Abstract
Pichia guilliermondii is a representative of a group of so-called flavinogenic yeast species that overproduce riboflavin (vitamin B2) in response to iron limitation. Using insertion mutagenesis, we isolated P. guilliermondii mutants overproducing riboflavin. Analysis of nucleotide sequence of recombination sites revealed that insertion cassettes integrated into the genome disrupting P. guilliermondii genes similar to the VMA1 gene of Ashbya gossypii and Saccharomyces cerevisiae and FES1 and FRA1 genes of S. cerevisiae. The constructed P. guilliermondii Δvma1–17 mutant possessed five- to sevenfold elevated riboflavin production and twofold decreased iron cell content as compared with the parental strain. Pichia guilliermondii Δfra1–45 mutant accumulated 1.8–2.2-fold more iron in the cells and produced five- to sevenfold more riboflavin as compared with the parental strain. Both Δvma1–17 and Δfes1–77 knockout strains could not grow at 37 °C in contrast to the wild-type strain and the Δfra1–45 mutant. Increased riboflavin production by the wild-type strain was observed at 37 °C. Although the Δfes1–77 mutant did not overproduce riboflavin, it showed partial complementation when crossed with previously isolated P. guilliermondii riboflavin-overproducing mutant rib80–22. Complementation analysis revealed that Δvma1–17 and Δfra1–45 mutants are distinct from previously reported riboflavin-producing mutants hit1-1, rib80-22 and rib81-31 of this yeast.
Keywords: riboflavin biosynthesis, iron acquisition, VMA1, FES1, FRA1, oxidative stress
Introduction
It is known that in certain yeast species, iron deprivation, in addition to the activation of iron transport, causes the activation of riboflavin biosynthesis (Tanner et al., 1945; Shavlovskii & Logvinenko, 1988). This group includes Candida albicans, Pichia guilliermondii, Schwanniomyces occidentalis, Debaryomyces hansenii and the industrially important species Candida famata (recently reidentified as Candida flareri and its teleomorph form as Debaryomyces subglobosus) (Shavlovskii & Logvinenko, 1988; Voronovsky et al., 2002; Santos et al., 2004; Dmytruk et al., 2006; Nguyen et al., 2009; Sibirny & Boretsky, 2009). This phenomenon was also reported in some bacteria and plants (Fassbinder et al., 2000; Crossley et al., 2007; Vorwieger et al., 2007). It should be noted that iron does not regulate riboflavin synthesis in the best-studied yeast Saccharomyces cerevisiae and as well as in the vast majority of other yeast species (Philpott & Protchenko, 2008).
Yeast P. guilliermondii (anamorph is also known as Candida guilliermondii) is a convenient model organism for studying the inter-relationships between iron and flavin metabolisms. A large collection of P. guilliermondii mutants defective in the regulation of riboflavin biosynthesis has been created in the past few years. It was demonstrated that P. guilliermondii mutants constitutively overproducing riboflavin (rib80-22, rib81-31, hit1-1, red1–6) also exhibit both increased ferrireductase activity and high levels of iron transport. These mutations are recessive, monogenic and are not linked to the structural genes of the riboflavin biosynthetic pathway (Shavlovskii et al., 1990, 1993; Fedorovich et al., 1999; Stenchuk & Kapustiak, 2003). However, the corresponding genes have not been identified, mostly due to the absence of a useful phenotype in the mutants for gene cloning. To explore the genetic mechanisms affecting riboflavin biosynthesis, we used insertion mutagenesis in order to isolate P. guilliermondii mutants defective in the regulation of this biosynthetic pathway. Based on this screen, three genes designated PgVMA1, PgFES1 and PgFRA1 were identified. Here, we describe the construction and properties of the corresponding P. guilliermondii insertion and deletion strains.
Materials and methods
Strains, growth conditions and media
Pichia guilliermondii strains used in this study are listed in Table 1. For plasmid construction and propagation, Escherichia coli strain DH5α [lacZΔM15 recA1 endA1 gyrA96 thi-1hsdR17(rK−mK+)supE44 relA1 deoR Δ(lacZYA-argF) U169] has been used. Orotidine 5′-monophosphate decarboxylase-deficient E. coli strain (pyrFcysB) was kindly provided by Dr J. M. Beckerich (Laboratoire de Microbiologie et Genetique Moleculaire, CNRS-Institut National Agronomique Paris-Grignon-INRA, Thiverval-Grignon, France).
Table 1.
Pichia guilliermondii strains used in this study
| Strains | Genotypes | Sources or references |
|---|---|---|
| R-66 | MAT− hisX ura3 | Pynyaha et al. (2009) |
| Δfes1–77 | Mat− fes1Δ::URA3ura3 hisX | This study |
| Δvma1–17 | Mat− vma1Δ::URA3ura3 hisX | This study |
| Δfra1–45 | Mat− fra1Δ::URA3ura3 hisX | This study |
| rib80-22 | MAT+rib80 metX | Shavlovskii et al. (1990) |
| rib81-31 | MAT+rib81 metX | Shavlovskii et al. (1993) |
| hit1-1 | MAT+ hit1 cytX | Fedorovich et al. (1999) |
Yeast cells were grown on the complete medium YPS (10 g yeast extract, 20 g peptone, 20 g sucrose, 20 g agar per litre) at 30 °C or synthetic Burkholder medium (40 mg L−1) and uridine (400 mg L−1) if required (Shavlovskii et al., 1990). Pichia guilliermondii insertional mutants were selected on a medium containing 0.67% yeast nitrogen base (YNB), 2% glucose, 0.5% vitamin-free casamino acids, 2% of agar. Yeast cells were grown in Erlenmeyer flasks on a gyro shaker (200 r.p.m.) at 30 °C. Yeast strain hybridization and subsequent spore progeny analysis were performed as described (Sibirnyi et al., 1977). Escherichia coli strains were grown in Luria–Bertani medium at 37 °C supplemented with ampicillin (100 μg mL−1) if necessary. Minimal medium M9 supplemented with 0.05% vitamin-free casamino acids was used for complementation experiments (Sambrook & Russell, 2001).
Plasmid construction and analysis
DNA manipulation and transformation of E. coli were carried out according to previously published procedures (Sambrook & Russell, 2001). To provide a high level of expression of the cassette-born modified URA3 gene of S. cerevisiae, it was placed under the control of P. guilliermondii strong constitutive promoter of glyceraldehyde 3-phosphate dehydrogenase (GAP1). Using plasmid pAGU34 as a template, a 0.9-kb DNA fragment bearing the URA3 gene (Boretsky et al., 2007a) was amplified by PCR with the primers JB25 and Ura32r, thereby introducing 5′-PstI and 3′-BamHI terminal sites (Table 2). Using P. guilliermondii chromosomal DNA, a 0.5-kb DNA fragment carrying promoter region of the GAP1 gene was amplified with the primers JB75 and JB76, thereby introducing 5′-BamHI and 3′-PstI terminal sites (Table 2). Both PCR products were purified, digested with BamHI and PstI restriction endonucleases and cloned into the BamHI site of the pUC57 vector. The resulting plasmid pGAPURA3 was used to generate a BamHI 1.4-kb DNA fragment carrying the modified URA3 gene of S. cerevisiae under the control of P. guilliermondii GAP1 gene promoter.
Table 2.
Primers used for this study
| Primers | Sequences (5′–3′) |
|---|---|
| JB 25 | ACCTGCAGGAAACGAAGATAAATC |
| Ura32r | CGGGATCCGGTAATAACTGATATAATT |
| JB75 | CTGGATCCAGTACTTGAAAAACGAACAATTATAG |
| JB76 | AACTGCAGTTTTCAATTCTGGTGAGTACCAGAT |
| JB67 | AAGGATCCTTGCGAGTTGACGGAATATCG |
| JB77 | TTGAATTCAAGCTTCACATAA |
| JB68 | GCAGATCTCGAAAATAATCCGATCGATTG |
| JB69 | TGAGATCTCAATTGTCACAGCTTAAATACC |
| JB 61 | TCGTGAGCTCGGGTGCAAATTG |
| JB62 | TTGGAGCTCCCAAACCATCGCAA |
| JB63 | CAGTTCACAATTGCAACCTAGGCT |
| JB64 | TAAGATCTATGATGCATTCTGTCCAAT |
| JB65 | CGAGATCTGCGTTAGTGTCTAAGACC |
| JB72 | TCGGATCCGATCATTTCGGTATAACG |
| UB1 | CTTCGTCGACGAAGAAAATCGTCCTG |
| UB2 | ATCAGTCGACTGATATAATTAAATTGAAGCTC |
| UB3 | TGTTGAATTCAACGTTTAATTGGGATGC |
| UB4 | CAAGAATTCTTGTGGTATGCTAAGTGAATG |
| UB5 | AAGTCTCGAGACTCTTCTGGTACTAATA |
| UB6 | ACTGCTCGAGCAGTGATACACTACAA |
| UB7 | TTCAACGTTTAATTGGGATGC |
| UB8 | ATCACTGTGATGGCCTTGTGCAC |
| JB42 | GACACCAAAGTGCCAGATTCGTTG |
To construct the fes1::URA3 deletion cassette, a 3.3-kb DNA fragment of P. guilliermondii chromosomal DNA bearing the FES1 gene was amplified by PCR using the primers JB67 and JB77 (Table 2), thereby introducing EcoRI and BamHI sites at the ends. The PCR product was purified, digested with EcoRI and BamHI restriction endonucleases and cloned into the same sites of the pUC57 vector. The pFES1 plasmid constructed carried the FES1 structural gene flanked with 1.5 and 0.9 kb of promoter and terminator sequences, respectively. This plasmid was used to substitute FES1 structural gene with the S. cerevisiae URA3 gene. Almost the entire sequence of the pFES1 plasmid, except for the FES1 structural gene, was amplified with the primers JB68 and JB69 (Table 2), thereby introducing BglII sites at the ends of the PCR product. The PCR product was purified, digested with BglII restriction endonuclease and ligated with the 1.4-kb BamHI fragment of pGAPURA3 plasmid carrying the modified S. cerevisiae URA3 gene. The resulting plasmid pFES1URA3 carried the modified S. cerevisiae URA3 gene inserted between 1.5 and 0.9 kb of promoter and terminator sequences of the P. guilliermondii FES1 gene, respectively. Then plasmid pFES1URA3 was digested with EcoRI and BamHI endonucleases, yielding a fes1::URA3 deletion cassette that was used for the transformation of the P. guilliermondii R-66 strain.
A 4.1-kb DNA fragment of P. guilliermondii chromosomal DNA bearing the VMA1 gene was amplified by PCR using the primers JB61 and JB62 (Table 2), thereby introducing SacI sites at the ends. The PCR product was purified, digested with SacI restriction endonuclease and cloned into the SacI site of the pUC57 vector. The pVMA1 plasmid constructed carried the VMA1 structural gene flanked with 1.1 and 1.3 kb of promoter and terminator sequences, respectively. This plasmid was used to substitute the VMA1 structural gene with the S. cerevisiae URA3 gene. Almost the entire sequence of the pVMA1 plasmid, except for the VMA1 structural gene, was amplified with the primers JB64 and JB65 (Table 2), thereby introducing BglII sites at the ends of the PCR product. The PCR product was purified, digested with BglII restriction endonuclease and ligated with the 1.5-kb BamHI fragment of pPGKURA3 plasmid carrying the modified S. cerevisiae URA3 gene (Pynyaha et al., 2009). The resulting plasmid pVMA1URA3 carried the modified S. cerevisiae URA3 gene inserted between 1.1 and 1.3 kb of the promoter and the terminator sequences of the P. guilliermondii VMA1 gene, respectively. Then plasmid pVMA1URA3 was digested with SacI endonuclease, yielding a vma1::URA3 deletion cassette, which was used for the transformation of the P. guilliermondii R-66 strain.
A 3.7-kb DNA fragment of P. guilliermondii chromosomal DNA bearing the FRA1 gene was amplified by PCR using primers UB3 and UB4 (Table 2), thereby introducing EcoRI sites at the ends. The PCR product was purified, digested with EcoRI restriction endonuclease and cloned into the EcoRI site of the pUC57 vector. The pFRA1 plasmid constructed carried the FRA1 structural gene flanked with 1.1 and 0.5 kb of promoter and terminator sequences, respectively. This plasmid was used to substitute 1.5 kb of the 5′-part of the FRA1 structural gene with the S. cerevisiae URA3 gene. The modified URA3 gene was amplified using plasmid pPGKURA3 as a template (Pynyaha et al., 2009) and primers UB1 and UB2, thereby introducing XhoI sites at the ends of the PCR product. Almost the entire sequence of the pFRA1 plasmid, except for the 5′-part of FRA1 structural gene, was amplified with the primers UB5 and UB6 (Table 2), thereby introducing XhoI sites at the ends of the PCR product. Both the PCR products were purified, digested with XhoI restriction endonuclease, mixed and ligated. The resulting plasmid pFRA1URA3 carried the modified S. cerevisiae URA3 gene inserted between the promoter and the terminator sequences (with adjacent 0.7 kb of the 3′-part of the FRA1 structural gene). Then this plasmid was digested with EcoRI endonuclease, yielding a fra1::URA3 deletion cassette, which was used for the transformation of the P. guilliermondii R-66 strain.
To identify knockout strains, PCR analysis was performed using total DNA purified from selected transformants as a template and primers JB76 with JB72 or JB63 with Ura32r or UB8 with JB42 in the case of fes1::URA3, vma1::URA3 or fra1::URA3 deletion cassette, respectively.
Miscellaneous procedures
Yeast transformation, PCR analysis and Southern blot analysis of transformants were performed as described previously (Boretsky et al., 2007b). Transformants were selected on an agar medium containing 0.67% YNB, 2% sucrose and 0.5% casamino acids (Difco) without uridine. Riboflavin was assayed fluorometrically using a solution of synthetic riboflavin as a standard with a Turner Quantech FM 109510–33 fluorometer. Thin-layer chromatography was carried out on Silufol (Chemapol) plates with systems n-butanol : acetic acid : water (10 : 3 : 7 v/v) or a 2.5% solution of Na2HPO4 in water. The cellular iron content was determined with 2,2-dipiridyl as described earlier (Fedorovich et al., 1999). Cells were disrupted by grinding with 0.4–0.5-mm-glass beads. The protein concentration was determined after dialysis using the Lowry method (Lowry et al., 1951). The activity of GTP cyclohydrolase II was determined using a fluorometric method as described earlier (Shavlovskii et al., 1983). Hydrogen peroxide (H2O2) sensitivity was examined as described (Pynyaha et al., 2009).
Results
Generation of insertional mutants
Previously, we have shown that linear DNA fragments introduced into P. guilliermondii cells integrate into the genome of the recipient by nonhomologous recombination (Pinyaga et al., 2002; Boretsky et al., 2007b). Taking advantage of this, we decided to apply random insertion mutagenesis for the selection of the riboflavin-overproducing P. guilliermondii mutants. The P. guilliermondii R-66 strain defective in the URA3 gene was used as the recipient strain for transformation by linear DNA fragment bearing the modified URA3 gene of S. cerevisiae as described (Pynyaha et al., 2009).
Plasmid p57URA3 was digested with endonuclease EcoRI, yielding an insertion cassette that consisted of the modified URA3 gene of S. cerevisiae placed under the control of the promoter of P. guilliermondii phosphoglycerate kinase gene and vector pUC57 without a poly-linker. The cassette obtained was transformed into the R-66 strain using the lithium acetate procedure.
Three insertion mutants IS12, IS19 and IS45 that had yellow-green fluorescence halo under UV light were selected among 6000 uracil prototrophic transformants obtained. They excreted a yellow substance in an iron-sufficient medium identified as riboflavin by means of both thin-layer chromatography and absorption spectra analysis (data not shown). Integration of a single copy of the cassette into the genome of selected transformants was confirmed by Southern blot analysis (data not shown).
Identification of insertion sites and construction of the corresponding deletion strains
To identify the site of cassette insertion, we digested the genomic DNA isolated from the selected mutant IS12 with restriction enzymes, which do not cleave the cassette, namely HindIII, SacI and XbaI. The DNA fragments obtained were purified, self-ligated and transformed into E. coli pyrF mutant anticipating to select ampicillin-resistant uracil prototrophs. Unfortunately, this approach did not allow us to obtain E. coli transformants, possibly due to the generation of very large DNA fragments that cannot be cloned into the pUC57 vector. To avoid this problem, genomic DNA isolated from the mutant was partially digested with Sau3A endonuclease and ligated into the BamHI site of the pUC19 vector. Several uracil prototrophic ampicillin-resistant transformants of E. coli pyrF were isolated successfully. All rescued plasmids were amplified and sequenced.
Sequencing results revealed that, in the selected strain IS12, the insertion cassette disrupted gene PGUG_00565.1 encoding a highly conserved protein homologous to catalytic subunits of vacuolar ATPase reported in Ashbya gossypii and S. cerevisiae. The P. guilliermondii gene designated as PgVMA1 encodes a 612-residue protein with a predicted Mr of 67.1 kDa. Alignment of the PgVma1p amino acid sequence with the corresponding sequences reported in A. gossypii (Förster et al., 1999) and S. cerevisiae (Milgrom et al., 2007) revealed that these proteins share 489 identical and 81 similar amino acid residues totalling 93% similarity. To confirm that the inactivation of this gene affected the regulation of riboflavin biosynthesis by P. guilliermondii, a deletion cassette vma1::URA3 (see Materials and methods) was introduced into the R66 recipient strain. Using PCR analysis, two of 40 checked transformants were found to bear the vma1::URA3 deletion cassette integrated into the genome by homologous recombination that led to a knockout of the PgVMA1 structural gene.
The same procedure was used to identify the site of integration of the insertion cassette in mutant IS19. Sequencing analysis revealed that in the insertion mutant IS19, the cassette disrupted a P. guilliermondii gene PGUG_03809.1 encoding protein homologous to the FES1 gene of S. cerevisiae, a nucleotide exchange factor required for the activity of the cytosolic molecular chaperon Ssa1p. This P. guilliermondii gene, designated as PgFES1, encodes a 286 residues protein with a theoretical Mr of 32.01 kDa that shares 113 identical and 67 similar amino acid residues (totalling 62% similarity) with the S. cerevisiae counterpart (Kabani et al., 2002). The deletion cassette fes1::URA3 was constructed (see Materials and methods) and introduced into the R66 recipient strain. By means of PCR analysis, recombinant clones bearing the fes1::URA3 deletion cassette integrated into the genome by homologous recombination that led to a knockout of the PgFES1 structural gene were identified.
Cloning of an integration site of the insertion cassette using total DNA of IS45 mutant was performed via Sau3A partial hydrolysis as described above. Sequence analysis of plasmids bearing the integration site revealed that the insertion cassette disrupted P. guilliermondii gene PGUG_02071.1 (designated PgFRA1) that encodes a protein-sharing homology with members of the aminopeptidase superfamily.
To confirm the role of this gene in the regulation of riboflavin biosynthesis in P. guilliermondii, a deletion cassette fra1::URA3 (see Materials and methods) was transformed into R66 recipient. Several transformants were found to bear the fra1::URA3 deletion cassette integrated into the genome by homologous recombination, resulting in a knockout of the 5′ 1.45-kb fragment of the PgFRA1 (PGUG_02071.1) structural gene, as verified by PCR analysis.
Phenotypic and genetic analysis of the deletion strains
To confirm the role of VMA1, FES1 and FRA1 genes in the regulation of riboflavin biosynthesis, at first, we evaluated the riboflavin production by deletion strains. Similar to the corresponding insertion mutants Δvma1–17 and Δfra1–45, knockout mutants produced five to seven times more riboflavin relative to the recipient parental strain when grown in the synthetic Burkholder medium under iron-repletion condition (Fig. 1). In contrast to the riboflavin-overproducing insertion mutant IS19, riboflavin production by the Δfes1–77 strain was only slightly higher when compared with the parental strain (Fig. 1). The activity of the key enzyme of riboflavin pathway GTP cyclohydrolase II in the knockout strains Δvma1–17 and Δfra1–45 was elevated 1.9 and 4.9 times, respectively, as compared with the parental strain grown under the same conditions. Again, the activity of GTP cyclohydrolase II in the Δfes1–77 strain did not differ from the parental strain (Table 3).
Fig. 1.

Riboflavin productivity of Pichia guilliermondii wild-type strain and mutants Δfes1–77, Δvma1–17 and Δfra1–45. Cultures of P. guilliermondii wild-type strain R-66 (WT) and mutants were grown for 5 days (stationary phase) aerobically at 30 °C in a synthetic Burkholder medium supplemented with 3.6 μM of iron added as ammonium ferrous sulphate. Values are means ± SE of three independent experiments.
Table 3.
GTP cyclohydrolase II activity in the Pichia guilliermondii wild-type strain and mutants Δfes1–77, Δvma1–17 and Δfra1–45
| Strains of P. guilliermondii | GTP cyclohydrolase II activity (μU mg−1 protein) |
|---|---|
| R-66 | 8.2 ± 0.9 |
| Δfes1–77 | 7.6 ± 0.8 |
| Δvma1–17 | 15.3 ± 1.1 |
| Δfra1–45 | 40.6 ± 3.5 |
Similar to the majority of P. guilliermondii mutants defective in the regulation of riboflavin biosynthesis, the Δfra1–45 strain possessed 1.8–2.2-fold increased cellular iron content as compared with the parental strain (Fig. 2). In contrast, the Δvma1–17 strain had a cellular iron content twofold lower than the parental strain, whereas the Δfes1–77 strain had an iron content just slightly higher than the parental strain. Decreasing iron content in the medium from 3.6 to 0.18 μM completely inhibited the growth of the Δvma1–17 mutant strain, whereas the parental strain and two other knockout strains grew well under such iron-depletion conditions (Table 4).
Fig. 2.

Iron content in the cells of the Pichia guilliermondii wild-type strain and mutants Δfes1–77, Δvma1–17 and Δfra1–45. Cultures of P. guilliermondii wild-type strain R-66 (WT) and mutants were grown aerobically at 30 °C in a synthetic Burkholder medium supplemented with 3.6 μM of iron added as ammonium ferrous sulphate. Cells from the middle exponential growth phase were used to measure the iron content. Values are means ± SE of three independent experiments.
Table 4.
Riboflavin production by insertion and knockout mutants of Pichia guilliermondii
| Strains of P. guilliermondii | Conditions of growth
|
|||
|---|---|---|---|---|
| 3.6 μM of iron
|
0.18 μM of iron
|
|||
| Growth (mg dry cells weight mL−1) | Riboflavin productivity (μg mg−1 dry cells weight) | Growth (mg dry cells weight mL−1) | Riboflavin productivity (μg mg−1 dry cells weight) | |
| R-66 | 6.0 | 0.11 | 4.0 | 4.45 |
| IS12 | 4.2 | 0.25 | 1.5 | 6.8 |
| Δvma1–17 | 1.5 | 0.50 | No growth | – |
| IS19 | 5.9 | 0.35 | 2.5 | 8.0 |
| Δfes1–77 | 2.0 | 0.15 | 1.3 | 3.6 |
| IS45 | 5.2 | 0.7 | 4.0 | 11.2 |
| Δfra1–45 | 5.4 | 0.72 | 4.3 | 11.7 |
Previously, we reported that P. guilliermondii mutants defective in the regulation of riboflavin biosynthesis are hypersensitive to the oxidative stress (Boretsky et al., 2007a). Hence, we tested the sensitivity of the new mutant strains to H2O2. Δfes1–77 and Δfra1–45 strains did not differ significantly from the wild type regarding H2O2 sensitivity. Contrary to this, the viability of the Δvma1–17 mutant was drastically reduced by this agent. Only 2–5% of the Δvma1–17 mutant cells survived after a 1.5-h exposure with 1 mM H2O2, while the viability of the parental strain was not affected (Fig. 3).
Fig. 3.
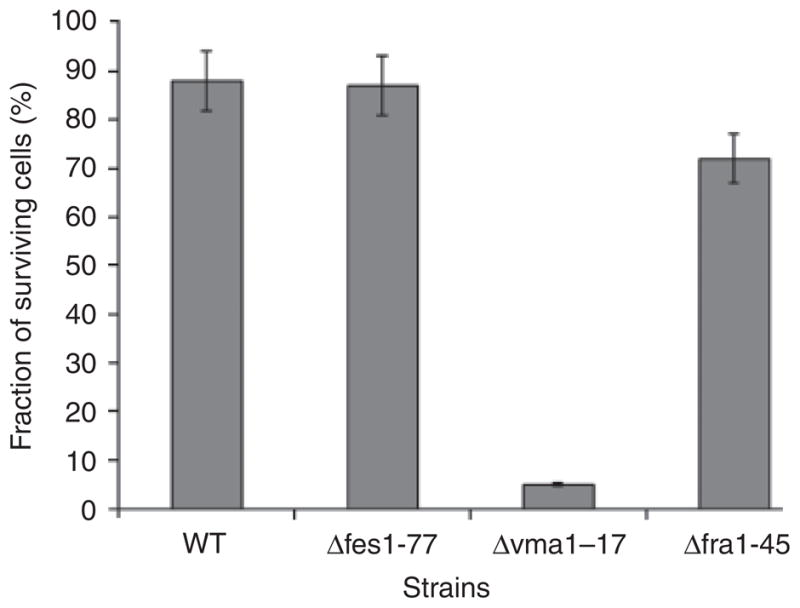
Sensitivity of Pichia guilliermondii wild-type strain and mutants Δfes1–77, Δvma1–17 and Δfra1–45 to H2O2. Cultures of P. guilliermondii wild-type strain R-66 (WT) and mutants were grown aerobically in YPD medium for 16 h, diluted to an OD600 nm of 0.2 and allowed to grow for 3.5 h. Aliquots (2 mL) were treated with 1 mM H2O2 for 1.5 h at 30 °C. Cells were pelleted at 3000 g for 10 min and resuspended in a fresh YPD medium. Suspensions were diluted 100–1000-fold in a complete medium and plated on YPD agar plates. Colonies were counted after 3 days of incubation at 30 °C. The quantity of colonies obtained with untreated cultures was assumed to be 100%. Values are means ± SE of three independent experiments.
The constructed Δvma1–17 and Δfes1–77 knockout strains both showed temperature-sensitive phenotypes similar to the corresponding insertion mutants IS19 and IS12 and Δfes1 mutants of S. cerevisiae (Kabani et al., 2002). In contrast to the parental strain, they could not grow at 37 °C, although they grew well at 30 °C, whereas the Δfra1–45 strain grew well at both temperatures (Fig. 4). Unexpectedly, we observed that the parental wild-type strain grown at 37 °C in an iron-sufficient medium excreted a yellow substance identified as riboflavin. Riboflavin production by wild-type cells grown in the synthetic liquid medium at 37 °C was twofold higher as compared with those grown at 30 °C. This was accompanied by a twofold increase of both the iron cell content and the activity of GTP cyclohydrolase II that catalyses the first step of riboflavin biosynthesis.
Fig. 4.

Growth of Pichia guilliermondii wild-type strain, and mutants Δfes1–77, Δvma1–17 and Δfra1–45 under different temperature conditions. Cultures of P. guilliermondii wild-type strain R-66 (WT) and mutants were grown aerobically in YPD medium for 48 h. Cells were harvested, washed with water and resuspended in water to an OD600 nm = 0.2. Serial fivefold dilutions were made. Five microlitre aliquots of each dilution were plated onto YPS medium. Plates were incubated at 30 and 37 °C for 4 days. The results of a typical representative experiment are shown.
Transformation of Δvma1–17 and Δfes1–77 mutants with 4.1- and 3.3-kb fragments of P. guilliermondii chromosomal DNA bearing correspondingly intact VMA1 and FES1 genes restored the wild-type phenotype in both cases. The transformants grew well at 37 °C and did not overproduce riboflavin under iron-repletion conditions (data not shown).
To confirm that these newly isolated mutations are different from those previously described mutations rib80, rib81 and hit1 (Shavlovskii et al., 1990, 1993; Fedorovich et al., 1999), we crossed P. guilliermondii mutants overproducing riboflavin rib80-22, rib81-31 and hit1-1 with newly isolated mutant strains Δfes1–77, Δvma1–17 and Δfra1–45. All diploid hybrids resulting from crossing the Δvma1–17 and Δfra1–45 strains with previously reported mutants possessed a wild-type phenotype: they did not overproduce riboflavin in an iron-sufficient medium and did not display temperature sensitivity (data not shown). Thus, neither of the previously reported mutations impairs the PgVMA1 or the PgFRA1 genes. However, the diploid hybrid obtained after crossing the Δfes1–77 strain with previously reported mutant P. guilliermondii rib80-22 (Shavlovskii et al., 1990) exhibited 2.5-fold increased riboflavin production relative to the wild-type strain, suggesting incomplete complementation.
Discussion
Pichia guilliermondii, C. famata and some other flavinogenic yeast species overproduce riboflavin under iron-limitation conditions. Whereas P. guilliermondii has been used more widely for basic research of riboflavin biosynthesis, the latter species C. famata is used as an industrial producer of this vitamin (Voronovsky et al., 2002). A few transformation systems were developed for P. guilliermondii (Boretsky et al., 1999, 2007a). It was observed that introduced DNA integrate into the genome of a recipient strain according to a nonhomologous mechanism of recombination (Pinyaga et al., 2002). This observation suggested that insertion mutagenesis can be a powerful tool for gene targeting experiments in P. guilliermondii. This technique was successfully applied to select and identify C. famata mutants that were unable to overproduce riboflavin (Dmytruk et al., 2006). In this study, we used a very similar approach to obtain P. guilliermondii mutants that possess increased riboflavin production. As a result of these experiments, three genes VMA1, FES1 and FRA1 that are required for the proper regulation of riboflavin biosynthesis by P. guilliermondii were identified. The data obtained further support the observation that the regulation of both riboflavin biosynthesis and iron acquisition by P. guilliermondii is tightly connected.
The P. guilliermondii Δvma1–17 mutant possesses decreased iron contents in the cells and hypersensitivity to H2O2 similar to that of S. cerevisiae mutants lacking vacuolar ATPase (Milgrom et al., 2007). Similar to the corresponding mutants of A. gossypii, this strain overproduces riboflavin. In addition, it possesses a temperature-sensitive phenotype. It cannot grow at 37 °C, whereas the parental strain grows well under the same conditions. Notably, wild-type cells grown in the synthetic liquid medium at 37 °C possess increased iron cell content and riboflavin production. Previously, it was hypothesized that riboflavin oversynthesis in the A. gossypii vma1 mutant occurs due to defects of riboflavin crystal accumulation inside the vacuoles (Förster et al., 1999). Our data on riboflavin oversynthesis by Δvma1–17 knockout and insertion mutants suggest another possible role of VMA1 in the regulation of riboflavin synthesis as the parental P. guilliermondii strain does not accumulate riboflavin in vacuoles. The data rather suggest that the overproduction of riboflavin is required for P. guilliermondii survival under stress conditions. In favour of this hypothesis, P. guilliermondii mutants hit1-1, rib80-22, Δyfh1–2 as well as the newly constructed Δvma1–17 strain that are continuously stressed all overproduce riboflavin (Boretsky et al., 2007a; Pynyaha et al., 2009).
Another P. guilliermondii insertion riboflavin-overproducing mutant IS19 has been shown to bear the cassette integrated into the genome disrupting the gene homologous to the S. cerevisiae FES1 gene. This gene encodes a nucleotide exchange factor required for modulating the function of Hsp70s proteins (Dragovic et al., 2006). Unexpectedly and in contrast to the IS19 insertion mutant, the deletion mutant did not overproduce riboflavin. Perhaps this inconsistency could be explained by the appearance of an unknown mutation generated during the transformation of the recipient strain (Rosenberg, 2001; Bouchonville et al., 2009). All diploid strains resulting from crossing of previously reported riboflavin overproducer P. guilliermondii rib80-22 strain with either the IS19 insertional mutant or the Δfes1–77 knockout mutant exhibit three to 4.2 times lower riboflavin production when compared with the rib80-22 mutant, but this value is increased 2.3–2.7 times relative to the wild-type strain. This incomplete complementation in the selected diploids suggests that the product of FES1 gene is required for the proper regulation of riboflavin biosynthesis by P. guilliermondii. Sequence analysis revealed that P. guilliermondii rib80-22 strain possesses the FES1 gene of the wild type (our unpublished data). One may assume that both genes are involved in the same regulatory mechanism, although additional studies are necessary to confirm this hypothesis.
The third P. guilliermondii insertion mutant IS45 was found to be defective in the PGUG_02071.1 gene potentially encoding a protein belonging to the superfamily of amino-peptidases. This protein shares 336 identical and 127 similar amino acid residues (totalling 63% similarity) with the S. cerevisiae counterpart FRA1 (YLL029W) that is involved in the regulation of iron acquisition (Kumánovics et al., 2008). The deletion of this gene in P. guilliermondii causes iron hyperaccumulation similar to that in S. cerevisiae (Kumánovics et al., 2008). In addition, the P. guilliermondii Δfra1–45 deleted strain exhibits riboflavin oversynthesis, apparently due to the elevated activity of enzymes of this biosynthetic pathway. Earlier, it has been shown that the regulation of riboflavin biosynthesis in P. guilliermondii occurred mainly at the transcriptional level (Boretsky et al., 2005; Sibirny & Boretsky, 2009). This current study did not reveal any putative transcription factor, but our screening experiment did not reach saturation. In addition, a P. guilliermondii gene encoding a potential transcription factor Sef1p highly homologous to C. famata Sef1p involved in the transcriptional regulation of riboflavin synthesis (Dmytruk et al., 2006) has been identified recently (our unpublished data). The existence of at least seven different P. guilliermondii complementation groups of the mutants that overproduce riboflavin in an iron-sufficient medium suggests that many genes are required to fine tune the level of the vitamin B2 synthesis according to the cellular needs. The exact role of three of them, presented here, is currently under examination and should be elucidated in the near future.
References
- Boretsky Y, Kapustyak K, Fayura L, Stasyk O, Stenchuk M, Bobak Y, Drobot L, Sibirny A. Positive selection of mutants defective in transcriptional repression of riboflavin synthesis by iron in the flavinogenic yeast Pichia guilliermondii. FEMS Yeast Res. 2005;5:829–837. doi: 10.1016/j.femsyr.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Boretsky YR, Protchenko OV, Prokopiv TM, Mukalov IO, Fedorovych DV, Sibirny AA. Mutations and environmental factors affecting regulation of riboflavin synthesis and iron assimilation also cause oxidative stress in the yeast Pichia guilliermondii. J Basic Microb. 2007a;47:371–377. doi: 10.1002/jobm.200610279. [DOI] [PubMed] [Google Scholar]
- Boretsky YR, Pynyaha YV, Boretsky VY, Kutsyaba VI, Protchenko OV, Philpott CC, Sibirny AA. Development of a transformation system for gene knock-out in the flavinogenic yeast Pichia guilliermondii. J Microbiol Meth. 2007b;70:13–19. doi: 10.1016/j.mimet.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Boretsky YR, Voronovsky AA, Liauta-Tehlivets OY, Hasslacher M, Kohlwein SD, Shavlovsky GM. Identification of an ARS element and development of a high efficiency transformation system for Pichia guilliermondii. Curr Genet. 1999;36:215–221. doi: 10.1007/s002940050493. [DOI] [PubMed] [Google Scholar]
- Bouchonville K, Forche A, Tang KE, Selmecki A, Berman J. Aneuploid chromosomes are highly unstable during DNA transformation of Candida albicans. Eukaryot Cell. 2009;10:1554–1566. doi: 10.1128/EC.00209-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley RA, Gaskin DJ, Holmes K, Mulholland F, Wells JM, Kelly DJ, van Vliet AH, Walton NJ. Riboflavin biosynthesis is associated with assimilatory ferric reduction and iron acquisition by Campylobacter jejuni. Appl Environ Microb. 2007;73:7819–7825. doi: 10.1128/AEM.01919-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmytruk KV, Voronovsky AA, Sibirny AA. Insertion mutagenesis of the yeast Candida famata (Debaryomyces hansenii) by random integration of linear DNA fragments. Curr Genet. 2006;3:183–191. doi: 10.1007/s00294-006-0083-0. [DOI] [PubMed] [Google Scholar]
- Dragovic Z, Shomura Y, Tzvetkov N, Hartl FU, Bracher A. Fes1p acts as a nucleotide exchange factor for the ribosome-associated molecular chaperone Ssb1p. Biol Chem. 2006;387:1593–1600. doi: 10.1515/BC.2006.198. [DOI] [PubMed] [Google Scholar]
- Fassbinder F, van Vliet AH, Gimmel V, Kusters JG, Kist M, Bereswill S. Identification of iron-regulated genes of Helicobacter pylori by a modified fur titration assay (FURTA-Hp) FEMS Microbiol Lett. 2000;184:225–229. doi: 10.1111/j.1574-6968.2000.tb09018.x. [DOI] [PubMed] [Google Scholar]
- Fedorovich DV, Protchenko OV, Lesuisse E. Iron uptake by the yeast Pichia guilliermondii. Flavinogenesis and reductive iron assimilation are co-regulated processes. Biometals. 1999;12:295–300. doi: 10.1023/a:1009298530145. [DOI] [PubMed] [Google Scholar]
- Förster C, Santos MA, Ruffert S, Kramer R, Revuelta JL. Physiological consequence of disruption of the VMA1 gene in the riboflavin overproducer Ashbya gossypii. J Biol Chem. 1999;274:9442–9448. doi: 10.1074/jbc.274.14.9442. [DOI] [PubMed] [Google Scholar]
- Kabani M, Beckerich JM, Brodsky JL. Nucleotide exchange factor for the yeast Hsp70 molecular chaperone Ssa1p. Mol Cell Biol. 2002;22:4677–4689. doi: 10.1128/MCB.22.13.4677-4689.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumánovics A, Chen OS, Li L, et al. Identification of FRA1 and FRA2 as genes involved in regulating the yeast iron regulon in response to decreased mitochondrial iron–sulfur cluster synthesis. J Biol Chem. 2008;283:10276–10286. doi: 10.1074/jbc.M801160200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Milgrom E, Diab H, Middleton F, Kane PM. Loss of vacuolar proton-translocating ATPase activity in yeast results in chronic oxidative stress. Biol Chem. 2007;282:7125–7136. doi: 10.1074/jbc.M608293200. [DOI] [PubMed] [Google Scholar]
- Nguyen HV, Gaillardin C, Neuvéglise C. Differentiation of Debaryomyces hansenii and Candida famata by rRNA gene intergenic spacer fingerprinting and reassessment of phylogenetic relationships among D. hansenii, C. famata, D. fabryi, C. flareri ( = D. subglobosus) and D. prosopidis: description of D. vietnamensis sp. nov. closely related to D. nepalensis. FEMS Yeast Res. 2009;9:641–662. doi: 10.1111/j.1567-1364.2009.00510.x. [DOI] [PubMed] [Google Scholar]
- Philpott C, Protchenko OV. Response to iron deprivation in Saccharomyces cerevisiae. Eukaryot Cell. 2008;7:20–27. doi: 10.1128/EC.00354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinyaga YuV, Prokopiv TM, Petrishin AV, Khalimonchuk OV, Protchenko OV, Fedorovich DV, Boretskii YuR. The reversion of Pichia guilliermondii transformants to the wild-type phenotype. Mikrobiologiia. 2002;71:368–372. (in Russian) [PubMed] [Google Scholar]
- Pynyaha YV, Boretsky YR, Fedorovych DV, Fayura LR, Levkiv AI, Ubiyvovk VM, Protchenko OV, Philpott CC, Sibirny AA. Deficiency in frataxin homologue YFH1 in the yeast Pichia guilliermondii leads to missregulation of iron acquisition and riboflavin biosynthesis and affects sulfate assimilation. Biometals. 2009;22:1051–1061. doi: 10.1007/s10534-009-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SM. Evolving responsively: adaptive mutation. Nat Rev Genet. 2001;2:504–515. doi: 10.1038/35080556. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell D. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2001. [Google Scholar]
- Santos R, Buisson N, Knight S, Dancis A, Camadro J, Lesuisse E. Candida albicans lacking the frataxin homologue: a relevant yeast model for studying the role of frataxin. Mol Microbiol. 2004;54:507–519. doi: 10.1111/j.1365-2958.2004.04281.x. [DOI] [PubMed] [Google Scholar]
- Shavlovskii G, Logvinenko E. Riboflavin oversynthesis in yeast and mechanisms of its regulation. Prikl Biokhim Mikrobiol. 1988;24:435–447. [PubMed] [Google Scholar]
- Shavlovskii G, Logvinenko E, Zakalskii A. Purification and properties of GTP cyclohydrolase II of the yeast Pichia guilliermondii. Biokhimiia. 1983;48:837–843. [PubMed] [Google Scholar]
- Shavlovskii G, Fedorovich D, Babyak L. The effect of carbon sources on the manifestation of rib80 and rib81 regulatory mutations in Pichia guilliermondii. Mikrobiologiia. 1990;59:404–410. [Google Scholar]
- Shavlovskii G, Fedorovich D, Babyak L. The effect rib81 mutation on riboflavin biosynthesis and iron transport in Pichia guilliermondii yeast. Mikrobiologiia. 1993;62:897–903. [Google Scholar]
- Sibirny AA, Boretsky YR. In: Pichia guilliermondii. Yeast Biotechnology: Diversity and Applications. Kunze G, Satyanarayana T, editors. Springer Science; New Delhi: 2009. pp. 113–134. [Google Scholar]
- Sibirnyi A, Zharova V, Kshanovskaia B, Shavlovskii G. Selection of a genetic line of Pichia guilliermondii yeasts capable of forming a significant quantity of spores. Tsitol Genet. 1977;11:330–333. [PubMed] [Google Scholar]
- Stenchuk NN, Kapustiak KY. The red mutations impair the regulation of flavinogenesis and metal homeostasis in yeast Pichia guilliermondii. Genetika. 2003;39:1026–1032. [PubMed] [Google Scholar]
- Tanner F, Vojnovich C, Lanee J. Riboflavin production by Candida species. Science. 1945;101:180–183. doi: 10.1126/science.101.2616.180. [DOI] [PubMed] [Google Scholar]
- Voronovsky AY, Abbas C, Fayura LR, Kshanovska BV, Dmytruk KV, Sybirna KA, Sibirny AA. Development of a transformation system for the flavinogenic yeast Candida famata. FEMS Yeast Res. 2002;2:381–388. doi: 10.1016/S1567-1356(02)00112-5. [DOI] [PubMed] [Google Scholar]
- Vorwieger A, Gryczka C, Czihal A, Douchkov D, Tiedemann J, Mock H-P, Jakoby M, Weisshaar B, Saalbach I, Bäumlein H. Iron assimilation and transcription factor controlled synthesis of riboflavin in plants. Planta. 2007;226:147–158. doi: 10.1007/s00425-006-0476-9. [DOI] [PubMed] [Google Scholar]


