Abstract
This laboratory showed that ethanol augments apoptosis in fetal rhombencephalic neurons and co-treatment with alpha-lipoic acid (LA) or one of several other antioxidants prevents ethanol-associated apoptosis. Because ethanol increases oxidative stress, which causes apoptosis, it is likely that some of the neuroprotective effects of LA and other antioxidants involve classical antioxidant actions. Considering the reported link of LA with pro-survival cell signaling, it is also possible that LA’s neuroprotective effects involve additional mechanisms.
The present study investigated the effects of LA on ethanol-treated fetal rhombencephalic neurons with regard to oxidative stress and up-regulation of the pro-survival genes Xiap and Bcl-2. We included parallel gene expression studies with N-acetyl cysteine (NAC) to determine whether LA’s effects on Xiap and Bcl-2 were shared by other antioxidants. We also used enzyme inhibitors to determine which signaling pathway(s) might be involved with the effects of LA.
The results of this investigation showed that LA treatment of ethanol-treated neurons exerted several pro-survival effects. LA blocked two pro-apoptotic changes, i.e., the ethanol-associated rise in ROS and caspase-3. LA also up-regulated the expression genes that encode the anti-apoptotic proteins Bcl-2 and Xiap by a mechanism that involves NF-κB. NAC also up-regulated Bcl-2 and Xiap. Thus, the neuroprotective effects of LA and NAC could involve up-regulation of pro-survival genes as well as their classical antioxidant actions.
Keywords: Ethanol, Apoptosis, Neuroprotection, Lipoic Acid, Xiap, Bcl-2, N-acetyl cysteine
1. Introduction
In utero ethanol exposure seriously damages the developing CNS, causing learning and behavioral disabilities in children with Fetal Alcohol Syndrome (FAS) and Fetal Alcohol Spectrum Disorders (FASD) (Mattson et al., 1996; 1999; Riley et al., 2003; reviewed in Wattendorf, and Muenke, 2005). Neuroimaging studies detect structural abnormalities in several brain areas of children affected with FAS and/or FASD (Roebuck et al., 1998; Norman et al., 2009). Brain morphological changes can be explained in part by the ethanol-associated reduction in developing CNS neurons in vivo (Miller, 1995; Bonthius et al., 1996; Tajuddin and Druse, 1999; 2001; Sari et al., 2004; Zhou et al., 2001). The reduction in CNS neurons appears to be caused by apoptosis (Liesi, 1997; Cheema et al., 2000; Dunty et al., 2001; Ramachandran et all., 2001; 2003; Ikonomidou et al., 2000; Druse et al. 2004; 2005; 2007; Antonio and Druse, 2008; Cherian et al., 2008), which is associated with an ethanol-associated increase in oxidative stress (Li et al., 2001; Ramachandran et al., 2003).
In light of the devastating effects of ethanol on the developing CNS, several laboratories investigated potential therapeutic agents that might be neuroprotective and the mechanisms that are linked with these neuroprotective effects. This laboratory previously reported that the serotonin-1A (5-HT1A) receptor agonists ipsapirone and buspirone prevented an ethanol-associated decline in 5-HT neurons in the dorsal and median raphe (Tajuddin and Druse, 1999; 2001). We also showed that ipsapirone prevents ethanol-associated apoptosis in cultured 5-HT and other CNS neurons found in the fetal rhombencephalon (Druse et al., 2004; 2005). The mechanism, which underlies the pro-survival effects of ipsapirone appears to involve an increase in the pro-survival protein pAkt and an up-regulation of Xiap and Bcl-XL.
Several different antioxidants also exert neuroprotection against ethanol-associated apoptosis (Li et al., 2001; Ramachandran et al., 2003; Heaton et al., 2004; Antonio and Druse, 2008; Sheth et al., 2009). These antioxidants include lipoic acid (LA), vitamin E, N-acetyl cysteine (NAC), epigallocatechin gallate (EGCG), curcumin, resveratrol, and melatonin. One mechanism by which antioxidants cause neuroprotection undoubtedly involves their ability to reduce oxidative stress. However, additional mechanisms might also be involved. Potentially, LA could up-regulate the NF-kB dependent pro-survival genes X-inhibitor of apoptosis protein (Xiap) and Bcl-2 by activating PI3K ->pAkt (Zhang et al., 2001; 2007). Alternatively, LA could increase the expression of these anti-apoptotic genes through additional signaling pathways that activate NF-κB. For example, NF-κB activity can be increased via activation of I Kappa Kinase (IKK), modulation of ROS levels (Schreck et al., 1991; Flohe et al., 1997; Nakano et al., 2006; Pantano et al., 2006), and/or activation of at least two forms of protein kinase C (PKC), i.e., PKCalpha and PKCdelta (Mut et al., 2010). Importantly, another antioxidant, vitamin E, elevates the levels of the pro-survival proteins Bcl-2, Bcl-XL and activated Akt kinase in cerebellar neurons (Heaton et al., 2004).
Presently, we investigated two potential mechanisms that could underlie the neuroprotective effects of LA in ethanol-treated neurons: reduction of oxidative stress and increased expression of two antiapoptotic/pro-survival genes, Xiap and Bcl-2. A limited number of additional experiments were done with NAC to determine whether another antioxidant would have similar effects on these genes. In addition, inhibitors of PI3K, IKK, and PKC were included to determine whether any of these pathways were involved with LA’s effects on Xiap and Bcl-2.
2. Results
The results presented in Figure 1 show that ethanol augments apoptosis in cultures of fetal rhombencephalic neurons as shown by the 50% elevation of levels of activated/cleaved caspase-3 (p < .05) and co-treatment with LA blocks this pro-apoptotic change (p < .05). In Figure 1 and all subsequent figures, data is presented as the mean ± SEM of values obtained from separate experiments. Activated/cleaved caspase-3 was analyzed at 16 hours, because we expected to find an elevation in the apoptotic enzyme prior to the detection of fragmented/apoptotic nuclei. Fragmented/apoptotic nuclei were observed in ethanol-treated cultures at 24 hours in several prior studies from this laboratory (Druse et al., 2004–2007; Antonio and Druse, 2008). Statistical analyses of these results identified a main effect ethanol [F(1,27)=4.4, p<.05] and LA [F(1,27)=5.9, p<.05] and an interaction between ethanol and LA [F(1,27)=5.0, p<.05].
Figure 1. Ethanol increases caspase-3 in cultures that were treated with ethanol for 16 hours, and lipoic acid prevents this pro-apoptotic effect of ethanol.
Figure 1 shows cleaved/caspase-3 bands from a representative western blot. Below the western blot are bands of selected coomassie blue-stained protein bands from a duplicate gel that were used as loading controls. The densities of caspase-3 bands were calculated relative to these loading controls. The graphic presentation depicts the mean ± SEM of the adjusted relative densities of cleaved/activated caspase-3 levels, using data that was collected from 7 separate experiments in which fetal rhombencephalic neurons were cultured in the presence or absence of ethanol (E) and/or lipoic acid (LA) for 16 hours. Control (C) neurons were cultured in the absence of both ethanol and LA. A 2-way ANOVA demonstrated that there was a main effect of ethanol [F(1,27) = 4.4, p<.05] and lipoic acid [F(1,27)=5.9, p<.05] and an interaction between ethanol and lipoic acid. F(1,27) = 5.0, p<.05. The * identifies values different from the control value atp<0.05, while # identifies a significant difference in ELA neurons relative to E neurons at p<0.05.
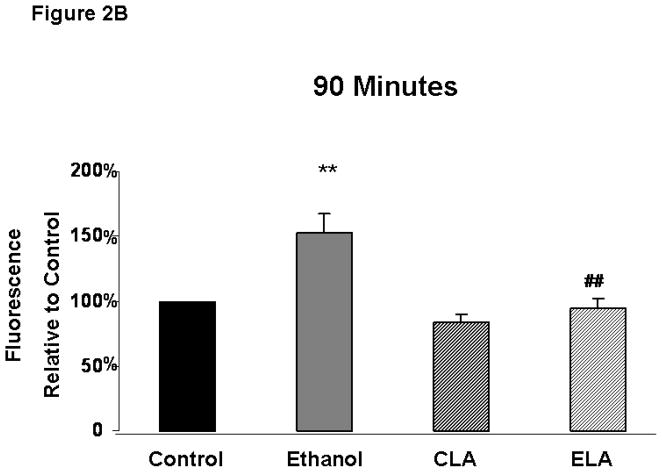
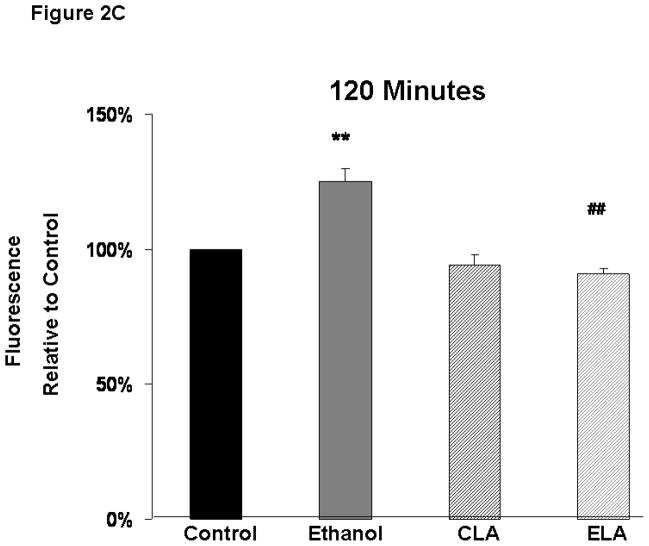
Ethanol treatment significantly increased ROS levels in fetal rhombencephalic neurons and LA blocked the ethanol-associated rise in ROS (Figure 2). Figure 2A depicts the results of a typical experiment in which relative levels of ROS were measured over a 2 hour time period using the fluorescent dye 2′,7′-dichlorofluorescein (DCF). In this and three similar replicate experiments 8–12 replicates were quantified at each time point. As shown in this experiment, ROS levels were elevated above those in the other treatment groups from 15 minutes through the 2-hour duration of the experiment. It can also be seen that there was a gradual increase in ROS in the control and other treatment groups, presumably because of the unfavorable culture conditions that had to be used with the DCF exerperiments, i.e., use of a serum-free and antioxidant-free medium during the 2-hour duration of ROS analyses. Figures 2B and 2C show that ethanol treatment significantly augmented ROS levels above those in control cultures (p < .01) at 90 and 120 minutes of treatment and LA blocked the ethanol-associated rise in ROS (p < .01). At both times of analyses, there was a main effect of ethanol [90 minutes: F(1,11)=14.0, p<.01; 120 minutes: F(1,17)=10.4, p<.01] and LA [90 minutes: F(1,11)=20.0, p<.001; 120 minutes: F(1,17)=36.5, p<.001], interaction between ethanol and LA [90 minutes: F(1,11)=6.5, p<.05; 120 minutes F(1,17)=18.3, p<.001]. ROS levels also increased over baseline values in control cultures over the 2-hour time period.
Figure 2. Ethanol increases reactive oxygen species (ROS) and lipoic acid prevents the ethanol-associated increase in ROS.
Figure 2A depicts the relative levels of ROS, detected by fluorescent dye 2′,7′-dichlorofluorescein (DCF), obtained in a typical experiment with 8–12 replicates of cultures maintained under control [no ethanol (E) or lipoic acid (LA)] conditions or treated with ethanol. Figures 2B and 2C are graphical depictions of the mean ± SEM of ROS levels at 90 and 120 minutes from values obtained from 3–5 separate experiments. The symbol ** identifies values that are significantly different from the control (C)) at p<0.01, while ## notes a significant difference between the ethanol (E) and ELA/ELA cultures at p<0.01. At both 90 and 120 minutes, there was a main effect of both ethanol [90 minutes: F(1,11)=14.0, p<.01; 120 minutes: F(1,17)=10.4, p<.01] and LA [90 minutes: F(1,11)=20.0, p<.001; 120 minutes: F(1,17)=36.5, p<.001], interaction between ethanol and LA [90 minutes: F(1,11)=6.5, p<.05; 120 minutes F(1,17)=18.3, p<.001].
LA caused a brisk and transient increase in pAkt at 20 minutes in ethanol-treated neurons. LA also prevented the ethanol-associated decrease in pAkt at 24 hours (Figure 3). pAkt levels were quantified at early (10′–30′) time points in order to determine whether LA activated the PI3K-> pAkt pro-survival pathway; measurements at the 24-hr time point were made to determine the levels of this pro-survival protein at a time when ethanol was known to augment apoptosis (Druse et al. 2004; 2005; 2007; Antonio and Druse, 2008). The mean pAkt levels from three to eight separate experiments at each time point are depicted relative to those of a loading control. At 20 minutes the mean in the group treated with both ethanol and LA was significantly greater than that in the control group (< .05) and in the ethanol-treated group (p < .01). At 24 hours ethanol decreased pAkt relative to control neurons (p < .05). Importantly, when cultures were co-treated with ethanol and LA for 24 hours, there was no significant difference in the amount of pAkt from that in control neurons (p > .05). Statistical analyses demonstrated that there was a main effect of ethanol [F(1,21)=5.5, p<.05] and LA [F(1,21)=7.1, p<.01] and an interaction of ethanol and LA [F(1,21)=5.5, p<.05] at 20 minutes. At 24 hours there was also a main effect of ethanol [F(1,11)=7.4, p<.01] and of LA F(1,11)=73.1 p<.001].
Figure 3. Lipoic acid increases pAkt in ethanol-treated neurons at 20 minutes (p < .05) and prevents an ethanol-associated decrease in pAkt at 24 hours )p < .05).
Figure 3 shows pAkt bands from a representative western blot. Below this the western blot are bands of selected coomassie blue-stained proteins from a duplicate gel that were used as loading controls. The density of pAkt bands was calculated relative to these loading controls. Each value represents the mean ± SEM of data obtained from 3 to 6 separate experiments in which fetal rhombencephalic neurons were cultured in the presence or absence of ethanol (E) and/or lipoic acid (LA). Values are presented relative to those from control cultures (no ethanol, no LA). The * identifies values which are significantly different from the control value at p<0.05 and ## identifies values from cultures treated with ethanol and Eth + LA that are different at p<0.01. A 2-way ANOVA detected a significant main effect of ethanol [F(1,21) = 5.5, p<.05] and lipoic acid [F(1,21) = 7.1, p<.01] and an interaction of ethanol and lipoic acid [F(1,21) = 5.5, p<.05] at the 2 hour time point. At 24 hours there was a main effect of ethanol [F(1,11) = 7.4, p<.01] and of LA [F(1,11) = 73.1, p<.001].
LA treatment significantly (p < .05) augmented Bcl-2 in both control and ethanol-treated neuronal cultures (Figure 4A). LA increased Bcl-2 in control neurons at 8, 16, and 24 hours. At 16 and 24 hours Bcl-2 was significantly increased in the group that was co-treated with ethanol and lipoic acid relative both to control and to ethanol-treated neurons. Statistical analyses showed that there was a main effect of LA on Bcl-2 at 8 hours [F(1,15)= 7.9, p <.05], 16 hours [F(1,49)= 25.4, p <.001], and 24 hours [F(1,25)= 15.0, p <.001]. Corresponding studies with Bcl-2 protein levels were not done, because the level of this protein was below detectability in cultures of fetal rhombencephalic neurons (data not shown).
Figure 4. Co-treatment with lipoic acid significantly increases Bcl-2 (Figure 4A) and Xiap (Figure 4B) mRNA in control- and/or ethanol-treated fetal rhombencephalic neurons.
Figure 4A depicts a time course in which fetal rhombencephalic neurons were maintained under control conditions [no ethanol (E) no lipoic acid (LA)] or treated with E and/or LA for periods of 2 to 24 hours. The 16-hour time point is noted with a box because additional experiments regarding this time point are described in Figure 5. Each value represents the mean ± SEM of values obtained from 4–10 separate experiments. Values are expressed as the fold change in Bcl-2 or Xiap mRNA as calculated by the 2−ΔΔCT method (Livak and Schmittgen, 2001). The symbols * and ** identify values that are significantly different from control values at p<0.05 and p<0.01, respectively, while # and ## identify values from the group treated with ethanol and lipoic acid (ELA) that are significantly different from the ethanol group at p<0.05 and p < 0.01, respectively. In each group, the SEM of the control value is typically 2–3% of the mean. For Bcl-2, there was a main effect of LA at 8 hours [F(1,15)= 7.9, p <.05], 16 hours [F(1,49)= 25.4, p <.001], and 24 hours [F(1,25)= 15.0, p <.001]. With regard to Xiap there was a main effect of ethanol at 2 hours [F(1,21)= 4.1, p=.05] and 24 hours [F(1,28)= 4.8, p < .05]. There was also a main effect of LA at 8 hours [F(1,15)= 6.6, p < .05], 16 hours [F(1,37)= 9.8, p < .01] and 24 hours [F(1,28)=24.9, p < .0001] and an interaction between ethanol × LA at 16 hours F(1,37)= 8.7, p < .01].
LA increased Xiap in control and ethanol-treated cultures (Figure 4B) at 8, 16, and 24 hours (p< .05). At 24 hours, Xiap was increased in neurons treated both with ethanol and LA relative to both control and ethanol-treated neurons. Interestingly, ethanol elevated Xiap at both an early (2 hours) and late (16 hours) time point (p < .05). There was a main effect of ethanol at 2 hours [F(1,21)= 4.1, p< .05] and 24 hours [F(1,28) = 4.8, p < .05 and a main effect of LA at 8 hours [F(1,15) = 6.6, p<.05], 16 hours [F(1,37)= 9.8, p < .01] and 24 hours [F(1,28)=24.9, p < .0001]. There was also an interaction between ethanol and LA at 16 hours [F(1,37)= 8.7, p < .01]. Corresponding studies with Xiap protein levels were not done, because the level of this protein was below detectability in cultures of fetal rhombencephalic neurons (data not shown).
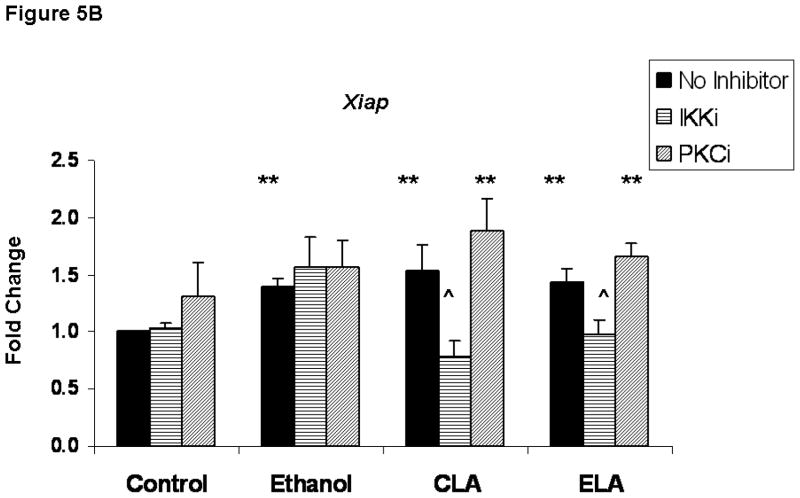
Inhibition of IκB kinase (IKKi) blocked the ability of LA to up-regulate Bcl-2 and Xiap, suggesting the NF-κB activity is involved with the LA-mediated changes in the expression of these genes (Figure 5) The data depicted in Figure 5 comes from ethanol. and LA-treated neuronal cultures that were maintained in the absence or presence of inhibitors of IKKi or protein kinase C (PKCi) for 16 hours. For each treatment group, the inhibitor data is presented relative to samples that were not treated with inhibitor. These studies were performed to assess whether either signaling pathway is involved with LA-mediated up-regulation of Bcl-2 and/or Xiap that was detected at 16 hours. The IKKi inhibitor of the NF-κB pathway significantly reduced the LA-mediated elevation of Bcl-2 (Figure 5A) and Xiap (Figure 5B) in both control and ethanol-treated cultures (p< .01). In contrast, PKCi blocked only the LA-mediated increase of Bcl-2 in control neurons (p < .05). Inhibitors of the PI3K and MAPKK pathways failed to attenuate the effects of LA at the 16-hour time point (data not shown). In addition, the ethanol-associated increase in Xiap at 16 hours was not blocked by either IKKi or PKCi.
Figure 5. The effects of lipoic acid on Bcl-2 and Xiap appear to involve the NF-κB pathway.
Figure 5 depicts the effects of inhibitors of IκB kinase (IKKi) and protein kinase C (PKCi) on the expression of Bcl-2 (Figure 5A) and Xiap (Figure 5B) in neurons that were maintained under control conditions (C) or co-treated with ethanol (E) and LA for 16 hours. For each treatment group, inhibitor values are presented relative to cultures that lacked inhibitors. Each value represents the mean ± SEM of values obtained from 4 separate experiments. The symbol ** identifies values that are significantly different from the control value at p < 0.01, while ^ identifies values in the inhibitor-treated group that are significantly different from the matched groups in the no-inhibitor group at p< 0.05.
Another antioxidant, NAC, also augmented Xiap and Bcl-2 at 16 hours (Figure 6). The results of four to five separate experiments showed that NAC augmented Bcl-2 (Figure 6A) and Xiap (Figure 6B) in control and/or ethanol-treated cultures after 16 hours of treatment, a time at which LA also increased the expression of both genes.
Figure 6. N-Acetylcysteine augments levels of Bc l-2 and Xiap in control and/or ethanol-treated fetal rhombencephalic neurons.
Levels of Bcl-2 (Figure 6A) and Xiap (Figure 6B) were determined in fetal rhombencephalic neurons that were maintained under control conditions (C)) or co-treated with ethanol (E) and/or NAC as described. Each value is presented as the mean ± SEM of values obtained from 4–7 separate experiments. The symbols * and ** identify values for which the NAC group significantly altered the level of Bcl-2 or Xiap at p < .05 and p< .01, respectively. ## identifies values in the Ethanol + NAC group that are significantly different from those in the ethanol group at p < .01. There was a main effect of NAC on Bcl-2 [F(1,16) = 19.5 p < .001] and on XIAP [F(1,16) = 17.8, p < .001].
3. Discussion
The increase in the pro-apoptotic enzyme caspase-3 in ethanol-treated fetal rhombencephalic neurons is consistent with prior evidence that ethanol augments apoptosis in neuronal cells (Liesi, 1997; Oberdoerster and Rabin, 1999; Dunty et al., 2001; Cherian et al., 2008; Ramachandran et all., 2001; 2003; Ikonomidou et al., 2000; Druse et al. 2004; 2005; 2007; Antonio and Druse, 2008). The ethanol-associated loss of CNS neurons that accompanies such apoptotic effects undoubtedly contributes to both the morphological (Roebuck et al., 1998; Norman et al., 2009) and functional abnormalities (Mattson et al., 1996; 1999; Riley et al., 2003; reviewed in Wattendorf, and Muenke, 2005) that are found in children with FAS and/or FASD.
Importantly, co-treatment with the antioxidant LA blocks the increase in apoptosis in ethanol-treated neurons. LA prevents the rise in caspase-3 (this study), fragmented/apoptotic nuclei (Antonio and Druse, 2008), and neurotoxicity (Pirlich et al., 2002). One mechanism by which LA could prevent the pro-apoptotic effects of ethanol is by blocking the rise in ROS (this study) and associated increase in oxidized proteins (Pirlich et al., 2002), because ethanol-associated oxidative stress augments apoptosis (Sun et al., 1997; Li et al., 2001; Ramachandran et al., 2001; 2003; Heaton et al., 2002; Watts et al., 2005; Chu et al., 2007). Additional antioxidants, including vitamin E, resveratrol, curcumin, ECGC, melatonin, and NAC, also prevent the neurotoxic effects of ethanol (Li et al., 2001; Ramachandran et al., 2003; Heaton et al., 2004; Marino et al., 2004; Siler-Marsiglio et al., 2005; Antonio and Druse, 2008; Sheth et al., 2009). Interestingly, while LA blocked an ethanol-associated rise in ROS and caspase-3 in ethanol cultures, ROS and caspase-3 levels in control + LA-treated neurons were comparable to those in control cultures. Thus, it appears that there are compensatory mechanisms in control/unstressed neurons which prevent them from unnecessary responses to an exogenous stimulus.
Another mechanism by which LA could mediate its neuroprotective effects in ethanol-treated cultures of fetal rhombencephalic neurons is by up-regulating the expression of Xiap and Bcl-2. LA augments both genes above the baseline levels found in control and ethanol-treated cultures NAC exerted similar effects. If these changes in. gene expression are paralleled by increased levels of the corresponding proteins, apoptosis would be attenuated in the ethanol-treated cultures. In fact, another group (Heaton et al., 2004) reported that treatment with the antioxidant vitamin E resulted in higher levels of the Bcl-2, Bcl-XL and Xiap proteins in ethanol-treated cerebellar neurons. Although the LA- and NAC-mediated increase in pro-survival gene expression could attenuate apoptosis in ethanol-treated cultures, a similar rise in Xiap and Bcl-2 in control neurons did not reduce the baseline level of apoptosis as measured by caspase-3 (this study) or fragmented nuclei (Antonio and Druse, 2008). As noted previously, such observations suggest that there are compensatory cellular mechanisms to prevent the unnecessary overproduction of pro-survival proteins in unstressed/control cells. In contrast to an earlier study (Druse et al., 2006), ethanol treatment did not reduce levels of Xiap or Bcl-2 below those in control cultures at later time points. The difference in findings can be explained by use of a higher seeding density of fetal rhombencephalic neurons in the present study. Comparative studies suggest that cultures seeded at a lower density are more vulnerable to the effects of ethanol such as these gene changes (data not shown).
The LA-mediated elevation of Bcl-2 and Xiap appears to involve NF- κB, which is downstream of IKK, because IKKi blocked this effect of LA in both control and ethanol-treated cultures. It is possible that additional signaling pathways, such as those involving PKC, PI3K, or MAPKK, could also up -regulate the expression of NF-κB pro-survival genes. In fact, LA increased pAkt in ethano l-treated neurons at 20 minutes. However, inhibitors of PI3K and MAPK (data not shown) as well as PKC (Figure 5) failed to block the increase in Xiap and Bcl-2 at 16 hours. We cannot rule out the possibility that analyses at additional time points might have implicated one or more of these pathways with the effects of LA.
Interestingly, Xiap was up-regulated by ethanol; ethanol increased expression of this gene at 2 and 16 hours. It is likely that ethanol’s effects were mediated directly by the ethanol-associated increase in ROS, because NF-κB is a ROS-sensitive (Schreck et al., 1991; Flohe et al., 1997; Nakano et al., 2006; Pantano et al., 2006) transcription factor. Moreover, neither IKKi nor PKCi blocked the ethanol-associated rise of Xiap at 16 hours. Such a transient increase in Xiap could reflect the attempts of stressed ethanol-treated neurons to survive.
In summary, LA treatment of ethanol-treated neurons exerted several effects which could promote the survival of these cells. Specifically, LA blocked two ethanol-associated pro-apoptotic changes: LA blocked the rise in ROS and caspase-3. LA also up-regulated the expression of two genes that encode the anti-apoptotic proteins Bcl-2 and Xiap by a mechanism that appears to involve NF-κB. The up-regulation of Bcl-2 and Xiap by LA is not limited to this antioxidant. Rather, similar changes accompanied treatment with NAC. Thus, the neuroprotective effects of LA, NAC and potentially additional antioxidants could involve up-regulation of pro-survival genes as well as their classical antioxidant actions.
4. Experimental Procedure
Neuronal cultures
Fetal rhomencephalic tissue from gestation day 14 (G14) fetuses of Sprague-Dawley rats was used as a source of neurons, because this laboratory previously demonstrated that developing 5-HT and other CNS neurons from this brain region are vulnerable to the pro-apoptotic effects of ethanol (Druse et al., 2004 – 2007). All procedures for the care and use of animals were approved by the Institutional Animal Care and Use Committee at Loyola University Chicago, Stritch School of Medicine.
Fetal rhombencephalic tissue was mechanically disaggregated using sterile procedures (Eriksen and Druse, 2001). For most studies tissue was seeded onto poly-D-lysine coated plates (55 cm2) (Corning, Corning, NY) at a density of 9 to 11 × 106 cells/plate. However, for the assessment of ROS levels, cells were plated onto 96-well plates at a similar density, which is equivalent to 60,000 cells/well. The day of seeding corresponds to the first day in vitro (DIV 1).
In all experiments, cells were initially maintained in Dulbecco’s Minimal Essential Media/F12 (DMEM/F12) media (Invitrogen, Carlsbad, CA), which was supplemented with hydrocortisone-21 sulfate (Sigma, St. Louis, MO), Basal Medium Eagles Vitamin Solution (Sigma, St. Louis, MO), B27 serum free medium supplement (Invitrogen, Carlsbad, CA), and 0.25% fetal bovine serum. After 24 h (DIV 2), 0.4 M cytosine arabinoside (Sigma, St. Louis, MO) was included in each culture to arrest gliogenesis. Media was changed on DIV 2 and DIV 5. Neurons were maintained in culture for 5 days (DIV 1 to DIV 6). In DIV6 cultures less than 5% of the cells were positive for glial fibrillary acidic protein, which is a marker of astrocytes.
Ethanol and antioxidant treatment
For western blot analyses and RT-PCR studies, media was replaced on DIV 5 with one that contained 0 or 75 mM ethanol and/or 0 or 10 μM (±)-α-LA (Sigma, St. Louis, MO) and an antioxidant-free B27 supplement (Invitrogen, Carlsbad, CA). The ethanol chamber system, used in this and prior studies, maintains the ethanol concentration in cultures at ≥85% of the initial concentrations (Eriksen and Druse, 2001). The dose of LA was selected because it was the lowest of three doses (1, 10 or 50 M LA) that exerted neuroprotection against ethanol-associated apoptosis (Antonio and Druse, 2008). Similar screening studies determined the dose of N-acetylcysteine (NAC, 1 mM) that was used in a limited number of additional quantitative real-time RT-PCR experiments.
Enzyme inhibitors were included to determine whether the effects of LA were linked to one of the common pro-survival pathways. Separate studies included either 10 μM of the PI3K inhibitor LY294002 (Cell Signaling, Danvers, MA); 10 μM of the mitogen activated protein kinase kinase (MAPKK) inhibitor PD98059 (Cell Signaling, Danvers, MA); 2.5 μM bisindolymaleimide I (Calbiochem/EMD, Gibbstown, NJ], which is an inhibitor of the alpha, beta, gamma, delta and epsilon forms of protein kinase C (PKC); or 0.2 μM of the IKK inhibitor VII (Calbiochem/EMD, Gibbstown, NJ].
Western blot analyses
Protein was extracted from cells after treatment with ethanol and/or LA as described previously (Druse et al., 2005). Briefly, media was removed, cells were rinsed with 0.01 M phosphate buffered saline (PBS), pH 7.6, and cell suspensions were sonicated in lysis buffer (Cell Signaling, Beverly, MA) that contained protease inhibitors (leupeptin, aprotinin, pepstatin A, phenylmethylsulfonyl fluoride). Denatured and reduced proteins were separated by SDS-PAGE on 12% polyacrylamide gels and transferred to nitrocellulose membranes (Druse et al., 2005). A solution containing 1% BSA in Tris-buffered saline containing 0.05% Tween-20 was used as a blocking agent. Membranes were probed with a primary antibody to either cleaved/activated caspase-3 or pAkt, (Cell Signaling, Beverly, MA) and a biotinylated secondary antibody conjugated with ABC-peroxidase (Pierce, Rockford, IL). Specific bands were visualized using the Super Signal West Pico Chemiluminescence Substrate (Pierce, Rockford, IL) and matched with molecular weight markers on gels. The relative optical densities of the bands on autoradiograms were quantified using NIH Image. A comparable amount of protein (~30 micrograms) was loaded onto each gel. Efficiency of protein transfer was verified by examining any residual proteins on coomassie blue stained gels. The density of the same randomly selected band from a replicate untransferred gel that was stained with coomassie blue was used to adjust for potential differences in loading. Western blot data is presented relative to the loading control. Protein content was determined (Lowry et al., 1951).
Measurement of ROS
ROS content was measured using a nonfluorescent dye, 2′7′-dichlorodihydrofluorescein diacetate (H2DCFDA) (Sigma, St. Louis, MO), which is oxidized by ROS to the fluorescent dye 2′,7′-dichlorofluorescin (DCF). On the day of analysis, DIV 6, neuronal cultures were pretreated for 1 hour with 10 μM LA in an antioxidant- and phenol red-free media, washed and then treated with culture media containing 50 μM H2DCFDA in 1X Dulbecco’s phosphate buffered saline with calcium and magnesium (Hyclone, Logan, Utah) for 20 minutes at 37 °C. Wells with no H2DCFDA (only cells) and wells with no cells (only H2DCFDA) were included to measure the background fluorescence. Cells were loaded with H2DCFDA, washed with PBS, and treated with 0 or 75 mM ethanol in PBS. DCF fluorescence was determined over the 2-hour treatment period using a fluorescent plate reader with an excitation filter (485 nm/20) and an emission filter (530 nm/25).
Quantitative real-time RT-PCR
Neuronal cultures were treated for 2, 4, 8, 16, and 24 on DIV 5 with 0 or 75 mM ethanol and/or 0 or 10 μM LA. As mentioned earlier, a limited number of additional cultures were cultured in the presence and absence of NAC or inhibitors of PI3K, MAPKK, PKC, or IKK.
RNA was isolated from neuronal cultures as previously described by this laboratory (Druse et al., 2006; Druse et al., 2007). cDNA was prepared and used in real time RT-PCR as previously described (Druse et al., 2006; Druse et al., 2007). For each target gene, PCR amplifications were performed in triplicate using a Perkin-Elmer Gene Amp 7300 Sequence Detector thermal cycler (Applied Biosystems, Foster City, CA). GAPDH was used to normalize sample inputs and to generate standard curves, because our prior studies found that GAPDH is not affected by ethanol at the treatment times examined in this study. The Primer Express program (Applied Biosystems, Foster City, CA) and the NCBI database were used to select specific primary sequences, and primers were synthesized by Life Technologies/Invitrogen, Carlsbad, CA. Data was analyzed by SDS software (Applied Biosystems, Foster City, CA). Results were then analyzed as described (Druse et al., 2007; Druse et al., 2006) using the 2−ΔΔCT method (Livak and Schmittgen, 2001), which determines values relative to the matched control. The number that gives rise to the fold change value (CT of gene of interest-CT of GAPDH) for control cultures was 2–3% of the mean.
Timing of Analyses
We selected specific times for each analysis based on the relative time at which LA- or ethanol-associated changes were expected to occur or on prior observations from this or another laboratory. The selected times of analyses reflect the temporal relationships of certain events and the evidence that some events are expected to be downstream of others. For example, preliminary studies showed that ethanol increased ROS quickly and earlier studies showed that ethanol augmented apoptosis (as measured by fragmented nuclei) at 24 hours (Druse et al., 2004; 2005; 2007; Antonio and Druse, 2008). Because caspase-3 would be activated before one could detect fragmented nuclei, caspase-3 was measured at an earlier time, i.e., 16 hours. pAkt was quantified after a brief treatment with ethanol and/or LA to reflect signaling changes and at a later time point (24 hours) to assess levels in surviving neurons. Gene expression was evaluated over a wide time course, because changes in ROS, pAkt and other factors could potentially alter this expression.
Statistical analyses
Data were analyzed using 2-way ANOVA [ethanol × antioxidant] we were investigating the separate and combined effects of two agents, ethanol and LA, and the interaction of these two agents. In studies using an inhibitor of a signaling pathway, a 3-way ANOVA [ethanol × antioxidant × inhibitor] and a Newmann-Keuls post-hoc analysis was used. p<0.05 was considered to be significant.
Acknowledgments
This research was supported by a grant from the USPHS - AA03490. As a pre-doctoral fellow, Angeline M. Antonio was supported by T32 AA13527 and 5F31AA017343.
List of Abbreviations
- DCF
dye 2′,7′-dichlorofluorescin (DCF)
- DIV
day in vitro
- H2DCFDA
2′7′-dichlorodihydrofluorescein diacetate
- IKK
I-kappa B kinase
- LA
(±)- alpha lipoic acid
- MAPKK
mitogen activated protein kinase kinase
- NAC
N- acetyl cysteine
- PKC
protein kinase C
- PI3K
phosphatidylinositol 3′kinase
- ROS
reactive oxygen species
- Xiap
X-inhibitor of apoptosis protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonio AM, Druse MJ. Antioxidants prevent ethanol-associated apoptosis in fetal rhombencephalic neurons. Brain Res. 2008;1204:16–23. doi: 10.1016/j.brainres.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthius DJ, Bonthius NE, Napper RM, Astley SJ, Clarren SK, West JR. Purkinje cell deficits in nonhuman primates following weekly exposure to ethanol during gestation. Teratology. 1996;53:230–236. doi: 10.1002/(SICI)1096-9926(199604)53:4<230::AID-TERA5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Cheema ZF, West JR, Miranda RC. Ethanol induces Fas/Apo [apoptosis]-1 mRNA and cell suicide in the developing cerebral cortex. Alcohol Clin Exp Res. 2000;24:535–543. [PubMed] [Google Scholar]
- Cherian PP, Schenker S, Henderson GI. Ethanol-mediated DNA damage and PARP-1 apoptotic responses in cultured fetal cortical neurons. Alcohol Clin Exp Res. 2008;32:1884–1892. doi: 10.1111/j.1530-0277.2008.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Tong M, de la Monte SM. Chronic ethanol exposure causes mitochondrial dysfunction and oxidative stress in immature central nervous system neurons. Acta Neuropathol. 2007;113:659–673. doi: 10.1007/s00401-007-0199-4. [DOI] [PubMed] [Google Scholar]
- Druse MJ, Tajuddin NF, Gillespie RA, Dickson E, Atieh M, Pietrzak CA, Le PT. The serotonin-1A agonist ipsapirone prevents ethanol-associated death of total rhombencephalic neurons and prevents the reduction of fetal serotonin neurons. Brain Res Dev Brain Res. 2004;150:79–88. doi: 10.1016/j.devbrainres.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Druse M, Tajuddin NF, Gillespie RA, Le P. Signaling pathways involved with serotonin1A agonist-mediated neuroprotection against ethanol-induced apoptosis of fetal rhombencephalic neurons. Brain Res Dev Brain Res. 2005;159:18–28. doi: 10.1016/j.devbrainres.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Druse M, Gillespie RA, Tajuddin NF, Rich M. S100B-mediated protection against the pro-apoptotic effects of ethanol on fetal rhombencephalic neurons. Brain Res. 2007;1150:46–54. doi: 10.1016/j.brainres.2007.02.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druse MJ, Tajuddin NF, Gillespie RA, Le P. The effects of ethanol and the serotonin(1A) agonist ipsapirone on the expression of the serotonin(1A) receptor and several antiapoptotic proteins infetal rhombencephalic neurons. Brain Res. 2006;1092:79–86. doi: 10.1016/j.brainres.2006.02.065. [DOI] [PubMed] [Google Scholar]
- Druse M, Gillespie RA, Tajuddin NF, Rich M. S100B-mediated protection against the pro-apoptotic effects of ethanol on fetal rhombencephalic neurons. Brain Res. 2007;1150:46–54. doi: 10.1016/j.brainres.2007.02.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunty WC, Chen SY, Zucker RM, Dehart DB, Sulik KK. Selective vulnerability of embryonic cell populations to ethanol-induced apoptosis: implications for alcohol-related birth defects and neurodevelopmental disorder. Alcohol Clin Exp Res. 2001;25:1523–1535. [PubMed] [Google Scholar]
- Eriksen JL, Druse MJ. Astrocyte-mediated trophic support of developing serotonin neurons: effects of ethanol, buspirone and S100B. Brain Res Dev Brain Res. 2001;131:9–15. doi: 10.1016/s0165-3806(01)00240-1. [DOI] [PubMed] [Google Scholar]
- Flohe L, Brigelius-Flohe R, Saliou C, Traber MG, Packer L. Redox regulation of NF-kappa B activation. Free Radic Biol Med. 1997;22:1115–1126. doi: 10.1016/s0891-5849(96)00501-1. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Mayer J, Miller R. Ethanol-mediated generation of reactive oxygen species in developing rat cerebellum. Neurosci Lett. 2002;334:83–86. doi: 10.1016/s0304-3940(02)01123-0. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Madorsky I, Paiva M, Siler-Marsiglio KI. Vitamin E amelioration of ethanol neurotoxicity involves modulation of apoptosis-related protein levels in neonatal rat cerebellar granule cells. Brain Res Dev Brain Res. 2004;150:117–124. doi: 10.1016/j.devbrainres.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Li Y, Walker DW, King MA. Peroxide mediates ethanol-induced cytotoxicity in PC12 cells. Free Radic Biol Med. 2001;30:389–392. doi: 10.1016/s0891-5849(00)00484-6. [DOI] [PubMed] [Google Scholar]
- Liesi P. Ethanol-exposed neurons fail to migrate and undergo apoptosis. J Neurosci Res. 1997;48:439–448. doi: 10.1002/(sici)1097-4547(19970601)48:5<439::aid-jnr5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- Marino MD, Aksenov MY, Kelly SJ. Vitamin E protects against alcohol-induced cell loss and oxidative stress in the neuronal rat hippocampus. Int J Dev Neurosci. 2004;22:363–377. doi: 10.1016/j.ijdevneu.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Delis DC, Stern C, Jones KL. Verbal learning and memory in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20:810–816. doi: 10.1111/j.1530-0277.1996.tb05256.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 1999;23:1808–1815. [PubMed] [Google Scholar]
- Miller MW. Generation of neurons in the rat dentate gyrus and hippocampus: effects of prenatal and postnatal treatment with ethanol. Alcohol Clin Exp Res. 1995;19:1500–1509. doi: 10.1111/j.1530-0277.1995.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Mut M, Amos S, Hussaini IM. PKC alpha phosphorylates cytosolic NF-kappaB/p65 and PCK delta delays nuclear translocation of NF-kappaB/p65 in U1242 glioblastoma cells. Turk Neurosurg. 2010;20:277–285. doi: 10.5137/1019-5149.JTN.3008-10.1. [DOI] [PubMed] [Google Scholar]
- Nakano H, Nakajima A, Sakon-Komazawa S, Piao JH, Xue X, Okumura K. Reactive oxygen species mediate crosstalk between NF-kappaB and JNK. Cell Death Differ. 2006;13:730–737. doi: 10.1038/sj.cdd.4401830. [DOI] [PubMed] [Google Scholar]
- Norman AL, Crocker N, Mattson SN, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Dev Disabil Res Rev. 2009;15:209–217. doi: 10.1002/ddrr.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdoerster J, Rabin RA. Enhanced caspase activity during ethanol-induced apoptosis in rat cerebellar granule cells. Eur J Pharmacol. 1999;385:273–282. doi: 10.1016/s0014-2999(99)00714-1. [DOI] [PubMed] [Google Scholar]
- Pantano C, Reynaert NL, van der Vliet A, Janssen-Heininger YM. Redox-sensitive kinases of the nuclear factor-kappaB signaling pathway. Antioxid Redox Signal. 2006;8:1791–806. doi: 10.1089/ars.2006.8.1791. [DOI] [PubMed] [Google Scholar]
- Pirlich M, Kiok K, Sandig G, Lochs H, Grune T. Alpha-lipoic acid prevents ethanol-induced protein oxidation in mouse hippocampal HT22 cells. Neurosci Lett. 2002;328:93–96. doi: 10.1016/s0304-3940(02)00415-9. [DOI] [PubMed] [Google Scholar]
- Ramachandran V, Perez A, Chen J, Senthil D, Schenker S, Henderson GI. In utero ethanol exposure causes mitochondrial dysfunction, which can result in apoptotic cell death in fetal brain: a potential role for 4-hydroxynonenal. Alcohol Clin Exp Res. 2001;25:862–871. [PubMed] [Google Scholar]
- Ramachandran V, Watts LT, Maffi SK, Chen J, Schenker S, Henderson G. Ethanol-induced oxidative stress precedes mitochondrially mediated apoptotic death of cultured fetal cortical neurons. J Neurosci Res. 2003;74:577–88. doi: 10.1002/jnr.10767. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Li TK, Jacobson SW, Coles CD, Kodituwakku PW, Adnams CM, Korkman MI. Neurobehavioral consequences of prenatal alcohol exposure: an international perspective. Alcohol Clin Exp Res. 2003;27:362–373. doi: 10.1097/01.ALC.0000052703.38558.B2. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. A review of the neurantaomical findings in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:339–344. doi: 10.1111/j.1530-0277.1998.tb03658.x. [DOI] [PubMed] [Google Scholar]
- Sari Y, Zhou FC. Prenatal alcohol exposure causes long-term serotonin neuron deficit in mice. Alcohol Clin Exp Res. 2004;28:941–948. doi: 10.1097/01.alc.0000128228.08472.39. [DOI] [PubMed] [Google Scholar]
- Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth DS, Tajuddin NF, Druse MJ. Antioxidant neuroprotection against ethanol-induced apoptosis in HN2–5 cells. Brain Res. 2009;1285:14–21. doi: 10.1016/j.brainres.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siler-Marsiglio KI, Pan Q, Paiva M, Madorsky I, Khurana NC, Heaton MB. Mitochondrially targeted vitamin E and vitamin E mitigate ethanol-mediated effects on cerebellar granule cell antioxidant defense systems. Brain Res. 2005;1052:202–211. doi: 10.1016/j.brainres.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Sun AY, Chen YM, James-Kracke M, Wixom P, Cheng Y. Ethanol-induced cell death by lipid peroxidation in PC12 cells. Neurochem Res. 1997;22:1187–1192. doi: 10.1023/a:1021968526696. [DOI] [PubMed] [Google Scholar]
- Tajuddin NF, Druse MJ. In utero ethanol exposure decreased the density of serotonin neurons. Maternal ipsapirone treatment exerted a protective effect. Brain Res Dev Brain Res. 2001;117:91–97. doi: 10.1016/s0165-3806(99)00102-9. [DOI] [PubMed] [Google Scholar]
- Tajuddin NF, Druse MF. A persistent deficit of serotonin neurons in the offspring of ethanol-fed dams: Protective effects of maternal ipsapirone treatment. Brain Res Dev Brain Res. 2001;129:181–188. doi: 10.1016/s0165-3806(01)00199-7. [DOI] [PubMed] [Google Scholar]
- Wattendorf DJ, Muenke M. Fetal alcohol spectrum disorders. Am Fam Physician. 2005;72:279–282. [PubMed] [Google Scholar]
- Watts LT, Rathinam ML, Schenker S, Henderson GI. Astrocytes protect neurons from ethanol-induced oxidative stress and apoptotic death. J Neurosci Res. 2005;80:655–666. doi: 10.1002/jnr.20502. [DOI] [PubMed] [Google Scholar]
- Zhang L, Xing GQ, Barker JL, Chang Y, Maric D, Ma W, Li BS, Rubinow DR. Alpha-lipoic acid protects rat cortical neurons against cell death induced by amyloid and hydrogen peroxide through the Akt signaling pathway. Neurosci Lett. 2001;312:125–128. doi: 10.1016/s0304-3940(01)02205-4. [DOI] [PubMed] [Google Scholar]
- Zhang WJ, Wei H, Hagen T, Frei B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci USA. 2007;104:4077–4082. doi: 10.1073/pnas.0700305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Zhang JK, Goodlett CR, Li T. Prenatal alcohol exposure retards the migration and development of serotonin neurons in fetal C57BL mice. Dev Brain Res. 2001;125:147–155. doi: 10.1016/s0165-3806(00)00144-9. [DOI] [PubMed] [Google Scholar]