Abstract
Background
The aim of this multinational (Canada, France, Germany, United Kingdom, and United States), task and interview-based study was to compare the ease of use and performance of the ClikSTAR® (sanofi-aventis, Paris, France) insulin pen with other commonly used reusable pens based on participant and interviewer assessments.
Methods
People with diabetes (n = 654) were asked to demonstrate four pens consecutively—ClikSTAR, Lilly Luxura® (Eli Lilly, Indianapolis, IN), and NovoPen® 3 and 4 (Novo Nordisk, Bagsvaerd, Denmark)—according to the respective instruction manuals. The endpoint was assessed by a rating from the participants and the interviewer. While the participants focused on the pen's ease of use, the interviewer considered the participants' difficulty in preparing and delivering a 40-unit dose and their requirement for help.
Results
Twenty percent of U.S. participants and 24% of participants from the other countries had type 1 diabetes. Approximately 50% of participants in each group had prior insulin pen experience. A higher proportion of participants, including those with dexterity or visual impairments, reported ClikSTAR as easier to use than other pens (P < 0.05). Participants using ClikSTAR did not experience any difficulty in completing the tasks. The proportion of participants not requiring help in completing the tasks with ClikSTAR was rated as numerically higher than, or similar to, that observed with Lilly Luxura or NovoPen 3 or 4 (75%, 74%, 62%, and 65%, respectively). According to participants, ClikSTAR and NovoPen 4 emerged as the most highly rated pens.
Conclusions
In comparison with other pens, ClikSTAR was significantly easier to use, which, when taken together with overall performance, meets the need of people with diabetes.
Introduction
Traditional methods of injecting insulin with a vial and manual syringe are being replaced by alternative insulin delivery methods, such as pen devices, which are a more convenient and flexible option and are more socially acceptable.1–3 Several studies have shown that people with diabetes who need to inject insulin tend to prefer pen devices to the vial and syringe.4,5 Insulin pens have repeatedly been demonstrated to exhibit greater dose accuracy compared with syringes.6,7
Reusable pen devices are estimated to be used by 29% of insulin users worldwide.8 However, the properties and design of some reusable pens, such as maximum dose, body color differentiation, injection force, and dose knob dial extension, may represent limitations to some patients. For example, because manual dexterity disorders are common in people with diabetes and may limit finger or hand strength and the ability to inject insulin without assistance,9 the issue of injection force is of particular concern. A pen with a reduced injection force would have several potential advantages.10 By allowing injection with one hand using less force, the device could potentially reduce discomfort, enhance safety aspects, and reduce the risk of over- or underdosing.
Similarly, there is a high prevalence of visual impairments in people with diabetes due to associated retinopathy or age-related maculopathy.11 In this population, the incorporation of pen design elements that enable differentiation between long- and short-acting insulin devices, as well as audible and tactile features that facilitate dose selection and delivery, is of particular importance.
ClikSTAR® (sanofi-aventis, Paris, France)12 is a new reusable insulin pen for injection of insulin glargine (long-acting insulin; LANTUS®, sanofi-aventis) or insulin glulisine (short-acting insulin; Apidra®, sanofi-aventis). The aim of this multinational study (Canada, France, Germany, United Kingdom, and United States) was to assess the extent to which the design of the ClikSTAR insulin pen meets the needs of people with diabetes and provide comparative data relative to other commonly used reusable pens.
Subjects and Methods
Study objectives
The primary objectives of the study were to provide comparative data on the ease of use and performance of ClikSTAR against three other reusable insulin pens—NovoPen® 3,13 NovoPen 414 (both Novo Nordisk, Bagsvaerd, Denmark), and Lilly Luxura® (Eli Lilly, Indianapolis, IN)15—and to assess the extent to which the design of the ClikSTAR pen meets the needs of people with type 1 and type 2 diabetes mellitus (T1DM and T2DM, respectively). The key performance metrics included operation of each pen with the assistance of the instruction manual, ease and comfort of dialing a dose, ease and comfort of delivering a dose, tactility and audibility of the click mechanism, ease of replacing the cartridge, and overall assessment and preference.
Participants
All participants were required to have T1DM or T2DM and to be treated with insulin or oral antidiabetes drugs (OADs). To capture a broad range of people with diabetes, a quota system was applied to ensure that people with T1DM and T2DM, with and without insulin (and pen device) experience as well as visual and dexterity impairments, were enrolled. Participants on insulin therapy had to have had experience with insulin injections or an insulin pen, be able to administer the therapy themselves, not be currently using an insulin pump, and not take fewer than three injections per day. Participants on non–insulin-based treatment had to be on an OAD, with no insulin injection or pen experience. In the United States, all participants with T1DM had to be a pen or vial and syringe user (or both), whereas all participants with T2DM were required to be on an OAD and/or be a pen or vial and syringe user. People using continuous subcutaneous insulin infusion or inhaled insulin were excluded from the study. A maximum of 23 participants each using NovoPen 3, NovoPen 4, or Lilly Luxura in the United States were enrolled. In the other countries, all participants with T1DM had to be insulin pen users, and all participants with T2DM were required to be on an OAD or be an insulin pen user. Furthermore, a maximum of 19 participants using Lilly Luxura, NovoPen 3, or NovoPen 4 were to be enrolled. Within each country, approximately 20% of participants had to have difficulty with dexterity and 25% had to have vision problems.
Participants were invited to take part in this research through local primary care practices. In response to invitations sent via their physician, participants contacted the research organization and were prescreened via telephone to assess their individual eligibility. All eligibility requirements were confirmed again on the day the participants attended the research center. The characteristics of the respondents who did not meet the eligibility criteria at the prescreening phase were not recorded.
A total of 654 people with diabetes were surveyed over a 6-week period via face-to-face interviews and a questionnaire; none of the participants dropped out during the participation phase. Interviews were conducted consecutively and were reviewed and validated during data collection and data entry.
Study design
Participants were assessed via a face-to-face questionnaire, requiring approximately 60 min for completion. Interviews were carried out by independent moderators from a research agency. The research agency developed the questionnaire with input from the principal investigator and sponsor. In the first phase of the interview, participants were asked to demonstrate consecutively the use of each of the four pens according to each pen's instruction manual. The participants were assessed on their ability to perform the following steps:
Unscrewing the cartridge holder and inserting the cartridge
Screwing the cartridge holder onto the pen (with the cartridge inside)
Attaching the needle
Performing a safety test, which consisted of dialing a small dose and injecting it into the air
Dialing a dose of 40 units
Delivering a dose of 40 units
Degree of reliance on the instruction manual
The interviewers assessed the level of difficulty that participants experienced in the completion of a specified sequence of steps and the level of help required on a 5-point scale (1 = got stuck to 5 = no difficulty).
In the second phase of the interview, participants were asked to provide their own assessment of the overall usability of the four pen devices based on a 7-point scale (1 = not easy at all to 7 = extremely easy). The four pens were compared with each other based on the study endpoints: evaluation of pen clicks (hearing and feeling the clicks), ease and precision of cartridge replacement, safety test performance, ease of using the dialing mechanism, and ease of attaching the needle. Participants were given the opportunity to change their scores for any pen if they so wished while using each pen. To avoid bias, the interviewers changed the order that the pens were presented between each participant. The order the pens were presented to an individual participant was kept the same between each phase of the interview.
Statistical analysis
Statistical significance was calculated at the 95% confidence level. The final dataset was analyzed at an aggregated level for each individual country and according to the participant subgroups that had been represented within the sample. To identify the level of significance for differences emerging between participant subgroups and between insulin pens, independent and paired-sample t tests were applied at the 95% confidence level. These tests were used to provide a clear insight into the relative strengths of the ClikSTAR pen versus the other pens. The first test was used for comparisons between the responses of mutually exclusive groups, for example, between countries. The second test was used for non-exclusive groups, for example, to compare responses for groups of participants between each pen. The number of patients to be enrolled was calculated on the basis of the margin of error for a statistical analysis at the 95% confidence level. For any given subgroup of patients, a minimum of 30 patients was required to provide a margin of error of ± 18%. The smallest subgroup analyzed in this study was for individuals with impaired dexterity (n = 74) providing a margin of error of ± 11%; the margin of error for analysis of the total population (n = 654) was ± 4%.
Results
Study population
A total of 654 people with diabetes from the United States, Canada, the United Kingdom, France, and Germany were included in the study, which was conducted from October to December 2008 (Table 1). In the United States, 20% and 80% of the participants had T1DM or T2DM, respectively; in the other four countries, the corresponding rates were 24% and 76%, respectively. The age distribution for all participants with T1DM was 50% 12–25 years old and 50% over 25 years old. For T2DM, the age distribution was 15% under 45 years old, 35% 45–64 years old, and 50% over 65 years old (Supplementary Table S1; Supplementary Data are available online at www.liebertonline.com/dia<http://www.liebertonline.com/dia>). Overall, 54% of participants now on insulin treatment had previously used OADs; the mean duration of OAD use among these participants was 4.7 years (Table 1).
Table 1.
Participant Demographics (n = 654)
| Parameter | Value |
|---|---|
| Participants with T1DM (%) | 23 |
| Participants with T2DM (%) | 77 |
| Age (years, mean) | |
| T1DM | 33 |
| T2DM | 61 |
| Age at diagnosis (years, mean) | |
| T1DM | 18 |
| T2DM | 49 |
| Sex (%) | |
| Male, T1DM | |
| 12–25 years (n = 68) | 56 |
| >25 years (n = 83) | 55 |
| Male, T2DM | |
| 18–44 years (n = 77) | 38 |
| 45–64 years (n = 176) | 52 |
| >65 years (n = 250) | 48 |
| Current insulin-based treatment (%) | |
| T1DM | |
| 12–25 years (n = 68) | 100 |
| >25 years (n = 83) | 100 |
| T2DM | |
| 18–44 years (n = 77) | 57 |
| 45–64 years (n = 176) | 52 |
| >65 years (n = 250) | 55 |
| Participants using (%; U.S. patients only) | |
| Syringe (n = 150) | 16 |
| Insulin pen (n = 150) | 88 |
| Insulin injection experience | |
| Mean number of units injected daily (n = 425) | |
| T1DM (n = 151) | 62 |
| T2DM (n = 274) | 63 |
| Number of injections taken daily (n = 425) | |
| T1DM (n = 151) | 4.1 |
| T2DM (n = 274) | 3.0 |
| Insulin users: history of oral medication (number of participants who previously used oral medication only, length of time taking oral medication) | |
| T1DM | |
| 12–25 years (n = 68) | 4%, 1.4 years |
| >25 years (n = 83) | 12%, 2.8 years |
| T2DM | |
| 18–44 years (n = 44) | 77%, 3.1 years |
| 45–64 years (n = 92) | 78%, 4.4 years |
| >65 years (n = 138) | 81%, 5.7 years |
| Insulin-naive participants, current use of oral medication (%; n = 229) | |
| One brand | 48 |
| Two brands | 44 |
| More than two brands | 8 |
Information is based on patient interviews and questionnaires. In line with the requested sample specifications, approximately three-quarters of the sample population had type 2 diabetes mellitus (T2DM), and 88% of the overall population used a pen. Data on use of vial and syringe were required only in the United States.
T1DM, type 1 diabetes mellitus.
Ease of use
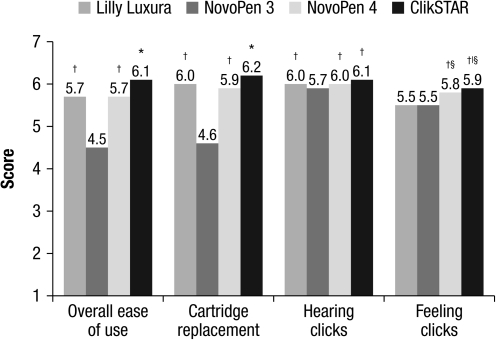
A higher proportion of participants reported that the ClikSTAR pen was significantly easier to use compared with other pens (P < 0.001 vs. all pens; Fig. 1). The mean overall score for ease of use was 6.1 for ClikSTAR versus 4.5, 5.7, and 5.7 for NovoPen 3, NovoPen 4, and Lilly Luxura, respectively (Fig. 1). Participants reported that it was significantly easier to replace the cartridge for the ClikSTAR pen than for all of the other insulin pens (P < 0.001 vs. all pens; Fig. 1). The cartridge holders of all the pens were fully closed in more than 96%, with no significant differences among pens.
FIG. 1.
Ease of completing tasks, as reported by the participants. Ratings were on a scale of 1–7, where 1 = not easy at all and 7 = extremely easy. *P < 0.001 versus all pens; †P < 0.001 versus NovoPen 3; §P < 0.001 versus Lilly Luxura; |P < 0.05 versus NovoPen 4.
The clicks of the ClikSTAR, NovoPen 4, and Lilly Luxura pens were heard with equal levels of clarity by the study participants. The clicks of the ClikSTAR pen, however, were reported to feel much clearer than those of other pens (P < 0.05 vs. NovoPen 4 and P < 0.001 vs. both Lilly Luxura and NovoPen 3; Fig. 1).
Participants with dexterity or visual impairments found cartridge replacement to be easier with the ClikSTAR pen than with the other pens (mean of 6.0 for ClikSTAR vs. 4.3 for NovoPen 3, 5.7 for NovoPen 4, and 5.7 for Lilly Luxura pens for participants with visual impairments [P < 0.001 for all] and mean of 6.2 for ClikSTAR vs. 4.4 for NovoPen 3 [P < 0.001], 5.8 for NovoPen 4 [P < 0.001], and 5.9 for Lilly Luxura [P < 0.01] pens for participants with dexterity impairments). Participants without visual or dexterity impairments rated the audible feedback of the clicks for ClikSTAR more highly than did participants with such impairments (mean of 6.2 vs. 5.8 [P < 0.01] for visual impairments and 6.1 vs. 5.9 [P = not significant] for dexterity impairments). Participants without visual and dexterity impairments rated the tactile feedback of the clicks for ClikSTAR more highly than did participants with such impairments (mean of 6.0 vs. 5.6 [P < 0.01] for visual impairments and 6.0 vs. 5.7 [P < 0.05] for dexterity impairments). Participants with visual impairments rated the sound of the clicks more highly for ClikSTAR than for the NovoPen 3 pen (mean of 5.8 vs. 5.7; P ≤ 0.05). Participants with dexterity impairment rated the ClikSTAR pen as significantly more tactile than the NovoPen 3 and Lilly Luxura pens (5.7 vs. 5.3 and 5.4, respectively; both P ≤ 0.05).
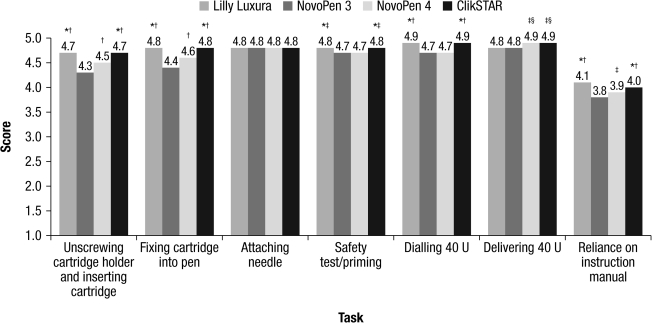
Overall, participants using the ClikSTAR pen did not experience any substantial difficulty in completing the tasks compared with the other pens according to the interviewer's rating (Fig. 2). Unscrewing the cartridge holder and inserting the cartridge were rated to be significantly easier with ClikSTAR and Lilly Luxura than with the NovoPens (all P < 0.001; Fig. 2); 80% of the participants experienced “no difficulty” for ClikSTAR and Lilly Luxura. Screwing the cartridge holder with the cartridge inside onto the pen was rated to be significantly easier with ClikSTAR and Lilly Luxura than with the NovoPens (all P < 0.001; Fig. 2). Overall, 86% of the participants experienced “no difficulty” with the ClikSTAR pen, and 98% screwed on the cartridge holder of the pen correctly. Attaching the needle was rated as being equally easy for all four pens tested (Fig. 2). The majority of participants (approximately two-thirds) attached the needle before setting the safety dose for all the pens.
FIG. 2.
Difficulty in completing tasks, as rated by the interviewers. Ratings were on a scale of 1–5, where 1 = got stuck and 5 = no difficulty. For the factor “Reliance on the instruction manual,” a scale of 1–5 was used, where 1 = relied completely and 5 = did not rely at all. *P < 0.001 versus NovoPen 4; †P < 0.001 versus NovoPen 3; ‡P < 0.05 versus NovoPen 3; §P < 0.001 versus Lilly Luxura. U, units.
The safety test was skipped by participants in approximately 43% of cases, with no appreciable differences between pens. Performing the test was rated to be significantly easier with ClikSTAR and Lilly Luxura than with the NovoPens (both P < 0.05 vs. NovoPen 3 and P < 0.001 vs. NovoPen 4; Fig. 2), with 88% of participants experiencing “no difficulty” with the ClikSTAR.
Dialing a dose of 40 units was rated as being significantly easier using the ClikSTAR and Lilly Luxura pens than the NovoPens (all P < 0.001; Fig. 2), with 93% of participants experiencing “no difficulty” with the ClikSTAR pen. Delivering a dose of 40 units was rated as being significantly easier with the ClikSTAR and NovoPen 4 pens than with the NovoPen 3 and Lilly Luxura pens (both P < 0.05 vs. NovoPen 3 and both P < 0.001 vs. Lilly Luxura; Fig. 2). The entire dose was delivered in 99% of cases with the ClikSTAR pen. The remaining participants delivered a dose of less than 40 units.
Almost half of the participants used the instruction manual, a practice that was strongly encouraged during training to minimize the risk of incorrectly using the insulin pens. However, the participants were rated as being significantly less reliant on the instruction manual of the ClikSTAR and Lilly Luxura pens than on those of the NovoPen devices (all P < 0.001; Fig. 2).
The interviewers rated the participants with dexterity or visual impairments as experiencing significantly more difficulty in screwing the cartridge holder onto the pen and performing the safety test than those with no difficulty with all four pens. Participants with limited dexterity encountered significantly more difficulty in dialing a dose of 40 units with NovoPen 3 and NovoPen 4 than participants with no such impairment. Participants with visual difficulties experienced significantly more difficulty in delivering a dose of 40 units with ClikSTAR, NovoPen 3, and Lilly Luxura than participants without such impairments (data not shown). Participants with prior insulin experience encountered significantly less difficulty in completing all of the tasks than insulin-naive participants (data not shown).
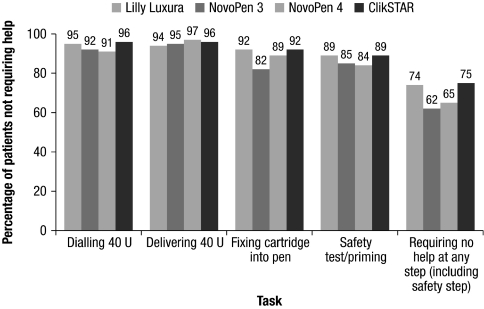
Requirement for help
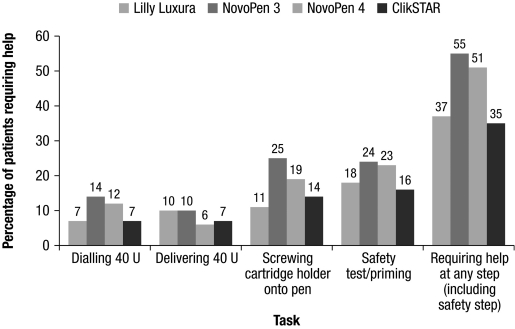
The proportion of participants who did not require assistance from the interviewer to complete the specific steps required to prepare and administer a 40-unit dose with the ClikSTAR pen was numerically higher than or similar to that observed with the other pens. More participants using the ClikSTAR and Lilly Luxura pens completed all steps without aid compared with NovoPen 3 and NovoPen 4 (Fig. 3). Regarding dialing and delivering a dose of 40 units, 96% of the participants did not require help with the ClikSTAR pen. Similarly, 92% of the participants did not require assistance to fix the cartridge in the ClikSTAR pen. Interviewers noted that no help was necessary in 89% of cases for the safety test with the ClikSTAR pen. Overall, 75% of participants needed no assistance at any step (including the safety test step) with the ClikSTAR pen; this increased to 80% when the safety test step was not included. A higher proportion of elderly participants (over 65 years old) required assistance from the interviewer compared with younger participants (Fig. 4). NovoPen 3 and NovoPen 4 proved particularly difficult in participants over 65 years of age; approximately half of this group required help (Fig. 4).
FIG. 3.
Percentage of participants not requiring help, as rated by the interviewers. U, units.
FIG. 4.
Percentage of participants over 65 years old requiring help, as rated by the interviewers. U, units.
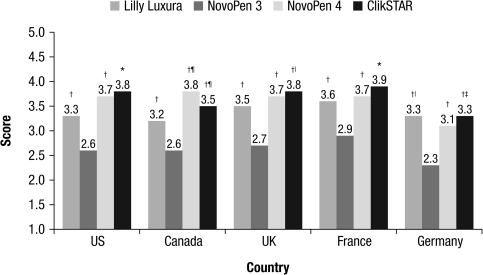
Results by country
The majority of participants in all countries found the ClikSTAR pen the easiest to use (Fig. 5). There were no significant differences between countries for most of the study endpoints regarding both ease of use and requirement for help. French and Canadian participants found the audibility and tactility of the four pens to be significantly better (P ≤ 0.05) than did participants from the United States, the United Kingdom, and Germany. Regardless of the device, participants in the United States, Canada, the United Kingdom, and France found the task of replacing the cartridge easier than those from Germany (P ≤ 0.05). The U.K. and German participants experienced significantly more difficulty than the Canadian participants in dialing a dose of 40 units (P ≤ 0.05), but there were no differences between participants from the other countries and those from the United States or France. There were no significant differences between any of the countries regarding the proportion of participants successfully delivering a full 40-unit dose.
FIG. 5.
Overall rating of each pen by country, as reported by the participants. Ratings were on a scale of 1–5, where 1 = poor and 5 = excellent. *P < 0.001 versus all pens; †P < 0.001 versus NovoPen 3; ‡P < 0.001 versus NovoPen 4; |P < 0.05 versus NovoPen 4; ¶P < 0.05 versus Lilly Luxura.
Overall rating
Of the four pens tested, ClikSTAR and NovoPen 4 were given the highest ratings by participants. There were no significant differences between these two pens for any endpoint. In addition, 59% of participants considered the ClikSTAR pen to be “very good” or “excellent.” Participants with dexterity or visual impairments reported that, overall, the ClikSTAR pen was significantly easier to use than the other three pens (mean of 3.6 for ClikSTAR vs. 2.5 for NovoPen 3 [P < 0.001], 3.5 for NovoPen 4 [P < 0.01], and 3.3 for Lilly Luxura [P < 0.01] for participants with dexterity impairments and 3.6 for ClikSTAR vs. 2.6 for NovoPen 3 [P < 0.001], 3.5 for NovoPen 4 [P < 0.01], and 3.3 for Lilly Luxura [P < 0.001] for participants with visual impairments). No significant differences emerged between the ratings for all four pens by participants with or without dexterity or visual impairments.
Discussion
The ease of use and overall performance of the ClikSTAR pen meet the needs of people with diabetes. Participants over 65 years of age rated the ClikSTAR pen as being easier to use than the other insulin pens. This finding is particularly important because the incidence of diabetes, particularly T2DM, is highest in this population. Interestingly, ClikSTAR was associated with greater overall ease of use, including ease of replacing the cartridge, feeling the clicks, and dialing a dose versus other pens in this group.
This study has some limitations that should be considered when interpreting the results. First, although care was taken in conducting the study to reduce bias (for example, changing the order the pens were presented between participants), this was not a randomized controlled study. Furthermore, the OptiClik® pen (sanofi-aventis) was not included in this study. OptiClik is another reusable insulin pen device that shares some features with ClikSTAR, including maximum dose (80 units), availability in different body colors for insulin glargine and insulin glulisine, and low injection force. Therefore, it might have been of interest to conduct a side-by-side comparison of these two pens.
All interviews were performed by an independent market research company with experience in performing similar surveys, and participants were not informed of the study sponsor in order to avoid unintentional bias or favor towards ClikSTAR. In addition, the sample size was large (much greater than that used in other similarly designed studies16,17), and the results were reproducible in each of the five countries involved. It is important to note that none of the participants had prior experience of using the ClikSTAR pen. In contrast, some participants may have had experience of the other pens. Finally, randomized controlled trials may not always reflect the reality of day-to-day self-management of diabetes for people using insulin, and observational studies such as this one are particularly useful in reflecting the real-world experiences of people with diabetes.
Conclusions
In comparison with other reusable insulin pens tested, ClikSTAR was rated as significantly easier to use overall by individuals with diabetes, including those with dexterity or visual impairment. Greater ease of completing tasks such as replacing an insulin cartridge, feeling the clicks, and dialing a dose suggest that ClikSTAR may facilitate insulin delivery for many people with diabetes.
Supplementary Material
Acknowledgments
This research was sponsored by sanofi-aventis and was performed for sanofi-aventis by an independent research company (P\S\L Research Europe, London, UK). Editorial support was provided by Anisha Mehra, Ph.D., Medicus International, and was funded by sanofi-aventis.
Author Disclosure Statement
A.P. has been an investigator, consultant, or speaker, attended advisory boards, and received honorariums, consulting fees, or research grants from Abbott, AstraZeneca, Boehringer-Ingelheim Pharmaceutical, Bristol-Myers Squibb, Eli Lilly, Medtronic, Merck-Serono, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi Aventis, Servier, and Takeda.
References
- 1.Coscelli C. Lostia S. Lunetta M. Nosari I. Coronel GA. Safety, efficacy, acceptability of a pre-filled insulin pen in diabetic patients over 60 years old. Diabetes Res Clin Pract. 1995;28:173–177. doi: 10.1016/0168-8227(95)01092-r. [DOI] [PubMed] [Google Scholar]
- 2.D'Eliseo P. Blaauw J. Milicevic Z. Wyatt J. Ignaut DA. Malone JK. Patient acceptability of a new 3.0 ml pre-filled insulin pen. Curr Med Res Opin. 2000;16:125–133. [PubMed] [Google Scholar]
- 3.Haak T. Edelman S. Walter C. Lecointre B. Spollett G. Comparison of usability and patient preference for the new disposable insulin device Solostar versus Flexpen, Lilly disposable pen, and a prototype pen: an open-label study. Clin Ther. 2007;29:650–660. doi: 10.1016/j.clinthera.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Korytkowski M. Bell D. Jacobsen C. Suwannasari R. A multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther. 2003;25:2836–2848. doi: 10.1016/s0149-2918(03)80337-5. [DOI] [PubMed] [Google Scholar]
- 5.Summers KH. Szeinbach SL. Lenox SM. Preference for insulin delivery systems among current insulin users and nonusers. Clin Ther. 2004;26:1498–1505. doi: 10.1016/j.clinthera.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Gnanalingham MG. Newland P. Smith CP. Accuracy and reproducibility of low dose insulin administration using pen-injectors and syringes. Arch Dis Child. 1998;79:59–62. doi: 10.1136/adc.79.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keith K. Nicholson D. Rogers D. Accuracy and precision of low-dose insulin administration using syringes, pen injectors, and a pump. Clin Pediatr (Phila) 2004;43:69–74. doi: 10.1177/000992280404300109. [DOI] [PubMed] [Google Scholar]
- 8.Polonsky WH. Fisher L. Guzman S. Villa-Caballero L. Edelman SV. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care. 2005;28:2543–2545. doi: 10.2337/diacare.28.10.2543. [DOI] [PubMed] [Google Scholar]
- 9.Aljahlan M. Lee KC. Toth E. Limited joint mobility in diabetes. Postgrad Med. 1999;105:99–101. doi: 10.3810/pgm.1999.02.536. 105–106. [DOI] [PubMed] [Google Scholar]
- 10.Clarke A. Spollett G. Dose accuracy and injection force dynamics of a novel disposable insulin pen. Expert Opin Drug Deliv. 2007;4:165–174. doi: 10.1517/17425247.4.2.165. [DOI] [PubMed] [Google Scholar]
- 11.Davidson JA. Ciulla TA. McGill JB. Kles KA. Anderson PW. How the diabetic eye loses vision. Endocrine. 2007;32:107–116. doi: 10.1007/s12020-007-0040-9. [DOI] [PubMed] [Google Scholar]
- 12.Liepmann D. Analysis: actuation force requirements for glucose pens. Diabetes Technol Ther. 2005;7:636–637. doi: 10.1089/dia.2005.7.636. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbloom AL. Limitation of finger joint mobility in diabetes mellitus. J Diabet Complications. 1989;3:77–87. doi: 10.1016/0891-6632(89)90016-0. [DOI] [PubMed] [Google Scholar]
- 14.Savas S. Koroglu BK. Koyuncuoglu HR. Uzar E. Celik H. Tamer NM. The effects of the diabetes related soft tissue hand lesions and the reduced hand strength on functional disability of hand in type 2 diabetic patients. Diabetes Res Clin Pract. 2006;77:77–83. doi: 10.1016/j.diabres.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Allahham A. Stewart P. Marriott J. Mainwaring DE. Flow and injection characteristics of pharmaceutical parenteral formulations using a micro-capillary rheometer. Int J Pharm. 2004;270:139–148. doi: 10.1016/j.ijpharm.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Asakura T. Seino H. Jensen KH. Patient acceptance and issues of education of two durable insulin pen devices. Diabetes Technol Ther. 2008;10:299–304. doi: 10.1089/dia.2007.0268. [DOI] [PubMed] [Google Scholar]
- 17.Reimer T. Hohberg C. Pfützner AH. Jørgensen C. Jensen KH. Pfützner A. Intuitiveness, instruction time, and patient acceptance of a prefilled insulin delivery device and a reusable insulin delivery device in a randomized, open-label, crossover handling study in patients with type 2 diabetes. Clin Ther. 2008;30:2252–2262. doi: 10.1016/j.clinthera.2008.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.