Abstract
Fluorescence-based assays and detection techniques are among the most highly sensitive and popular biological tests for researchers. To match the needs of research and the clinic, detection limits and specificities need to improve, however. One mechanism is to decrease non-specific background signals, which is most efficiently done by increasing fluorescence quenching abilities. Reports in the literature of theoretical and experimental work have shown that metallic gold surfaces and nanoparticles are ultra-efficient fluorescence quenchers. Based on these findings, subsequent reports have described gold nanoparticle fluorescence-based activatable probes that were designed to increase fluorescence intensity based on a range of stimuli. In this way, these probes can detect and signify assorted biomarkers and changes in environmental conditions. In this review, we explore the various factors and theoretical models that affect gold nanoparticle fluorescence quenching, explore current uses of activatable probes, and propose an engineering approach for future development of fluorescence based gold nanoparticle activatable probes.
1. Introduction
Fluorescence detection is the basis for many biological assays and medical diagnostics because of its highly sensitive nature. However, fluorescence-based assays and other fluorescence detection techniques, both in vitro and in vivo, require increased sensitivity to determine smaller concentrations of the target and provide more information about biomolecules to researchers and clinicians. Although advanced optical imaging instrumentation exists, current limitations of fluorescence detection lie with the fluorescence probe, including low photostability and high background fluorescence.1 A practical way to increase fluorescence sensitivity is to either increase the emission strength (utilizing high quantum yield, increasing percentage of emission collected) or to decrease the non-specific background signals by using efficient quenchers.
Among the various techniques to improve fluorescence sensitivity, activatable probes have been designed with high specificity to the target of interest and with superior signal-to-background ratio in the imaging signal.2 Activatable probes are specifically designed to amplify imaging signals in response to a target, such as a particular protein, oligonucleotide, or product of an environmental change. Primarily, efforts to develop activatable probes have focused on optical imaging applications. Fluorescence activatable probes are made up of at least two components: the fluorophore that acts as the donor and the quencher that acts as the acceptor. In the native state, the components are in close proximity, causing the fluorescence to be quenched by various energy transfer mechanisms, as will be discussed in this review. The fluorescence is dependent on the distance between these two components. When the distance is short, quenching occurs. When the distance is increased, as in response to external stimuli, fluorescence is effectively restored. Activatable probes, also referred to as molecular beacons, are designed to increase the physical distance between the donor and acceptor in response to a specific chemical stimulus or biomolecule. In this way, the fluorescence signal is quenched when no target is detected and amplified when the specific stimulus is reached. With additional components such, as peptides, oligo-nucleotides and polymers, activation can occur due to specific binding among complementary molecules. Activatable probes have been designed with various quenchers, fluorophores, and biomolecules.
Inorganic gold nanoparticles (AuNPs) show the highest quenching efficiency (up to 99%) and therefore the highest sensitivity in the development of activatable probes. Metallic gold, both on the macro and nanoscales, is well-known for ultrahigh fluorescence quenching ability, as predicted theoretically and observed experimentally.3,4 Gold thin films and AuNPs have revealed extremely high quenching efficiencies, making them useful for numerous fluorescence-based assays and in vivo probes. Specifically AuNPs have versatility in applications due to their interesting nanoscale properties, including high surface-area-to-volume ratio, significant surface plasmon resonance, tunable dimensions and ease of functionalization. Therefore, fluorophore-modified AuNPs are effective activatable probes with high fluorescence activation and very effective fluorescence quenching.
AuNPs are one of the most studied nanomaterials in biology.5,6 In addition to exhibiting highly efficient fluorescence quenching,5 AuNPs can be synthesized with high degrees of precision for diameters between 2–300 nm. Their absorbance is dependent on their size and shape; hence they are highly tunable absorbing agents. As their diameters increase, their plasmon band red shifts. AuNP surfaces are also easily chemically conjugated with various biomolecules and targeting agents, useful in preventing non-specific interactions and false-positive detection signals. Moreover, AuNPs have high conductivity, plasmonic coupling, and biocompatibility.
In the following review, we provide an overview of AuNP-based fluorescence systems, including key energy transfer mechanisms, the main factors affecting fluorescence quenching and enhancement, and unique concepts and features specific to biomedical applications. Finally, we will present a guideline for the design of effective fluorescence-based AuNP activatable probe systems.
2. Fluorescence energy transfer mechanisms with AuNPs
Various models have been designed to gain understanding of fluorophore–gold interactions. When fluorophores are placed near metal surfaces, the resonant energy transfer (RET) rate and the radiative lifetime of the fluorophores are changed depending on various physical features of the metals and fluorophores.1,7–9 However, the mechanism of quenching by AuNPs, and not just thin films, has not been thoroughly investigated. Theory has predicted that energy transfer rates and radiative decay rates are affected by AuNPs differently than by metal thin films.
Quenching efficiency depends on the measure of the fluorescence decay rate (Rfluo), radiative decay rate (Rrad), nonradiative decay rate (Rnonrad), and the fluorescence quantum efficiency (η). The fluorescence decay rate is the inverse of the fluorescence lifetime (τ), Rfluo = 1/τ and can be expressed as the sum of the radiative and nonradiative decay rates:
| (1) |
The quantum efficiency is the portion of fluorescence that is radiative:
| (2) |
Radiative decay occurs when the dye molecule releases a photon that returns to the ground state and is detected. An increase in the radiative decay rate accounts for an enhancement in fluorescence.10 Non-radiative decay occurs when the excited photon cannot return to its ground state due to various processes, such as intersystem crossings or heat dissipation. An increase in the non-radiative decay decreases the quantum efficiency as seen in eqn (2). Radiative and nonradiative rates can depend on the size and shape of the NP, the distance between the fluorophores and NP, the orientation of the dye molecule binding onto the AuNP, and also on the overlap of the fluorophore’s emission and NP absorption.7
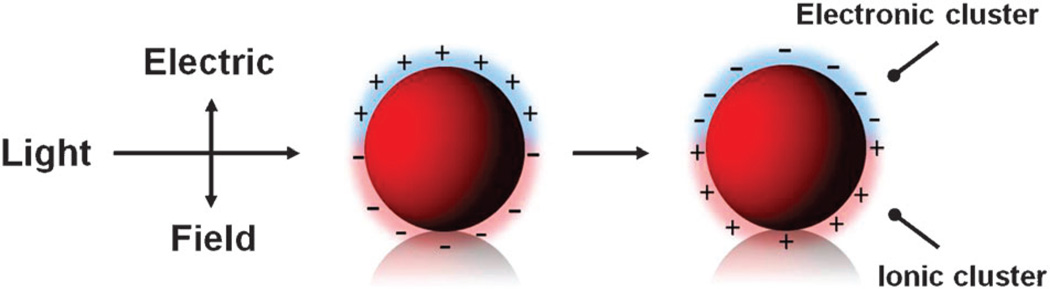
A major feature of AuNPs that has been attributed to their interesting fluorescence effects is a strong interaction with the radiation field in the visible light region. This interaction causes the excited conducting electrons to oscillate in a collective manner, called the surface plasmon resonance (SPR) as seen in Fig. 1. Surface plasmons of AuNPs occur with direct optical excitement and lead to visibly deep red colors. The dielectric dispersion equation, which explains the interaction of the metal NP with the electric field, is made up of an adsorption and a scattering term. Depending on the metal NP size, the adsorption and scattering terms can be real or imaginary components. The magnitude of the electric field at the surface and the dielectric dispersion of the material are two key features that affect the radiative or non-radiative decay.
Fig. 1.
Schematic illustrating the excitation of the dipole surface plasmon oscillation. The electric field of the light induces polarization of free electrons of the nanoparticle (NP) surface, but not of the electrons making up the NP core. Therefore, a net charge difference acts on the surface of the particles and serves as a restoring force to cause dipolar oscillations of the electrons. These oscillations are known as the surface plasmon absorption. Image adapted from Link and El-Sayed.27
Although AuNPs near fluorophores have shown extremely high quenching efficiencies,3,4,11–16 fluorescence enhancement has also been explored.7,17 The rational design of AuNP activatable probes is dependent on the factors that affect fluorescence quenching and enhancement. The determinants of fluorescence quenching or enhancement and the various models of energy transfer between gold and fluorophores will be discussed below.
2.1 AuNP size
Due to the advancement in NP synthesis techniques, tunable AuNPs with various sizes and shapes can be developed. The size of AuNPs can have different effects on the SPR band, color, and extent of functionalization. More importantly, AuNP size affects the adsorption and scattering cross section, which affects its fluorescence quenching or enhancement abilities.
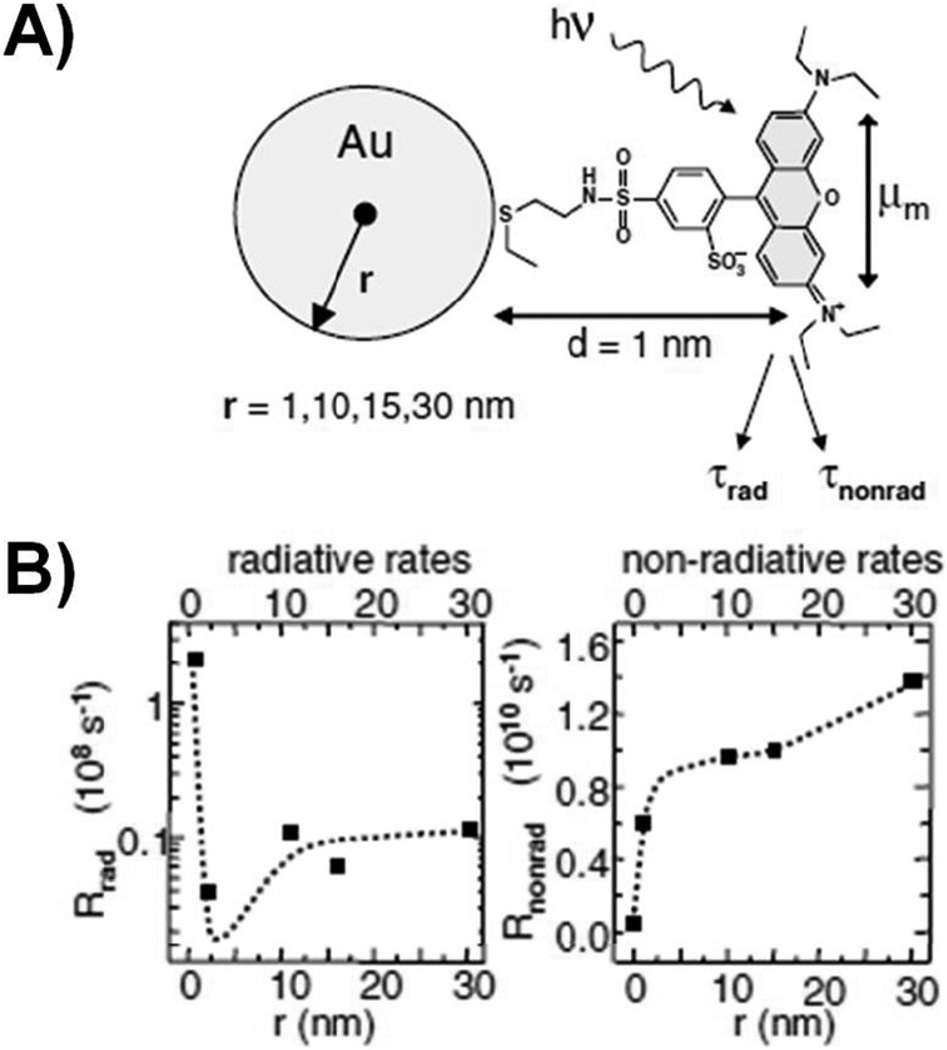
Dulkeith et al. studied fluorescence quenching of lissamine dye molecules by AuNPs with diameters ranging from 2 nm to 60 nm, as seen in Fig. 2.13 The distance between the fluorophores and the AuNP remained constant at 1 nm and the dye covered 50% of the NP surface. This type of design reduced the effect of molecular absorption changes, because the excitation wavelength did not coincide with any of the plasmon resonance peaks. The fluorescence lifetime decreased from 169 ps to 72 ps as the AuNP diameter changed from 2 nm to 60 nm. (This compared to the free dye fluorescence lifetime of 1.54 ns.13) When the dye was bound to the AuNP, the radiative rate decreased by an order of magnitude and the non-radiative rate increased by an order of magnitude (Fig. 2B). The radiative rate was the lowest for AuNPs with a diameter of 8 nm, which was attributed to destructive interference between the molecular and metal dipole of the dye chosen for this study.13 Based on the experimental data and theoretical modeling, energy transfer rates were found to be on the pico-second scale and to decrease as the particle size increased. Most importantly, the dye radiative rate was reduced by an order of magnitude when near AuNPs, even at the smallest diameter. Both effects have been predicted by the Gersten–Nitzan model.18 This study implies that fluorescence quenching by AuNPs is mainly due to the decrease in the radiative rate over the increase in the nonradiative rate.
Fig. 2.
(A) Model used by Dulkeith et al.13 to demonstrate gold nanoparticle (AuNP) fluorescence quenching by radiative and non-radiative effects. A lissamine dye molecule is attached to the AuNPs of different diameters (2, 20, 30, 60 nm). (B) The radiative and nonradiative rates as a function of particle radius. At r = 0, the measurement is for dye alone. The dye’s radiative rate lowers by more than an order of magnitude and the nonradiative rate increases by more than an order of magnitude in the presence of AuNPs. This suggests that both pathways play an important role in fluorescence quenching by AuNPs. Image adapted from Dulkeith et al.13
Lakowicz introduced the radiating plasmon (RP) model to explain how a rationally designed fluorophore and metal nanoparticle system can lead to fluorescence enhancement or quenching, mainly based on the size of the AuNP and distance between the fluorophore and AuNP.3,4,7,11,19–23 Using this model, emission or quenching of a fluorophore near metals can be predicted solely by electrodynamics, Mie theory, and Maxwell’s equations, that is without knowing chemical or electron-transfer molecular interactions. According to Mie theory, small colloids up to 40 nm in diameter are expected to quench fluorescence, because absorption is the dominant mechanism, while larger colloids above 40 nm are expected to enhance fluorescence, because scattering becomes the dominant mechanism. The RP model for NPs explains that their induced plasmon will radiate when the scattering cross section rules over the absorption cross-section. This can be seen in detail by taking into account Mie theory for NPs in the shape of spheres. The particle cross section for extinction (CE) with a dielectric constant ε1 is dependent on the cross section due to absorption (CA) and scattering (CS) by:
| (3) |
where k1 is the wavevector of the incident light in medium. Polarizability (α) of a sphere with a radius r is
| (4) |
where εm is the complex dielectric constant of the metal. The absorption term, CA, is responsible for quenching, while the scattering term, CS, can cause fluorescence enhancement. As seen by this model, the NP size plays a more significant role in CS (r6) over CA (r3). Therefore, smaller metal nanoparticles are preferred for quenching.
Before the RP model was developed, Yguerabide et al. measured the scattering efficiency of nanoparticles using a custom built modular instrument made up of a horizontal beam of monochromatic light as the excitation source and a photomultiplier tube as the scattering detector.24 This system found scattering efficiencies smaller than expected by Mie theory. Using silica nanoparticles as the calibrators, Yguerabide et al. demonstrated Cs dependency on nanoparticle size. For diameters ranging from 40–90 nm, Cs increases according to r5.2, while for diameters between 87–118 nm Cs increases according to r5.2. Although there are some discrepancies between the Mie theory calculated and experimentally determined values, both methods agree that particles in the 40–90 nm range have high scattering properties.
Overall, particles above 50 nm have high scattering intensities, but particles above 100 nm start to decrease in the Mie extinction coefficient.24 This suggests that more efficient fluorophore quenchers are gold nanoparticles with diameters below 40 nm, in accordance with the RP model.
2.2 Shape of AuNPs
Different AuNP shapes, such as nanospheres, nanorods, and nanoshells, have different scattering and absorption coefficients that can affect fluorescence enhancement and quenching. Nanospheres have only one size variable, the diameter. Nanorods have a cylindrical shape that can be defined by the aspect ratio, which is the ratio of the dimension along the long axis to the short axis or the effective radius, which is dependent on the volume of the rod dimensions. Nanoshells can be described by their inner and outer diameters, and the inner core can be hollow or made up of different materials.
Jain et al. reported absorption and scattering properties of AuNPs of different sizes and shapes using Mie theory and discrete dipole approximation methods.25 They found that gold nanorods (AuNRs) have per micron absorption and scattering coefficients that are an order of magnitude greater than those of nanospheres and nanoshells. A dominant scattering coefficient exists in the AuNR’s effective radius range of 8 to 22 nm, but is not affected by a change in the aspect ratio from 3 to 5. Scattering coefficients of gold nano-shells made with a silica core increase with an increase in the nanoshell thickness or a decrease in the ratio of the inner to the outer diameter. As seen by this study, AuNPs can be fine-tuned to increase their scattering or adsorption coefficients. Additionally, AuNRs can be utilized as fluorescence enhancers. As a side note, AuNRs have also shown utility as photo-thermal agents, due to their high scattering, and proof-of-concept studies have shown local tumor ablation, due to the rapid heating caused by AuNRs.26–28
Fu et al. demonstrated that covalently linking of fluorophores to AuNRs can increase the optical signal of the dye and therefore improve signal sensitivity.17 The AuNRs were 80 nm in length and 13 nm in diameter with a plasmon band at 980 nm. Cy5 dye was held at approximately 8 nm from the AuNR by oligonucleotides. Compared to the oligonucleotide-labeled dye alone, the AuNR–oligonucleotide hybrid exhibited a 40-times higher fluorescence emission rate with a seven times shorter fluorescence decay rate. AuNR fluorescence enhancers were designed by choosing NRs that display a dominant scattering component in the same wavelength range as the fluorophore emission.
More recently, Tam et al. demonstrated molecular fluorescence enhancement by 50 times for indocyanine green (ICG) when in proximity to gold nanoshells.21 As suggested by Dulkeith et al.,3 local field enhancement of dyes with quantum efficiencies (QE) around 1%, such as ICG, can improve fluorescence. Gold nanoshells are different than spherical NPs as they have a core center and inner and outer radii. The advantage of gold nanoshells in this study is that plasmon resonance energy can be adjusted by the inner and outer radii of the shell, and the scattering cross section can be adjusted by the absolute particle size. Therefore, by adjusting the inner radius, outer radius and absolute particle size, fluorescence enhancement is possible by increasing the particle scatter efficiency, usually by making larger particles, and tuning the plasmon resonance to the dye emission wavelength, even at dye–NP separations of only around 4 nm.21 Theoretical studies performed by Enderlein of single molecule fluorescence within a metallic nanoparticle, like a nanoshell, suggest that fine tuning the nanoparticle radius can enhance the fluorescence emission rate by a factor of 25.29,30 By adjusting their size, nanoparticles can be created as effective fluorescence quenchers or enhancers.
2.3 Distance between AuNP and photoluminescence material
The fluorophore distance from the AuNP plays an important role in the quenching efficiency. Previously, radiative decay rates could only be increased in proportion to the square of the refractive index.31 However, in recent findings the distance between the fluorophores and metal surface can affect the radiative decay rate as well.3,11–14,32
Dulkeith et al. studied fluorophore quenching at distances between 2–16 nm from the surface of the AuNPs (Fig. 3).3 To control the distance between the AuNP and a Cy5 dye molecule, different concentrations of dye-labeled single-stranded DNA were coated per AuNP. As more Cy5-labeled single-stranded DNA was bound per AuNP, the DNA elongated and produced a large spacer between the AuNP and Cy5. Time-resolved spectra were used to find the fluorescence and radiative rates. The radiative rate increased by two orders of magnitude as the distance increased from 2.2 to 16.2 nm, as seen in Fig. 3B. At a distance of 16.2 nm, the radiative rate was about the same as that of the unbound Cy5–DNA molecules. QE was seen to increase to almost 10% as the distance between the AuNP and dye increased from 2 to 16 nm. Therefore, as predicted, the nonradiative rate increased as the distance decreased. However, this effect due to resonance energy transfer was weaker than expected and may imply that energy transfer does not play an important role in quenching with large distances between dye molecules and AuNPs. In other words, the reduced QE may be due to the reduced radiative rate and not energy transfer.
Fig. 3.
(A) Model used by Dulkeith et al.3 to demonstrate AuNP fluorescence quenching of Cy5 dye molecules. The distance between the dye molecule and AuNP surface was regulated by varying the concentration of single stranded DNA molecules per AuNP. The more DNA particles on the surface, the larger the distance between the dye and AuNP. d2 < d1. (B) The radiative rate (i), nonradiative rate (ii), and quantum efficiency effect (iii) as a function of the distance between AuNP and Cy5 molecules. The dotted lines are calculated values for the molecular dipole perpendicular orientation, while the dash dotted lines are for the tangential orientation. It is evident that the experimental results suggest that phase-induced suppression of the radiative rate causes Cy5 fluorescence quenching. Image adapted from Dulkeith et al.3
However, a question remains about whether larger separation distances can lead to increases in fluorescence, because such enhancements have been seen for fluorophores near silver metal films at distances above 10 nm.33 Anger et al. demonstrated Nile blue dye fluorescence shifts from a quenched state to an enhanced state when the distance between the dye and 80 nm AuNPs is adjusted.11 In this study, the dye was coated onto a substrate, a 2 nm layer of polymethyl methacrylate was overcoated onto the dye layer, and a single 80 nm AuNP was attached to an optical fiber. The optical fiber was adjusted in height from the surface to obtain distance curves of the dye fluorescence rate. The maximum fluorescence enhancement was found at a distance of 5 nm from the surface, which was where the excitation frequencies red-shifted from the AuNP surface plasmon resonance. Quenching occurred at distances below 5 nm. Anger et al. note that this transition from fluorescence quenching to enhancement as a function of distance cannot be explained by simply using dipole approximation as this over estimates the quantum yield at shorter distances and does not predict quenching. Higher multipole orders are needed for accurate description of nanoparticle-molecule fluorescence effects.
Surface-plasmon-coupled emission (SPCE), modeled by Lakowicz, implies that fluorescence enhancement occurs because radiation from the metal surface plasmon induces the nearby excited fluorophores at 10–80 nm distances above the metal surface.1 By coupling excited fluorophores with SPR of thin metal films or NPs, 50% of the light is collected. Without coupling, fluorescence emits in all directions, making it difficult to collect all the photons emitted. It has been stated that typically less than 1% of the total emission is detected.34 SPCE is detected for any fluorophores that are not quenched and at least 1 nm above the metal surface. For example, fluorophores near silver particles experienced increased fluorescence intensity, quantum yield, and photostability.8,9 Theory suggests that SPCE is highest when the fluorophores are beyond 20 nm and closer to the surface than 500 nm. This theory specifically deals with metallic surfaces, however such findings can provide clues to the physics of gold nanoparticle based activatable probes.1 The effect of fluorescence quenching at smaller distances between the donor and acceptor has also been studied.20,22,35 These studies are discussed in the following section, since they help to illustrate energy transfer mechanisms.
2.4 Energy transfer mechanisms
Although the RP model provides an understanding of scattering and absorption balance necessary for fluorescence quenching and enhancement of AuNPs, there is little to describe the mechanism of quenching by energy transfer. Therefore, theoretical predictions, such as Fluorescence Resonance Energy Transfer (FRET),35,36 Gersten–Nitzan (GN),37 Chance–Prock–Silbey (CPS)-Kuhn,38,39 and Nanometal Surface Energy Transfer (NSET)22,40 models, have been used to explain energy transfer between a donor and AuNP. The generic energy transfer efficiency, E, is dependent on the distance between the donor and acceptor, R, and the 50% quenching distance R0:
| (5) |
where R0 and n are dependent on the energy transfer model. To determine the transition rate from one energy state to another, Fermi’s Golden Rule governs,27 indicating that the separation distance, energy overlap between the fluorophore photoluminescence and metal extinction, and orientation of the fluorophore dipole to the gold nanoparticle play important roles.
FRET occurs when a fluorophore in an excited state, acting as a donor, transfers its excitation energy to a nearby acceptor through non-radiative dipole–dipole interactions. For AuNP–dye interactions, the AuNP acts as the acceptor. For such an energy transfer to occur, the absorbance spectrum of the donor and acceptor should overlap. An indication of this energy transfer occurs when the donor photoluminescence is quenched and the acceptor emission is increased. FRET distances typically occur at 15–60 Å. The energy transfer efficiency equation is dependent on the inverse sixth power of the distance between the donor and acceptor.35 In eqn (5), n = 6 and R0 is the Forster distance:
| (6) |
where κ is the dipole orientation factor, Φdye is the quantum yield of the donor, NA is Avogadro’s number, n is the medium refractive index, and J(λ) is the overlap integral between the donor emission and acceptor absorption. The rate of energy transfer is related to the lifetime of the donor in the presence or absence of the acceptor.35 In FRET, the acceptor, AuNP, is estimated to be molecular with little disruption placed on it by the donor.20 Therefore, this energy transfer model does not describe the strong effect of dipole interactions towards the AuNP SPR.
The GN Model approximates molecular dipoles and particle plasmons in a transient manner, modeling small dielectric spheroidal grains with fluorescent molecules adsorbed on the surface.37 In eqn (5), n = 6 just as for FRET, and R0 is:
| (7) |
where ωdye is the frequency of the donor dye, Φdye is the quantum yield of the donor, a is the radius of the metal nanoparticle, ε1 and ε2 are the real and imaginary components of the dielectric constant of the metal, respectively, and c is the speed of light. Both radiative (fluorescence enhancement) and non-radiative (fluorescence quenching) rates are taken into account under this model.37 As opposed to FRET, GN attributes a strong electric field to AuNPs and describes coupling of the fluorophore and metal electric field. The GN model is able to show that a small dipole from the fluorophore can induce a large dipole in the NP.41 Such an enhancement in the dipole increases energy transfer efficiencies by 104–105.37 However, such strong interactions may underestimate the quenching abilities of AuNPs due to the rapid damping of the electric field on their surface.20
In the Kuhn model (CPS-Kuhn), a luminescent molecule is modeled as a classical linear harmonic oscillator near an ideal metal mirror.38 In eqn (5), n = 4 and R0 is:
| (8) |
where A is the absorptivity of the mirror which is (4πkd2)/λ for a perpendicularly orientated dipole to the metal surface and (1/(4π))(9/2)1/2 when parallel to the metal surface, α is the dipole orientation factor, λ is the emission wavelength of the donor dipole, ε1 and ε2 are the real and imaginary components of the dielectric constant, respectively, nr and k are the real and imaginary components of the refractive index, respectively, n is the refractive index of the medium, and d2 is the thickness of the mirror, which is the diameter of the nanoparticle in this case. Deactivation of the luminescence is modeled by thermal deactivation, rate of radiation emission, and the rate of absorption in the mirror.38 The quenching mechanism is attributed to a retardation effect of fluorescence when near a mirror. The emitter dipole induces oscillation in the acceptor and then the donor interacts with the radiation field of the acceptor.38 CPS-Kuhn predicted a 4th order distance dependence.20 This model may not be accurate for AuNP–fluorophore interactions, since an AuNP does not act as a perfect mirror.20
NSET models energy transfer from a molecular dipole to a nanometal surface at twice the FRET range.22 In eqn (5), n = 4 and R0 is replaced with d0, because the distance is between the donor and surface, not the acceptor. The value is calculated as:
| (9) |
where Φ is the quantum yield of the donor, ωdye is the angular frequency of the donor emission, and the other values are constants: c = 3 × 108, the speed of light, kF = 1.2 × 108 cm−1, and ωF = 8.4 × 1015 rad s−1. This model takes into account the limitation that FRET has in explaining fluorescence quenching by metal NPs, namely the small length of energy transfer. While FRET depends on the 6th power of distance dependence, NSET depends on the 4th power, as derived from Person and Lang.16 These changes show that energy transfer to a surface undergoes different interactions, such as the dependence of magnitude on distance. In this model, the donor electromagnetic field interacts with almost free conducting electrons of a metal thin film, where the conducting electrons interact strongly with the oscillating dipole.16 The NSET model has therefore been applied to AuNP–dye studies, since it can be used for longer distance ranges and for a variety of particle sizes as opposed to FRET.22 NSET can be used as an optical ruler to measure distances twice the Forster range of 220 Å; for example, using a 1.4 nm AuNP conjugated to fluorescein and separated by a DNA strand, energy transfer over 50–250 Åhas been measured.22 NSET was found to have lower sensitivity but a larger detection range than FRET-based optical rulers. Above 70 Å, NSET provides better resolution of distance than FRET.
Recently, Singh and Strouse compared the efficiencies of these models for describing energy transfer between fluorescence dyes of varying emission wavelengths and 2 nm AuNPs.20 Each theory was compared based on three experimental terms: 50% quenching distance, QE, and the total quenching range. The distance between the AuNP and dye was varied from 68 to 171 Å by the conjugation of synthetic DNA sequences of increasing base pairs. Both FRET and GN under-predicted the distance dependence, while CPS-Kuhn over-predicted it. Based on efficiency curve fits of experimental photoluminescence and lifetime data, Singh and Strouse found that the NSET model best described the quenching behavior for a 2 nm AuNP across all distances from the dye and for all dyes tested.
Singh and Strouse also found experimentally that no quenching occurred for a dye that did not emit within the spectral region of the 2 nm AuNP localized SPR.20 Localized SPR occurs when ultrasmall nanoparticles localize their SPR at the surface and are therefore defined as skin-depth oscillations of the electric field (Fig. 1). This finding suggests that energy transfer occurs from a dipole of the fluorophore to the AuNP surface only when within the LSPR frequency range. NSET is therefore dependent on the type of fluorophore– AuNP pair used. Singh and Strouse provided NSET constants for dyes ranging in wavelength emission from 519–775 nm.20 This leads to a question of whether NSET or the decrease in the radiative rate dominates fluorophore quenching by AuNP for particles that do not exhibit SPR bands. Ultra-small AuNPs below 2 nm in diameter do not exhibit an SPR band42 and therefore can only quench fluorophores by changing its radiative rate or by changing its energy transfer to the metal NP.40 Jennings et al. calculated the experimental oscillator strength of a dye molecule, which is directly related to its radiative rate, after quenching occurred at close distances between the 1.5 nm AuNP and fluorophore.43 At high quenching efficiencies, the oscillator strength only contributed to 5–10% of the effect, thus did not account for the actual 50–70% fluorescence intensity decrease. Therefore, fluorescence quenching of fluorescein and Cy5 by AuNPs occurred because of NSET and was not due to changes in the radiative rate.43
2.5 Adsorption features affecting quenching by AuNPs
Although the NSET model is highly applicable to the design of efficient AuNP-based activatable probes, chemical functionalization factors affecting the quenching ability of AuNPs should also be well understood. Functionalized AuNPs conjugated with fluorescence polymers have been demonstrated as efficient detection systems. The adsorbed fluorophore is quenched and, upon disruption of this adsorption by the target, fluorescence recovers.44 Due to the ease of functionalization of AuNPs, different chemical groups and recognition elements can be adsorbed to make a wide array of sensors. With such an activatable probe system, additional adsorption and chemical features can be taken into account to understand quenching mechanisms. What concentrations of dye to AuNPs are required? Can the bonding characteristics between a dye and AuNP provide different quenching efficiencies? Can fluorophore–AuNP complexes differ in fluorescence quenching and enhancement abilities based on environmental changes? Such effects make the design of wide-array protein detection systems by AuNP activatable probes possible, as described in an informative review by Bunz and Rotello.44
These types of effects introduce a different understanding of fluorescence quenching beyond energy transfer mechanisms. Quenching can occur by different means, such as by: (a) static quenching, where it occurs in the ground state, (b) collisional quenching, or (c) trivial matters, such as attenuation of incident light by other absorbing species. As opposed to RET, which can occur across small distances, collisional quenching occurs when the fluorophore in the excited state is deactivated because of direct contact with a molecule in solution, such as oxygen, a halogen or an amine. Although the mechanism of such quenching is dependent on the specific fluorophore–quencher pair, the quenching fluorescence intensity can be described by the Stern–Volmer equation:
| (10) |
where KSV is the Stern–Volmer quenching constant, kq is the bimolecular quenching constant, τ0 is the unquenched lifetime and [Q] is the quencher concentration. As seen by this relationship, the higher the Stern–Volmer constant (KSV) the less quencher is required for a significant fluorescence change. It has been demonstrated that minute amounts of AuNPs are required for high quenching efficiencies of polyfluorene.4 Fan et al., following recent literature,45,46 reported KSV values of polymer repeat units per quencher at 50% quenching.4 AuNPs quench the fluorescence of cationic polymers with KSV values in the range of 107–1011. KSV values of 1011 are about four orders of magnitude greater than those of small molecule dye–inorganic quencher pairs, such as [Ru(bpy)3]2+.12 KSV value for 2 nm AuNPs with a fluorine phenylene fluorophore are 4 orders of magnitude lower than for 20 nm AuNPs, but each AuNP had about the same quenching efficiency.4 Even though the KSV is diameter dependent, the high constant explains quenching abilities that occur at less than picomolar AuNP concentrations as well as demonstrates that AuNPs are superior quenching agents in comparison to other quenchers (whether organic or inorganic).
AuNPs can quench or enhance fluorescence based on specific donor–acceptor matching and AuNP physical features. Quenching mechanisms are dependent on SPR, particle size, shape, and distance from the donor fluorophore. Spherical NPs below 40 nm in diameter have the lowest scattering constant and therefore have the highest potential to quench fluorescence. An effective distance below 2 nm between the AuNP and fluorophore demonstrates the best quenching ability. NSET and a decrease in the radiative rate most closely predict AuNP–fluorophore systems. NSET energy transfer efficiency directly correlates to experimental data for AuNPs below 2 nm in diameter. Additional effects such as collisional quenching can be optimized to enhance fluorescence quenching and enhancement. In the next section, these principles are applied to design Au-NP-based fluorescence activatable probes that are used to sense oligonucleotides, proteins, and other biomolecules.
3. AuNP-based fluorescence activatable probes
Fluorescence is used in many biological assays and staining, but is highly dependent on a low nonspecific background signal. Therefore fluorescence quenching is a key component for the design of fluorescence detection methods, such as molecular beacons47 and activatable nanoprobes.2 Metallic surfaces are known to quench fluorophores at close proximity.3,7 AuNPs, in particular, have high fluorescence quenching abilities experimentally and have therefore found great application as molecular beacons, activatable probes, and chemical sensors.
3.1 AuNPs for oligonucleotide detection
A molecular beacon is a nucleic acid probe that is able to detect specific nucleic acid sequences of interest.48 Upon hybridization, the probe changes its conformation and provides a fluorescence signal. This allows the probe to be used in real time and for various ex vivo applications, such as nucleic acid amplification assays, and in vitro applications, such as detecting the fate of messenger RNA (mRNA) in living cells.48 Molecular beacons are designed where a fluorophore and organic quencher are attached on opposite ends of a looped oligonucleotide. Once the oligonucleotide complementary pair is reached, the dye and quencher are forced apart, causing an “on” fluorescence signal to occur. In other words, hybridization causes the quencher and dye to separate and prevent fluorescence from being quenched. Dubertret et al. designed the first molecular beacon using 1.4 nm AuNPs as the quenchers (Fig. 4A).49 The probe was in a stem-looped configuration, with four different fluorophores—fluorescein, Rhodamine 6G, Texas Red, Cy5— attached on one end and an AuNP on the opposite end. This work introduced the application of AuNPs for organic fluorophore quenching or, more specifically, fluorescence activation. The AuNP was found to have a quenching efficiency a hundred times higher than the organic quencher, 4-((4′-(dimethyl-amino)-phenyl)azo)benzoic acid (DABCYL), giving higher sensitivity for detection of oligonucleotide single mismatches.49
Fig. 4.
(A) Schematic drawing of a molecular beacon conformation with a fluorophore, oligonucleotide, and gold nanoparticle designed by Dubertret et al.49 The oligonucleotide is made of 15 thymidines, which produces a hairpin structure, quenching fluorescence. When the target is reached, the hairpin unwraps causing fluorescence emission. Image adapted from Dubertret et al.49 (B) Schematic drawing of a molecular beacon conformation using oligonucleotide self-assembly on a gold nanoparticle surface, as demonstrated by Maxwell et al.41 When the dye is adsorbed on the NP surface, the fluorescence is quenched. Upon targeting, the conformation is constrained and the fluorophore distances itself from the dye due to the rigid double-stranded DNA. Au—gold nanoparticle, F—fluorophore, S—sulfur atom. Image adapted from Maxwell et al.41
Another group designed a new molecular beacon configuration utilizing the unique feature of fluorophore adsorption on AuNPs. Maxwell et al. designed a molecular beacon where a 2.5 nm AuNP served as a scaffold for fluorophore (either fluorescein or tetramethylrhodamine)-tagged oligonucleotides (Fig. 4B).41 The fluorophore self-assembles onto the AuNP surface in an arch-like configuration, due to the flexible single-stranded chain of DNA. When the fluorophore is adsorbed onto the AuNP surface, fluorescence is quenched in an “off” state.41,49 Once the oligonucleotide binds to its target, the double-stranded DNA becomes more rigid than the single-stranded DNA, the arch-like configuration is lost and the fluorophore and AuNP separate. This state turns the fluorescence “on”, since the distance between the fluorophore and AuNP reaches about 10 nm.41,49 A QE of almost 100% was seen when the fluorophore was adsorbed on the particle. This type of study can give insight into the distance necessary between the fluorophore and AuNP for ideal fluorescence quenching. Further optimization of this system has involved the use of larger AuNPs of about 15 nm in diameter, which have stronger SPR absorption over smaller particles.50 The strong SPR absorption can be used to quench a broad range of fluorophores. Fan et al. reported that AuNPs between 5–20 nm diameters absorb strongly between 300–500 nm wavelength range, the range in which 2 nm AuNPs absorb hundreds of times less intensely.4 In addition, the greater surface area of the particle is utilized to functionalize a larger number of oligonucleotides as well as different types of oligonucleotides.
Utilizing the advantages of larger particles, Song et al. designed multicolor molecular beacons, based on 15 nm AuNPs conjugated to multiple molecular beacons with different fluorophores.50 As a proof-of-principle study, hairpin probes consisting of complementary sequences to three different tumor-suppressor genes were labeled with FAM, Cy5, and Rox, respectively, and bound to 15 nm AuNPs. Approximately 40–50 probes were conjugated per AuNP. Using different targets on single particles, these molecular beacons were able to detect each specific gene as well as detect single-base-mismatched DNA.50
Due to their efficient cellular internalization and low cytotoxicity,51 AuNP molecular beacons can be used in vitro. Recently, live cell imaging of mRNA using hairpin DNA-functionalized AuNPs was achieved.52 Targets for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA and Respiratory Syncytical Virus (RSV) mRNA were functionalized onto 15 nm AuNPs and used in live normal and RSV-infected HEp-2 cells, respectively, with high target signal to background ratio. The detection of GAPDH and RSV mRNA enabled real-time analysis of mRNA transport and processing in live cells.
3.2 AuNP for protein detection
A rational choice of functionalized AuNPs and fluorophores allows the binding constant, and hence the quenching of fluorescence, to be adjusted for different responses to analytes.44,53 KSV can be correlated to the binding constant (KA). By modulating KA of the dye to the AuNP, the fluorescence quenching is modulated making it possible to design indicator displacement assays and, hence, effective sensors and activatable probes.44 The KSV of AuNPs is in the range of 107–1011. Assuming that the lifetimes of certain fluorophores are in the range of 0.3–0.7 nm, the observed KSV is assumed to be equal to KA. Diffusion and dynamic quenching are assumed to be small at micromolar polymer concentrations. Another interesting feature seen by conjugated polymers on AuNPs is the change in quenching efficiency based on polymer charge. Static quenching occurs due to strong Coulomb interactions between the anionic AuNPs and cationic polyfluorene.4,14,54 Increases in ionic strength reduce quenching efficiency as demonstrated by the increase in fluorescence of 2-OF–AuNP conjugates when the ionic strength becomes greater than 0.6 M.4 Electrostatic complex formation can affect quenching, and therefore the KSV, by enhancing aggregation of the complex.4
Rotello and colleagues have used this strategy to design array-based sensor platforms, so-called ‘chemical noses’, to identify proteins, differentiate bacteria, and discriminate between healthy and cancerous cells.42,55–58 The most popular and effective mechanism to functionalize AuNPs is via gold–sulfur binding. By coating AuNPs with molecular recognition agents, numerous AuNP quenchers can be created with different sensory abilities. In addition, the AuNP can be functionalized and coated with different polyvalent monolayers upon which fluorophores can complex. Binding constants between monolayer-functionalized AuNPs with alkyl, cycloalkyl, aromatic, and polar groups and different anionic poly(p-phenyleneethynylene) type fluorophores and green fluorescence proteins have been studied to determine the strongest quenching abilities.44 When these fluorophore–AuNP complexes bind with other proteins, the binding interactions between the donor and acceptor are changed and therefore the fluorescence intensity increases or decreases depending on the specific protein interaction. In this manner, Rotello has been able to design self-assembled indicator displacement assays to detect numerous proteins.
You et al. developed chemical nose sensors using an array made of AuNPs–fluorescent polymer conjugates.59 The fluorescence generation per well in this sensor array was dependent on the interactions of the six cationic nanoparticles and the one anionic fluorescent polymer, poly(p-phenyleneethynylene)-CO2. The polymer fluorescence was quenched when in close contact with the AuNP and restored when displaced from the NP surface. The disruption of the fluorescent polymer was specific to individual proteins at nanomolar concentrations and was quantified by linear discriminant analysis (LDA). The fluorescence intensity pattern was highly repeatable. The array identified bovine serum albumin, cytochrome c, lipase, subtilisin A, alkaline phosphatase, acid phosphatase, and β-galactosidase on 52 unknown samples with 94.2% accuracy in PBS.
Furthermore, De et al. utilized green fluorescent protein (GFP) and AuNP interactions to detect proteins in more biologically relevant matrices, such as buffer and serum with 100% and 97% accuracy, respectively (Fig. 5).55 This was a huge feat, as these NPs were able to ‘sniff out’ specific proteins like fibrinogen, human serum albumin, α-antitrypsin, transferrin and immunoglobin G in serum that contained tens of thousands different proteins. GFP was found to bind to polar functionalized AuNPs at a binding constant to the 9th order. The use of GFP, with a defined size and molecular weight, was chosen to prevent nonspecific interactions and aggregation. Using LDA, the increases and decreases in fluorescence intensity were correlated with specific proteins of interest. Such chemical noses, apply understanding of fluorescence quenching along with electrostatic and hydrophobic interactions to sense proteins with over 97% accuracy.
Fig. 5.
Schematic of competitive binding of green fluorescent protein (GFP) onto monolayer-protected AuNPs. The competitive binding of GFP protein to the AuNP is affected by specific types of proteins, which, in turn, affects the efficacy of fluorescence quenching. Different proteins can therefore be identified based on the shifts in fluorescence intensity. Image adapted from De et al.55
3.3 AuNP and quantum dot pair
AuNP quenching has also been investigated with nontraditional photoluminescence materials, most notably quantum dots (QDs).60,61 QDs are semiconductors that are confined on the nanometre scale. At this confined space, the oscillation strength is focused to a reduced number of transitions with electronic excitation shifts at higher energies allowing for unique optical properties. QDs have narrow, typically 20 to 30 nm full width at half maximum, and tunable emission spectra in the range of 400 nm to 2 µm. The emission spectrum is tuned by the size of the confinement. For example, when the diameter of CdSe QD is reduced from 20 nm to 2.0 nm the band gap shifts from a deep red to a green color.62 QDs can therefore be used for efficient single-molecule detection methods, because of their photostability, broad absorption sections, wide absorption spectra, and narrow emission spectra.63
Pons et al. investigated the AuNP quenching efficiency of CdSe–ZnS core–shell QDs by functionalizing twelve 1.4 nm AuNPs around a QD surface with a separation distance between 50–200 Å controlled by rigid polypeptides made up of a different number of repeat units.60 The quenching efficiency measured by steady-state and time-resolved fluorescence spectroscopy was compared with theoretical models based on Forster dipole–dipole resonance energy transfer, dipole–metal particle energy transfer, and NSET. The QD excited-state lifetime was decreased greatly when conjugated to AuNPs. The radiative decay rates of QDs remained unchanged with or without AuNPs. Yet the presence of AuNPs introduced a nonradiative pathway for QD excitation. The AuNP driven quenching for QDs occurred over a larger distance range of 200 Å, similar to NSET. Based on modeling, the nonradiative quenching of QD photoluminescence emission was attributed to long distance dipole–metal interactions. The AuNPs are credited with the nonradiative energy dissipation without modification of the QD radiative rate.60
Utilizing the QD and AuNP donor and quenching pair, Oh et al. created an inhibition assay that could be applied for high throughput screening of inhibitors.61 In this proof-of-concept study, avidin concentration was determined by the photo-luminescence quenching of streptavidin conjugated to 5 nm QDs and biotinylated 2 nm AuNPs. AuNPs were bound to the QDs by strong streptavidin–biotin binding upon which fluorescence was quenched. When another molecule, such as avidin, binds to AuNPs, conjugation to QDs is inhibited and the QDs restore their photoluminescence. In this study, QD photo-luminescence was quenched by 80% when in close proximity of AuNPs and the detection limit of avidin was 10 nM.61
QD–AuNP conjugates as donor–acceptor pairs can also be used for ultra-sensitive detection of cellular prion protein (PrPc).64 Prion disease is a rare progressive neurodegenerative disorder that is associated with neuronal loss and causes no inflammatory response. It is triggered by a prion infection that causes proteins to undergo conformational changes in the brain leading to rapid progression of the disease that is fatal.65 Carboxylated QDs were first functionalized with nickel– nitrilotriacetic acid (NTA) and Ni2+ which easily binds to PrPc with high specificity. AuNPs were functionalized with a thiolated anti-prion protein aptamer.When the QD and AuNP conjugates were mixed, the aptamer on the AuNP surface interacted with the PrPc protein on the QD surface forming a PrPc–aptamer duplex with distances between 9–22 nm. This complex caused long range quenching between the AuNP and QD up to 88.7%. The degree of quenching was linearly correlated to the concentration of PrPc in the range of 0.82–3.30 fM with a correlation coefficient of 0.973. The limit of determination for this highly sensitive system was 33 aM. Furthermore AuNP quenching of QDs can be applied to detect other histidine-tagged proteins with long distances as well as for in vitro protein detection since both QDs and AuNPs have shown low cytotoxicity and high cellular internalization.51,63
3.4 AuNP activatable probes for in vivo applications
AuNP activatable probe studies have mainly been fixed to microtiter plates to be used as diagnostic tools. Yet AuNPs are widely accepted to be noncytotoxic, biocompatible and show good cellular uptake.51,66 Therefore, fluorescence-based activatable probes can be used in vitro and in vivo as cellular and clinical bioimaging agents. However, this remains a little explored field. Controlling fluorescent optical properties, aggregation and quantifiable signals in vitro and in vivo are challenges for bioimaging applications. For example, near-infrared fluorescent dyes are required for in vivo use in order to obtain deep tissue penetration and prevent fluorescence scattering from surrounding tissue and blood.
A new 16 nm AuNP probe designed with a near-infrared fluorescence (NIRF) dye and hyaluronic acid (HA) has shown ultrasensitive detection of reactive oxygen species (ROS) and hyaluronidase (HAdase) in vivo.67 HA is a non-immunogenic carbohydrate that is easily functionalized with multiple NIR dyes like HiLyte Fluort 647 amine. HA is commonly degraded by HAdase and has shown degradability by certain types of ROS, such as superoxide and hydroxyl radical. HAdase and ROS expression was found to be present in the soft tissue of joints during arthritis.68–70 Enhanced ROS production is also known to activate various cell signaling pathways, such as inducing tumor cell invasion and generating vascular endothelial growth.71 Therefore fluorescent signals of AuNPs functionalized to dye-conjugated HA can be shifted when in the presence of degradation-inducing biomolecules, such as HAdase and ROS. Lee et al. found that the dye-HA–AuNP probe remained intact under a wide range of environmental condition at different pHs and salt concentrations, and in serum.67 Once HAdase was detected, the HA was degraded and the dye was released from the proximity of the AuNPs. This caused the probe to induce NIR fluorescence. In vitro, the probe was able to detect different concentrations of ROS and HAdase. In vivo in a rheumatoid arthritis animal model, the probe was used to localize arthritic inflammation. The probe was fully activated three hours after intra-articular injection. Background signals in the healthy joints were considered negligible. The enhancement fluorescence level was 3.6 times greater at three hours than at 30 minutes post injection. This type of probe shows potential for future applications with a more rational design of AuNP quenchers and with multifunctional activities, such as drug delivery.
The design of protease-sensitive AuNP-quenched NIRF probes by Lee et al. is a unique combination of diagnostic screening tools and in vivo probes (Fig. 6).2,32 Protease and protease inhibitors are involved in cancer, inflammation, and vascular disease.72 Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases that play key roles in tumor invasiveness, metastasis, and angiogenesis. Because of their unique and significant roles in cancer biology, MMPs have been investigated as an important target for tumor imaging and therapy.73 MMP-activatable AuNPs can serve as sensitive and simple protease-detection systems for high throughput screening of drugs and early diagnosis of this wide array of diseases. The NIRF dye, Cy5.5, was conjugated to the matrix MMP substrate, GPLGVRGC, and then functionalized onto a 20 nm AuNP surface. In this conformation, the AuNP successfully quenched the NIR dye, giving a minimal background signal for in vitro and in vivo imaging. Yet, the probe was highly activated when the substrate was selectively degraded by MMPs. When the fluorescently-quenched AuNP probe was injected into MMPs-positive SCC7 tumor-bearing mice, the AuNP probe demonstrated strong NIR fluorescence signals only in the tumor regions by targeting active MMPs. This study can be applied to various peptide substrates by replacing the spacer between the AuNP and dye.
Fig. 6.
(A) A schematic of the matrix metalloproteinase (MMP)-sensitive in vivo AuNP fluorescent probe that activates upon targeting to specific MMPs. (B) Near-infrared fluorescence (NIRF) images of tumor-bearing mice after injection of the AuNP probe (i) with and (ii) without an MMP inhibitor. Image adapted from Lee et al.32
As seen by these numerous biomedical applications of fluorescence quenching by AuNPs, highly sensitive and specific detection is possible for both ex vivo, in vitro, and even in vivo uses. Thanks to the highly versatile AuNP surface and its biocompatibility, various targeting agents, such as oligonucleotides, aptamers, and polypeptides, and different fluorescence molecules, such as proteins and inorganic fluorophores, can be conjugated in order to perform specific tasks. Future applications of these activatable fluorescence probes can include point-of-care detection devices, image guided surgery, and theranostics.
4. Conclusions
AuNPs exhibit ultra-quenching properties that have been applied for biomedical sensing, diagnostics, and imaging. These applications can further be enhanced with the rational design of AuNPs and fluorophore interaction. To do this, the mechanisms of fluorescence quenching and enhancement need to be addressed. Photoluminescence materials near AuNPs can undergo fluorescence ultra-quenching or enhancement, depending on numerous factors. For efficient quenching, AuNPs should have: (1) plasmon resonances that overlap dye emission, (2) diameters below 50 nm, (3) highly controlled spherical particle shapes, and (4) distances from the photo-luminescence molecule below 2 nm. Although these factors have been examined experimentally and theoretically, no standards have been set for specific fluorophore and AuNP pairs. Currently, the NSET model is accepted as explaining AuNP quenching over a wide range of fluorophores and over a large range of distances between the dye and AuNP. However, this model will need to pass rigorous testing for larger AuNP diameters, which can be challenging as the distance from the dye cannot be as easily controlled with larger particles, and intrinsic SPR may interfere with fluorescence signals.
Designs using larger AuNPs can benefit from the larger surface areas and incorporate more dye and functional group conjugation. As described above, rigorous experiments have been reported that study AuNP quenching ability over a wide range of lengths from the dye molecules. Yet, such experiments have mainly focused on AuNPs below 5 nm. Also, unique fluorescence activation techniques could be utilized, such as a recent light-triggered release of dyes from Au nanoshells.74 In addition, unique shapes and sizes can now be studied due to the easily tunable AuNP synthesis techniques.
If AuNPs are thoroughly engineered and accurately/ precisely synthesized, one can foresee high quenching efficiencies for all currently existing fluorescence-based assays. This may lead to a more quantitative approach to development of AuNP activatable probes for single molecule detection.75 By utilizing the growing understanding of nanotechnology, it will be possible to devise fluorescence based detection systems with 100% quenching efficiency.
Acknowledgements
This work was supported in part by the Intramural Research Program (IRP) of the National Institute of Biomedical Imaging and Bioengineering (NIBIB), NIH and the International Cooperative Program of the National Science Foundation of China (NSFC) (81028009). We thank Dr Henry S. Eden for proofreading the manuscript.
List of abbreviations
- AuNPs
gold nanoparticles
- AuNRs
gold nanorods
- CPS-Kuhn
Chance–Prock–Silbey (CPS)-Kuhn
- FRET
fluorescence resonance energy transfer
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GFP
green fluorescent protein
- GN
Gersten–Nitzan
- HA
hyaluronic acid
- HAdase
hyaluronidase
- ICG
indocyanine green
- LDA
linear discriminant analysis
- MMPs
matrix metalloproteinases
- mRNA
messenger RNA
- NIRF
near-infrared fluorescence
- NPs
nanoparticles
- NSET
nanometal surface energy transfer
- NTA
nickel–nitrilotriacetic acid
- PrPc
prion protein
- QDs
quantum dots
- QE
quantum efficiencies
- RET
resonant energy transfer
- ROS
reactive oxygen species
- RP
radiating plasmon
- RSV
respiratory syncytical virus
- SPR
surface plasmon resonance
Biographies

Magdalena Swierczewska is participating in a Graduate Partnership Program (GPP) between the Biomedical Engineering Department at Stony Brook University and Dr Xiaoyuan Chen’s Laboratory of Molecular Imaging and Nanomedicine (LOMIN) of the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH). Her previous work includes the development and characterization of novel nanomaterials utilizing the properties of inorganic particles. Using her background in nanotechnology and material science, Maggie’s work toward her thesis is focused on the development of novel nanoplatforms for ultrasensitive diagnostics.

Seulki Lee is Group Leader of the Theranostic Nanomedicine Section in LOMIN. He received his PhD in biomedical engineering at Gwangju Institute of Science and Technology in Korea. He focused his training on nanomedicine and molecular imaging at the Korea Institute of Science and Technology and then moved to the United States and joined the Molecular Imaging Program at Stanford under the supervision of Dr Xiaoyuan Chen. In 2009, he joined LOMIN. With a background in nanomedicine and molecular imaging, his research aims to develop smart nanoplatforms for future diagnosis and therapy of various diseases with the emphasis on theranostics.

Xiaoyuan Chen received his PhD in chemistry from the University of Idaho in 1999 and is currently a tenured Senior Investigator and Chief of the Laboratory of Molecular Imaging and Nanomedicine (LOMIN), NIBIB/ NIH. He is interested in developing a molecular imaging toolbox for better understanding of biology, early diagnosis of disease, monitoring therapy response, and guiding drug discovery/development. His lab also puts special emphasis on high-sensitivity nanosensors for biomarker detection and theranostic nanomedicine for imaging, gene and drug delivery, and monitoring of treatment.
References
- 1.Lakowicz JR. Anal. Biochem. 2004;324:153–169. doi: 10.1016/j.ab.2003.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee S, Park K, Kim K, Choi K, Kwon IC. Chem. Commun. 2008:4250–4260. doi: 10.1039/b806854m. [DOI] [PubMed] [Google Scholar]
- 3.Dulkeith E, Ringler M, Klar TA, Feldmann J, Muñ oz Javier A, Parak WJ. Nano Lett. 2005;5:585–589. doi: 10.1021/nl0480969. [DOI] [PubMed] [Google Scholar]
- 4.Fan C, Wang S, Hong JW, Bazan GC, Plaxco KW, Heeger AJ. Proc. Natl. Acad. Sci. U. S. A. 2003;100:6297–6301. doi: 10.1073/pnas.1132025100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song S, Qin Y, He Y, Huang Q, Fan C, Chen H-Y. Chem. Soc. Rev. 2010;39:4234–4243. doi: 10.1039/c000682n. [DOI] [PubMed] [Google Scholar]
- 6.Wilson R. Chem. Soc. Rev. 2008;37:2028–2045. doi: 10.1039/b712179m. [DOI] [PubMed] [Google Scholar]
- 7.Lakowicz JR. Anal. Biochem. 2005;337:171–194. doi: 10.1016/j.ab.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakowicz JR, Shen Y, D’Auria S, Malicka J, Fang J, Gryczynski Z, Gryczynski I. Anal. Biochem. 2002;301:261–277. doi: 10.1006/abio.2001.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lakowicz JR. Anal. Biochem. 2001;298:1–24. doi: 10.1006/abio.2001.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weitz DA, Garoff S, Gersten JI, Nitzan A. J. Chem. Phys. 1983;78:5324–5338. [Google Scholar]
- 11.Anger P, Bharadwaj P, Novotny L. Phys. Rev. Lett. 2006;96:113002. doi: 10.1103/PhysRevLett.96.113002. [DOI] [PubMed] [Google Scholar]
- 12.Huang T, Murray RW. Langmuir. 2002;18:7077–7081. [Google Scholar]
- 13.Dulkeith E, Morteani AC, Niedereichholz T, Klar TA, Feldmann J, Levi SA, van Veggel FCJM, Reinhoudt DN, Möller M, Gittins DI. Phys. Rev. Lett. 2002;89:203002. doi: 10.1103/PhysRevLett.89.203002. [DOI] [PubMed] [Google Scholar]
- 14.Wang D, Wang J, Moses D, Bazan GC, Heeger AJ. Langmuir. 2001;17:1262–1266. [Google Scholar]
- 15.Arkin MR, Stemp EDA, Turro C, Turro NJ, Barton JK. J. Am. Chem. Soc. 1996;118:2267–2274. [Google Scholar]
- 16.Persson BNJ, Lang ND. Phys. Rev. B: Condens. Matter. 1982;26:5409. [Google Scholar]
- 17.Fu Y, Zhang J, Lakowicz JR. J. Am. Chem. Soc. 2010;132:5540–5541. doi: 10.1021/ja9096237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gersten J, Nitzan A. J. Chem. Phys. 1981;75:1139. [Google Scholar]
- 19.Narband N, Uppal M, Dunnill CW, Hyett G, Wilson M, Parkin IP. Phys. Chem. Chem. Phys. 2009;11:10513–10518. doi: 10.1039/b909714g. [DOI] [PubMed] [Google Scholar]
- 20.Singh MP, Strouse GF. J. Am. Chem. Soc. 2010;132:9383–9391. doi: 10.1021/ja1022128. [DOI] [PubMed] [Google Scholar]
- 21.Tam F, Goodrich GP, Johnson BR, Halas NJ. Nano Lett. 2007;7:496–501. doi: 10.1021/nl062901x. [DOI] [PubMed] [Google Scholar]
- 22.Yun CS, Javier A, Jennings T, Fisher M, Hira S, Peterson S, Hopkins B, Reich NO, Strouse GF. J. Am. Chem. Soc. 2005;127:3115–3119. doi: 10.1021/ja043940i. [DOI] [PubMed] [Google Scholar]
- 23.Zhang CX, Zhang Y, Wang X, Tang ZM, Lu ZH. Anal. Biochem. 2003;320:136–140. doi: 10.1016/s0003-2697(03)00353-1. [DOI] [PubMed] [Google Scholar]
- 24.Yguerabide J, Yguerabide EE. Anal. Biochem. 1998;262:157–176. doi: 10.1006/abio.1998.2760. [DOI] [PubMed] [Google Scholar]
- 25.Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. J. Phys. Chem. B. 2006;110:7238–7248. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]
- 26.Huang XH, El-Sayed IH, Qian W, El-Sayed MA. J. Am. Chem. Soc. 2006;128:2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 27.Link S, El-Sayed MA. Int. Rev. Phys. Chem. 2000;19:409–453. [Google Scholar]
- 28.O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Cancer Lett. 2004;209:171–176. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Enderlein J. Appl. Phys. Lett. 2002;80:315–317. [Google Scholar]
- 30.Enderlein J. Phys. Chem. Chem. Phys. 2002;4:2780–2786. [Google Scholar]
- 31.Strickler SJ, Berg RA. J. Chem. Phys. 1962;37:814–822. [Google Scholar]
- 32.Lee S, Cha E-J, Park K, Lee S-Y, Hong J-K, Sun I-C, Kim SY, Choi K, Kwon IC, Kim K, Ahn C-H. Angew. Chem., Int. Ed. 2008;47:2804–2807. doi: 10.1002/anie.200705240. [DOI] [PubMed] [Google Scholar]
- 33.Malicka J, Gryczynski I, Gryczynski Z, Lakowicz JR. Anal. Biochem. 2003;315:57–66. doi: 10.1016/S0003-2697(02)00702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Orden A, Machara NP, Goodwin PM, Keller RA. Anal. Chem. 1998;70:1444–1451. doi: 10.1021/ac970545k. [DOI] [PubMed] [Google Scholar]
- 35.Herman B. Fluorescence Microscopy. Oxford: BIOS Scientific Publishers; 1998. [Google Scholar]
- 36.Stryer L, Haugland RP. Proc. Natl. Acad. Sci. U. S. A. 1967;58:719–726. doi: 10.1073/pnas.58.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gersten J, Nitzan A. J. Chem. Phys. 1981;75:1139–1152. [Google Scholar]
- 38.Kuhn H. J. Chem. Phys. 1970;53:101–108. [Google Scholar]
- 39.Chance RR, Prock A, Silbey R. J. Chem. Phys. 1975;62:771–772. [Google Scholar]
- 40.Jennings TL, Schlatterer JC, Singh MP, Greenbaum NL, Strouse GF. Nano Lett. 2006;6:1318–1324. doi: 10.1021/nl052458a. [DOI] [PubMed] [Google Scholar]
- 41.Maxwell DJ, Taylor JR, Nie S. J. Am. Chem. Soc. 2002;124:9606–9612. doi: 10.1021/ja025814p. [DOI] [PubMed] [Google Scholar]
- 42.Logunov SL, Ahmadi TS, El-Sayed MA, Khoury JT, Whetten RL. J. Phys. Chem. B. 1997;101:3713–3719. [Google Scholar]
- 43.Jennings TL, Singh MP, Strouse GF. J. Am. Chem. Soc. 2006;128:5462–5467. doi: 10.1021/ja0583665. [DOI] [PubMed] [Google Scholar]
- 44.Bunz UHF, Rotello VM. Angew. Chem., Int. Ed. 2010;49:3268–3279. doi: 10.1002/anie.200906928. [DOI] [PubMed] [Google Scholar]
- 45.Jones RM, Lu L, Helgeson R, Bergstedt TS, McBranch DW, Whitten DG. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14769–14772. doi: 10.1073/pnas.251555298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu L, Helgeson R, Jones RM, McBranch D, Whitten D. J. Am. Chem. Soc. 2001;124:483–488. doi: 10.1021/ja011517t. [DOI] [PubMed] [Google Scholar]
- 47.Wang K, Tang Z, Yang CJ, Kim Y, Fang X, Li W, Wu Y, Medley CD, Cao Z, Li J, Colon P, Lin H, Tan W. Angew. Chem.,Int. Ed. 2009;48:856–870. doi: 10.1002/anie.200800370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tyagi S, Kramer FR. Nat. Biotechnol. 1996;14:303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- 49.Dubertret B, Calame M, Libchaber AJ. Nat. Biotechnol. 2001;19:365–370. doi: 10.1038/86762. [DOI] [PubMed] [Google Scholar]
- 50.Song SP, Liang ZQ, Zhang J, Wang LH, Li GX, Fan CH. Angew. Chem., Int. Ed. 2009;48:8670–8674. doi: 10.1002/anie.200901887. [DOI] [PubMed] [Google Scholar]
- 51.Shukla R, Bansal V, Chaudhary M, Basu A, Bhonde RR, Sastry M. Langmuir. 2005;21:10644–10654. doi: 10.1021/la0513712. [DOI] [PubMed] [Google Scholar]
- 52.Jayagopal A, Halfpenny KC, Perez JW, Wright DW. J. Am. Chem. Soc. 2010;132:9789–9796. doi: 10.1021/ja102585v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia F, Zuo X, Yang R, Xiao Y, Kang D, Vallee-Belisle A, Gong X, Yuen JD, Hsu BBY, Heeger AJ, Plaxco KW. Proc. Natl. Acad. Sci. U. S. A. 2010;107:10837–10841. doi: 10.1073/pnas.1005632107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Wang D, Miller EK, Moses D, Bazan GC, Heeger AJ. Macromolecules. 2000;33:5153–5158. [Google Scholar]
- 55.De M, Rana S, Akpinar H, Miranda OR, Arvizo RR, Bunz UHF, Rotello VM. Nat. Chem. 2009;1:461–465. doi: 10.1038/nchem.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verma A, Rotello VM. Chem. Commun. 2005:303–312. doi: 10.1039/b410889b. [DOI] [PubMed] [Google Scholar]
- 57.Phillips RL, Miranda OR, You C-C, Rotello VM, Bunz UHF. Angew. Chem., Int. Ed. 2008;47:2590–2594. doi: 10.1002/anie.200703369. [DOI] [PubMed] [Google Scholar]
- 58.Bajaj A, Miranda OR, Kim I-B, Phillips RL, Jerry DJ, Bunz UHF, Rotello VM. Proc. Natl. Acad. Sci. U. S. A. 2009;106:10912–10916. doi: 10.1073/pnas.0900975106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.You C-C, Miranda OR, Gider B, Ghosh PS, Kim I-B, Erdogan B, Krovi SA, Bunz UHF, Rotello VM. Nat. Nanotechnol. 2007;2:318–323. doi: 10.1038/nnano.2007.99. [DOI] [PubMed] [Google Scholar]
- 60.Pons T, Medintz IL, Sapsford KE, Higashiya S, Grimes AF, English DS, Mattoussi H. Nano Lett. 2007;7:3157–3164. doi: 10.1021/nl071729+. [DOI] [PubMed] [Google Scholar]
- 61.Oh E, Hong M-Y, Lee D, Nam S-H, Yoon HC, Kim H-S. J. Am. Chem. Soc. 2005;127:3270–3271. doi: 10.1021/ja0433323. [DOI] [PubMed] [Google Scholar]
- 62.Alivisatos AP. Science. 1996;271:933–937. [Google Scholar]
- 63.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu PP, Chen LQ, Liu C, Zhen SJ, Xiao SJ, Peng L, Li YF, Huang CZ. Chem. Commun. 2010;46:8285–8287. doi: 10.1039/c0cc02600j. [DOI] [PubMed] [Google Scholar]
- 65.Belay ED. Annu. Rev. Microbiol. 1999;53:283–314. doi: 10.1146/annurev.micro.53.1.283. [DOI] [PubMed] [Google Scholar]
- 66.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Small. 2005;1:325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 67.Lee H, Lee K, Kim IK, Park TG. Biomaterials. 2008;29:4709–4718. doi: 10.1016/j.biomaterials.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 68.Bresnihan B. J. Rheumatol. 1999;26:717–719. [PubMed] [Google Scholar]
- 69.McInnes IB, Schett G. Nat. Rev. Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 70.Liu D, Pearlman E, Diaconu E, Guo K, Mori H, Haqqi T, Markowitz S, Willson J, Sy MS. Proc. Natl. Acad. Sci. U. S. A. 1996;93:7832–7837. doi: 10.1073/pnas.93.15.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schumacker PT. Cancer Cell. 2006;10:175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 72.Turk B. Nat. Rev. Drug Discovery. 2006;5:785–799. doi: 10.1038/nrd2092. [DOI] [PubMed] [Google Scholar]
- 73.Kessenbrock K, Plaks V, Werb Z. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huschka R, Neumann O, Barhoumi A, Halas NJ. Nano Lett. 2010;10:4117–4122. doi: 10.1021/nl102293b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roy R, Hohng S, Ha T. Nat. Methods. 2008;5:507–516. doi: 10.1038/nmeth.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]