Abstract
The mechanisms of increased rectal stiffness in women with fecal incontinence (FI) and rectal urgency are not understood. Our hypothesis was that distention-induced activation of mechanosensitive L-type calcium channels in smooth muscle contributes to increased rectal stiffness in FI. Anal pressures, rectal distensibility (compliance, capacity, and contractile response to sinusoidal oscillation), and rectal sensation were assessed before and after oral nifedipine (30 + 10 mg) or placebo in 16 women with FI and 16 asymptomatic women. At baseline, FI patients had a lower anal pressure increment during squeeze (health, 66.9 ± 7.6: FI, 28.6 ± 5.9, mean ± SE, P ≤ 0.01), lower rectal capacity (P = 0.052), and higher rectal pressures during sinusoidal oscillation (health, 13.7 ± 3.2: FI, 21.7 ± 1.4, mean ± SE, P = 0.02) than the healthy women, which suggests an exaggerated rectal contractile response to distention. Nifedipine decreased mean BP, increased heart rate (P = 0.01 vs. placebo), and reduced anal resting pressure (P ≤ 0.01) but did not significantly modify rectal distensibility in health or FI. Plasma nifedipine concentrations (health, 103 ± 21 ng/ml: FI, 162 ± 34 ng/ml) were correlated with increased rectal compliance (r = 0.6, P = 0.02) in all study participants and, in healthy subjects, with decreased rectal pressures during sinusoidal oscillation (r = 0.86, P = 0.01), indicative of reduced stiffness. No consistent effects on rectal perception were observed. These observations confirm that FI is associated with anal weakness and increased rectal stiffness. At therapeutic plasma concentrations, nifedipine reduced anal resting pressure but did not improve rectal distensibility in FI, outcomes that argue against a predominant contribution of myogenic L-type calcium channels to reduced rectal distensibility in FI.
Keywords: rectal compliance, sensation, calcium channel blockers, rectal capacity, pressure
fecal incontinence (FI) is a relatively common problem, particularly among women and the elderly, which can significantly impair daily functioning and also contribute to institutionalization (9). While most attention has focused on anal sphincter injury and weakness, there is increasing evidence for rectal sensorimotor dysfunctions in FI. For example, we observed reduced rectal capacity in 27% and reduced rectal compliance in 23% of women with FI (1, 4). Reduced rectal capacity was more common in women with (i.e., 36%) than without (i.e., 8%) the symptom of rectal urgency. Moreover, rectal hypersensitivity was observed in 18% of patients with normal and 50% of patients with reduced rectal capacity. These observations have been confirmed by other groups (12, 29).
The mechanisms of reduced rectal capacity and compliance in FI have not been investigated; conceivably “active” (e.g., abnormal mechanosensitivity or intercellular communication) or “passive” (e.g., tensile properties of cells, fibrosis) mechanisms may be involved. Mechanosensitivity of the gut is a complex process. In humans, enteric nerves, smooth muscle cells, and interstitial cells of Cajal are all mechanosensitive. Human gastrointestinal smooth muscle cells and interstitial cells of Cajal express mechanosensitive calcium and sodium channels (19). Of particular relevance to this study, distention activates mechanosensitive L-type calcium channels that are the main calcium entry pathway in gastrointestinal smooth muscle (13, 16), and nifedipine blocks these L-type calcium channels (13). Hence, this study investigated the hypothesis that an exaggerated myogenic response to distention contributes to increased rectal stiffness in FI by evaluating the effects of nifedipine on rectal mechanical properties (13). Because L-type calcium channels also contribute to internal anal sphincter function, which is primarily responsible for maintaining anal resting pressure (11, 21), the effects of nifedipine on anal pressures were also evaluated.
MATERIALS AND METHODS
Study design.
After obtaining written informed consent, bowel symptoms and anorectal sensorimotor functions were assessed before and after nifedipine in 16 controls [age 37 ± 3 (SE) yr, body mass index (BMI) 25.2 ± 1.0 kg/m2] and 16 women (age 52 ± 3 yr, BMI 30.3 ± 1.8 kg/m2) with urge-predominant FI in this study, which was approved by the Institutional Review Board at Mayo Clinic. This study was initiated before publicly available registries were developed. After a clinical assessment and completion of questionnaires, anorectal functions were assessed before and after nifedipine or placebo on the same day.
Participants.
To be included in the study, patients had to complain of FI for 6 mo or longer. Exclusion criteria included diabetes mellitus with neuropathy, congenital anorectal malformations, previous rectal surgery (such as rectopexy or rectal resection), chronic inflammatory bowel disease, pelvic radiation, active anal abscesses and fistulae, and neurologic diseases such as clinically significant peripheral neuropathy or spinal cord injury. Except for the following conditions (i.e., hypothyroidism with euthyroid status on pharmacological supplementation, degenerative joint disease, urinary incontinence, clinically stable bronchial asthma, hypertension, hyperlipidemia, or anxiety/depression), patients with chronic medical conditions were also excluded from the study. One control and eight patients were taking oral tricyclic antidepressants or selective serotonin reuptake inhibitors (SSRIs), i.e., citalopram (40 mg daily, 3 patients), fluoxetine (20 mg daily, 2 patients), amitriptyline (25 mg daily or less, 2 patients), escitalopram (10 mg daily, 1 patient), or sertraline (20 mg daily, 1 control).
Medication.
Subjects randomized to nifedipine received an initial oral dose of 30 mg since this dose is safe and inhibited esophageal, antral, and colonic contractility in healthy subjects (2, 23–25). The bioavailability of oral nifedipine is between 45 and 75% (18, 31). Because plasma concentrations decline rapidly between 60 and 120 min after a dose, a second dose of oral nifedipine (10 mg) was administered at 60 min after the first dose (30 mg) (Fig. 1). To avoid carryover effects, nifedipine (or placebo) was administered after baseline anorectal assessments were performed. Nifedipine capsules (Purepac Pharmaceutical, Totowa, NJ) were overencapsulated by size “00” capsules to match placebo capsules. The placebo capsules contained lactose monohydrate while nifedipine capsules contained other inactive ingredients (e.g., glycerin, peppermint oil, and polyethylene glycol). Because plasma concentrations of nifedipine peak at 30 min after a dose, postnifedipine anorectal assessments commenced at 20 min after the first dose. Plasma nifedipine concentrations were measured by high-performance liquid chromatography (National Medical Services, Willow Grave, PA), at 40 min after the first and 25 min after the second dose.
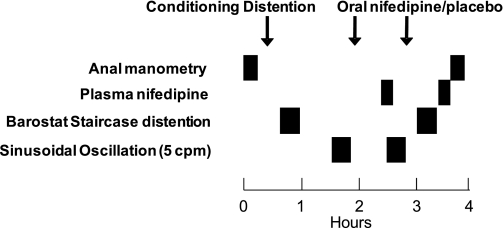
Fig. 1.
Study design. Anal manometry, rectal pressure-volume relationships with a barostat, and sinusoidal oscillation were assessed before and after nifedipine (2 doses, 30 and 10 mg orally) or placebo. Plasma nifedipine concentrations were assessed two times.
Symptom assessments.
At baseline, all patients completed a validated questionnaire pertaining to bowel symptoms, abdominal discomfort, as well as severity and circumstances surrounding FI (5, 8). These responses were used to grade the severity of FI by a validated scoring system [i.e., Fecal Incontinence and Continence Assessment (FICA)] incorporating the type, frequency, amount, and characteristics of FI (i.e., urge, passive, or combined) (5). Those patients who reported they were “often” or “usually” incontinent because they had “great urgency and could not reach the toilet on time” were considered to have urge incontinence. Those patients who reported they were often or usually “unaware when the leakage was actually happening” were considered to have “passive” incontinence.
Assessment of anorectal sensorimotor functions.
After two sodium phosphate enemas (Fleets; C.B. Fleet, Lynchburg, VA), anorectal testing (i.e., anal manometry and assessment of rectal compliance and sensation by a barostat) was conducted in the left lateral position. Average anal resting and squeeze pressures were measured using the station pull-through technique and summarized as described previously (6). Rectal compliance and sensation were recorded using previously validated techniques by an “infinitely” compliant 7-cm-long balloon with a maximum volume of 500 ml (Hefty Baggies; Mobil Chemical, Pittsford, NY) linked to an electronic rigid piston barostat (Mayo Clinic, Rochester, MN) (4, 20). An initial or conditioning distention followed by a rectal staircase distention (from 0 to 44 mmHg or to maximum tolerated pressure, whichever came first, in 4-mmHg steps at 1-min intervals) was performed. Rectal pressure-volume relationships were analyzed by a power exponential function and summarized by the pressure corresponding to half-maximum volume (Prhalf) and rectal capacity (i.e., maximum volume). Rectal compliance and sensory thresholds for first sensation, desire to defecate, and urgency were recorded during the staircase distention; the threshold was the first sensation of each symptom.
Rectal elasticity was also assessed using validated techniques by sinusoidal oscillation (3). After distending the balloon to 125 ml, it was oscillated at 5 counts/min for 20 min. Because the Institutional Review Board required that rectal pressures not exceed 48 mmHg during sinusoidal oscillation, the initial distending volume was reduced to 75 ml before pre- and postdrug sinusoidal oscillations in three patients with FI (3). Although the colonic response to sinusoidal oscillation is frequency-dependent and higher at 10 than at 5 or 20 counts/min (3), unpublished data from our laboratory suggest that the rectal response to sinusoidal oscillation is not frequency dependent. Hence, sinusoidal oscillation was only performed at one frequency (i.e., 5 counts/min) in this study.
Statistical analysis.
Anal manometry and rectal barostat data were summarized as anal resting and squeeze pressures, Prhalf during rectal compliance, rectal capacity, pressure and volume sensory thresholds for first sensation, desire to defecate, and urgency. During sinusoidal oscillation, average rectal pressures and the variability in pressures over 20 min were summarized. All response variables were analyzed separately before and after nifedipine.
Associations between baseline values of anorectal parameters and disease status (i.e., FI vs. control) were examined by an analysis of covariance incorporating age and BMI as covariates. The analyses of treatment effects were based on analysis of covariance models for the differences (baseline vs. postintervention) for all responses, except sensory thresholds (see below), and included subject status as a covariate. Tests of specific differences (e.g., delta Prhalf in those treated with nifedipine) were based on the ANCOVA model pooled residual variation. The interaction term (i.e., status × treatment) was not significant in any of the models for treatment effects. Posttreatment pressure and volume sensory thresholds were analyzed by proportional hazard regression models for each threshold type separately, adjusting for baseline threshold value and subject status. The pressure level values for sensory thresholds that were not perceived by a given subject were considered as “censored” using an approach described previously (1). Hazard ratios (HRs) for treatment effects were expressed as nifedipine relative to placebo. The association between plasma concentrations of nifedipine and the changes (before vs. after nifedipine) in anorectal functions were assessed using Spearman's correlation coefficient. The association of plasma nifedipine concentrations with subject status (healthy subjects vs. FI) was assessed by the Wilcoxon rank sum test.
RESULTS
Clinical features.
Of 37 subjects who were screened for this study, two were ineligible because of medication or cardiovascular issues while three had personal conflicts and were unable to complete the study. All 32 enrolled subjects completed the study. The 16 FI patients had FI for two or more years with symptoms of urge (1 patient), combined (i.e., passive and urge FI, 14 patients), and/or passive FI (1 patient). The frequency of FI ranged from less than once per month (1 patient), once a week (9 patients), or daily (6 patients). Patients were incontinent for only liquid stools (9 patients), only formed stools (1 patient), or both liquid and formed stools (6 patients). The typical amount of leakage was small (i.e., staining only, n = 7) or moderate (i.e., more than staining but less than a full bowel movement, n = 9). Thus, the FICA incontinence symptom severity score indicated moderate (12 patients) or severe (4 patients) FI.
Among controls, seven had at least one vaginal delivery (range 1–4 deliveries) and two had a hysterectomy. Fourteen of 16 patients had a vaginal delivery (range 1–7 deliveries). No controls but 10 patients had one or more known obstetric risk factors for FI [i.e., more than 4 vaginal deliveries (2 patients), 3rd or 4th degree perineal tear (2 patients), or a forceps-assisted delivery (8 patients)]. Three patients reported anal sphincteroplasty, and eight had a hysterectomy. Seven patients had anorectal imaging with endoanal ultrasound or magnetic resonance imaging. Imaging revealed normal-appearing internal and external anal sphincters (3 patients), only internal sphincter abnormalities (i.e., atrophy or scar, 2 patients), only external sphincter abnormalities (1 patient), or internal and external anal sphincter abnormalities (1 patient).
Effects of nifedipine on hemodynamic parameters.
Blood pressure (BP) declined and heart rate increased after nifedipine but not placebo. For example, at 20 min after the first dose, the mean BP and heart rate after nifedipine were 70 ± 4 vs. 81 ± 5 mmHg at baseline (P < 0.01 for drug effect vs. placebo) and 71 ± 5 vs. 64 ± 5 beats/min at baseline, respectively (P = 0.02 for drug effect vs. placebo). Thereafter, these effects were sustained throughout the study. The effects of nifedipine on hemodynamic parameters were not significantly influenced by subject status (FI vs. controls).
Because samples were not appropriately processed in 3 subjects, of whom 2 received nifedipine, nifedipine plasma concentrations were measured in 14 of 16 subjects who received nifedipine. Among healthy subjects who received nifedipine, plasma concentrations were 103 ± 21 ng/ml (therapeutic range 25–100 ng/ml) at 40 min and 99 ± 19 ng/ml at 85 min after the first dose. Plasma concentrations at corresponding times in FI were 162 ± 34 and 142 ± 28 ng/ml, respectively. The reduction in mean BP at 40 but not 85 min was correlated (r = −0.64, P = 0.02) with the plasma concentration of nifedipine. Among subjects randomized to placebo, plasma nifedipine concentrations were undetectable.
Effects on anorectal functions.
Baseline anal resting pressure was associated (i.e., lower) with age (P = 0.03) but not with FI (Table 1). In contrast, the anal pressure increment during squeeze was lower (P < 0.01) in FI than controls and not associated with age. Nifedipine reduced (P = 0.0002 vs. placebo) anal resting but not squeeze pressures; the reduction in resting pressure was not significantly influenced by subject status (controls vs. FI). However, drug effects on anal resting pressure and the pressure increment during the squeeze maneuver were not correlated with the plasma concentration of nifedipine.
Table 1.
Effects of nifedipine on anal pressures
| Resting Pressure |
Squeeze Pressure Increment |
|||
|---|---|---|---|---|
| Before | After | Before | After | |
| Health | ||||
| Placebo | 38.9 ± 4.5 | 41.1 ± 5.1 | 56.4 ± 10 | 44.7 ± 3.2 |
| Nifedipine | 37.5 ± 4.6 | 25.7 ± 2.6† | 77.4 ± 10.9 | 69.4 ± 9.8 |
| Fecal incontinence | ||||
| Placebo | 26.7 ± 4.0 | 23.5 ± 0.09 | 25.2 ± 10.4* | 32.1 ± 9.8 |
| Nifedipine | 29.6 ± 3.3 | 19.2 ± 1.0† | 31.9 ± 6.1* | 28.7 ± 4.1 |
Values are means ± SE.
P = 0.01 for Fecal incontinence (FI) vs. health (pooled baseline).
P = 0.0002 for treatment effect vs. placbo.
Effect on rectal mechanical properties during barostat and sinusoidal distention.
While rectal compliance (Prhalf) was lower in FI than in healthy subjects, associations with subject status were not significant after correcting for age (P < 0.0003) and BMI (Table 2). In contrast, baseline rectal capacity was associated with subject status (P =0.052); values were lower in FI (235 ± 13 ml) than in controls (262 ± 12 ml) even after adjusting for age and BMI. While nifedipine increased (P = 0.02) rectal compliance (i.e., reduced Prhalf from 17.8 ± 1.2 to 16 ± 0.6 mmHg) in FI but not controls, overall treatment effects (nifedipine vs. placebo) were not significant (p = 0.09). However, among subjects who received nifedipine, plasma concentrations of nifedipine were inversely correlated (r = 0.6, P = 0.02) with Prhalf, indicating that a lower Prhalf (i.e., increased compliance) was associated with higher plasma nifedipine concentrations. In contrast, nifedipine did not significantly increase rectal capacity in FI. Moreover, correlations between nifedipine's effects on rectal capacity and plasma nifedipine concentrations were not significant.
Table 2.
Effects of nifedipine on rectal stiffness during pressure-volume relationships
| Prhalf |
Rectal Capacity |
|||
|---|---|---|---|---|
| Before | After | Before | After | |
| Health | ||||
| Placebo | 14.5 ± 1.5 | 15.3 ± 1.1 | 268.5 ± 17.2 | 289.5 ± 11.9 |
| Nifedipine | 12.9 ± 1.2 | 12.3 ± 1.0 | 254.5 ± 16.2 | 263.8 ± 19.0 |
| Fecal incontinence | ||||
| Placebo | 18.0 ± 0.82 | 17.9 ± 2.7 | 232.0 ± 55.2* | 250.9 ± 20.7 |
| Nifedipine | 17.8 ± 1.2 | 16.0 ± 0.6 | 238.1 ± 17.8* | 252.3 ± 17.0 |
Values are means ± SE. Prhalf, pressure corresponding to half-maximal volume.
P = 0.052 for FI vs. health (pooled baseline).
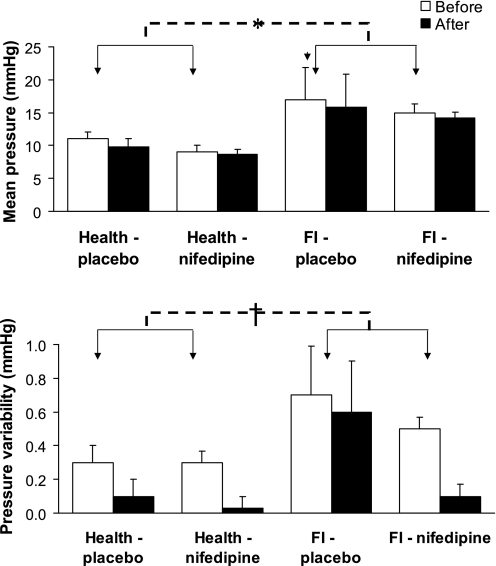
During sinusoidal oscillation, the balloon pressure waveform was generally sinusoidal. Balloon pressures were higher for ∼2–4 min after oscillation commenced and declined thereafter (Fig. 2), punctuated by intermittent high-pressure peaks. Mean rectal pressures and variability in rectal pressures during sinusoidal oscillations at baseline were associated with subject status (P = 0.024 and P = 0.051, respectively) and higher in FI, indicating increased rectal stiffness and irritability, respectively (Fig. 3). While nifedipine's effects on mean pressures or the variability in pressures during sinusoidal oscillation were not significant, plasma concentrations of nifedipine were correlated (r = 0.86, P = 0.01) with the difference (after − before) in mean pressures in healthy subjects but not FI. However, effects on the variability in rectal pressures during sinusoidal oscillation were not correlated with plasma concentrations of nifedipine.
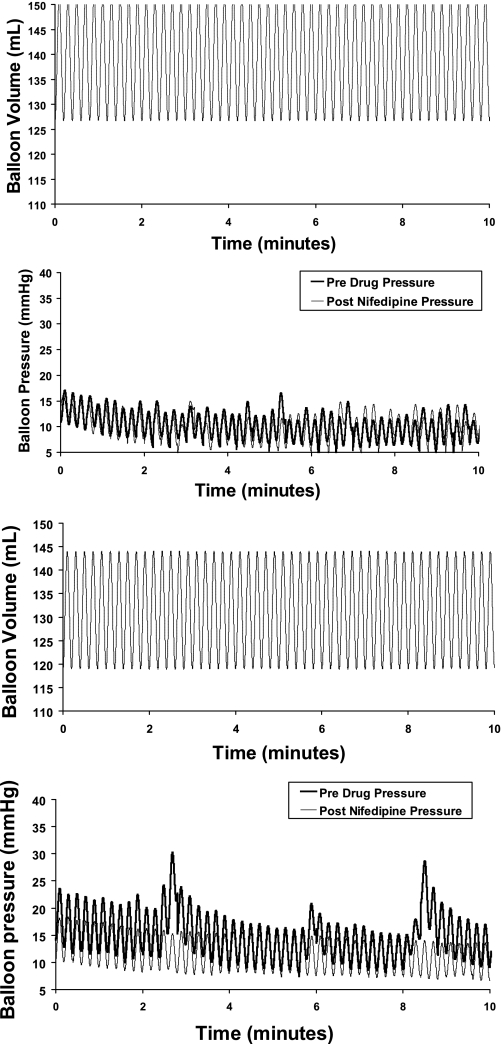
Fig. 2.
Balloon pressure waveform during sinusoidal oscillation (25 ml) at 5 counts/min in a healthy subject (2 panels on top) and a patient with fecal incontinence (FI) (2 panels on bottom). Data were acquired and depicted at 10 Hz. While sinusoidal oscillation was performed for 20 min, data for the first 10 min are shown for clarity. For simplicity, pre- and postdrug volumetric oscillations are superimposed. Rectal pressures during sinusoidal oscillation before drug were substantially higher in the FI patient than the healthy subject. While rectal pressures were comparable before and after placebo in the healthy subject, nifedipine reduced rectal pressure and its variability in the FI patient.
Fig. 3.
Effects of nifedipine and placebo on mean pressures (top) and pressure variability (bottom) in health and FI. Data are averaged across sinusoidal oscillation epoch at 5 counts/min for 20 min. *P = 0.02 for FI vs. health (pooled baseline). †P = 0.051 for FI vs. health (pooled baseline).
Effects on rectal sensation.
At baseline, pressure and volume thresholds for first sensation, desire to defecate, and urgency were not significantly associated with subject status (controls vs. FI) after adjusting for age and BMI (Table 3). In the placebo group, all pressure and volume thresholds were higher after than before placebo treatment. Compared with placebo, nifedipine was associated with a lower volume threshold for first sensation (HR, 2.5; 95% confidence interval, 1.001–6.09) and a lower pressure threshold for desire to defecate (HR, 3.0; 95% confidence interval, 1.3–7.1). However, treatment effects on other pressure and volume thresholds were not significant.
Table 3.
Effects of nifedipine on rectal sensation
| First Sensation |
Desire to Defecate |
Urgency |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pressure |
Volume |
Pressure |
Volume |
Pressure |
Volume |
|||||||
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | |
| Health | ||||||||||||
| Placebo | 9 ± 2 | 11 ± 2 | 63 ± 15 | 76 ± 16 | 14 ± 2 | 17 ± 3 | 107 ± 21 | 131 ± 29 | 23 ± 4 | 27 ± 4 | 174 ± 24 | 204 ± 22 |
| Nifedipine | 13 ± 3 | 11 ± 1 | 109 ± 28 | 98 ± 17* | 14 ± 3 | 13 ± 1† | 129 ± 26 | 119 ± 19 | 24 ± 4 | 26 ± 3 | 191 ± 29 | 217 ± 25 |
| Fecal incontinence | ||||||||||||
| Placebo | 11 ± 2 | 15 ± 4 | 62 ± 12 | 106 ± 26 | 14 ± 2 | 21 ± 2 | 86 ± 14 | 131 ± 16 | 22 ± 3 | 27 ± 2 | 125 ± 25 | 172 ± 22 |
| Nifedipine | 16 ± 5 | 14 ± 1 | 87 ± 29 | 78 ± 14* | 24 ± 4 | 16 ± 2† | 135 ± 36 | 113 ± 21 | 27 ± 3 | 23 ± 3 | 163 ± 32 | 161 ± 34 |
Values are means ± SE.
Hazard ratio (HR) 2.5 [95% confidence interval (CI) 1.001, 6.09].
HR 3.0 (95% CI 1.3, 7.1).
DISCUSSION
This study confirms that women with urge-predominant FI have anal weakness and an exaggerated rectal contractile response to distention. Extending previous studies, nifedipine reduced anal resting pressure in health (10) and FI. In comparison, the effects of nifedipine on rectal mechanical properties were relatively modest. Within the constraints of administering a higher dose of nifedipine to humans, these data suggest it is unlikely that L-type calcium channels substantially contribute to increased rectal stiffness in women with urge-predominant FI.
Rectal mechanosensitivity was evaluated by barostat-driven staircase distentions and by sinusoidal oscillation, which has been extensively used to understand normal airway function and mechanisms of bronchoconstriction in asthma, and in the human colon but not in the rectum (3, 15, 27, 28). Rectal distention can be performed for longer durations during sinusoidal oscillation than with a barostat, providing a more robust estimate of rectal mechanical properties and the response to rectal distention. Within 5 min after the onset of sinusoidal oscillation, rectal pressures decline and stabilize thereafter, punctuated by pressure peaks, which probably reflect the contractile response to oscillation. Compared with health, patients with FI had higher average rectal pressures, which reflects increased rectal stiffness, and more variability during sinusoidal oscillation, which reflects increased “irritability.” While rectal capacity and compliance (Prhalf) were also lower in FI than in healthy subjects, differences between health and FI were either not (Prhalf) or of only borderline statistical significance (rectal capacity) after correcting for age. These observations are consistent with our previous observations that rectal compliance but not rectal capacity declines with age (14).
After correcting for age and BMI, nifedipine did not significantly affect any parameter of rectal distensibility. However, higher plasma concentrations of nifedipine were associated with lower pressures (i.e., increased compliance) during barostat distention in all subjects and during sinusoidal oscillation in healthy subjects only. Together, these data suggest that L-type calcium channels contribute to distention-induced rectal contractility and perhaps to a greater extent in health than in FI. Plasma nifedipine concentrations in this study were comparable to those that inhibited the lower esophageal sphincter and the amplitude of esophageal peristalsis (17). Analogous to that study, where a larger dose was necessary to inhibit the esophageal body than the lower esophageal sphincter, we observed that nifedipine had more pronounced effects on anal resting pressure than rectal mechanical properties. However, drug effects on anal pressures were not correlated with plasma concentrations of nifedipine. Conceivably, a higher dose or local delivery (e.g., suppositories) of nifedipine may significantly increase rectal distensibility. However, safety considerations limit the use of higher doses, particularly since there are interindividual pharmacokinetic variations with plasma nifedipine (33).
Nifedipine had modest and inconsistent effects on rectal pressure and volume sensory thresholds. The raw values suggest that, among healthy subjects and FI patients randomized to placebo, pressure and volume sensory thresholds were higher after than before drug administration, perhaps reflecting habituation of visceral perception to repeated distention (22). We cannot explain why the volume threshold for first sensation and pressure threshold for desire to defecate were lower after nifedipine. However, nifedipine did not affect other sensory thresholds, which is similar to the lack of effects on perception of esophageal distention (30). Eight patients and one control subject were taking a low-dose tricyclic agent (i.e., amitriptyline) or a SSRI. It is conceivable but unlikely that reduced rectal distensibility in FI is explained by these medications, since amitriptyline reduced the frequency of rectal motor complexes and urgency in FI while citalopram increased colonic compliance in healthy subjects (26, 32).
Given these findings, further studies to ascertain active and/or passive mechanisms contributing to increased rectal stiffness in patients with urge-predominant FI are necessary. It is conceivable that passive rather than active mechanisms contribute to increased rectal stiffness in women with FI. Indeed, in an uncontrolled study, the α2-adrenergic receptor agonist clonidine had less prominent effects on rectal sensorimotor functions in FI than in health, suggesting perhaps that rectal sensorimotor functions may be less amenable to pharmacological modulation in FI than in healthy people (7, 34).
In summary, this controlled study confirms that nifedipine reduces anal resting pressure in health and FI. Also, women with FI had reduced rectal capacity and exaggerated responses to rectal sinusoidal oscillation. While plasma concentrations of nifedipine were correlated with increased rectal compliance during barostat and sinusoidal distention, nifedipine did not restore rectal compliance, capacity, or responses to sinusoidal oscillation in FI, arguing against a predominant contribution of myogenic L-type calcium channels to reduced rectal distensibility in FI.
GRANTS
This project was supported by National Institutes of Health (NIH) Grant R01 DK-78924 and Grant No. 1 UL1 RR-024150 from the National Center for Research Resources (NCRR), a component of the NIH, and the NIH Roadmap for Medical Research.
DISCLOSURES
The authors have no conflict of interest. The contents of this project are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov.
REFERENCES
- 1. Andrews C, Bharucha AE, Camilleri M, Low PA, Seide B, Burton D, Baxter K, Zinsmeister AR. Rectal sensorimotor dysfunction in women with fecal incontinence. Am J Physiol Gastrointest Liver Physiol 292: G282–G289, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bassotti G, Calcara C, Annese V, Fiorella S, Roselli P, Morelli A. Nifedipine and verapamil inhibit the sigmoid colon myoelectric response to eating in healthy volunteers. Dis Colon Rectum 41: 377–380, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Bharucha AE, Dhamija S, Japp A, Seide B, Walters B, Stroetz R, Zinsmeister A, Hubmayr R. Contractile response to colonic distention is influenced by oscillation frequency. Neurogastroenterol Motil 17: 64–75, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bharucha AE, Fletcher JG, Harper CM, Hough D, Daube JR, Stevens C, Seide B, Riederer SJ, Zinsmeister AR. Relationship between symptoms and disordered continence mechanisms in women with idiopathic fecal incontinence. Gut 54: 546–555, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bharucha AE, Locke GR, Seide B, Zinsmeister AR. A new questionnaire for constipation and fecal incontinence. Alimen Pharmacol Therapeut 20: 355–364, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Bharucha AE, Seide B, Fox JC, Zinsmeister AR. Day-to-day reproducibility of anorectal sensorimotor assessments in healthy subjects. Neurogastroenterol Motil 16: 241–250, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Bharucha AE, Seide BM, Zinsmeister AR. The effects of clonidine on symptoms and anorectal sensorimotor function in women with faecal incontinence. Alimentary Pharmacol Therapeut 32: 681–688, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bharucha AE, Zinsmeister AR, Locke GR, Schleck C, McKeon K, Melton LJ. Symptoms and quality of life in community women with fecal incontinence. Clin Gastroenterol Hepatol 4: 1004–1009, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Bharucha AE, Zinsmeister AR, Locke GR, Seide B, McKeon K, Schleck CD, Melton LJI. Prevalence and burden of fecal incontinence: a population based study in women. Gastroenterology 129: 42–49, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Brisinda G, Maria G. Oral nifedipine reduces resting anal pressure and heals chronic anal fissure (Abstract). Br J Surg 87: 251, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Chakder S, Sarma DN, Rattan S. Mechanism of internal anal sphincter smooth muscle relaxation by phorbol 12,13-dibutyrate. Am J Physiol Gastrointest Liver Physiol 280: G1341–G1350, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Deutekom M, Dobben AC, Terra MP, Engel AF, Stoker J, Bossuyt PM, Boeckxstaens GE. Clinical presentation of fecal incontinence and anorectal function: what is the relationship? Am J Gastroenterol 102: 351–361, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Farrugia G, Holm AN, Rich A, Sarr MG, Szurszewski JH, Rae JL. A mechanosensitive calcium channel in human intestinal smooth muscle cells. Gastroenterology 117: 900–905, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Fox JC, Fletcher JG, Zinsmeister AR, Seide B, Riederer SJ, Bharucha AE. Effect of aging on anorectal and pelvic floor functions in females [erratum appears in Dis Colon Rectum 50: 404, 2007]. Dis Colon Rectum 49: 1726–1735, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Fredberg JJ, Inouye D, Miller B, Nathan M, Jafari S, Raboudi SH, Butler JP, Shore SA. Airway smooth muscle, tidal stretches, and dynamically determined contractile states. Am J Respir Crit Care Med 156: 1752–1759, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Holm AN, Rich A, Sarr MG, Farrugia G. Whole cell current and membrane potential regulation by a human smooth muscle mechanosensitive calcium channel. Am J Physiol Gastrointest Liver Physiol 279: G1155–G1161, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Hongo M, Traube M, McAllister RG, Jr, McCallum RW. Effects of nifedipine on esophageal motor function in humans: correlation with plasma nifedipine concentration. Gastroenterology 86: 8–12, 1984 [PubMed] [Google Scholar]
- 18. Kelly JG, O'Malley K. Clinical pharmacokinetics of calcium antagonists. An update. Clin Pharmacokinet 22: 416–433, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Kraichely RE, Farrugia G. Mechanosensitive ion channels in interstitial cells of Cajal and smooth muscle of the gastrointestinal tract. Neurogastroenterol Motil 19: 245–252, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Law NM, Bharucha AE, Undale AS, Zinsmeister AR. Cholinergic stimulation enhances colonic motor activity, transit, and sensation in humans. Am J Physiol Gastrointest Liver Physiol 281: G1228–G1237, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Mutafova-Yambolieva VN, O'Driscoll K, Farrelly A, Ward SM, Keef KD. Spatial localization and properties of pacemaker potentials in the canine rectoanal region. Am J Physiol Gastrointest Liver Physiol 284: G748–G755, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Naliboff BD, Berman S, Suyenobu B, Labus JS, Chang L, Stains J, Mandelkern MA, Mayer EA. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology 131: 352–365, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Narducci F, Bassotti G, Gaburri M, Farroni F, Morelli A. Nifedipine reduces the colonic motor response to eating in patients with the irritable colon syndrome. Am J Gastroenterol 80: 317–319, 1985 [PubMed] [Google Scholar]
- 24. Richter JE, Dalton CB, Buice RG, Castell DO. Nifedipine: a potent inhibitor of contractions in the body of the human esophagus. Studies in healthy volunteers and patients with the nutcracker esophagus. Gastroenterology 89: 549–554, 1985 [DOI] [PubMed] [Google Scholar]
- 25. Santander R, Mena I, Gramisu M, Valenzuela JE. Effect of nifedipine on gastric emptying and gastrointestinal motility in man. Dig Dis Sci 33: 535–539, 1988 [DOI] [PubMed] [Google Scholar]
- 26. Santoro GA, Eitan BZ, Pryde A, Bartolo DC. Open study of low-dose amitriptyline in the treatment of patients with idiopathic fecal incontinence. Dis Colon Rectum 43: 1676–1681, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Sasaki H, Hoppin FG., Jr Hysteresis of contracted airway smooth muscle. J Appl Physiol 47: 1251–1262, 1979 [DOI] [PubMed] [Google Scholar]
- 28. Shen X, Gunst SJ, Tepper RS. Effect of tidal volume and frequency on airway responsiveness in mechanically ventilated rabbits. J Appl Physiol 83: 1202–1208, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Siproudhis L, El Abkari M, El Alaoui M, Juguet F, Bretagne JF. Low rectal volumes in patients suffering from fecal incontinence: what does it mean? Alimentary Pharmacol Therapeut 22: 989–996, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Smout AJ, Devore MS, Dalton CB, Castell DO. Effects of nifedipine on esophageal tone and perception of esophageal distension. Dig Dis Sci 37: 598–602, 1992 [DOI] [PubMed] [Google Scholar]
- 31. Sorkin EM, Clissold SP, Brogden RN. Nifedipine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy, in ischaemic heart disease, hypertension and related cardiovascular disorders. Drugs 30: 182–274, 1985 [DOI] [PubMed] [Google Scholar]
- 32. Tack J, Broekaert D, Corsetti M, Fischler B, Janssens J. Influence of acute serotonin reuptake inhibition on colonic sensorimotor function in man. Alimentary Pharmacol Therapeut 23: 265–274, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Traube M, Lange RC, McAllister RG, Jr, McCallum RW. Effect of nifedipine on gastric emptying in normal subjects. Dig Dis Sci 30: 710–712, 1985 [DOI] [PubMed] [Google Scholar]
- 34. Viramontes BE, Malcolm A, Camilleri M, Szarka LA, McKinzie S, Burton DD, Zinsmeister AR. Effects of an alpha(2)-adrenergic agonist on gastrointestinal transit, colonic motility, and sensation in humans. Am J Physiol Gastrointest Liver Physiol 281: G1468–G1476, 2001 [DOI] [PubMed] [Google Scholar]