Abstract
Peripheral inflammation or nerve injury induces a primary afferent barrage into the spinal cord, which can cause N-methyl -aspartate (NMDA) receptor-dependent alterations in the responses of dorsal horn sensory neurons to subsequent afferent inputs. This plasticity, such as “wind-up” and central sensitization, contributes to the hyperexcitability of dorsal horn neurons and increased pain-related behavior in animal models, as well as clinical signs of chronic pain in humans, hyperalgesia and allodynia. Binding of NMDA receptor subunits by the scaffolding protein postsynaptic density protein-95 (PSD-95) can facilitate downstream intracellular signaling and modulate receptor stability, contributing to synaptic plasticity. Here, we show that spinal delivery of the mimetic peptide Tat-NR2B9c disrupts the interaction between PSD-95 and NR2B subunits in the dorsal horn and selectively reduces NMDA receptor-dependent events including wind-up of spinal sensory neurons, and both persistent formalin-induced neuronal activity and pain-related behaviors, attributed to central sensitization. Furthermore, a single intrathecal injection of Tat-NR2B9c in rats with established nerve injury-induced pain attenuates behavioral signs of mechanical and cold hypersensitivity, with no effect on locomotor performance. Thus, uncoupling of PSD-95 from spinal NR2B-containing NMDA receptors may prevent the neuronal plasticity involved in chronic pain and may be a successful analgesic therapy, reducing side effects associated with receptor blockade.
Introduction
Elevated responsiveness of spinal dorsal horn neurons to sensory inputs, a phenomenon termed central sensitization, is one mechanism proposed to contribute to chronic pain states.1,2 Such plasticity occurs following prolonged noxious peripheral stimulation and involves activation of dorsal horn glutamatergic N-methyl -aspartate (NMDA) receptors, triggering intracellular signaling cascades within dorsal horn sensory neurons and inducing events such as “wind-up.” Many human descriptions of this are reported.3,4 Antagonists of NMDA receptors, such as ketamine, dextromethorphan and MK-801, block wind-up and display good analgesic performance in animal models of chronic pain,5,6,7 but their clinical use is limited by numerous intolerable side effects due to the essential functional contribution of NMDA receptors throughout the central nervous system.8,9,10 Subtype-specific drugs, such as those which selectively target NR2B subunit-containing receptors, have a significantly better side effect profile, while showing great promise as analgesics for neuropathic pain states.11,12 Exploring novel ways of modulating NMDA receptor activity without receptor blockade may be an even better analgesic strategy.
Postsynaptic density protein-95 (PSD-95) is a synaptic scaffolding protein containing three PDZ (postsynaptic density 95, discs large, and zonula occludens-1) domains, which allow for protein complex formation. The second PDZ domain can bind NR2 subunits of the NMDA receptor, which contain a PDZ-binding motif (tSXV) at the C-terminus.13 PSD-95 connects the receptor to intracellular effector proteins, such as neuronal nitric oxide synthase (nNOS),14 which has been shown to be involved in NMDA-dependent pain.15 Knockdown of PSD-95 in the spinal cord using antisense has demonstrated a role for this protein in both the development and maintenance of nerve injury-induced pain,16,17 while a cyclic peptide, which blocks binding interactions at each of the three PDZ domains of PSD-95, also reduces behavioral hypersensitivity following nerve injury.18
Although it is clear that NR2B-subtype NMDA receptors and PSD-95 are both individually involved in abnormal pain states, it remains to be seen whether a specific physical interaction between these two synaptic proteins is required for dorsal horn neuronal plasticity and the establishment of a chronic pain state, particularly following nerve injury. Here, for the first time, we have investigated the electrophysiological and behavioral effects of perturbing the specific interaction between spinal NR2B-containing NMDA receptors and PSD-95, using a peptide-mimetic strategy, in dorsal horn wind-up and formalin-induced central sensitization, two models of spinal nociceptive plasticity. In addition, we tested this exciting novel analgesic strategy within integrated systems in a rat model of chronic neuropathic pain.
Results
Tat-NR2B9c perturbs the interaction between NR2B subunits and PSD-95
Protein expression of PSD-95 was confirmed by western immunoblotting in hippocampus and spinal cord dorsal horn tissue. As expected, PSD-95 protein was not present in dorsal root ganglion (Figure 1a). Thus, in the spinal cord, PSD-95 is exclusively found in intrinsic dorsal horn sensory neurons and not in terminating primary afferent fibers.
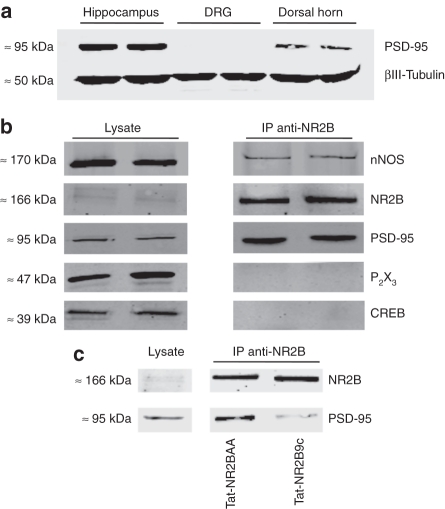
Figure 1.
Postsynaptic density protein-95 (PSD-95) forms a complex with NR2B-subtype receptors in dorsal horn neurons and binding to NR2B subunits is perturbed by Tat-NR2B9c. (a) Western immunoblot showing expression of PSD-95 in hippocampus (two left lanes), dorsal root ganglion (DRG) (two centre lanes), and spinal dorsal horn (two right lanes). βIII-tubulin served as a loading control. (b) Western immunoblots of normal lumbar spinal dorsal horn lysates (left two lanes) and coimmunoprecipitates from lumbar spinal dorsal horn lysate obtained using an antibody against NR2B (right two lanes), probed with antibodies against NR2B, neuronal nitric oxide synthase (nNOS), PSD-95, CREB, and P2X3. (c) Western immunoblots showing immunoprecipitates (IP, right lanes) from the lumbar dorsal horn of rats pretreated intrathecally with Tat-NR2BAA (125 ng) or Tat-NR2B9c (125 ng), 20 minutes before being sacrificed, obtained using an antibody against NR2B subunits (representative of four independent experiments). Note the marked reduction in PSD-95 coimmunoprecipitated with NR2B subunits from the dorsal horn of rats pretreated with Tat-NR2B9c. Left lane shows normal dorsal horn lysates with no immunoprecipitation, acting as positive controls.
We used immunoprecipitation to reveal physical coupling between spinal dorsal horn NR2B subunits and associated proteins using a specific antibody against this subunit (Figure 1b). As expected, PSD-95 protein was coimmunoprecipitated by the anti-NR2B antibody, as was nNOS. Normal spinal lysate was run alongside immunoprecipitation samples to act as positive controls, whereas CREB and P2X3 were probed as two negative control proteins, to ensure that the immunoprecipitation step had worked correctly. CREB is a nuclear protein and the P2X3 receptor is located exclusively on the central terminals of primary afferents, thus these two proteins should not be found in a postsynaptic, membrane-associated NR2B/PSD-95 complex. Accordingly, both CREB and P2X3 were only detected in normal spinal tissue lysates and not in immunoprecipitated samples, confirming that nonspecific detection of proteins in the immunoprecipitation sample had not occurred. Thus, PSD-95 and nNOS are physically coupled to NR2B-containing NMDA receptors in the dorsal horn.
In a separate experiment, rats were pretreated via intrathecal injection with either Tat-NR2BAA (125 ng) or Tat-NR2B9c (125 ng), 20 minutes before collection of lumbar dorsal horn tissue. PSD-95 protein was coimmunoprecipitated with NR2B subunits by the anti-NR2B antibody, as expected, from the dorsal horn of rats pretreated with the control peptide Tat-NR2BAA (Figure 1c), similar to that from naive rats. In contrast, coimmunoprecipitation of PSD-95 with NR2B subunits was markedly decreased in rats pretreated with the disrupting peptide Tat-NR2B9c (Figure 1c).
Thus, spinal delivery of Tat-NR2B9c perturbs interactions between PSD-95 and NR2B-containing NMDA receptors in the dorsal horn within 20 minutes. Since PSD-95 is only expressed within intrinsic dorsal horn neurons, the presence of PSD-95 protein in the immunoprecipitated sample indicates that the NR2B-associated protein complex is present within the same intrinsic dorsal horn neurons, suggesting that Tat-NR2B9c has an exclusively postsynaptic action in the dorsal horn.
Spinal administration of Tat-NR2B9c does not alter afferent-evoked responses but inhibits postdischarge of deep dorsal horn WDR neurons
Electrically evoked responses of deep dorsal horn wide dynamic range (WDR) neurons due to primary afferent activity were unaltered following spinal Tat-NR2B9c (12.5 ng, n = 6). No changes were seen in Aβ-fiber, Aδ-fiber or C-fiber evoked responses (Figure 2a). The control peptide Tat-NR2BAA (1.25 µg, n = 6) also had no effect on these evoked responses, at a dose 100× greater than that of the active peptide. WDR neurons continue to fire action potentials (APs) once the afferent barrage has ceased, a measure known as postdischarge, indicating the hyperexcitability of the cell. Tat-NR2B9c produced a significant and robust reduction of postdischarge (Figure 2a; percentage of predrug baseline = 45 ± 4 %, P < 0.01), whereas Tat-NR2BAA had no effect. Inhibitory effects of Tat-NR2B9c were evident by 20 minutes following spinal administration, peaked at ~40 minutes and persisted for the duration of the experiment. Predrug control responses did not differ between treatment groups (Supplementary Table S1).
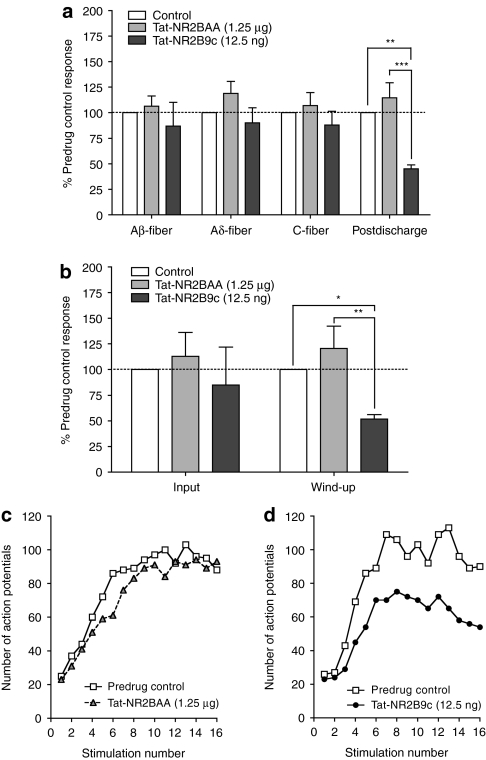
Figure 2.
Spinal Tat-NR2B9c decreases hyperexcitability of wide dynamic range (WDR) neurons. (a) Afferent-evoked responses and postdischarge of WDR neurons following transcutaneous electrical stimulation of the hindpaw receptive field, with spinal application of Tat-NR2BAA (1.25 µg) or Tat-NR2B9c (12.5 ng). (b) Effect of spinal Tat-NR2BAA (1.25 µg) or Tat-NR2B9c (12.5 ng) on input and wind-up of WDR neurons. White bars represent predrug control. Data presented as mean ± SEM of predrug baseline responses; *P < 0.05, **P < 0.01, ***P < 0.001 versus predrug baseline or control peptide; n = 6 in each group. (c,d) Examples of the wind-up of single WDR neurons following repetitive stimulation, and in the presence of spinal (c) Tat-NR2BAA (1.25 µg) or (d) Tat-NR2B9c (12.5 ng).
Wind-up of deep dorsal horn WDR neurons is reduced by spinal Tat-NR2B9c
The presynaptic “input” (nonpotentiated response) as well as the “wind-up” of WDR neurons were both assessed (Figure 2b). Wind-up is the NMDA-dependent increasing response of WDR neurons to repetitive noxious peripheral stimulation at constant intensity and frequency (Figure 2c,d). Both Tat-NR2B9c and Tat-NR2AA had no effect on input (Figure 2b). In contrast, Tat-NR2B9c significantly decreased wind-up of WDR neurons (Figure 2b; percentage of predrug baseline = 52 ± 4 %, P < 0.05), whereas Tat-NR2BAA had no effect.
Therefore, Tat-NR2B9c, believed to exert its effects by disruption of binding between PSD-95 and NR2B subunits, selectively reduces NMDA receptor-mediated postdischarge and wind-up of deep dorsal horn WDR neurons, while having no effect on afferent-evoked responses and input, indicating a postsynaptic mechanism of action.
Formalin-induced central sensitization of deep dorsal horn WDR neurons is reduced by spinal pretreatment with Tat-NR2B9c
Formalin was injected into the hindpaw receptive field and the firing response of single WDR neurons was recorded (Figure 3c). This stimulus produces an afferent drive with a delayed central NMDA component to the response. In control recordings, where no drug was applied, formalin induced a characteristic biphasic neuronal firing response (Figure 3a; 1st phase: 0–10 minutes, total APs = 9,632 ± 1,678; 2nd phase: 10–70 minutes, total APs = 64,071 ± 16,220; n = 11). Control pretreatment with spinal Tat-NR2BAA (1.25 µg, n = 11), 20 minutes before formalin injection, did not alter this response (Figure 3a–c; 1st phase total APs = 13,970 ± 2,228, P > 0.05; 2nd phase total APs = 54,483 ± 13,673, P > 0.05). Spinal pretreatment with Tat-NR2B9c (12.5 ng, n = 10) did, however, significantly and selectively reduce 2nd phase neuronal firing (Figure 3a–c; total APs = 9,695 ± 5,386, P < 0.001), attributed to central sensitization of spinal dorsal horn neurons.19,20 Only a small nonsignificant reduction of neuronal firing during the 1st phase was observed following Tat-NR2B9c pretreatment, likely reflecting the inhibitory effects seen previously on postdischarge and wind-up. All cells recorded were characterized before injection of drug and formalin to ensure that cells were comparable between treatment groups (Supplementary Table S2).
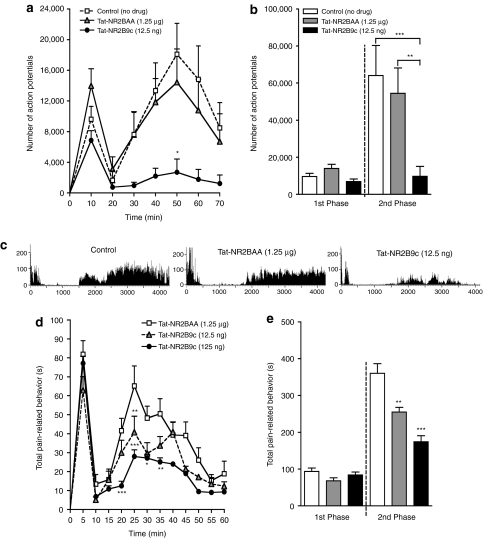
Figure 3.
Spinal Tat-NR2B9c reduces formalin-induced central sensitization. (a) Time course of firing of wide dynamic range (WDR) neurons following subcutaneous injection of formalin into the hindpaw receptive field with either no drug (control, n = 11) or following spinal pretreatment with Tat-NR2BAA (1.25 µg, n = 11) or Tat-NR2B9c (12.5 ng, n = 10, *P < 0.05 at 50 minutes versus Tat-NR2BAA). (b) Total neuronal activity during the 1st phase (0–10 minutes) and 2nd phase (10–70 minutes) of the response to formalin with no drug (control) or following spinal pretreatment with Tat-NR2AA or Tat-NR2B9c (2nd phase, ***P < 0.001, **P < 0.01 versus no drug and Tat-NR2BAA, respectively). (c) Example rate recordings of firing responses of WDR neurons to formalin with no drug (control) or following spinal pretreatment with Tat-NR2BAA (1.25 µg) or Tat-NR2B9c (12.5 ng). (d) Time course of pain-related behaviors following subcutaneous injection of formalin into the hindpaw following intrathecal pretreatment with Tat-NR2BAA (1.25 µg, n = 11) or Tat-NR2B9c (12.5 ng, n = 10, **P < 0.01 at 25 minutes versus Tat-NR2BAA; 125 ng, n = 6, ***P < 0.001 at 20, 25 minutes, *P < 0.05 at 30 minutes, **P < 0.01 at 35 minutes versus Tat-NR2BAA). (e) Total pain-related behavior during the 1st phase (0–10 minutes) and 2nd phase (10–60 minutes) of the response to formalin following spinal pretreatment with Tat-NR2AA or Tat-NR2B9c (2nd phase, 12.5 ng, **P < 0.01, 125 ng, ***P < 0.001 versus Tat-NR2BAA). All data presented as mean ± SEM.
Thus, spinal application of Tat-NR2B9c reduces NMDA-mediated components of formalin-induced central sensitization of deep dorsal horn WDR neurons.
Spinal Tat-NR2B9c inhibits pain-related behaviors due to formalin-induced central sensitization
Having observed the inhibitory effects of Tat-NR2B9c on neuronal activity related to spinal plasticity, we next sought to see whether similar effects could be found in a behavioral assay, again using formalin injected into the hindpaw of freely moving and awake rats. As with neuronal activity, formalin induced a biphasic response of pain-related behaviors in rats pretreated intrathecally with the control peptide Tat-NR2BAA (1.25 µg, n = 11) 20 minutes before formalin injection (Figure 3d,e; 1st phase: 0–10 minutes, total time of pain-related behavior = 94 ± 9 seconds; 2nd phase: 10–60 minutes, total time of pain-related behavior = 360 ± 26 seconds). Pretreatment with Tat-NR2Bc, however, significantly decreased pain-related behavior during the 2nd phase of the response in a dose-dependent manner (Figure 3d,e; 12.5 ng, n = 10, 2nd phase total time of pain-related behavior = 255 ± 13 seconds, P < 0.01; 125 ng, n = 6, 2nd phase total time of pain-related behavior = 174 ± 16 seconds, P < 0.001). No difference was seen during the 1st phase between either dose of Tat-NR2B9c and the control peptide (Figure 3d,e; 12.5 ng, 1st phase total time of pain-related behavior = 68 ± 8 seconds, P > 0.05; 125 ng, 1st phase total time of pain-related behavior = 84 ± 8 seconds, P > 0.05).
Further analysis of the different pain behaviors showed that rats injected with formalin spent most time licking and biting the injured paw, rather than lifting and flinching (Supplementary Figure S1). Licking and biting behavior alone also shows a strong biphasic response following pretreatment with Tat–NR2BAA (Supplementary Figure S1a; 1st phase: 0–10 minutes, total time of licking and biting behavior = 83 ± 10 seconds; 2nd phase: 10–60 minutes, total time of licking and biting behavior = 285 ± 23 seconds), which correlates exactly with the biphasic neuronal activity. Pretreatment with Tat-NR2B9c produced a significant and dose-dependent reduction of this licking and biting behavior during the 2nd phase of the response (Supplementary Figure S1b; 12.5 ng, 2nd phase total time of licking and biting behavior = 200 ± 18 seconds, P < 0.05; 125 ng, 2nd phase total time of licking and biting behavior = 124 ± 19 seconds, P < 0.001). In contrast, rats spent little time lifting and flinching and this behavior did not follow such an obvious biphasic time course (Supplementary Figure S1c). There was no difference in total lifting and flinching behavior in either the 1st or 2nd phase between Tat-NR2BAA and Tat-NR2B9c groups (Supplementary Figure S1d).
Thus, Tat-NR2B9c reduces formalin-induced pain-related behaviors that are associated with spinal central sensitization via a selective action on the active licking and biting responses rather than the more reflexive lifting and flinching.
Spinal Tat-NR2B9c does not alter afferent-evoked responses but inhibits postdischarge of deep dorsal horn WDR neurons in SNL and sham-operated rats
Spinal nerve ligation (SNL) induces mechanical and cold hypersensitivity of the injured paw,21 indicative of a neuropathic pain state. The effects of spinal administration of Tat-NR2B9c on electrically evoked responses of deep dorsal horn WDR neurons were compared in SNL and sham-operated rats (n = 8 in each group). Responses of WDR neurons due to primary afferent activity were unaltered in both groups following spinal Tat-NR2B9c (12.5 ng). No changes were seen in Aβ-fiber Aδ-fiber or C-fiber evoked responses (Figure 4a). Postdischarge, however, was reduced in both SNL and sham rats to a similar extent (Figure 4a; SNL: percentage of predrug baseline = 60 ± 11%, P < 0.01; sham: percentage of predrug baseline = 52 ± 13.0%, P < 0.01). This inhibitory effect of Tat-NR2B9c on the postdischarge of WDR neurons was similar to that seen in naive rats (Figure 2a). Predrug control responses did not differ between surgical groups (Supplementary Table S3).
Figure 4.
Spinal Tat-NR2B9c decreases hyperexcitability of wide dynamic range (WDR) neurons in both sham and spinal nerve ligation (SNL) rats. (a) Afferent-evoked responses and postdischarge of WDR neurons induced by transcutaneous electrical stimulation of the hindpaw receptive field in sham and SNL rats and following spinal application of Tat-NR2B9c (12.5 ng). (b) Effect of spinal application of Tat-NR2B9c (12.5 ng) on input and wind-up of WDR neurons in sham and SNL rats. White bars represent predrug control. Data presented as mean ± SEM of predrug control responses; **P < 0.01 versus predrug baseline; n = 8 in each group. (c,d) Examples of the wind-up of single WDR neurons following repetitive electrical stimulation, and in the presence of spinal Tat-NR2B9c (12.5 ng) in (c) sham and (d) spinal nerve ligation (SNL) rats.
Wind-up of deep dorsal horn WDR neurons is reduced by spinal Tat-NR2B9c in both SNL and sham-operated rats
As in naive rats, no effect was seen with Tat-NR2B9c on input (nonpotentiated response) in either SNL or sham-operated rats (Figure 4b). In contrast, Tat-NR2B9c produced a significant inhibition of wind-up in both surgery groups (Figure 4b–d; SNL: 12.5 ng, percentage of predrug baseline = 56 ± 10%, P < 0.01, n = 8; sham: 12.5 ng, percentage of predrug baseline = 54 ± 12%, P < 0.01, n = 8). Inhibition of wind-up was comparable between surgical groups and to that observed in naive rats (Figure 3b).
Therefore, Tat-NR2B9c selectively reduces NMDA receptor-mediated postdischarge and wind-up of deep dorsal horn WDR neurons, while having no effect on afferent-evoked responses and input, in both SNL and sham-operated rats. The inhibitory effect of Tat-NR2B9c on postdischarge and wind-up is not altered in neuropathy.
Responses of deep dorsal horn WDR neurons evoked by natural stimuli are reduced by spinal application of Tat-NR2B9c in SNL and sham-operated rats
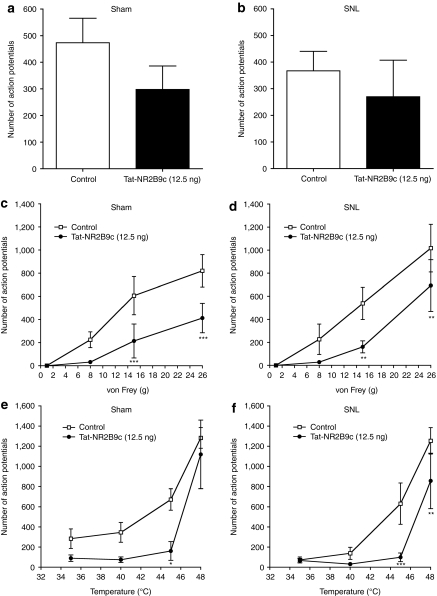
Next, the responses of deep dorsal horn WDR neurons to natural stimuli applied to the hindpaw receptive field were assessed. The predrug brush-evoked response was higher in sham rats compared to SNL rats, though this difference was not statistically significant (Supplementary Table S4; sham: mean number of APs = 473 ± 92, n = 6, SNL: mean number of APs = 367 ± 73, n = 6; P > 0.05). Spinal application of Tat-NR2B9c minimally reduced the response to brush in both surgical groups to a similar level, but this effect did not reach statistical significance (Figure 5a,b; 12.5 ng, n = 6 in each group, sham: APs = 298 ± 88, P > 0.05; SNL: APs = 270 ± 137; P > 0.05).
Figure 5.
Spinal Tat-NR2B9c reduces responses of wide dynamic range (WDR) neurons evoked by mechanical and thermal stimulation in both sham and spinal nerve ligation (SNL) rats. (a,b) Responses of WDR neurons to brush stimulation of the hindpaw receptive field in (a) sham and (b) SNL rats and following spinal application of Tat-NR2B9c (12.5 ng). (c,d) Responses of WDR neurons to mechanical stimulation of the hindpaw receptive field with von Frey hairs (1, 8, 15 and 26 g) in (c) sham and (d) SNL rats and following spinal application of Tat-NR2B9c (12.5 ng). (e,f) Responses of WDR neurons to thermal stimulation of the hindpaw receptive field with different temperatures of water (35, 40, 45 and 48 °C) in (e) sham and (f) SNL rats and following spinal application of Tat-NR2B9c (12.5 ng). All data presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001; n = 6 in each group.
Mechanical punctate stimulation of the hindpaw was delivered by different von Frey filaments which produced graded responses of WDR neurons that did not differ significantly between SNL and sham-operated rats (Supplementary Table S4; n = 6 in each group; 1 g, sham: mean number of APs = 0.7 ± 0.5, SNL: APs = 0.3 ± 0.3; 8 g, sham: APs = 225 ± 68, SNL: APs = 227 ± 132; 15 g, sham: APs = 606 ± 165, SNL: APs = 539 ± 140; 26 g, sham: APs = 821 ± 140, SNL: APs = 1,017 ± 207). Spinal application of Tat-NR2B9c (12.5 ng, n = 6 in each group) reduced graded responses to mechanical stimulation in both SNL and sham rats (Figure 5c,d; 1 g, sham: APs = 0.0 ± 0.0, P > 0.05, SNL: APs = 0.2 ± 0.2, P > 0.05; 8 g, sham: APs = 32 ± 13, P > 0.05, SNL: APs = 28 ± 12, P > 0.05; 15 g, sham: APs = 214 ± 147, P < 0.001, SNL: APs = 161 ± 52, P < 0.01; 26 g, sham: APs = 413 ± 127, P < 0.001, SNL: APs = 693 ± 224, P < 0.01). The effects of Tat-NR2B9c were similar in both surgical groups.
Graded responses of WDR neurons to thermal stimulation, via a water jet of different temperatures, were similar in SNL and sham-operated rats (Supplementary Table S4; n = 6 in each group; 35 °C, sham: APs = 284 ± 97, SNL: APs = 73 ± 30; 40 °C, sham: APs = 346 ± 98, SNL: APs = 138 ± 60; 45 °C, sham: APs = 672 ± 106, SNL: APs = 631 ± 205; 48 °C, sham: APs = 1,282 ± 102, SNL: APs = 1,254 ± 131). Responses in both surgical groups were reduced by spinal application of Tat-NR2B9c (12.5 ng, n = 6 in each group) to a similar degree (Figure 5e,f; 35 °C, sham: APs = 89 ± 32, P > 0.05, SNL: APs = 67 ± 22, P > 0.05; 40 °C, sham: APs = 74 ± 27, P > 0.05, SNL: APs = 31 ± 11, P > 0.05; 45 °C, sham: APs = 161 ± 93, P < 0.05, SNL: APs = 99 ± 43, P < 0.001; 48 °C, sham: APs = 1,119 ± 340, P > 0.05, SNL: APs = 857 ± 274, P < 0.01).
Therefore, spinal administration of Tat-NR2B9c reduces graded responses of deep dorsal horn WDR neurons to mechanical and thermal stimulation in both SNL and sham-operated rats. Brush-evoked responses are minimally reduced.
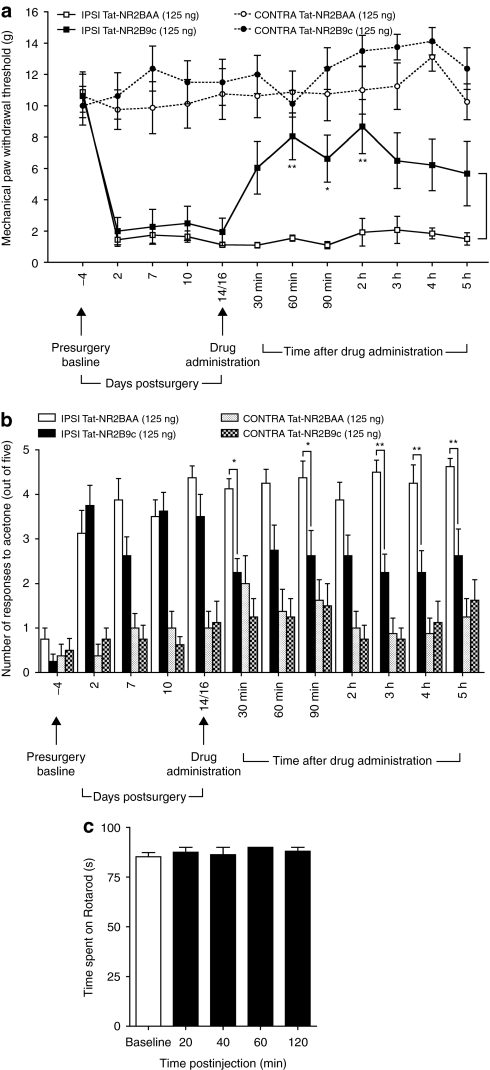
Nerve injury-induced behavioral mechanical and cold hypersensitivity is attenuated by spinal Tat-NR2B9c
In a behavioral study, paw withdrawal thresholds (PWTs) and number of responses to acetone of both hindpaws were measured in rats, 4 days before SNL surgery. Rats were assigned to Tat-NR2B9c (n = 8) or Tat-NR2BAA (n = 8) treatment groups in a randomized and blinded fashion. Baseline thresholds of ipsilateral and contralateral paws of rats in each group were identical (Figure 6a; Tat-NR2BAA group: ipsilateral PWT = 10.9 ± 1.3 g, contralateral PWT = 10.6 ± 1.4 g; Tat-NR2B9c group: ipsilateral PWT = 10.6 ± 1.4 g, contralateral PWT = 10.0 ± 1.2 g). Number of responses to acetone (out of five) was also comparable between hindpaws of rats in each group (Figure 6b; Tat-NR2BAA group: ipsilateral acetone responses = 0.8 ± 0.3, contralateral acetone responses = 0.4 ± 0.3; Tat-NR2B9c group: ipsilateral acetone responses = 0.3 ± 0.2, contralateral acetone responses = 0.5 ± 0.3).
Figure 6.
Intrathecal Tat-NR2B9c attenuates nerve injury-induced mechanical and cold hypersensitivity but does not affect locomotor performance. (a) Mechanical paw withdrawal thresholds (PWTs) of spinal nerve ligation (SNL) rats and (b) responses to acetone at both the ipsilateral (IPSI) and contralateral (CONTRA) hindpaws measured 4 days before SNL surgery and on postsurgical days 2, 7, 10, and 14 or 16. Rats received intrathecal Tat-NR2B9c (125 ng) or Tat-NR2BAA (125 ng) on day 14 or 16 and PWTs were reassessed at 30 minutes, 60 minutes, 90 minutes, 2 hours, 3 hours, 4 hours and 5 hours following drug administration. n = 8 in each group; *P < 0.05, **P < 0.01 versus ipsilateral Tat-NR2BAA group. (c) Time spent on the accelerating rotarod (i.e., latency to fall) before (baseline, white bar) and after intrathecal injection of Tat-NR2B9c (125 ng, n = 4, black bars). A cutoff time of 90 seconds was used. All data presented as mean ± SEM.
Assessment of PWTs and responses to acetone continued throughout the postoperative period. On postoperative day 2, PWTs of the ipsilateral paw of rats in each treatment group had significantly decreased, indicating mechanical hypersensitivity, whereas PWTs of the contralateral paws remained at baseline levels (Figure 6a; Tat-NR2BAA group: ipsilateral PWT = 1.5 ± 0.4 g, contralateral PWT = 9.8 ± 1.3 g, P < 0.001; Tat-NR2B9c group: ipsilateral PWT = 2.0 ± 0.9 g, contralateral PWT = 10.6 ± 1.5 g, P < 0.001). Similarly, rats developed cold hypersensitivity of the ipsilateral paw by day 2, as indicated by the greater number of responses to acetone compared to the contralateral paw (Figure 6b; Tat-NR2BAA group: ipsilateral acetone responses = 3.1 ± 0.5, contralateral acetone responses = 0.4 ± 0.3, P < 0.001; Tat-NR2B9c group: ipsilateral acetone responses = 3.8 ± 0.5, contralateral acetone responses = 0.8 ± 0.3, P < 0.001). These differences in PWTs and responses to acetone between ipsilateral and contralateral paws were maintained throughout the postoperative period, measured up to day 14 or 16, in both treatment groups (Figure 6a,b).
Following measurement of PWTs and acetone responses on day 14 or 16, either Tat-NR2BAA (125 ng) or Tat-NR2B9c (125 ng) was delivered spinally to rats via intrathecal lumbar puncture. PWTs and responses to acetone were then remeasured at various time points after treatment (Figure 6a,b). In the Tat-NR2BAA treatment group, there were no changes in PWTs or responses to acetone such that mechanical and cold hypersensitivity persisted throughout the testing period. In contrast, treatment with Tat-NR2B9c produced a reversal in both mechanical PWTs and responses to acetone of the ipsilateral hindpaw toward presurgery baseline levels and those of the contralateral paw. The ipsilateral PWTs of rats treated with Tat-NR2B9c were higher than those of rats treated with Tat-NR2BAA throughout the 5-hour testing period, with statistically significant differences seen from 60 minutes until 2 hours after treatment (Figure 6a; 60 minutes: Tat-NR2B9c group ipsilateral PWT = 8.1 ± 1.5 g, Tat-NR2BAA group ipsilateral PWT = 1.6 ± 0.2 g, P < 0.01; 90 minutes: Tat-NR2B9c group ipsilateral PWT = 6.6 ± 1.5 g, Tat-NR2BAA group ipsilateral PWT = 1.1 ± 0.2 g, P < 0.05; 2 hours: Tat-NR2B9c group ipsilateral PWT = 8.7 ± 1.7 g, Tat-NR2BAA group ipsilateral PWT = 1.9 ± 0.9 g, P < 0.01). Acetone responses of the ipsilateral hindpaw were lower in the Tat-NR2B9c group than the Tat-NR2BAA following treatment throughout the 5-hour testing period, with statistically significant differences at 30 minutes, 90 minutes, 3 hours, 4 hours and 5 hours after treatment (Figure 6b; 30 minutes: Tat-NR2B9c group ipsilateral acetone response = 2.3 ± 0.3, Tat-NR2BAA group ipsilateral acetone response = 4.1 ± 0.2, P < 0.05; 90 minutes: Tat-NR2B9c group ipsilateral acetone response = 2.6 ± 0.6, Tat-NR2BAA group ipsilateral acetone response = 4.4 ± 0.4, P < 0.05; 3 hours: Tat-NR2B9c group ipsilateral acetone response = 2.3 ± 0.4, Tat-NR2BAA group ipsilateral acetone response = 4.5 ± 0.3, P < 0.01; 4 hours: Tat-NR2B9c group ipsilateral acetone response = 2.3 ± 0.5, Tat-NR2BAA group ipsilateral acetone response = 4.3 ± 0.4, P < 0.01; 5 hours: Tat-NR2B9c group ipsilateral acetone response = 2.6 ± 0.6, Tat-NR2BAA group ipsilateral acetone response = 4.6 ± 0.2, P < 0.01).
Thus, intrathecal Tat-NR2B9c attenuates SNL-induced mechanical and cold behavioral hypersensitivity, suggesting that the physical interaction between NR2B-containing NMDA receptors and PSD-95 is required for the maintenance of nerve injury-induced neuropathic pain.
Spinal Tat-NR2B9c does not alter locomotor performance in the rotarod test
Performance on the rotarod was used to assess any adverse effects of spinal administration of Tat-NR2B9c on locomotor function of rats (Figure 6c). Rats were initially trained to remain on the rotarod for a minimum of 60 seconds and up to 90 seconds. Prior to administration of drug, rats spent an average of 85.3 ± 2.1 seconds on the rotarod. Intrathecal lumbar puncture treatment with Tat-NR2B9c (125 ng, n = 4) had no effect on rotarod performance during the 2-hour testing period following treatment (mean time spent on rotarod: 20 minutes = 87.5 ± 2.5 seconds, 40 minutes = 86.3 ± 3.8, 60 minutes = 90.0 ± 0.0, 2 hours = 88.0 ± 2.0).
Therefore, spinal delivery of Tat-NR2B9c does not affect locomotor performance in rats at a dose which reverses mechanical and behavioral hypersensitivity following spinal nerve ligation and also pain-related behaviors due to formalin-induced central sensitization. The analgesic effects of Tat-NR2B9c may thus be attributed to alterations specifically in sensory processing.
Discussion
Repetitive and prolonged noxious primary afferent stimulation, as elicited by transcutaneous electrical stimulation, formalin-induced inflammation and peripheral nerve damage, leads to NMDA receptor-mediated plastic changes in spinal neuronal activity, producing wind-up and central sensitization of dorsal horn sensory neurons, which may contribute to the manifestation of chronic pain states. We show that binding of PSD-95 to spinal NMDA receptors, composed specifically of NR2B subunits, is important for these sensitizing effects of NMDA receptor activation. Perturbing such interactions with the mimetic peptide, Tat-NR2B9c, reduces neuronal hyperexcitability and plasticity and also abnormal pain-related behaviors (Figure 7). In particular, our findings suggest that physical coupling between spinal NR2B-containing NMDA receptors and PSD-95 is crucial to the maintenance of nerve injury-induced neuropathic pain.
Figure 7.
Disruption of N-methyl -aspartate (NMDA)-mediated intracellular signaling via postsynaptic density protein-95 (PSD-95) by Tat-NR2B9c. Ca2+ influx via NMDA receptors can activate intracellular effectors located in the vicinity of the channel pore. Such effectors, for example neuronal nitric oxide synthase (nNOS), are physically linked to the receptor via the scaffolding protein PSD-95, which directly binds to NR2B subunits of the NMDA receptor via its second PDZ domain. The cytoplasmic tail of NR2B subunits contains the PSD-95-binding motif ESDV. Activation of specific effectors and pathways downstream of the NMDA receptor lead to mechanisms of spinal plasticity such as wind-up and central sensitization, and perhaps manifestation of chronic pain states such as nerve injury-induced neuropathic pain. The intracellular introduction of the peptide Tat-NR2B9c uncouples NMDA receptor activation from such potentially pathological downstream signaling pathways. Tat-NR2B9c binds to PSD-95, thus preventing PSD-95 from binding to NR2B subunits. However, receptor activation and Ca2+ influx can still occur, thereby possibly minimizing side effects.
Our novel electrophysiological data provide a neuronal substrate for the behavioral effects of disrupting interactions between NMDA receptors and PSD-95. Tat-NR2B9c inhibits postdischarge and wind-up, two measures of dorsal horn neuronal hyperexcitability and plasticity, identically in naive, sham and SNL rats. Primary afferent-evoked responses and presynaptic input were unaltered, suggesting a postsynaptic mechanism of action. These highly selective electrophysiological results are similar to those achieved by blocking NR2B-subtype receptors.11,22 Responses of WDR neurons evoked by mechanical and thermal stimulation of the hindpaw were inhibited by Tat-NR2B9c to the same extent in SNL and sham rats. These effects resemble those exerted by the NMDA antagonist MK-801 which inhibits postdischarge and wind-up as well as mechanically and thermally evoked responses of WDR neurons equally in both SNL and sham rats.23 A more recent study showed that the specific NR2B antagonist, Ro-256981, reduced C-fiber-evoked neuronal responses and blocked spinal LTP identically in naive and SNL rats.12 Since the neuronal effects of Tat-NR2B9c do not differ following neuropathy, it may seem that coupling between NR2B subunits and PSD-95 is also not altered. However, major changes in connectivity between primary afferents and spinal neurons have occurred following neuropathy. Two thirds of the input from the sciatic nerve has effectively been eliminated in the SNL model. Even within the L4 spinal segment, although afferent inputs are maintained, these are reduced because the ligated L5 and L6 spinal nerves cross-innervate this segment.24,25 Despite this, baseline responses of WDR neurons were similar in SNL and sham rats, suggesting that some compensation in spinal excitability has occurred in neuropathic rats to counter the loss of peripheral input. Whether such compensation involves altered interactions between NR2B-subtype receptors and PSD-95 remains to be elucidated.
Behavioral analyses confirmed that unilateral mechanical and cold hypersensitivity develops by day 2 following surgery in SNL rats and is maintained for the 2 weeks of testing. Spinal administration of Tat-NR2B9c, 2 weeks after surgery, via a single intrathecal injection, attenuated both mechanical and cold hypersensitivity of the injured paw, while the control peptide, Tat-NR2BAA, had no effect at all. PWTs were increased and responses to acetone were reduced toward baseline levels and those of the uninjured paw. These effects began 30 minutes after drug administration, consistent with the onset of biochemical disruption between PSD-95 and NR2B subunits, and lasted for the full 5-hour testing period. Reversal of nerve injury-induced mechanical and thermal hypersensitivity by an NR2B-selective antagonist also begins ~30 minutes after administration.12,26 Together, these results confirm the key role of spinal NR2B-subtype receptors in chronic neuropathic pain.
The contribution of PSD-95-mediated protein interactions to inflammatory pain was indicated through the use of an alternative peptide, Tat-PSD-95 PDZ2, which mimics the second PDZ domain of PSD-95.27 However, the analgesic effect of this peptide may involve disruption of binding of PSD-95 to any number of signaling proteins, including Shaker-type K+ channels28 and nNOS,29 which interact with PSD-95 via its second PDZ domain. A cyclic peptide, CN2097, which blocks interactions at all PDZ domains of PSD-95, has been shown to reduce neuropathic pain, though again, the particular binding proteins involved in this inhibitory effect were not identified.18 We are confident that our results are due to a more specific disruption of NR2B and PSD-95 interactions, since our Tat-NR2B9c peptide mimics the specific C-tail of NR2B subunits, so having no effect on the binding of PSD-95 to even NR2A subunits.30 We expect that other PSD-95 interactions would also remain intact.
The formalin model31 produces a biphasic response, electrophysiologically in single neurons, as well as behaviorally in awake animals. Tat-NR2B9c selectively reduced the 2nd phase of the formalin response, thought to represent central sensitization,19,20 an effect which mimics that of NR2B-subtype receptor antagonism and genetic knockdown.26,32 Surprisingly, mutant mice expressing a PSD-95 protein truncated at the third PDZ domain were found to display normal behavioral responses to formalin.33 However, the truncated protein, with the first two PDZ domains intact, may still be present at synapses since these first two domains of PSD-95 are required for synaptic targeting, while domain three is dispensable.34 In addition, the binding capacity at each individual PDZ domain independently contributes to synaptic clustering of the protein35 so that any loss of the third PDZ domain of PSD-95 may not affect its binding to NMDA receptors. Alternatively, if a truncated PSD-95 protein can no longer bind NR2 subunits or be clustered in synaptic regions, other related proteins may compensate for this loss. For example, PSD-93 is known to be important for inflammatory pain.36 Notably, Garry and colleagues33 measured only formalin-induced lifting and flinching behavior, a spinally mediated reflex response which we found to be infrequent and which was unaltered by Tat-NR2B9c. Licking and biting behavior, more of an active response requiring higher processing, predominated and this behavior exactly paralleled the neuronal firing and, significantly, was sensitive to inhibition by Tat-NR2B9c. Therefore, the seemingly conflicting results of these studies may simply reflect a difference in the behavioral end point measured. Interestingly, pain induced by intrathecal NMDA or nerve injury was reduced in these PSD-95 mutant mice,33 suggesting that individual PDZ domains of PSD-95 may contribute to different pain states.
Both the NR2B subunit and PSD-95 are distributed throughout the gray matter of the dorsal horn but are most densely concentrated in superficial laminae,37,38 ideally positioned to receive inputs from terminating nociceptors. We confirmed coimmunoprecipitation of PSD-95 and NR2B subunits in spinal neurons in accordance with previous work.39 The deep dorsal horn neurons we recorded most likely receive nociceptive inputs via interneurons,40,41 whereas a relatively smaller number may synapse directly with terminating nociceptors via their dorsally extending dendrites.42
Our electrophysiological studies showed that 12.5 ng of Tat-NR2B9c reduced wind-up by ~50% in all groups, suggesting that this dose was perhaps submaximal. Even at this dose, however, we saw some significant effects in the behavioral formalin test. Indeed a higher dose (125 ng) produced an even larger behavioral effect; therefore, we used this higher dose in the neuropathic pain behavioral study. Higher drug doses are often required in behavioral studies compared to electrophysiological experiments, since in behavioral studies smaller injection volumes, lower spatial access of the drug and movement of the animal limits the time for the drug to reach its target. In contrast, drug administration during electrophysiology is into a static system, i.e., a laminectomy exposing the surface of the cord with the dura removed, where minimal movement of fluid occurs for the whole duration of the recording and thus less removal of drug. Even at the higher behavioral dose, though, Tat-NR2B9c did not alter the performance of rats on the rotarod, indicating a lack of adverse locomotor effects which are commonly associated with conventional NMDA antagonists. Such drugs, for example MK-801 and ketamine, produce motor dysfunction at doses which are analgesic in the formalin test and neuropathic pain models.6,11
At present, the precise downstream signaling mechanisms by which coupling between NR2B-containing NMDA receptors and PSD-95 promotes nociceptive hypersensitivity are unknown. However, previous studies employing Tat-NR2B9c may suggest which intracellular molecules and pathways are involved. Tat-NR2B9c is neuroprotective and prevents the NMDA-dependent generation of nitric oxide in cortical cultures,30 while reducing glutamate-induced phosphorylation of the extracellular signal-regulated kinase in striatal neurons.43 Additionally, PSD-95 mutant mice fail to exhibit increased Ca2+/calmodulin-dependent kinase II activity following nerve injury.33 Both extracellular signal-regulated kinase and Ca2+/calmodulin-dependent kinase II have key roles in central sensitization.44,45 Phosphatidylinositol 3-kinase, another mediator of central sensitization,46 has also been shown to bind to PSD-95 in contributing to formalin-induced pain.47 Alternatively, binding of PSD-95 to NMDA subunits stabilizes the receptor in the cell membrane.48 Thus Tat-NR2B9c may decrease neuronal hyperexcitability by interfering with membrane insertion of NMDA receptors. However, the fact that Tat-NR2B9c does not affect NMDA-mediated excitatory postsynaptic currents or Ca2+ influx in the hippocampus may suggest otherwise.30 Disruption of downstream intracellular signaling is the more likely mechanism of action.
Importantly, we have demonstrated that NMDA receptor-dependent phenomena, such as wind-up and central sensitization, can be prevented without receptor blockade, a likely cause of the neurological side effects associated with NMDA antagonists. Tat-NR2B9c, a mimetic peptide which disrupts binding specifically between NR2B-containing NMDA receptors and PSD-95, reversed nerve injury-induced neuropathic pain without adverse locomotor effects. Therefore, mimetic peptides, particularly those targeting interactions within the NMDA receptor complex, should be investigated further as novel analgesics for the clinical treatment of chronic pain.
Materials and Methods
Animals. Experiments were conducted in adult male Sprague–Dawley rats (Central Biological Services, University College London, or Harlan, UK), housed in standard laboratory conditions with free access to food and water. Experimental procedures were approved by the UK Home Office and followed guidelines under the International Association for the Study of Pain.49
Synthesis of Tat-NR2B9c and Tat-NR2BAA peptides. Tat-NR2B9c peptide comprises the final nine amino acids of the NR2B subunit cytoplasmic tail (KLSSIESDV), including the PSD-95 PDZ domain-binding motif (ESDV). This sequence is conjugated to a HIV-1 virus coat protein, Tat, transduction domain (YGRKKRRQRRR), for intracellular delivery.50 The full peptide is 20 amino acids (YGRKKRRQRRRKLSSIESDV) in length. A control peptide, Tat-NR2BAA, was synthesized with a double substitution in the PDZ domain-binding motif (EADA), thereby rendering it unable to bind to its target and being inactive (YGRKKRRQRRRKLSSIEADA). Peptides were synthesized using an ACT 396 peptide synthesizer to >95% purity with high-performance liquid chromatography and mass spectrometry analysis (Wolfson Institute for Biomedical Research, University College London). Peptides were dissolved in phosphate-buffered saline.
In vivo electrophysiology—set-up. Rats (220–250 g) were anesthetized using 4–5% isoflurane (66% N2O and 33% O2) and, once areflexic, a tracheal cannula was inserted. Rats were placed in a stereotaxic frame and core body temperature was maintained at 37 °C using a feedback-heating blanket. Anesthesia was reduced to 2.5% isoflurane, and a laminectomy was performed at the L1–L3 vertebral level to expose the L4–L5 segments of the spinal cord. For the remainder of the experiment anesthesia was maintained at 1.5% isoflurane.
Extracellular recordings from single convergent deep dorsal horn (>600 µm) WDR neurons, identified by tapping at the hindpaw receptive field, were made using parylene-coated tungsten electrodes (A-M Systems, Sequim, WA). WDR neurons respond to both innocuous and noxious stimulation in a graded manner, and can respond to various stimulus modalities including mechanical, thermal, electrical and chemical. Data were captured and analyzed by a CED 1401 interface coupled to a Pentium computer with Spike 2 software (Cambridge Electronic Design, Cambridge, UK; PSTH and rate functions). In all electrophysiological experiments, “n” refers to the total number of cells recorded.
Electrical stimulation of hindpaw to induce wind-up. A train of 16 transcutaneous electrical stimuli (2 ms wide pulses, 0.5 Hz) was applied at three times the threshold current for C-fibers via two stimulating needles inserted under the skin of the hindpaw. A poststimulus histogram (Supplementary Figure S2) was constructed and Aβ- (0-20 ms), Aδ- (20–90 ms), and C-fiber (90–300 ms) evoked responses were separated and quantified on a basis of latency. Responses occurring after the C-fiber latency band were taken to be the postdischarge of the cell (300–800 ms). Input (nonpotentiated response) was quantified as the response after the first stimulus × 16. Wind-up was calculated as: (number of APs after 16 stimuli)—[number of AP after the first stimulus × 16 (i.e., input)]. Stable control responses to electrical stimuli were established at 10 minute intervals before drug administration. Following application of the drug, tests were continued at 10 minute intervals for a further 2 hours.
In vivo electrophysiology—SNL model. Electrophysiological recordings of WDR neurons were conducted in the left side of the dorsal horn, ipsilateral to the site of nerve injury, in both SNL and sham-operated animals on postoperative days 15–18. Rats weighed between 250 and 300 g at this point. Electrical and natural tests were conduced in separate animals to avoid overstimulation and sensitization of the sensory system. Only one cell was recorded per animal.
Transcutaneous electrical stimuli were applied as above. The latency band for C-fibers was increased to 90–350 ms to allow for the larger size of the animals. The latency for postdischarge was 350–800 ms. Input and wind-up were calculated as before. Drug was applied to the cord following the establishment of stable control responses and electrical tests were continued at 10 minute intervals for 1 hour.
In a separate set of SNL- and sham-operated animals, neuronal responses to natural stimuli were assessed. The hindpaw peripheral receptive field was stimulated using natural stimuli, which extended from the non-noxious to noxious range. Brushing of the receptive field for 10 seconds was used to produce a dynamic mechanical stimulus. Static mechanical stimulation was achieved by application of von Frey filaments 1, 8, 15, and 26 g (9.8, 78.5, 147.1 and 255 mN, respectively; Touch-Test, North Coast Medical, San Jose, CA). Filaments 1 and 8 g are considered innocuous while filaments 15 and 26 g are noxious. Each filament was applied in ascending order for 10 seconds. Next, the receptive field was stimulated thermally with the following temperatures: 35, 40, 45 and 48 °C. Temperatures including 45 °C and above are considered noxious. Heat was applied with a constant water jet onto the centre of the receptive field, again in ascending order for a period of 10 seconds/stimulus. Drug administration occurred following the establishment of stable control responses and natural tests (brush, von Frey filaments and heat) were continued at 20 minute intervals for 1 hour.
Neuronal formalin test. WDR neurons from adult rats (220–250 g) were characterized before formalin administration. Transcutaneous stimulating needles were used to electrically stimulate the receptive field (as above) and responses were monitored to thermal stimulation using a constant jet of water onto the centre of the receptive field to indicate a strong C-fiber innervation of the WDR neuron, which is required for the response to subcutaneous formalin.19
Following cell characterization, rats were pretreated with Tat-NR2B9c or Tat-NR2BAA, 20 minutes before the injection of formalin. A separate group of control animals received no drug. Formalin (5%, 50 µl) was prepared from a 37% formaldehyde solution and then injected subcutaneously into the hindpaw receptive field. The firing response of the WDR neuron was recorded for the subsequent 70 minutes after formalin injection. Activity was displayed as a rate recording and quantified in 10 minute time bins.
Behavioral formalin test. Rats (220–250 g) were placed in a Plexiglas box and were allowed to acclimatize for 30 minutes. Rats were then lightly anesthetized with isoflurane and injected intrathecally (lumbar puncture method) with either 10 µl of Tat-NR2B9c (12.5 ng, n = 10 or 125 ng, n = 6) or 10 µl of Tat-NR2BAA (1.25 µg, n = 11). Experimenters were blind to treatment for the whole testing period. Twenty minutes later, rats received a subcutaneous injection of 50 µl of formalin (5%) into the plantar surface of the right hindpaw. Lifting, flinching, licking, and biting of the injected paw were monitored by measuring the total duration of the response in seconds during the 60 minute period following formalin administration. Data was captured in 5 minute time bins.
SNL model of neuropathic pain. Male Sprague–Dawley rats (130–150 g; Central Biological Services, University College London, UK) underwent surgery for ligation of L5 and L6 spinal nerves, or sham operation21. Under isoflurane anesthesia (1:1 O2:N2O, 5% isoflurane for induction, 2% for maintenance), rats were placed in a prone position and the left paraspinal muscles were separated from the spinal processes at the L4–S2 vertebral level. Part of the L6 transverse process was carefully removed and the left L4–L6 spinal nerves were identified. The L5 and L6 spinal nerves were isolated and tightly ligated with 6–0 silk thread, distal to the dorsal root ganglion and proximal to the formation of the sciatic nerve. The procedure for sham operation was identical, except for the ligation of spinal nerves, which was omitted. Animals were allowed to recover in a warm chamber and were checked for signs of motor impairment, such as dragging or nonweight bearing of the affected hindpaw.
Behavioral assessment of mechanical and cold hypersensitivity. Four days before SNL surgery, PWTs and cold sensitivity were assessed for both hindpaws of rats. Following SNL surgery, testing was repeated in the postoperative period on days 2, 7, 10, and 14/16 to observe the development of mechanical and cold hypersensitivity in the ipsilateral injured paw versus the contralateral paw. Drug effects were assessed on days 14/16.
PWT. Mechanical hypersensitivity of SNL rats was detected by measuring PWTs. von Frey filaments were applied 10 times to the plantar surface of each hindpaw from below, in ascending order. The PWT was determined by recording the first von Frey filament to produce 5 positive responses out of 10. A positive response was withdrawal as well as licking and/or biting of the stimulated hindpaw, indicative of nocifensive behavior. A cutoff was set at 15 g (147.1 mN) to avoid tissue damage.
Acetone drop test. Cold hypersensitivity of SNL rats was assessed by the application of a single drop of acetone, five times, to the plantar surface of the ipsilateral and contralateral hindpaws. Acetone was delivered from below through a 10 cm piece of tubing connected to a 0.5 ml syringe. Each rat was given a score out of 5 for the number of positive responses for each hindpaw. A positive response was the same as with the application of von Frey filaments except that a short delay would often be noted between application of the acetone drop and foot withdrawal. This is due to the fact that the cooling stimulus is brought about by evaporation of the acetone drop.
On day 14/16, following initial sensory testing, animals were treated with either Tat-NR2B9c or Tat-NR2BAA (both 125 ng in a volume of 10 µl, n = 8 in each group) via intrathecal lumbar puncture. Sensory testing then continued and PWTs and responses to acetone were assessed at 30 minutes, 60 minutes, 90 minutes, 2 hours, 3 hours, 4 hours and 5 hours after intrathecal injection. Experimenters were blind to treatment for the full duration of the experiment.
Assessment of locomotor function—rotarod. Locomotor function and motor effects of drugs were assessed using an accelerating rotarod device (Rotarod 7750; Ugo Basile, Comerio, Italy). The apparatus was set to accelerate from 0 to 20 rpm over 60 seconds and the latency to fall was monitored with a maximum cutoff of 90 seconds. Rats (200–220 g) underwent a training period on days −3 and −1 before testing of drug effects in order to ensure that they could remain on the rotarod for a minimum of 60 seconds. Locomotor function was assessed following intrathecal administration of Tat-NR2B9c (125 ng, 10 µl, n = 4) via lumbar puncture. Tests were carried out before injection (baseline) and at 20, 40, 60 and 120 minutes postinjection.
Drug administration—in vivo electrophysiology. In all electrophysiological experiments, drugs were applied directly to the surface of the spinal cord of rats, after removal of any residual cerebrospinal fluid, in a volume of 50 µl, using a Hamilton syringe. Before drug administration, control values were obtained by averaging the responses from the last three neuronal tests.
Intrathecal drug administration for behavioral studies—lumbar puncture method. In all behavioral pharmacological studies, drugs were delivered via intrathecal injections performed by direct lumbar puncture. Briefly, rats were lightly anesthetized with isoflurane. Their backs were shaved and the skin was cleansed with gauze soaked in an antimicrobial solution. A 25-gauge needle connected to a syringe was then inserted into the subarachnoidal space between the spinal processes of the L5 and L6 lumbar vertebrae. Drug was injected in a volume of 10-µl, eliciting a tail-flick. The syringe was held in position for a few seconds after the injection.
Western immunoblotting and immunoprecipitation. Rats anesthetized with urethane were sacrificed by decapitation and fresh tissues (spinal dorsal horn, dorsal root ganglion, or hippocampus) were dissected out and snap frozen. Two additional groups of rats received Tat-NR2BAA (125 ng) or Tat-NR2B9c (125 ng) via intrathecal lumbar puncture, 20 minutes before being sacrificed and dissection of tissue, under urethane anesthesia. Tissue was subsequently homogenized in RIPA (radioimmunoprecipitation assay) buffer (50 mmol/l Tris–HCl pH 7.5, 150 mmol/l NaCl, 1 mmol/l EDTA, 1% NP-40, 0.1% SDS + 0.5% deoxycholic acid + complete protease inhibitor cocktail) using a glass homogenizer. Homogenates were then centrifuged at 14,000 rpm for 10 minutes at 4 °C and supernatants containing tissue lysates were collected. Spinal whole cell lysates were next titrated to determine their protein concentrations using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Cramlington, UK).
For western immunoblotting, laemmli loading buffer was added to dorsal horn, dorsal root ganglion and hippocampus protein lysates (40 µg) and samples were incubated at 70 °C for 30 minutes. Samples were then loaded onto 8% gels and separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis.
For immunoprecipitation, magnetic Dynabeads Protein G (Invitrogen, Paisely, UK) were washed and coupled with 5 µg of rabbit anti-NR2B antibody (06-600; Upstate, Lake Placid, NY) by incubation at room temperature for 40 minutes. Next 500 µg of spinal cord dorsal horn lysate was added to the antibody–dynabeads complex and allowed to incubate overnight for capture of target antigen. Captured protein was eluted from the dynabeads by resuspension in 40 µl of laemmli loading buffer and heating at 70 °C for 30 minutes. Samples were then separated on 8% gels by sodium dodecyl sulphate-polyacrylamide gel electrophoresis. Normal spinal lysate samples (40 µg) were run alongside immunoprecipitated samples to act as positive controls.
Following electrophoresis, proteins were transferred to nitrocellulose membranes, which were then incubated with primary antibody overnight at 4 °C. Antibodies used were mouse anti-NR2B (1:500, 75–101; Neuromab, Davis, CA), rabbit anti-PSD-95 (1:1,000, ab18258; Abcam, Cambridge, UK), rabbit anti-nNOS (1:3,000, 07-571; Millipore, Billerica, MA), rabbit anti-CREB (1:500, ab5803; Abcam), rabbit anti-P2X3 (1:500, ab10269; Abcam) and rabbit anti-neuronal βIII-tubulin (1:3,000, ab18207; Abcam), which served as a loading control. Following incubation with dye-linked donkey anti-rabbit IR800 or goat anti-mouse IR600 secondary antibody, proteins were revealed using the Odyssey fluorescence detection system (Licor, Cambridge, UK).
Statistical analysis. Data are presented as mean ± SEM. Effects of Tat-NR2B9c and Tat-NR2BAA following electrical stimulation of the hindpaw were assessed by one-way repeated measures analysis of variance (one-way RM ANOVA) followed by Bonferroni's multiple comparison post-tests. Cell characteristics were compared by Student's unpaired t-tests (wind-up studies and brush-evoked responses), one-way ANOVA followed by Bonferroni's post-test (neuronal formalin test) or two-way RM ANOVA (evoked responses to graded mechanical and thermal stimulation). Formalin neuronal and behavioral response time course data was compared between treatment groups and analyzed by two-way RM ANOVA followed by Bonferroni's post-test. Total activity in the 1st and 2nd phases was compared between treatment groups by quantifying the area under each curve and analyzed by one-way ANOVA followed by Bonferroni's post-test. Effects of Tat-NR2B9c following natural stimulation (von Frey and heat) of the hindpaw were assessed by two-way RM ANOVA followed by Bonferroni post-tests. Effects on brush-evoked responses were assessed using one-way RM ANOVA followed by Bonferroni post-tests. Two-way RM ANOVA followed by Bonferroni post-tests was used to compare PWTs and responses to acetone between ipsilateral and contralateral paws in SNL rats as well as effects of Tat-NR2B9c and Tat-NR2BAA. One-way RM ANOVA followed by Dunnett's post-test was used to assess effects of Tat-NR2B9c in the rotarod locomotor function test. Statistical analyses were carried out using GraphPad Prism v.4 software (GraphPad Software, La Jolla, CA).
SUPPLEMENTARY MATERIAL Figure S1. Intrathecal Tat-NR2B9c selectively reduces active pain-related licking and biting behaviors following intraplantar formalin. (a) Time course of licking and biting behavior after injection of formalin into the hindpaw following intrathecal pre-treatment with Tat-NR2BAA (1.25 μg, n = 10) or Tat-NR2B9c (12.5 ng, n = 10; 125 ng, n = 6). (b) Total licking and biting behavior during the 1st phase (0-10 min) and 2nd phase (10-60 min) of the response to formalin following spinal pre-treatment with Tat-NR2AA or Tat-NR2B9c (2nd phase, 12.5 ng, *P < 0.05, 125 ng, ***P < 0.001 versus Tat-NR2BAA). (c) Time course of lifting and flinching behavior after injection of formalin into the hindpaw following intrathecal pre-treatment with Tat-NR2BAA or Tat-NR2B9c. (d) Total licking and biting behavior during the 1st phase (0-10 min) and 2nd phase (10-60 min) of the response to formalin following spinal pre-treatment with Tat-NR2AA or Tat-NR2B9c. Note that due to the low incidence of lifting and flinching behaviors the vertical axes are scaled differently. All data presented as mean ± SEM. Figure S2. Representative poststimulus histogram (PSTH) of electrically evoked responses of a WDR neuron to electrical stimulation of the hindpaw. A train of 16 transcutaneous electrical stimuli (2 ms wide pulses, 0.5 Hz, 3 x C-fiber threshold) was applied to the receptive field via two stimulating needles inserted under the skin of the hindpaw. Aβ- (0-20 ms), Aδ- (20-90 ms) and C-fiber (90-300 ms) evoked responses were separated and quantified on a basis of latency. Responses occurring after the C-fiber latency band were taken to be the post-discharge (PD) of the cell (300-800 ms). Table S1. Predrug control responses induced by transcutaneous electrical stimulation of the hindpaw receptive field of WDR neurons in naive rats. There were no differences between cells recorded in each treatment group for any measure prior to drug delivery. All data are expressed as mean ± SEM. Table S2. Formalin electrophysiology experiment WDR cell characteristics. Cells were characterized prior to drug administration and formalin injection. There was no difference between cells recorded in any of the treatment groups. All data are expressed as mean ± SEM. Table S3. Predrug control responses induced by transcutaneous electrical stimulation of the hindpaw receptive field of WDR neurons in sham and SNL rats. There were no differences between cells recorded in each surgical group for any measure prior to treatment. All data are expressed as mean ± SEM. Table S4. Predrug control responses induced by natural stimulation of the hindpaw receptive field of WDR neurons in sham and SNL rats. There were no differences between cells recorded in each surgical group for any measure prior to drug treatment. All data are expressed as mean ± SEM.
Acknowledgments
This work was supported by the Wellcome Trust-funded London Pain Consortium (R.D., S.B.M. and A.H.D.). The authors declared no conflict of interest.
Supplementary Material
Intrathecal Tat-NR2B9c selectively reduces active pain-related licking and biting behaviors following intraplantar formalin. (a) Time course of licking and biting behavior after injection of formalin into the hindpaw following intrathecal pre-treatment with Tat-NR2BAA (1.25 μg, n = 10) or Tat-NR2B9c (12.5 ng, n = 10; 125 ng, n = 6). (b) Total licking and biting behavior during the 1st phase (0-10 min) and 2nd phase (10-60 min) of the response to formalin following spinal pre-treatment with Tat-NR2AA or Tat-NR2B9c (2nd phase, 12.5 ng, *P < 0.05, 125 ng, ***P < 0.001 versus Tat-NR2BAA). (c) Time course of lifting and flinching behavior after injection of formalin into the hindpaw following intrathecal pre-treatment with Tat-NR2BAA or Tat-NR2B9c. (d) Total licking and biting behavior during the 1st phase (0-10 min) and 2nd phase (10-60 min) of the response to formalin following spinal pre-treatment with Tat-NR2AA or Tat-NR2B9c. Note that due to the low incidence of lifting and flinching behaviors the vertical axes are scaled differently. All data presented as mean ± SEM.
Representative poststimulus histogram (PSTH) of electrically evoked responses of a WDR neuron to electrical stimulation of the hindpaw. A train of 16 transcutaneous electrical stimuli (2 ms wide pulses, 0.5 Hz, 3 x C-fiber threshold) was applied to the receptive field via two stimulating needles inserted under the skin of the hindpaw. Aβ- (0-20 ms), Aδ- (20-90 ms) and C-fiber (90-300 ms) evoked responses were separated and quantified on a basis of latency. Responses occurring after the C-fiber latency band were taken to be the post-discharge (PD) of the cell (300-800 ms).
Predrug control responses induced by transcutaneous electrical stimulation of the hindpaw receptive field of WDR neurons in naive rats. There were no differences between cells recorded in each treatment group for any measure prior to drug delivery. All data are expressed as mean ± SEM.
Formalin electrophysiology experiment WDR cell characteristics. Cells were characterized prior to drug administration and formalin injection. There was no difference between cells recorded in any of the treatment groups. All data are expressed as mean ± SEM.
Predrug control responses induced by transcutaneous electrical stimulation of the hindpaw receptive field of WDR neurons in sham and SNL rats. There were no differences between cells recorded in each surgical group for any measure prior to treatment. All data are expressed as mean ± SEM.
Predrug control responses induced by natural stimulation of the hindpaw receptive field of WDR neurons in sham and SNL rats. There were no differences between cells recorded in each surgical group for any measure prior to drug treatment. All data are expressed as mean ± SEM.
REFERENCES
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Dickenson AH., and, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987;26:1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- Price DD, Mao J, Frenk H., and, Mayer DJ. The N-methyl-D-aspartate receptor antagonist dextromethorphan selectively reduces temporal summation of second pain in man. Pain. 1994;59:165–174. doi: 10.1016/0304-3959(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Petersen-Felix S, Fischer M, Bak P, Bjerring P., and, Zbinden AM. The effect of N-methyl-D-aspartate antagonist (ketamine) on single and repeated nociceptive stimuli: a placebo-controlled experimental human study. Anesth Analg. 1995;81:63–68. doi: 10.1097/00000539-199507000-00013. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Hayes RL, Lu J, Mayer DJ., and, Frenk H. Intrathecal treatment with dextrorphan or ketamine potently reduces pain-related behaviors in a rat model of peripheral mononeuropathy. Brain Res. 1993;605:164–168. doi: 10.1016/0006-8993(93)91368-3. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Malmberg AB., and, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997;280:829–838. [PubMed] [Google Scholar]
- Ren K., and, Dubner R. NMDA receptor antagonists attenuate mechanical hyperalgesia in rats with unilateral inflammation of the hindpaw. Neurosci Lett. 1993;163:22–26. doi: 10.1016/0304-3940(93)90220-f. [DOI] [PubMed] [Google Scholar]
- Backonja M, Arndt G, Gombar KA, Check B., and, Zimmermann M. Response of chronic neuropathic pain syndromes to ketamine: a preliminary study. Pain. 1994;56:51–57. doi: 10.1016/0304-3959(94)90149-X. [DOI] [PubMed] [Google Scholar]
- McQuay HJ, Carroll D, Jadad AR, Glynn CJ, Jack T, Moore RA.et al. (1994Dextromethorphan for the treatment of neuropathic pain: a double-blind randomised controlled crossover trial with integral n-of-1 design Pain 59127–133. [DOI] [PubMed] [Google Scholar]
- Jørum E, Warncke T., and, Stubhaug A. Cold allodynia and hyperalgesia in neuropathic pain: the effect of N-methyl-D-aspartate (NMDA) receptor antagonist ketamine–a double-blind, cross-over comparison with alfentanil and placebo. Pain. 2003;101:229–235. doi: 10.1016/S0304-3959(02)00122-7. [DOI] [PubMed] [Google Scholar]
- Boyce S, Wyatt A, Webb JK, O'Donnell R, Mason G, Rigby M.et al. (1999Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: correlation with restricted localisation of NR2B subunit in dorsal horn Neuropharmacology 38611–623. [DOI] [PubMed] [Google Scholar]
- Qu XX, Cai J, Li MJ, Chi YN, Liao FF, Liu FY.et al. (2009Role of the spinal cord NR2B-containing NMDA receptors in the development of neuropathic pain Exp Neurol 215298–307. [DOI] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB., and, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Hillier BJ, Lim WA., and, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274:27467–27473. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- Kitto KF, Haley JE., and, Wilcox GL. Involvement of nitric oxide in spinally mediated hyperalgesia in the mouse. Neurosci Lett. 1992;148:1–5. doi: 10.1016/0304-3940(92)90790-e. [DOI] [PubMed] [Google Scholar]
- Tao F, Tao YX, Gonzalez JA, Fang M, Mao P., and, Johns RA. Knockdown of PSD-95/SAP90 delays the development of neuropathic pain in rats. Neuroreport. 2001;12:3251–3255. doi: 10.1097/00001756-200110290-00022. [DOI] [PubMed] [Google Scholar]
- Tao F, Tao YX, Mao P., and, Johns RA. Role of postsynaptic density protein-95 in the maintenance of peripheral nerve injury-induced neuropathic pain in rats. Neuroscience. 2003;117:731–739. doi: 10.1016/s0306-4522(02)00801-1. [DOI] [PubMed] [Google Scholar]
- LeBlanc BW, Iwata M, Mallon AP, Rupasinghe CN, Goebel DJ, Marshall J.et al. (2010A cyclic peptide targeted against PSD-95 blocks central sensitization and attenuates thermal hyperalgesia Neuroscience 167490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson AH., and, Sullivan AF. Subcutaneous formalin-induced activity of dorsal horn neurones in the rat: differential response to an intrathecal opiate administered pre or post formalin. Pain. 1987;30:349–360. doi: 10.1016/0304-3959(87)90023-6. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Vaccarino AL., and, Melzack R. Central nervous system plasticity in the tonic pain response to subcutaneous formalin injection. Brain Res. 1990;535:155–158. doi: 10.1016/0006-8993(90)91835-5. [DOI] [PubMed] [Google Scholar]
- Kim SH., and, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kovács G, Kocsis P, Tarnawa I, Horváth C, Szombathelyi Z., and, Farkas S. NR2B containing NMDA receptor dependent windup of single spinal neurons. Neuropharmacology. 2004;46:23–30. doi: 10.1016/s0028-3908(03)00339-3. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Matthews EA., and, Dickenson AH. Comparison of the effects of MK-801, ketamine and memantine on responses of spinal dorsal horn neurones in a rat model of mononeuropathy. Pain. 2001;91:101–109. doi: 10.1016/s0304-3959(00)00423-1. [DOI] [PubMed] [Google Scholar]
- Pinto V, Szûcs P, Derkach VA., and, Safronov BV. Monosynaptic convergence of C- and Adelta-afferent fibres from different segmental dorsal roots on to single substantia gelatinosa neurones in the rat spinal cord. J Physiol (Lond) 2008;586 Pt 17:4165–4177. doi: 10.1113/jphysiol.2008.154898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehab SA, Al-Marashda K, Al-Zahmi A, Abdul-Kareem A., and, Al-Sultan MA. Unmyelinated primary afferents from adjacent spinal nerves intermingle in the spinal dorsal horn: a possible mechanism contributing to neuropathic pain. Brain Res. 2008;1208:111–119. doi: 10.1016/j.brainres.2008.02.089. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Gilbert H, McCabe RT., and, Basbaum AI. Powerful antinociceptive effects of the cone snail venom-derived subtype-selective NMDA receptor antagonists conantokins G and T. Pain. 2003;101:109–116. doi: 10.1016/s0304-3959(02)00303-2. [DOI] [PubMed] [Google Scholar]
- Tao F, Su Q., and, Johns RA. Cell-permeable peptide Tat-PSD-95 PDZ2 inhibits chronic inflammatory pain behaviors in mice. Mol Ther. 2008;16:1776–1782. doi: 10.1038/mt.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Niethammer M, Rothschild A, Jan YN., and, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR.et al. (1996Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and α1-syntrophin mediated by PDZ domains Cell 84757–767. [DOI] [PubMed] [Google Scholar]
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW.et al. (2002Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions Science 298846–850. [DOI] [PubMed] [Google Scholar]
- Dubuisson D., and, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Tan PH, Yang LC, Shih HC, Lan KC., and, Cheng JT. Gene knockdown with intrathecal siRNA of NMDA receptor NR2B subunit reduces formalin-induced nociception in the rat. Gene Ther. 2005;12:59–66. doi: 10.1038/sj.gt.3302376. [DOI] [PubMed] [Google Scholar]
- Garry EM, Moss A, Delaney A, O'Neill F, Blakemore J, Bowen J.et al. (2003Neuropathic sensitization of behavioral reflexes and spinal NMDA receptor/CaM kinase II interactions are disrupted in PSD-95 mutant mice Curr Biol 13321–328. [DOI] [PubMed] [Google Scholar]
- Craven SE, El-Husseini AE., and, Bredt DS. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron. 1999;22:497–509. doi: 10.1016/s0896-6273(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Nonaka M, Doi T, Fujiyoshi Y, Takemoto-Kimura S., and, Bito H. Essential contribution of the ligand-binding β B/β C loop of PDZ1 and PDZ2 in the regulation of postsynaptic clustering, scaffolding, and localization of postsynaptic density-95. J Neurosci. 2006;26:763–774. doi: 10.1523/JNEUROSCI.2489-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX, Rumbaugh G, Wang GD, Petralia RS, Zhao C, Kauer FW.et al. (2003Impaired NMDA receptor-mediated postsynaptic function and blunted NMDA receptor-dependent persistent pain in mice lacking postsynaptic density-93 protein J Neurosci 236703–6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy GG, Watanabe M, Fukaya M., and, Todd AJ. Synaptic distribution of the NR1, NR2A and NR2B subunits of the N-methyl-d-aspartate receptor in the rat lumbar spinal cord revealed with an antigen-unmasking technique. Eur J Neurosci. 2004;20:3301–3312. doi: 10.1111/j.1460-9568.2004.03798.x. [DOI] [PubMed] [Google Scholar]
- Polgár E, Watanabe M, Hartmann B, Grant SG., and, Todd AJ. Expression of AMPA receptor subunits at synapses in laminae I-III of the rodent spinal dorsal horn. Mol Pain. 2008;4:5. doi: 10.1186/1744-8069-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX, Huang YZ, Mei L., and, Johns RA. Expression of PSD-95/SAP90 is critical for N-methyl-D-aspartate receptor-mediated thermal hyperalgesia in the spinal cord. Neuroscience. 2000;98:201–206. doi: 10.1016/s0306-4522(00)00193-7. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Malmberg AB., and, Basbaum AI. PKCγ contributes to a subset of the NMDA-dependent spinal circuits that underlie injury-induced persistent pain. J Neurosci. 2001;21:5321–5327. doi: 10.1523/JNEUROSCI.21-14-05321.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Nassar MA, Wood JN., and, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Woolf CJ., and, King AE. Physiology and morphology of multireceptive neurons with C-afferent fiber inputs in the deep dorsal horn of the rat lumbar spinal cord. J Neurophysiol. 1987;58:460–479. doi: 10.1152/jn.1987.58.3.460. [DOI] [PubMed] [Google Scholar]
- Yang L, Mao L, Tang Q, Samdani S, Liu Z., and, Wang JQ. A novel Ca2+-independent signaling pathway to extracellular signal-regulated protein kinase by coactivation of NMDA receptors and metabotropic glutamate receptor 5 in neurons. J Neurosci. 2004;24:10846–10857. doi: 10.1523/JNEUROSCI.2496-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Baba H, Brenner GJ., and, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- Fang L, Wu J, Lin Q., and, Willis WD. Calcium-calmodulin-dependent protein kinase II contributes to spinal cord central sensitization. J Neurosci. 2002;22:4196–4204. doi: 10.1523/JNEUROSCI.22-10-04196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet S, Marchand F, D'Mello R, Grist J, Clark AK, Malcangio M.et al. (2008Phosphatidylinositol 3-kinase is a key mediator of central sensitization in painful inflammatory conditions J Neurosci 284261–4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle MI, Komiyama NH, Delaney A, Coba M, Garry EM, Rosie R.et al. (2010The SH3 domain of postsynaptic density 95 mediates inflammatory pain through phosphatidylinositol-3-kinase recruitment EMBO Rep 11473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD., and, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A., and, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intrathecal Tat-NR2B9c selectively reduces active pain-related licking and biting behaviors following intraplantar formalin. (a) Time course of licking and biting behavior after injection of formalin into the hindpaw following intrathecal pre-treatment with Tat-NR2BAA (1.25 μg, n = 10) or Tat-NR2B9c (12.5 ng, n = 10; 125 ng, n = 6). (b) Total licking and biting behavior during the 1st phase (0-10 min) and 2nd phase (10-60 min) of the response to formalin following spinal pre-treatment with Tat-NR2AA or Tat-NR2B9c (2nd phase, 12.5 ng, *P < 0.05, 125 ng, ***P < 0.001 versus Tat-NR2BAA). (c) Time course of lifting and flinching behavior after injection of formalin into the hindpaw following intrathecal pre-treatment with Tat-NR2BAA or Tat-NR2B9c. (d) Total licking and biting behavior during the 1st phase (0-10 min) and 2nd phase (10-60 min) of the response to formalin following spinal pre-treatment with Tat-NR2AA or Tat-NR2B9c. Note that due to the low incidence of lifting and flinching behaviors the vertical axes are scaled differently. All data presented as mean ± SEM.
Representative poststimulus histogram (PSTH) of electrically evoked responses of a WDR neuron to electrical stimulation of the hindpaw. A train of 16 transcutaneous electrical stimuli (2 ms wide pulses, 0.5 Hz, 3 x C-fiber threshold) was applied to the receptive field via two stimulating needles inserted under the skin of the hindpaw. Aβ- (0-20 ms), Aδ- (20-90 ms) and C-fiber (90-300 ms) evoked responses were separated and quantified on a basis of latency. Responses occurring after the C-fiber latency band were taken to be the post-discharge (PD) of the cell (300-800 ms).
Predrug control responses induced by transcutaneous electrical stimulation of the hindpaw receptive field of WDR neurons in naive rats. There were no differences between cells recorded in each treatment group for any measure prior to drug delivery. All data are expressed as mean ± SEM.
Formalin electrophysiology experiment WDR cell characteristics. Cells were characterized prior to drug administration and formalin injection. There was no difference between cells recorded in any of the treatment groups. All data are expressed as mean ± SEM.
Predrug control responses induced by transcutaneous electrical stimulation of the hindpaw receptive field of WDR neurons in sham and SNL rats. There were no differences between cells recorded in each surgical group for any measure prior to treatment. All data are expressed as mean ± SEM.
Predrug control responses induced by natural stimulation of the hindpaw receptive field of WDR neurons in sham and SNL rats. There were no differences between cells recorded in each surgical group for any measure prior to drug treatment. All data are expressed as mean ± SEM.