Abstract
Drosophila melanogaster is ideal for studying lifespan modulated by dietary restriction (DR) and oxidative stress, and also for screening prolongevity compounds. It is critical to measure food intake in the aforementioned studies. Current methods, however, overlook the amount of the food excreted out of the flies as feces or deposited in eggs. Here we describe a feeding method using a radioactive tracer to measure gender-specific food intake, retention and excretion in response to DR and oxidative stress to account for all the ingested food. Flies were fed a full, restricted or paraquat-containing diet. The radioactivity values of the food in fly bodies, feces and eggs were measured separately after a 24-hr feeding. Food intake was calculated as the sum of these measurements. We found that most of the tracer in the ingested food was retained in the fly bodies and <8% of the tracer was excreted out of the flies as feces and eggs in the case of females during a 24-hr feeding. Under a DR condition, flies increased food intake in volume to compensate for the reduction of calorie content in the diet and also slightly increased excretion. Under an oxidative stress condition, flies reduced both food intake and excretion. Under all the tested dietary conditions, males ingested and excreted 3- to 5-fold less food than females. This study describes an accurate method to measure food intake and provides a basis to further investigate prandial response to DR and prolongevity interve ntions in invertebrates.
Key words: food intake, excretion, aging, dietary restriction, oxidative stress
Introduction
Dietary restriction (DR) has been shown to extend lifespan in a number of diverse organisms ranging from yeast, worms and flies, rodents and probably primates.1–6 Much of our understanding of molecular mechanisms of lifespan extension by DR comes from genetic analysis of the single-cell organism, Saccharomyces cerevisiae and invertebrates, which include Caenorhabditis elegans and Drosophila melanogaster.1,2,4,5,7 In these small model organisms, DR is generally imposed by diluting or changing the amounts of ingredients in the food but allowing ad libitum feeding, since it is hard if not impossible to control the amount of food intake mainly due to their tiny body size.1,2,4,5 This is different from rodents and primates where DR or calorie restriction (CR) can be achieved by limiting the amount of food available to the animals and the actual food consumption can be measured directly.3
In D. melanogaster, several methods have been developed to indirectly and directly quantify food intake. By taking advantage of the fact that flies extend their proboscis when they eat, Partridge and her colleagues have described a method to indirectly assess food intake by counting the frequency of proboscis protrusion.8 However, a drawback of this approach is that it is based on the assumption that a fly ingests a similar amount of the food each time its proboscis extends. The second method developed by Tatar and his colleagues is to directly measure the calorie and nutrient contents of flies to assess food intake.9 They found that restriction of yeast, the main protein source for flies, in the diet resulted in a reduction of calorie content in the flies. This suggests that lifespan extension by protein restriction is partly due to the reduction of calorie intake. A confounding factor in this method is that food intake, metabolism and excretion all contribute to the measuring results, which makes it difficult to assess actual food intake.
The third approach is the tracer method which quantifies the amount of food intake by directly measuring the amount of the tracer, which is spiked in the food, in the fly bodies.10–17 The tracers can be either fluorescent dyes or more sensitive radioactive compounds, such as 32P, 3H or 14C. However, the published tracer methods actually measure food retention rather than intake and miss the amount of the tracer excreted out of the flies through feces and eggs in the case of females, which leads to underestimation of the amount of food intake. Using only yeast as the food source and radiophosphorus as the tracer, King and Wilson found that significant amount (∼25%) of radiophosphorus was excreted out as feces in a 24-hr feeding.18 This amount is likely to be variable when a different diet is used. Therefore, it is critical to account for the tracer that has passed through the flies in order to reliably measure food intake. Benzer and his colleague published an elegant approach to directly measure food intake in D. melanogaster, called the Capillary Feeder (CAFE) assay,19 which is similar to the glass tube feeding method in blowflies.20 Food intake is quantified by measuring consumption of liquid food in a calibrated capillary by a single or a few flies. The CAFE method is direct and relatively accurate even at the single fly level. However, this method requires providing liquid food to flies, which is different from the standard laboratory fly culture conditions that use solid agar food. The feeding behavior may not be the same toward liquid and solid food especially under DR conditions.
To address the issues related to prandial response, we evaluate the limitation and accuracy of the tracer method in this study. We describe a method to separately measure the tracer retained in the fly bodies and excreted from the flies in order to accurately assess food intake. Using this approach, we also investigate sex-specific prandial response to DR and oxidative stress, a factor underlying the aging processes.
Results
The method to measure food intake.
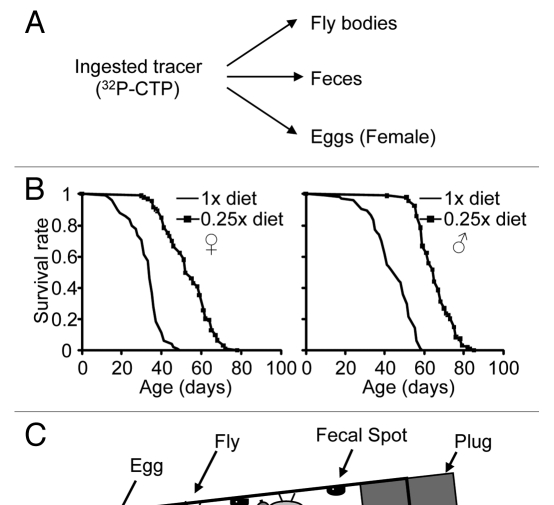
We postulated that the ingested tracer should be the sum of the amounts that were retained in the fly, excreted as feces and deposited in eggs in the case of females (Fig. 1A). Previous methods did not account for which fraction of the ingested food contributes to the excretion and egg production.10–12 It is also not known whether the retention-to-excretion ratios are significantly different under DR and oxidative conditions. Dilution of a standard sugar-yeast extract (1x SY) diet by four fold extended lifespan in wild-type Canton-S flies when compared to the full diet (Fig. 1B). To account for all the ingested food, we designed a feeding method using radioactive (32P-dCTP) food, which was provided to flies in a 500 µl eppendorf tube (Fig. 1C). The tube was inserted upside down into a hole created in a plug, which was then fitted to a fly vial. To collect eggs laid by females, the bottom of the vial was filled with approximately 5 ml of 1.5% agar. After a 24-hr feeding, the plug with the radioactive food was removed and flies were transferred to scintillation vials for measuring the radioactivity accumulated in their bodies. The radioactivity in feces or eggs was measured as described in the Materials and Methods. This approach allows measuring the entire ingested tracer as well as its distribution among fly bodies, feces and laid eggs. In addition, the small food surface in this method minimizes the amount of the tracer attached to the outside of the flies' bodies due to walking and grooming on the food surface.
Figure 1.
Measurement of food intake. (A) Distribution of the ingested food tracer. 32P-dCTP was used as the tracer. (B) Lifespan of male and female flies fed the full (1x SY) or DR diet (0.25x SY). (C) Feeding setup. Food was provided to flies in a tube inserted into the plug of a tilted vial. 1.5% agar in the bottom of the vial was provided for females to lay eggs. (D) Percentage of eggs laid on the agar only surface. “f” represents female; “1x” refers to the full diet; “0.25x” refers to the DR diet.
Prandial response of flies fed the full diet.
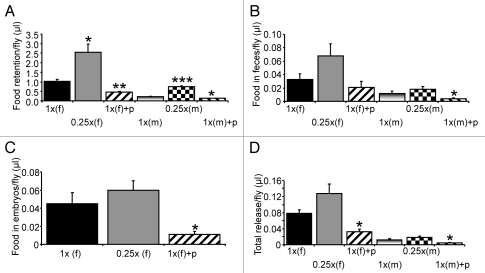
Using the technique described above, we assessed prandial response of flies fed the full diet (1x SY) by measuring food retention and excretion (Figs. 2 and 3). We found that a male ingested approximately 0.227 µl of food on average over a 24-hr period. Approximately 95% of the tracer (equivalent to ∼0.216 µl food/male) remained in the body, while approximately 5% of tracer was excreted out as feces (equivalent to ∼0.012 µl food/male). On the other hand, food intake of a female was almost five fold of that of a male and reached approximately 1.115 µl on average in 24 hours. Similarly to males, approximately 93% of ingested tracer was retained in females. The rest was excreted out as feces (equivalent to 0.033 µl food/female), which was ∼3% of ingested tracer, or deposited into eggs (equivalent to 0.045 µl food/female), which was ∼4% of ingested tracer. Therefore, the total food release, referring to the tracer in both feces and eggs, was ∼7% of ingested tracer in females. These findings indicate that most of the ingested food tracer 32P-dCTP is retained in the fly during a 24-hr feeding under our experimental conditions.
Figure 2.
Prandial response of flies to the full, DR and paraquat diets. (A) Food retention in a fly. (B) Tracer in excretion. (C) Tracer in eggs from a female. (D) Total food tracer release. Each measurement was repeated three times with three vials each housing approximately 20 flies. For the full diet represented by 1x, total number of males (N-male) was 60 for the three replicates and total number of females (N-female) was 57; for the DR diet (0.25x), N-male = 60; N-female = 58; for the paraquat diet, N-male = 59; N-female = 58; “f” represents female and “m” refers to male. “+p” represents addition of paraquat to the diet. The amount of food in µl shown on Y axis was calculated based on the amount of food tracer. *p < 0.05; **p < 0.01; ***p < 0.001.
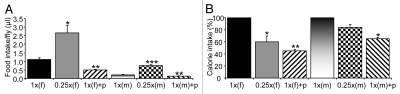
Figure 3.
Total food intake. (A) Volume of ingested food. (B) Percentage of calorie intake for flies under DR and oxidative stress when compared to that under the full diet, which was normalized to 100% separately for males and females. The comparison was conducted within each sex. The abbreviations are the same as those in Figure 2. *p < 0.05; **p < 0.01.
Prandial response of flies under DR.
We also measured the prandial response of flies under DR. DR was imposed by diluting all the nutrients to 25% of the full diet, called 0.25x SY, in this study. We found that both males and females increased the volume of food intake under DR by more than 2-fold when compared to the corresponding full diet (p < 0.05 for males and p < 0.001 for females) (Fig. 3). Food ingestion of a male and a female under DR was approximately 0.501 µl and 2.68 µl respectively. The excretion in males and food release in females were equivalent to approximately 0.018 µl and 0.128 µl of the food. Similarly to flies under the full diet, 93–95% of ingested tracer was retained in the fly and the remaining 5–7% was excreted out of the fly as feces and deposited in eggs in the case of females (Figs. 2 and 3). DR appeared to increase the amount of total food released from the fly bodies by more than 50% for both males and females, which, however, did not reach statistically significance (p = 0.23 for males; p = 0.10 for females). Considering the increase of food intake in volume, the difference of calorie intake for flies under the full and restriction diets was not as large as the degree of food dilution. The calorie intake of a male under the restricted diet (0.25x SY) was approximately 84.1% of calorie taken by a male under the full diet (1x SY), which was far larger than the 25% predicted from the diet difference (Fig. 3B). Similarly, but to a less extent, the calorie intake of a female under the DR diet was approximately 60.0% of calorie taken by a female under the full diet (Fig. 3B). This confirmed a previous observation made by Benzer and his colleagues using the CAFE method that the flies under DR can compensate for the calorie reduction in the food source by increasing the volume of food intake.12
Prandial response of flies under oxidative stress.
To assess the impact of stress on fly behavior, we measured food retention and release of flies fed an oxidative stress inducing agent, paraquat.21 Paraquat suppressed food intake of males and females by approximately 35% (p < 0.01) and 55% (p < 0.01) respectively (Fig. 3). The calorie intake of males and females fed the paraquat diet was approximately 65% (p < 0.05) and 45% (p < 0.01) of those under the full diet respectively. Total excretion of food under oxidative stress was significantly reduced compared to the full diet for both males and females (p < 0.05) (Fig. 2D). However, the percentages of food accumulated in the fly and released from the fly (94–97% and 3–6% respectively) were similar to flies under the full diet and DR. This indicates that oxidative stress reduces food intake without changing much the pattern of tracer retention and release.
Changes of fecal spot numbers in response to DR and oxidative stress.
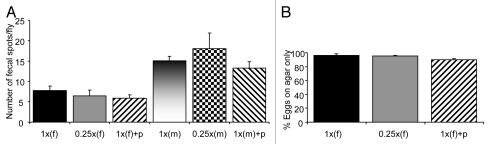
Food intake was previously estimated by counting the number of feces generated by flies based on the assumption that when food intake is higher, more feces should be produced.13 To test this assumption, we counted the fecal spots of flies under the full diet, DR and oxidative stress (Fig. 4A). Under the full diet, each male and female on average generated approximately 15 and eight fecal spots respectively in 24 hrs. In this study, the fecal counts between males and females could not be compared since only females were provided with agar on the bottom of the vials and the fecal spots on the agar surface were not accounted for. We found that DR and oxidative stress did not significantly alter the number of fecal spots for both males and females (Fig. 4A). This suggests that fecal count is not a reliable indicator of food intake under our experimental conditions.
Figure 4.
Distribution of fecal spots and eggs. (A) Fecal spot counts of flies under the diet, DR and paraquat diets. (B) Percentage of total eggs laid on the agar only surface under three dietary conditions. The numbers of eggs laid by one female on the bottom agar only surface and the top food surface on average are 17.4 ± 2.2 and 0.6 ± 0.3 respectively for the full diet (1x), 20.8 ± 8.8 and 1.1 ± 0.4 respectively for the DR diet (0.25x), and 12.5 ± 0.6 and 1.4 ± 0.3 respectively for the paraquat diet (1 x +p). Each measurement was repeated three times with three vials each containing approximately 20 females. The abbreviations are the same as those in Figure 2.
Distribution of eggs in the vial.
In our protocol, the radioactive food in a 500 µl eppendorf tube was removed before any measurement, and therefore any eggs laid on the radioactive food surface were excluded from the measurement. To evaluate the effect of diet and agar surface on egg laying and determine whether the removal of the food in the plug significantly affected the measurement for eggs, we counted the number and distribution of eggs laid to the agar only surface on the bottom of the vial and to the food agar surface on the top of the vial by females on the three diets. We found that each female on average laid a total of 12–20 eggs in 24 hrs under the three dietary conditions tested here (Fig. 4B). There was no statistical difference in egg laying among these diets (Fig. 4B legend). We also found that 89%–97% of the eggs were deposited on the agar only surface (Fig. 4B). This indicates that only small percentage of eggs (<11%) is on the food source, which is not accounted for in our method.
Discussion
Food consumption has profound effects on the health and longevity of animals. Over consumption of food has been linked to many diseases in human.22 Genetically tractable model organisms, such as D. melanogaster, are ideal for studying various dietary issues. However, it is hard if not impossible to control the food intake for the tiny invertebrate models. It is, therefore, critical to accurately measure the amount of food intake as well as excretion or release.23 Here we describe a simple but accurate method using the radioactive tracer 32P-dCTP to account for the amount of food retained in the fly, excreted as feces, and deposited in eggs in the case of females in Drosophila melanogaster. We have employed this tracer method to estimate food intake and excretion or release in flies under standard diet, DR and oxidative stress conditions. Our findings reveal several gender-specific patterns of prandial response in Drosophila. First, most of the ingested tracer (>93%) is retained in the fly bodies during a 24-hr feeding and only a small percentage of tracer (<7%) is released from the flies. Our findings support that the food intake method using the tracer 32P-dCTP previously described by Carvalho et al. has more than 90% accurate although it only measures the trace inside the flies.12 However, if other tracers are used in the food intake assay, their absorption and partition by flies will not necessarily be the same once they are ingested and metabolized by flies under different experimental conditions.24 Therefore, it is critical to evaluate a tracer before its usage for the food intake method, which only accounts for the tracer inside the flies. This type of evaluation can be easily performed by our method to measure both retention and release of a tracer. Other patterns of prandial response observed in our study include that DR increases the volume of food intake to compensate the reduction of calorie in the diet, while paraquat-induced oxidative stress reduces food intake, when compared to the standard full diet. However, DR and oxidative stress do not significantly alter the pattern of food retention and release. Furthermore, females tend to ingest more food than males under all dietary conditions tested in this study, but have similar pattern of food retention and release as males. These findings provide a foundation for future studying molecular mechanisms underlying prandial response under various dietary conditions.
The method described in this study has accounted for most but not all the tracer ingested by the flies. However, we estimate that those exclusions do not significantly compromise the accuracy of any of our measurements. In our method, feces on the plug for both males and females, and eggs on the food were not collected for measurement to avoid contamination from the strongly radioactive food source. To determine the impact of these omissions, we measured the relative distribution of feces on the vial and the plug, which provided an estimation of the percentage of feces that were excluded from our food intake measurements. Specifically, after feeding the flies the full or restricted diet containing the green food dye overnight in the same fashion as depicted in Figure 1, we transferred the flies to a new vial containing the same diet without the food dye and allow defecation for 10 min. We then counted the number of fecal spots. We found that less than 7% of the fecal spots were on the plug for both males and females under the full or restricted diet (data not shown), suggesting that the exclusion of fecal spots on the plug has relatively little effect on the measurement of food tracer in feces. For the egg measurement, as shown in our egg counting experiments approximately 90% of the total eggs laid in each vial were found on the agar only surface under all three diet conditions (Fig. 4B). Omitting less than 10% eggs laid on the food surface should not significantly skew the measurement of the tracer in eggs. Another source of measurement error in our method may potentially stem from the fact that we did not account for the tracer taken up externally by flies when they walk across the food surface. However, we estimate that this carry-over is negligible to affect any of our measurements for at least three reasons. One reason is that flies tend to spend most of their time away from the food source and the opening surface of the food tube consists of <2% of the total internal surface of a vial. The amount of time that flies spend on the food should be fairly low. Second, typically a only tiny part of flies, i.e., the bottoms of fly legs, touches any surface in the vial. Third, the flies will typically walk across the surface of the plug after they walk across the food surface. The tracer carried externally by flies should be mostly scratched away by the plug. Therefore, it is reasonable to assume that the amount of the tracer externally taken by flies should be present at an extremely low level in any measurements of food retention and release. Taken all together, missing the amount of tracer present on the plug and food and externally taken by flies has negligible impacts on accuracy of any measurements in this study.
Previous DR experiments have presented contradicting results on whether flies under restricted diets would compensate for the reduction of calorie content or a specific nutrient in the food source by increasing the volume of food intake.9,10,12,14 Under the experimental condition of the current study, we have observed a significant compensation in food intake in volume for flies fed diets with a 4-fold difference in calorie content. As a result, the actual calorie intake differs by only 15–40% between flies fed a full and a restricted diet, which is consistent with what Benzer and his colleagues observed.12 This observation of compensation is also consistent with a study by Lee et al., which involves a long-term measurement of food intake using the CAFE method.25 On the contrary, Bross et al. did not observe compensatory feeding in their DR paradigm,9,14 and Min and Tatar even observed an increase of food intake volumes for females that were fed diets with a higher percentage of protein,14 suggesting the lack of compensatory feeding in their experimental conditions. The discrepancy may be due to employment of different tracers, length of feeding and dietary composition. Our and Benzer's labs fed flies the radioactive tracer for at least 24 hrs, which may be more sensitive than the 4-hr feeding of a fluorescent food dye used by Bross et al.10,11 Both yeast and sugar are diluted while the ratio of yeast to sugar remains unchanged in the DR diets used by our and Benzer's labs. Min and Tatar have altered only the yeast content (from 1% to 16%) in the diets they used while maintaining the sugar at a constant level (11%). This implies that dietary conditions have a significant impact on food intake and the mechanisms of lifespan extension by different DR paradigms operate through distinct pathways. Further experiments are needed to fully address the different responses in feeding behavior.
It has been demonstrated in numerous experiments that DR or CR in mammals results in increased resistance to oxidative stress.26–28 In D. melanogaster, however, a recent study showed that DR did not render the fly more resistant to paraquat, and even appeared to decrease the resistance.29 Based on our studies of food intake of flies fed the DR or paraquat-containing diet, we postulate that the discrepancy between mammalian and fly studies lies in the food intake. Although paraquat reduces food intake in flies, DR dramatically increases food intake in volume, when compared to the standard full diet. It is conceivable that flies may uptake more paraquat in volume after DR treatment than the full diet control. The excessive amount of paraquat taken by the DR flies could counteract the increased resistance induced by DR, potentially rendering the DR flies more sensitive to paraquat, when compared to the controls fed the full diet. Therefore, it would be important to directly measure paraquat intake when assessing the resistance to oxidative stress. It is worth pointing out here that paraquat was found not to significantly alter the patterns of food retention and release and neither did it significantly reduce the number of eggs laid by females in this study when compared to the controls on the regular diet. This suggests that observed changes of prandial patterns in flies fed the paraquat containing diet are likely due to oxidative stress instead of general sickness that paraquat may cause in flies.
An interesting area of aging research is the hunt for prolongevity compounds and investigation of the underlying mechanisms.30 Compounds often work in a dosage dependent fashion.31–34 Direct measurement of food intake is critical to evaluate the dosage effect for flies fed a compound dissolved in food since a compound may significantly alter food intake. Difference in food intake will result in different calorie intake, which may in turn affect lifespan and mask the direct effect of the compound on lifespan. In addition, we have observed a marked difference of food intake between males and females (3- to 5-fold), which may lead to intake of different amount of a prolongevity compound. This may explain why some of the prolongevity compounds only work for one sex, and why males and females have different optimal dosages.31,35–38 The excretion should also be considered in these studies. We have observed a significant reduction of food tracer excretion or release by paraquat and a slight increase by DR. It is conceivable that certain compounds may dramatically increase excretion by affecting metabolic and excretion systems. For example, addition of dinitrophenol to the fly diet was found to significantly reduce incorporation of exogenous phosphorus into flies.39 Very little is known about how dietary conditions affect food excretion or release. Taken all together, the method and findings described in this study provide a basis to investigate how gender-specific food retention and release are affected by genetic and environmental factors, such as DR, and to affect prolongevity compounds in genetically tractable D. melanogaster. Our method can also be adopted to study food intake, retention and release in other small invertebrate models.
Materials and Methods
Fly culture condition.
D. melanogaster wild-type strain, Canton-S, was obtained for the Bloomington Stock Center and maintained with the standard cornmeal food40 or a sugar-yeast extract (SY) diet41 described below. Flies were maintained at 25 ± 1°C, 60 ± 5% humidity and under a 12 hr light/dark cycle.
Dietary restriction assay.
Adult flies were transferred to food with 10% sugar and 10% yeast extract, called 1x SY diet, within 24 hrs after eclosion, and were allowed to mate for another 24 hrs. Males and females were sorted out and placed separately in the vials (20 flies per vial) with 1x SY food for 24 hrs. Flies were then transferred daily to fresh vials for three days with 1x SY food as the full diet or a diluted SY food with 2.5% sugar and 2.5% yeast extract, the latter of which was called 0.25x SY diet as the DR diet. All food also contained 1.5% agar and 0.2% Tegosept. The diet was provided in a 500 µl eppendorf tube inserted upside down in the cotton plug (Flug™, Genesee Scientific Corporation, San Diego, CA). The plug was then fitted to a 25 mm (diameter) × 95 mm (length) fly vial. For females, approximately 5 ml of 1.5% agar was provided on the bottom of each vial for egg laying.
Oxidative stress assay.
Flies used in the oxidative stress assay were handled in the same way as described above for the DR assay except that flies were fed 1x SY food with 15 mM paraquat for 24 hrs before the measurement of food intake.
Food intake assay.
After treatment under a full or restricted diet for three days, the flies were transferred to new vials with the tracer food. The tracer food was prepared by mixing 32P-dCTP evenly with 1x SY, 0.25x SY or 1x SY-paraquat respectively. The final concentration of radioactivity in all the diets was approximately 5.36 nCi/µl food. After feeding for 24 hrs, flies were anesthetized on ice and transferred to scintillation vials with 10 ml of scintillation liquid. To measure the amount of radioactivity in the feces for males, 10 ml of scintillation liquid was added to each vial to rinse the feces off the vials after flies and the food in the plug were removed. For females, the feces and eggs that were scraped out of the agar medium were washed off into 10 ml of scintillation liquid. The radioactivity in the mixture of eggs and feces was measured first, and then the radioactivity of the scintillation liquid without eggs was measured after transferring only the liquid to another vial, which represented the radioactivity in the feces. The radioactivity in the eggs was calculated by subtracting the radioactivity of the feces from that of the mixture. Approximately 20 flies were present in each vial and each measurement was repeated three times with three biologically independent samples. In the mean time, 100 nCi of 32P-dCTP was taken to make serial dilutions for generating the standard curve, which was used to correlate the amount of radioactivity to the amount of food. Since flies feed by sucking up food stuff including soluble ingredients on the agar surface,42 it is expected that the water soluble tracer and other dietary nutrients, such as protein and sugar, are ingested by flies at the same ratio as they are in the food. Therefore, the amount of the trace ingested by flies reflects the amount of food intake.
Statistical analysis.
Statistical parameters were calculated with the StatView software version 5.0 (SAS Institute Inc., Cary, NC). p values were calculated by unpaired Student t-test. p < 0.05 was considered as statistically significant.
Acknowledgements
We thank Dr. Donald Ingram, Jason Sinclair and Dr. Toshimitsu Komatsu for discussion, Sharron Daly for assistance with the radioactive measurements and Drs. Kevin Pearson and Min Zhu for critical reading of the manuscript. This study was funded by the Intramural Research Program of the National Institute on Aging, NIH, to Sige Zou and the Shaw Scientist Foundation of Milwaukee to Chaoyang Zeng.
Abbreviations
- SY
sugar-yeast extract
- DR
dietary restriction
References
- 1.Guarente L. Calorie restriction and SIR2 genes—towards a mechanism. Mech Ageing Dev. 2005;126:923–928. doi: 10.1016/j.mad.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Partridge L, Pletcher SD, Mair W. Dietary restriction, mortality trajectories, risk and damage. Mech Ageing Dev. 2005;126:35–41. doi: 10.1016/j.mad.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Min KJ, Flatt T, Kulaots I, Tatar M. Counting calories in Drosophila diet restriction. Experimental Gerontology. 2007;42:247–251. doi: 10.1016/j.exger.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bross TG, Rogina B, Helfand SL. Behavioral, physical and demographic changes in Drosophila populations through dietary restriction. Aging Cell. 2005;4:309–317. doi: 10.1111/j.1474-9726.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho GB, Kapahi P, Anderson DJ, Benzer S. Allocrine modulation of feeding behavior by the sex peptide of Drosophila. Curr Biol. 2006;16:692–696. doi: 10.1016/j.cub.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carvalho GB, Kapahi P, Benzer S. Compensatory ingestion upon dietary restriction in Drosophila melanogaster. Nat Methods. 2005;2:813–815. doi: 10.1038/nmeth798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edgecomb RS, Harth CE, Schneiderman AM. Regulation of feeding behavior in adult Drosophila melanogaster varies with feeding regime and nutritional state. J Exp Biol. 1994;197:215–235. doi: 10.1242/jeb.197.1.215. [DOI] [PubMed] [Google Scholar]
- 14.Min KJ, Tatar M. Drosophila diet restriction in practice: do flies consume fewer nutrients? Mech Ageing Dev. 2006;127:93–96. doi: 10.1016/j.mad.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Tanimura T, Isono K, Takamura T, Shimada I. Genetic dimorphism in the taste sensitivity to trehalose in Drosophila melanogaster. J Comp Physiol. 1982;147:433–437. [Google Scholar]
- 16.Thompson ED, Reeder BA. Method for selecting exposure levels for the Drosophila sex-linked recessive lethal assay. Environ Mol Mutagen. 1987;10:357–365. doi: 10.1002/em.2850100405. [DOI] [PubMed] [Google Scholar]
- 17.Thompson ED, Reeder BA, Bruce RD. Characterization of a method for quantitating food consumption for mutation assays in Drosophila. Environ Mol Mutagen. 1991;18:14–21. doi: 10.1002/em.2850180104. [DOI] [PubMed] [Google Scholar]
- 18.King RC, Wilson LP. Studies with radiophosphorus in Drosophila V. The phosphorus incorporation by adult Drosophila melanogaster. J Exp Zool. 1955;130:71–82. [Google Scholar]
- 19.Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, et al. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci USA. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson SJ, Barton Browne L, van Gerwen ACM. The patterning of compensatory sugar feeding in the Australia sheep blowfly. Physiological Entomology. 1989;14:91–105. [Google Scholar]
- 21.Arking R, Buck S, Berrios A, Dwyer S, Baker GT., 3rd Elevated paraquat resistance can be used as a bioassay for longevity in a genetically based long-lived strain of Drosophila. Dev Genet. 1991;12:362–370. doi: 10.1002/dvg.1020120505. [DOI] [PubMed] [Google Scholar]
- 22.Popkin BM. What can public health nutritionists do to curb the epidemic of nutrition-related noncommunicable disease? Nutr Rev. 2009;67:79–82. doi: 10.1111/j.1753-4887.2009.00165.x. [DOI] [PubMed] [Google Scholar]
- 23.Tatar M. Diet restriction in Drosophila melanogaster. Design and analysis. Interdiscip Top Gerontol. 2007;35:115–136. doi: 10.1159/000096559. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien DM, Min KJ, Larsen T, Tatar M. Use of stable isotopes to examine how dietary restriction extends Drosophila lifespan. Curr Biol. 2008;18:155–156. doi: 10.1016/j.cub.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, et al. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc Natl Acad Sci USA. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harper ME, Bevilacqua L, Hagopian K, Weindruch R, Ramsey JJ. Ageing, oxidative stress and mitochondrial uncoupling. Acta Physiol Scand. 2004;182:321–331. doi: 10.1111/j.1365-201X.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- 27.Lass A, Sohal BH, Weindruch R, Forster MJ, Sohal RS. Caloric restriction prevents age-associated accrual of oxidative damage to mouse skeletal muscle mitochondria. Free Radic Biol Med. 1998;25:1089–1097. doi: 10.1016/s0891-5849(98)00144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward WF, Qi W, Van Remmen H, Zackert WE, Roberts LJ, 2nd, Richardson A. Effects of age and caloric restriction on lipid peroxidation: measurement of oxidative stress by F2-isoprostane levels. J Gerontol A Biol Sci Med Sci. 2005;60:847–851. doi: 10.1093/gerona/60.7.847. [DOI] [PubMed] [Google Scholar]
- 29.Burger JM, Hwangbo DS, Corby-Harris V, Promislow DE. The functional costs and benefits of dietary restriction in Drosophila. Aging Cell. 2007;6:63–71. doi: 10.1111/j.1474-9726.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 30.Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, deCabo R. Calorie restriction mimetics: an emerging research field. Aging Cell. 2006;5:97–108. doi: 10.1111/j.1474-9726.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- 31.Jafari M, Khodayari B, Felgner J, Bussel II, Rose MR, Mueller LD. Pioglitazone: an anti-diabetic compound with anti-aging properties. Biogerontology. 2007;8:639–651. doi: 10.1007/s10522-007-9105-7. [DOI] [PubMed] [Google Scholar]
- 32.Lee KS, Lee BS, Semnani S, Avanesian A, Um CY, Jeon HJ, et al. Curcumin extends life span, improves health span and modulates the expression of age-associated aging genes in Drosophila melanogaster. Rejuvenation Res. 2010;13:561–570. doi: 10.1089/rej.2010.1031. [DOI] [PubMed] [Google Scholar]
- 33.Sun X, Seeberger J, Alberico T, Wang C, Wheeler CT, Schauss AG, Zou S. Açai palm fruit (Euterpe oleracea Mart.) pulp improves survival of flies on a high fat diet. Experimental gerontology. 2010;45:243–251. doi: 10.1016/j.exger.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 35.Jafari M, Felgner JS, Bussel II, Hutchili T, Khodayari B, Rose MR, Mueller LD. Rhodiola: a promising anti-aging Chinese herb. Rejuvenation Res. 2007;10:587–602. doi: 10.1089/rej.2007.0560. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Y, Sun H, Lu J, Li X, Chen X, Tao D, et al. Lifespan extension and elevated hsp gene expression in Drosophila caused by histone deacetylase inhibitors. J Exp Biol. 2005;208:697–705. doi: 10.1242/jeb.01439. [DOI] [PubMed] [Google Scholar]
- 37.Zou S, Sinclair J, Wilson MA, Carey JR, Liedo P, Oropeza A, et al. Comparative approaches to facilitate the discovery of prolongevity interventions: effects of tocopherols on lifespan of three invertebrate species. Mech Ageing Dev. 2007;128:222–226. doi: 10.1016/j.mad.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou S, Carey JR, Liedo P, Ingram DK, Yu B, Ghaedian R. Prolongevity effects of an oregano and cranberry extract are diet dependent in the Mexican fruit fly (Anastrepha ludens) J Gerontol A Biol Sci Med Sci. 2010;65:41–50. doi: 10.1093/gerona/glp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson LP, King RC. Studies with radiophosphorus in Drosophila VI. The effect of DNP on phosphorus incorporation by adult Drosophila melanogaster. J Exp Zool. 1955;130:341–352. [Google Scholar]
- 40.Ashburner M. Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory Press: Plainview, NY; 1989. [Google Scholar]
- 41.Mair W, Goymer P, Pletcher SD, Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301:1731–1733. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- 42.Miller A. The internal anatomy and histology of the imago of Drosophila melanogaster. In: Demerec M, editor. Biology of Drosophila. New York: Cold Spring Harbor Laboratory Press; 1994. pp. 420–531. [Google Scholar]