Abstract
Because renal function in newborns is immature, the pharmacokinetics of drugs administered to neonates vary significantly from adult patients. The establishment of drug transport systems is a key process in the functional maturation of the nephron. However, a thorough examination of the expression of the main drug transporters in the kidney throughout all stages of development (embryonic, postnatal, and mature) has yet to be carried out, and the functional (physiological) impact is not well understood. Using time-series microarray data, we analyzed the temporal behavior of mRNA levels for a wide range of SLC and ABC transporters in the rodent kidney throughout a developmental time series. We find dynamic increases between the postnatal and mature stages of development for a number of transporters, including the proximal tubule-specific drug and organic anion transporters (OATs) OAT1 (SLC22a6) and OAT3 (SLC22a8). The OATs are the major multispecific basolateral drug, toxin, and metabolite transporters in the proximal tubule responsible for handling of many drugs, as well as the prototypical OAT substrate para-aminohippurate (PAH). We therefore performed specific in vivo pharmacokinetic analysis of the transport of PAH in postnatal and maturing rodent kidney. We show that there is a 4-fold increase in PAH clearance during this period. Clearance studies in Oat1 and Oat3 knockouts confirm that, as in the adult, Oat1 is the principle transporter of PAH in the postnatal kidney. The substantial differences observed supports the need for better understanding of pharmacokinetics in the newborn and juvenile kidney compared with the adult kidney at the basic and clinical level.
Introduction
At birth, the fetus transitions from an aqueous environment in utero to a terrestrial environment. The physiological changes that are initiated at birth affect all organ systems to accommodate the rapidly shifting environment. A key issue has to do with developmental changes in drug and toxin transporters in the kidney and other organs that function at epithelial interfaces between the newborn and the outside world. Transporters involved in the maintenance of homeostatic balance within this new environment must be adequately expressed and functionally regulated so that rapid environmental shifts and changes can be accommodated or recovered from safely (Ahn and Nigam, 2009; Wu et al., 2011).
The kidneys play a particularly critical role in the elimination of toxins and drugs that may be encountered by or administered to the newborn. For example, various transporters, including the ATP-binding cassette (ABC) and solute carrier (SLC) families of transporters, together, are responsible for the transport of a majority of the drugs handled by the kidney. In particular, the transporters of SLC22 subfamily mediate the secretion of both endogenous and exogenous organic anions, including a number of xenobiotics and pharmacological drugs. Endogenous and exogenous organic anions, including β-lactam antibiotics, HIV antiviral drugs, and diuretics, are excreted from the kidney by glomerular filtration and by secretion across the proximal tubule via these transporters (Nigam et al., 2007). The gene products of both Slc22a6 (OAT1) and Slc22a8 (OAT3) are localized to the basolateral membrane of the proximal tubule (Kojima et al., 2002) and contribute to the secretion of a number of therapeutic agents and endogenous organic anions (Burckhardt and Burckhardt, 2003; Eraly et al., 2006; Vallon et al., 2008a,b).
It is known that the newborn kidney has a lower glomerular filtration rate compared with the adult and that the concentration of urine and secretion of organic compounds is reduced (Hook and Bailie, 1979), and that renal expression of Oats increases substantially in postnatal rats and mice (Buist et al., 2002; Buist and Klaassen, 2004). Neonatal pharmacokinetics differ from adult parameters because of this functional renal immaturity, and it cannot be assumed that drug treatment of a newborn or premature infant can be extrapolated from the kinetics or therapeutic efficacy observed in adult patients (Warner, 1986). In addition, the immature nature of the drug transport system at birth not only makes the kidney itself prone to toxic injury (Sekine and Endou, 2009) but also leaves other organ systems vulnerable to toxicants when the pharmacological agent has lower clearance rates. Although toxic injury due to the misdosing of drugs is a concern regardless of age, based on the immaturity of drug transport mechanisms, newborns can be particularly susceptible to overdosing from prescription errors (Chappell and Newman, 2004).
In light of this, pharmacokinetic and dynamic studies specifically targeting the newborn are of critical importance for effective therapeutic treatment of this vulnerable population. However, little is known about the temporal expression of the main drug transporters throughout kidney development into adulthood. Using microarray analysis, we carried out a comprehensive analysis of the profile of expression of SLC and ABC transporter families in the rodent kidney from early embryonic time points through newborn stages and into maturity. Given the importance of OAT1 and OAT3 function in the elimination of a number of clinically important drugs, we also performed a pharmacokinetic analysis using the prototypic substrate p-aminohippurate (PAH) during the neonatal period to gain insight into the functional state and maturation of the renal drug transport system.
Materials and Methods
Microarray Analysis.
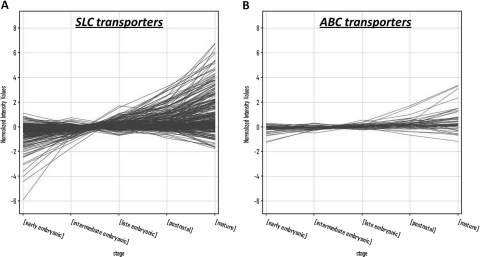
Microarray analysis was carried out using Genespring GX 11 software (Agilent Technologies, Santa Clara, CA). The whole embryonic rat kidney time course data were obtained from previously published data sets (Tsigelny et al., 2008), and the mouse data sets from GUDMAP were downloaded (www.gudmap.org). All microarray data sets were processed using robust multiarray analysis normalization. The average expression for each time point in each stage was calculated. SLC, ABC, and SLC22 gene lists were compiled based on the probe set annotations on the Affymetrix rat 230 2.0 chip (Affymetrix, Santa Clara, CA). The translate function in Genespring GX11 was used to translate probe set IDs between the Affymetrix rat 230 2.0 chip and the mouse 430 2.0 chip. Homology was based on Entrez gene ID annotations. For each graph of gene expression, the y-axis represents normalized intensity of expression in log2 scale. Full lists and expression data corresponding to all gene expression profiles (Figs. 1, A and B; 2, A and C; 3A; and 4A) are provided in Supplemental Tables 1 to 4.
Fig. 1.
Expression profile of SLC and ABC transporters during kidney development. A, microarray expression profile of probe sets for the SLC family of transporters were plotted for each stage of kidney development. B, expression profiles were also plotted for the ABC family of transporters. The x-axis represents the developmental stages of kidney development (Tsigelny et al., 2008).
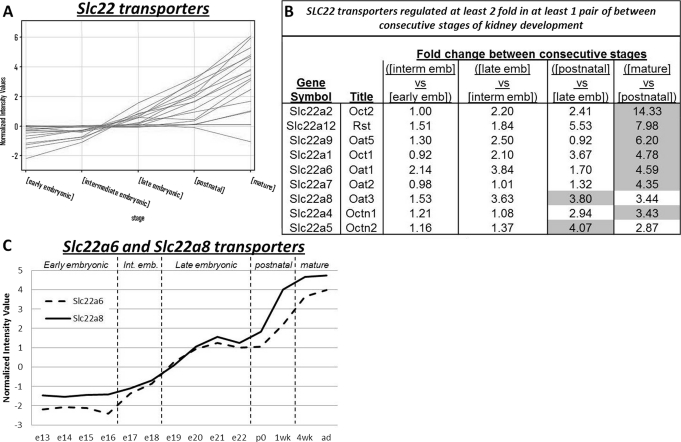
Fig. 2.
The SLC22 family of transporters show overall increases in mRNA expression over the course of kidney development. A, the expression of Slc22 (Oat) transporters throughout kidney development highlights a number of transporters that show large increases in expression around the postnatal to mature stage. B, 2-fold filtering identifies the Slc22 genes that have a 2-fold change in expression between the consecutive stages of kidney development. The shaded cells highlight the stage comparison with the largest change in expression. Early emb, early embryonic stage; interm emb, intermediate embryonic stage; late emb, late embryonic stage. C, specific expression of Slc22a6 (broken line) and Slc22a8 (solid line) from e13 through to adulthood highlight maximal expression at adulthood.
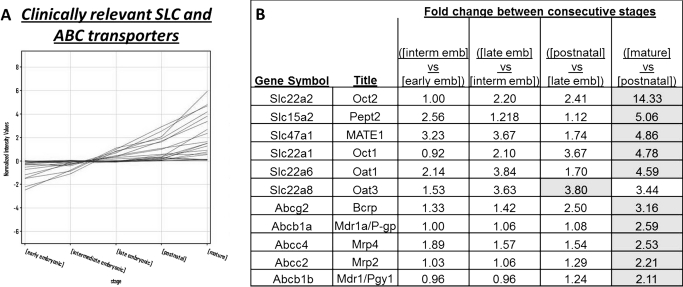
Fig. 3.
Clinically relevant SLC and ABC transporters show increased expression throughout the stages of kidney development, with highest expression at the mature stage. A, the expression profiles for the SLC transporters and ABC transporters with emerging clinical importance (Giacomini et al., 2010) were examined during kidney development. B, Fold-change of expression in “clinically relevant” drug transporters. Two-fold filtering identifies the clinically relevant drug transporters, which have a 2-fold change in expression between the consecutive stages of kidney development. The shaded cells indicate the stage comparison with the largest fold change. Early emb, early embryonic stage; interm emb, intermediate embryonic stage; late emb, late embryonic stage.
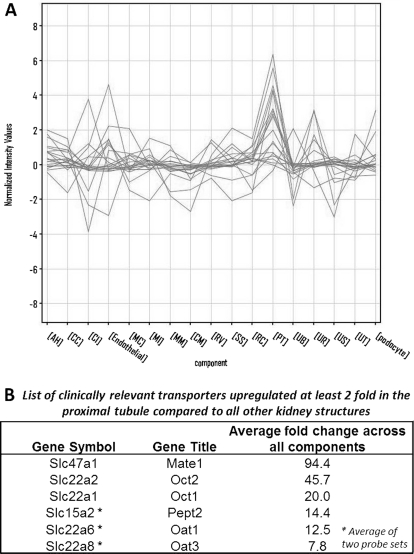
Fig. 4.
A number of clinically important drug transporters are specifically up-regulated in the developing proximal tubule. A, using the GUDMAP consortium microarray data sets profiling the substructures of the developing mouse kidney, 2-fold filtering was carried out to identify which clinically relevant transporters are specifically up-regulated in the developing proximal tubule. Six transporters show fold changes greater than 2-fold in the developing proximal tubule compared with other structures. GUDMAP substructure abbreviations are listed under Materials and Methods. B, the average fold change of transporters shown in B relative to the proximal tubule sample is tabulated. Average fold change is based on the geometric mean of fold change across all substructures.
Oat1 and Oat3 Knockout Mice [Oat1(−/−), Oat3(−/−)].
Oat1(−/−) mice (Eraly et al., 2006) and Oat3(−/−) mice (Sweet et al., 2002) were back-crossed to C57BL/6J mice for 10 generations. Heterozygous mice from the final back-cross were bred to each other to generate knockout (−/−) and wild-type (WT) mice, from which all of the animals used in the experiments described were descended. Female and male mice, at 1, 2, 3, and 8 weeks of age were used in the present experiments. Mice were genotyped by polymerase chain reaction. All experimental protocols were in accordance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD) and were approved by the Institutional Animal Care and Use Committee.
PAH Clearance Experiments in Wild-Type Mice at 1, 2, and 3 Weeks of Age.
During brief isoflurane anesthesia, PAH solution (77 μmol/kg; including unlabeled and 14C-labeled PAH in 0.85% NaCl) was injected into the retro-orbital plexus (2 μl/g of body weight). At defined times after injection (3, 7, 10, 20, or 40 min in 2-week-old mice; 5, 10, 15, and 20 min in 1- and 3-week-old mice), trunk blood was collected in heparinized capillaries after euthanization. After centrifugation, 10 μl of plasma was analyzed for PAH by scintillation counting. PAH volume of distribution, elimination constant, and clearance were calculated using a two-compartment model of two-phase exponential decay (Prism software; GraphPad Software Inc., San Diego, CA) (Vallon et al., 2009), using data from five animals to generate one decay curve.
Two-Week Postnatal Inulin and PAH Clearance in Oat1(−/−), Oat3(−/−), and Wild-Type.
Studies were performed as described above with addition of [3H]inulin (20 μg/kg) to the injection solution to also determine inulin clearance as described previously (Vallon et al., 2009).
Statistical Analysis.
Data are shown as means ± S.E.M. Unpaired Student's t test was performed to analyze for statistical differences between knockout and WT mice with P < 0.05 being considered to be statistically significant.
Results
Expression of SLC and ABC Transporters in the Embryonic, Neonatal, and Mature Kidney.
The SLC and ABC transporter families comprise the two large families of drug transporters in the genome (He et al., 2009; Wu et al., 2009). We sought to determine the expression profile of a comprehensive set of these transporters using microarray expression data from rodent (rat) kidneys. Data published previously profiling the whole kidney transcriptome from its initial formation at e13 through each consecutive day of embryonic development (e14–e22) and time points profiling the kidney at birth, week 1, week 4, and adulthood was obtained (Tsigelny et al., 2008). A stage model as described in Tsigelny et al. (2008) was used to focus on the main transitional changes in gene expression across the data set. In brief, this stage model was computed based on the similarity of gene expression of the time points from cluster analysis. The resultant five stages of kidney development were as follows: the early embryonic stage of kidney development consisted of e13, e14, e15, and e16 time points; the intermediate embryonic stage consisted of e17 and e18; the late embryonic stage was inclusive of e19, e20, e21, and e22; the postnatal stage consisted of birth and week 1; and the mature stage was a grouping of week 4 and adult kidney. Using this stage model of gene expression of the whole embryonic kidney, SLC and ABC transporter genes were examined (Fig. 1). In general there is an upward trend in expression for the SLC (Fig. 1A; Supplemental Table 1) and ABC (Fig. 1B; Supplemental Table 2) transporters, with a number of the transporters showing maximal expression at the mature stage.
Analyzing the Expression of SLC22 Family of Transporters in the Embryonic, Neonatal, and Mature Kidney.
Next we examined the expression of the SLC22 family of transporters. This family of transporters is responsible for the transport of organic cationic as well as anionic (and zwitterionic) molecules across the cell membrane and so has relevance as potential transporters of drug substances of similar chemical nature. Although there are some data on embryonic expression (Pavlova et al., 2000; Sweet et al., 2006), detailed time-series analysis of the subfamily has not been done. Of the 17 probe sets for the SLC22 genes examined (profile plot as in Fig. 2A; Supplemental Table 3), 12 showed a 2-fold change or greater between two temporally consecutive stages of kidney development (Fig. 2B). Comparison of consecutive stages (according to (Tsigelny et al., 2008) were performed between the early embryonic and the intermediate embryonic stages, the intermediate embryonic and late embryonic stages, the late embryonic and postnatal stage and finally between the postnatal stage and mature stage. It is noteworthy that in almost all cases (except for Oat3 and Octn2), the largest fold change in expression occurred between the postnatal and mature stage of kidney development. Specific expression of the drug transporters Oat1 (Slc22a6) and Oat3 (Slc22a8) were also examined across the individual time points from embryonic days 13 to 22, newborn, week 1, week 4, and adult to further highlight the progressive increase in mRNA expression levels over the entire developmental time course (Fig. 2C).
Investigating Clinically Important Drug Transporters in the Developing Kidney and Developing Nephron Segments.
Our findings indicate that there is a general tendency for members of the SLC, ABC, and SLC22 families of transporters to have naturally increased expression as renal development of the kidney proceeds and that the largest increase in expression is often observed between the postnatal and mature stage of kidney development. However, it is unclear which subsets of these transporters have specific and potentially therapeutic roles in drug secretion and/or retention in the kidney. A review published by the International Transporter Consortium has outlined a select list of SLC and ABC transporters, which have emerging clinical relevance for the absorption and disposition of drugs across epithelial interfaces (International Transporter Consortium et al., 2010). Accordingly, we determined the expression profiles of these clinically important transporters during kidney development (Fig. 3A; Supplemental Table 4). Of the 22 probe sets examined for these transporters, 11 showed a 2-fold increase in at least one consecutive stage comparison (Fig. 3B). By comparing the expression profiles between temporally sequential stages, it is evident that for the majority of these transporters, the greatest fold change in expression occurs between the transition between birth to maturity (the comparison between the postnatal stage and the mature stage).
Although it is clear that the largest increase in “pharmaceutically” relevant transporters also seems to occur between birth to maturity in the kidney, it was also important to determine whether the localization of these transporters was specific to a developing nephron segment of the embryonic kidney. The embryonic kidney comprises a number of developing nephron and collecting duct segments such as the ureteric bud, proximal tubules, and renal corpuscles (Fig. 4A), each of which have individual transcriptomic expression profiles as differentiation proceeds. To facilitate the examination of the localization of the transcripts for transporters in these developing nephron segments, the GUDMAP consortium microarray data sets were used (Brunskill et al., 2008). Microarray data sets were obtained for a number of developing mouse nephron segments, including the e11.5 metanephric mesenchyme (MM), e15.5 cap mesenchyme (CM), e12.5 renal vesicle (RV), e15.5 S-shaped body (SS), endothelial cells, podocytes, e15.5 renal corpuscle (RC), e15.5 proximal tubules (PT), e15.5 anlage of and immature loop of Henle (AH), e11.5 ureteric bud (UB), e15.5 ureteric tip region (UT), e15.5 cortical collecting duct (CC), e15.5 medullary collecting duct (MC), e15.5 urothelium (UR), e15.5 ureteral smooth muscle layer (US), e15.5 medullary interstitium (MI), and e15.5 cortical and nephrogenic interstitium (CI). We applied 2-fold filtering in pairwise comparisons between each of these nephron components on the list of clinically important drug transporters. There was a propensity for high expression of a number of transporters in the developing proximal tubule (Fig. 4A). Specifically, six of the drug transporters show significant up-regulation in the developing proximal tubule over all other structures (Fig. 4B), including the basolateral transporters Oat1 and Oat3. This implies that the drug transport function may be partially established, even in the e15.5 proximal tubule, and that drug transporters already show distinct segmental localization along the nephron even at this relatively early time point during kidney development. These transporters may be functional even at this time (Sweet et al., 2006; Rosines et al., 2007; Truong et al., 2008; Nagle et al., 2011).
Investigating the Functional Maturation of OAT1 and OAT3 in the Postnatal Kidney Using PAH as a Representative Organic Anion for In Vivo Physiological Studies.
Both Oat1 and Oat3 are up-regulated in the maturing kidney and are specifically up-regulated in the developing proximal tubule. These transporters mediate the basolateral uptake of a plethora of drugs, including nonsteroidal anti-inflammatory drugs, antivirals, β-lactams, and methotrexate (Nigam et al., 2007; Vanwert et al., 2007; Nagle et al., 2011). Our previous studies in adult knockout mice for these two genes revealed that OAT1 but not OAT3 mediates the proximal tubular secretion of the prototypic organic anion p-aminohippurate and endogenous organic anions (Eraly et al., 2006; Vallon et al., 2008). OAT1 and OAT3 are also essential for diuretic delivery into the lumen of the proximal tubule, and OAT3 may be involved in blood pressure regulation (Vallon et al., 2008a,b). Whereas some information is available on the OAT1 and OAT3 systems in adult rats, mice, and humans, very little is known about the newborn and juvenile situation, yet many of these drugs are also applied in the neonatal period.
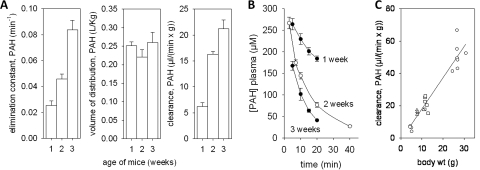
Because the increased expression of transporters of the rodent kidney from 1 to 3 weeks may reflect the functional maturation of the human kidney during the first year of life (Holtbäck and Aperia, 2003) we sought to determine the functional state of the OAT transporters in the postnatal rat kidney. Using PAH as a representative organic anion, PAH clearance experiments were carried out in wild-type mice at 1, 2, and 3 weeks of age (two to three sets of female and two to three sets of male mice per time point). Whereas the volume of distribution for PAH was not different for WT mice at 1, 2, or 3 weeks of age, there was an age-dependent increase in the elimination constant and in the clearance of PAH (Fig. 5, A and B). Including data from previous analyses of PAH clearance in adult WT mice (8–10 weeks) (Eraly et al., 2006; Vallon et al., 2008) revealed a further increase in PAH clearance between 3 and 8 to 10 weeks of age (Fig. 5C). This suggests that, although organic anion transport is functional in the postnatal pups, its activity is immature and is dependent on the age of the neonate. The data seem generally consistent with the expression profiling described earlier.
Fig. 5.
Age-dependent increase of PAH clearance in wild-type mice. A, elimination constant, volume of distribution, and clearance of PAH were determined by plasma kinetics after intravenous bolus injection of PAH for mice at 1, 2, and 3 weeks of age. Data are means ± S.E.M. in four to six sets of mice per age (four to five mice/set); results were similar for male and female and therefore were pooled. B, plasma kinetics of PAH. C, lower left, ○, ▵, and □ are 1, 2, and 3 weeks of age, respectively. Upper right, circles are from published data for PAH clearance derived from adult mice (8–10 weeks of age) (Eraly et al., 2006; Vallon et al., 2008) and are included for comparison.
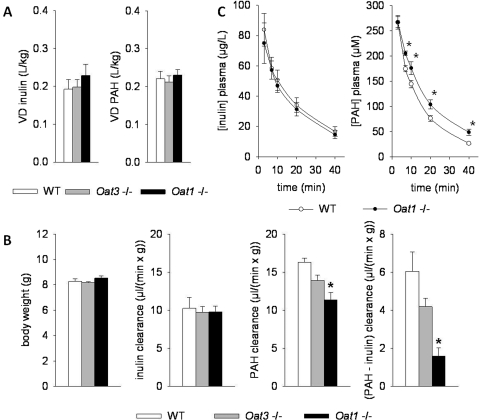
The In Vivo Clearance of PAH by OAT1 and OAT3 in the Postnatal Kidney.
Studies in knockout mice revealed that Oat1 is the principle transporter of PAH in the adult (Eraly et al., 2006; Vallon et al., 2008). However, it is unclear whether this is the case in the newborn, which is in a dynamic state, at least based on the expression data presented here. This is an important issue because newborns and juveniles may be dosed with organic anion drugs based upon extrapolations from adult studies. To investigate this further, we carried out inulin and PAH clearance in Oat1(−/−), Oat3(−/−), and wild-type mice at 2 weeks of age (Fig. 6). Body weight and the volume of distribution for PAH and inulin were similar between WT and Oat1(−/−) and Oat3(−/−) mice (Fig. 6, A and B). Whereas the clearance of inulin was comparable between genotypes, the clearance of PAH and the difference between PAH and inulin clearance (as an indirect measure of renal PAH secretion) were significantly lower in Oat1(−/−) than in WT mice (Fig. 6, B and C). Figure 6B presents the combined data for male and female mice because parameters were not significantly different between the sexes (e.g., PAH clearance and PAH secretion in WT female versus male were 16 ± 1 versus 17 ± 1 μl · min−1 · g−1 and 7 ± 2 versus 5 ± 1 μl · min−1 · g−1; n = 3/sex; NS). Likewise, PAH clearance and PAH secretion were not different between Oat3(−/−) female versus male (14 ± 2 versus 14 ± 1 and 4 ± 1 versus 4 ± 1 μl · min−1 · g−1; n = 3–4/sex; NS) or Oat1(−/−) female versus male (10 ± 1 versus 12 ± 2 and 2 ± 1 versus 2 ± 1 μl · min−1 · g−1; n = 3/sex; NS). The PAH clearance tended to be lower in Oat3(−/−) mice, but this did not reach statistical significance. This suggests that OAT1 mediates the proximal tubular secretion of PAH in the postnatal kidney.
Fig. 6.
PAH clearance is attenuated at 2 weeks of age in Oat1(−/−) mice. A, the volume of distribution for PAH and inulin was similar between WT, Oat1(−/−), and Oat3(−/−) mice. B, whereas body weight and the clearance of inulin were similar between genotypes, the clearance of PAH and the difference between PAH and inulin clearance (as an indirect measure of renal secretion) were significantly lower in Oat1(−/−) than in WT mice. C, plasma kinetics of inulin and PAH in WT versus Oat1(−/−) mice. Data are means ± S.E.M. in six to seven sets of mice per genotype (five mice/set); *, P < 0.05 versus WT.
Discussion
The kidney is comprised of a number of epithelial cell types, which carry out various essential roles including metabolite excretion, electrolyte homeostatic maintenance, and drug and toxin transport. Although pharmacokinetic studies often focus on the fate of drugs in the adult situation, there is a critical need to understand the kinetics of pharmacological agents in newborns and infants given that their renal functional capacity is still undergoing maturation (Hook and Bailie, 1979). Using rat and mouse mRNA microarray expression data published previously, we describe the expression of many components of the major drug transport systems during kidney development, through the postnatal period, and on to adulthood. Overall, there seems to be an up-regulation of the SLC and ABC transporters with developmental time. The expression of SLC22 family of transporters also increases with time, but perhaps of particular interest are the clinically relevant transporters, of which 11 transporters are up-regulated at least 2-fold across the development stages analyzed.
The specific expression of a large proportion of these clinically relevant transporters (Slc47a1, Slc22a2, Slc15a2, Slc22a1, Slc22a6, and Slc22a8) in the e15.5 developing proximal tubule suggests that drug transport systems are present early in renal development. In fact, it has been shown previously using whole embryonic kidney culture in vitro that OAT1-mediated uptake of the fluorescent specific substrate fluorescein can be observed after a week of culture of an e13 rat kidney, a time at which proximal tubule formation should be well under way (Sweet et al., 2006; Truong et al., 2008; Nagle et al., 2011).
The expression of Oats in postnatal development has been measured in mice and rats previously (Buist et al., 2002; Buist and Klaassen, 2004) and shown to increase to mature levels in the weeks after birth. Oat1 and Oat3 proteins localize to proximal tubule (Hwang et al., 2010), and increases in staining are visible until 3 weeks postnatally, when the tissue is similar in protein level to the adult organ but remains early enough in development that sex-based differences are negligible. Although there exist some differences in the expression and or localization of Oat drug transporters along the nephron in different species (Enomoto et al., 2002; Kojima et al., 2002; Ljubojevic et al., 2004; Wu et al., 2009; Nagle et al., 2011), rodents provide an accessible model to carry out pharmacodynamic and kinetic studies. In light of these divergences, experimental studies using human tissues and cells would be ideally relevant for clinical benefit. However, the study of the subtle differences between rodent and human may be key to understanding the vulnerabilities and susceptibilities to drug/metabolite toxicity that have evolved in humans. Although previous analyses of expression and protein localization corroborate a dramatic increase in expression, there has yet to be a functional analysis of the transport systems to examine the crucial postnatal developmental phase.
The maturation of the organic anion transport systems in the postnatal mouse kidney was studied using the prototypic organic anion PAH. The kinetics of PAH reflect the kinetic behavior of any pharmacological agent transported by OAT1. Clearance of PAH increases nearly 4-fold between weeks 1 and 3 in the mouse, and, by using data from mature mice, it is clear that this increase continues from 3 weeks to adulthood. Therefore, the drug transport system can be considered relatively immature even at 3 weeks of age in the mouse. The attenuation of PAH clearance in the Oat1 knockout animal confirms that PAH is indeed transported via OAT1 in the young kidney similar to the adult situation.
Although there were increases in both mRNA levels of clinical drug transporters and in PAH clearance in the kidney in the postnatal to mature period, it is still unclear what triggers these increases. Previous studies suggest that hormonal regulation may be responsible for the expression of the transporters. Studies have shown that sex differences in Oat 1 expression are influenced by the sex steroids testosterone and estradiol (Ljubojevic et al., 2004). It is also known that the corticosteroid dexamethasone, increases PAH uptake in both rat and human renal slices (Fleck et al., 2002). Thyroid hormones have also been implicated in the regulation of OAT activity in the postnatal kidney, using assessment of PAH clearance (Bräunlich, 1984). Given that there is a surge in the levels of catecholamine, glucocorticoids, and thyroid hormones at birth (Freemark, 1999; Challis et al., 2000; Blackburn, 2007), it is possible that circulating hormone levels are responsible for the maturational increases in the mRNA levels of the drug transporters. The impetus for up-regulation in expression may also be linked to the feeding and fasting behavior that is newly established after birth, as may be occurring with the regulation of liver transporters (Eder and Ringseis, 2010; Kok et al., 2003).
The fold-change increase in expression and function could also be attributed to morphological changes that are occurring in the maturing kidney. In humans, nephrogenesis is complete by 34 weeks of gestation, whereas in the rat, nephrogenesis is complete between postnatal days 7 and 10 (Dickinson et al., 2005). Although the additional development of new nephrons could be responsible for the increases in transporter mRNA levels, it is more likely that the postnatal changes in segmental maturation of the individual S1, S2, and S3 segments of the proximal tubule could be a major contributing factor. In the rabbit, it is not until postnatal day 40 that all segments of the outer cortical tubules have reached maturity (Evan et al., 1983). Over this period of time, all segments of the proximal tubule show increases in length with the S1 segment undergoing a 6-fold increase in segment length (Evan et al., 1983). In light of this, it should be considered that the formation of new nephron units and the extensive growth of established nephrons may also contribute to the high up-regulation of Oat1 and Oat3 in the postnatal kidney.
In summary, this study provides a comprehensive overview of the maturation of drug transporter expression throughout the embryonic, postnatal, and maturation stages of renal development. Specific transporters are up-regulated over the developmental time course, which parallels the functional maturation observed in PAH clearance rates. It is vital that neonatal-specific drug-transport studies continue to be carried out if pharmacological safety is to be enhanced and overdose or toxicity issues are to be avoided. Understanding how epithelial drug transporters, present at multiple interfaces (not only the blood-nephron barrier but also transepidermal, gut, and blood-brain barrier), are regulated pre- and postnatally is a key component to this endeavor, as is understanding how intracellular drug-metabolizing enzymes are working in concert with the transport mechanisms to detoxify or activate the pharmacological compounds. To this end, this study should serve as a basis for investigations into the pharmacokinetics of neonatally relevant drugs transported by the renal drug transport system in the future.
Supplementary Material
Acknowledgments
We thank Shamara Closson and Jana Schroth for expert technical assistance and Megan Bettilyon for editorial assistance.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant R01-HL94728]; the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants P30-DK079337, DK079784]; the National Institutes of Health National Institute of General Medical Sciences [Grant GM088824]; the Department of Veterans Affairs; the American Heart Association [Grant 10SDG2610034]; and the American Society of Nephrology Carl W. Gottschalk Research Scholar Grant.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.070680.
- ABC
- ATP-binding cassette
- OAT
- organic anion transporter
- PAH
- para-aminohippurate
- SLC
- solute carrier
- GUDMAP
- genitourinary development molecular anatomy project
- UT
- ureteric tip
- PT
- proximal tubules
- RC
- renal corpuscle
- MM
- metanephric mesenchyme
- CM
- cap mesenchyme
- RV
- renal vesicle
- SS
- S-shaped body
- AH
- loop of Henle
- UB
- ureteric bud
- CCD
- cortical collecting duct
- MC
- medullary collecting duct
- UR
- urothelium
- US
- ureteral smooth muscle
- MI
- medullary interstitium
- P0
- birth
- CI
- cortical interstitium
- e
- embryonic day
- WT
- wild type
- NS
- not significant.
Authorship Contributions
Participated in research design: Sweeney, Vallon, Rieg, Wu, and Nigam.
Conducted experiments: Sweeney, Vallon, Rieg, and Gallegos.
Performed data analysis: Sweeney, Vallon, and Rieg.
Wrote or contributed to the writing of the manuscript: Sweeney, Vallon, Rieg, Wu, Gallegos, and Nigam.
Other: Sweeney and Vallon contributed equally and are considered co-first authors.
References
- Ahn SY, Nigam SK. (2009) Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Mol Pharmacol 76:481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn ST. (2007) Pituitary, adrenal, and thyroid function, in Maternal, Fetal & Neonatal Physiology: a Clinical Perspective, p. 669–699, Saunders, St. Louis, MO [Google Scholar]
- Bräunlich H. (1984) Postnatal development of kidney function in rats receiving thyroid hormones. Exp Clin Endocrinol 83:243–250 [DOI] [PubMed] [Google Scholar]
- Brunskill EW, Aronow BJ, Georgas K, Rumballe B, Valerius MT, Aronow J, Kaimal V, Jegga AG, Yu J, Grimmond S, et al. (2008) Atlas of gene expression in the developing kidney at microanatomic resolution. Dev Cell 15:781–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buist SC, Cherrington NJ, Choudhuri S, Hartley DP, Klaassen CD. (2002) Gender-specific and developmental influences on the expression of rat organic anion transporters. J Pharmacol Exp Ther 301:145–151 [DOI] [PubMed] [Google Scholar]
- Buist SC, Klaassen CD. (2004) Rat and mouse differences in gender-predominant expression of organic anion transporter (Oat1–3; Slc22a6–8) mRNA levels. Drug Metab Dispos 32:620–625 [DOI] [PubMed] [Google Scholar]
- Burckhardt BC, Burckhardt G. (2003) Transport of organic anions across the basolateral membrane of proximal tubule cells. Rev Physiol Biochem Pharmacol 146:95–158 [DOI] [PubMed] [Google Scholar]
- Challis JRG, Matthews SG, Gibb W, Lye SJ. (2000) Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 21:514–550 [DOI] [PubMed] [Google Scholar]
- Chappell K, Newman C. (2004) Potential tenfold drug overdoses on a neonatal unit. Arch Dis Child Fetal Neonatal Ed 89:F483–F484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson H, Walker DW, Cullen-McEwen L, Wintour EM, Moritz K. (2005) The spiny mouse (Acomys cahirinus) completes nephrogenesis before birth. Am J Physiol Renal Physiol 289:F273–F279 [DOI] [PubMed] [Google Scholar]
- Eder K, Ringseis R. (2010) The role of peroxisome proliferator-activated receptor alpha in transcriptional regulation of novel organic cation transporters. Eur J Pharmacol 628:1–5 [DOI] [PubMed] [Google Scholar]
- Enomoto A, Takeda M, Shimoda M, Narikawa S, Kobayashi Y, Kobayashi Y, Yamamoto T, Sekine T, Cha SH, Niwa T, et al. (2002) Interaction of human organic anion transporters 2 and 4 with organic anion transport inhibitors. J Pharmacol Exp Ther 301:797–802 [DOI] [PubMed] [Google Scholar]
- Eraly SA, Vallon V, Vaughn DA, Gangoiti JA, Richter K, Nagle M, Monte JC, Rieg T, Truong DM, Long JM, et al. (2006) Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. J Biol Chem 281:5072–5083 [DOI] [PubMed] [Google Scholar]
- Evan AP, Gattone VH, 2nd, Schwartz GJ. (1983) Development of solute transport in rabbit proximal tubule. II. Morphologic segmentation. Am J Physiol 245:F391–F407 [DOI] [PubMed] [Google Scholar]
- Fleck C, Hilger R, Jurkutat S, Karge E, Merkel U, Schimske A, Schubert J. (2002) Ex vivo stimulation of renal transport of the cytostatic drugs methotrexate, cisplatin, topotecan (Hycamtin) and raltitrexed (Tomudex) by dexamethasone, T3 and EGF in intact human and rat kidney tissue and in human renal cell carcinoma. Urol Res 30:256–262 [DOI] [PubMed] [Google Scholar]
- Freemark M. (1999) The fetal adrenal and the maturation of the growth hormone and prolactin axes. Endocrinology 140:1963–1965 [DOI] [PubMed] [Google Scholar]
- He L, Vasiliou K, Nebert DW. (2009) Analysis and update of the human solute carrier (SLC) gene superfamily. Hum Genomics 3:195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtbäck U, Aperia AC. (2003) Molecular determinants of sodium and water balance during early human development. Semin Neonatol 8:291–299 [DOI] [PubMed] [Google Scholar]
- Hook JB, Bailie MD. (1979) Perinatal renal pharmacology. Annu Rev Pharmacol Toxicol 19:491–509 [DOI] [PubMed] [Google Scholar]
- Hwang JS, Park EY, Kim WY, Yang CW, Kim J. (2010) Expression of OAT1 and OAT3 in differentiating proximal tubules of the mouse kidney. Histol Histopathol 25:33–44 [DOI] [PubMed] [Google Scholar]
- International Transporter Consortium, Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, et al. (2010) Membrane transporters in drug development. Nat Rev Drug Discov 9:215–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima R, Sekine T, Kawachi M, Cha SH, Suzuki Y, Endou H. (2002) Immunolocalization of multispecific organic anion transporters, OAT1, OAT2, and OAT3, in rat kidney. J Am Soc Nephrol 13:848–857 [DOI] [PubMed] [Google Scholar]
- Kok T, Wolters H, Bloks VW, Havinga R, Jansen PL, Staels B, Kuipers F. (2003) Induction of hepatic ABC transporter expression is part of the PPARalpha-mediated fasting response in the mouse. Gastroenterology 124:160–171 [DOI] [PubMed] [Google Scholar]
- Ljubojevic M, Herak-Kramberger CM, Hagos Y, Bahn A, Endou H, Burckhardt G, Sabolic I. (2004) Rat renal cortical OAT1 and OAT3 exhibit gender differences determined by both androgen stimulation and estrogen inhibition. Am J Physiol Renal Physiol 287:F124–F138 [DOI] [PubMed] [Google Scholar]
- Nagle MA, Truong DM, Dnyanmote AV, Ahn SY, Eraly SA, Wu W, Nigam SK. (2011) Analysis of three-dimensional systems for developing and mature kidneys clarifies the role of OAT1 and OAT3 in antiviral handling. J Biol Chem 286:243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam SK, Bush KT, Bhatnagar V. (2007) Drug and toxicant handling by the OAT organic anion transporters in the kidney and other tissues. Nat Clin Pract Nephrol 3:443–448 [DOI] [PubMed] [Google Scholar]
- Pavlova A, Sakurai H, Leclercq B, Beier DR, Yu AS, Nigam SK. (2000) Developmentally regulated expression of organic ion transporters NKT (OAT1), OCT1, NLT (OAT2), and Roct. Am J Physiol Renal Physiol 278:F635–F643 [DOI] [PubMed] [Google Scholar]
- Rosines E, Sampogna RV, Johkura K, Vaughn DA, Choi Y, Sakurai H, Shah MM, Nigam SK. (2007) Staged in vitro reconstitution and implantation of engineered rat kidney tissue. Proc Natl Acad Sci USA 104:20938–20943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine T, Endou H. (2009) Children's toxicology from bench to bed–Drug-induced renal injury (3): Drug transporters and toxic nephropathy in childhood. J Toxicol Sci 34:SP259–SP265 [DOI] [PubMed] [Google Scholar]
- Sweet DH, Eraly SA, Vaughn DA, Bush KT, Nigam SK. (2006) Organic anion and cation transporter expression and function during embryonic kidney development and in organ culture models. Kidney Int 69:837–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK. (2002) Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J Biol Chem 277:26934–26943 [DOI] [PubMed] [Google Scholar]
- Truong DM, Kaler G, Khandelwal A, Swaan PW, Nigam SK. (2008) Multi-level analysis of organic anion transporters 1, 3, and 6 reveals major differences in structural determinants of antiviral discrimination. J Biol Chem 283:8654–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigelny IF, Kouznetsova VL, Sweeney DE, Wu W, Bush KT, Nigam SK. (2008) Analysis of metagene portraits reveals distinct transitions during kidney organogenesis. Sci Signal 1:ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Eraly SA, Wikoff WR, Rieg T, Kaler G, Truong DM, Ahn SY, Mahapatra NR, Mahata SK, Gangoiti JA, et al. (2008) Organic anion transporter 3 contributes to the regulation of blood pressure. J Am Soc Nephrol 19:1732–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Rieg T, Ahn SY, Wu W, Eraly SA, Nigam SK. (2008) Overlapping in vitro and in vivo specificities of the organic anion transporters OAT1 and OAT3 for loop and thiazide diuretics. Am J Physiol Renal Physiol 294:F867–F873 [DOI] [PubMed] [Google Scholar]
- Vallon V, Schroth J, Satriano J, Blantz RC, Thomson SC, Rieg T. (2009) Adenosine A(1) receptors determine glomerular hyperfiltration and the salt paradox in early streptozotocin diabetes mellitus. Nephron Physiol 111:p30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanwert AL, Bailey RM, Sweet DH. (2007) Organic anion transporter 3 (Oat3/Slc22a8) knockout mice exhibit altered clearance and distribution of penicillin G. Am J Physiol Renal Physiol 293:F1332–F1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner A. (1986) Drug use in the neonate: interrelationships of pharmacokinetics, toxicity, and biochemical maturity. Clin Chem 32:721–727 [PubMed] [Google Scholar]
- Wu W, Baker ME, Eraly SA, Bush KT, Nigam SK. (2009) Analysis of a large cluster of SLC22 transporter genes, including novel USTs, reveals species-specific amplification of subsets of family members. Physiol Genomics 38:116–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Dnyanmote AV, Nigam SK. (2011) Remote Communication through Solute Carriers and ATP Binding Cassette Drug Transporter Pathways: An Update on the Remote Sensing and Signaling Hypothesis. Mol Pharmacol 79:795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.