Abstract
BACKGROUND AND PURPOSE
rhuMAb Beta7 is a humanized anti-human β7 monoclonal antibody currently in phase I in inflammatory bowel disease. rhuMAb Beta7 binds the β7 subunit of the integrins α4β7 and αEβ7, blocking interaction with their ligands. These integrins play key roles in immune cell homing to and retention in mucosal sites, and are associated with chronic inflammatory diseases of the gastrointestinal tract. The goal of this study was to evaluate the mucosal specificity of rhuMAb Beta7.
EXPERIMENTAL APPROACH
We assessed the effect of murine anti-Beta7 on lymphocyte homing in mouse models of autoimmune disease. We also compared the effect of rhuMAb Beta7 on circulating mucosal-homing versus peripheral-homing T cells in naïve non-human primates.
KEY RESULTS
In cynomolgus monkeys, occupancy of β7 integrin receptors by rhuMAb Beta7 correlated with an increase in circulating β7+ mucosal-homing lymphocytes, with no apparent effect on levels of circulating β7- peripheral-homing lymphocytes. rhuMAb Beta7 also inhibited lymphocyte homing to the inflamed colons of severe combined immunodeficient mice in CD45RBhigh CD4+ T-cell transfer models. Consistent with a lack of effect on peripheral homing, in a mouse model of experimental autoimmune encephalomyelitis, anti-β7 treatment resulted in no amelioration of CNS inflammation.
CONCLUSIONS AND IMPLICATIONS
The results presented here suggest that rhuMAb Beta7 selectively blocks lymphocyte homing to the gastrointestinal tract without affecting lymphocyte trafficking to non-mucosal tissues. rhuMAb Beta7 provides a targeted therapeutic approach with the potential for a more attractive benefit : risk ratio than currently available inflammatory bowel disease therapies.
Keywords: integrin α4β7, integrins αEβ7, ulcerative colitis, Crohn's disease, CD4+ T cells, intestinal homing, gastrointestinal tract, lymphocyte trafficking
Introduction
Integrins are cell surface glycoprotein receptors that play key roles in leucocyte adhesion, signalling, proliferation and migration by binding as heterodimers to specific ligands (Hynes, 2002). Integrin receptors function in a highly regulated manner in tissue-specific cell adhesion, aiding in the recruitment of leucocytes from blood into tissue sites, and contributing to the homing of leucocytes to normal tissue and to sites of inflammation (Von Andrian and MacKay, 2000). Tissue-specific preferences of subsets of leucocytes reflect differential interactions of leucocytes with homing receptors on vascular endothelium in lymphoid tissue. Adhesion pathways play a dominant role in trafficking, helping to segregate intestinal/mucosal homing from non-intestinal/peripheral trafficking networks (Butcher et al., 1999; Cheroutre and Madakamutil, 2005).
The β7 integrin forms heterodimers with both the α4 and αE integrins. The α4β7 integrin is a pivotal mediator of leucocyte infiltration into the gastrointestinal tract through its interactions with mucosal addressin cell adhesion molecule-1 (MAdCAM-1) on high endothelial venules within the vessels of mucosal tissue (Butcher et al., 1999; Gurish et al., 2001). α4β7 is expressed on monocytes, lymphocytes, eosinophils, basophils, progenitor mast cells, macrophages and follicular dendritic cells, but not on neutrophils. The αEβ7 integrin binds selectively to E-cadherin and has been shown to mediate the adhesion of intraepithelial T cells to epithelial cells (Cepek et al., 1993; Karecla et al., 1995; Higgins et al., 1998). Only approximately 1–2% of circulating lymphocytes in human peripheral blood express the αEβ7 integrin (Parker et al., 1992; Cepek et al., 1993). However, in humans, more than 90% of intraepithelial lymphocytes and 50% of T cells in the human intestinal lamina propria express αEβ7 integrin, suggesting a distinctive role in mucosal immunology (Parker et al., 1992; Higgins et al., 1998). In addition, αEβ7 is expressed on intestinal dendritic cells, which are probably involved in the generation of gut-tropic effector T cells (Johansson-Lindbom et al., 2005).
In human ulcerative colitis (UC) and Crohn's disease (CD), up-regulation of MAdCAM-1 has been demonstrated in the inflamed colonic mucosa (Briskin et al., 1997; Souza et al., 1999). αEβ7 has also been shown to be up-regulated in the inflamed colonic mucosa of human UC and CD in the active phase of disease only (Elewaut et al., 1998; Pang et al., 1998), and data suggest that E-cadherin is up-regulated in the inflamed bowel mucosa in CD and UC (Demetter et al., 2000). Efficacy and/or biological activity in non-clinical and clinical studies has been demonstrated with anti-α4β7 and anti-MAdCAM-1 monoclonal antibodies (MAbs) (Hesterberg et al., 1996; Picarella et al., 1997; Feagan et al., 2005; 2008; Pullen et al., 2009; Vermeire et al., 2009). Antibodies to the αEβ7 integrin have been shown to be effective in a mouse IL-2−/− model of colitis; the onset and persistence of the inflammation were associated with increases in lamina propria CD4+ lymphocytes expressing αEβ7 (Lúdvíksson et al., 1999). Amelioration of the disease was associated with the disappearance of these cells from the lamina propria, These data point to the importance of α4β7/MAdCAM-1 and αEβ7/E-cadherin interactions in directing lymphocytes to and retaining lymphocytes in the mucosa in inflammatory conditions, and point to the possibility of therapeutic intervention by blocking these interactions, thereby reducing the leucocyte infiltration to the inflamed gut.
Inhibition of α4β7 binding to its ligand, MAdCAM-1, using an anti-α4 molecule, natalizumab (Tysabri®), has shown efficacy in CD (Sandborn et al., 2005; Targan et al., 2007). However, natalizumab blocks both α4β7:MAdCAM-1 and α4β1:vascular cell adhesion molecule-1 (VCAM-1) interactions; the latter have been shown to mediate leucocyte homing to the CNS (Yednock et al., 1992; Butcher et al., 1999; Rice et al., 2005). Treatment with natalizumab has been associated with confirmed cases of progressive multifocal leucoencephalopathy (PML) in patients treated for relapsing remitting multiple sclerosis (MS) (Kleinschmidt-DeMasters and Tyler, 2005; Langer-Gould et al., 2005) and in one patient treated for CD (Van Assche et al., 2005). The exact mechanism for the development of PML following treatment with natalizumab remains unclear, but may be related to the systemic effect on leucocyte trafficking mediated through inhibition of α4β1:VCAM-1 interactions. While the natalizumab efficacy data in CD confirm that targeting α4β7/MAdCAM-1 interactions is a valid strategy for combating autoimmunity in mucosal tissues, specifically targeting only mucosal-restricted integrins for the treatment of inflammatory bowel disease (IBD) may reduce the risk of PML.
We have developed a humanized monoclonal antibody (rhuMAb Beta7) that inhibits the binding of the β7 integrins (α4β7 and αEB7) to their ligands (MAdCAM-1 and E-cadherin respectively). rhuMAb Beta7 does not interfere with lymphocyte trafficking into non-mucosal tissues, and is therefore expected to provide comparable efficacy to α4 blockade without decreasing lymphocyte surveillance of non-target tissues. In addition, the blocking of αEB7 binding to E-cadherin and subsequent disruption of lymphocyte retention in the lamina propria has the potential to provide improved efficacy over inhibition of α4β7 alone and this hypothesis will be tested in forthcoming clinical trials. Thus, rhuMAb Beta7 provides a mechanism of action for the treatment of IBD that inhibits both trafficking and retention of leucocytes in mucosal tissues.
The pharmacokinetic (PK) and pharmacodynamic (PD) properties of rhuMAb Beta7 were assessed in mice and non-human primates. The specificity of rhuMAb Beta7 in non-human primates was assessed by examining subsets of circulating lymphocytes according to their homing properties, comparing PD effects on lymphocyte subsets that home preferentially to the mucosal tissue of the gastrointestinal tract versus non-mucosal tissues. To further examine the specificity of rhuMAb Beta7, we determined the effects of rhuMAb Beta7 on recruitment and homing of lymphocytes to the inflamed colon and spleen in the CD45RBhigh T cell-reconstituted severe combined immunodeficient (SCID) mouse model of colitis. In addition, we compared the effects of anti-β7 with anti-α4 in myelin basic protein T-cell receptor (MBP-TCR) transgenic mice with experimental autoimmune encephalomyelitis (EAE), a murine model of MS. We found that rhuMAb Beta7 selectively inhibits lymphocyte homing to the gastrointestinal tract, with no apparent effect on lymphocyte trafficking to non-mucosal tissues or the CNS. Based on these preclinical data, rhuMAb Beta7 exhibits favourable PK, PD and safety profiles, and provides potential for a targeted therapeutic approach in the treatment of IBD.
Methods
Test material
rhuMAb Beta7 is a humanized monoclonal IgG1κ antibody against the integrin subunit β7 that was derived from the rat anti-mouse/human monoclonal antibody FIB504 (Andrew et al., 1994). The humanization of this antibody has been described previously (Dennis, 2010). Briefly, a complementarity-determining region (CDR) graft of FIB504 into a consensus VLkappaI/VHIII framework resulted in a complete loss of antigen binding. To restore binding, a framework toggle phage library (Baca et al., 1997) was generated, which identified framework position 78 in VH (L78F) as important. This single framework change, incorporated into the CDR graft, restored binding to within 23-fold of the chimera. CDR repair, an approach that seeks to restore proper CDR-framework packing interactions (Dennis, 2010), was then used to identify two CDR changes: T31D and Y32L in CDR-L1 that fully restored β7 binding when compared with the parent antibody.
rhuMAb Beta7 was produced by transfected Chinese hamster ovary cells. Short-term dosing studies (such as the SCID mouse model of colitis) were performed with rhuMAb Beta7; however, as rhuMAb Beta7 is highly immunogenic in mice, chronic dosing studies in mice with EAE were performed using the less immunogenic rat–mouse chimeric anti-murine β7 (muFIB504), which is derived from the same parent antibody as rhuMAb Beta7 and shares the same CDR sequences but is much less immunogenic in mice. rhuMAb Beta7 binds the human, cynomolgus monkey and mouse β7 receptors with high affinity and high specificity. The drug and molecular target nomenclature used in this paper are in agreement with the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2009).
Animal studies
Studies in mice were conducted according to institutional guidelines for animal care and welfare. All study protocols were approved by institutional review committees and were consistent with current standards in research.
In studies involving cynomolgus monkeys, treatment of the animals was in accordance with regulations outlined in the USDA Animal Welfare Act and the conditions specified in The Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Research, 1996).
Single dose distribution study in BALB/c mice
Eighteen normal female BALB/c mice weighing 18–21 g were given single i.p. bolus doses of 5 mg sodium iodide (NaI) at 24 and 1 h before administration of the test material to block the non-specific uptake of iodine in the thyroid. Nine of the mice received a single i.v. bolus dose of 125I-rhuMAb Beta7 (5 µCi) in a volume of 100 µL, and the other nine mice received a single i.v. bolus dose of [125I]-rhuMAb Beta7 (5 µCi) plus 200 µg unlabelled rhuMAb Beta7 in a volume of 100 µL. Selected tissues were collected from three mice in each group at 30 min, 4 h and 24 h post dose. The tissues were rinsed in saline, blotted dry, weighed and frozen at −70°C until they were analysed with a Wallac 1470 gamma counter (Perkin Elmer Life Sciences; Downers Grove, IL, USA) to determine the amount of radioactivity associated with each tissue.
Single dose PK/PD study in BALB/c mice
Forty-eight normal female BALB/c mice weighing 17–21 g (four mice per time point) were given a single i.v. injection of 5 mg·kg−1 rhuMAb Beta7. Blood was collected through day 11 post dosing for PK analysis of anti-rhuMAb Beta7 antibody in serum, and for flow cytometry analysis of occupation and total expression of β7 on peripheral blood CD4+ and CD8+ T cells. Occupation of rhuMAb Beta7 and total expression of β7 receptors on intraepithelial T cells isolated from the small intestine were assessed from six untreated mice and four treated mice 24 h after rhuMAb Beta7 treatment. Intraepithelial lymphocytes were isolated from the small intestine by cell straining of the tissue and 44% Percoll gradient centrifugation and resuspended in 0.4 mL ice-cold PBS and 1% BSA buffer.
SCID mouse model of colitis
The effect of rhuMAb Beta7 on lymphocyte homing in vivo was evaluated in a mouse model of colitis in which SCID mice were reconstituted with CD45RBhigh CD4+ T cells (Morrissey et al., 1993). CD45RBhigh CD4+ T cells were isolated from the spleens of female BALB/c mice (mouse weight: 20–25 g), washed, resuspended at 3000 cells µl−1, and 100 µL(3 × 105 cells) were injected i.v. into CB-17.SCID female mice (mouse weight: 18–21 g). In this colitis model, weight loss is indicative of colitic inflammation. Recipient SCID mice (n = 50) were checked for donor T-cell reconstitution based on weight loss for four consecutive weeks of either 10% compared with baseline or 15% compared with peak weight. When a sufficient number of mice met these enrolment criteria, animals were randomly assigned to groups. One group had no colitis (group 1, n = 4); groups 2 and 3 had colitis and included nine and eight mice respectively.
Mesenteric lymph node cells from 100 BALB/c donor mice were radiolabelled with Cr51 and 4 × 106 Cr51-labelled mesenteric lymph node cells (100 µL total volume) were i.v. injected into animals from each of the three groups. Thirty minutes prior to i.v. injection of Cr51-labelled mesenteric lymph node cells, antibodies were administered by i.p. injection in a total volume of 100 µL; 200 µg of anti-gp 120 (a humanized IgG1 isotype control; group 2) or 200 µg rhuMAb Beta7 (group 3). One hour following the injection of the labelled cells, the mice were killed; spleens and colons were collected, weighed, and the total radioactivity for colon and spleen was determined using a gamma counter.
MBP-TCR transgenic mouse EAE model
Female MBP-TCR Tg mice on B10.Pl background that were 8–14 weeks old were used for this study. These mice overexpress the TCR for MBP, a known encephalitogenic peptide, and were immunized with MBP (20 µg Ac1-11) in the presence of complete Freund's adjuvant. Pertussis toxin was administered on days 1 and 2 following immunization to facilitate breakdown of the blood-brain barrier. Ten mice in each of three groups were given s.c. injections of anti-β7 (200 µg muFIB504), anti-α4 (200 µg mPS/2, positive control) or anti-gp120 (200 µg mouse IgG1 antibody, negative control) three times each week, starting on the day of immunization. Mice were evaluated daily using the following grading system:
0 = Normal mouse, no overt signs of disease; 1 = Limp tail or hind limb weakness but not both; 2 = Limp tail and hind limb weakness; 3 = Partial hind limb paralysis; 4 = Complete hind limb paralysis; 5 = Moribund state from EAE; killed. A disease score of 4 for seven consecutive days resulted in a severity score of 5 and subsequent death.
At the end of the study, brains and spinal cords from each animal were fixed in 10% neutral buffered formalin and embedded in paraffin (FFPE); four representative regions of brain and four representative regions of each of the three spinal cord segments (cervical, thoracic and lumbar, for a total of 12 regions) were grossly dissected and embedded in paraffin. FFPE sections were stained with haematoxylin and eosin and analysed for inflammatory cellular infiltration. Sections were scored on a scale of 0 (no inflammation) to 4 (severe inflammation, infiltration of the majority of the histological tissue section). One mouse in the group given the control (anti-gp120) died on day 20, before collection of the CNS, and therefore was not included in the histological analysis.
Single dose PK study in cynomolgus monkeys
The study was conducted at Covance Laboratories Inc. (Alice, TX, USA). Three naïve cynomolgus male monkeys (weight: 2–4 kg) in each of four groups were given a single i.v. injection of vehicle, or 1, 3 or 10 mg·kg−1 rhuMAb β7 at 0.25 mL·kg−1. Blood (approximately 1.2 mL) was collected from each animal and serum was harvested for PK analysis at baseline and through to day 44.
Multiple dose PK/PD/toxicology study in cynomolgus monkeys
This study was conducted at SNBL USA (Everett, WA, USA). Twenty-six (13 males and 13 females) naïve cynomolgus monkeys that weighed 2–6 kg were randomly allocated into one of three groups and received four weekly i.v. doses of vehicle or rhuMAb Beta7 at 5 or 25 mg·kg−1. Three males and three females from each group were killed on day 29, 7 days after the final dose. Two males and two females from groups 1 and 3 were followed for a recovery period of approximately 23 weeks prior to their death. Standard toxicity endpoints, PK, PD and anti-therapeutic antibodies (ATA) were evaluated at baseline and at various intervals through the dosing and recovery phases.
rhuMAb Beta7 elisa
Mouse and cynomolgus monkey serum samples were analysed by enzyme-linked immunosorbent assay (elisa) using plates coated with F(ab′)2 rabbit or sheep anti-human IgG Fc (Jackson ImmunoResearch, West Grove, PA, USA). Plate-bound rhuMAb Beta7 was detected with horseradish peroxidase (HRP) tagged goat (mouse elisa) or sheep (cyno elisa) anti-human Fc (Jackson ImmunoResearch, West Grove, PA, USA). The signal was generated using tetramethyl benzidine peroxidase as substrate for HRP. The lower limit of quantification of the mouse assay was determined to be 1.56 ng·mL−1. The minimum quantifiable concentration for neat cynomolgus monkey serum was determined to be 20 ng·mL−1, based on the assay limit of 1 ng·mL−1 and the minimum dilution of 1:20.
Anti-rhuMAb Beta7 antibody assay
Mouse serum samples were analysed by elisa using plates coated with rhuMAb Beta7. Plate-bound anti-rhuMAb Beta7 antibodies were detected using goat anti-mouse IgG Fc-HRP (Jackson ImmunoResearch, West Grove, PA, USA). The data for the elisa were reported as log titre values. The minimum quantifiable titre was determined to be 2 in neat serum (accounting for a minimum sample dilution of 1–100).
Serum samples from cynomolgus monkeys were analysed by a bridging electrochemiluminescence assay to determine the level of antibody response against rhuMAb Beta7. Polyclonal antibodies directed against human IgG (H&L, pre-absorbed with monkey serum) were obtained from The Binding Site and were used as surrogate positive control. Streptavidin-coated Dynabeads M280 (Invitrogen Corp., Carlsbad, CA, USA) were used for detection.
FACS analysis of occupancy and total expression of β7 on T cells in peripheral blood and intraepithelial T cells in BALB/c mice
For the analysis, 0.05 mL of either blood with lithium heparin as an anticoagulant or intraepithelial lymphocyte suspension was labelled with 10 µg·mL−1 of fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD4 (clone RM4 4), R-phycoerythrin (PE)-conjugated anti-mouse β7 (clone M293), Peridinin chlorophyll (PerCP)-conjugated anti-mouse CD3 (clone 145 2C11) and allophycocyanin (APC)-conjugated anti-mouse β7 (clone FIB504) monoclonal antibodies (BD Biosciences, San Jose, CA) for 45 min at 2–8°C. The samples were analysed on a FACSort cytofluorimeter (BD Biosciences, San Jose, CA, USA). Total β7 expression on CD3+CD4+ or CD3+CD4- lymphocytes was determined using clone M293, which binds a different epitope from rhuMAb Beta7. Available β7 expression on T cells was determined using clone FIB504 that binds the same epitope as rhuMAb Beta7. For each sample, total and available β7 were expressed as the geometric mean fluorescent intensity (GMFI). Changes in total β7 (down modulation) and unoccupied β7 (saturation) for each mouse treated with anti-Beta7 MAb were expressed as a percentage of the average GMFI of control mice.
Analysis of β7 occupancy and expression on CD4+ and CD8+ lymphocyte subsets in peripheral blood of cynomolgus monkeys
Whole cynomolgus monkey blood was collected in tubes containing heparin coagulant and shipped to Genentech for analysis. The panel configuration for expression analysis is presented in Table 1; the panel configuration for occupancy analysis is presented in Table 2.
Table 1.
Whole blood panel configuration for total β7-expressing CD4+ and CD8+ T-cell subsets in cynomolgus monkeys
| Tube no. | Antigen markers: fluorochrome | Cell types identified |
|---|---|---|
| 1 | IgG1-FITC, IgG1-PE, CD4-PerCP, SA-APC | Isotype used for setting quadrant markers when gating on lymphocytes from SSC versus FSC plots |
| 2 | CD45RA-FITC, CD49d-PE, CD4-PerCP or CD4-PerCP-Cy5.5, 9D8-Biotin/SA-APCa | α4 and β7 expressing subsets of CD4 lymphocytes |
| 3 | CD45RA-FITC, CD49d-PE, CD8-PerCP or CD8-PerCP-Cy5.5, 9D8-Biotin/SA-APCa | α4 and β7 expressing subsets of CD8 lymphocytes |
9D8-biotin was incubated first, before SA-APC was added.
CD4 (BD Biosciences, clone L200); CD8 (BD Biosciences, clone SK1); CD45RA (BD Biosciences, clone 5H9); CD49d (BD Biosciences, clone 9F10).
APC, allophycocyanin; FITC, fluorescein isothiocyanate; FSC, forward scatter; IgG, immunoglobulin G; PE, phycoerytherin; PerCP-Cy5.5, peridinin chlorophyll protein–cyanine-5.5; SA, streptavidin; SSC, side scatter.
Table 2.
Whole blood panel configuration for occupancy of β7 on CD4+ and CD8+ T-cell subsets in cynomolgus monkeys
| Tube no. | Antigen markers: fluorochrome | Cell types identified |
|---|---|---|
| 1 | CD4-FITC, CD45RA-PE, CD8-PerCP, Her-Alx647 | Negative control |
| 2 | CD4-FITC, CD45RA-PE, CD8-PerCP, rhuMAb β7-Alx647 | Detect β7 occupancy |
| 3 | CD4-FITC, CD45RA-PE, CD8-PerCP, rhuMAb β7-Alx647 | Control for tube #2 that contains saturating concentration (10 µg·mL−1) of rhuMAb Beta7 |
CD4 (BD Biosciences, clone M-T477); CD45RA (BD Biosciences, clone 5H9); CD8 PerCP (BD Biosciences, clone SK1).
Alx647, AlexHerceptina 647; FITC, fluorescein isothiocyanate; Her, Herceptin; PE, phycoerytherin; PerCP, peridinin chlorophyll protein.
The total β7-expressing CD4+ and CD8+ lymphocytes were measured using 9D8, an in-house Genentech anti-β7 antibody targeting a different epitope than rhuMAb Beta7. Flow cytometric acquisition was performed on a FACSCalibur™ (BD Biosciences; San Jose, CA, USA). Twenty-five thousand lymphocyte events were acquired using a forward scatter (FSC)/side scatter (SSC) plot. Data analysis was based on both FSC/SSC gated lymphocytes and, depending on the cocktail combination, CD4+/SSClow- or CD8+/SSClow-gated lymphocytes using CellQuest Pro software (BD CellQuest Pro, version 5.2). Flow cytograms were generated to establish the fraction of cells positive for each cell surface marker. 9D8-expressing sub-populations were analysed as a percentage of gated CD4+ and CD8+ lymphocytes, and separately, as GMFI. For each sample at each time point, molecules of equivalent soluble fluorescence (MOEF) were calculated by multiplying the GMFI of each population with a standard curve, generated by the SpheroTech Beads standard. GMFI and the percentage of gated cells for each of the CD4+ and CD8+ lymphocyte populations (CD45RA-9D8intermediate, CD45RA-9D8high, CD45RA-9D8low, CD45RA+ and CD45RA-) were monitored throughout the study to examine the effect of drug on each subset. For each sample at each time point for each lymphocytes subset, the absolute cell count was calculated based on the lymphocyte count per µL of peripheral blood (the latter provided by SNBL).
The CD4+ and CD8+ lymphocytes showing β7high/CD45RA-, β7low/CD45RA- and β7intermediate/CD45RA+ phenotypes were identified for the tubes with and without saturating concentrations (10 µg·mL−1) of rhuMAb Beta7. The GMFI and the percentage of gated cells were assessed, and the GMFI was then expressed as MOEF using the slope of the APC channel where MOEF = GMFI × slope of the FL4 channel.
Statistical analysis
SCID mouse model of colitis: the group means for the amount of radioactivity in the colon were analysed by anova followed by Dunnett's test.
MBP-TCR transgenic mouse EAE model: the group means for the disease severity scores and inflammation scores were analysed by anova followed by Dunnett's test. The survival test was a Kaplan–Meier survival analysis.
Pharmacokinetic analysis
Pharmacokinetic analysis was performed using the WinNonlin Professional Edition computer software, Version 3.2 (Pharsight Corp., Mountain View, CA, USA). Serum concentration–time profiles were assessed using a non-compartment model with bolus input. Calculations of rate constants and additional parameters including AUC (area under the rhuMAb Beta7 serum concentration–time curve), Cmax (model-predicted maximum serum rhuMAb Beta7 concentration), tmax (time to maximum serum rhuMAb Beta7 concentration), Vss (estimated steady-state volume of distribution), CL (clearance) and (t1/2, elim) terminal half-life have been described previously (Gibaldi and Perrier, 1982).
Results
Down-modulation of Beta7 integrins on T lymphocytes following a single dose of rhuMAb Beta7 in mice
Following a single i.v. bolus dose of ∼ 5 mg·kg−1 of rhuMAb Beta7 in a PK/PD study in mice, serum rhuMAb Beta7 concentrations exhibited biphasic disposition with a rapid initial distribution phase followed by a slower elimination phase (data not shown). Serum rhuMAb Beta7 concentrations dropped rapidly 6 days after dosing, which was probably due to the presence of anti-rhuMAb Beta7 antibodies, as all mice developed anti-rhuMAb Beta7 antibodies by day 5 after dosing.
β7 integrin receptors were down-modulated on CD8+ and CD4+ T cells to approximately 50–60% of control values following dosing with rhuMAb Beta7 as early as 6 h post dose (Figure 1A and B); 90–97% of peripheral blood T-cell β7 receptors were saturated by rhuMAb Beta7 as early as 6 h post dose (Figure 1C and D). On day 10 after dosing, unoccupied β7 integrin receptors were detected on peripheral blood CD8+ and CD4+ T cells (Figure 1C and D), which was consistent with decreased serum rhuMAb Beta7 concentrations (data not shown). At 24 h post dose, a similar down-modulation of β7 integrin receptors on intraepithelial T cells from the small intestine was observed (62.2 ± 14.9% of control for CD8+ T cells and 41.5 ± 31.0% for CD4+ T cells). Also, 98.3 ± 0.091% of intraepithelial CD8+ T-cell β7 integrin receptors and 90.0 ± 1.64% of intraepithelial CD4+ T-cell β7 integrin receptors were saturated 24 h after rhuMAb Beta7 administration.
Figure 1.

The total β7 integrin receptor expression on peripheral blood CD8+ (A) or CD4+ lymphocytes (B) and unoccupied β7 receptor expression on peripheral blood CD8+ (C) or CD4+ lymphocytes (D) following single i.v. bolus administration of 5 mg·kg−1 rhuMAb Beta7 to BALB/c mice (n = 4 per time point). The data are presented as the mean (±SD) percentage for each group normalized to the percentage in the control group (Time 0). Samples were analysed by flow cytometry, and 20 000 lymphocyte events were acquired using a FSC/SSC plot. Total β7 receptor expression on lymphocytes was determined using anti-β7 (clone M293), a non-competing antibody that binds a different epitope from rhuMAb Beta7. Changes in total β7 receptor expression (down-modulation) for each mouse were expressed as per cent of control GMFI, which was determined from the average of six untreated mice. Unoccupied β7 receptor expression on lymphocytes was determined using anti-β7 (clone FIB504), which binds the same epitope as rhuMAb Beta7. Changes in unoccupied β7 receptor expression (saturation) for each mouse were expressed as % of control GMFI, which was determined from the average of six untreated mice.
Tissue distribution of [125I]-rhuMAb Beta7
Two groups of animals were used to investigate rhuMAb Beta7 binding to mouse tissues; one group was dosed with [125I]-rhuMAb Beta7, and the other was dosed with [125I]-rhuMAb Beta7 plus excess unlabelled rhuMAb Beta7. The purpose of these two groups was to determine which organs in the group dosed with [125I]-rhuMAb Beta7 had radioactivity that was displaced in the respective organs of the group co-dosed with excess unlabelled rhuMAb Beta7. Similar to a competition assay, this would indicate target-specific uptake (or binding to β7-expressing cells) in those organs. The presence of radioactive signal in organs that was not displaced by co-administration of excess unlabelled rhuMAb Beta7 suggests non-specific uptake, probably due to blood circulating through those particular organs.
Thirty minutes following administration of [125I]-rhuMAb Beta7 alone to mice, the percentage of total injected drug per gram of tissue was highest in spleen, followed by blood and lungs. Radioactivity in the spleen was reduced in the presence of excess unlabelled rhuMAb Beta7, consistent with binding to β7-expressing cells. In contrast, 125I uptake in the blood and lungs was not reduced in the presence of excess unlabelled rhuMAb Beta7. The presence of radioactive signal at these sites that was not displaced by co-administration of excess unlabelled rhuMAb Beta7 is consistent with non-specific uptake (in the lungs, probably due to blood circulating through the organ) (Figure 2A). At 24 h post dose, [125I]-rhuMAb Beta7 signal was detected in the spleen, mesenteric lymph nodes and small intestine. Radioactive signal in these organs was reduced in the presence of excess unlabelled rhuMAb Beta7, consistent with target-specific binding of rhuMAb Beta7 in these tissues. Conversely, an increase in the amount of [125I]-rhuMAb Beta7 in the blood at 24 h in the presence of excess unlabelled rhuMAb Beta7 is consistent with displacement of [125I]-rhuMAb Beta7 from specific interactions with β7-expressing cells in tissues (Figure 2B).
Figure 2.

Single dose distribution study in BALB/c mice. (A) At 30 min post injection, a strong rhuMAb Beta7 signal was observed in the spleen; signal was decreased in the presence of unlabelled (cold) rhuMAb Beta7. This is consistent with specific binding of rhuMAb Beta7 to splenic β7-expressing cells. In contrast, signal in the lung was not decreased in the presence of excess unlabelled rhuMAb Beta7, suggesting that signal in this organ was due to the presence of blood rather than specific binding of rhuMAb Beta7 to cells in the lung. (B) At 24 h post injection, a strong radioactive rhuMAb Beta7 signal was observed in the spleen, lymph node and intestine; this signal was decreased by cold rhuMAb Beta7, consistent with specificity of rhuMAb Beta7 binding in these tissues. Columns represent the mean of n = 3 mice and vertical lines show SD.
Inhibition of lymphocyte homing by rhuMAb Beta7 in the CD45RBhigh T cell-reconstituted SCID mouse model of colitis
In the CD45RBhigh CD4+ T cell-reconstituted SCID mouse model of colitis, rhuMAb Beta7 significantly blocked lymphocyte recruitment and homing to the inflamed colon (Figure 3A) compared with a control Ig. In mice without colitis, lymphocyte homing to the colon was minimal (Figure 3A). In contrast, rhuMAb Beta7 had no apparent effect on lymphocyte homing to the spleen, a non-mucosal lymphoid organ (Figure 3B). These results indicate that rhuMAb Beta7 can reduce recruitment of activated T cells to the inflamed mucosa, without altering circulation of T cells to non-mucosal tissues. These results are similar to those reported for rat anti-murine-β7 (FIB504) in a previous study (Picarella et al., 1997).
Figure 3.
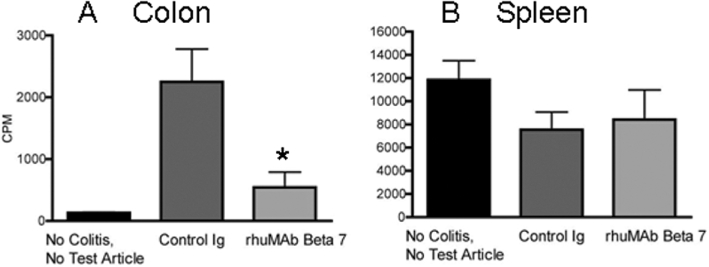
In vivo homing of lymphocytes to the inflamed colon of CD45RBhigh reconstituted SCID mice. SCID mice were reconstituted with Cr51-labelled CD45RBhigh CD4+ T cells. In this model of colitis, rhuMAb Beta7 significantly blocked lymphocyte recruitment and homing to the inflamed colon (as measured by gamma count of harvested organs) when compared with a control Ig (A, control IgG vs. rhuMAb Beta7, P < 0.001), but had no effect on lymphocyte homing to the spleen, a non-mucosal lymphoid organ (B). Untreated mice without colitis had essentially no homing to the colon (A, see group 1); nearly all labelled lymphocytes trafficked to the spleen (B, see group 1). Group 1 = non-colitis, no test article (n = 4 mice); group 2 = control Ig (gp120; n = 9 mice); group 3 = rhuMAb Beta7 (n = 8 mice); Ig, immunoglobulin; anti-gp120, control mouse IgG1 antibody. Values shown are group mean counts per minute (CPM) and vertical lines show SD. Asterisk indicates significant P-value.
Anti-β7, unlike anti-α4, has no therapeutic effect in MBP-TCR transgenic mice with EAE
Experimental autoimmune encephalomyelitis is a T cell-mediated autoimmune demyelinating disease of the CNS that serves as a useful mouse model for human MS. In human MS lesions, T cells, macrophages and activated microglial cells are the predominant inflammatory cells. MBP-TCR transgenic mice develop EAE in response to MBP immunization; approved treatments such as anti-α4 are efficacious in this MS model, indicating the importance of lymphocyte homing in establishment of disease (Hardardottir et al., 1995).
In order to compare the effects of anti-β7 with anti-α4 in peripheral (CNS) homing, we investigated whether anti-β7 (muFIB504) could reduce the severity of EAE, compared with an anti murine-α4 (mPS/2), in the MBP-TCR transgenic model (Hardardottir et al., 1995). The mean (±SD) terminal disease severity scores for groups given anti-β7 (muFIB504), anti-α4 (mPS/2) and anti-gp120 were 3.9 ± 1.8, 1.2 ± 2.1 and 4.0 ± 1.5 respectively. Analysis by Dunnett's test indicated that terminal disease severity scores in the group given anti-α4 (mPS/2) were significantly lower than those of the group given anti-gp120 (control; P = 0.003), whereas those of the anti-β7 (muFIB504) group showed no statistically significant difference compared with the group given control antibodies (P = 0.989) (Figure 4A).
Figure 4.

Anti-Beta7 in mouse model of EAE. (A) Anti-Beta7 had no effect on lymphocyte homing to brain; while treatment with anti-murine α4 (mPS/2) MAb significantly improved the mean EAE disease severity score (P = 0.003) compared with control anti-gp120, anti-murine β7 (muFIB504) had no effect (P = 0.989) on disease severity compared with control anti-gp120. MBP-TCR mice with EAE were evaluated daily using an EAE disease severity scoring system between 0 and 5; mean scores are plotted. Statistical analysis was performed on terminal disease severity scores using Dunnett's test. (B) Histological sections from each region of brain and spinal cord were scored on a scale of 0 (no inflammation) to 4 (severe inflammation involving the majority of the histological section); mean scores (and SD) were plotted. Anti-murine α4 MAb (mPS/2) significantly improved mean CNS histological inflammation (P = 0.009) compared with control anti-gp120, whereas anti-murine β7 (muFIB504) had no effect on histological inflammation in the CNS compared with control anti-gp120. Statistical analysis was performed on terminal histological inflammation scores using Dunnett's test. Asterisk indicates significant P-value. (C) % survival of MBP-TCR transgenic mice with severe EAE. Anti-murine α4 MAb (PS/2) significantly improved survival from EAE compared with control anti-gp120, while anti-murine β7 (muFIB504) had no effect on EAE survival compared with control anti-gp120. The difference between these survival curves is significant (log–rank test, P = 0.0005). MBP-TCR, myelin basic protein T-cell receptor; anti-gp120, control mouse IgG1 antibody; mPS/2, anti-murine α4; muFIB504, anti-murine β7 (rat–mouse chimeric antibody). n = 10 mice per group.
The corresponding mean (±SD) terminal histological inflammation scores for groups given anti-β7 (muFIB504), anti-α4 (mPS/2) and anti-gp120 were 1.38 ± 0.77, 0.69 ± 0.48 and 1.47 ± 0.45 respectively (Figure 4B). Animals in the anti-α4 (mPS/2) group exhibited statistically significant levels of improvement in both disease severity scores (P = 0.003) and histological inflammation scores (P = 0.009). Anti-α4 (mPS/2) significantly ameliorated EAE severity in this model, including inhibition of inflammatory cellular infiltration into the CNS, whereas anti-β7 (muFIB504) had no effect on disease severity or CNS inflammatory cellular infiltration. Furthermore, 90% of the animals in the group given anti-α4 (mPS/2) were alive on day 21, but only three of the 10 animals (30%) in the group given anti-β7 (muFIB504) and four of the 11 animals (36%) in the control group (anti-gp120) were alive on day 21 (Figure 4C). The difference between these survival curves is significant (log–rank test, P = 0.0005).
Characterization of rhuMAb Beta7 PK in non-human primates following a single dose to cynomolgus monkeys
The serum drug concentration–time profiles for animals administered a single dose of rhuMAb Beta7 are presented in Table 3. Following a single i.v. dose to cynomolgus monkeys at 1, 3 or 10 mg·kg−1, rhuMAb Beta7 exhibited biphasic disposition, characterized by a short distribution phase followed by a longer terminal elimination phase. Dose-normalized exposure from day 0 to day 7 (AUC0−7) was non-dose proportional between the 1 and 3 mg·kg−1 dose groups, and dose proportional between the 3 and 10 mg·kg−1 dose groups. The non-proportional AUC at lower doses suggests non-linear pharmacokinetics and is probably due to binding to β7 integrin receptors, with saturation of the receptor probably occurring at doses ≥ 3 mg·kg−1 in cynomolgus monkeys. This non-linear PK due to receptor-mediated clearance (CL) is consistent with the mechanism of action of rhuMAb Beta7, which is blocking the integrin β7 receptor. The majority of the monkeys developed anti-rhuMAb Beta7 antibodies (ATA) by day 14, which probably contributed to the rapid clearance of rhuMAb Beta7 in serum after day 7, which was observed in most of the monkeys (data not shown).
Table 3.
Non-compartmental PK parameters of rhuMAb Beta7 following a single i.v. bolus dose to cynomolgus monkeys
| Dose (mg·kg-1) | AUC0−7 (day ·µg·mL-1) | AUC0−7/dose (day ·µg·kg·mL-1·mg-1) |
|---|---|---|
| 1 | 54.3 ± 7.94 | 54.3 ± 7.94 |
| 3 | 310 ± 22.0 | 103 ± 7.32 |
| 10 | 922 ± 134 | 92.2 ± 13.4 |
Data are presented as mean ± SD.
AUC0−7, area under concentration–time curve from day 0 to day 7; PK, pharmacokinetic.
rhuMAb Beta7 safety, PK and PD following multiple doses to cynomolgus monkeys
In a 4-week repeat dose study in cynomolgus monkeys, rhuMAb Beta7 was well-tolerated following weekly i.v. doses (a total of four) ranging from 5 to 25 mg·kg−1. No toxic effects were observed in any of the endpoints evaluated, including clinical signs, body weights, food consumption, clinical pathology parameters, gross necropsy observations, organ weights or microscopic evaluation (data not shown). rhuMAb Beta7 exhibited a relatively slow CL of 3.05 mL·kg−1·day−1 and long elimination half-life (t1/2, elim) of 10.1 days following i.v. administration of four weekly doses of 25 mg·kg−1 (Table 4). The exposure appeared dose proportional between the 5 and 25 mg·kg−1 dose groups suggesting linear PK at these dose levels. These data suggest that receptors on the subset of β7-expressing lymphocytes were saturated with this dose regimen, resulting in linear PK.
Table 4.
Non-compartmental toxicokinetic (TK) parameter estimates following i.v. administration of 25 mg·kg−1 rhuMAb Beta7 to cynomolgus monkeys (four doses, one dose per week)
| TK parameters | |
|---|---|
| AUCall(day ·µg·mL−1) | 33 400 ± 4 940 |
| CL (mL·kg−2·day−1) | 3.05 ± 0.481 |
| Cmax (µg·mL−1) | 1 700 ± 131 |
| tmax(days) | 21.1 ± 0.120 |
| t1/2, elim(day) | 10.1 ± 3.66 |
| Vss (mL·kg−1) | 90.6 ± 4.99 |
AUCall, area under the serum concentration–time curve from first day of dosing (day 0) to the last measurable concentration at the end of study (study day 191); Cmax, maximum observed concentration; CL, clearance; t1/2, elim, elimination half-life; tmax, time (days) to maximum observed concentration; Vss, volume of distribution at steady state. Data are presented as mean ± SD, n = 10 cynomolgus monkeys.
To examine the PD effects of rhuMAb Beta7, subsets of CD4+ T cells in the peripheral blood of cynomolgus monkeys were assessed. To subdivide and assess lymphocytes according to their homing and immunological properties, CD4+ T cells were gated according to their expression of CD45RA and β7. CD45RA-β7high CD4+ T cells and CD45RA-β7low CD4+ T cells are phenotypically similar to mucosal-homing T cells and peripheral-homing T cells respectively (Rott et al., 1996; 1997; Williams and Butcher, 1997; Roséet al., 1998; Williams et al., 1998; Butcher et al., 1999) (see Figure 5), while CD45RA+ CD4+ T cells (phenotypically similar to naïve helper T cells) traffic well to both intestinal and peripheral lymph nodes and tissues (Rott et al., 1996; 1997; Williams and Butcher, 1997; Roséet al., 1998; Williams et al., 1998; Butcher et al., 1999).
Figure 5.

Pharmacodynamic (PD) biomarker analysis of rhuMAb Beta7 treatment. Three subsets of peripheral blood CD4+ lymphocytes, subdivided according to their homing properties, were monitored by flow cytometry as PD markers: CD45RA-β7high (‘gut/mucosal-homing memory’), CD45RA-β7low (‘peripheral-homing memory’) and CD45RA+β7intermediate (‘naïve’) CD4+ cells. The figure shows a FACS scatter plot of CD45RA on the x-axis and β7 on the y-axis, gated on CD4+ cells. The figure is representative of staining pattern seen in untreated cynomolgus monkeys.
Group mean absolute numbers of peripheral blood CD45RA-β7high CD4+ T cells increased approximately fivefold to sixfold over baseline levels following four weekly doses of 5 or 25 mg·kg−1 rhuMAb Beta7 (Figure 6). In contrast, animals dosed with vehicle showed no substantial changes in this cell population. The PD results are consistent with the proposed mechanism of action of rhuMAb β7: inhibition of homing of β7 positive lymphocytes to the gut. This mechanism is expected to lead to accumulation of CD45RA-β7high CD4+ T cells in circulation. Levels of circulating CD45RA+β7intermediate CD4+ T cells and CD45RA-β7low CD4+ T cells showed no substantial differences in cynomolgus monkeys dosed with vehicle versus cynomolgus monkeys dosed with rhuMAb Beta7 (Figures 7 and 8). These results taken together suggest that rhuMAb Beta7 has no apparent effect on non-mucosal-homing subsets and specifically targets cells that home to mucosal sites such as the gastrointestinal tract.
Figure 6.

Mean (±SD) absolute peripheral blood CD45RA-β7high CD4+ T cell counts following four weekly i.v. bolus doses of vehicle or 5 or 25 mg·kg−1 rhuMAb Beta7 to cynomolgus monkeys. Absolute CD45RA-β7high CD4+ T cell counts are shown as a percentage of predose baseline. Groups dosed with vehicle and 25 mg·kg−1 had 10 animals per group from days 0 to 28, and four animals per group after day 28. There were six cynomolgus monkeys in the 5 mg·kg−1 group.
Figure 7.

Mean (±SD) absolute peripheral blood CD45RA+β7intermediate CD4+ T cell counts following four weekly i.v. bolus doses of vehicle or 5 or 25 mg·kg−1 rhuMAb Beta7 to cynomolgus monkeys. Absolute CD45RA+β7intermediate CD4+ T cell counts are shown as a percentage of predose baseline. Groups dosed with vehicle and 25 mg·kg−1 had 10 animals per group from days 0 to 28, and four animals per group after day 28. There were six cynomolgus monkeys in the 5 mg·kg−1 group.
Figure 8.

Mean (±SD) absolute peripheral blood CD45RA-β7low CD4+ T cell counts following four weekly i.v. bolus doses of vehicle or 5 or 25 mg·kg−1 rhuMAb Beta7 to cynomolgus monkeys. Absolute CD45RA-β7low CD4+ T cell counts are expressed as a percentage of predose baseline. Groups dosed with vehicle and 25 mg·kg−1 had 10 animals per group from days 0 to 28, and four animals per group after day 28. There were six cynomolgus monkeys in the 5 mg·kg−1 group.
The β7 receptors on peripheral blood CD45RA-β7high CD4+ T cells were fully saturated following the first of four weekly doses of 25 mg·kg−1 rhuMAb Beta7 to cynomolgus monkeys (Figure 9) and maintained saturation throughout the dosing phase and for up to 5 months following the last dose. This was not due to a loss of β7 receptor as total β7 receptor expression levels were comparable following rhuMAb Beta7 administration relative to their respective baseline levels (data not shown).
Figure 9.

Individual cynomolgus monkey profiles of rhuMAb Beta7 serum concentrations (left y-axis) and unoccupied peripheral blood CD45RA-β7high CD4+ T cells (right y-axis) following four weekly i.v. bolus doses of 25 mg·kg−1 rhuMAb β7. Unoccupied CD45RA-β7high CD4+ T cells are shown as a percentage of predose baseline. Conc., concentration.
Receptor saturation correlated with an increase in CD45RA-β7high CD4+ T cells in peripheral blood (Figure 10). The loss of receptor occupancy of rhuMAb Beta7 correlated with return to predose levels of CD45RA-β7high CD4+ T cells in peripheral blood in cynomolgus monkeys (Figure 10). The timing of the loss of receptor occupancy and the return to baseline of CD45RA-β7high CD4+ T cells varied between the individual cynomolgus monkeys, and correlated with decreases in serum rhuMAb Beta7 concentrations below 1–10 µg·mL−1 (Figure 9). The individual variability in the PK profiles for rhuMAb Beta7 appeared to be associated with anti-rhuMAb Beta7 antibodies (data not shown). Anti-rhuMAb Beta7 antibodies were detectable in three out of four animals that were followed through recovery, but in general all animals maintained exposure in the range expected as measured by AUC (Table 4 and Figure 9).
Figure 10.

Individual cynomolgus monkey profiles of absolute peripheral blood CD45RA-β7high CD4+ T cell counts (left y-axis) and unoccupied peripheral blood CD45RA-β7high CD4+ T cells (right y-axis) following four weekly i.v. bolus doses of 25 mg·kg−1 rhuMAb Beta7. Absolute CD45RA-β7high CD4+ T cell counts (total) and unoccupied CD45RA-β7high CD4+ T cells are shown as a percentage of predose baseline.
Discussion
The treatment of moderate to severe IBD poses significant challenges to treating physicians, as conventional therapy with corticosteroids and immunomodulator therapy (e.g. azathioprine, 6-mercaptopurine and methotrexate) is associated with side effects and intolerance, and corticosteroids have not shown benefit in maintenance therapy. Monoclonal antibodies targeting TNF-α, such as infliximab (a chimera), adalimumab (a fully human antibody) and certolizumab pegol (pegylated antibody Fab′ fragment) are currently approved for the management of CD. Infliximab has also shown efficacy and has been approved for use in UC. However, approximately 10–20% of patients with CD are primary non-responders to anti-TNF therapy, and another ∼20–30% of CD patients lose response over time (Schnitzler et al., 2009). Other adverse events associated with anti-TNFs include elevated rates of bacterial infection, including tuberculosis, and, more rarely, lymphoma and demyelination (Chang and Lichtenstein, 2006). No currently available therapy achieves sustained remission in more than 20–30% of IBD patients with chronic disease (Hanauer et al., 2002; Sandborn et al., 2005). Therefore, there is a need to develop a more targeted therapy in IBD that is optimized for chronic use in terms of effecting an improved safety profile, sustained remission in a greater proportion of patients with a view to preventing long-term complications and that can be effective in patients who either never respond to an anti-TNF or lose response over time.
Observations from the non-clinical studies presented here suggest that rhuMAb Beta7 has the potential to provide selective targeting of lymphocyte homing to mucosal sites, with limited to no effects on non-mucosal cells. First, rhuMAb Beta7 significantly blocked lymphocyte homing to the inflamed colon of CD45RBhigh cell-reconstituted SCID colitic mice without any observed effects on lymphocyte homing to spleen, a non-mucosal lymphoid organ. In addition, in the MBP-TCR transgenic mouse model of EAE, anti-β7 (muFIB504) had no apparent effect on either the clinical severity of disease or the histological degree of inflammatory cellular infiltration into the CNS. In contrast, an anti-murine α4 MAb significantly decreased both clinical severity and inflammatory infiltration in this model. These results support the conclusion that anti-β7 antibodies, unlike anti-α4 antibodies, do not impair lymphocyte trafficking to the CNS.
In agreement with these data, two previous studies addressed the effects of anti-β7 integrin antibodies in murine EAE. In SJL/J mice with EAE, induced by phospholipoprotein-specific T cells, antibodies against α4 (PS/2) inhibited or diminished clinical and histopathological signs of EAE while antibodies against β7 (FIB504 and FIB30) or α4β7 (DATK-32) had no effect, although encephalitogenic T cells expressed both α4β1 and α4β7 integrins (Engelhardt et al., 1998). In C57BL/6 mice with severe established EAE induced by adoptive transfer of MOG35-55 stimulated lymphoblasts, anti-α4 was able to effect full remission of disease. Anti-β7 (FIB504) did not induce remission and had only a moderate effect on disease severity. This moderate effect was considered to be due to altered homing due to the severity of the disease, rather than to a disruption of normal homing signals in the absence of severe inflammation. This suggests a role for β7 solely in the maintenance of inflammation rather than specific homing to the CNS, which appears to be dominated by α4β1: VCAM interactions (Engelhardt et al., 1998; Kanwar et al., 2000; Engelhardt, 2006). Taken together, these data suggest that rhuMAb Beta7 should provide a more selective approach to targeting the intestinal mucosa compared with anti-α4 integrin molecules such as natalizumab, which blocks homing to non-mucosal and mucosal tissues (Baker, 2007).
In agreement with the observations in mice, rhuMAb Beta7 provided targeted specificity for mucosal-homing lymphocytes in cynomolgus monkeys. Administration of rhuMAb Beta7 induced a moderate increase in peripheral blood lymphocytes that was largely due to a marked increase in CD45RA-β7high CD4+ and CD45RA-β7high CD8+ T cells (data not shown), subsets phenotypically similar to the memory/effector T cells in humans and mice that preferentially home to mucosal lymph nodes and tissues (Rott et al., 1996; 1997;) and have been shown to contribute to immunity to mucosal-specific antigens (Roséet al., 1998). These observations are consistent with the proposed mechanism of action of rhuMAb β7: inhibition of homing of β7 positive lymphocytes to the gut, through blockade of α4β7 binding to its ligand, MAdCAM-1. This mechanism is expected to lead to the observed accumulation of CD45RA-β7high CD4+ T cells in the peripheral circulation. As this subset represents ∼2–5% of the total lymphocyte count, a change in the total lymphocyte count in the peripheral blood is not expected and was not observed (data not shown). In contrast, rhuMAb Beta7 had no apparent effect on CD45RA-β7low peripheral blood T cells, a subset phenotypically similar to the memory/effector T cells that preferentially home to non-mucosal tissues and provide functional immunity to systemic antigens (Rott et al., 1996; Roséet al., 1998; Butcher et al., 1999). The binding of rhuMAb Beta7 to this limited population of CD45RA-β7high CD4+ T cells is similar to what has been reported for vedolizumab (anti-α4β7) in human blood (Soler et al., 2009). This supports the hypothesis that rhuMAb Beta7 selectively targets the α4β7 gut-homing cells. The impact of rhuMAb Beta7 treatment on cells expressing αEβ7 was not assessed because this cell population is represented at an extremely low frequency in the peripheral blood.
The increase in CD45RA-β7high (‘mucosal-homing’) peripheral blood T cells correlated with occupation of β7 receptors on these cells and serum concentrations of rhuMAb Beta7. The levels of the mucosal-homing T cells returned to baseline when rhuMAb Beta7 serum concentrations dropped below ∼1–10 µg·mL−1. Taken together, these data support the conclusion that rhuMAb Beta7 specifically inhibits the trafficking of β7 positive lymphocytes to, and potentially their retention in, the gastrointestinal tract, with little to no effect on lymphocytes homing to non-mucosal tissues.
In mice, modulation and saturation of β7 receptors on T cells by rhuMAb Beta7 in blood show parallel effects on T cells in intestinal tissues, as treatment of normal mice with rhuMAb Beta7 resulted in decreased β7 integrin receptors on peripheral blood T cells and intraepithelial T cells in the intestine. Saturation and down-modulation of β7 receptors on blood and intraepithelial T cells correlated with serum concentrations of rhuMAb Beta7. Similar to cynomolgus monkeys, the serum concentration of rhuMAb Beta7 required to maintain saturation of β7 receptors on T cells in peripheral blood in mice was approximately 1–10 µg·mL−1. Interestingly, unlike the results seen in mice, there was no apparent down-modulation of total β7 receptor expression levels on blood T cells following administration of rhuMAb Beta7 to cynomolgus monkeys (data not shown). This may be due to varying levels of β7 receptor expression in the two species, which may affect receptor internalization.
The observations from the murine study, as well as the study in cynomolgus monkeys (despite the differences in profile), support the measurement of drug occupancy, modulation of β7 receptors and absolute numbers of β7-expressing T cells as potential exploratory PD biomarkers in humans. Evidence exists to support the use of these circulating T cell subsets as biomarkers of altered T-cell homing in human disease. Studies have suggested that there are increased numbers of circulating activated gut-homing T cells (CD4+ CD25+α4β7+) in Crohn's and UC patients (Meenan et al., 1997), while there is an overall decrease in circulating gut-homing memory T cells (CD4+ CD45RO+α4β7+) in Crohn's, UC and active coeliac disease patients (Meenan et al., 1997; Di Sabatino et al., 2009). Similarly, further studies have shown a decrease in circulating α4β7+ gut-homing T cells in CD, which is associated with increased expression of MAdCAM-1 in the gastrointestinal tracts of patients (Di Sabatino et al., 2009).
Inhibition of lymphocyte homing to the CNS via α4β1:VCAM-1 interactions and release of progenitor cells from the bone marrow via VCAM-1 blockade have been suggested as potential mechanisms for the development of PML in patients treated with natalizumab (Major, 2009; Allison, 2010). rhuMAb Beta7 does not target α4β1:VCAM-1 interactions, does not block homing to CNS or non-mucosal tissues and shows no efficacy in the EAE mouse model of MS. rhuMAb Beta7 therefore provides a more targeted approach to the inhibition of leucocyte trafficking to and retention in the gut mucosa and is a more attractive option for IBD therapy.
As rhuMAb Beta7 blocks both α4β7 and αEβ7 interactions with their ligands and disrupts homing to mucosal tissues expressing these ligands, the gastrointestinal, respiratory and urogenital tracts could potentially have increased susceptibility to opportunistic infections. However, this is a similar risk for any T cell directed or anti-cytokine therapy. Published reports suggest that the urogenital tract may potentially be a reservoir for the JC virus (Ferrante et al., 1997); however, a role of αEβ7-expressing cells in normal immune surveillance of the urogenital tract has not been demonstrated. αE-expressing dendritic cells in the lung have been shown to be players in the immune response to viral infection in mouse (GeurtsvanKessel et al., 2008); however, the importance of αE in immune monitoring of the respiratory system is not fully understood. There is high turnover of αE+ dendritic cells in the lung, with continual replenishment from blood monocytes (Jakubzick et al., 2008). While we cannot exclude the possibility that disruption of the αEβ7:E-cadherin interaction may impact immune protection against respiratory viruses, we did not observe any pulmonary toxicity, including any evidence of pulmonary infections, in cynomolgus monkeys or mice given anti-β7 at doses as high as 50 mg·kg−1 for as long as 26 weeks (unpublished data).
In healthy humans and mice, αEβ7, another molecule targeted by rhuMAb Beta7, is expressed on T cells and dendritic cells in the mucosal immune system (gastrointestinal, respiratory and urogenital tracts), and is prominent on intraepithelial lymphocytes in the gut (Kilshaw and Murant, 1990; Kilshaw, 1993; Parker et al., 1992; Erle et al., 1994). In normal human tissues, expression of αEβ7 is restricted to the mucosal immune system, spleen and thymus (Shaw et al., 1994). In inflammatory diseases, αE expression can be detected on T cells in non-mucosal tissues. For example, αE is expressed on T cells in the joints of RA patients, in plaques of psoriatic patients (but not in lesion-free skin), in alveolar T cells in interstitial lung disease, and in CD8+ T cell infiltrates associated with kidney tubular epithelium during acute rejection of renal allografts (Baumgart et al., 1996; Lohmeyer et al., 1999; Robertson et al., 1999; Teraki and Shiohara, 2002). In Sjogren's syndrome, αE is associated with the ductal epithelium of salivary and lachrymal glands (Kroneld et al., 1998; Fujihara et al., 1999). While the role of αEβ7 is not well understood, experiments with αE null/null mice suggest that the integrin is involved in retention of T cells in or near the mucosal epithelia, probably through interactions with E-cadherin (Schön et al., 1999).
rhuMAb Beta7 targets both α4β7:MAdCAM-1 and αEβ7:E-cadherin interactions. Clinical trial data in UC from drugs targeting the α4β7:MAdCAM-1 interaction have shown efficacy (vedolizumab) and/or biological activity (anti-MAdCAM-1) and are well tolerated (Feagan et al., 2005; 2008; Scholz et al., 2009; Vermeire et al., 2009). By inhibiting both αEβ7 and α4β7 binding to their respective ligands, MAdCAM-1 and E-cadherin, rhuMAb Beta7 may be more effective at reducing inflammation at the site of disease activity than blocking α4β7 alone. Although a recent study has suggested that inhibition of αEβ7 interactions with E-cadherin would have systemic effects (Soler et al., 2009), the results described here in the murine EAE model – as well as data from αEβ7 knockout mice (Kilshaw, 1999; Schön et al., 1999) and expression patterns in human (Shaw et al., 1994) – support the hypothesis that the effects of rhuMAb Beta7 are mucosa-specific and not systemic. Additionally, no adverse effects were observed in any of the parameters examined (body weights, food consumption, clinical signs, clinical pathology, immunophenotyping and anatomical pathology) in cynomolgus monkeys administered rhuMAb Beta7 weekly for 12 weeks at doses up to 50 mg·kg−1 (unpublished observations).
The studies in mice and cynomolgus monkeys presented here are consistent with selective targeting of rhuMAb Beta7 for subsets of lymphocytes involved in homing and retention in mucosal sites, while leaving non-mucosal lymphocytes unaffected. These data suggest that rhuMAb Beta7 has the potential to provide superior safety profiles compared with therapeutic agents that block leucocyte trafficking to both non-mucosal and mucosal lymph nodes and tissues, as well as potential superior efficacy to agents that inhibit only α4β7. The selective targeting, along with the observed strong safety profiles in non-human primates, suggests that rhuMAb Beta7 may provide a better therapeutic window for UC and CD patients than currently available therapies. By blocking β7-expressing cells from entering intestinal sites, without affecting trafficking of leucocytes to non-mucosal sites, rhuMAb Beta7 could provide specific down-modulation of the mucosal inflammatory response that is characteristic of IBD, thereby allowing re-establishment of homeostasis in the intestinal immune system.
Acknowledgments
The authors acknowledge the financial support of Genentech for these studies, and the contributions of Mark Dennis in generating rhuMAb Beta7.
Glossary
Abbreviations
- APC
allophycocyanin
- ATA
anti-therapeutic antibody
- AUC
area under the curve
- CD
Crohn's disease
- CDR
complementarity-determining region
- CL
clearance
- EAE
experimental autoimmune encephalomyelitis
- FACS
fluorescence-activated cell sorting
- FITC
fluorescein isothiocyanate
- FSC
forward scatter
- GMFI
geometric mean fluorescence intensity
- HRP
horseradish peroxidase
- IBD
inflammatory bowel disease
- IgG
immunoglobulin G
- MAb
monoclonal antibody
- MAdCAM-1
mucosal addressin cell adhesion molecule-1
- MBP
myelin basic protein
- MOEF
molecules of equivalent fluorescence
- MS
multiple sclerosis
- PD
pharmacodynamic
- PE
R-phycoerythrin
- PerCP
peridinin chlorophyll
- PML
progressive multifocal leucoencephalopathy
- PK
pharmacokinetic
- SCID
severe combined immunodeficient
- SSC
side scatter
- TCR
T-cell receptor
- UC
ulcerative colitis
- VCAM-1
vascular cell adhesion molecule-1
- VH
heavy chain variable region
- VL
light chain variable region
Conflict of interest
The authors are current or former employees of Genentech, a member of the Roche group.
Supporting Information
Teaching Materials; Figs 1–10 as PowerPoint slide.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edition. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison M. PML problems loom for Rituxan. Nat Biotechnol. 2010;28:105–106. doi: 10.1038/nbt0210-105. [DOI] [PubMed] [Google Scholar]
- Andrew DP, Berlin C, Honda S, Yoshino T, Hamann A, Holzmann B, et al. Distinct but overlapping epitopes are involved in α4β7-mediated adhesion to vascular cell adhesion molecule-1, mucosal adhesion molecule-1, fibronectin, and lymphocyte aggregation. J Immunol. 1994;153:3847–3861. [PubMed] [Google Scholar]
- Baca M, Presta LG, O'Connor SJ, Wells JA. Antibody humanization using monovalent phage display. J Biol Chem. 1997;272:10678–10684. doi: 10.1074/jbc.272.16.10678. [DOI] [PubMed] [Google Scholar]
- Baker DE. Natalizumab: overview of its pharmacology and safety. Rev Gastroenterol Disord. 2007;7:38–46. [PubMed] [Google Scholar]
- Baumgart M, Witt C, Hüge W, Müller B. Increase in the expression of alpha E beta 7, characteristic of intestinal intraepithelial lymphocytes, on cells in the lung epithelium of patients with interstitial lung diseases and in synovial fluid of patients with rheumatic diseases. Immunobiology. 1996;196:415–424. doi: 10.1016/s0171-2985(96)80063-5. [DOI] [PubMed] [Google Scholar]
- Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- Cepek KL, Parker CM, Madara JL, Brenner MB. Integrin αEβ7 mediates adhesion of T lymphocytes to epithelial cells. J Immunol. 1993;150:3459–3470. [PubMed] [Google Scholar]
- Chang JT, Lichtenstein GR. Drug insight: antagonists of tumor-necrosis factor-α in the treatment of inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol. 2006;3:220–228. doi: 10.1038/ncpgasthep0447. [DOI] [PubMed] [Google Scholar]
- Cheroutre H, Madakamutil L. Mucosal effector memory T cells: the other side of the coin. Cell Mol Life Sci. 2005;62:2853–2866. doi: 10.1007/s00018-005-5232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetter P, De Voss M, Van Damme N, Baeten D, Elewaut D, Vermeulen S, et al. Focal upregulation of E-Cadherin-Catenin complex in inflammed bowel mucosa but reduced expression in ulcer-associated cell lineage. Am J Clin Pathol. 2000;114:364–370. doi: 10.1093/ajcp/114.3.364. [DOI] [PubMed] [Google Scholar]
- Dennis MS. Humanization by CDR repair in pharmaceutical aspects of monoclonal antibodies. In: Shire S, Gombotz W, Bechtold-Peters K, Andya J, editors. Current Trends in Monoclonal Antibody Development and Manufacturing. New York: Association for Pharmaceutical Scientists & Springer; 2010. pp. 9–28. Co-published by the. [Google Scholar]
- Di Sabatino A, Rovedatti L, Rosado MM, Carsetti R, Croazza GR, MacDonald TT. Increased expression of mucosal addressin cell adhesion molecule 1 in the duodenum of patients with active celiac disease is associated with depletion of intergrin α4β7-positive T cells in blood. Human Path. 2009;40:699–704. doi: 10.1016/j.humpath.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Elewaut D, De Keyser F, Cuvelier C, Lazarovits AI, Mielants H, Verbruggen G, et al. Distinctive activated cellular subsets in colon from patients with Crohn's disease and ulcerative colitis. Scand J Gastroenterol. 1998;33:743–748. doi: 10.1080/00365529850171693. [DOI] [PubMed] [Google Scholar]
- Engelhardt B. Molecular mechanisms involved in T cell migration across the blood-brain barrier. J Neural Transm. 2006;113:477–485. doi: 10.1007/s00702-005-0409-y. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Laschinger M, Schulz M, Samulowitz U, Vestweber D, Hoch G. The development of experimental autoimmune encephalomyelitis in the mouse requires α4-integrin, but not α4β7-integrin. J Clin Invest. 1998;102:2096–2105. doi: 10.1172/JCI4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erle DJ, Brown T, Christian D, Aris R. Lung epithelial lining fluid T cell subsets defined by distinct patterns of beta 7 and beta 1 intergrin expression. Am J Respir Cell Mol Biol. 1994;10:237–244. doi: 10.1165/ajrcmb.10.3.7509610. [DOI] [PubMed] [Google Scholar]
- Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, et al. Treatment of ulcerative colitis with a humanized antibody to the α4β7 integrin. N Engl J Med. 2005;352:2499–2507. doi: 10.1056/NEJMoa042982. [DOI] [PubMed] [Google Scholar]
- Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, et al. Treatment of active Crohn's disease with MLN0002, a humanized antibody to the α4β7 integrin. Clin Gastroenterol Hepatol. 2008;6:1370–1377. doi: 10.1016/j.cgh.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Ferrante P, Caldarelli-Stefano R, Omodeo-Zorini E, Cagni AE, Cocchi L, Suter F, et al. Comprehensive investigation of the presence of JC virus in AIDS patients with and without progressive multifocal leukoencephalopathy. J Med Virol. 1997;52:235–242. doi: 10.1002/(sici)1096-9071(199707)52:3<235::aid-jmv1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Fujihara T, Fujita H, Tsubota K, Saito K, Tsuzaka K, Abe T, et al. Preferential localization of CD8+ alpha E beta 7+ T cells around acinar epithelial cells with apoptosis in patients with Sjogren's syndrome. J Immunol. 1999;163:2226–2235. [PubMed] [Google Scholar]
- GeurtsvanKessel CH, Willart MA, van Rijt LS, Muskens F, Kool M, Baas C, et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J Exp Med. 2008;7:1621–1634. doi: 10.1084/jem.20071365. Humanization by CDR Repair in Pharmaceutical Aspects of Monoclonal Antibodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibaldi M, Perrier D. Pharmacokinetics, 2nd edn (Drugs and the Pharmaceutical Sciences) Vol. 15. New York: Marcel Dekker; 1982. [Google Scholar]
- Gurish MF, Tao H, Abonia JP, Arya A, Friend DS, Parker CM, et al. Intestinal mast cell progenitors require CD49dβ7 (α4β7 integrin) for tissue-specific homing. J Exp Med. 2001;194:1243–1252. doi: 10.1084/jem.194.9.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomized trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- Hardardottir F, Baron JL, Janeway CA., Jr T cells with two functional antigen-specific receptors. Proc Natl Acad Sci USA. 1995;92:354–358. doi: 10.1073/pnas.92.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesterberg PE, Winsor-Hines D, Briskin MJ, Soler-Ferran D, Merrill C, Mackay CR, et al. Rapid resolution of chronic colitis in the cotton-top tamarin with an antibody to a gut-homing integrin alpha 4 beta 7. Gastroenterology. 1996;111:1373–1380. doi: 10.1053/gast.1996.v111.pm8898653. [DOI] [PubMed] [Google Scholar]
- Higgins JM, Mandlebrot DA, Shaw SK, Russell GJ, Murphy EA, Chen Y, et al. Direct and regulated interaction of integrin αEβ7 with E-cadherin. J Cell Biol. 1998;140:197–210. doi: 10.1083/jcb.140.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bi-directional, allosteric, signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Institute for Laboratory Animal Research Commission on Life Sciences. Guide for the Care and use of Laboratory Animals. Washington, DC: National Academies Press; 1996. [Google Scholar]
- Jakubzick C, Tacke F, Ginhoux F, Wagers AJ, van Rooijen N, Mack M, et al. Blood monocyte subsets differentially give rise to CD103+ and CD103- pulmonary dendritic cell populations. J Immunol. 2008;180:3019–3027. doi: 10.4049/jimmunol.180.5.3019. [DOI] [PubMed] [Google Scholar]
- Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Förster R, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar JR, Harrison JE, Wang D, Leung E, Mueller W, Wagner N, et al. Beta7 integrins contribute to demyelinating disease of the central nervous system. J Neuroimmunol. 2000;103:146–152. doi: 10.1016/s0165-5728(99)00245-3. [DOI] [PubMed] [Google Scholar]
- Karecla PI, Bowden SJ, Green SJ, Kilshaw PJ. Recognition of E-cadherin on epithelial cells by the mucosal T cell integrin alpha M290 beta 7 (alpha E beta 7) Eur J Immunol. 1995;25:852–856. doi: 10.1002/eji.1830250333. [DOI] [PubMed] [Google Scholar]
- Kilshaw PJ. Expression of the mucosal T cell integrin aM290b7 by a major subpopulation of dendritic cells in mice. Eur J Immunol. 1993;23:3365–3368. doi: 10.1002/eji.1830231246. [DOI] [PubMed] [Google Scholar]
- Kilshaw PJ. Alpha E beta 7. J Clin Pathol. 1999;52:203–207. doi: 10.1136/mp.52.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilshaw PJ, Murant SJ. A new surface antigen on intraepithelial lymphocytes in the intestine. Eur J Immunol. 1990;20:2201–2207. doi: 10.1002/eji.1830201008. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- Kroneld U, Jonsson R, Carlsten H, Bremell T, Johannessen AC, Tarkowski A. Expression of the mucosal lymphocyte integrin αEβ7 and its ligand E-cadherin in salivary glands of patients with Sjogren's syndrome. Scand J Rheumatol. 1998;27:215–218. doi: 10.1080/030097498440831. [DOI] [PubMed] [Google Scholar]
- Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- Lohmeyer J, Friedrich J, Grimminger F, Maus U, Tenter R, Morr H, et al. Expression of mucosa-related integrin alphaEbeta7 on alveolar T cells in interstitial lung diseases. Clin Exp Immunol. 1999;116:340–346. doi: 10.1046/j.1365-2249.1999.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lúdvíksson BR, Strober W, Nishikomori R, Hasan SK, Ehrhardt RO. Administration of mAb against αEβ7 prevents and ameliorates immunization-induced colitis in IL-2 -/- mice. J Immunol. 1999;162:4975–4982. [PubMed] [Google Scholar]
- Major EO. Reemergence of PML in natalizumab-treated patients – new cases, some concerns. N Engl J Med. 2009;361:1041–1043. doi: 10.1056/NEJMp0906248. [DOI] [PubMed] [Google Scholar]
- Meenan J, Spaans J, Grool TA, Pals ST, Tytgat GNJ, van Deventer SJ. Altered expression of a gut homing integrin, by circulating and mucosal T cells in colonic mucosal inflammation. Gut. 1997;40:241–246. doi: 10.1136/gut.40.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med. 1993;178:237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang M, Abe T, Fujihara T, Mori S, Tsuzaka K, Amano K, et al. Up-regulation of αEβ7, a novel integrin adhesion molecule, on T cells from systemic lupus erythematosus patients with specific epithelial involvement. Arthritis Rheum. 1998;41:1456–1463. doi: 10.1002/1529-0131(199808)41:8<1456::AID-ART16>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Parker CM, Cepek K, Russell GJ, Shaw SK, Posnett DN, Schwarting R, et al. A family of beta 7 integrins on human mucosal lymphocytes. Proc Natl Acad Sci USA. 1992;89:1924–1928. doi: 10.1073/pnas.89.5.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picarella D, Hurlbut P, Rottman J, Shi X, Butcher E, Ringler DJ. Monoclonal antibodies specific for beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of SCID mice reconstituted with CD45RBhigh CD4+ T cells. J Immunol. 1997;158:2099–2106. [PubMed] [Google Scholar]
- Pullen N, Molloy E, Carter D, Syntin P, Clemo F, Finco-Kent D, et al. Pharmacological characterization of PF-00547659, an anti-human MAdCAM monoclonal antibody. Br J Pharmacol. 2009;157:281–293. doi: 10.1111/j.1476-5381.2009.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GP, Hartung HP, Calabresi PA. Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology. 2005;64:1336–1342. doi: 10.1212/01.WNL.0000158329.30470.D0. [DOI] [PubMed] [Google Scholar]
- Robertson H, Wong WK, Burt AD, Kirby JA. Renal allograft rejection: does CD103 hold the clue to tissue-specific cytotoxicity? Immunology. 1999;98:125. [Google Scholar]
- Rosé JR, Williams MB, Rott LS, Butcher EC, Greenberg HB. Expression of the mucosal homing receptor α4β7 correlates with the ability of CD8+ memory T cells to clear rotavirus infection. J Virol. 1998;72:726–730. doi: 10.1128/jvi.72.1.726-730.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott LS, Briskin MJ, Andrew DP, Berg EL, Butcher EC. A fundamental subdivision of circulating lymphocytes defined by adhesion to mucosal addressin cell adhesion molecule-1. Comparison with vascular cell adhesion molecule-1 and correlation with β7 integrins and memory differentiation. J Immunol. 1996;156:3727–3736. [PubMed] [Google Scholar]
- Rott LS, Rosé JR, Bass D, Williams MB, Greenberg HB, Butcher EC. Expression of mucosal homing receptor α4β7 by circulating CD4+ cells with memory for intestinal rotavirus. J Clin Invest. 1997;100:1204–1208. doi: 10.1172/JCI119633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, et al. Natalizumab induction and maintenance therapy for Crohn's disease. N Engl J Med. 2005;353:1912–1925. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
- Schnitzler F, Fidder H, Ferrante M, Norman M, Arijs I, Van Assche G, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn's disease: results from a single-centre cohort. Gut. 2009;58:492–500. doi: 10.1136/gut.2008.155812. [DOI] [PubMed] [Google Scholar]
- Scholz C, Wyant T, Leach T, Sankoh S, Mould DR, Patella M, et al. Clinical pharmacology of vedolizumab (MLN0002) in patients with active ulcerative colitis. J Crohns Colitis. 2009;3:S75–S76. [Google Scholar]
- Schön MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin αE (CD103)-deficient mice. J Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- Shaw SK, Cepek KL, Murphy EA, Russell GJ, Brenner MB, Parker CM. Molecular cloning of the human mucosal lymphocyte integrin aE subunit. Unusual structure and restricted RNA distribution. J Biol Chem. 1994;269:6016–6025. [PubMed] [Google Scholar]
- Soler D, Chapman T, Yang LL, Wyant T, Eagan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, and anti-α4β7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330:864–875. doi: 10.1124/jpet.109.153973. [DOI] [PubMed] [Google Scholar]
- Souza HS, Elia CC, Spencer J, MacDonald TT. Expression of lymphocyte-endothelial receptor-ligand pairs, α4β7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut. 1999;45:856–863. doi: 10.1136/gut.45.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targan SR, Feagan BG, Fedorak RN, Lashner BA, Panaccione R, Present DH, et al. Natalizumab for the treatment of active Crohn's disease: results of the ENCORE trial. Gastroenterology. 2007;5:1672–1683. doi: 10.1053/j.gastro.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Teraki Y, Shiohara T. Preferential expression of αEβ7 integrin (CD103) on CD8+ T cells in the psoriatic epidermis: regulation by interleukins 4 and 12 and transforming growth factor-β. Br J Dermatol. 2002;147:1118–1126. doi: 10.1046/j.1365-2133.2002.05005.x. [DOI] [PubMed] [Google Scholar]
- Van Assche G, Van Ranst M, Sciot R, Dubois B, Vermeire S, Noman M, et al. Progressive Multifocal Leukoencephalopathy after natalizumab therapy for Crohn's disease. N Engl J Med. 2005;353:362–368. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- Vermeire S, Ghosh S, Panes J, Dahlerup J, Luegering A, Sirotiakova J, et al. Safety and efficacy of PF-00547,659, a fully human anti-MAdCAM antibody, in ulcerative colitis. Results of a first-in-human study. Gastroenterology. 2009;136(Suppl 1):A–132. [Google Scholar]
- Von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- Williams MB, Butcher EC. Homing of naïve and memory T lymphocyte subsets to Peyer's patches, lymph nodes, and spleen. J Immunol. 1997;159:1746–1752. [PubMed] [Google Scholar]
- Williams MB, Rosé JR, Rott LS, Franco MA, Greenberg HB, Butcher EC. The memory B cell subset responsible for the secretory IgA response and protective humoral immunity to rotavirus expresses the intestinal homing receptor, α4β7. J Immunol. 1998;161:4227–4235. [PubMed] [Google Scholar]
- Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4β1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


