair. Annu Rev Biochem. 1996;65:43–81. doi: 10.1146/annurev.bi.65.070196.000355. [DOI] [PubMed] [Google Scholar]
- 16.de Boer J, Hoeijmakers JH. Nucleotide excision repair and human syndromes. Carcinogenesis. 2000;21:453–60. doi: 10.1093/carcin/21.3.453. [DOI] [PubMed] [Google Scholar]
- 17.Setlow RB, Grist E, Thompson K, Woodhead AD. Wavelengths effective in induction of malignant melanoma. Proc Natl Acad Sci U S A. 1993;90:6666–70. doi: 10.1073/pnas.90.14.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Setlow RB, Woodhead AD, Grist E. Animal model for ultraviolet radiation-induced melanoma: platyfish-swordtail hybrid. Proc Natl Acad Sci U S A. 1989;86:8922–6. doi: 10.1073/pnas.86.22.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell DL, Nairn RS, Johnston DA, Byrom M, Kazianis S, Walter RB. Decreased levels of (6-4) photoproduct excision repair in hybrid fish of the genus Xiphophorus. Photochem Photobiol. 2004;79:447–52. doi: 10.1562/ca-03-14.1. [DOI] [PubMed] [Google Scholar]
- 20.Schartl M, Hornung U, Gutbrod H, Volff JN, Wittbrodt J. Melanoma loss-of-function mutants in Xiphophorus caused by Xmrk-oncogene deletion and gene disruption by a transposable element. Genetics. 1999;153:1385–94. doi: 10.1093/genetics/153.3.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell DL. Radioimmunoassay of DNA damaged by ultraviolet ligh. In: Pfeifer GP, editor. Technologies for Detection of DNA Damage and Mutations. Plenum Press; New York: 1996. pp. 73–85. [Google Scholar]
- 22.Mitchell DL. DNA Repair Protocols. Humana Press; Totowa: 2006. Quantification of photoproducts in mammalian cell DNA using radioimmunoassay; pp. 239–249. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell DL, Byrom M, Chiarello S, Lowery MG. Effects of chronic exposure to ultraviolet B radiation on DNA repair in the dermis and epidermis of the hairless mouse. J Invest Dermatol. 2001;116:209–15. doi: 10.1046/j.1523-1747.2001.01192.x. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell DL, Greinert R, de Gruijl FR, Guikers KL, Breitbart EW, Byrom M, Gallmeier MM, Lowery MG, Volkmer B. Effects of chronic low-dose ultraviolet B radiation on DNA damage and repair in mouse skin. Cancer Res. 1999;59:2875–84. [PubMed] [Google Scholar]
- 25.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK. SEER Cancer Statistics Review, 1975–2006. National Cancer Institute; Bethesda, MD: 2009. pp. based on November 2008 SEER data submission, posted to the SEER web site, 2009. [Google Scholar]
- 26.Desai A, Krathen R, Orengo I, Medrano EE. The Age of Skin Cancers. Sci Aging Knowl Environ. 2006;2006:pe13. doi: 10.1126/sageke.2006.9.pe13. [DOI] [PubMed] [Google Scholar]
- 27.Li C, Wang LE, Wei Q. DNA repair phenotype and cancer susceptibility--a mini review. Int J Cancer. 2009;124:999–1007. doi: 10.1002/ijc.24126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rass K, Reichrath J. UV damage and DNA repair in malignant melanoma and nonmelanoma skin cancer. Adv Exp Med Biol. 2008;624:162–78. doi: 10.1007/978-0-387-77574-6_13. [DOI] [PubMed] [Google Scholar]
- 29.Jemal A, Devesa SS, Hartge P, Tucker MA. Recent trends in cutaneous melanoma incidence among whites in the United States. J Natl Cancer Inst. 2001;93:678–83. doi: 10.1093/jnci/93.9.678. [DOI] [PubMed] [Google Scholar]
- 30.Lachiewicz A, Berwick M, Wiggins C, Thomas N. Epidemiologic support for melanoma heterogeneity using the Surveillance, Epidemiology, and End Results Program. Journal of Investigative Dermatology. 2007;128:243–245. doi: 10.1038/sj.jid.5701028. [DOI] [PubMed] [Google Scholar]
- 31.Gaddameedhi S, Kemp MG, Reardon JT, Shields JM, Smith-Roe SL, Kaufmann WK, Sancar A. Similar nucleotide excision repair capacity in melanocytes and melanoma cells. Cancer Res. 2010;70:4922–30. doi: 10.1158/0008-5472.CAN-10-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, Varela I, Lin ML, Ordonez GR, Bignell GR, Ye K, Alipaz J, Bauer MJ, Beare D, Butler A, Carter RJ, Chen L, Cox AJ, Edkins S, Kokko-Gonzales PI, Gormley NA, Grocock RJ, Haudenschild CD, Hims MM, James T, Jia M, Kingsbury Z, Leroy C, Marshall J, Menzies A, Mudie LJ, Ning Z, Royce T, Schulz-Trieglaff OB, Spiridou A, Stebbings LA, Szajkowski L, Teague J, Williamson D, Chin L, Ross MT, Campbell PJ, Bentley DR, Futreal PA, Stratton MR. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–6. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savery NJ. The molecular mechanism of transcription-coupled DNA repair. Trends Microbiol. 2007;15:326–33. doi: 10.1016/j.tim.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Whiteman D, Watt P, Purdie D, Hughes M, Hayward N, Green A. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. JNCI Journal of the National Cancer Institute. 2003;95:806–812. doi: 10.1093/jnci/95.11.806. [DOI] [PubMed] [Google Scholar]
- 35.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Brocker EB, LeBoit PE, Pinkel D, Bastian BC. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 36.Wang LE, Xiong P, Strom SS, Goldberg LH, Lee JE, Ross MI, Mansfield PF, Gershenwald JE, Prieto VG, Cormier JN, Duvic M, Clayman GL, Weber RS, Lippman SM, Amos CI, Spitz MR, Wei Q. In vitro sensitivity to ultraviolet B light and skin cancer risk: a case-control analysis. J Natl Cancer Inst. 2005;97:1822–31. doi: 10.1093/jnci/dji429. [DOI] [PubMed] [Google Scholar]
- 37.Wang LE, Hsu TC, Xiong P, Strom SS, Duvic M, Clayman GL, Weber RS, Lippman SM, Goldberg LH, Wei Q. 4-Nitroquinoline-1-oxide-induced mutagen sensitivity and risk of nonmelanoma skin cancer: a case-control analysis. J Invest Dermatol. 2007;127:196–205. doi: 10.1038/sj.jid.5700481. [DOI] [PubMed] [Google Scholar]
- 38.Wei Q, Lee JE, Gershenwald JE, Ross MI, Mansfield PF, Strom SS, Wang LE, Guo Z, Qiao Y, Amos CI, Spitz MR, Duvic M. Repair of UV light-induced DNA damage and risk of cutaneous malignant melanoma. J Natl Cancer Inst. 2003;95:308–15. doi: 10.1093/jnci/95.4.308. [DOI] [PubMed] [Google Scholar]
- 39.Kazianis S, Gan L, Della Coletta L, Santi B, Morizot DC, Nairn RS. Cloning and comparative sequence analysis of TP53 in Xiphophorus fish hybrid melanoma models. Gene. 1998;212:31–8. doi: 10.1016/s0378-1119(98)00144-9. [DOI] [PubMed] [Google Scholar]
- 40.Noonan FP, Otsuka T, Bang S, Anver MR, Merlino G. Accelerated ultraviolet radiation-induced carcinogenesis in hepatocyte growth factor/scatter factor transgenic mice. Cancer Res. 2000;60:3738–43. [PubMed] [Google Scholar]
- 41.Mitchell DL, Paniker L, Douki T. DNA damage, repair and photoadaptation in a Xiphophorus fish hybrid. Photochem Photobiol. 2009;85:1384–90. doi: 10.1111/j.1751-1097.2009.00591.x. [DOI] [PubMed] [Google Scholar]
- 42.Collins AR, Gaivao I. DNA base excision repair as a biomarker in molecular epidemiology studies. Mol Aspects Med. 2007;28:307–22. doi: 10.1016/j.mam.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Gaivao I, Piasek A, Brevik A, Shaposhnikov S, Collins AR. Comet assay-based methods for measuring DNA repair in vitro; estimates of inter- and intra-individual variation. Cell Biol Toxicol. 2009;25:45–52. doi: 10.1007/s10565-007-9047-5. [DOI] [PubMed] [Google Scholar]
- 44.Tyson J, Caple F, Spiers A, Burtle B, Daly AK, Williams EA, Hesketh JE, Mathers JC. Inter-individual variation in nucleotide excision repair in young adults: effects of age, adiposity, micronutrient supplementation and genotype. Br J Nutr. 2009;101:1316–23. doi: 10.1017/S0007114508076265. [DOI] [PubMed] [Google Scholar]
- 45.Mocellin S, Verdi D, Nitti D. DNA repair gene polymorphisms and risk of cutaneous melanoma: a systematic review and meta-analysis. Carcinogenesis. 2009;30:1735–43. doi: 10.1093/carcin/bgp207. [DOI] [PubMed] [Google Scholar]