Abstract
Diabetic retinopathy is a leading cause of blindness. The purpose of this study is to identify novel genetic loci associated with the sight threatening complications of diabetic retinopathy. We performed a meta-analysis of genome-wide association data for severe diabetic retinopathy as defined by diabetic macular edema or proliferative diabetic retinopathy in unrelated cases ascertained from two large, type I diabetic cohorts: the Genetics of Kidney in Diabetes (GoKinD) and the Epidemiology of Diabetes Intervention and Control Trial (EDIC) studies. Controls were other diabetic subjects in the cohort. A combined total of 2829 subjects (973 cases, 1856 controls) were studied on 2 543 887 single nucleotide polymorphisms (SNPs). Subjects with nephropathy were excluded in a sub-analysis of 281 severe retinopathy cases. We also performed an association analysis of 1390 copy number variations (CNVs) using tag SNPs. No associations were significant at a genome-wide level after correcting for multiple measures. The meta-analysis did identify several associations that can be pursued in future replication studies, including an intergenic SNP, rs476141, on chromosome 1 (P-value 1.2 × 10−7). The most interesting signal from the CNV analysis came from the sub-group analysis without nephropathy subjects and is rs10521145 (P-value 3.4 × 10−6) in the intron of CCDC101, a histone acetyltransferase. This SNP tags the copy number region CNVR6685.1 on chromosome 16 at 28.5 Mb, a gain/loss site. In summary, this study nominates several novel genetic loci associated with the sight-threatening complications of diabetic retinopathy and anticipates future large-scale consortium-based validation studies.
INTRODUCTION
Diabetic retinopathy is the number one cause of irreversible vision loss in working age adults in the developed world (1). Over time, almost all diabetic individuals will go on to develop diabetic retinopathy (2). By 2020, the prevalence of diabetic retinopathy is expected to almost double to ∼7.2 million within the USA alone (3). In raw numbers, it is currently estimated that over 300 000 people in the USA have vision-threatening complications from severe diabetic retinopathy (4). As the leading cause of irreversible vision loss in the working age population of the USA, diabetic retinopathy has an enormous impact from an economic perspective due to both healthcare and labor costs (5).
Most of the visual morbidity and attendant health care expense due to diabetic retinopathy can be attributed to its two severe manifestations: diabetic macular edema (DME) and proliferative diabetic retinopathy (PDR). The hallmark clinical manifestation of DME is a thickening of the central retina produced by extravascular transudation through breakdown of the blood retinal barrier. Focal laser photocoagulation treatment in DME generally is not able to improve vision but only decreases the rate of vision loss (6,7). PDR is heralded by the presence of secondary pathologic retinal angiogenesis induced by retinal hypoxia. PDR is treated by pan-retinal photocoagulation (PRP) that results in a reduction of hypoxic retinal tissue. While efficacious in preventing severe vision loss in PDR, PRP has a number of serious side effects (6,8). Thus, molecular approaches, by elucidating the underlying genetic determinants of diabetic retinopathy, should suggest more elegantly targeted forms of intervention and provide one of the fundamental keys for its secondary prevention.
Epidemiologic studies suggest that the severity of diabetic retinopathy closely correlates with the glycemic level and diabetes duration (9,10). There is mounting evidence indicating a large genetic contribution to diabetic retinopathy severity as well. For example, in a familial aggregation study of Mexican-Americans with type 2 diabetes, Hallman et al. (11) found that severe retinopathy in the proband was significantly associated with severe retinopathy in the siblings. A correlation also exists between the severity of retinopathy among affected family members and an increased risk of sight-threatening complications in relatives of affected subjects with an odds ratio close to 3 (12). Heritability estimates for the severe manifestations of diabetic retinopathy range from 25 to 50% or more (13,14). When a cohort of identical twins with diabetes was examined, 35 of 37 were found to have the same degree of retinopathy (15). Hence, these findings suggest that genetic variation significantly contributes to the severity of diabetic retinopathy.
For the study of diabetic retinopathy in both type 1 and type 2 diabetes, candidate gene studies were initially performed to evaluate molecules in the major mechanistic pathways thought to be important in the development of microvascular complications associated with diabetes. To date, candidate gene studies of these pathways have been inconclusive in linking them to the severity of diabetic retinopathy (16) with the exception of aldose reductase (17) and vascular endothelial growth factor (18).
Strong evidence exists that there is a significant genetic component to diabetic retinopathy severity; yet to date, few genes or pathways have reliably been associated with the development of this disorder. We employed a genome-wide association (GWA) approach to agnostically implicate those critical genes and biological pathways dysregulated in this condition. Analysis was performed in all subjects regardless of NEPHROPATHY status. Herein, we report a GWA meta-analysis for severe diabetic retinopathy in two large independent cohorts of type 1 diabetic subjects, presenting results for both the single nucleotide polymorphism (SNP) and copy number variation (CNV) association.
RESULTS
Between the two cohorts, we had access to genotypic and phenotypic information for 2829 Caucasian subjects with type 1 diabetes (Table 1). Individuals were stratified according to the presence of severe diabetic retinopathy. Subjects were examined for population substructure and no evidence of admixture was observed (19).
Table 1.
Comparison of GoKinD and EDIC cohorts
| Subjects | GoKinD | EDIC |
|---|---|---|
| Severe retinopathy total | 815 | 158 |
| Severe retinopathy and nephropathy | 672 | 15 |
| Severe retinopathy only | 143 | 138 |
| Controls | 803 (701) | 1053 (1014) |
| Basis for retinopathy phenotype | Self-reported laser treatment for diabetic eye disease | 7-standard field stereoscopic retinal photos graded by reading center according to ETDRS grading scale |
| Follow-up | None | 16 years |
Severe retinopathy cases were identified on the basis of prior laser photocoagulation for either DME or PDR. All individuals with documented nephropathy had persistent proteinuria or ESRD. ESRD status was unknown in five severe retinopathy cases from EDIC. Controls are all remaining subjects in the cohort. Controls in parentheses are the number of control subjects used in the sub-analysis that excluded individuals with nephropathy.
Table 1 presents data on Caucasian type 1 diabetic patients. Severe retinopathy is defined by the presence of focal laser treatment for DME or panretinal photocoagulation for PDR (20). All individuals with nephropathy were defined by the presence of persistent proteinuria or documented end-stage renal disease (ESRD). ESRD was defined by a history of kidney transplantation and/or the use of dialysis. Cases for each cohort were defined as those individuals with severe retinopathy. Controls were all other probands from the cohort for whom phenotypic and genotypic data were obtained. Epidemiology of Diabetes Intervention and Control Trial (EDIC) subjects received gold standard retinal phenotyping of Early Treatment Diabetes Retinopathy Study (ETDRS) photos along with substantial longitudinal follow-up.
We analyzed the data set for correlations between Genetics of Kidney in Diabetes (GoKinD) study variables and the presence of severe retinopathy (Table 2). As anticipated, duration of diabetes had a significant effect on the risk for severe retinopathy (P = 2.2 × 10−16). The subjects with severe retinopathy have a longer duration of diabetes (mean duration 30.7 versus 23.5 years). The distributions of HbA1c (glycated hemoglobin %) (cases: 7.510 ± 1.826; controls: 7.485 ± 1.282) also differ between subjects with and without severe retinopathy. Note that the difference is not in the mean (P = 0.88 for a two-tailed t-test) but in the variance (P < 10−10 for an F-test).
Table 2.
Demographic table
| GOKIND controls | GOKIND cases | GOKIND_pval | EDIC controls | EDIC cases | EDIC_pval | |
|---|---|---|---|---|---|---|
| Age | 37.846 ± 8.805 | 43.318 ± 6.670 | <2.1 × 10−16 | 33.243 ± 6.992 | 34.379 ± 6.814 | 0.06 |
| Gender | 57.8 ± 33.3% | 49.9 ± 50.0% | 4.2 × 10−3 | 47.6 ± 49.9% | 48.4 ± 50.1% | 0.87 |
| HbA1c | 7.485 ± 1.282 | 7.510 ± 1.826 | 0.88 | 8.064 ± 1.495 | 9.422 ± 1.722 | 2.8 × 10−16 |
| Duration of diabetes | 24.233 ± 7.410 | 32.164 ± 7.223 | <2.2 × 10−16 | 11.604 ± 4.713 | 15.273 ± 4.612 | 2.2 × 10−16 |
| Nephropathy | 4.2 ± 20.1% | 58 ± 49.4% | <2.2 × 10−16 | 8 ± 8.8% | 9.8 ± 29.8% | 7.2 × 10−7 |
Age and duration presented in years. Gender expressed as percentage of female subjects. HbA1c is expressed as a percentage of glycated hemoglobin. All individuals with documented nephropathy were defined by persistent proteinuria or ESRD.
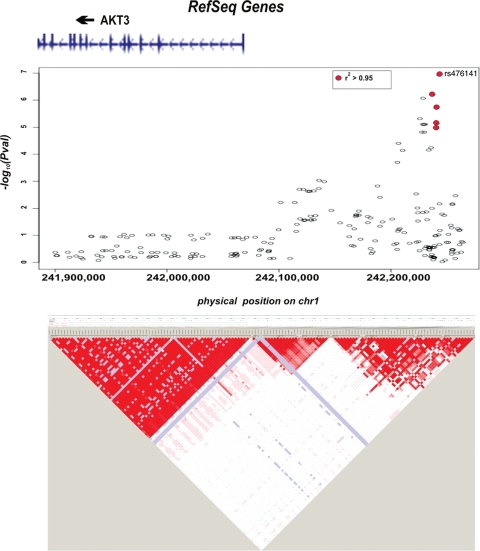
The GWA revealed three loci at a P-value < 10−6 (Fig. 1 and Table 3). Top signals in this study were at the level of 10−7 falling below the conventional genomic threshold of 5 × 10−8. The most significant SNP associated with severe diabetic retinopathy in the meta-analysis is rs476141 (P-value of 1.2 × 10−7) at 242Mb on chromosome 1 (Fig. 2).
Figure 1.
Manhattan plot summarizing results of the genome-wide association meta-analysis. Each dot represents a SNP, plotted by its chromosomal location (x-axis) and its associated P-value (y-axis). Hence, each point signifies an examined SNP's genomic location in the imputed data set plotted in relation to its P-value.
Table 3.
Severe retinopathy (all cases) GWA meta-analysis top associations
| Alleles | Chromosome | Genomic location | Gene | Left gene 3′-> 5′ | Right gene 3′-> 5′ | GoKind reference allele frequency | GoKinD OR | EDIC reference allele frequency | EDIC OR | Z-score | Meta OR | GOKIND_pval | EDIC_pval | META_pval |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A/C | 1 | 242243047 | NA | AKT3 | ZNF238 | 0.51 | 1.27 | 0.52 | 1.72 | 5.3 | 1.37 | 1.5 × 10−3 | 1.6 × 10−5 | 1.2 × 10−7 |
| A/G | 1 | 242236817 | NA | AKT3 | ZNF238 | 0.51 | 1.25 | 0.52 | 1.69 | 4.99 | 1.35 | 3.9 × 10−3 | 3.1 × 10−5 | 6.2 × 10−7 |
| A/G | 16 | 7355553 | A2BP1 | LOC100131413 | LOC100131080 | 0.83 | 0.73 | 0.81 | 0.59 | −4.98 | 0.68 | 8.0 × 10−4 | 2.1 × 10−4 | 6.4 × 10−7 |
| A/G | 3 | 158337436 | NA | LEKR1 | CCNL1 | 0.96 | 1.82 | 0.95 | 0.44 | −0.46 | 0.98 | 1.5 × 10−3 | 1.3 × 10−4 | 7.1 × 10−7 |
| A/G | 1 | 242228605 | NA | AKT3 | ZNF238 | 0.49 | 1.21 | 0.51 | 1.71 | 4.92 | 1.32 | 7.3 × 10−3 | 1.8 × 10−5 | 8.5 × 10−7 |
| C/T | 3 | 158433273 | NA | KRT18P34 | VEPH1 | 0.96 | 1.74 | 0.91 | 0.43 | −0.61 | 0.98 | 2.3 × 10−3 | 9.3 × 10−5 | 8.8 × 10−7 |
Top association signals from meta-analysis (P-value < 10−6) for severe retinopathy. Information for the SNP, genomic location, gene designation with most proximal upstream and downstream genes, associated meta P-values, Z-scores and odds ratios provided. Risk allele is given first with respective allele frequencies in each cohort. OR is odds ratio. Genomic location based on genome build 36.
Figure 2.
Plot of genomic region around the SNP rs476141. The figure reveals the location of most significant SNPs in relation to neighboring genes. SNPs in LD with the SNP rs476141 (r2 0.8–1.0) are colored in red. Gene location and exons are indicated above figure. Association in the region generated by Haploview is shown by LD plot below the figure. Figure created using genome build 36.
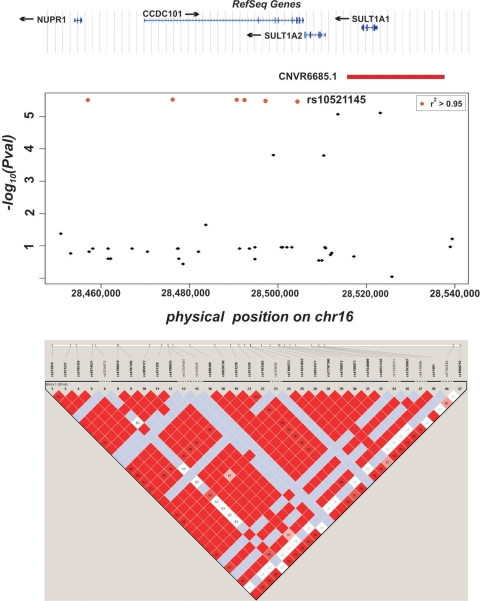
Sub-analysis was performed by removing the 828 total study subjects with nephropathy between cases and controls in order to enrich for a retinopathy-specific signal and control for the potential confounding effect of nephropathy (Table 4). The analysis included 281 severe retinopathy-only subjects and 1715 controls. It was compared with the main analysis in which the nephropathy subjects were included. The comparison was conducted in these groups in order to assess for genetic heterogeneity and pleiotropy. No overlap of signals was seen among the top SNPs (P < 10 × 10−5). Of the top signals (P-value < 10 × 10−5), no SNPs became smaller or achieved genome-wide significance with the exclusion of the subjects with nephropathy (Supplementary Material, Table S2). Likewise, of the top signals (P-value < 10 × 10−5) from the sub-analysis, no SNPs became smaller or achieved genome-wide significance by including the nephropathy subjects (Supplementary Material, Table S3). The most significant SNP associated with severe diabetic retinopathy in the sub-analysis is rs227455 (P-value of 1.6 × 10−7) at 165 Mb on chromosome 6. This SNP is in an intergenic region more than 200 kb from two undesignated genes, LOC728275 and LOC728316. The CNV analysis in the 281 severe retinopathy cases without nephropathy cases yielded an association at rs10521145 (Fig. 3) (P-value 3.4 × 10−6). It is in very strong linkage disequilibrium (LD) (r2 = 0.995) with the copy number region CNVR6685.1 on chromosome 16 at 28.5 Mb based on NCBI human genome build 36, a gain/loss site.
Table 4.
Top severe retinopathy associations when nephropathy subjects removed
| RS number | Alleles | Chromosome | Genomic location | Gene | Left gene 5′ -> 3′ | Right gene 3′->5′ | GoKinD reference allele frequency | GoKinD OR | EDIC reference allele frequency | EDIC OR | Z-score | Meta OR | GoKinD, P-value | EDIC, P-value | META, P-values |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs227455 | C/T | 6 | 165398041 | NA | LOC728275 | LOC728316 | 0.8599 | 0.561 | 0.8649 | 0.506 | −5.24234 | 0.53038 | 1.2 × 10−3 | 3.0 × 10−5 | 1.6 × 10−7 |
| rs227453 | A/T | 6 | 165398788 | NA | LOC728275 | LOC728316 | 0.856 | 0.582 | 0.8538 | 0.508 | −5.10967 | 0.54265 | 1.6 × 10−3 | 4.9 × 10−5 | 3.2 × 10−7 |
| rs151320 | A/G | 16 | 28476179 | CCDC101 | NUPR1 | SULT1A2 | 0.8492 | 0.571 | 0.8516 | 0.58 | −4.66382 | 0.57546 | 8.2 × 10−4 | 1.2 × 10−3 | 3.1 × 10−6 |
| rs151227 | C/T | 16 | 28457009 | NUPR1 | IL27 | CCDC101 | 0.8492 | 0.572 | 0.8517 | 0.579 | −4.65957 | 0.57547 | 8.4 × 10−4 | 1.1 × 10−3 | 3.2 × 10−6 |
| rs151229 | C/T | 16 | 28492438 | CCDC101 | NUPR1 | SULT1A2 | 0.849 | 0.57 | 0.8516 | 0.581 | −4.65710 | 0.57547 | 8.1 × 10−4 | 1.2 × 10−3 | 3.2 × 10−6 |
| rs151230 | C/T | 16 | 28490716 | CCDC101 | NUPR1 | SULT1A2 | 0.8491 | 0.57 | 0.8516 | 0.581 | −4.65709 | 0.57547 | 8.1 × 10−4 | 1.2 × 10−3 | 3.2 × 10−6 |
| rs11641853 | C/T | 16 | 28497220 | CCDC101 | NUPR1 | SULT1A2 | 0.849 | 0.57 | 0.8513 | 0.583 | −4.64733 | 0.57646 | 8.1 × 10−4 | 1.3 × 10−3 | 3.4 × 10−6 |
| rs10521145 | C/T | 16 | 28504385 | CCDC101 | NUPR1 | SULT1A2 | 0.8486 | 0.569 | 0.8514 | 0.582 | −4.64328 | 0.57546 | 8.3 × 10−4 | 1.3 × 10−3 | 3.4 × 10−6 |
| rs11871508 | A/G | 17 | 18644756 | FAM18B | FBXW10 | LOC100132391 | 0.9824 | 0.177 | 0.9776 | 0.269 | −4.57157 | 0.23806 | 3.0 × 10−3 | 4.7 × 10−4 | 4.8 × 10−6 |
| rs238252 | C/G | 13 | 41813654 | NA | AKAP11 | FABP3P2 | 0.1726 | 1.943 | 0.175 | 1.414 | 4.51521 | 1.66417 | 2.4 × 10−5 | 3.1 × 10−2 | 6.3 × 10−6 |
| rs11736136 | A/G | 4 | 83237298 | NA | COX5BL1 | LOC441026 | 0.8557 | 0.435 | 0.9035 | 0.635 | −4.51436 | 0.50705 | 1.1 × 10−5 | 4.7 × 10−2 | 6.4 × 10−6 |
| rs17684886 | A/T | 16 | 73644376 | ZNRF1 | WDR59 | LDHD | 0.8693 | 0.5 | 0.8892 | 0.628 | −4.50131 | 0.55931 | 1.3 × 10−4 | 1.1 × 10−2 | 6.8 × 10−6 |
| rs737141 | A/G | 20 | 17277725 | PCSK2 | OTOR | TCTE1P | 0.8594 | 1.866 | 0.8464 | 2.408 | 4.48800 | 2.11632 | 7.8 × 10−3 | 2.3 × 10−4 | 7.2 × 10−6 |
| rs238250 | A/C | 13 | 41812646 | NA | AKAP11 | FABP3P2 | 0.1631 | 1.988 | 0.1696 | 1.39 | 4.47351 | 1.65677 | 2.1 × 10−5 | 3.8 × 10−2 | 7.7 × 10−6 |
| rs11074904 | C/T | 16 | 28523209 | SULT1A1 | SULT1A2 | SULT1A1 | 0.8624 | 0.535 | 0.861 | 0.578 | −4.47057 | 0.55736 | 1.1 × 10−3 | 2.3 × 10−3 | 7.8 × 10−6 |
| rs4941432 | A/G | 13 | 41996347 | NA | FABP3P2 | TNFSF11 | 0.2785 | 1.884 | 0.2802 | 1.41 | 4.46850 | 1.62069 | 5.5 × 10−5 | 2.2 × 10−2 | 7.9 × 10−6 |
| rs17670074 | A/C | 10 | 19745389 | C10orf112 | RP11-49L2.2 | LOC100131155 | 0.407 | 2.047 | 0.4149 | 1.577 | 4.46360 | 1.73963 | 5.2 × 10−4 | 4.5 × 10−3 | 8.1 × 10−6 |
| rs12267418 | A/G | 10 | 19754560 | C10orf112 | RP11-49L2.2 | LOC100131155 | 0.9077 | 0.343 | 0.902 | 0.574 | −4.45932 | 0.49570 | 5.3 × 10−4 | 4.5 × 10−3 | 8.2 × 10−6 |
| rs11647881 | G/T | 16 | 28513674 | SULT1A2 | CCDC101 | SULT1A1 | 0.8763 | 0.496 | 0.8707 | 0.585 | −4.45032 | 0.54208 | 5.6 × 10−4 | 4.4 × 10−3 | 8.6 × 10−6 |
| rs4942139 | A/G | 13 | 41996328 | NA | FABP3P2 | TNFSF11 | 0.2786 | 1.872 | 0.281 | 1.392 | 4.44663 | 1.60165 | 5.4 × 10−5 | 2.4 × 10−2 | 8.7 × 10−6 |
| rs2025650 | A/T | 13 | 41996125 | NA | FABP3P2 | TNFSF11 | 0.2788 | 1.862 | 0.2814 | 1.38 | 4.43208 | 1.58697 | 5.4 × 10−5 | 2.6 × 10−2 | 9.3 × 10−6 |
| rs9888035 | C/T | 10 | 19755024 | C10orf112 | RP11-49L2.2 | LOC100131155 | 0.9081 | 0.34 | 0.9015 | 0.573 | −4.43169 | 0.49585 | 6.3 × 10−4 | 4.4 × 10−3 | 9.3 × 10−6 |
| rs17063155 | C/G | 13 | 41763197 | AKAP11 | DGKH | FABP3P2 | 0.1516 | 1.964 | 0.168 | 1.398 | 4.42051 | 1.64826 | 3.6 × 10−5 | 3.4 × 10−2 | 9.8 × 10−6 |
Top association signals from meta-analysis excluding nephropathy subjects (P-value < 10−5). Information for the SNP, genomic location, gene designation with most proximal upstream and downstream genes, associated meta P-values, Z-scores and odds ratios provided. Risk allele is given first with respective allele frequencies in each cohort. OR is odds ratio. Genomic location based on genome build 36.
Figure 3.
Plot of the genomic region around CNVR6685.1. The figure reveals the location of the SNP rs10521145 (arrow) in relation to SULT1A1, SULT1A2, CCDC101 and CNVR6685.1. SNPs in LD with rs10521145 (r2 0.8–1.0) are colored in red. Gene location and exons are indicated above figure. Association in the region generated by Haploview is shown by LD plot below the figure. Figure created using genome build 36.
DISCUSSION
The most significant association for severe diabetic retinopathy generated by the meta-analysis is the intergenic SNP rs476141 (P-value of 1.2 × 10−7). It is in a region between two genes, AKT3 and ZNF238 (Fig. 2). AKT3 is a particularly intriguing candidate gene. It is a serine/threonine kinase regulating cell survival, insulin signaling and angiogenesis. In particular, AKT3 has been shown to be activated by both platelet-derived growth factor and insulin-like growth factor 1 both of which have been implicated in proliferative retinopathy (21).
In the sub-analysis of the 281 severe diabetic retinopathy subjects without nephropathy, an association was identified with an intergenic SNP, rs227455 (P-value of 1.6 × 10−7) on chromosome 6. To date, 43% of all trait-associated SNPs from GWA studies are found in intergenic regions (22). Intergenic DNA often harbors transcriptional regulatory sites that include enhancers, repressors and miRNA. rs227455 has been associated with expression levels of other genes (eQTL) (23). Variation at this SNP is associated with expression of several genes in lymphoblastoid cell lines that include: BRCC3, P = 3 × 10−5; F2RL3, P = 4 × 10−5; IGLV6-57, P = 5 × 10−5; DGAT2, P = 9 × 10−5 and KRT8, P = 9 × 10−5 (24).
A recent study genotyped 3432 polymorphic CNVs to construct a database of tag SNPs for many of the known structural variants in the human genome (25). The information in the database allows the direct interrogation of CNVs using observed and imputed SNP genotypes (26). The CNV analysis in the retinopathy-only sub-analysis yielded an association at CNVR6685.1. An increased allele frequency of 1.5-fold for CNVR6685.1 is present in the severe retinopathy cases (SNP allele frequency: cases = 0.22 versus controls = 0.15). Two sulfotransferase genes, SULT1A1 and SULT1A2, that function in post-translational modifications of proteins lie inside the region. Also, there is an eQTL inside the copy number variant region (CNVR) that affects expression of CFL2 (P = 2 × 10−5) and PRSS33 (P = 8 × 10−5). The genes CCDC101 and NUPR1 are upstream and in LD with the region. In fact, rs10521145 which tags CNVR6685.1 lies within an intron of CCDC101, while rs151227 another tag SNP is found in an intron of NUPR1. Many other SNPs (P-value 10−6) from the meta-analysis (Fig. 3) are found in this interval. CCDC101 is involved in transcriptional regulation through histone acetyltransferase activity. NUPR1 has pleiotropic functions that relate to cellular stress response, including apoptosis, cell cycle progression and chromatin accessibility (27). A proximal gene to this locus is IL-27 (Table 2) that has been implicated in both type 1 diabetes as well as inflammatory bowel disease (28,29). No association was found between SNPs implicated in these conditions and severe retinopathy in this study (rs4788084, P = 0.04359; rs1968752, P = 0.10957; rs8049439, P = 0.10955). Moreover, while these three SNPs are all in high LD with each other (r2 > 0.69), none is in LD with rs10521145 (r2 < 0.08).
A major advantage of the study design is the comparability between cases and controls between the cohorts. A study composed of subjects all of the same ethnicity and diabetes type should help minimize heterogeneity, exposure to environmental differences and population substructure. In addition, by controlling for nephropathy in both cohorts, the ability to detect a retinopathy-specific genetic signal was increased and the potential confounding role of nephropathy was minimized. The presence of a second cohort allows the generation of a more accurate determination of the true effect of the variation by addressing the issue of the ‘winner's curse' (30). The second cohort also provides an extremely valuable resource from the standpoint of potentially refuting any positive associations identified in only one cohort. In this study, all of the top signals (meta-analysis P-value <10−6) reported in Tables 2 and 3 demonstrate a level of reproducibility in both cohorts (P-value < 0.05) increasing support for the validity of the associations.
There are a number of limitations of this study. Effect size for most significant associations for GWA studies to date has been on the order of a genetic relative risk (GRR) of 1.2. Hence, successful GWA studies typically require cohort sizes of close to 10 000 subjects (31). In comparison, this study had limited power to detect an allele whose GRR is <1.5. A greater number of subjects may also be necessary to further refine the regions associated with diabetic retinopathy than are currently available from the GoKinD and EDIC samples. Moreover, the size of this cohort limits the extent to which we can evaluate genome-wide the relationship between genetic variation and environmental factors as well as epistasis. This study was conducted only in subjects with type 1 diabetes. Its relevance to type 2 diabetes is debatable, as the underlying genetic architecture for type 1 and type 2 diabetes differs substantially. It remains to be seen whether this will also be the case for diabetic retinopathy as well. Hence, more extensive future studies will require the ascertainment of additional diabetes cohorts for the replication and extension of these findings.
MATERIALS AND METHODS
We performed two independent GWA studies. The GoKinD data set has phenotypic information from 1900 individuals with type 1 diabetes, who self-reported laser treatment for severe retinopathy. The cohort from the EDIC has 1441 subjects classified according to ETDRS scores. Each study participant in GoKinD was genotyped using the Affymetrix SNP Array 5.0. In EDIC, the Illumina platform was used for which 550K SNPs were available. Quality control steps ensured sample integrity, identified highly related samples and excluded population stratification. Allelic association tests were performed. The top SNPs from the analysis were based on statistical, bioinformatic and biologic criteria.
Institutional Review Board (IRB) approval
The samples were obtained from all subjects through an approved Institutional Review Board (IRB) protocol at the consenting institution. The IRB at the University of Chicago has declared this research is not human subject research as all patient health information was de-identified prior to genotyping and analysis. As such, this study falls under the purview of non-human subjects research.
Data repository
The data sets used for the analyses described in this paper can be obtained from the database of Genotypes and Phenotypes (dbGaP) at http://www.ncbi.nlm.nih.gov/gap/.
Phenotypic characterization
We restricted the analysis to Caucasian type 1 diabetic subjects in both cohorts thereby controlling for ethnicity and type of diabetes.
Severe retinopathy cases were identified on the basis of prior laser photocoagulation for either DME or PDR. Details are given below for each cohort. Controls are all remaining subjects in the cohort.
All individuals with the presence of nephropathy were removed from the retinopathy-only sub-group analysis. In GoKinD, nephropathy was defined by persistent proteinuria or documented ESRD. Persistent proteinuria was defined as at least two out of three tests positive for albuminuria (at least 1 month apart), i.e. dipstick (Albustix or Multistix) at least 1+ or an albumin/creatinine ratio value exceeding 300 μg albumin/mg of urine creatinine. ESRD was defined by a history of kidney transplantation and/or the use of dialysis. All subjects with ESRD were removed from EDIC for the sub-group analysis. Subjects with nephropathy were removed to reduce possible confounding given the close correlation between nephropathy and retinopathy and to facilitate assessment for genetic heterogeneity and pleiotropy (32,33).
GoKinD
All subjects were administered a detailed medical questionnaire that specifically inquired about a history of laser treatment for diabetic retinopathy. Patients reliably recall past laser treatment to their eyes (20). Individuals with severe retinopathy, as defined by prior laser treatment for either clinically significant macular edema or threshold PDR, are typically evaluated by an ophthalmologist prior to laser treatment to determine whether treatment criteria are met. Such examinations are presumed to have occurred in the course of routine clinical care external to this study. In a previous study, we validated the correlation of patient self-report for prior laser treatment with ‘severe' diabetic retinopathy as defined by the presence of either PDR or DME (20). We found that 99% of the participants were accurate in reporting whether they had prior laser treatment for diabetic eye disease (sensitivity 96.0%, specificity 99.5%, positive predictive value 95.6%). Our findings suggest that patient self-reported laser treatment for diabetic eye disease is a valid approach to identifying individuals with the sight-threatening manifestations of diabetic retinopathy.
We analyzed the data set for correlations between these GoKinD study variables and the presence of severe retinopathy as defined by subject self-reported laser treatment for diabetic eye disease.
EDIC
Retinopathy was assessed by fundus photography according to the DCCT/EDIC protocols semi-annually during the DCCT and during EDIC on the 8th, 12th and 16th anniversary of DCCT randomization, and at EDIC years 4 and 10. All photographs were graded centrally according to the final Early Treatment Diabetic Retinopathy Study (ETDRS) grading scale (34) and DCCT methods (35).
We analyzed the data set for correlations between these EDIC study variables and the presence of severe retinopathy as defined by photographic and clinical evidence of prior laser treatment for diabetic eye disease, including focal laser treatment and PRP.
Genotyping and quality control
Genotyping for all GoKinD subjects was conducted on the Affymetrix 5.0 platform. The platform has probe-sets for 500 568 SNPs and 420 000 additional non-polymorphic probes that can measure other variation such as CNV. The genotyping was done at the Broad Institute and the SNP genotypes were called in batches using Birdseed (36). This calling algorithm was run plate by plate on the data originally released from NCBI through dbGaP. Extensive QC analyses were performed on the data downloaded from NCBI, including detecting relatedness, sample contamination, deviations from the self-reported ethnicity and SNP level test such as the test for Hardy–Weinberg equilibrium, and analysis of plate effects. All related individuals were removed from the analysis (pi-hat threshold < 0.1). The detailed description of the QC steps can be found in the previously published paper (19) and in Supplementary Material, Figure S1. The QC process revealed a substantial plate effect caused by contaminated or degraded DNA samples that could be eliminated only by recalling the whole plate with contaminated/degraded samples (19).
In the EDIC cohort, subjects were genotyped on the Illumina HumanHap550 composed of ∼550 000 SNPs. The data were released through dbGap (http://www.ncbi.nlm.nih.gov/sites/entrez?db=gap) and quality control analyses were performed in the same fashion as for the GoKinD cohort as described above.
Statistical analyses
Association testing for severe diabetic retinopathy was conducted by comparing allele frequency between cases and controls using Chi-square tests. In both GoKinD and EDIC cohorts, the allelic association tests were done in two groups: (i) including subjects with nephropathy and (ii) excluding subjects with nephropathy.
The conventionally accepted threshold of 5 × 10−8 was used as the correction for multiple testing to account for all common variants in the genome (37).
To combine the results from the data on two different platforms, we performed a genome-wide imputation of Phase II HapMap SNPs using MACH (www.sph.umich.edu/csg/abecasis/Mach/). All the imputed SNPs were initially used in the analysis. The analysis of the imputed genotypes was done with the association software MACH2DAT (www.sph.umich.edu/csg/abecasis/Mach/). We have checked our top associations (P > 10−5) for the quality of imputation and kept only accurately imputed SNPs with allelic r2 value >0.98. For each SNP, in each data set, a Z-statistic having a standard normal distribution under the null hypothesis of no association was calculated. The Z-statistics preserve the direction of the effect, and they were used in a linear combination to calculate a meta-analysis test statistic. A normal approximation was then used to calculate the meta-analysis P-values. All meta-analysis P-values demonstrating an association with P < 10−5 for severe retinopathy are reported in either Table 3 or Supplementary Material, Table S1.
In an attempt to study CNVRs contributing to severe diabetic retinopathy, we compiled a list of CNVRs reported in the Wellcome Trust (WTCCC) (25). In all the analyses reported by WTCCC, human genome build 36 was used. We selected the CNVR tagging SNPs (with LD threshold of r2 = 0.8) reported by WTCCC that have been interrogated in our study in both GoKinD and EDIC cohorts. We found 1364 SNPs included in our analyses that tag 1390 CNVs previously reported in the WTCCC study.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by the National Institutes of Health (K08 EY019089-02, R01 DK077489); Diabetes Research and Training Center (P60 DK020595-32); Illinois Society for the Prevention of Blindness; Fight for Sight; OneSight; the Louis Block fund; and the Genetic Association Information Network.
Supplementary Material
ACKNOWLEDGEMENTS
We are indebted to the subjects and their families for their participation in the study; to Dr Graeme Bell for many helpful discussions; to two excellent anonymous reviewers whose critical input substantially improved the original manuscript and to Anish Patel, Mary Potkonjak and Fran Lietz for administrative and technical assistance.
We gratefully acknowledge support from the following organizations for this research: National Eye Institute, National Institute of Diabetes and Digestive and Kidney Diseases, Diabetes Research and Training Center P60 DK020595-32, Illinois Society for the Prevention of Blindness, Fight for Sight, OneSight, the Louis Block fund and GAIN (Genetic Association Information Network). The Genetics of Kidneys in Diabetes (GoKinD) Study was conducted by the GoKinD Investigators and supported by the Juvenile Diabetes Research Foundation, the CDC, and the Special Statutory Funding Program for type 1 Diabetes Research administered by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). This manuscript was not prepared in collaboration with Investigators of the GoKinD study and does not necessarily reflect the opinions or views of the GoKinD study or of the NIH.
Conflict of Interest statement. None declared.
REFERENCES
- 1.National Estimates on Diabetes. 2007. in National Diabetes Fact Sheet ed. CDC.
- 2.Klein R., Klein B.E.K., Moss S.E., Davis M.D., DeMets D.L. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch. Ophthalmol. 1984;102:527–532. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 3.National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) NIH; 2006. National Diabetes Information Clearinghouse: National Diabetes Statistics. [Google Scholar]
- 4.The Eye Diseases Prevalence Research Group. The prevalence of diabetic retinopathy among adults in the United States. Arch. Ophthalmol. 2004;122:552–563. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- 5.Prevention, C.f.D.C.a. 2007. National Estimates on Diabetes, in National Diabetes Fact Sheet CDC, Editor.
- 6.The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology. 1981;88:583–600. [PubMed] [Google Scholar]
- 7.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch. Ophthalmol. 1985;103:1796–1806. [PubMed] [Google Scholar]
- 8.Anonymous. Photocoagulation treatment of proliferative diabetic retinopathy: the second report of diabetic retinopathy study findings. Ophthalmology. 1978;85:82–106. doi: 10.1016/s0161-6420(78)35693-1. [DOI] [PubMed] [Google Scholar]
- 9.Klein B.E., Davis M.D., Segal P., Long J.A., Harris W.A., Haug G.A., Magli Y.L., Syrjala S. Diabetic retinopathy: assessment of severity and progression. Ophthalmology. 1984;91:10–17. doi: 10.1016/s0161-6420(84)34374-3. [DOI] [PubMed] [Google Scholar]
- 10.Klein R., Klein B.E.K., Moss S.E., Davis M.D., DeMets D.L. The Wisconsin Epidemiologic Study of Diabetic Retinopathy, III: prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch. Ophthalmol. 1984;102:527–532. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 11.Hallman D.M., Huber J.C., Jr, Gonzalez V.H., Klein B.E., Klein R., Hanis C.L. Familial aggregation of severity of diabetic retinopathy in Mexican Americans from Starr County Texas. Diabetes Care. 2005;28:1163–1168. doi: 10.2337/diacare.28.5.1163. [DOI] [PubMed] [Google Scholar]
- 12.Diabetes Control and Complications Trial Research Group. Clustering of long-term complications with diabetes in the diabetes control and complications trial. Diabetes. 1997;46:1829–1839. [PubMed] [Google Scholar]
- 13.Arar N.H., Freedman B.I., Adler S.G., Iyengar S.K., Chew E.Y., Davis M.D., Satko S.G., Bowden D.W., Duggirala R., Elston R.C., et al. Heritability of the severity of diabetic retinopathy: the FIND-Eye study. Invest. Ophthalmol. Vis. Sci. 2008;49:3839–3845. doi: 10.1167/iovs.07-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hietala K., Forsblom C., Summanen P., Groop P.H. Heritability of proliferative diabetic retinopathy. Diabetes. 2008;57:2176–2180. doi: 10.2337/db07-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leslie R.D., Pyke D.A. Diabetic retinopathy in identical twins. Diabetes. 1982;31:19–21. doi: 10.2337/diab.31.1.19. [DOI] [PubMed] [Google Scholar]
- 16.Warpeha K.M., Chakravarthy U. Molecular genetics of microvascular disease in diabetic retinopathy. Eye. 2003;17:305–311. doi: 10.1038/sj.eye.6700348. [DOI] [PubMed] [Google Scholar]
- 17.Ko B.C., Lam K.S., Wat N.M., Chung S.S. An (A-C)n dinucleotide repeat polymorphic marker at the 5′ end of the aldose reductase gene is associated with early-onset diabetic retinopathy in NIDDM patients. Diabetes. 1995;44:727–732. doi: 10.2337/diabetes.44.7.727. [DOI] [PubMed] [Google Scholar]
- 18.Al-Kateb H., Mirea L., Xie X., Sun L., Liu M., Chen H., Bull S.B., Boright A.P., Paterson A.D. DCCT/EDIC Research Group. Multiple variants in vascular endothelial growth factor (VEGFA) are risk factors for time to severe retinopathy in type 1 diabetes: the DCCT/EDIC genetics study. Diabetes. 2007;56:2161–2168. doi: 10.2337/db07-0376. [DOI] [PubMed] [Google Scholar]
- 19.Pluzhnikov A., Below J., Konkashbaev A., Tikhomirov A., Kistner-Griffin E., Roe C., Nicolae D.L., Cox N. Spoiling the whole bunch: quality control to preserve the integrity of high throughput genotyping. Am. J. Hum. Genet. 2010;87:123–128. doi: 10.1016/j.ajhg.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grassi M.A., Mazzulla D.A., Knudtson M.D., Huang W.W., Lee K.E., Klein B.E., Nicolae D.L., Klein R. Patient self-report of prior laser treatment reliably indicates presence of severe diabetic retinopathy. Am. J. Ophthalmol. 2009;147:501–504. doi: 10.1016/j.ajo.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandirasegarane L., Kester M. Enhanced stimulation of Akt-3/protein kinase B-gamma in human aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 2001;283:158–163. doi: 10.1006/bbrc.2001.4739. [DOI] [PubMed] [Google Scholar]
- 22.Hindorff L.A., Sethupathy P., Junkins H.A., Ramos E.M., Mehta J.P., Collins F.S., Manolio T.A. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc. Natl Acad. Sci. USA. 2009;106:9362–9367. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veyrieras J.B., Kudaravalli S., Kim S.Y., Dermitzakis E.T., Gilad Y., Stephens M., Pritchard J.K. High-resolution mapping of expression-QTLs yields insight into human gene regulation. PLoS Genet. 2008 doi: 10.1371/journal.pgen.1000214. e1000214 [Epub 10 October 2008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gamazon E.R., Zhang W., Konkashbaev A., Duan S., Kistner E.O., Nicolae D.L., Dolan M.E., Cox N.J. SCAN: SNP and copy number annotation. Bioinformatics. 2002;26:259–262. doi: 10.1093/bioinformatics/btp644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conrad D.F., Pinto D., Redon R., Feuk L., Gokcumen O., Zhang Y., Aerts J., Andrews T.D., Barnes C., Campbell P., et al. The Wellcome Trust Case Control Consortium. Origins and functional impact of copy number variation in the human genome. Nature. 2010;464:704–712. doi: 10.1038/nature08516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Wellcome Trust Case Control Consortium. Genome-wide association study of CNVs in 16,000 cases of eight common diseases and 3,000 shared controls. Nature. 2010;464:713–720. doi: 10.1038/nature08979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cano C.E., Hamidi T., Sandi M.J., Iovanna J.L. Nupr1: the swiss-knife of cancer. J. Cell Phys. 2010 doi: 10.1002/jcp.22324. doi:10.1002/jcp.22324. [DOI] [PubMed] [Google Scholar]
- 28.Barrett J.C., Clayton D.G., Concannon P., Akolkar B., Cooper J.D., Erlich H.A., Julier C., Morahan G., Nerup J., Nierras C., et al. Type 1 Diabetes Genetics Consortium. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 2009;41:703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mielinski M., Baldassano R.N., Griffiths A., Russell R.K., Annese V., Dubinsky M., Kugathasan S., Bradfield J.P., Walters T.D., Sleiman P., et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat. Genet. 2009;41:1335–1340. doi: 10.1038/ng.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zollner S., Pritchard J.K. Overcoming the Winner's Curse: estimating penetrance parameters from case-control data. Am. J. Hum. Genet. 2007;80:605–615. doi: 10.1086/512821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarthy M.I., Abecasis G.R., Cardon L.R., Goldstein D.B., Little J., Ioannidis J.P.A., Hirschhorn J.N. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat. Rev. Genet. 2008;9:356–369. doi: 10.1038/nrg2344. [DOI] [PubMed] [Google Scholar]
- 32.Barnett A.H., Dallinger K., Jennings P., Fletcher J., Odugbesan O. Microalbuminuria and diabetic retinopathy. Lancet. 1985;1:53–54. doi: 10.1016/s0140-6736(85)91008-6. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert R.E., Tsalamandris C., Allen T.J., Colville D., Jerums G. Early nephropathy predicts vision-threatening retinal disease in patients with type I diabetes mellitus. J. Am. Soc. Nephrol. 1998;9:85–89. doi: 10.1681/ASN.V9185. [DOI] [PubMed] [Google Scholar]
- 34.Early Treatment Diabetic Retinopathy Study (ETDRS) Manual of Operations. Baltimore, MD: National Technical Information Service; 1985. pp. 1–49. [Google Scholar]
- 35.The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 36.Korn J.M., Kuruvilla F.G., McCarroll S.A., Wysoker A., Nemesh J., Cawley S., Hubbell E., Veitch J., Collins P.J., Darvishi K., et al. Integrated genotype calling and association analysis of SNPs, common copy number polymorphisms and rare CNVs. Nat. Genet. 2008;40:1253–1260. doi: 10.1038/ng.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pe'er I., Yelensky R., Altshuler D., Daly M.J. Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet. Epidemiol. 2008;32:381–385. doi: 10.1002/gepi.20303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.