Summary
Advances in imaging technology have provided powerful tools for dissecting the angiogenic and inflammatory aspects of atherosclerosis. Improved technology along with multi-modal approaches has expanded the utilisation of imaging. Recent advances provide the ability to better define structure and development of angiogenic vessels, identify relationships between inflammatory mediators and the vessel wall, validate biological effects of anti-inflammatory and anti-angiogenic drugs, delivery and/or targeting specific molecules to inflammatory regions of atherosclerotic plaques.
Keywords: Atherosclerosis, inflammation, angiogenesis, imaging
Introduction
Angiogenesis and inflammation are co-workers in the reparative and remodelling processes following tissue damage as well as in the destructive process of disease. Cues from within the local environment help determine if the processes will be beneficial or damaging in their response to injury. Scleroderma, atherosclerosis, diabetes, abdominal aortic aneurysm and ischaemic injury are aberrant vascular conditions that exemplify the detrimental effects of the relationship between angiogenesis and inflammation. Advances in imaging technology have provided powerful tools for dissecting the angiogenic and inflammatory components of atherosclerosis. This review will discuss the advances with emphasis placed on modalities that have provided the most insight into angiogenesis and inflammation in the atherosclerotic disease process.
A dysfunctional endothelium is one of the initial signatures of early atherosclerosis (see [1] for review). It is stimulated by multiple factors that include elevated and modified low-density lipoproteins (LDL), shear stress, free radicals and hypertension (see [2] for review). The altered endothelium induces an inflammatory response that results in formation of lesions in large and medium sized arteries (3, 4). The lesions are composed of macrophages/monocytes, T lymphocytes, endothelial cells (EC), and vascular smooth muscle cells (VSMC). The inflammatory cells secrete cytokines that stimulate EC activation, proliferation and migration, which are characteristics of angiogenesis (5, 6).
Vasa vasorum, a network of microvasculature that originate primarily in the adventitia of large arteries, become activated during atherosclerosis in humans (7, 8) and in mouse models of atherosclerosis (9, 11). Vasa vasorum supply oxygen and nutrients to the outer layers of the arterial wall that are beyond the limit of diffusion from the luminal surface (12). The increase in vasa vasorum density was originally thought to be in response to the thickened arterial wall that occurs with plaque development. However, there is evidence that their expansion occurs prior to endothelial dysfunction, intimal thickening or plaque development (13–15). Other studies show that angiogenesis is not sufficient to induce neointima thickening. Once the event is initiated, pro-angiogenic molecules can promote growth of intimal lesions (16). Despite the reported differences surrounding the sequence of events, the fact remains that intraplaque vasa vasorum are an indicator of plaque growth, progression, haemorrhage, instability and rupture (17–19). Anti-angiogenic molecules have proven to be effective inhibitors of vasa vasorum density and plaque progression (9–11).
Adventitial vasa vasorum are thought to be a conduit for entry of inflammatory mediators and cells to the vessel wall and eventually to the developing plaque where they contribute significantly to disease progression (20, 21). This is made possible by vascular permeability factors such as VEGF. The permeability leads to edema, which attracts inflammatory cells to the permeable vessels (see [22] for review).
There are two theories about the mechanism by which inflammatory cells enter the vessel wall to initiate an inflammatory response to vascular injury (Fig. 1) (23). The original “inside-out” theory supports the concept that various risk factors stimulate T lymphocyte and monocyte association with the area of endothelial injury. Adhesion molecules and integrins stabilise the cellular interactions and inflammatory cells infiltrate the subendothelial space at EC junctions (24, 25). These events promote endothelial production of reactive oxygen species (ROS). Accumulation of oxidized lipids on the intimal surface causes injury to the endothelium that initiates an inflammatory response (26). Oxidised LDL taken up by macrophages stimulates the release of numerous pro-inflammatory mediators that recruit inflammatory cells to the lesion, activate EC and VSMC that release more inflammatory mediators (27). In this theory, the process would begin in the intima and work its way outward into the adventitia.
Figure 1. Depiction of “inside-out” and “outside-in” theories of vascular inflammation.

The “outside-in” theory assumes that inflammatory cell enter the vessel wall from the luminal side. The “outside-in” theory predicts that the inflammatory cells gain entry to the vessel wall via adventitial vasa vasorum. Printed with permission by Oxford University Press: Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res 2007; 75: 640–648. (23).
The more recent “outside-in” theory advocates initiation of vessel wall inflammation in the adventitia that works its way into the media and intima. Data that support this theory are increased vasa vasorum neovascularisation, increased numbers of adventitial leukocytes (28), proliferation and differentiation of adventitial fibroblasts into myofibroblasts (29, 30). The adventitial response to vascular injury was first shown in restenosis following balloon angioplasty. Almost half of the injury-induced neointima originated from adventitial cell proliferation (31); this opposed earlier theories that the neointima is composed of VSMC originating in the media (32). T and B lymphocytes have been identified in the adventitia prior to intimal lesion development (33, 34). The combined evidence is beginning to make the “outside-in” theory plausible.
Although it is widely accepted that angiogenesis and inflammation play significant roles in the development of atherosclerosis, the data are not definitive with respect to how or when each of these processes is involved in initiation and progression of the disease. Rapidly evolving advances in imaging technology are enabling better methods to address these important topics. Magnetic resonance imaging (MRI), micro positron emission tomography (mPET), micro computed tomography (mCT), ultrasound and intravital microscopic imaging (IVM) are among the tools used to study the vasa vasorum and inflammation in the context of atherosclerosis. This review will discuss the unique advantages each modality provides in the context of angiogenesis and inflammation in atherosclerosis with greater emphasis on microCT, microPET and MRI.
Imaging angiogenesis and inflammation in atherosclerosis
Micro computed tomography (mCT)
mCT is particularly useful for structural imaging, because it can differentiate contrast-enhanced tissues or structures from non-enhanced soft tissues with high attenuation. Its high spatial resolution makes it an important imaging modality for vascular structural applications (35). High resolution three-dimensional (3D) representation of vascular structures directly reflects the level of vessel expansion or regression/collapse (9) and provides quantitative assessment (36), vessel volume, vascular surface area (37), vessel density (36), and branching patterns (38, 39). These capabilities are exemplified by Kwon et al. (15), who were able to determine that adventitial vasa vasorum consist of a 1st and 2nd order; the 2nd order are associated with neovascularisation related to atherosclerosis. He was also able to demonstrate with mCT imaging that adventitial vasa vasorum form a plexus in hypercholesterolaemic pigs that is not present in non-diseased pigs (36). mCT imaging performed by Gossl et al. showed that vasa vasorum in non-diseased pigs are endarteries (38). Together these studies provide insight into potential differences in the vasa vasorum branching pattern in the disease setting. However, limitations of contrast agents, resolution of imaging hardware and tools for visualisation and quantification restrict further exploration.
mCT is an excellent tool for acquiring and quantifying high resolution (1–30 μm) images of ex vivo vascular structure. The drawback is that animals must be sacrificied to perform the imaging; therefore, the same animal cannot be imaged over time. mCT can not be used to obtain in vivo vessel measurements because of the lower resolution (50 μm) of in vivo scanners. Additionally, it gives a significant dose of radiation to the animal that may interfere with the study.
mCT technology has advanced recently to even higher resolution imaging using nanotomography. The scanner has the capability of imaging internal structures of small objects down to 400 nanometer spatial resolution with a scan time of 15–90 minutes (min), but it too is limited to ex vivo use due to high levels of radiation.
Despite the limitations, nanotomography opens a new avenue for exploration of the structure and development of vasa vasorum along with plaque development. It has the potential to provide much needed insight into how the two processes proceed in relationship to each other. This potential was demonstrated by Kampschulte et al. who examined ex vivo the spatial distribution of vasa vasorum from the ascending to the descending aorta and compared it with progression of atherosclerotic plaques in ApoE−/− LDL−/− mice at 25 and 80 weeks of age (40). The entire heart and thoracic aorta were scanned at 14 μm voxel resolution. The aorta was removed, cut into 10 mm segments and scanned at <400 nm. Serial sections of the aorta were analysed for plaque content. The study showed that vasa vasorum density decreased along the ascending aorta and aortic arch with age, but increased along the descending aorta. The plaque area increased with age in all examined aortic regions.
Micro positron emission tomography (mPET)
mPET provides 3D image volumes representing the temporal and spatial distribution of a contrast agent (radioisotope) localised at a target. It can be used for visualisation and quantitative assessment of angiogenesis (41–43) and inflammation (44, 45) in vivo. The high sensitivity of mPET imaging enables very low concentrations of a radioisotope to delineate, with a high signal-to-noise ratio, a plaque based on its physiological characteristics. The isotope can be combined with targeting agents, thus making it a useful modality for antibody targeted imaging.
PET imaging is widely used to image metabolic activity in cells. Inflammatory cells utilise glucose for their metabolic activity, in the case of macrophages that would include phagocytosis and cell motility (46, 47). Fluorodeoxyglucose (FDG), a glucose analog, competes with glucose for uptake into metabolically active cells, becomes phosphorylated upon entry into cells, but can not be metabolised. FDG is labelled with F-18 isotope and PET measures its accumulation in tissues (48). Caution must be used when interpreting data since FDG can be taken up by the arterial wall in the absence of vascular inflammatory disease.
18F-FDG PET imaging is used to detect neoangiogenesis in atherosclerotic plaques. It has not been as successful as other imaging modalities mainly because of its low spatial resolution and the need for co-registration with other structural modalities (MRI or CT). In an attempt to use alternative imaging techniques to assess plaque inflammantion, Calcagno et al. (44) utilised PET imaging to measure 18F-FDG accumulation into tissue. Since macrophage infiltration is associated with neoangiogenesis in plaque, they considered 18F-FDG uptake as a measurement of risk associated with plaque progression. They compared the PET imaging with dynamic contrast-enhanced (DCE) MRI of vessels and validated the results with histological counts of vessels in New Zealand White rabbits and a black blood turbo spin echo sequence (Fig. 2). CT in the absence of contrast agent was used for anatomic co-registration. Analysis of the DCE MRI images was based on calculations of the area under the signal intensity versus time curve (AUC) at 2 min and 7 min after injection of the contrast agent. There was a significant AUC correlation between the DCE MRI image analysis and intima vessel counts. However, there was a positive, but not significant correlation between 18F-FDG accumulation and vessel count. These studies indicate that using more than one imaging modality provides a means of correlating neoangiogenesis and macrophage content with the severity of atherosclerotic disease progression.
Figure 2. Correlation of multi-modal imaging of neoangiogenesis in atherosclerotic plaque.

Three imaging techniques were used to correlate and validate data. a) DCE-MRI map of area under the curve following gadolinium injection; b) CD31 probed cross-sections of abdominal aorta; c) FDG PET image of 18F-FDG uptake in abdominal aorta of a hypercholesterolaemic rabbit. Printed with permission from Wollters Kluwer Health: Calcagno C, et al. Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced MRI and 18F-FDG PET. Arterioscler Thromb Vasc Biol 2008; 28: 1311–1317. (44).
Others have used 18F-FDG PET imaging to examine the effects of anti-inflammatory simvastatin (45). Forty-three subjects, who had undergone 18F-FDG PET imaging in a voluntary screening for cancer, showed an uptake of 18F-FDG in the thoracic aorta or carotid arteries. The same subjects consented to a simvastatin clinical trial where all received diet management for three months and 21 also received statin treatment for the same period of time. Baseline images were acquired by co-registered CT and 18F-FDG PET imaging to identify areas of inflammation in plaque. Following the treatment regimen, a second image analysis showed significant reduction in the standard uptake value (SUV) of 18F-FDG in the simvastatin treatment group, but not in those who only received dietary management. Furthermore, the simvastatin treatment group had a significant reduction in LDL cholesterol and a significant increase in high-density lipoprotein (HDL) cholesterol that correlated with the SUV. These alterations in cholesterol were not detected in the group receiving dietary control only (Fig. 3). This study demonstrates another approach to testing therapeutic effectiveness of drugs for atherosclerosis treatment. Combining the effects of nanotechnology used in mCT with CT/18F-FDG PET provides a means of validation.
Figure 3. Imaging the effects of simvastatin on plaque inflammation.
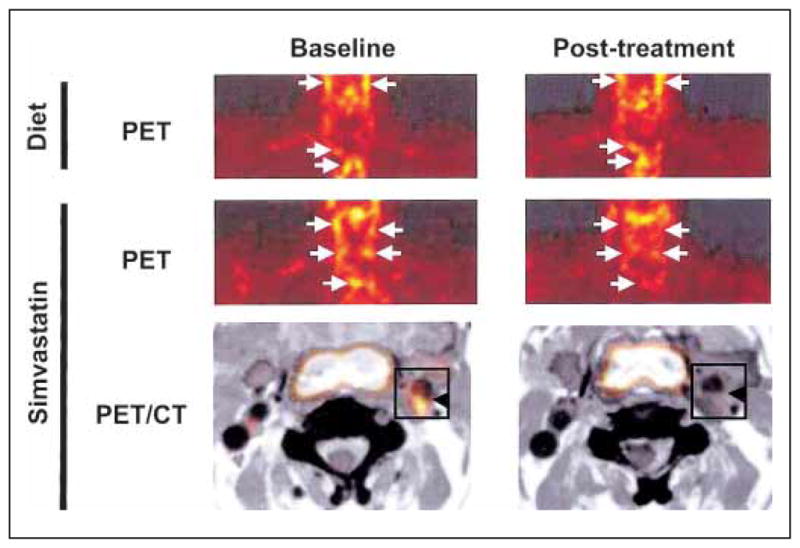
PET images pre- and post- 18FDG uptake in human subjects with potential risk of atherosclerosis, who received diet management or diet management + simvastatin treatment. Arrows indicate 18FDG uptake into plaque. Printed with permission from Elsevier Inc.: Tahara N, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 2006; 48: 1825–1831 (45).
Nahrendorf et. al. developed a novel magnetic nanoparticle that takes advantage of macrophage phagocytic properties (49). An iron oxide nanoparticle was coated with dextran, crosslinked and labelled with a near-infrared fluorochrome and incubated with a metal chelator for labelling with Cu-64. This design allows the nanoparticle to be used for MRI, fluorescence and PET imaging. Coating promotes internalisation and trapping of the nanoparticle into phagocytic cells. The nanoparticles were injected into ApoE−/− or wild-type mice and imaged in all three modalities. Nanoparticles were found primarily in macrophages with accumulation to a significantly higher level in the aorta and carotid arteries of ApoE−/− mice compared to the wild-type control. This nanoparticle has potential for delivery and/or targeting of specific molecules to inflammatory regions of atherosclerotic plaques.
Contrast-enhanced CT
Contrast-enhanced CT uses iodine-based contrast agents to alter the attenuation properties of their corresponding targets, thus enhancing their visibility in the reconstructed volume. Contrast-enhanced CT has pre-clinical and clinical application, which provides it with a distinct advantage. It can potentially be used as a means of analyzing therapeutic effects (see [50] for review). It has provided accurate detection of arterial stenosis, direct measurement of atherosclerotic plaque size, characterisation of plaque density and calcification (51–56).
Recent development of an iodinated nanoparticulate contrast agent, N1177, has enabled CT imaging of macrophages in hypercholesterolaemic rabbits. N1177 was compared to a conventional clinical contrast agent; intravenous injection of N1177 increased the lumen density and enhanced the macrophage uptake of the nanoparticles compared to the standard contrast agent, thus providing a clear delineation of the vessels relative to the macrophage-laden plaque (57). In a later study, the same group compared CT detected intensity of enhancement following N1177 injection with 18F-FDG PET detection of macrophage density in atherosclerotic rabbits (58). Using the same rabbit for measurements with both imaging modalities, they detected strong CT enhancement 2 hours (h) after intravenous administration of N1177 that correlated with PET measurements 3 h after injection of 18F-FDG (r=0.61, p<0.001); this was validated by immunohistochemistry analysis of plaque macrophages in vessel cross sections. Others have expanded the utilisation of the N1177-enhanced CT detection of macrophages in an attempt to identify ruptured plaque in hypercholesterolaemic rabbits (59).
Magnetic resonance imaging (MRI)
DCE MRI has been used as a non-invasive approach to examine atherosclerotic plaque neo-vascularisation (44, 60). In this approach, gadolinium (Gd) -based contrast agents are used to acquire serial, high temporal resolution MR images both pre- and post-Gd delivery (61). Contrast agent concentration is determined by change in signal intensity over time of the scan. Permeability of vessels is based on the kinetics of tissue uptake of the contrast agent from the vasculature (62, 63), which is dependent upon vessel characteristics. In the case of angiogenic vessels, concentration curves of contrast agent retention in the vessel versus extravasation into the surrounding tissue are calculated, which is proportional to the permeable surface area (61). Importantly, the contrast agent can pass through the permeable vessel wall, but not through cell membranes, thus providing reliable measurements.
Methods for detection of more advanced, neovascularised plaques are improved by using multi-contrast MRI. This technique is based on acquisition of pre-contrast images followed by injection of a contrast agent and post-contrast imaging. Intravascular MR contrast agents circulate in the blood without diffusing into the extracellular matrix. This property makes them suitable for vessel imaging in general. Imaging of angiogenic vessels, however, is quite different from imaging of large, well formed vasculature, because they are immature, leaky and tortuous. That combined with the extremely small size of the imaging target, and the relatively low resolution of the imaging hardware, presents a significant challenge for most MR intravascular agents with respect to imaging vessel structure.
Improved MRI capabilities incorporate paramagnetic Gd-chelates or supermagnetic iron particle derivatives coupled antibodies or peptides specific for a target antigen (64). Gadofluorine is a Gd-chelate containing hydrophobic fluorinated side chains that cause the contrast agent to form nanometer sized lipid micelles when exposed to water. These particles accumulate in lipid rich areas of plaque in hypercholesterolaemic rabbits (65). Further amplification of signal enhancement utilizes nanoparticles, which provide a large surface area for incorporation of Gd-chelates or target-specific ligands (66). The distinct advantage of gadolinium-based contrast agents is their application to both pre-clinical and clinical studies. Other MRI agents, such as superparamagnetic iron oxide (SPIO) nanoparticles have been used in clinical trials (67).
DCE MRI was used by Kerwin et al. (68) to measure inflammation in carotid atherosclerotic plaque in a prospective in vivo study of 30 patients scheduled to undergo endarterectomy. A standard two-tissue compartment model was applied to estimate the uptake of a Gd-based contrast agent in intra- and extra-vascular spaces. The obtained transfer rates were correlated with results from histology. Strong correlation was found between the extra-vascular transfer rate of Gd and macrophage and neovasculature content.
The same technique was used to visualise vasa vasorum (69). Adventitia perfusion properties were probed by DCE MRI to generate a parametric image of the vasa vasorum. The image shows the plasma volume and the transfer constant, which is considered to be the quantitative value assigned to the vasa vasorum. The study was done with 20 subjects who had 50–79% carotid stenosis and were asymptomatic for carotid artery disease. An additional 25 subjects, who had 80–90% stenosis or 50–79% stenosis accompanied by ischaemic symptoms, participated in the study. This group had a carotid endarterectomy following the MRI. A 1.5T MR scanner was used to acquire 2D slices at predefined locations with a T1 weighted fast spin echo sequence with double inversion recovery. Lumen and vessel wall boundaries were identified by the user with the help of an active B-Spline contour. Cluster centres derived from a blood signal curve were used to initialise the two-tissue kinetic compartment model. A strong correlation between contrast transfer rate, neovasculature and amount of macrophages in excised plaque was established.
Nanotechnology combined with MRI was used by Nahrendorf et al. (49) to examine biological processes. They identified a peptide expressed on an M13 bacteriophage that binds to vascular cell adhesion molecule-1 (VCAM-1), an immuglobulin superfamily glycoprotein that is associated with inflammation in atherosclerosis (70). VCAM is not generally expressed in physiologic conditions, but is stimulated by cytokines in diseased endothelium. The peptide was attached to a magnetofluorescent nanoparticle that is internalised primarily by ECs and secondarily by SMC and macrophages. In a mouse model of atherosclerosis, the peptide-linked nanoparticle accumulated at a high level in the aortic root accompanied by a change in MRI signal. This was validated in fluorescent reflectance and fluorescent microscopy images of the dissected aortas. Next, they used the peptide in hypercholesterolaemic mice to detect the effect of statin treatment on the atherosclerotic disease process. The statin-treated mice showed very little MRI enhancement of the peptide-linked nanoparticle compared to the control mice; thus demonstrating that this technique can be used as a method to validate biological effects of a drug.
Ultrasound Imaging
Spectral and power Doppler ultrasounds are used to measure blood flow velocities. Ultrasound contrast agents have been developed to take advantage of this technique to visualise angiogenesis (71). The contrast agents are gas-filled microbubbles that are administered intravenously to the systemic circulation. Their size of 1–5 μm allows them to circulate in the capillary network. This modality has the advantage of being non-invasive thus allowing repeated measurements over time.
Albumin and lipid-shelled microbubbles have been used to detect activated leukocytes that are adhering to the endothelium in a non-specific manner (72, 73). Advances in the technology enable targeted contrast-enhanced ultrasound to improve the signal of microbubbles. This approach conjugates microbubbles to ligands that bind to targets expressed on the vascular endothelium, thus enabling the microbubble complex to accumulate selectively in areas of interest. The binding efficiency is dependent upon the number of ligands per surface area of a microbubble and the circulatory shear stress. Unfortunately the attachment of the micro-bubbles to the endothelium is reduced with increasing sheer stress, thus eliminating its application to many cardiovascular diseases. Additional problems involve the non-specific interaction of microbubbles with activated leukocytes and endothelium versus the specific target, which complicates interpretation and quantification of the data (72, 73). This imaging modality would have very limited application to the vasa vasorum due to its low level of resolution that would be better achieved with mCT.
Intravital microscopy (IVM)
IVM is a method for studying “real-time” angiogenesis using a chamber to continuously monitor angiogenesis in vivo with high temporal and spatial resolution (74–75). The technology enables the use of fluorescent nanoparticles to enhance visualisation of the angiogenic process. Much of the technology was tested in tumour models (76), but has now been integrated into atherosclerosis studies (77–81).
One study used IVM to examine the anti-inflammatory role of peroxiredoxins (Prdx1) in ApoE−/−/Prdx1−/− mice (78). Peroxiredoxin 1 is an antioxidant that scavenges H2O2 to result in suppression of the κB pathway and the associated inflammatory response (82). Antibodies to either P-selectin or VCAM-1 were coupled to microspheres. Intravital microscopy was used to visualise the interaction of the antibodies with their respective targets on the endothelium of mesenteric vessels (Fig. 4) (78). The data very clearly demonstrate a highly significant increase in rolling leukocytes per minute in the Prdx1−/− mice compared to the wild type. Others have used IVM to visualise similar interactions of inflammatory mediators with the endothelium in animal models of atherosclerosis (77, 78, 80, 81, 83, 84). The nanotechnology combined with IVM is a very powerful tool for examining the relationships between inflammatory mediators and the vessel wall. However, it is doubtful that IVM will be useful for studying the vasa vasorum since it’s projection imaging will lack the detail to detect differences in structure that can be achieved by mCT.
Figure 4. Intravital microscopy imaging (IVM) of leukocyte rolling mechanisms.

P-selectin dependent leukocyte rolling in mesenteric veins of Prdx1−/− mice was detected by IVM. Mice were infused with either (a) a P-selectin aptamer inhibitor vs. a scrambled aptamer or (b) an anti-P-selectin Ig vs. control Ig. Endogenous leukocytes are indicated by arrows. Printed with permission from Lippincott Williams & Wilkins: Kisucka J, et al. Peroxiredoxin1 prevents excessive endothelial activation and early atherosclerosis. Circ Res 2008; 103: 598–605 (79).
Multi-photon laser scanning microscopy (MPLSM) utilises the IVM system to achieve 3D images of live tissue. MPLSM technology is able to achieve tissue penetration in a 400–1,000 μm range due to its use of lasers that can excite fluorophores in the near infrared range. Deeper tissue penetration and reduced photobleaching provide MPLSM with advantages over confocal laser scanning microscopy (85, 86).
Conclusion
Imaging angiogenesis and inflammation in atherosclerosis requires a multi-modal imaging approach capable of imaging both structure and function of vessels. Ideally, the multi-modal choice would be capable of spanning pre-clinical to clinical studies. Despite the significant progress in imaging technology, there is still a need for better contrast agents and delivery vehicles for more detailed exploration of small angiogenic vessels. Imaging hardware with much higher spatial resolution is necessary to address questions related to vessel structure and formation. Availability of these tools will provide the capability of imaging specific processes involved in initiation and progression of angiogenesis and inflammation in atherosclerosis.
Acknowledgments
Financial support:
Supported in part by National Institutes of Health, National Heart, Lung, and Blood Institute Grant HL-69948 (M. J. Mulligan-Kehoe).
References
- 1.Sadeghi MM. The pathobiology of the vessel wall: implications for imaging. J Nucl Cardiol. 2006;13:402–414. doi: 10.1016/j.nuclcard.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109:II27–33. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 4.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 5.Naldini A, Carraro F. Role of inflammatory mediators in angiogenesis. Curr Drug Targets Inflamm Allergy. 2005;4:3–8. doi: 10.2174/1568010053622830. [DOI] [PubMed] [Google Scholar]
- 6.Ward JR, Wilson HL, Francis SE, Crossman DC, Sabroe I. Translational mini-review series on immunology of vascular disease: inflammation, infections and Toll-like receptors in cardiovascular disease. Clin Exp Immunol. 2009;156:386–394. doi: 10.1111/j.1365-2249.2009.03886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien ER, Garvin MR, Dev R, et al. Angiogenesis in human coronary atherosclerotic plaques. Am J Pathol. 1994;145:883–894. [PMC free article] [PubMed] [Google Scholar]
- 8.Virmani R, Kolodgie FD, Burke AP, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 9.Drinane M, Mollmark J, Zagorchev L, et al. The antiangiogenic activity of rPAI-1(23) inhibits vasa vasorum and growth of atherosclerotic plaque. Circ Res. 2009;104:337–345. doi: 10.1161/CIRCRESAHA.108.184622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation. 1999;99:1726–1732. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- 11.Moulton KS, Vakili K, Zurakowski D, et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci USA. 2003;100:4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heistad DD, Marcus ML. Role of vasa vasorum in nourishment of the aorta. Blood Vessels. 1979;16:225–238. doi: 10.1159/000158209. [DOI] [PubMed] [Google Scholar]
- 13.Fleiner M, Kummer M, Mirlacher M, et al. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation. 2004;110:2843–2850. doi: 10.1161/01.CIR.0000146787.16297.E8. [DOI] [PubMed] [Google Scholar]
- 14.Herrmann J, Lerman LO, Rodriguez-Porcel M, et al. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc Res. 2001;51:762–766. doi: 10.1016/s0008-6363(01)00347-9. [DOI] [PubMed] [Google Scholar]
- 15.Kwon HM, Sangiorgi G, Ritman EL, et al. Enhanced coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Clin Invest. 1998;101:1551–1556. doi: 10.1172/JCI1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khurana R, Zhuang Z, Bhardwaj S, et al. Angiogenesis-dependent and independent phases of intimal hyperplasia. Circulation. 2004;110:2436–2443. doi: 10.1161/01.CIR.0000145138.25577.F1. [DOI] [PubMed] [Google Scholar]
- 17.Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 18.Kolodgie FD, Virmani R, Burke AP, et al. Pathologic assessment of the vulnerable human coronary plaque. Heart. 2004;90:1385–1391. doi: 10.1136/hrt.2004.041798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 20.de Boer OJ, van der Wal AC, Teeling P, Becker AE. Leucocyte recruitment in rupture prone regions of lipid-rich plaques: a prominent role for neovascularization? Cardiovasc Res. 1999;41:443–449. doi: 10.1016/s0008-6363(98)00255-7. [DOI] [PubMed] [Google Scholar]
- 21.Kaartinen M, Penttila A, Kovanen PT. Accumulation of activated mast cells in the shoulder region of human coronary atheroma, the predilection site of atheromatous rupture. Circulation. 1994;90:1669–1678. doi: 10.1161/01.cir.90.4.1669. [DOI] [PubMed] [Google Scholar]
- 22.Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11:109–119. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res. 2007;75:640–648. doi: 10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook-Mills JM, Deem TL. Active participation of endothelial cells in inflammation. J Leukoc Biol. 2005;77:487–495. doi: 10.1189/jlb.0904554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res. 2009;105:223–230. doi: 10.1161/CIRCRESAHA.109.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janabi M, Yamashita S, Hirano K, et al. Oxidized LDL-induced NF-kappa B activation and subsequent expression of proinflammatory genes are defective in monocyte-derived macrophages from CD36-deficient patients. Arterioscler Thromb Vasc Biol. 2000;20:1953–1960. doi: 10.1161/01.atv.20.8.1953. [DOI] [PubMed] [Google Scholar]
- 27.Virella G, Lopes-Virella MF. Atherogenesis and the humoral immune response to modified lipoproteins. Atherosclerosis. 2008;200:239–246. doi: 10.1016/j.atherosclerosis.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Y, O’Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94:1655–1664. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- 30.Shi Y, Pieniek M, Fard A, O’Brien J, Mannion JD, Zalewski A. Adventitial remodeling after coronary arterial injury. Circulation. 1996;93:340–348. doi: 10.1161/01.cir.93.2.340. [DOI] [PubMed] [Google Scholar]
- 31.Wilcox JN, Waksman R, King SB, Scott NA. The role of the adventitia in the arterial response to angioplasty: the effect of intravascular radiation. Int J Radiat Oncol Biol Phys. 1996;36:789–796. doi: 10.1016/s0360-3016(96)00299-4. [DOI] [PubMed] [Google Scholar]
- 32.Clowes AW, Schwartz SM. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res. 1985;56:139–145. doi: 10.1161/01.res.56.1.139. [DOI] [PubMed] [Google Scholar]
- 33.Houtkamp MA, de Boer OJ, van der Loos CM, van der Wal AC, Becker AE. Adventitial infiltrates associated with advanced atherosclerotic plaques: structural organization suggests generation of local humoral immune responses. J Pathol. 2001;193:263–269. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH774>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 34.Moos MP, John N, Grabner R, et al. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:2386–2391. doi: 10.1161/01.ATV.0000187470.31662.fe. [DOI] [PubMed] [Google Scholar]
- 35.Marxen M, Thornton MM, Chiarot CB, et al. MicroCT scanner performance and considerations for vascular specimen imaging. Med Phys. 2004;31:305–313. doi: 10.1118/1.1637971. [DOI] [PubMed] [Google Scholar]
- 36.Kwon HM, Sangiorgi G, Ritman EL, et al. Adventitial vasa vasorum in balloon-injured coronary arteries: visualization and quantitation by a microscopic three-dimensional computed tomography technique. J Am Coll Cardiol. 1998;32:2072–2079. doi: 10.1016/s0735-1097(98)00482-3. [DOI] [PubMed] [Google Scholar]
- 37.Kennel SJ, Davis IA, Branning J, Pan H, Kabalka GW, Paulus MJ. High resolution computed tomography and MRI for monitoring lung tumor growth in mice undergoing radioimmunotherapy: correlation with histology. Med Phys. 2000;27:1101–1107. doi: 10.1118/1.598974. [DOI] [PubMed] [Google Scholar]
- 38.Gossl M, Malyar NM, Rosol M, Beighley PE, Ritman EL. Impact of coronary vasa vasorum functional structure on coronary vessel wall perfusion distribution. Am J Physiol Heart Circ Physiol. 2003;285:H2019–2026. doi: 10.1152/ajpheart.00399.2003. [DOI] [PubMed] [Google Scholar]
- 39.Gossl M, Rosol M, Malyar NM, et al. Functional anatomy and hemodynamic characteristics of vasa vasorum in the walls of porcine coronary arteries. Anat Rec A Discov Mol Cell Evol Biol. 2003;272:526–537. doi: 10.1002/ar.a.10060. [DOI] [PubMed] [Google Scholar]
- 40.Kampschulte M, Brinkmann A, Stieger P, et al. Quantitative CT imaging of the spatio-temporal distribution patterns of vasa vasorum in aortas of apoE(−/−)/LDL(−/−) double knockout mice. Atherosclerosis. 2010;212:444–450. doi: 10.1016/j.atherosclerosis.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 41.McQuade P, Knight LC, Welch MJ. Evaluation of 64Cu- and 125I-radiolabeled bitistatin as potential agents for targeting alpha v beta 3 integrins in tumor angiogenesis. Bioconjug Chem. 2004;15:988–996. doi: 10.1021/bc049961j. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Cai W, Chen K, et al. A new PET tracer specific for vascular endothelial growth factor receptor 2. Eur J Nucl Med Mol Imaging. 2007;34:2001–2010. doi: 10.1007/s00259-007-0524-0. [DOI] [PubMed] [Google Scholar]
- 43.Wu Y, Zhang X, Xiong Z, et al. microPET imaging of glioma integrin {alpha}v{beta}3 expression using (64)Cu-labeled tetrameric RGD peptide. J Nucl Med. 2005;46:1707–1718. [PubMed] [Google Scholar]
- 44.Calcagno C, Cornily JC, Hyafil F, et al. Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced MRI and 18F-FDG PET. Arterioscler Thromb Vasc Biol. 2008;28:1311–1317. doi: 10.1161/ATVBAHA.108.166173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tahara N, Kai H, Ishibashi M, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48:1825–1831. doi: 10.1016/j.jacc.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 46.Evans WH, Karnovsky ML. The biochemical basis of phagocytosis. IV. Some aspects of carbohydrate metabolism during phagocytosis. Biochemistry. 1962;1:159–166. doi: 10.1021/bi00907a024. [DOI] [PubMed] [Google Scholar]
- 47.Weisdorf DJ, Craddock PR, Jacob HS. Granulocytes utilize different energy sources for movement and phagocytosis. Inflammation. 1982;6:245–256. doi: 10.1007/BF00916406. [DOI] [PubMed] [Google Scholar]
- 48.Shepherd PR, Kahn BB. Glucose transporters and insulin action--implications for insulin resistance and diabetes mellitus. N Engl J Med. 1999;341:248–257. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- 49.Nahrendorf M, Zhang H, Hembrador S, et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sadeghi MM, Glover DK, Lanza GM, Fayad ZA, Johnson LL. Imaging atherosclerosis and vulnerable plaque. J Nucl Med. 51(Suppl 1):51S–65S. doi: 10.2967/jnumed.109.068163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Achenbach S, Moselewski F, Ropers D, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004;109:14–17. doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 52.Hoffmann U, Moselewski F, Cury RC, et al. Predictive value of 16-slice multidetector spiral computed tomography to detect significant obstructive coronary artery disease in patients at high risk for coronary artery disease: patient-versus segment-based analysis. Circulation. 2004;110:2638–2643. doi: 10.1161/01.CIR.0000145614.07427.9F. [DOI] [PubMed] [Google Scholar]
- 53.Leber AW, Becker A, Knez A, et al. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J Am Coll Cardiol. 2006;47:672–677. doi: 10.1016/j.jacc.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 54.Leber AW, Knez A, Becker A, et al. Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques: a comparative study with intracoronary ultrasound. J Am Coll Cardiol. 2004;43:1241–1247. doi: 10.1016/j.jacc.2003.10.059. [DOI] [PubMed] [Google Scholar]
- 55.Leber AW, Knez A, von Ziegler F, et al. Quantification of obstructive and non-obstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46:147–154. doi: 10.1016/j.jacc.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 56.Viles-Gonzalez JF, Poon M, Sanz J, et al. In vivo 16-slice, multidetector-row computed tomography for the assessment of experimental atherosclerosis: comparison with magnetic resonance imaging and histopathology. Circulation. 2004;110:1467–1472. doi: 10.1161/01.CIR.0000141732.28175.2A. [DOI] [PubMed] [Google Scholar]
- 57.Hyafil F, Cornily JC, Feig JE, et al. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med. 2007;13:636–641. doi: 10.1038/nm1571. [DOI] [PubMed] [Google Scholar]
- 58.Hyafil F, Cornily JC, Rudd JH, Machac J, Feldman LJ, Fayad ZA. Quantification of inflammation within rabbit atherosclerotic plaques using the macrophage-specific CT contrast agent N1177: a comparison with 18F-FDG PET/CT and histology. J Nucl Med. 2009;50:959–965. doi: 10.2967/jnumed.108.060749. [DOI] [PubMed] [Google Scholar]
- 59.Van Herck JL, De Meyer GR, Martinet W, et al. Multi-slice computed tomography with N1177 identifies ruptured atherosclerotic plaques in rabbits. Basic Res Cardiol. 2010;105:51–59. doi: 10.1007/s00395-009-0052-0. [DOI] [PubMed] [Google Scholar]
- 60.Kerwin W, Hooker A, Spilker M, et al. Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation. 2003;107:851–856. doi: 10.1161/01.cir.0000048145.52309.31. [DOI] [PubMed] [Google Scholar]
- 61.Yankeelov TE, Gore JC. Dynamic Contrast Enhanced Magnetic Resonance Imaging in Oncology: Theory, Data Acquisition, Analysis, and Examples. Curr Med Imaging Rev. 2009;3:91–107. doi: 10.2174/157340507780619179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 63.Tofts PS, Kermode AG. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med. 1991;17:357–367. doi: 10.1002/mrm.1910170208. [DOI] [PubMed] [Google Scholar]
- 64.Winter PM, Caruthers SD, Lanza GM, Wickline SA. Quantitative cardiovascular magnetic resonance for molecular imaging. J Cardiovasc Magn Reson. 2010;12:62. doi: 10.1186/1532-429X-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sirol M, Itskovich VV, Mani V, et al. Lipid-rich atherosclerotic plaques detected by gadofluorine-enhanced in vivo magnetic resonance imaging. Circulation. 2004;109:2890–2896. doi: 10.1161/01.CIR.0000129310.17277.E7. [DOI] [PubMed] [Google Scholar]
- 66.Caruthers SD, Cyrus T, Winter PM, Wickline SA, Lanza GM. Anti-angiogenic per-fluorocarbon nanoparticles for diagnosis and treatment of atherosclerosis. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:311–323. doi: 10.1002/wnan.9. [DOI] [PubMed] [Google Scholar]
- 67.Kooi ME, Cappendijk VC, Cleutjens KB, et al. Accumulation of ultrasmall super-paramagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–2458. doi: 10.1161/01.CIR.0000068315.98705.CC. [DOI] [PubMed] [Google Scholar]
- 68.Kerwin WS, O’Brien KD, Ferguson MS, Polissar N, Hatsukami TS, Yuan C. Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology. 2006;241:459–468. doi: 10.1148/radiol.2412051336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kerwin WS, Oikawa M, Yuan C, Jarvik GP, Hatsukami TS. MR imaging of adventitial vasa vasorum in carotid atherosclerosis. Magn Reson Med. 2008;59:507–514. doi: 10.1002/mrm.21532. [DOI] [PubMed] [Google Scholar]
- 70.Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med. 2008;18:228–232. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lindner JR. Molecular imaging of myocardial and vascular disorders with ultrasound. JACC Cardiovasc Imaging. 2010;3:204–211. doi: 10.1016/j.jcmg.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 72.Lindner JR. Contrast ultrasound molecular imaging of inflammation in cardiovascular disease. Cardiovasc Res. 2009;84:182–189. doi: 10.1093/cvr/cvp302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lindner JR. Contrast ultrasound molecular imaging: harnessing the power of bubbles. Cardiovasc Res. 2009;83:615–616. doi: 10.1093/cvr/cvp243. [DOI] [PubMed] [Google Scholar]
- 74.Hak S, Reitan NK, Haraldseth O, de Lange Davies C. Intravital microscopy in window chambers: a unique tool to study tumor angiogenesis and delivery of nanoparticles. Angiogenesis. 2010;13:113–130. doi: 10.1007/s10456-010-9176-y. [DOI] [PubMed] [Google Scholar]
- 75.Koehl GE, Gaumann A, Geissler EK. Intravital microscopy of tumor angiogenesis and regression in the dorsal skin fold chamber: mechanistic insights and preclinical testing of therapeutic strategies. Clin Exp Metastasis. 2009;26:329–344. doi: 10.1007/s10585-008-9234-7. [DOI] [PubMed] [Google Scholar]
- 76.Fukumura D, Jain RK. Imaging angiogenesis and the microenvironment. APMIS. 2008;116:695–715. doi: 10.1111/j.1600-0463.2008.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang K, Francis SA, Aikawa E, et al. Pioglitazone Suppresses Inflammation In Vivo in Murine Carotid Atherosclerosis. Novel Detection by Dual-Target Fluorescence Molecular Imaging Arterioscler Thromb Vasc Biol. 2010;30:1933–1939. doi: 10.1161/ATVBAHA.110.206342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kato S, Amano H, Ito Y, et al. Effect of erythropoietin on angiogenesis with the increased adhesion of platelets to the microvessels in the hind-limb ischemia model in mice. J Pharmacol Sci. 2010;112:167–175. doi: 10.1254/jphs.09262fp. [DOI] [PubMed] [Google Scholar]
- 79.Kisucka J, Chauhan AK, Patten IS, et al. Peroxiredoxin1 prevents excessive endothelial activation and early atherosclerosis. Circ Res. 2008;103:598–605. doi: 10.1161/CIRCRESAHA.108.174870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McClelland S, Gawaz M, Kennerknecht E, et al. Contribution of cyclooxygenase-1 to thromboxane formation, platelet-vessel wall interactions and atherosclerosis in the ApoE null mouse. Atherosclerosis. 2009;202:84–91. doi: 10.1016/j.atherosclerosis.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 81.Rotzius P, Soehnlein O, Kenne E, et al. ApoE(−/−)/lysozyme M(EGFP/EGFP) mice as a versatile model to study monocyte and neutrophil trafficking in atherosclerosis. Atherosclerosis. 2009;202:111–118. doi: 10.1016/j.atherosclerosis.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 82.Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 83.Missiou A, Rudolf P, Stachon P, et al. TRAF5 Deficiency Accelerates Atherogenesis in Mice by Increasing Inflammatory Cell Recruitment and Foam Cell Formation. Circ Res. 2010;107:757–766. doi: 10.1161/CIRCRESAHA.110.219295. [DOI] [PubMed] [Google Scholar]
- 84.Ohman MK, Wright AP, Wickenheiser KJ, Luo W, Russo HM, Eitzman DT. Monocyte chemoattractant protein-1 deficiency protects against visceral fat-induced atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:1151–1158. doi: 10.1161/ATVBAHA.110.205914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kiessling F, Razansky D, Alves F. Anatomical and microstructural imaging of angiogenesis. Eur J Nucl Med Mol Imaging. 2010;37 (Suppl 1):S4–19. doi: 10.1007/s00259-010-1450-0. [DOI] [PubMed] [Google Scholar]
- 86.Tozer GM, Ameer-Beg SM, Baker J, et al. Intravital imaging of tumour vascular networks using multi-photon fluorescence microscopy. Adv Drug Deliv Rev. 2005;57:135–152. doi: 10.1016/j.addr.2004.07.015. [DOI] [PubMed] [Google Scholar]


