Abstract
Background and Aims
Rice is one of the few crops able to withstand periods of partial or even complete submergence. One of the adaptive traits of rice is the constitutive presence and further development of aerenchyma which enables oxygen to be transported to submerged organs. The development of lysigenous aerenchyma is promoted by ethylene accumulating within the submerged plant tissues, although other signalling mechanisms may also co-exist. In this study, aerenchyma development was analysed in two rice (Oryza sativa) varieties, ‘FR13A’ and ‘Arborio Precoce’, which show opposite traits in flooding response in terms of internode elongation and survival.
Methods
The growth and survival of rice varieties under submergence was investigated in the leaf sheath of ‘FR13A’ and ‘Arborio Precoce’. The possible involvement of ethylene and reactive oxygen species (ROS) was evaluated in relation to aerenchyma formation. Cell viability and DNA fragmentation were determined by FDA/FM4-64 staining and TUNEL assay, respectively. Ethylene production was monitored by gas chromatography and by analysing ACO gene expression. ROS production was measured by using Amplex Red assay kit and the fluorescent dye DCFH2-DA. The expression of APX1 was also evaluated. AVG and DPI solutions were used to test the effect of inhibiting ethylene biosynthesis and ROS production, respectively.
Key Results
Both the varieties displayed constitutive lysigenous aerenchyma formation, which was further enhanced when submerged. ‘Arborio Precoce’, which is characterized by fast elongation when submerged, showed active ethylene biosynthetic machinery associated with increased aerenchymatous areas. ‘FR13A’, which harbours the Sub1A gene that limits growth during oxygen deprivation, did not show any increase in ethylene production after submersion but still displayed increased aerenchyma. Hydrogen peroxide levels increased in ‘FR13A’ but not in ‘Arborio Precoce’.
Conclusions
While ethylene controls aerenchyma formation in the fast-elongating ‘Arborio Precoce’ variety, in ‘FR13A’ ROS accumulation plays an important role.
Keywords: Oryza sativa, rice, submergence, Sub1A, ACO expression, lysigenous aerenchyma, ethylene, reactive oxygen species, ROS
INTRODUCTION
Flooding events of various depths and durations are the principal environmental cause of a shortage of oxygen (O2) in plants. Rice is one of the few crops able to withstand periods of partial or even complete submergence (Colmer and Voesenek, 2009; Licausi and Perata, 2009; Nagai et al., 2010). One of the adaptive traits of rice is the constitutive presence of aerenchyma which enables O2 to be transported to the submerged organs (Armstrong, 1971; Kawase and Whitmoyer, 1980). Although this tissue is present even in well-drained conditions, it develops further when the soil becomes waterlogged (Armstrong, 1971; Pradhan et al., 1973; Das and Jat, 1977). In rice, aerenchymatous areas are formed by cell lysis and seem to be co-ordinated by a programmed cell death (PCD) mechanism (Kawai et al., 1998). In mature leaf sheaths of rice, lysigenous aerenchyma is linked to stomata, allowing gases to flow from leaves to the basal part of the plants (Matsukura et al., 2000), and it is interrupted by septa composed of stellate cells that have large intercellular spaces and which thus allow the passage of O2 (Hoshikawa, 1989).
The mechanisms responsible for aerenchyma formation have not yet been fully elucidated (Shiono et al., 2008); however, it is known that this process involves ethylene, which accumulates in submerged organs (Kavase, 1972, 1978; Könings and Jackson, 1979; Justin and Armstrong, 1991; He et al., 1996; Zhou et al., 2002; Geisler-Lee et al., 2010; Lenochova et al., 2009). In hypoxic roots of maize, exogenous ethylene applications induce the aerenchymatous areas and ethylene inhibitors repress their formation (Drew et al., 1981; Jackson et al., 1985; Könings, 1982). In addition, both 1-aminocyclopropane-1-carboxylate synthase (ACC) synthase activity and ACC concentrations are high in hypoxic maize roots (Atwell et al., 1988; He et al., 1994; Geisler-Lee et al., 2010).
Aerenchyma formation, however, does not always require ethylene, as is described for lysigenous aerenchyma formation in the root of the wetland plant Juncus effusus (Visser and Bögemann, 2006).
Although the formation of maize root aerenchyma under waterlogging and hypoxia is stimulated by enhanced ethylene biosynthesis and increased endogenous ethylene concentration (Drew et al., 1981; Könings, 1982; Jackson et al., 1985; Atwell et al., 1988; He et al., 1994; Geisler-Lee et al., 2010), this is not the case when aerenchyma formation is induced by nutrient-starvation (Drew et al., 1989; He et al., 1992). While temporary deprivation of N or P greatly enhances the sensitivity of ethylene-responsive cells of the maize root cortexin leading to cell lysis and aerenchyma formation (He et al., 1992, 1994), sulfate deprivation shows altered levels of reactive oxygen species (ROS) in the aerenchymatous areas (Bouranis et al., 2003, 2006), suggesting a role for ROS in inducing aerenchyma formation.
In rice, early studies reported that aerenchyma formation in adventitious roots was not controlled by ethylene (Jackson et al., 1985). Ethylene was later shown to have a role in promoting aerenchyma, but differences were found in cultivar responses (Justin and Armstrong, 1991). Recently, a study by Steffens et al. (2011) on aerenchyma formation in rice stems in response to ethylene and hydrogen peroxide (H2O2) showed that both compounds promote its formation in a dose-dependent manner. The production of lysigenous aerenchyma in Arabidopsis thaliana under hypoxia requires both ethylene and H2O2 signalling (Mühlenbock et al., 2007). ROS have been proposed to be the central component of a plant's adaptation to both biotic and abiotic stresses, by exacerbating oxidative damage and by acting as signal molecules in protective responses (Mittler et al., 2004). ROS signalling is involved in the metabolic adaptation to low O2 (Baxter-Burrel et al., 2002) and also acts as a trigger for the induction of HsfA2 transcription factors, thus conferring anoxia tolerance (Banti et al., 2010).
The ability to grow when submerged is another trait which may help to allow the efficient transport of O2 to the submerged organs. The formation of aerenchyma is crucial for submerged organs, but if the growth of the plant is unable to compete with rises in water levels, the plant dies. In rice, the type of growth response to submergence strongly depends on the genotype; rice varieties containing the Sub1A gene adopt a quiescence strategy with growth restriction, while other varieties grow very rapidly, to attempt to maintain at least the leaf tips above the water level (Bailey-Serres and Voesenek, 2008; Colmer and Voesenek, 2009; Bailey-Serres et al., 2010; Nagai et al., 2010). Extraordinary internode elongation induced under deep water via gibberellin is observed in deepwater rice varieties, and is triggered by the ethylene responsive factors SNORKEL (SK) 1 and 2 (Hattori et al., 2009).
Sub1A is an ethylene responsive factor that is induced by ethylene signalling under submergence (Fukao et al., 2006; Xu et al., 2006). Sub1A represses further ethylene synthesis (Bailey-Serres and Voesenek, 2010), and may hamper aerenchyma formation in Sub1A varieties. Sub1A varieties do not grow in response to submergence. This would not make aerenchyma important in deep flooding to have access to O2 above the water level, but rather to rely on O2 produced and stored during photosynthesis in the underwater organs, or retained by the leaf surface gas film (Colmer and Pedersen, 2008; Pedersen et al., 2009). However, under shallow submergence it is unclear how to reconcile the Sub1A-dependent inhibition of ethylene synthesis with the formation of aerenchyma.
In this paper, aerenchyma formation was examined in ‘FR13A’, a Sub1A variety, and in ‘Arborio Precoce’ (‘AP’), a non-Sub1A variety displaying fast shoot elongation when submerged. The results showed the presence of constitutive aerenchyma in both ‘AP’ and ‘FR13A’ varieties, which further increased under submergence. Submergence-induced ethylene synthesis was observed in ‘AP’ only, ‘FR13A’ did not show any increase in ethylene production. The results suggest that aerenchyma formation in ‘FR13A’ is independent of ethylene signalling, and ROS appear to be important to regulate aerenchyma formation in this Sub1A variety.
MATERIALS AND METHODS
Plant material and submergence treatment
Oryza sativa seeds of the varieties ‘FR13A’ and ‘AP’ were water-soaked in Petri dishes for 3 d (28 ± 2 °C, dark conditions). Germinated seedlings were grown in 50-mL plastic pots filled with sand and transferred to a growth chamber for 7 d (26 ± 2 °C, 15-h light photoperiod; PAR approx. 50 µmol m−2 s−2 provided by white fluorescence lamps). The following complete nutrient solution was used: Ca(NO3)2.4H2O (4·5 mm), MgSO4 (0·8 mm), KH2PO4 (2·6 mm), KNO3 (13·5 mm), K2SO4 (0·2 mm) and Chelamix (30 mg L−1; Valagro, Chieti, Italy). Submergence treatments were carried out for up to 21 d, as detailed in Fig. 1 (26 ± 2 °C, 15-h light photoperiod; PAR approx. 50 µmol m−2 s−2).
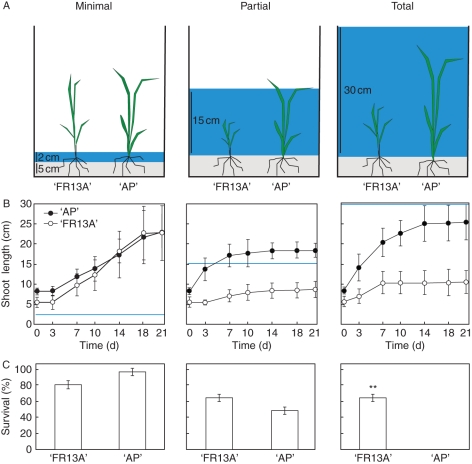
Fig. 1.
‘Arborio Precoce’ (‘AP’) and ‘FR13A’ plants under submergence. (A) Diagram showing how the rice varieties were submerged in water. One-week-old rice seedlings were grown in pots and flooded with water, 2 cm (minimal), 15 cm (partial) or 30 cm (total) above soil surface. The drawing depicts the plants at the end of the experiment. (B) Shoot length of the rice plants under different submergence conditions. The blue lines indicate the water level. Data are expressed as mean ± s.d., n = 12. (C) Percentage of plant survival after 21 d of submersion followed by 7 d of recovery under well-drained conditions. Data are expressed as mean ± s.e., n = 12; **, P < 0·01according to Student's t-test.
Fresh leaf sheath samples were harvested after 2 and 3 d of complete submergence to observe sampling and microscopic events following the treatment. For each time-point, three biological repetitions, each consisting of a composite sample from three different plant leaf sheaths, were immediately processed or stored at –80 °C for further molecular and biochemical analysis. Other analyses were repeated as described in figure legends.
Aerenchyma observation and quantification
Transverse sections (80 µm) of leaf sheaths, were prepared using samples from rice plants that had been flooded for 3 d as well as from control plants using a vibratory microtome (Vibratome 1000 Plus; Vibratome, St Louis, MO, USA). Sections were observed for aerenchyma formation and photographed with a Nikon Eclipse Ti-S microscope (Nikon, Tokyo, Japan). The percentage of aerenchyma was determined using the Imag-pro Plus version 6·2 (Media Cybernetics, Bethesda, MD, USA) and was calculated on total tissue cross-sectional area.
Viability staining
The cell viability of rice leaf sheath sections was determined by staining with fluorescein diacetate (FDA) (2 µg mL−1 in phosphate buffer saline; Sigma-Aldrich, St Louis, MO, USA) for 15 min followed by FM4-64 (20 µm in phosphate buffer saline; Molecular Probes, Carlsbad, CA, USA) for 3 min (Fath et al., 2001). The sections were examined using a Nikon Eclipse Ti-S microscope (Nikon) equipped with EGFP (λex 450–490, dichroic 495, λem 500–550 nm) and TRITC (λex 505–565, dichroic 550, λem 580–630 nm) filter blocks for FDA (λex 488, λem 502–540 nm) and FM4-64 (λex 515, λem 625 nm) signals, respectively. Images were captured by a QICAM digital CCD camera (QImaging, Surrey, BC, Canada).
In situ detection of DNA fragmentation (TUNEL assay)
‘FR13A’ and ‘AP’ leaf sheath from 3-d flooded and control plants were fixed in 4 % (w/v) paraformaldehyde in a phosphate buffer saline (pH 7·4). After dehydration through an ethanol series, samples were embedded in Paraplast Plus (Paraplast, Sherwood Medical Industries, St Louis, MO, USA). Sections (10 µm) were cut and stretched onto poly-lysine-coated slides. The sections were then dewaxed in xylene and rehydrated before examination. A TUNEL assay was performed using the ‘In situ cell death detection kit’ (Promega, Madison, WI, USA), according to the manufacturer's instructions. To facilitate the introduction of the TdT enzyme into the tissue sections, the slides were treated with proteinase K (20 mg mL−1) for 20 min. The labelling reaction was performed at 37 °C in a humidified chamber in the dark for 1 h. A negative control was included in each experiment by omitting TdT from the reaction mixture. As a positive control, permeabilized sections were incubated with DNase I (10 U mL−1) for 10 min before the TUNEL assay. The yellow-green fluorescence of incorporated fluorescein-12-dUTP was examined using the microscope equipment previously described, using filter blocks for EGFP (λex 450–490, dichroic 495, λem 500–550 nm). Experiments were repeated three times and each time, five slides were labelled for both the control and treated plants. A counter stain was done with DAPI (1 mg mL−1).
Ethylene experiments
Ethylene production was measured by enclosing samples in airtight glass containers (30 mL). Each sample consisted of three rice leaf sheaths picked from separate plants submerged for 3 d before performing ethylene measurements. Gas samples (2 mL each) were taken from the headspace of the containers with a hypodermic syringe after 1 h incubation at room temperature. The ethylene concentration in the sample was measured by gas chromatography (HP5890; Hewlett-Packard, Menlo Park, CA, USA) using a flame ionization detector, a stainless steel column (150 × 0·4 cm diameter packed with Hysep T), column and detector temperatures of 70 °C and 350 °C, respectively, and nitrogen carrier gas at a flow rate of 30 mL min−1. Quantification was performed against an external standard and results were expressed on a fresh weight basis (nL g−1 f. wt h−1).
For the experiments designed to test how inhibiting ethylene biosynthesis affects aerenchyma formation, rice leaf sheaths were brushed with 500 µm aminoethoxyvinylglycine (AVG) (Fluka, Sigma-Aldrich, St Louis, MO, USA) in an aqueous solution every 24 h for 3 d before and during the water submersion.
ROS experiments
Leaf sheaths excised from control and 3-d flooded plants were used to measure H2O2 production using the Amplex Red H2O2/peroxidase assay kit (Molecular Probes) according to the manufacturer's instructions. Fifty milligrams of frozen ground tissue was used for the extraction, and was mixed with 200 µL of 20 mm sodium phosphate buffer (pH 6·5), according to the protocol developed by the Schachtman Laboratory (Shin and Schachtman, 2004). After centrifugation at 9000 g for 10 min (4 °C), 50 µL of supernatant were used for the Amplex Red assay.
ROS visualization was performed on ‘FR13A’ and ‘AP’ leaf sheath sections using the fluorescent dye 2′-7′-dichlorodihydrofluorescein diacetate (DCFH2-DA; Sigma-Aldrich) (λex 488, λem 525 nm). Sections were obtained and immediately immersed in 10 mm DCFH2-DA in 10 mm Tris-KCl buffer (pH 7·4) in dark conditions for 30 min, followed by a 15-min washing step with a buffer. Negative controls were only incubated with the buffer. Imaging was performed with the microscope equipment previously described using filter blocks for EGFP (λex 450–490, dichroic 495, λem 500–550 nm).
For the experiments designed to test how inhibiting ROS production affects aerenchyma formation, 10 µm diphenyleneiodonium (DPI) (Sigma-Aldrich) in an aqueous solution (3 % DMSO) was injected in the central cavity of the leaf sheaths of rice every 24 h for 3 d before and during the water submersion.
Molecular analysis
Genomic DNA of ‘FR13A’ and ‘AP’ leaf sheaths was prepared using GenEluteTM Plant Genomic DNA Miniprep Kit (Sigma-Aldrich), following the manufacturer's protocol. The PCR reaction mixture was prepared in 20 µL total volume using Red Taq Master mix (Invitrogen, Carlsbad, CA, USA), 0·25 mm primers and 100 ng genomic DNA. PCR was performed using Sub1A, SK1 and SK2 specific primers according to Fukao et al. (2006) and Hattori et al. (2009; Table S1 in Supplementary data, available online).
For gene expression analysis, total RNA was extracted using a RNAqueous kit (Applied Biosystems/Ambion, Foster City, CA, USA), according to the manufacturer's instructions, and subjected to DNase treatment using TURBO DNA-free kit (Ambion). Five micrograms of RNA were reverse-transcribed using the High-Capacity cDNA Archive kit (Applied Biosystems). Transcript abundance was analysed by real-time reverse transcription PCR, using qPCR MasterMix Plus for SYBR® green I (Eurogentec, Liège, Belgium) with specifically designed primers (Table S1 in Supplementary data), using an ABI Prism 7000 Sequence Detection System (Applied Biosystems). The relative expression level of each gene was quantified with the comparative threshold cycle method, as described in the ABI Prism 7000 Sequence Detection System User Bulletin No 2 (Applied Biosystems), using rice glyceraldehyde-3-phosphate dehydrogenase as internal reference. PCR reactions for each of the three biological replicates were performed in duplicate.
RESULTS
‘AP’ and ‘FR13A’ showed a different sensitivity to partial and total submergence
Sub1A varieties such as ‘FR13A’ can tolerate complete, short duration submergence thanks to their growth restriction strategy. The rapid growth of submerged plants can, however, be an advantage when growth is fast enough to allow the plant to reach the water surface and transport air to the submerged organs through aerenchyma. ‘FR13A’ and ‘AP’ differ greatly in their growth responses to submergence and we therefore tested whether their tolerance to submergence changed depending on the water level used to submerge the plants (Fig. 1A).
When plants were submerged with only 2 cm of water, most of the aerial parts of the plants were aerobic and both varieties survived well (Fig. 1B). However, ‘FR13A’ showed a percentage of survival lower than ‘AP’ (approx. 20 %), due probably to the sensitivity of this variety to stagnant water (Datta and Banerji, 1973). Submergence in 15 cm of water implies that both ‘AP’ and ‘FR13A’ were completely submerged at time 0. In this case, while ‘FR13A’ elongation was not sufficient for emergence, rapid growth allowed ‘AP’ plants to emerge between day 3 and day 7 of submergence. This variety did not show amplification with SK1 and SK2 primers (data not shown), suggesting the absence of the genes as already described for other japonica rice species (Hattori et al., 2009). The growth of ‘AP’ was very rapid when submerged with 15 cm of water and slowed down after the leaf tip of ‘AP’ had reached the water surface (Fig. 1B). Both varieties suffered from submergence; however, approx. 50 % of plants survived, without any significant differences between the two varieties (Fig. 1C). When submerged under 30 cm water, ‘AP’ plants grew longer than plants submerged under 15 cm of water, but growth was not enough to reach the water surface (Fig. 1B). ‘FR13A’ growth was, as expected (Xu et al., 2006), minimal (Fig. 1B), but survival was good (Fig. 1C), whereas ‘AP’ plants were all dead by the end of the treatment (Fig. 1C).
‘AP’ and ‘FR13A’ leaf sheath aerenchyma formation under complete submergence
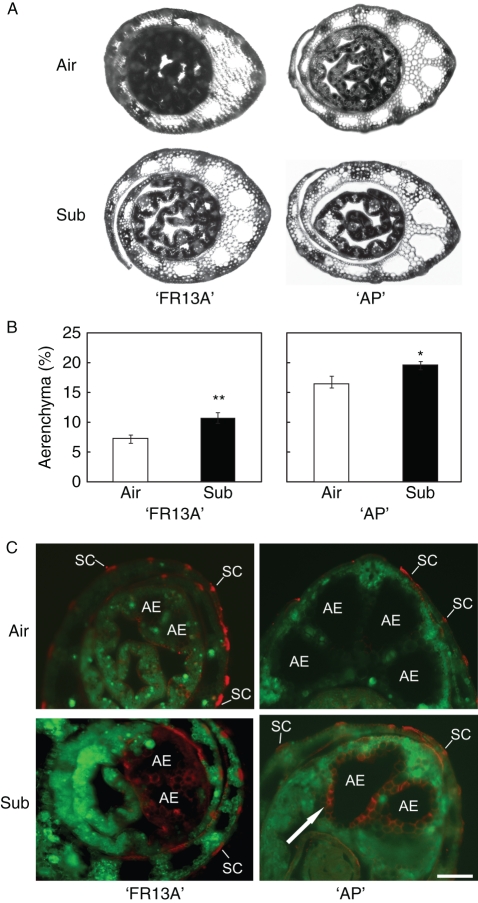
‘AP’ leaf sheaths showed more constitutive aerenchyma than ‘FR13A’ (Fig. 2A, B; Air), that increased following submergence in both ‘AP’ and ‘FR13A’ (Fig. 2A, B; Sub).
Fig. 2.
Aerenchyma formation in leaf sheath of ‘AP’ and ‘FR13A’ under air and after 3 d of total plant submergence (Sub). (A) Representative fresh cross-sections of the leaf sheath rice varieties under air and submergence. (B) Percentage of aerenchymatous area in leaf sheath sections of the two varieties. Data are expressed as mean ± s.e., n = 9; * P < 0·05 and ** P < 0·01 according to Student's t-test. (C) Viability of ‘AP’ and ‘FR13A’ leaf sheath cells showing aerenchyma formation using double staining with FM4-64 and FDA. Viable cells stained green by FDA indicate intact plasma membranes. Absence of green colour and substantial red cell staining with FM4-64 indicates the loss of membrane integrity (arrow). Abbreviations: AE, aerenchyma; SC, schlerenchyma. Scale bar = 0·2 mm.
In rice, aerenchyma develops through lysigeny (Hoshikawa, 1989; Matsukura et al., 2000), resulting from the selective death of root and shoot cortex cells (Kawai et al., 1998).
The viability of leaf sheath cells was examined using a double-labelling procedure with FDA/FM4-64 (green/red) fluorescent dyes (Lombardi et al., 2007; Schapire et al., 2008). FDA stains viable cells green, while FM4-64 stains cell plasma membranes, with increased red fluorescence in the cells where the membrane is damaged (Samaj et al., 2005). Both ‘FR13A’ and ‘AP’ showed dead cells (stained red) localized on the border region of the constitutive aerenchyma, mostly in samples from submerged plants (Fig. 2C). The cell death pattern (Fig. 2C) indicated progressive enlargement of aerenchyma following submergence (Fig. 2C; AE). Red fluorescent cells were also observed along schlerenchyma (Fig. 2C; SC).
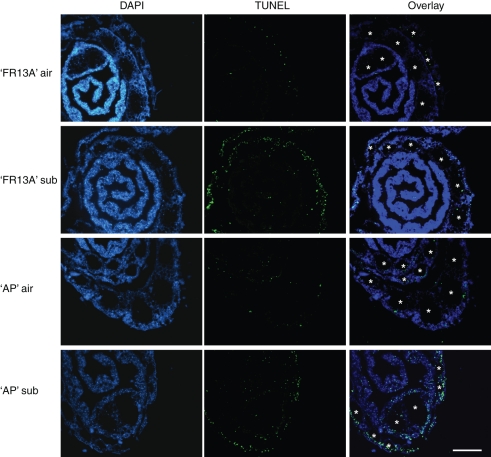
To determine whether DNA fragmentation, a process associated with PCD (Wang et al., 1996), occurred in the nuclei, thus revealing dying cells, transverse sections of ‘FR13A’ and ‘AP’ leaf sheaths were processed for a TUNEL assay. TUNEL-positive nuclei were present in both ‘FR13A’ and ‘AP’ aerobic sections, in the tissue surrounding the expanded dead regions of the parenchymatic cells detected with the FDA/FM4-64 dyes (Fig. 3). DNA degradation became more evident in the submerged samples of both varieties, as demonstrated by the presence of a higher quantity of green-fluorescent TUNEL-positive nuclei (Fig. 3). The blue-fluorescent nuclei detected with DAPI staining showed the presence of an intact nucleus in the other regions of the leaf sheath sections (Fig. 3).
Fig. 3.
DNA fragmentation in ‘AP’ and ‘FR13A’ leaf sheaths under air and after 3 d of total plant submergence (sub), detected by TUNEL staining. DAPI-stained nuclei were used as positive controls. Scale bar = 0·2 mm.
Only ‘AP’ showed submergence-dependent ethylene production
The growth responses described in Fig. 1 suggested that ‘AP’ does not contain the Sub1A gene. This prediction was experimentally confirmed by the lack of Sub1A PCR product in DNA samples of ‘AP’, a japonica group rice variety (data not shown). In ‘FR13A’, a typical Sub1A variety, the mRNA level of Sub1A-1 increased rapidly during submergence (Fig. 4).
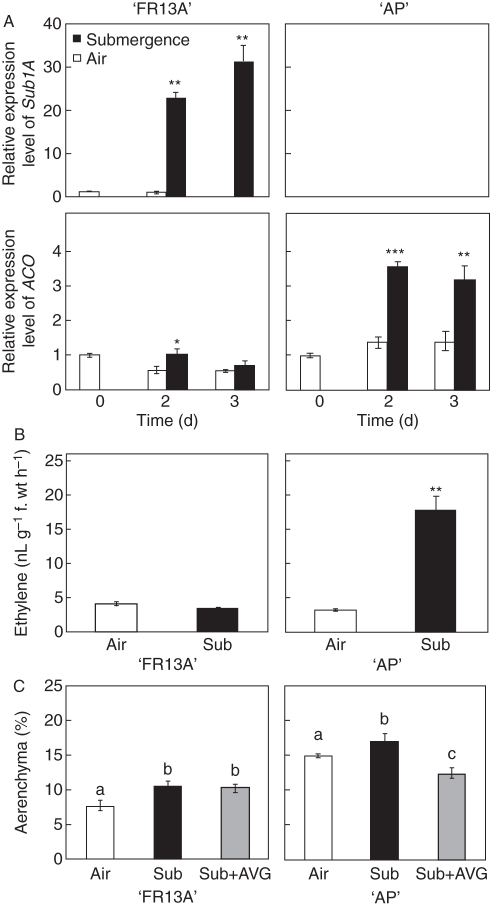
Fig. 4.
Ethylene synthesis in ‘AP’ and ‘FR13A’ leaf sheaths during aerenchyma formation. (A) Expression of Sub1A and ACO genes. The expression level was measured based on ‘FR13A’ air control at day 0 = 1. Data are mean ± s.d., n = 3. *** P < 0·001, ** P < 0·01 and * P < 0·05 according to Student's t-test. (B) Ethylene production after 3 d air and after 3 d of submergence treatment. Data are mean ± s.d., n = 3. ** P < 0·01 according to Student's t-test. (C) Percentage of aerenchymatous area in leaf sheath sections of ‘AP’ and ‘FR13A’ after the submergence treatment, with or without the ethylene biosynthesis inhibitor AVG (500 µm), every 24 h for 3 d before and during submergence. Data are mean ± s.e., n = 9. Different lower-case letters indicate significant differences between treatments (0·05 significance level) based on LSD multiple pairwise comparison test.
Since Sub1A has been reported to reduce ethylene synthesis (Fukao et al., 2006), a plant hormone with a predominant role in aerenchyma formation (Shiono et al., 2008), we checked whether ‘AP’ and ‘FR13A’ differed in their ability to produce ethylene when submerged.
The mRNA level of ACC oxidase (ACO), a key enzyme for ethylene biosynthesis, increased in ‘AP’ under flooding stress, but not in ‘FR13A’ (Fig. 4A). In agreement with the molecular evidence, ethylene production was significantly higher in submerged ‘AP’ leaf sheaths (Fig. 4B), while in ‘FR13A’ no increased ethylene synthesis/entrapment (Voesenek et al., 1993) was observed following submergence (Fig. 4B).
Treating the plants with the ethylene biosynthesis inhibitor AVG prevented the submergence-dependent increase in aerenchyma formation in ‘AP’ but no change was observed in ‘FR13A’ (Fig. 4C), suggesting that ethylene is not involved in leaf sheath underwater aerenchyma formation in this Sub1A variety.
‘FR13A’ displayed increased ROS accumulation under submergence
The lack of ethylene synthesis as a consequence of submergence (Fig. 4B), together with the increased aerenchymatous areas in ‘FR13A’ (Fig. 2) prompted us to verify whether an increased ROS production could compensate for the lack of ethylene as a signal triggering underwater aerenchyma formation in ‘FR13A’.
A significantly increased level of H2O2 was detected in submerged ‘FR13A’ leaf sheaths (Fig. 5A), while in ‘AP’ the H2O2 level was unchanged (Fig. 5A).
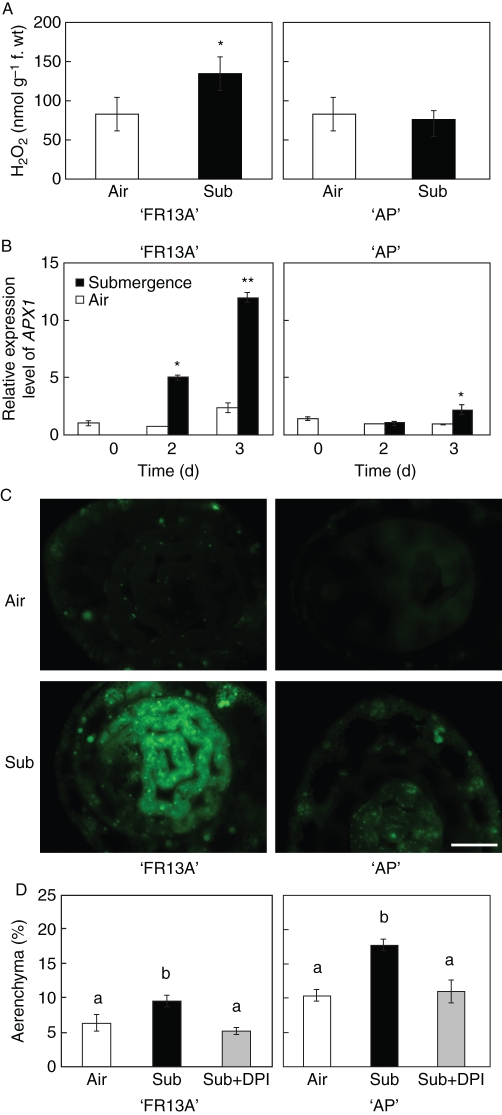
Fig. 5.
H2O2 production in ‘AP’ and ‘FR13A’ leaf sheaths. (A) H2O2 production after 3 d of submergence treatment and in well-drained conditions (air). Data are the mean ± s.d., n = 3; * P < 0·05 according to Student's t-test. (B) Expression pattern of APX1. The expression level was measured based on ‘FR13A’ control air at day 0 = 1. Data are the mean ± s.d., n = 3; ** P < 0·01 and * P < 0·05 according to Student's t-test. (C) Leaf sheath sections of ‘AP’ and ‘FR13A’ treated with the green fluorescent dye H2-DCFDA for the detection of ROS. Scale bar = 0·2 mm. (D) Percentage of aerenchymatous area in leaf sheath sections of ‘AP’ and ‘FR13A’ after the submergence treatment, with or without DPI (10 µm), every 24 h for 3 d before and during submergence. Data are mean ± s.e., n = 3. Different lower-case letters indicate significant differences between treatments (0·05 significance level) based on LSD multiple pairwise comparison.
A remarkable induction of ascorbate peroxidase 1 (APX1), a H2O2-induced mRNA (Karpinsky et al., 1997, 1999), was also observed in ‘FR13A’ under submergence, while no variation in the APX1 expression was detectable in ‘AP’ (Fig. 5B).
ROS production, detected using the fluorescent dye H2-DCFDA, was highest in ‘FR13A’ leaf sheath sections in submerged plants, in the internal immature tissue that had not yet been directly affected by the aerenchymatous areas (Fig. 5C). A H2-DCFDA green-fluorescence signal, indicating ROS accumulation, was also observed in both the varieties around the vascular tissue system towards the xylem (Fig. 5C).
Treating the plants with DPI, an inhibitor of the ROS-producing NADPH oxidase, prevented the submergence-dependent increase in aerenchyma formation in both ‘AP’ and ‘FR13A’ (Fig. 5C). This suggests that a NADPH oxidase-dependent ROS signalling is involved in leaf sheath aerenchyma formation under submergence.
DISCUSSION
Rice is a semi-aquatic plant, which is able to survive prolonged submergence. The molecular basis behind two adaptation mechanisms for surviving flooding have been identified in different rice genotypes, highlighting the existence of profound intra-specific variations in plant survival strategies to submergence (for reviews, see Bailey-Serres and Voesenek, 2008, 2010; Colmer and Voesenek, 2009; Bailey-Serres et al., 2010; Nagai et al., 2010).
Lowland rice varieties, belonging to the indica group and harbouring the ethylene-responsive factor Sub1A, respond to submergence by adopting a quiescence strategy, which includes reduced growth (Fukao et al., 2006; Xu et al., 2006). Deepwater rice varieties display an opposite strategy of fast internode elongation when submerged (Kende et al., 1998), in an attempt to reach the water surface and avoid O2 deprivation (Hattori et al., 2009). This strong growth is controlled by the two ethylene-responsive factors, SK1 and SK2, which are absent in all the non-deepwater rice varieties evaluated to date, but are present in some wild Oryza species that show deepwater responses (Hattori et al., 2009). The escape strategy is also activated in lowland rice lacking Sub1A; however, it is often unsuccessful if the plant is unable to reach the water surface early enough to avoid prolonged O2 deprivation, before a rapid depletion of carbohydrate reserves has taken place (Bailey-Serres and Voesenek, 2008).
It was observed that both ‘AP’, a variety lacking Sub1A, SK1 and SK2, and ‘FR13A’, a variety containing Sub1A, can survive moderate submergence, while ‘FR13A’ can additionally survive complete submergence, through the Sub1A tolerance mechanism. Under partial and total submergence, ‘AP’ showed fast growth with an energy cost which is probably exceedingly high, as demonstrated by the low percentage of survival (Fig. 1). Although the two varieties differ profoundly in their tolerance mechanisms, they both display increased aerenchyma formation when submerged, indicating that not only fast-elongating varieties, but also Sub1A varieties rely on O2 transport to the underwater organs when submergence is shallow. Aerenchyma is very important in deep flooding conditions, allowing the plant to access the alternative O2 sources, i.e. submergence water, underwater photosynthesis and leaves surface gas film (Colmer and Pedersen, 2008; Pedersen et al., 2009).
The major problem that a plant has to confront when submerged is a dramatic reduction in gas exchanges (Bailey-Serres and Voesenek, 2008). A decline in O2 to a concentration that limits aerobic respiration, leads to reduced ATP synthesis (for a review, see Gibbs and Greenway, 2003). Aerenchyma formation is one of the plant's morphological adaptations that helps to increase gas circulation inside the underwater organs (Evans, 2003).
By analysing the leaf sheaths of the two rice varieties ‘FR13A’ and ‘AP’, it was found that both displayed constitutive aerenchyma when not submerged which increased following submergence (Fig. 2). Increased aerenchyma likely helps both ‘AP’ and ‘FR13A’ to survive minimal submergence better (Fig. 1). The mechanism of aerenchyma formation appears to be similar in ‘AP’ and ‘FR13A’ leaf sheaths and is based on progressive cell-death along the peripheral tissues of the aerenchymatous tubes under submersion (Figs 2C and 3). TUNEL-positive nuclei that are peripherical to the FM4-64 fluorescent dead cells suggest the activation of a DNA-degradation mechanism prior to the final disruption of the nucleus under autolysis (Bouranis et al., 2003).
The signalling events leading to aerenchyma formation were very different. In ‘FR13A’ the activation of the Sub1A gene (Xu et al., 2006) probably limited the submergence-induced ethylene production. On the other hand, ‘AP’, which does not posses the Sub1A gene, displayed a remarkable induction of ACO, probably leading to the observed high ethylene synthesis in submerged plants. Therefore, although ethylene is known to be a key component in the lysigenous aerenchyma formation under waterlogging through PCD (for a review, see Shiono et al., 2008), it does not explain the formation of aerenchyma in ‘FR13A’. In agreement with our hypothesis that ethylene plays only a minor role in aerenchyma formation in ‘FR13A’, treatment with the ethylene biosynthesis inhibitor AVG resulted in a significant reduction in aerenchyma in ‘AP’ only (Fig. 4C).
Recently, aerenchyma formation in rice stems was found to be dependent on both ethylene and ROS accumulation (Steffens et al., 2011). Moreover, in arabidopsis hypocotyls, both ethylene and ROS are involved in aerenchyma formation under hypoxic conditions (Mühlenbock et al., 2007). A clear increase in H2O2 concentrations was found in submerged ‘FR13A’ plants (Fig. 5A), together with the up-regulation of APX1 expression (Fig. 5B), H2O2 regulated in higher plants (Karpinsky et al., 1997, 1999; Volkov et al., 2006). In addition, the analysis of ROS presence in the ‘AP’ and ‘FR13A’ leaf sheath sections revealed a higher H2O2 level in submerged ‘FR13A’, preferentially localized in the internal tissues that had not yet been affected by aerenchyma development (Fig. 5C). Similarly, high fluorescence due to the ROS-staining DCFH2-DA was observed in intact, sulfate-starved cells in maize roots forming aerenchyma (Bouranis et al., 2003). In the present experiments, treating the leaf sheaths with DPI, an inhibitor of the the ROS-producing NADPH oxidase enzyme, resulted in reduced aerenchyma formation in both ‘FR13A’ and ‘AP’. It has been proposed that the timing of ROS signalling occurs ahead to ethylene signalling in aerenchyma development in arabidopsis (Mühlenbock et al., 2007). In our hypothesis, a ROS transient production occurs ahead to ethylene signalling in rice as well and is not detectable in ‘AP’ after 3 d of flooding.
In ‘FR13A’, the ethylene synthesis response is blocked when plants are submerged (Fukao et al., 2006) and ROS-dependent signalling is likely to substitute it in promoting further aerenchyma production.
Jung et al. (2010) reported the activation of genes related to the removal of ROS in M202(Sub1) plants after submergence, suggesting that Sub1A is a positive regulator in ROS scavenging, in agreement with the higher APX1 expression that was observed in ‘FR13A’ (Fig. 5B).
It is concluded that ethylene signalling is not involved in aerenchyma formation in ‘FR13A’. The two cultivars investigated do not differ in the presence of Sub1A only, thus the observed difference can be due to several factors. However, it is likely that Sub1A rice varieties, that rely on a limited production of ethylene when submerged to restrict their growth response, have developed alternative signals to enhance aerenchyma formation, which presumably help the plant to survive shallow flooding. The production of ROS in submerged Sub1A rice varieties, such as ‘FR13A’, may be that signal. Further analyses are under way to evaluate the possible role of the Sub1A gene in activating ROS signalling and to determine its relationship to aerenchyma formation under submergence.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was supported by the Scuola Superiore Sant'Anna. N. P. Kudahettige and S. Parlanti were each supported by a PhD fellowship (N.P.K., PhD School on Biomolecular Sciences, University of Pisa; S.P., Scuola Superiore Sant'Anna-University of Pisa joint PhD course on Crop Science).
LITERATURE CITED
- Armstrong W. Radial oxygen losses from intact rice roots as affected by distance from the apex, respiration, and waterlogging. Physiologia Plantarum. 1971;25:192–197. [Google Scholar]
- Atwell BJ, Drew MC, Jackson MB. The influence of oxygen deficiency on ethylene synthesis, l-aminocyclopropane-l-carboxylic acid levels and aerenchyma formation in roots of Zea mays L. Physiologia Plantarum. 1988;72:15–22. [Google Scholar]
- Bailey-Serres J, Voesenek LACJ. Flooding stress: acclimations and genetic diversity. Annual Review of Plant Biology. 2008;59:313–339. doi: 10.1146/annurev.arplant.59.032607.092752. [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Voesenek LACJ. Life in the balance: a signaling network controlling survival of flooding. Current Opinion in Plant Biology. 2010;13:489–494. doi: 10.1016/j.pbi.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Fukao T, Ronald P, Ismail A, Heuer S, Mackill D. Submergence tolerant rice: SUB1's journey from landrace to modern cultivar. Rice. 2010;3:138–147. [Google Scholar]
- Banti V, Mafessoni F, Loreti E, Alpi A, Perata P. The heat-inducible transcription factor HsfA2 enhances anoxia tolerance in Arabidopsis. Plant Physiology. 2010;152:1471–1483. doi: 10.1104/pp.109.149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter-Burrell A, Yang Z, Springer P, Bailey-Serres J. RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science. 2002;296:2026–2028. doi: 10.1126/science.1071505. [DOI] [PubMed] [Google Scholar]
- Bouranis DL, Chorianopoulou SN, Siyiannis VF, Protonotarios VE, Hawkesford MJ. Aerenchyma formation in roots of maize during sulphate starvation. Planta. 2003;217:382–391. doi: 10.1007/s00425-003-1007-6. [DOI] [PubMed] [Google Scholar]
- Bouranis DL, Chorianopoulou SN, Kollias C, et al. Dynamics of aerenchyma distribution in the cortex of sulfate-deprived adventitious roots of maize. Annals of Botany. 2006;97:695–704. doi: 10.1093/aob/mcl024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD, Pedersen O. Oxygen dynamics in submerged rice (Oryza sativa) New Phytologist. 2008;178:326–334. doi: 10.1111/j.1469-8137.2007.02364.x. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Voesenek LACJ. Flooding tolerance: suites of plant traits in variable environments. Functional Plant Biology. 2009;36:665–681. doi: 10.1071/FP09144. [DOI] [PubMed] [Google Scholar]
- Das DK, Jat RL. Influence of three soil-water regimes on root porosity and growth of four rice varieties. Agronomy Journal. 1977;69:197–200. [Google Scholar]
- Datta SK, Banerji B. Behaviour of rice varieties under varying nature of flooded and non flooded conditions. Indian Journal of Agricultural Sciences. 1973;43:1040–1045. [Google Scholar]
- Drew MC, Jackson MB, Giffard SC, Campbell R. Inhibition by silver ions of gas space (aerenchyma) formation in adventitious roots of Zea mays L. subjected to exogenous ethylene or to oxygen deficiency. Planta. 1981;153:217–224. doi: 10.1007/BF00383890. [DOI] [PubMed] [Google Scholar]
- Drew MC, He CJ, Morgan PW. Decreased ethylene biosynthesis, and induction of aerenchyma, by nitrogen- or phosphate-starvation in adventitious roots of Zea mays L. Plant Physiology. 1989;91:266–271. doi: 10.1104/pp.91.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE. Aerenchyma formation. New Phytologist. 2003;161:35–49. [Google Scholar]
- Fath A, Bethke PC, Jones RL. Enzymes that scavenge reactive oxygen species are down-regulated prior to gibberellic acid-induced programmed cell death in barley aleurone. Plant Physiology. 2001;126:156–166. doi: 10.1104/pp.126.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Xu K, Ronald PC, Bailey-Serres J. A variable cluster of ethylene response factor- like genes regulates metabolic and developmental acclimation responses to submergence in rice. The Plant Cell. 2006;18:2021–2034. doi: 10.1105/tpc.106.043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler-Lee J, Caldwell C, Gallie DR. Expression of the ethylene biosynthetic machinery in maize roots is regulated in response to hypoxia. Journal of Experimental Botany. 2010;61:857–871. doi: 10.1093/jxb/erp362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J, Greenway H. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology. 2003;30:1–47. doi: 10.1071/PP98095. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Nagai K, Furukawa S, et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature. 2009;460:1026–1030. doi: 10.1038/nature08258. [DOI] [PubMed] [Google Scholar]
- He CJ, Morgan PW, Drew MC. Enhanced sensitivity to ethylene in nitrogen- or phosphate-starved roots of Zea mays L. during aerenchyma formation. Plant Physiology. 1992;98:137–142. doi: 10.1104/pp.98.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CJ, Morgan PW, Drew MC. Induction of enzymes associated with lysigenous aerenchyma formation in roots of Zea mays during hypoxia or nitrogen starvation. Plant Physiology. 1994;105:861–865. doi: 10.1104/pp.105.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CJ, Finlayson SA, Drew MC, Jordan WR, Morgan PW. Ethylene biosynthesis during aerenchyma formation in roots of maize subjected to mechanical impedance and hypoxia. Plant Physiology. 1996;112:1679–1685. doi: 10.1104/pp.112.4.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshikawa K. The growing rice plant. Tokyo: Noubunkyo; 1989. Leaf, stem, and root; pp. 87–121. [Google Scholar]
- Jackson MB, Fenning TM, Drew MC, Saker LR. Stimulation of ethylene production and gas-space (aerenchyma) formation in adventitious roots of Zea mays L. by small partial pressures of oxygen. Planta. 1985;165:486–492. doi: 10.1007/BF00398093. [DOI] [PubMed] [Google Scholar]
- Jung KH, Seo YS, Walia H, et al. The submergence tolerance regulator Sub1A mediates stress-responsive expression of AP2/ERF transcription factors. Plant Physiology. 2010;152:1674–1692. doi: 10.1104/pp.109.152157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justin SHFW, Armstrong W. Evidence for the involvement of ethene in aerenchyma formation in adventitious roots of rice (Oryza sativa L.) New Phytologist. 1991;118:49–62. [Google Scholar]
- Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux P. Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. The Plant Cell. 1997;9:627–640. doi: 10.1105/tpc.9.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P. Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science. 1999;284:654–657. doi: 10.1126/science.284.5414.654. [DOI] [PubMed] [Google Scholar]
- Kawai M, Samarajeewa PK, Barrero RA, Nishiguchi M, Uchimiya H. Cellular dissection of the degradation pattern of cortical cell death during aerenchyma formation of rice roots. Planta. 1998;204:277–287. [Google Scholar]
- Kawase M, Whitmoyer RE. Aerenchyma development in waterlogged plants. American Journal of Botany. 1980;67:18–22. [Google Scholar]
- Kawase M. Effect of flooding on ethylene concentration in horticultural plants. Journal of American Society of Horticulture Science. 1972;97:584–588. [Google Scholar]
- Kawase M. Anaerobic elevation of ethylene concentration in waterlogged plants. American Journal of Botany. 1978;65:736–740. [Google Scholar]
- Kende H, Van der Knaap E, Cho HT. Deepwater rice: a model plant to study stem elongation. Plant Physiology. 1998;118:1105–1110. doi: 10.1104/pp.118.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Könings H. Ethylene-promoted formation of aerenchyma in seedling roots of Zea mays L. under aerated and non-aerated conditions. Physiologia Plantarum. 1982;54:119–124. [Google Scholar]
- Könings H, Jackson MB. A relationship between rates of ethylene production by roots and the promoting or inhibiting effects of exogenous ethylene and water on root elongation. Zeitschrift fuer Pflanzenphysiologie. 1979;92:385–397. [Google Scholar]
- Lenochova Z, Soukup A, Votrubova O. Aerenchyma formation in maize roots. Biologia Plantarum. 2009;53:263–270. [Google Scholar]
- Licausi F, Perata P. Low oxygen signaling and tolerance. Advances in Botanical Research. 2009;50:139–198. [Google Scholar]
- Lombardi L, Casani S, Ceccarelli N, Galleschi L, Picciarelli P, Lorenzi R. Programmed cell death of the nucellus during Sechium edule Sw. seed development is associated with activation of caspase-like proteases. Journal of Experimental Botany. 2007;58:2949–2958. doi: 10.1093/jxb/erm137. [DOI] [PubMed] [Google Scholar]
- Matsukura C, Kawai M, Toyofuku K, Barrero RA, Uchimiya H, Yamaguchi J. Transverse vein differentiation associated with gas space formation fate of the middle cell layer in leaf sheath development of rice. Annals of Botany. 2000;85:19–27. [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends in Plant Science. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Mühlenbock P, Plaszczyca M, Plaszczyca M, Mellerowicz E, Karpinski S. Lysigenous aerenchyma formation in Arabidopsis is controlled by LESION SIMULATING DISEASE1. The Plant Cell. 2007;19:3819–3830. doi: 10.1105/tpc.106.048843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K, Hattori Y, Ashikari M. Stunt or elongate? Two opposite strategies by which rice adapts to floods. Journal of Plant Research. 2010;123:303–309. doi: 10.1007/s10265-010-0332-7. [DOI] [PubMed] [Google Scholar]
- Pedersen O, Rich SM, Colmer TD. Surviving floods: leaf gas films improve O2 and CO2 exchange, root aeration, and growth of completely submerged rice. The Plant Journal. 2009;58:147–156. doi: 10.1111/j.1365-313X.2008.03769.x. [DOI] [PubMed] [Google Scholar]
- Pradhan SK, Varade SB, Kar S. Influence of soil water conditions on growth and root porosity of rice. Plant Soil. 1973;38:501–507. [Google Scholar]
- Samaj J, Read ND, Volkmann D, Menzel D, Baluska F. The endocytic network in plants. Trends in Cell Biology. 2005;15:425–433. doi: 10.1016/j.tcb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Schapire AL, Voigt B, Jasik J, et al. Arabidopsis synaptotagmin 1 is required for the maintenance of plasma membrane integrity and cell viability. The Plant Cell. 2008;20:3374–3388. doi: 10.1105/tpc.108.063859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin R, Schachtman DP. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proceedings of the National Academy of Sciences of the USA. 2004;101:8827–8832. doi: 10.1073/pnas.0401707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiono K, Takahashi H, Colmer TD, Nakazono M. Role of ethylene in acclimations to promote oxygen transport in roots of plants in waterlogged soils. Plant Science. 2008;175:52–58. [Google Scholar]
- Steffens B, Geske T, Sauter M. Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytologist. 2011;190:369–378. doi: 10.1111/j.1469-8137.2010.03496.x. [DOI] [PubMed] [Google Scholar]
- Visser EJW, Bögemann GM. Aerenchyma formation in the wetland plant Juncus effusus is independent of ethylene. New Phytologist. 2006;171:305–314. doi: 10.1111/j.1469-8137.2006.01764.x. [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Banga M, Thier RH, et al. Submergence-induced ethylene synthesis, entrapment, and growth in two plant species with contrasting flooding resistances. Plant Physiology. 1993;103:783–791. doi: 10.1104/pp.103.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov RA, Panchuk II, Mullineaux PM, Schöffl F. Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Molecular Biology. 2006;61:733–746. doi: 10.1007/s11103-006-0045-4. [DOI] [PubMed] [Google Scholar]
- Wang M, Oppedijk BJ, Xin L, Van Duijn B, Schilperoort RA. Apoptosis in barley aleurone during germination and its inhibition by abscissic acid. Plant Molecular Biology. 1996;32:1125–1134. doi: 10.1007/BF00041396. [DOI] [PubMed] [Google Scholar]
- Xu K, Xu X, Fukao T, et al. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature. 2006;442:705–708. doi: 10.1038/nature04920. [DOI] [PubMed] [Google Scholar]
- Zhou Z, de Almeida Engler J, Rouan D, Michiels F, Van Montagu M, Van Der Straeten D. Tissue localization of a submergence-induced 1-aminocyclopropane-1-carboxylic acid synthase in rice. Plant Physiology. 2002;129:72–84. doi: 10.1104/pp.001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.