Abstract
Proteins encoded by the epidermal growth factor receptor (EGFR/HER1/ERBB1) gene are being studied as diagnostic, prognostic, and theragnostic biomarkers for numerous human cancers. The clinical application of these tissue/tumor biomarkers has been limited, in part, by discordant results observed for EGFR expression using different immunological reagents. Previous studies have used EGFR-directed antibodies that cannot distinguish between full-length vs. soluble EGFR (sEGFR) expression. We have generated and characterized an anti-sEGFR polyclonal antiserum directed against a 31-mer peptide (residues 604–634) located within the unique 78 amino acid carboxy-terminal sequence of sEGFR. Here, we use this antibody to demonstrate that sEGFR is co-expressed with EGFR in a number of carcinoma-derived cell lines. In addition, we show that a second protein of ~140-kDa (p140) also is detected by this antibody. Rigorous biochemical characterization identifies this second protein to be α5-integrin. We show that a 26 amino acid peptide in the calf domain of α5-integrin (residues 710–35) shares 35% sequence identity with a 31-mer carboxy-terminal sEGFR peptide, and exhibits approximately 5 times lower affinity for anti-sEGFR than does the homologous 31-mer sEGFR peptide. We conclude that the carboxy-terminus of sEGFR and the calf-1 domain of α5-integrin share a region of sequence identity, which results in their mutual immunological reactivity with anti-sEGFR. We also demonstrate that anti-sEGFR promotes 3D tissue cohesion and compaction in vitro, further suggesting a functional link between sEGFR and α5-integrin and a role of the calf-1 domain in cell adhesion. These results have implications for the study of both EGFR and sEGFR as cancer biomarkers, and also provide new insight into the mechanisms of interaction between cell surface EGFR isoforms and integrin in complex processes such as cell adhesion and survival signaling.
The EGFR/HER/ErbB family of receptor tyrosine kinases consists of EGFR/HER1, ErbB2/HER2/neu, ErbB3/HER3 and ErbB4/HER4. These receptors signal via both ligand-dependent (1) and -independent (2) pathways to regulate cell growth, differentiation, survival, migration, and adhesion, as well as oncogenic transformation. Consequently, it is not surprising that ErbB/HER overexpression and/or mutational activation play major roles in the etiology and progression of human cancer. In support of this concept, a number of HER receptor-targeted therapeutics have been FDA-approved (e.g., cetuximab, gefitinib, trastuzumab) for the treatment of adult solid malignancies, while many others are under clinical investigation (3).
EGFR is the prototypic member of the ErbB/HER receptor family; it is a ~170-kDa glycoprotein consisting of an amino-terminal extracellular domain (ECD), a short transmembrane domain, and a carboxy-terminal intracellular domain (Figure 1). The ECD is divided into four subdomains, I–IV; subdomains I and III regulate ligand binding, while subdomains II and IV regulate receptor homo- or hetero-dimerization. In addition to full-length EGFR, we have reported the isolation of a cDNA clone encoding a soluble EGFR isoform that contains most of the ECD, but which lacks the transmembrane and intracellular domains. This EGFR isoform, sEGFR, arises from an alternate splice event, and incorporates a short region of novel coding sequence, including a termination codon and two polyadenylation sequences (4). Specifically, sEGFR contains sequences encompassing EGFR ECD subdomains I–III, most of subdomain IV, and the unique 78 amino acid carboxy-terminus (Figure 1) (5). This alternatively spliced sEGFR protein product is co-expressed with EGFR in diverse human tissues and also is the precursor for the major circulating isoform of EGFR in human blood, as discussed below.
FIGURE 1.
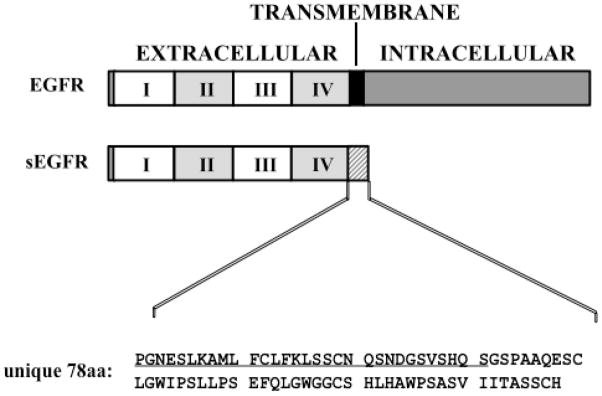
Schematic diagram illustrating the structure of EGFR compared to sEGFR. The extracellular domain is divided into four subdomains (I–IV); the transmembrane domain is indicated by the black box; the alternate C-terminal amino acid sequence of sEGFR is indicated by the hatched box. A peptide matching the first 31 amino acids (residues 604–634) of the unique C-terminus of sEGFR (underlined) was used to generate anti-sEGFR as described by Christensen et al. (10).
While sEGFR does not contain a canonical transmembrane domain, it is, nevertheless, associated with the cell surface (6), and we recently have shown that cell surface sEGFR is released into the extracellular milieu via a complex process that is regulated by the extracellular matrix protein fibronectin (Wilken et al., unpublished experiments). Purification of anti-EGFR reactive protein(s) from human blood reveals one major protein species which contains peptides derived from the unique carboxy-terminus of sEGFR as determined by mass spectrometry (7). Taken together, these observations support the hypothesis that sEGFR released from the cell surface enters the bloodstream via the lymphatic system and thoracic duct. Translational studies have shown that low serum sEGFR concentrations are associated with the presence of ovarian cancer (7), poor overall survival in endometrial cancer patients (8), and responsiveness to aromatase inhibitor therapy in breast cancer patients (9). To develop further the clinical utility of sEGFR as a serum and tumor biomarker we have generated polyclonal antisera directed against a 31-mer peptide (residues 604–634) located within the unique carboxy-terminal sequence of sEGFR (Figure 1) (10). This affinity-purified antibody (anti-sEGFR) is specific for sEGFR, and does not detect the full-length EGFR, or any other HER receptor family member (10). Here, we report the further characterization of this anti-sEGFR antibody, including an apparent evolutionary convergence between the unique sEGFR carboxy-terminal sequence and a short bioactive sequence within the extracellular region of α5-integrin, i.e., the calf-1 domain, which coordinates ligand-dependent associations between the α and β subunits of the integrin heterodimer (11).
MATERIALS AND METHODS
Reagents and cell lines
The JEG-3 choriocarcinoma cell line was purchased from American Type Culture Collection (Manassas, VA). Chinese hamster ovary (CHO) cells that stably express EGFR (CHO-EGFR), CHO cells that stably express sEGFR (CHO-sEGFR), antisera generated toward a 31-mer carboxy-terminal peptide of sEGFR (Figure 1, residues 604–634), and methods to affinity purify these polyclonal antibodies have been described previously (10). CHO cells that express low levels of endogenous α5-integrin (CHO B2) and CHO B2 cells stably expressing human α5-integrin (CHO-α5) have been described previously (12, 13). CHO-P3 cells were generated by transfecting CHO-B2 cells with pcDNA3 vector followed by G418 selection as described previously (14). Supersignal West Femto enhanced chemiluminescent luminol (ECL) substrate, goat anti-rabbit secondary antibody, and protein A/G agarose beads were purchased from Thermo Scientific (Rockford, Il). SP Sepharose was purchased from GE Healthcare (Piscataway, NJ). Hydroxylapatite was purchased from Bio-Rad (Hercules, CA). Rabbit anti-BASP-1 (AB9306) purified antibody, α5-integrin (AB1949) antiserum, and β1-integrin (AB1952) antiserum were purchased from Millipore (Billerica, MA). Rabbit anti-EGFR (cytoplasmic domain; sc-03) purified antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit IgG was purchased from Sigma (St. Louis, MO). Recombinant α5/β1 integrin and αv/β3 integrin were purchased from R&D Systems, Inc (Minneapolis, MN). Trypsin was purchased from Promega (Madison, WI). Cyanogen bromide (CNBr; 5M in acetonitrile) was purchased from Sigma. Peptide N-Glycosidease F (PNGaseF) was purchased from New England Biolabs (Ipswitch, MA). All cell culture reagents were purchased from Mediatech (Manassas, VA), except as indicated below. A2780 and A2780 CP-R cells were maintained in RPMI-1640, supplemented with 10% fetal bovine serum (FBS), 10 μg/ml insulin, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. ES-2 cells were maintained in McCoy’s 5A media, supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. HEY cells were maintained in DMEM/F12, supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. IGROV-1, OVCAR-5, OVCAR-8, and JEG-3 cells were maintained in DMEM supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. CHO-EGFR and CHO-sEGFR cells were maintained in F12 media with supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 800 μg/ml G418. CHO B2 cells were maintained in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (Hyclone, Logan, UT), 1 mM MEM non-essential amino acids, and 100 mg/ml streptomycin sulfate (Invitrogen); CHO-P3 and CHO-α5 cells were maintained in the same medium supplemented with 250 μg/ml G418.
Immunoblot analysis of protein expression
Confluent or near-confluent cells were rinsed three times with ice cold potassium phosphate buffered saline (K-PBS; 10 mM KH2PO4/K2HPO4, 150 mM NaCl, pH 7.2) and harvested by scraping into K-PBS followed by centrifugation at 500g for 5 min. Cell pellets were lysed by boiling with 2.5% sodium dodecyl sulfate (SDS), 0.5% sodium deoxycholate (DOC), and 0.5% Triton X-100 (TX-100) for 10 min. Total protein content of cell lysates was determined by Bio-Rad DC assay. Cell lysates were boiled for 5 min with 4x Laemmli reducing buffer (0.25 M Tris, pH 6.8, 40% glycerol, 8% SDS, 2% β-mercaptoethanol, 0.2% bromophenol blue) or 4x Laemmli non-reducing buffer (omit β-mercaptoethanol), equally loaded into gel lanes based on total protein, and resolved by SDS-PAGE (7.5% acrylamide). Resolved proteins were transferred to polyvinylidene difluoride (PVDF) membrane by semi-dry immunoblot (Millipore), followed by blocking for one hr with Trizma® buffered saline (TBS; 10 mM Trizma®, 150 mM NaCl, pH 7.4) supplemented with 5% non-fat dry milk. Membranes were rinsed six times for five min each with TBS with 0.1% Tween 20 (TBS-TW20), and incubated with TBS containing 1% BSA and anti-sEGFR (168 ng/ml) or anti-EGFR (cytoplasmic domain; 50 ng/ml) overnight at 4°C. Membranes were rinsed six times for ten min each with TBS-TW20, incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (Pierce, 2.5 ng/ml) for one hr at room temperature, rinsed six times for ten min each, reacted with ECL substrate, and visualized with a NucleoVISION camera station.
Purification of p140
Confluent JEG-3 cells were harvested, lysed, extracted, and subjected to cation exchange and hydroxylapatite chromatography to purify p140.
Pilot extraction studies
Cell pellets were lysed by boiling for 10 min in one of the following lysis solutions: i) 2.5% SDS, 0.5% NP-40, and 0.5% DOC; ii) 1% TX-100 and 1 mM EDTA in 10 mM Trizma®, pH 7.4; iii) 0.5% DOC; iv) 2.5% SDS; v) 0.5% TX-100; vi) 0.5% DOC in TBS; vii) 2.5% SDS in TBS; viii) or 0.5% TX-100 in TBS. Lysates were cooled to 4°C and centrifuged at 10,000g for 30 min to pellet cell debris. Supernatants were assessed for p140 content by SDS-PAGE (7.5% acrylamide) and immunoblot methods.
Cation exchange pH optimization
Aliquots of SP Sepharose beads (0.5 ml each) were equilibrated (3 washes each) with either 40 mM malonic acid (pH 2.5), 40 mM citric acid (pH 3.0 or 3.5), 100 mM formic acid (pH 4.0), 100 mM succinic acid (pH 4.5), 100 mM acetic acid (pH 5.0), 100 mM malonic acid (pH 5.5 or 6.0), 100 mM sodium phosphate (pH 7.0 or 7.5), or 100 mM HEPES (pH 8.0). JEG-3 cell lysates (0.5 ml each) prepared with unbuffered 1% TX-100 were added to each aliquot of SP Sepharose, mixed for one hr on a rotary shaker, and briefly centrifuged to pellet the SP Sepharose. Each supernatant (100 μl) was assessed by immunoblot analysis for the binding of p140 to SP Sepharose.
Cation exchange chromatography
JEG-3 cell lysates prepared with unbuffered 1% TX-100 were bound to SP Sepharose equilibrated with formic acid buffer (50 mM formic acid, pH 4.0, 0.01% TX-100) using a batch method. Unbound proteins were removed with three washes of formic acid buffer. The SP Sepharose slurry was divided into ten 0.5 ml aliquots, to which 2M NaCl and H2O were added to give a total volume of 1.0 ml each and final concentrations of 100, 200, 300, 400, 500, 600, 700, 800, 900, and 1000 mM NaCl. Each aliquot was mixed for 15 min on a rotary shaker, briefly centrifuged to pellet the SP Sepharose, and assessed by immunoblot analysis for p140 enriched fractions.
Hydroxylapatite chromatography
Sodium phosphate buffer (1 M NaH2PO4/Na2HPO4, pH 6.8) was added to JEG-3 lysate prepared with unbuffered 1% TX-100 to give a final concentration of 20 mM sodium phosphate. Lyates were applied to a hydroxylapatite column pre-equilibrated with 20 mM sodium phosphate buffer, pH 6.8, 0.01% TX-100 and washed with 10 column volumes of this equilibration buffer. p140 was eluted from the hydroxylapatite column with a 20–400 mM sodium phosphate buffer gradient.
Final purification scheme
JEG-3 cells were prepared with unbuffered 1% TX-100. Formic acid (1M, pH 4.0) was added to a final concentration of 50 mM, and the solution was mixed overnight at 4°C on a rotary shaker, followed by centrifugation at 10,000g for 30 min to pellet precipitated protein. The supernatant was applied and eluted from a 5 ml SP Sepharose column using the buffers described above. Fractions containing p140 were pooled, adjusted to pH 6.8 with 20 mM dibasic sodium phosphate and NaOH, applied to a 5ml hydroxylapatite column, and eluted with a 20–400 mM sodium phosphate buffer gradient. Fractions containing p140 were pooled, concentrated by Speed-Vac, and analyzed by liquid chromatography electrospray-ionization tandem mass spectrometry (LC ESI MS/MS).
Peptide identification by LC ESI MS/MS
Excised gel bands were washed sequentially for 5 min each with 50 mM ammonium bicarbonate and 50% acetonitrile in 50 mM ammonium bicarbonate, dehydrated with 100% acetonitrile, and rehydrated with 25 mM ammonium bicarbonate buffer containing 12.5 ng/μl trypsin. Each sample was digested at 4ºC for 1 hr, decanted to remove excess solution, overlaid with 25 mM ammonium bicarbonate buffer, digested overnight at 37ºC, quenched by adding 1 μl 50% formic acid, and analyzed by LC ESI MS/MS on a ThermoFinnigan LTQ linear ion trap mass spectrometer. Spectra were searched against the SwissProt database using the MASCOT (Matrix Science) search algorithm to identify the proteins of each corresponding gel band.
Miniblot analysis of JEG-3 and HEY lysates
JEG-3 or HEY cell lysates were loaded on a single well gel, resolved by SDS-PAGE, and transferred to PVDF membrane as described above. Following blocking with 5% NFDM in TBS, the PVDF membrane was placed in a miniblotter (Immunetics) and rinsed with TBS-TW20 using a vacuum manifold. Primary rabbit antibodies (65 μl per channel) directed against BASP-1, α5-integrin, β1-integrin, sEGFR, or EGFR were loaded into separate miniblotter channels, and incubated for 1 hr at 37°C with gentle rocking. Each primary antibody was diluted in TBS with 1% BSA as follows: BASP-1 (1:400–1:1600 dilution), α5-integrin (1:400–1:1600 dilution), β1-integrin (1:400–1:1600 dilution), sEGFR (1:400–1:1600 dilution), and EGFR (1:200–1:400 dilution). The PVDF membrane was washed with TBS-TW20, incubated with secondary antibody (1:4000 dilution), and reacted with ECL reagent.
Immunoprecipitation of p140
TX-100 JEG-3 lysate was diluted 1:9 with immunoprecipitation buffer (50 mM Trizma®, pH 7.4, 190 mM NaCl, 6 mM EDTA, 2.5% TX100) and divided into 1.2 ml aliquots. Aliquots were incubated with 1 μl of anti-α5-integrin antiserum or no antibody, and mixed on a rotary shaker at 4°C overnight. Protein A/G agarose beads (100 μl slurry) were added to each tube and mixed for an additional three hrs. The beads were rinsed three times each with immunoprecipitation wash buffer (10 mM Trizma®, pH 8.3, 150 mM NaCl, 5 mM EDTA) and then TBS with intervening centrifugation to pellet the beads. The final Protein A/G agarose pellet was boiled with 100 μl of Laemmli sample buffer for 10 min, resolved by SDS-PAGE, and processed for immunoblot analysis with anti-sEGFR.
Hydrolysis of integrin
CNBr
Recombinant α5/β1 integrin in 0.05% BSA (50 μl of 1 mg/ml stock) was lyophilized to dryness, and hydrolyzed with 120 μl of CNBr solution (70% formic acid, 15% H2O, 5% 5M CNBr in acetonitrile) in the dark at room temperature. Hydrolysis was terminated by adding 250 μl H2O to 20 μl of reaction solution followed by snap-freezing at 0 min, 5 min, 30 min, 1 hr, 4 hr, and 24 hr time points. Samples were lyophilized and resolved on a 15% acrylamide gel by SDS-PAGE.
PNGaseF
Recombinant α5/β1 integrin in 0.05% BSA (50 μl of 1 mg/ml stock) was denatured according to manufacturer’s instructions. Both PNGaseF-treated and untreated samples were lyophilized to dryness (note, the glycerol content of PNGaseF prevented complete lyophilization of the treated sample) and hydrolyzed with CNBr solution for 2 hrs before terminating the reaction.
Homology alignment
Homology alignment of sEGFR residues 604–634 (31-mer) to human α5-integrin (NCBI Accession entry NP_002196, 1008 residues) was performed using BLOSUM 62 and BLOSUM 30 alignment algorithms developed by the Genetics Computer Group (Madison, WI) and Myers and Miller (15). Gap open/extension penalties were set to standard default values (−12/−4) included in the program. The alignment output from both algorithms was essentially the same with the finding that BLOSUM 30 often identified longer sequence runs of residue identity. The BLOSUM 30 output indicated several regions of identity between the sEGFR 31-mer and α5-integrin, comprised of amino acid residues 637–649 (30% identity), 642–649 (38% identity) and 710–735 (35% identity). Synthetic peptides of these three homologous regions of α5-integrin and the sEGFR 31-mer were made by solid-phase peptide synthesis.
Solid phase peptide synthesis
Three human α5-integrin peptides comprising residues 637–649 (DKAQILLDCGEDN), 642–649 (LLDCGEDN), and 710–735 (PGNFSSLSCDYFAVNQSRLLVCDLGN); and sEGFR 31-mer peptide comprising residues 604–634 (PGNESLKAMLFCLFKLSSCNQSNDGSVSHQS) were synthesized by the Peptide Synthesis Facility of Mayo Clinic (Rochester, MN) using orthogonal solid phase methods on RINK amide resin (EMD Chemicals/Novabiochem, Gibbstown, NJ) as described previously (16). Briefly, peptides linked to RINK resin were assembled on a Liberty Microwave Peptide Synthesizer (0.1 mmol, CEM Corp., Matthews, NC) or APEX 396 Multiple Peptide Synthesizer (0.04 mmol, AAPPTEC, Louisville, KY) using Nα-9-fluorenyl-methoxycarbonyl protected L-amino acids and synthesis protocols provided by each instrument’s manufacturer. After synthesis, peptides were cleaved in-situ (Liberty synthesizer) from the resin support in 90% trifluoroacetic acid /5% water/5% triisopropylsilane (v/v/v) for 30 min at 55ºC. Each peptide was then purified to >90% homogeneity by reverse-phase HPLC (Beckman/Coulter 126P) from a C18 column (Phenomonex, 2.1 cm × 25 cm column) with a gradient elution of 40% n-propanol/water containing 0.1 % TFA. The molecular mass of each HPLC-purified peptide was confirmed by electrospray-ionization mass spectrometry on an MSQ single quad instrument (Thermo Fisher Scientific, Rockford, IL) and homogeneity was assessed by analytical reversed-phase HPLC (Agilent 1100 series, Santa Clara, CA).
Peptide quantification
Total net peptide content of each lyophilized synthetic peptide was determined by Waters AccQ-Tag amino acid analysis method (Waters, Inc., Milford, MA). Briefly, precise dilutions of accurately weighed stock peptide solutions were prepared and 1 μg of each stock peptide was added to a dried glass reaction vial (ashed 6×50 mm). Each peptide was hydrolyzed with 200 μL of constant boiling HCl and a crystal of phenol at 115ºC for 20 hrs under a vacuum seal. The vacuum dried samples were derivatized with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate according to the vendor's instructions. 6-aminoquinolyl-derivatized amino acids were separated on a Waters AccQ-Tag column using the manufacturer’s prescribed gradient on a Shimadzu 10AD HPLC system, and the integrated peak area of each amino acid was determined. Peak integration data was transferred to a Microsoft Office Excel spreadsheet and net peptide content was calculated using a template provided on the Association of Biomolecular Resource Facilities web site (http://www.abrf.org). Standard peak areas for each derivatized amino acid was performed with a known amino acid mixture (Pierce Standard H, Thermo Scientific), which was processed in parallel with the unknown peptide samples. Values for net peptide content of each peptide were reported as % peptide content per mg dry weight.
Surface plasmon resonance
Binding of α5-integrin and sEGFR peptides to affinity-purified anti- sEGFR was evaluated by Precision Antibody using a Biacore 3000 surface plasmon resonance system. Briefly, goat anti-rabbit Fc was directly immobilized to a CM5 chip by amine coupling chemistry and used to capture anti-sEGFR. Binding of anti-sEGFR to α5-integrin peptide 637–649, α5-integrin peptide 710–735, and sEGFR 31-mer peptide 604–634 was first assessed by a scouting analysis (yes or no) with peptide concentrations of 200–2000 nM in assay buffer (10 mM HEPES, pH 7.4, 150 mM NaCl, 3 mM EDTA, 0.005% polyoxyethylenesorbitan). Based on 1st order Kd approximation, serial 1:1 dilutions of each peptide were evaluated to calculate the equilibrium binding constant (KD), which is the ratio of the dissociation constant (Kd) to the association constant (Ka). CM5 chips were regenerated after each assay by washing with 50 mM Trizma®, pH 7.3, 2M NaCl.
Aggregate compaction assay
We have previously described methods for establishing CHO cell aggregates and quantifying CHO cell compaction (13, 17). Briefly, near-confluent monolayers were dissociated from 10-cm tissue culture plates with trypsin/EDTA (Invitrogen). Dispersed cells were washed in complete CHO B2 medium and centrifuged to pellet the cells. The pellet was washed with PBS and suspended in tissue culture medium containing 10% fibronectin-depleted fetal calf serum (18). Rat plasma fibronectin was then added to the medium to a final concentration of 100 μg/ml. Cells were counted using a BioRad TC10 automated cell counter and adjusted to a final concentration of 3.0×106 cells/ml. 10 μl aliquots of cell suspension with PBS, 20 μg/ml control rabbit IgG, or 20 μg/ml anti- sEGFR were deposited on the underside of the lid of a 60-mm tissue culture dish. The bottom of the dish contained 5ml PBS, and served to prevent evaporation of the drops by forming a hydration chamber. The drops were incubated overnight at 37°C, 5% CO2, and 95% humidity allowing cells to coalesce. Images were captured and analyzed using IP Lab imaging and analysis software. Each image was adjusted for optimum contrast. To measure aggregate size, outlines were automatically traced and the number of pixels within the outlines was calculated. Data points representing the mean and standard error for aggregate size expressed in pixels (a measure of aggregate compaction) were calculated from 9 aggregates of each cell line. Aggregate size was compared by one-way ANOVA and Tukey’s Multiple Comparisons Test. Since both CHO-P3 and CHO-α5 cells form circular sheets in the presence of anti- sEGFR, it was possible to calculate differences in surface area by converting pixel diameter to actual diameter and calculating area using standard equations. Statistical analysis in this case was performed using an unpaired Student’s t-test.
RESULTS
Anti-sEGFR polyclonal antibody recognizes two proteins
To evaluate the expression of EGFR and sEGFR in a panel of human carcinoma-derived cell lines, whole cell lysates were resolved by SDS-PAGE and processed for immunoblot analysis using a commercial antibody directed against the EGFR intracellular domain or an affinity-purified anti-sEGFR 31-mer antibody previously generated and characterized by our laboratory (10). CHO cells stably expressing EGFR or sEGFR were used as positive controls (10). Several cell lines (i.e., JEG-3, ES-2, HEY, IGROV-1, and OVCAR-5) expressed full-length EGFR, and several cell lines (i.e., JEG-3, A2780, A2780 CP-R, ES-2, and OVCAR-8) expressed an immunoreactive band migrating at the same apparent molecular weight (~90-kDa) as sEGFR expressed exogenously in CHO cells (Figure 2). Unexpectedly, some carcinoma-derived cell lines (i.e., JEG-3, A2780, A2780 CP-R, ES-2, HEY, and IGROV-1) also expressed a higher apparent molecular weight protein of ~140-kDa (i.e., ‘p140’) reactive with anti-sEGFR. Notably, p140 is highly expressed in the choriocarcinoma-derived JEG-3 and ovarian cystadenocarcinoma-derived HEY cell lines.
FIGURE 2.
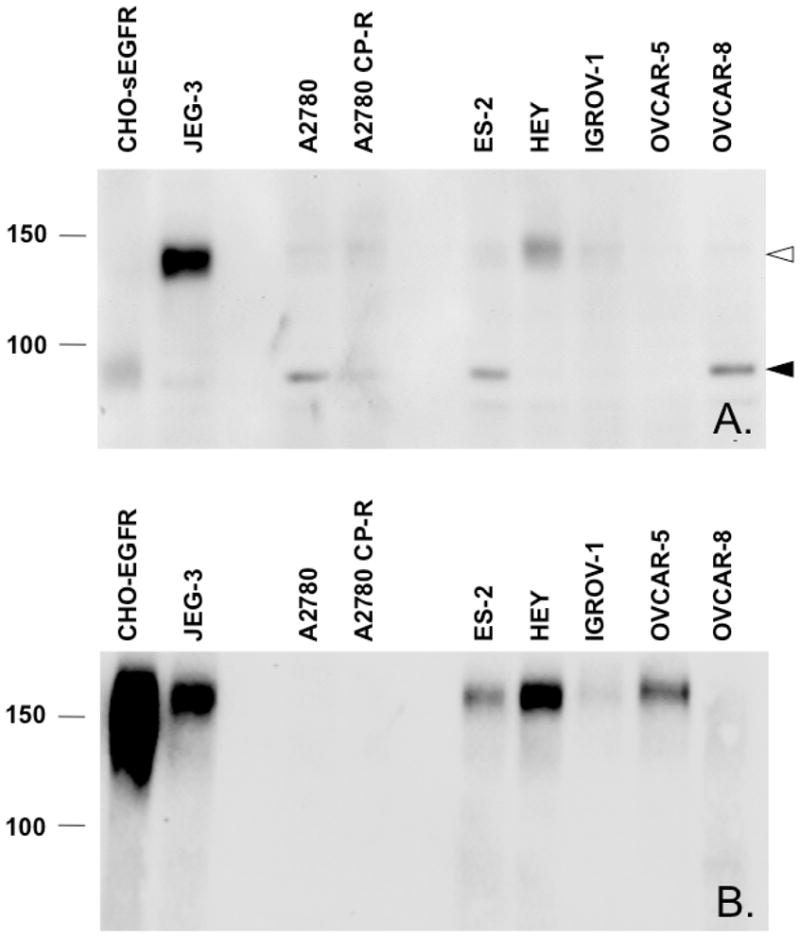
Expression of EGFR, sEGFR and p140 in carcinoma-derived cell lines is detected by immunoblot analysis with anti-sEGFR. Panel A. An anti-sEGFR reactive band of 90-kDa, which is also reactive with a monoclonal antibody against EGFR extracellular domain (not shown) is detected in the lysates of CHO cells stably expressing sEGFR (CHO-sEGFR), as well as in A2780, ES-2, and OVCAR-8 cells (closed arrowhead). A second immunoreactive band of 140-kDa is detected in JEG-3, HEY, A2780, A2780-CP-R, ES-2, and IGROV-1 cells (open arrowhead). Panel B. An anti-EGFR (intracellular domain specific) reactive band of 170 kDa is detected in the lysates of CHO cells stably expressing EGFR (CHO-EGFR), as well as in JEG-3, ES-2, HEY, IGROV-1, OVCAR-5 cells.
p140 and sEGFR are biochemically distinct species: differential detergent solubility
To determine whether p140 is related to sEGFR, we first compared the biochemical characteristics of these two proteins. JEG-3 cells were used for the purpose of purifying p140 because they express this protein in relative abundance (Figure 2). Cells were lysed to test the solubility of p140 vs. sEGFR in various detergents and buffers (Figure S1). JEG-3 cells lysed with an SDS/NP-40/DOC solution liberated both p140 and sEGFR into solution (Figure S1, lane 1). In contrast, JEG-3 cells lysed with buffer containing 1% TX-100 and 1 mM EDTA in PBS did not liberate p140 or sEGFR (Figure S1, lane 2). JEG-3 cells lysed with 0.5% DOC (Figure S1, lanes 3, 6) or 2.5% SDS prepared without buffer or with TBS (Figure S1, lanes 4, 7) also did not liberate p140. JEG-3 cells lysed with unbuffered 0.5% TX-100 (Figure S1, lane 5), but not with TX-100 buffered with TBS (Figure S1, lane 8) liberated p140 into solution. Additional experiments revealed that 1% TX-100 was the optimal concentration for solubilizing p140 from JEG-3 cell pellets in unbuffered solution, and that 0.01% TX-100 was sufficient for maintaining p140 solubility (data not shown). In contrast, sEGFR was released with SDS (Figure S1, lane 4), but not with TX-100 (Figure S1, lane 5). This observation was the first indication that sEGFR and p140 were biochemically distinct membrane-associated proteins.
p140 is α5-integrin
To ascertain the identity of p140, we used conventional chromatographic methods to partially purify p140 from TX-100 JEG-3 lysates, and then interrogated p140 enriched chromatography fractions by LC ESI MS/MS. Purification of p140 was monitored by immunoblot analysis of chromatographic fractions with anti-sEGFR. We observed that p140 is efficiently depleted from JEG-3 lysates by SP Sepharose using binding buffers adjusted to pH ≤ 5.0; i.e., p140 binds strongly to SP Sepharose at pH ≤ 5.0, weakly between pH 5.5 and 6.5, and not at all at pH > 7.0 (Figure 3A). Interestingly, most TX-100-solubilized proteins precipitate at pH 4.0 (by addition of 50 mM formic acid); however, p140 remains soluble, thus resulting in substantial p140 purification (data not shown). Consequently, we selected 50 mM formic acid (pH 4.0) as the binding buffer for SP Sepharose chromatography, and NaCl as the eluant. Under these conditions, we observed that p140 elutes from SP Sepharose at ≥ 300 mM NaCl (Figure 3B). Additional studies demonstrated that p140 also binds to hydroxylapatite in 20 mM sodium phosphate buffer (pH 6.8), and elutes with 90–110 mM sodium phosphate (Figure 3C). The final purification protocol for subsequent LC ESI MS/MS analysis thus consisted of TX-100/formic acid extraction followed by preparative chromatography with SP Sepharose and hydroxylapatite; fractions eluted with 200–370 mM NaCl and 80–160 mM sodium phosphate were pooled, respectively. LC ESI MS/MS analysis of p140 enriched chromatographic fractions identified peptides corresponding to α5-integrin, β1-integrin, and brain acid soluble protein-1 (BASP1/NAP-22/CAP-23), which exhibit apparent molecular weights of ~140-kDa, ~130-kDa, and ~55-kDa, respectively by SDS PAGE analysis (Table 1). Immunoblot analysis of JEG-3 and HEY cell lysates probed with antibodies specific for α1-integrin, β1-integrin, BASP-1, sEGFR, and EGFR detected proteins of approximately 140-kDa, 130-kDa, 52-kDa, 140-kDa, and 170-kDa, respectively (Figure 4). Remarkably, p140 reacted with antibodies specific for both sEGFR and α5-integrin.
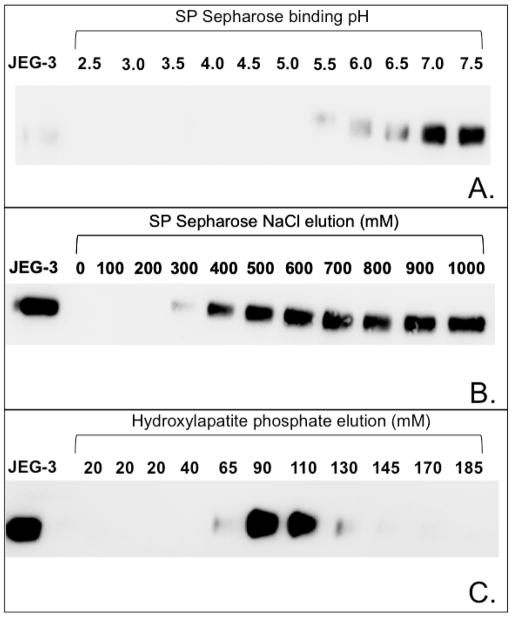
FIGURE 3.
Chromatographic purification steps for p140 as determined by immunoblot analysis with anti-sEGFR. Panel A. Depletion of p140 from JEG-3 lysates with SP Sepharose at each indicated pH. After incubation of SP Sepharose at the indicated pH, supernatants were assayed for unbound p140 by immunoblot analysis with anti-sEGFR. SP Sepharose efficiently bound p140 in buffers at pH ≤ 5.0. Panel B. Elution of p140 from SP Sepharose equilibrated with 50 mM formic acid (pH 4.0) using 0 to 1 M NaCl gradient. After incubation of SP Sepharose with cell lysate (pH 4.0) with the indicated NaCl solution, supernatants were assayed for unbound p140 by immunoblot analysis with anti-sEGFR. p140 was efficiently eluted from SP Sepharose at pH 4.0 by ≥ 400 mM NaCl. Panel C. Elution of p140 from a hydroxylapatite column equilibrated with 20 mM sodium phosphate buffer (pH 6.8) using a gradient of 20 to 185 mM sodium phosphate buffer. Eluted fractions were assayed for the presence of p140 by immunoblot analysis with anti-sEGFR. p140 was eluted from hydroxylapatite with 90–110 mM sodium phosphate, pH 6.8.
Table 1.
Summary of amino acid sequences as determined by LC ESI MS/MS using fractions enriched for p140, as described in the text.
| Observed SDS-PAGE Mass | Predicted m/z | ESI MS/MS protein identity | MOWSE score | Peptides |
|---|---|---|---|---|
| 140-kDa | 111-kDa* | α5-integrin | 260 | 120–137 LLESSLSSSEGEEPVEYK |
| 164–174 SLGTDLMNEMR | ||||
| 695–708 VTAPPEAEYSGLVR | ||||
| 760–770 TIQFDFQILSK | ||||
| 130-kDa | 88-kDa* | β1-integrin | 692 | 125–134 SGEPQTFTLK |
| 138–163 AEDYPIDLYYLMDLSYSMKDDLENVK | ||||
| 191–202 TVMPYISTTPAK | ||||
| 221–238 NVLSLTNKGEVFNELVGK | ||||
| 229–238 GEVFNELVGK | ||||
| 273–289 LLVFSTDAGFHFAGDGK | ||||
| 326–346 LSENNIQTIFAVTEEFQPVYK | ||||
| 775–784 WDTGENPIYK | ||||
| 785–794 SAVTTVVNPK | ||||
| 52-kDa and130-kDa | 23-kDa* | BASP-1 | 371 | 39–52 ESEPQAAAEPAEAK |
| 98–121 AEPPKAPEQEQAAPGPAAGGEAPK | ||||
| 122–149 AAEAAAAPAESAAPAAGEEPSKEEGEPK | ||||
| 185–198 ETPAATEAPSSTPK | ||||
| 199–227 AQGPAASAEEPKPVEAPAANSDQTVTVKE |
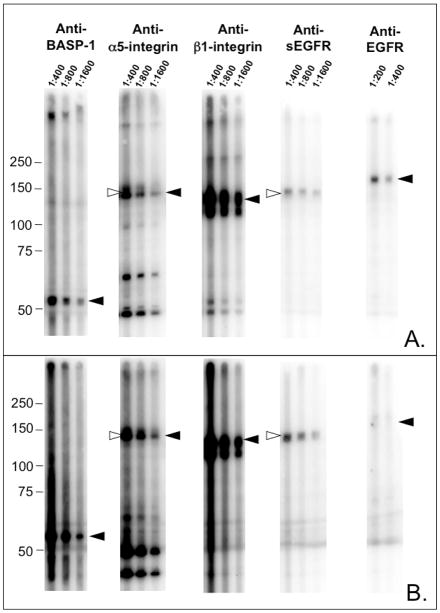
FIGURE 4.
Expression of BASP-1, α5-integrin, and β1-intergin in JEG-3 and HEY cell lysates as detected by immunoblot analysis. JEG-3 (panel A) and HEY (panel B) cell lysates were resolved by SDS-PAGE as a single band spanning the width of the gel, and transferred to PVDF membrane. The membrane was immunoblotted using a multichannel Immentics blotter, with antibodies directed against BASP-1, α5-integrin, β1-integrin, sEGFR, and EGFR (cytoplasmic domain) at the indicated dilutions. Solid arrowheads indicate migration of BASP-1, α5-integrin, β1-integrin, and EGFR, respectively. Open arrowheads indicate migration of p140. Of the tested species, only a band reactive with anti-α5-integrin co-migrated with p140.
To confirm that p140 reacts with both anti-α5-integrin and anti-sEGFR, JEG-3 lysates were immunoprecipitated with an anti-α5-integrin antibody followed by immunoblot analysis with anti-sEGFR. As illustrated in Figure 5A, anti-α5-integrin antibody immunoprecipitates a 140-kDa protein that is cross-reactive with anti-sEGFR. Furthermore, anti-sEGFR recognizes a commercial preparation of purified recombinant α5/β1 integrin (Figure 5B), but not purified recombinant αv/β3 integrin (Figure S2). Additionally, as shown in Figure 6, a 140-kDa protein cross-reactive with anti-sEGFR is detectable in lysates of CHO cells engineered to express human α5-integrin (CHO-α5) (13), but is not observed in lysates of CHO parental cells which express only low levels of endogenous (hamster) α5-integrin (CHO B2). Together with the identity of the p140 peptides presented in Table 1 (which correspond to α5-integrin), these immunoblot results demonstrate that p140 is α5-integrin, and also that α5-integrin harbors an epitope(s) that is immunoreactive with affinity-purified anti-sEGFR.
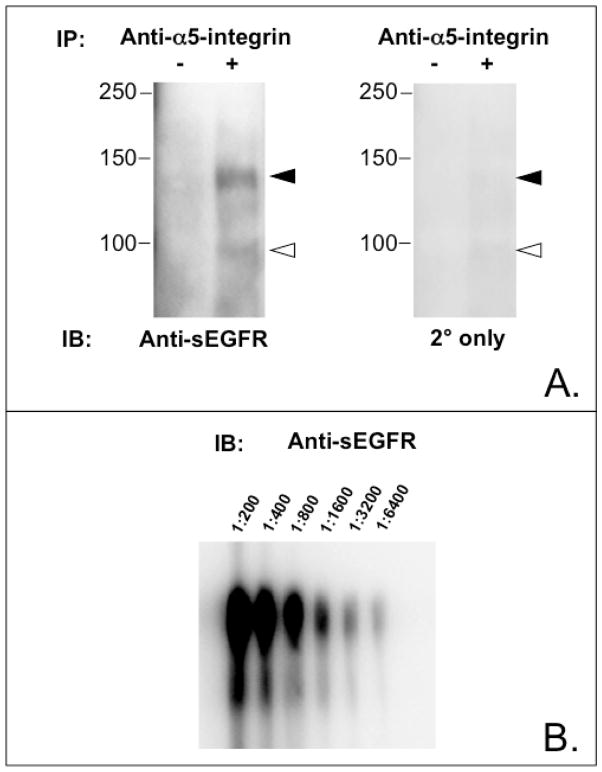
FIGURE 5.
Anti-sEGFR recognizes α5-integrin. Panel A. Immunoblot of p140 immunoprecipitated from JEG-3 cell lysates with anti-α5-integrin antibody and reacted with anti-sEGFR (right panel) or secondary antibody alone (left panel). Solid arrowhead indicates mobility of p140. Open arrowhead indicates mobility of a minor ~95 kDa band, which we postulate is a product of α5-integrin. Panel B. Immunoblot of recombinant α5/β1-integrin reacted with different dilutions of anti-sEGFR, as indicated, confirming that anti-sEGFR recognizes α5-integrin.
FIGURE 6.
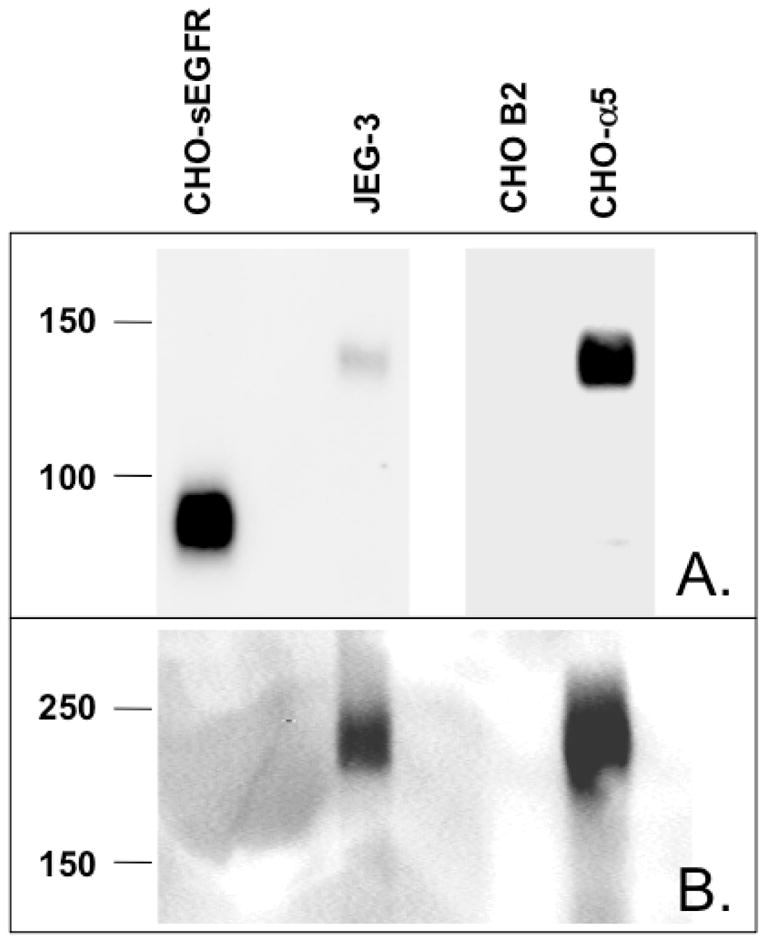
Expression of α5-integrin is detected by immunoblot analysis with anti-sEGFR and anti-α5-integrin antibodies. α5-integrin deficient CHO B2 cells express very low endogenous α5-integrin as detected by anti-sEGFR (Panel A) or anti-α5-integrin (Panel B). Lysates of CHO B2 cells stably expressing human α5-integrin (CHO-α5) contain species reactive as a band by reducing SDS-PAGE followed by anti-sEGFR immunoblot, or a higher molecular mass band by non-reducing SDS-PAGE followed by anti-α5-integrin immunoblot (following manufacturer’s instructions for this antibody). CHO-sEGFR and JEG-3 cells are included as negative and positive controls for p140/α5-integrin, respectively. Note different exposure lengths for CHO-sEGFR and JEG-3 vs. CHO B2 and CHO-α5 lanes in Panel A for clarity.
Anti-sEGFR binds to conserved sequences in the calf-1 domain of α5-integrin
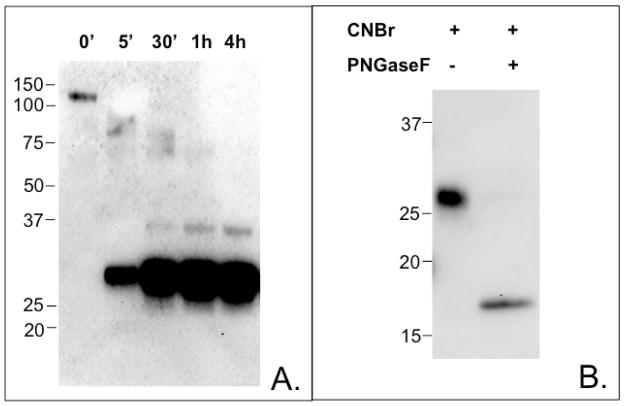
To identify the epitope(s) of α5-integrin recognized by anti-sEGFR, α5-integrin was subjected to CNBr hydrolysis. Figure 7A demonstrates the predicted CNBr cleavage sites of α5-integrin. CNBr treatment of recombinant α5/β1 integrin yielded one major band of 27-kDa that is detected by anti-sEGFR using immunoblot analysis (Figure 8A). This reactive band was excised from the gel, digested with trypsin, and analyzed by LC ESI MS/MS, which identified two α5-integrin peptides: 695VTAPPEAEYSGLVR708 and 739AGASLWGGLR748 (Figure 7A, underlined). Conspicuously, peptides 695–708 and 739–748 reside on distinct CNBr cleavage products consisting of residues 588–737 and 738–966, respectively, thus restricting the location of the anti-sEGFR binding epitope to α5-integrin residues 588–966. Based on amino acid sequence inspection, peptides 588–737 and 738–966 have predicted molecular masses of ~17-kDa and ~27-kDa, respectively. However, peptide 588–737 contains at least three potential sites of N-linked glycosylation, whereas peptide 738–966 contains only one potential glycosylation site (http://www.cbs.dtu.dk/services/NetNGlyc). Depending on the nature of the carbohydrate moiety(ies) involved, such glycosylation events could increase the observed molecular mobility of peptide 588–737 by as much as 9-kDa (based on a predicted ~3-kDa per complex triantennary glycan). As such, peptide 588–737 is of the correct apparent molecular weight to correspond to a glycosylated, 27-kDa CNBr fragment of α5-integrin. To confirm the identity of the 27- kDa anti-sEGFR immunoreactive band as α5-integrin peptide 588–737 (vs. 738–966), recombinant α5/β1-integrin was treated with PNGaseF, a pan-N-glycosidase, prior to CNBr hydrolysis. This combination treatment yielded a deglycosylated anti-sEGFR immunoreactive band of ~17-kDa, the predicted deglycosylated mass of α5-integrin peptide 588–737 (Figure 8B), further restricting the location of the anti-sEGFR binding epitope(s).
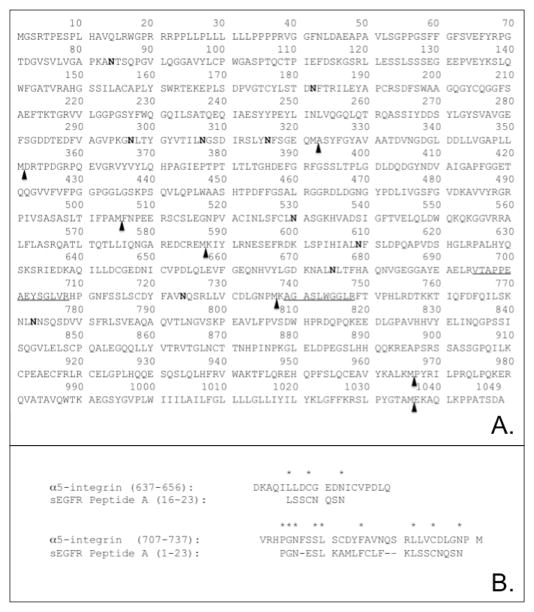
FIGURE 7.
Panel A. Diagram summary of α5-integrin amino acid sequence. Predicted sites of CNBr cleavage are shown with arrowheads. Peptides recovered by LC ESI MS/MS from a anti-sEGFR 31-mer immunoreactive 27-kDa gel band generated by CNBr hydrolysis are underlined: 695VTAPPEAEYSGLVR708 and 739AGASLWGGLR748. Potential sites of N-linked glycosylation are highlighted in bold. Potential sites of cyanogen bromide cleavage are indicated with solid arrowheads. Panel B. Sequence comparison of α5-integrin residues 588–737 with the sEGFR 31-mer peptide. Regions of α5-integrin that have >35% identity with sEGFR were aligned according to SIM analysis (http://www.expasy.org/tools/sim-prot.html). Amino acids sharing sequence identity are highlighted with an asterisk.
FIGURE 8.
Immunoblots of recombinant α5/β1 integrin digested by CNBr hydrolysis. Panel A. Time-course of CNBr hydrolysis of recombinant α5/β1 integrin. CNBr hydrolysis was terminated at the times indicated. A precursor-product relationship is evident resulting in an anti-sEGFR-reactive band of ~27-kDa. Panel B. CNBr hydrolysis of deglycosylated α5-integrin. Prior to CNBr hydrolysis, recombinant α5/β1-integrin was treated with PNGaseF. An anti-sEGFR-reactive protein of ~17-kDa was generated by this procedure; this peptide corresponds to α5-integrin peptide 588–737 (see Figure 7), indicating that the anti-sEGFR epitope of α5-integrin is located between amino acids 588–737.
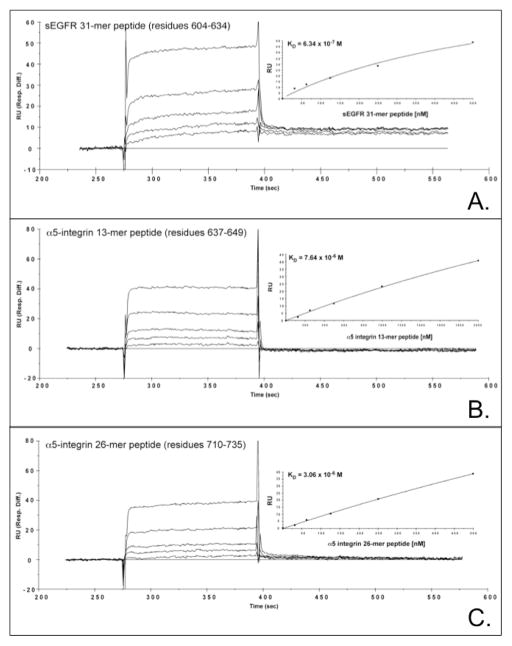
The SIM alignment tool (http://www.expasy.ch/tools/sim-prot.html) was then used to compare the sEGFR 31-mer peptide to the CNBr-cleaved α5–integrin peptide 588–737. Using 30% identity as the minimum cutoff threshold, two regions of sequence identity were identified between the sEGFR and α5–integrin peptides, further restricting the binding epitope(s) of anti-sEGFR. Notably, α5-integrin residues 710–735 (26-mer) and 642–649 (8-mer) share ~35% and ~38% identity with the 31-mer sEGFR peptide, respectively (Figure 7B), and both of these epitopes reside within α5-integrin’s bioactive calf-1 domain (19). To compare the binding of anti-sEGFR to its cognate 31-mer immunogen vs. these two α5-integrin peptides (note that residues 637–641 were included in the ‘642–649’ peptide to improve its solubility), all three peptides were analyzed by plasmon resonance in the presence of anti-sEGFR to determine their equilibrium binding constants (KD). Sensorgrams illustrate that antibody binding to the 31-mer sEGFR peptide is highest (KD = 6.34 × 10−7 M), and exhibits a non-linear relationship as predicted for a polyclonal antibody (Figure 9 and Table 2). In contrast, the anti-sEGFR binding affinities of the two α5-integrin peptides 637–649 (KD = 7.64 × 10−6 M) and 710–735 (KD = 3.06 × 10−6 M) are 12 and 5 times lower (respectively) than observed for the 31-mer sEGFR peptide. In addition, peptides 637–649 and 710–73 have a linear binding relationship with anti-sEGFR that is consistent with the presence of a single immunoglobulin binding epitope in each of these two α5-integrin peptides. Interestingly, both of these putative anti-sEGFR binding sites are located within a critical regulatory region of α5-integrin referred to as the calf–1 domain (19). BLAST homology search reveals that the peptide “LLDCGEDN” (peptide 642–649) is perfectly repeated in only one other protein, αv-integrin; however, anti-sEGFR does not recognize αv-integrin by immunoblot analysis (Figure S2). We conclude that there is a strong interaction between anti-sEGFR and α5-integrin in cell-free systems.
FIGURE 9.
Surface plasmon resonance sensorgrams of anti-sEGFR binding to a sEGFR vs. two α5-integrin peptides is indicated. Absorbance in relative units (RU) is plotted vs. time (sec) for immobilized anti-sEGFR in the presence of a sEGFR 31-mer peptide 604–634 (panel A), a α5-integrin peptide 637–649 (panel B), and a α5-integrin peptide 710–735 (panel C). Graphs of RU vs. peptide concentration are included as insets.
Table 2.
Summary of surface plasmon resonance of sEGFR and α5-integrin peptide interactions with anti-sEGFR.
| Peptide | Amino Acid Sequence | Anti-sEGFR antibody equilibrium binding constant (KD) |
|---|---|---|
| sEGFR 31-mer | PGNESLKAMLFCLFKLSSCNQSNDGSVSHQS | 6.34 × 10−7 M |
| α5-integrin 637–649 | DKAQILLDCGEDN | 7.64 × 10−6 M |
| α5-integrin 710–735 | PGNFSSLSCDYFAVNQSRLLVCDLGN | 3.06 × 10−6 M |
Anti-sEGFR promotes cell compaction
The interaction between anti-sEGFR and α5-integrin raised the possibility that this antibody might influence cell surface integrin-ECM interactions. We previously have demonstrated an interaction between α5-integrin and its ligand, fibronectin, that regulates the intrinsic ability of cells to form intercellular connections, resulting in the formation of cellular aggregates and 3D tissue cohesion in vitro (17). Fibronectin is a secreted protein that has been shown, through direct interaction with α5-integrin, to promote such cell-cell interactions by playing a direct role in aggregate compaction (13, 14). We, therefore, hypothesized that anti-sEGFR might similarly regulate 3D assembly, thereby implicating the calf-1 domain in α5-integrin-mediated cell aggregation and tissue assembly.
To address this hypothesis, the ability of anti-sEGFR to regulate 3D cell assembly was evaluated using an aggregate compaction assay. Figure 10A illustrates several images of hanging drop cultures of CHO-P3 parental cells and CHO cells transfected to express α5-integrin (CHO-α5) captured after overnight incubation in PBS, rabbit IgG, or anti-sEGFR. In agreement with previous observations (17), CHO-P3 parental cells (panel A) form loose, dispersed clusters, whereas CHO-α5 cells form tight, more compact clusters (panel B). The addition of rabbit IgG to either CHO-P3 (panel C) or CHO-α5 (panel D) cells had no effect on compaction. Addition of anti-sEGFR however, resulted in markedly more compact aggregates of both CHO-P3 (panel E) and CHO-α5 (panel F) cells. However, the degree of anti-sEGFR-mediated compaction in CHO-α5 cells is much greater than observed in parental CHO-P3 cells, suggesting pleiotropic regulation of tissue organization by anti-sEGFR in this in vitro model. Quantification and comparison of the aggregate size of each cell cluster using ANOVA and Tukey's Multiple Comparisons Test confirmed these effects of anti-sEGFR on compaction. Figure 10B shows that incubating CHO-α5 cells in anti-sEGFR gives rise to aggregates that are 50% smaller and more compact than those incubated in rabbit IgG (p<0.01) or in PBS (p<0.001), whereas no difference in cluster size was evident between CHO-α5 cells incubated in PBS or in rabbit IgG (p>0.05). Moreover, α5-integrin expressing CHO cells formed aggregates with surface area of 0.0171±0.0007 mm2 when incubated in anti-sEGFR, whereas those of CHO-P3 incubated under the same conditions formed aggregates that were significantly larger with a surface area of 0.045±0.001 mm2 (Student’s t-test, p<0.0001). Collectively, these data indicate that anti-sEGFR can regulate aggregate compaction, and that this effect is markedly enhanced in α5-integrin expressing cells. As discussed below, these observations may reveal a novel mechanism of α5-integrin-mediated cohesion transduced via its intrinsic calf-1 domain.
FIGURE 10.
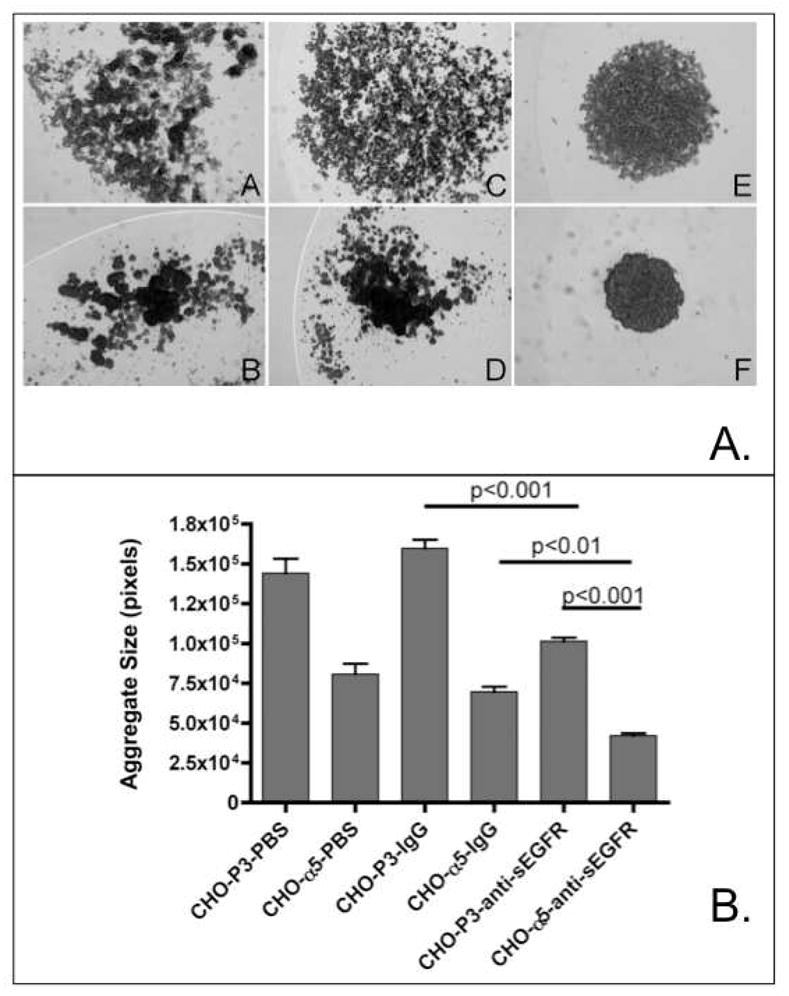
(A) Anti-sEGFR promotes compaction of suspended CHO B2 cells. Compaction of CHO-P3 and CHO-α5 was compared by suspending cells in hanging drops in the presence of PBS (panels A, B), rabbit IgG (panels C, D) or anti-sEGFR (panels E, F). In general, anti-sEGFR appears to markedly enhance aggregate compaction. This effect is greater for α5-integrin expressing cells than for control cell lines. (B) Quantification of aggregate size. Image analysis was used to calculate average aggregate size for parental and α5-integrin expressing cells incubated in hanging drop cultures in PBS, rabbit IgG, and anti-sEGFR. Statistical analysis by ANOVA revealed a significant difference in aggregate size in response to anti-sEGFR treatment (p<0.0001). Tukey’s Multiple Comparisons Test revealed a pair-wise difference between the following groups of cells: CHO-P3-anti-sEGFR and CHO- α5-anti-sEGFR (p<0.001); CHO-α5-IgG and CHO-α5-anti-sEGFR (p<0.01); CHO-P3-IgG and CHO-P3-anti-sEGFR (p<0.001). As expected, no difference in size was detected between CHO-P3 and CHO- P3-IgG (p>0.05) or CHO-α5 and CHO-α5 IgG (p>0.05).
DISCUSSION
The role of EGFR in the etiology and progression of a variety of human cancers is well established (20). However, the utility of this receptor as a clinically useful biomarker has been limited by the considerable disparity that exists among published reports regarding ‘EGFR expression’ in human tissues and tumors as determined by immunohistochemistry. For example, in ovarian cancer, over forty published studies have been conducted, and only half conclude that EGFR is an independent prognostic factor (reviewed in (21)). We and others have proposed that this discordance is attributable, at least in part, to the co-expression of sEGFR with EGFR in ovarian tumors (22).
To develop immunological reagents that can distinguish between sEGFR and EGFR expression, we have generated a rabbit polyclonal antiserum directed against the unique carboxy-terminal sequence of sEGFR; this antiserum does not cross-react with full-length EGFR or other HER family receptors, and is useful for the detection of sEGFR using immunoblot, immunoprecipitation, and immunohistochemical methods (10). Initial characterization of this anti-sEGFR antibody using human carcinoma-derived cell lines revealed unexpected cross-reactivity with an unknown 140-kDa protein, which we here identify as human α5-integrin. This observation was unanticipated since previous BLAST searches indicated no significant homology between the unique sEGFR 78 amino acid carboxy-terminal sequence and any known protein (5). Here we show that α5-integrin harbors two immunoreactive epitopes that share 35% (26-mer) and 38% (8-mer) identity with the unique carboxy-terminal sequence of sEGFR. However, we demonstrate that only the 26-mer epitope of intact α5-integrin is immunoreactive with anti-sEGFR.
The significance of these observations is several-fold. First, these observations will inform the development of next generation antibodies truly specific for either sEGFR or α5-integrin. Second, these results suggest that studies using anti-α5-integrin antibodies should be viewed cautiously until the specificity of such antibodies is empirically established, since sEGFR expression is ubiquitous in human tissues, including human blood. Identification of p140 as human α5-integrin was only possible through rigorous biochemical evaluation, and could not have been predicted via typical sequence comparison algorithms. Finally, while the functional implications of these short regions of sequence homology between sEGFR and α5-integrin have not yet been fully determined, previous studies of antibody cross-reactivity among structurally divergent adhesion molecules suggest that such regions of structural identify may reflect aspects of shared functions that arise via convergent evolution (23).
By analogy, we propose that the homologous regions reported here might have functional significance, perhaps providing contact regions characteristic of the homophillic and/or even homotypic interactions defined by other cell surface adhesion molecules. In support of this concept, we have shown that anti-sEGFR induces aggregation and compaction of suspended cells in culture, and that the ability of anti-sEGFR to induce cell compaction is correlated with the level of α5-integrin expression. While the mechanism underlying anti-sEGFR induced 3D cell assembly is not yet known, it is possible that by analogy to our previous studies on fibronectin (13, 14) anti-sEGFR activates α5-integrin by mediating its cross-linking, thereby promoting aggregation between CHO cells; alternatively, this antibody may inhibit cell sheet compaction in favor of higher order coalescence (allowing for greater overall tissue spherical compaction). This newly identified function of an antibody directed against sEGFR, that also recognizes α5-integrin, is consistent with the emerging role of sHER3, a soluble isoform of the HER3 receptor, in prostate cancer cell adhesion and metastasis (24, 25). Given that serum sEGFR concentrations are dysregulated in diverse malignancies (26), proteolysis of cell surface sEGFR also may regulate cell adhesion, proliferation, and/or migration responses in a manner paralleling proteolysis of homophilic cell-cell CAM complexes (27). These studies, therefore, not only identify anti-sEGFR as a novel probe for future studies of integrin function, they also specifically implicate the calf-1 domain of α5-integrin as a transducer of 3D tissue compaction, and possibly, cohesion.
In addition to the shared homology between α5-integrin and sEGFR, α-integrin is a cell surface co-receptor (along with β1-integrin) for fibronectin, and in its ligated form this complex acts in concert with full-length EGFR to promote EGF-independent cell survival signaling (28–30). The precise nature of this cell surface protein complex has been studied extensively, but the role of sEGFR in the function of this complex has yet to be elucidated. The new findings presented here suggest a potential structural basis for such interactions, and may provide a useful focus for future structure-function analyses. In particular, the calf-1 domain of α5-integrin is known to play a critical role in the conformational shift of integrin to its ligand binding state (31). These results may similarly provide an initial focus for structure-function analysis of the unique carboxy-terminus of sEGFR, as well as for further mapping of the integrin-EGFR complex that mediates cell adhesion and survival signaling.
It is noteworthy that, while both α5/β1 integrin and αv/β1 integrin function as receptors for fibronectin, α5/β1 integrin but not αv/β1 integrin transduces survival signals following fibronectin ligation (32). In this regard, α5-integrin expression has been correlated with progression-free and overall survival in ovarian cancer patients, although to date, α5-integrin has not been a reliable prognostic indicator in the clinic (33). Moreover, α5/β1-integrin-directed cancer therapeutics such as volociximab are in clinical trials for the treatment of patients with diverse solid tumors, including epithelial ovarian cancer, pancreatic cancer, and non small cell lung cancer (clinicaltrials.gov identifiers NCT00635193, NCT00401570, and NCT00654758, respectively). The clear diagnostic and theragnostic potential of EGFR, sEGFR, and α5-integrin, therefore, not only underscores the need for a more complete understanding of the functional attributes of these proteins, both alone and in complex, but also makes it imperative that the immunological reagents to be used for the clinical detection of these proteins be reliably mono-specific.
In conclusion, we speculate that the short regions of limited sequence homology between sEGFR and α5-integrin identified here may be of functional and biological significance. Moreover, the identification of an antibody that has the potential to detect both proteins with high affinity reveals the need for caution in interpreting either sEGFR or α5-integrin expression data that rely solely on immunohistochemical methods. The results presented here are consistent with recent studies which suggest that antibodies directed against various EGFR isoforms can exhibit unanticipated complexities, particularly in cancer cells (34, 35), and also that these complexities provide windows of opportunity for increasing our understanding of the structure and function of these important growth regulatory proteins, as well as their clinical utility.
Supplementary Material
Acknowledgments
We thank Drs. Elliott Bedows (University of Nebraska Medical Center) and Nathan Vanderford (University of Kentucky) for their editorial assistance and critical review of this manuscript, Dr. Jack Goodman (University of Kentucky) for LC ESI MS/MS technical assistance, Ms. Jane Peterson (Mayo Clinic) for synthesis and purification of synthetic peptides, Dr. Jun Hiyashi (Precision Antibody) for surface plasmon resonance technical assistance, and KJ Studio (www.kjstudio.com) for the Table of
Abbreviations
- BASP-1
brain acid soluble protein 1
- BSA
bovine serum albumin
- CHO
Chinese hamster ovary
- CNBr
cyanogen bromide
- DOC
deoxycholate
- ECD
extracellular domain
- ECL
enhanced chemiluminescence
- EGFR
epidermal growth factor receptor
- FDA
Food and Drug Administration
- K-PBS
potassium phosphate buffered saline
- LC ESI MS/MS
liquid chromatography electrospray-ionization tandem mass spectrometry
- NFDM
non-fat dry milk
- PAGE
polyacrylamide gel electrophoresis
- PVDF
polyvinylidene difluoride
- RU
relative units
- SDS
sodium dodecyl sulfate
- sEGFR
soluble epidermal growth factor receptor
- TBS
Trizma-buffered saline
- TBS-TW20
Trizma-buffered saline with 0.1% Tween 20
- TX-100
Triton X-100
Footnotes
This work was supported by grants from Susan G. Komen for the Cure and the Marsha Rivkin Center for Ovarian Cancer Research to J.A. Wilken, the Kentucky Lung Cancer Research Program and Markey Cancer Center Translational Research grant awards to A.T. Baron, NIH CA 118755 to R. A. Foty, NIH CA 15083-35 to D.J. McCormick, and NIH CA 79808 and a “Senior Women in Medicine Professorship” from Yale University School of Medicine to N.J. Maihle.
SUPPORTING INFORMATION AVAILABLE
Supplemental Figures S1 and S2 demonstrate the solubility of p140 in various buffers and confirm the specificity of anti-sEGFR for α5 integrin. This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
Jason A. Wilken, Email: jason.wilken@yale.edu.
Andre T. Baron, Email: A.BARON@uky.edu.
Ramsey A. Foty, Email: fotyra@umdnj.edu.
Daniel J. McCormick, Email: mccormick.daniel@mayo.edu.
Nita J. Maihle, Email: nita.maihle@yale.edu.
References
- 1.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 2.Boerner JL, Danielsen A, Maihle NJ. Ligand-independent oncogenic signaling by the epidermal growth factor receptor: v-ErbB as a paradigm. Exp Cell Res. 2003;284:111–121. doi: 10.1016/s0014-4827(02)00096-4. [DOI] [PubMed] [Google Scholar]
- 3.Flynn JF, Wong C, Wu JM. Anti-EGFR Therapy: Mechanism and Advances in Clinical Efficacy in Breast Cancer. J Oncol. 2009;2009:526963. doi: 10.1155/2009/526963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maihle NJ, Baron AT, Barrette BA, Boardman CH, Christensen TA, Cora EM, Faupel-Badger JM, Greenwood T, Juneja SC, Lafky JM, Lee H, Reiter JL, Podratz KC. EGF/ErbB receptor family in ovarian cancer. Cancer Treat Res. 2002;107:247–258. doi: 10.1007/978-1-4757-3587-1_11. [DOI] [PubMed] [Google Scholar]
- 5.Reiter JL, Threadgill DW, Eley GD, Strunk KE, Danielsen AJ, Sinclair CS, Pearsall RS, Green PJ, Yee D, Lampland AL, Balasubramaniam S, Crossley TD, Magnuson TR, James CD, Maihle NJ. Comparative genomic sequence analysis and isolation of human and mouse alternative EGFR transcripts encoding truncated receptor isoforms. Genomics. 2001;71:1–20. doi: 10.1006/geno.2000.6341. [DOI] [PubMed] [Google Scholar]
- 6.Reiter JL, Maihle NJ. Characterization and expression of novel 60-kDa and 110-kDa EGFR isoforms in human placenta. Ann N Y Acad Sci. 2003;995:39–47. doi: 10.1111/j.1749-6632.2003.tb03208.x. [DOI] [PubMed] [Google Scholar]
- 7.Baron AT, Cora EM, Lafky JM, Boardman CH, Buenafe MC, Rademaker A, Liu D, Fishman DA, Podratz KC, Maihle NJ. Soluble epidermal growth factor receptor (sEGFR/sErbB1) as a potential risk, screening, and diagnostic serum biomarker of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:103–113. [PubMed] [Google Scholar]
- 8.Leslie KK, Sill MW, Darcy KM, Baron AT, Wilken JA, Godwin AK, Cook J, Schilder RJ, Schilder JM, Maihle NJ. Efficacy and safety of gefitinib and potential prognostic value of soluble EGFR, EGFR mutations, and tumor markers in a Gynecologic Oncology Group phase II trial of persistent or recurrent endometrial cancer. J Clin Oncol. 2009;27:abstr e16542. [Google Scholar]
- 9.Lafky JM, Baron AT, Cora EM, Hillman DW, Suman VJ, Perez EA, Ingle JN, Maihle NJ. Serum soluble epidermal growth factor receptor concentrations decrease in postmenopausal metastatic breast cancer patients treated with letrozole. Cancer Res. 2005;65:3059–3062. doi: 10.1158/0008-5472.CAN-05-0067. [DOI] [PubMed] [Google Scholar]
- 10.Christensen TA, Reiter JL, Baron AT, Maihle NJ. Generation and characterization of polyclonal antibodies specific for human p110 sEGFR. Hybrid Hybridomics. 2002;21:183–189. doi: 10.1089/153685902760173908. [DOI] [PubMed] [Google Scholar]
- 11.Xiong JP, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–155. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- 12.Schreiner CL, Bauer JS, Danilov YN, Hussein S, Sczekan MM, Juliano RL. Isolation and characterization of Chinese hamster ovary cell variants deficient in the expression of fibronectin receptor. J Cell Biol. 1989;109:3157–3167. doi: 10.1083/jcb.109.6.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson EE, Zazzali KM, Corbett SA, Foty RA. Alpha5beta1 integrin mediates strong tissue cohesion. J Cell Sci. 2003;116:377–386. doi: 10.1242/jcs.00231. [DOI] [PubMed] [Google Scholar]
- 14.Robinson EE, Foty RA, Corbett SA. Fibronectin matrix assembly regulates alpha5beta1-mediated cell cohesion. Mol Biol Cell. 2004;15:973–981. doi: 10.1091/mbc.E03-07-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myers EW, Miller W. Optimal alignments in linear space. Comput Appl Biosci. 1988;4:11–17. doi: 10.1093/bioinformatics/4.1.11. [DOI] [PubMed] [Google Scholar]
- 16.Brown NK, McCormick DJ, David CS, Kong YC. H2E-derived Ealpha52–68 peptide presented by H2Ab interferes with clonal deletion of autoreactive T cells in autoimmune thyroiditis. J Immunol. 2008;180:7039–7046. doi: 10.4049/jimmunol.180.10.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caicedo-Carvajal CE, Shinbrot T, Foty RA. Alpha5beta1 integrin-fibronectin interactions specify liquid to solid phase transition of 3D cellular aggregates. PLoS One. 2010;5:e11830. doi: 10.1371/journal.pone.0011830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corbett SA, Lee L, Wilson CL, Schwarzbauer JE. Covalent cross-linking of fibronectin to fibrin is required for maximal cell adhesion to a fibronectin-fibrin matrix. J Biol Chem. 1997;272:24999–25005. doi: 10.1074/jbc.272.40.24999. [DOI] [PubMed] [Google Scholar]
- 19.Kamata T, Handa M, Sato Y, Ikeda Y, Aiso S. Membrane-proximal {alpha}/{beta} stalk interactions differentially regulate integrin activation. J Biol Chem. 2005;280:24775–24783. doi: 10.1074/jbc.M409548200. [DOI] [PubMed] [Google Scholar]
- 20.Uberall I, Kolar Z, Trojanec R, Berkovcova J, Hajduch M. The status and role of ErbB receptors in human cancer. Exp Mol Pathol. 2008;84:79–89. doi: 10.1016/j.yexmp.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Lafky JM, Wilken JA, Baron AT, Maihle NJ. Clinical implications of the ErbB/epidermal growth factor (EGF) receptor family and its ligands in ovarian cancer. Biochim Biophys Acta. 2008;1785:232–265. doi: 10.1016/j.bbcan.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Wilken JA, Baron AT, Maihle NJ. The epidermal growth factor receptor conundrum. Cancer. 2010 doi: 10.1002/cncr.25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grumet M, Hoffman S, Edelman GM. Two antigenically related neuronal cell adhesion molecules of different specificities mediate neuron-neuron and neuron-glia adhesion. Proc Natl Acad Sci U S A. 1984;81:267–271. doi: 10.1073/pnas.81.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vakar-Lopez F, Cheng CJ, Kim J, Shi GG, Troncoso P, Tu SM, Yu-Lee LY, Lin SH. Up-regulation of MDA-BF-1, a secreted isoform of ErbB3, in metastatic prostate cancer cells and activated osteoblasts in bone marrow. J Pathol. 2004;203:688–695. doi: 10.1002/path.1568. [DOI] [PubMed] [Google Scholar]
- 25.Chen N, Ye XC, Chu K, Navone NM, Sage EH, Yu-Lee LY, Logothetis CJ, Lin SH. A secreted isoform of ErbB3 promotes osteonectin expression in bone and enhances the invasiveness of prostate cancer cells. Cancer Res. 2007;67:6544–6548. doi: 10.1158/0008-5472.CAN-07-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baron AT, Wilken JA, Haggstrom DE, Goodrich ST, Maihle NJ. Clinical implementation of soluble EGFR (sEGFR) as a theragnostic serum biomarker of breast, lung and ovarian cancer. IDrugs. 2009;12:302–308. [PubMed] [Google Scholar]
- 27.Craig SE, Brady-Kalnay SM. Cancer cells cut homophilic cell adhesion molecules and run. Cancer Res. 2011;71:303–309. doi: 10.1158/0008-5472.CAN-10-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu D, Aguirre Ghiso J, Estrada Y, Ossowski L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell. 2002;1:445–457. doi: 10.1016/s1535-6108(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 29.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 30.Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci U S A. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark K, Pankov R, Travis MA, Askari JA, Mould AP, Craig SE, Newham P, Yamada KM, Humphries MJ. A specific alpha5beta1-integrin conformation promotes directional integrin translocation and fibronectin matrix formation. J Cell Sci. 2005;118:291–300. doi: 10.1242/jcs.01623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z, Vuori K, Reed JC, Ruoslahti E. The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci U S A. 1995;92:6161–6165. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawada K, Mitra AK, Radjabi AR, Bhaskar V, Kistner EO, Tretiakova M, Jagadeeswaran S, Montag A, Becker A, Kenny HA, Peter ME, Ramakrishnan V, Yamada SD, Lengyel E. Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer research. 2008;68:2329–2339. doi: 10.1158/0008-5472.CAN-07-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garrett TP, Burgess AW, Gan HK, Luwor RB, Cartwright G, Walker F, Orchard SG, Clayton AH, Nice EC, Rothacker J, Catimel B, Cavenee WK, Old LJ, Stockert E, Ritter G, Adams TE, Hoyne PA, Wittrup D, Chao G, Cochran JR, Luo C, Lou M, Huyton T, Xu Y, Fairlie WD, Yao S, Scott AM, Johns TG. Antibodies specifically targeting a locally misfolded region of tumor associated EGFR. Proc Natl Acad Sci U S A. 2009;106:5082–5087. doi: 10.1073/pnas.0811559106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swami M. Immunotherapy: A hidden target. Nature Reviews Cancer. 2009;9:318–319. [Google Scholar]
- 36.Sato Y, Isaji T, Tajiri M, Yoshida-Yamamoto S, Yoshinaka T, Somehara T, Fukuda T, Wada Y, Gu J. An N-glycosylation site on the beta-propeller domain of the integrin alpha5 subunit plays key roles in both its function and site-specific modification by beta1,4-N-acetylglucosaminyltransferase III. J Biol Chem. 2009;284:11873–11881. doi: 10.1074/jbc.M807660200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mosevitsky MI, Capony JP, Skladchikova G, Novitskaya VA, Plekhanov A, Zakharov VV. The BASP1 family of myristoylated proteins abundant in axonal termini. Primary structure analysis and physico-chemical properties. Biochimie. 1997;79:373–384. doi: 10.1016/s0300-9084(97)80032-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.