Abstract
Rationale
Mast cells (MCs) contribute to formation of abdominal aortic aneurysms (AAAs) by producing biologically active mediators. Tryptase is the most abundant MC granule protein and participates in MC activation, protease maturation, leukocyte recruitment, and angiogenesis — all processes critical to AAA pathogenesis.
Objective
To test the hypothesis that tryptase functions directly in AAA formation.
Methods and Results
Immunoreactive tryptase localized in the media and adventitia of human and mouse AAA lesions. Serum tryptase levels correlated significantly with the annual expansion rate of AAA before (r=0.30, P=0.003) and after (r=0.29, P=0.005) adjustment for common AAA risk factors in a patient follow-up study, and associated with risks for later surgical repair or overall mortality before (P=0.009, P=0.065) and after (P=0.004, P=0.001) the adjustment. Using MC protease-6–deficient mice (Mcpt6−/−) and experimental AAAs induced by aortic elastase perfusion, we proved a direct role of this tryptase in AAA pathogenesis. While all wild-type (WT) mice developed AAA at 14 or 56 days post-perfusion, Mcpt6−/− mice had full protection. AAA lesions from Mcpt6−/− mice contained fewer inflammatory and apoptotic cells, and lower chemokine levels than those from WT mice. MC from WT mice restored reduced AAA lesions and lesion inflammatory cell content in MC–deficient KitW-sh/W-sh mice, but those prepared from Mcpt6−/− mice did not. Mechanistic studies demonstrated that tryptase deficiency affected endothelial cell (EC) chemokine and cytokine expression, monocyte transmigration, smooth-muscle cell apoptosis, and MC and AAA lesion cysteinyl cathepsin expression and activities.
Conclusions
This study establishes the direct participation of MC tryptase in the pathogenesis of experimental AAAs, and suggests that levels of this protease can serve as a novel biomarker for abdominal aortic expansion.
Keywords: abdominal aortic aneurysm, tryptase, mMCP-6, macrophage, T cell, apoptosis
Introduction
Tryptase1 is a mast cell (MC) restricted serine protease stored in abundance in the secretory granules, as an enzymatically active tetramer ionically bound to serglycin proteoglycan. The serum tryptase level is normally <1 ng/mL, but it increases in patients with systemic anaphylaxis and other inflammatory disorders when their tissue MCs become activated.2 Mouse MC protease 6 (mMCP-6) is the ortholog of human tryptase.3
Tryptases have pathophysiologic functions pertinent to the development of abdominal aortic aneurysms (AAAs). Tryptase induces vascular leakage4 and chemotaxis of eosinophils and neutrophils.5,6 In support of these data, the injection of recombinant mMCP-6 in the mouse's peritoneal cavity or lungs results in a marked infiltration of granulocytes at those tissue sites.7,8 Moreover, mMCP-6–null (Mcpt6−/−) mice have a diminished ability to combat bacteria and helminth infections due to a defect in the rapid recruitment of granulocytes to infected tissue sites.9,10 Mouse and human tryptases induce endothelial cells (ECs) and other cell types to increase their expression of numerous chemokines (e.g., IL-8) and cytokines (e.g., IL-1β)11 by unknown mechanisms. Mouse and human tryptases also activate protease zymogens [e.g., pro-matrix metalloproteinase-3 (pro-MMP-3)12 and pro-urokinase13] that have been implicated in AAA formation.14,15 In turn, these tryptase-activated proteases can trigger a more extensive pro-enzyme activation cascade12 that likely contributes to arterial wall remodeling.
MCs are numerous in the media16 and adventitia17 of human or murine AAA lesions. These cells often localize adjacent to the thrombosed vessels and neovessels,16,18 and release factors that promote thrombolysis, prevent coagulation, and enhance neovessel growth.18,19 The absence of MCs protects mice and rats from experimental AAA formation,17,19 and the pharmacological activation or stabilization of MCs can differentially alter AAA growth in mice.19 In addition to recruiting leukocytes and activating pro-enzymes, tryptase can act as an autocrine mediator, provoking the release of histamine and other mediators from nearby MCs.20,21 Thus, tryptase and its mouse ortholog mMCP-6 might be important MC-derived effectors in AAA pathogenesis. We now report that the tryptase levels in the serum of AAA patients associate with aortic expansion. Using Mcpt6−/− mice, we also show that this tryptase participates in experimental AAA formation in vivo.
Materials and Methods
See Supplemental Materials online for details.
Tryptase Detection in AAA Lesions
Paraffin-embedded human aortic sections were prepared from 10 AAA donors (5 females and 5 males; mean age, 78.80±2.05 years) and 10 non-AAA heart-transplant patients (5 females and 5 males; mean age, 41.90±4.19 years) without detectable vascular diseases, from the Department of Surgery, Washington University in St. Louis. These sections were evaluated for the presence of tryptase protein (mouse anti-human tryptase monoclonal antibody, 1:1500, Chemicon International, Inc., Billerica, MA) and elastin fiber (Verhoeff-Van Gieson staining). Human aortic tissue extracts were prepared from three female AAA patients and three female heart-transplant donors with no detectable vascular disease from the Department of Medicine, Brigham and Women's Hospital. Lysates of the resulting tissue samples were used for immunoblot analysis (30 μg/lane) with the same tryptase antibody (1:1000). The same protein blot was reprobed using a β-actin antibody (1:2000, Santa Cruz Biotechnology) to affirm equal protein loading. Separate human protocols were pre-approved by the Human Investigation Review Committees at Washington University in St. Louis and at Brigham and Women's Hospital.
Results
Association of serum tryptase level with aneurysmal progression
MC degranulation and inflammatory mediator release associate with Murine AAA formation.19 We hypothesized that patients with AAA have elevated serum MC tryptase levels. To test this hypothesis, we developed an ELISA to measure human serum tryptase levels. In this study, cases of AAA and controls are from the Viborg Study — a population-based randomized screening trial of men 65 to 73 years of age.22 Of the patients in this study, 100 had defined AAA, and 35 age-matched men did not. Characteristics of cases and controls are listed in Table 1. Controls had a maximal diameter below 25 mm, with an average of 17 mm, as the background population. Compared with controls, AAA patients had more coexisting atherosclerotic manifestations, including previous acute myocardial infarction and angina pectoris. AAA patients also had lower ankle–brachial blood pressure indices, more frequent smoking, higher body-mass index (BMI), and lower pulmonary function than control subjects. Serum tryptase levels (ng/ml) had a left-skewed distribution in these populations, and were therefore log-transformed. The mean transformed serum tryptase levels of the AAA group and the control group, and those with and without coexisting cardiovascular and pulmonary diseases, were compared by the Student t test. The mean log-transformed serum tryptase levels in men with and without AAA were 1.80±0.35 and 1.69±0.20 (mean ± SD, ng/ml), respectively (P= 0.041) (Table 1). High tryptase levels in the control group may be due to the high percentage of current smokers (42.9%), and because many in the control group had acute myocardial infarction, angina pectoris, stroke, or hypertension (total 25.7%), although these subjects did not have AAA (Table 1). Patients with coexisting cardiovascular and pulmonary diseases, however, had levels of mean log-transformed serum tryptase similar to those without coexisting cardiovascular and pulmonary diseases (1.78±0.37 ng/ml vs. 1.76±0.26 ng/ml, P=0.714).
Table 1.
Baseline characteristics concerning cases and controls.
| Dichotomous variables | AAA (%) | Control (%) | P Value† |
|---|---|---|---|
| Current smoking* | 59/100 (59.0) | 15/35 (42.9) | 0.17 |
| Acute myocardial infarction* | 26/100 (26.0) | 2/35 (5.7) | 0.01 |
| Angina pectoris | 22/100 (22.0) | 2/35 (5.7) | 0.01 |
| Stroke or transcallosal inhibition* | 5/100 (5.0) | 1/35 (2.9) | 0.64 |
| Lower limb ischemia* | 7/100 (7.0) | 0/35 (0.0) | 0.12 |
| Chronic obstructive pulmonary disease* | 6/100 (6.0) | 0/35 (0.0) | 0.15 |
| Hypertension* | 15/100 (15.0) | 3/35 (8.6) | 0.39 |
|
| |||
| Continuous variables | AAA Mean (SD) | Control Mean (SD) | P Value‡ |
| Serum tryptase (ng/mL) | 103.6 (188.1) | 56.2 (55.6) | 0.03 |
| Log-transformed serum tryptase (ng/mL) | 1.80 (0.35) | 1.69 (0.20) | 0.04 |
| Age (year) | 67.9 (2.96) | 67.9 (2.68) | 0.74 |
| AAA-size (mm) | 33.9 (4.61) | 17.3 (2.14) | <0.01 |
Hospital discharge diagnosis;
Chi-square test or Fisher's exact test;
Student's t test
Elevated serum tryptase levels in AAA patients suggest an association of this MC protease with AAA development. We performed Pearson's correlation analysis to test whether serum tryptase levels associate with initial AAA size at baseline and/or the mean annual expansion rate. Initial AAA size and tryptase levels correlated weakly and insignificantly (r=0.14, P=0.106). The mean annual expansion rate also correlated weakly, but significantly with serum tryptase levels (r=0.30, P=0.003) (Figure 1A). These findings did not change after adjustment for other potential AAA risk factors, including AAA size, use of glucocorticoids, BMI, diastolic blood pressure, use of low-dose aspirin, current smoking, lowest ankle–brachial blood pressure index, coexisting cardiovascular and pulmonary diseases, age, and aneurysm wall calcification of more or less than 50% of the circumference23 at the maximal diameter. Serum tryptase levels still associated weakly and insignificantly with initial AAA size (r=0.12, P=0.299), but significantly with AAA expansion rate (r=0.29, P=0.005).
Figure 1.
Tryptase in human and mouse AAA. A. Correlation of serum tryptase level with annual AAA expansion rate, before (r=0.30, P=0.003) or after (r=0.29, P=0.005) adjustment for AAA confounders, Pearson's correlation test. B. Tryptase and elastin immunostaining in normal (left two panels) and aneurysmal (right two panels) human aortas. Bar: 400 μm; in inset (bottom panels), bar: 100 μm. C. Human AAA and normal aortic tissue extract immunoblot for human tryptase. Tryptase monomer (30-kDa) is indicated. Beta-actin serves as a protein loading control. D. Mouse mMCP-6 polyclonal antibody immunostaining in frozen sections from normal mouse aorta, AAA lesions from Mcpt6−/− (as negative control) and WT mice. Lumen, media, and adventitia are indicated. Bar: 100 μm.
Due to our relatively small sample sizes, we classified serum tryptase levels into tertiles and performed Cox regression analysis to assess whether serum tryptase levels associated with subsequent AAA surgical repair or overall mortality. The crude relative risk for later surgical repair increased 1.74 times between the tertiles (HR, 1.74; 95% C.I., 1.15; 2.62, P=0.009), and further increased to 2.15 (95% C.I., 1.27; 3.62, P=0.004) after adjustment for AAA size, use of glucocorticoids, BMI, diastolic blood pressure, use of low-dose aspirin, current smoking, lowest ankle–brachial blood pressure index, coexisting cardiovascular and pulmonary diseases, age, and AAA wall calcification (Table 2). When the subgroups were based upon the median, quartiles, or quintiles, the significant association between serum tryptase levels and the need for later repair persisted: median (HR, 1.94; 95% C.I., 1.001; 3.741, P=0.049), quartiles (HR, 1.43; 95% C.I., 1.07; 1.94, P=0.017), and quintiles (HR, 1.39; 95% C.I., 1.09; 1.765, P=0.008). We also found that serum tryptase levels correlated with overall mortality in this population, although less profoundly. The crude relative risk for dying increased 43% between the tertiles (HR, 1.43; 95% C.I., 0.98; 2.10, P=0.065), but further increased to more than three times between the tertiles (HR, 3.17; 95% C.I.,1.60; 6.30, P=0.001) after adjustment for the mentioned potential AAA risk factors (Table 3).
Table 2.
Cox regression analysis for later need for surgery.
| Variables | B | SE | Wald | df | Sig | Exp(B) (95.0% CI) |
|---|---|---|---|---|---|---|
| Before adjustment | ||||||
| Tryptase (ng/mL) | 0.551 | 0.211 | 6.800 | 1 | 0.009 | 1.735 (1.147∼2.624) |
|
| ||||||
| After adjustment | ||||||
| Tryptase (ng/mL) | 0.718 | 0.321 | 5.004 | 1 | 0.004 | 2.151 (1.273∼3.618) |
| MAPD (cm) | 0.264 | 0.061 | 18.614 | 1 | 0.000 | 1.302 (1.155∼1.467) |
| Use of glucocorticoids | -2.446 | 1.239 | 3.897 | 1 | 0.048 | 0.087 (0.008∼0.983) |
| Body mass index (kg/mm2) | -0.028 | 0.060 | 0.209 | 1 | 0.648 | 0.973 (0.864∼1.095) |
| Diastolic blood pressure (mmHg) | -0.001 | 0.019 | 0.002 | 1 | 0.968 | 1.001 (0.964∼1.039) |
| Low dose aspirin | -0.709 | 0.501 | 2.002 | 1 | 0.157 | 0.492 (0.184∼1.314) |
| Current smoking | 0.089 | 0.471 | 0.036 | 1 | 0.850 | 1.093 (0.434∼2.753) |
| Abi | 0.094 | 1.136 | 0.007 | 1 | 0.934 | 1.098 (0.118∼10.181) |
| Coex | 0.715 | 0.517 | 1.911 | 1 | 0.167 | 2.044 (0.742∼5.633) |
| Age (year) | 0.122 | 0.096 | 1.632 | 1 | 0.201 | 1.130 (0.937∼1.364) |
| AAA wall calcification | -0.656 | 0.458 | 2.051 | 1 | 0.152 | 0.519 (0.212∼1.273) |
B: unstandardized regression coefficient; SE: standard error of B; Wald: Wald test significance value; df: degrees of freedom; Sig: the significance value of the coefficient; Exp(B): the predicted change in the hazard for each unit increase in the covariate. MAPD: Maximal aneurysmal anteroposterior diameter; Abi: Ankle–brachial blood pressure index (sensitive marker for coexisting lower limb atherosclerosis); Coex: Hospital-recorded coexisting hypertension, pulmonary obstructive disease, and cardiovascular disease; AAA wall calcification: Degree of wall calcification at the maximal circumference of the AAA.
Table 3.
Cox regression analysis for relative risk of dying later.
| Variables | B | SE | Wald | df | Sig | Exp(B) (95.0% CI) |
|---|---|---|---|---|---|---|
| Before adjustment | ||||||
| Tryptase (ng/mL) | 0.359 | 0.195 | 3.406 | 1 | 0.065 | 1.432 (0.978∼2.097) |
|
| ||||||
| After adjustment | ||||||
| Tryptase (ng/mL) | 1.154 | 0.350 | 10.854 | 1 | 0.001 | 3.172 (1.596∼6.303) |
| MAPD (cm) | 0.158 | 0.054 | 8.454 | 1 | 0.004 | 1.171 (1.053∼1.302) |
| Use of glucocorticoids | -1.860 | 1.219 | 2.330 | 1 | 0.127 | 0.156 (0.014∼1.696) |
| Body mass index (kg/mm2) | -0.002 | 0.028 | 0.003 | 1 | 0.956 | 0.998 (0.945∼1.055) |
| Diastolic blood pressure (mmHg) | -0.040 | 0.021 | 3.465 | 1 | 0.063 | 0.961 (0.921∼1.002) |
| Use of aspirin | 0.987 | 0.588 | 2.815 | 1 | 0.093 | 2.682 (0.847∼8.494) |
| Current smoking | 1.119 | 0.554 | 4.079 | 1 | 0.043 | 3.063 (1.034∼9.074) |
| ABI | -2.134 | 1.641 | 1.692 | 1 | 0.193 | 0.118 (0.005∼2.950) |
| Coex | 0.049 | 0.509 | 0.009 | 1 | 0.924 | 1.050 (0.387∼2.845) |
| Age (year) | 0.258 | 0.098 | 6.883 | 1 | 0.009 | 1.295 (1.068∼1.570) |
| AAA wall calcification | -1.114 | 0.483 | 5.328 | 1 | 0.021 | 0.328 (0.127∼0.845) |
B: unstandardized regression coefficient; SE: standard error of B; Wald: Wald test significance value; df: degrees of freedom; Sig: the significance value of the coefficient; Exp(B): the predicted change in the hazard for each unit increase in the covariate. MAPD: Maximal aneurysmal anteriorposterior diameter; Abi: Ankle–brachial blood pressure index (sensitive marker for coexisting lower limb atherosclerosis); Coex: Hospital-recorded coexisting hypertension, pulmonary obstructive disease, and cardiovascular disease; AAA wall calcification: Degree of wall calcification at the maximal circumference of the AAA.
Increased tryptase expression in human AAA lesions
Significantly higher serum tryptase levels in AAA patients, compared with non-AAA subjects, suggested that AAA lesions might have higher tryptase expression than normal aortas. Immunostaining of aortic sections from AAA lesions and from non-AAA donors showed high tryptase immunoreactivity in the adventitia and media (as determined by Verhoeff-Van Gieson elastin staining) of human AAA lesions. The adventitia of healthy aortas contained only a few tryptase-positive MCs (Figure 1B). Immunoblot analysis of aortic tissue extracts revealed similar tryptase expression patterns. AAA tissue extracts contained more of the 30-kDa human tryptase than those prepared from normal aortas (Figure 1C). These observations agree with the elevated serum tryptase levels in AAA patients and their significant correlations with subsequent AAA surgery (Table 2) or death (Table 3).
Tryptase deficiency reduced AAA formation in mice
As discussed, tryptases trigger MC activation, stimulate inflammatory cell infiltration, and activate AAA-pertinent proteases, compatible with their participation in AAA formation. Experiments in mice subjected to aortic elastase perfusion showed increased expression of mMCP-6 and an essential role of this MC tryptase in AAA development. At 14 days post-perfusion, all WT mice developed AAA, but no Mcpt6−/− mice did. Aortic sections from healthy mice or those from Mcpt6−/− mice 14 days post-elastase perfusion immunostained with a rabbit anti-mMCP-6 polyclonal antibody,9,24 showed no mMCP-6 protein. The same antibody, however, detected mMCP-6 expression in both the media and adventitia in aortic sections from WT mice 14 days post-elastase perfusion (Figure 1D) as in human AAA lesions (Figure 1B). Tryptase mMCP-6-positive mast cells in the media in aortic section from WT mice also stained with an anti-mouse c-Kit (CD117) monoclonal antibody (data not shown). We extended the time point to 56 days. Aortic expansion increased by >150% in WT mice, but the aortas of Mcpt6−/− mice showed less ectasia (Figure 2A). Compared to WT mice, at 7 days, 14 days, and 56 days, Mcpt6−/− mice had significantly reduced AAA lesion accumulation of macrophages (Figure 2B) and T cells (Figure 2C). Lesions of Mcpt6−/− mice contained more media smooth-muscle cells (SMC) than those of WT mice at the 7-day time point (Figure 2D). Reduced inflammatory cell numbers correlated with low MHC class II-positive areas in Mcpt6−/− mouse lesions at all three time points (Figure 2E). Thus, tryptase deficiency impaired inflammatory cell accumulation in AAA lesions. This conclusion agrees with the finding of significantly reduced levels of the chemokine MCP-1 in AAA lesions from Mcpt6−/− mice compared with WT mice (Figure 2F). Elastin fragmentation characterizes human or murine AAA.25 While elastin fragmentation increased over time in WT mice, it did not in Mcpt6−/− mice. At 56 days post-perfusion, aortas from WT mice showed more elastin fragmentation than did those from Mcpt6−/− mice (Supplemental Figure 1A).
Figure 2.
Characterizations of AAA lesions from WT and Mcpt6−/− mice. A. Aortic diameter increase (%). B. Mac-3+ macrophage area (%). C. CD3+ T cell numbers. D. Alpha-actin–positive SMC grade. E. MHC class II-positive area (%). F. MCP-1–positive area (%). Representative images for panels B, C, E, F, H, and I are shown on the right. The number of mice in each experimental group is shown within each bar. Data are mean ± SEM. P<0.05 was considered statistically significant, Mann-Whitney U test.
We have previously shown that MCs participate in AAA formation by promoting aortic SMC apoptosis.19 At 7 days post-perfusion, lesions of Mcpt6−/− mice had fewer total apoptotic cells (Supplemental Figure 1B, mainly infiltrated inflammatory cells) and media apoptotic cells (Supplemental Figure 1C, mainly SMC) than did lesions in WT mice. These data agree with the increase of lesional SMC content in Mcpt6−/− mice at the same time point (Figure 2D), suggesting that most of these medial apoptotic cells were SMC, as previously described in human AAA lesions.26 The two genotypes did not have significant differences in lesion cell apoptosis or SMC content at the 14-day or 56-day time points (Figures 2D and Supplemental Figure 1B).
Tryptase can stimulate endothelial tube formation in vitro and promote angiogenesis,27 which may contribute to reduced AAA formation in Mcpt6−/− mice. Immunostaining AAA lesion sections for CD31 to visualize microvascular endothelial cells did not show significant differences in CD31-positive microvessel numbers between WT and Mcpt6−/− mice at any time point tested (Supplemental Figure 1D), suggesting a negligible role of mMCP-6 in angiogenesis in this AAA model. An in vitro aortic ring angiogenesis assay yielded similar observations. Bone marrow–derived MCs (BMMC) from WT mice or Mcpt6−/− mice, used as angiogenic stimuli, showed similar microvessel sprouting from the aortic rings (data not shown).
Reduced AAA formation, lesion inflammatory cell infiltration, or cell apoptosis in Mcpt6−/− mice did not result from less MC accumulation. Enumeration of CD117+ MCs showed no significant differences between the genotypes from all three time points (Supplemental Figure 1E), suggesting that reduced AAA formation in Mcpt6−/− mice resulted from the absence of mMCP-6 from MCs. To examine this hypothesis further, we reconstituted MC–deficient KitW-sh/W-sh mice with BMMC from WT and Mcpt6−/− mice. We have previously shown reduced AAA in KitW-sh/W-sh mice compared with WT mice in this preparation, and reconstitution of KitW-sh/W-sh mice with BMMC from WT mice restored AAA phenotypes.20 Reconstitution of KitW-sh/W-sh with BMMC from Mcpt6−/− mice conferred protection from AAA formation at the 56-day post-perfusion time point (Figure 3A). Macrophage and T-cell content also increased when KitW-sh/W-sh mice received BMMC from WT mice but not from Mcpt6−/− mice at the 14-day time point, although this difference between WT mice and Mcpt6−/− mice subsided at the 56-day time point (Figure 3B and 3C). In contrast, BMMC from WT and Mcpt6−/− mice behaved similarly in regulating CD31+ microvessel growth in AAA lesions at both time points (Figure 3D).
Figure 3.
MC reconstitution in KitW-sh/W-sh mice. WT, MC-deficient KitW-sh/W-sh mice, and those receiving BMMC from WT and Mcpt6−/− mice were induced to produce AAA. Aortic diameter increase (%) (A), Mac-3+ macrophage areas (B), CD3+ T cell numbers (C), and CD31+ microvessel numbers (D) were analyzed at 14 days and 56 days post-perfusion. Numbers of mice for each experimental group are shown in each bar. Data are mean ± SEM. P<0.008 was considered statistically significant, Mann-Whitney U test.
MCs interact with inflammatory and vascular cells
The finding of reduced leukocytes in Mcpt6−/− mouse AAA lesions suggested a role of mMCP-6 in leukocyte homing. To test this hypothesis in vitro, we first examined whether the absence of mMCP-6 affects MC chemokine and cytokine expression. RT-PCR analysis showed no significant differences in TNF-α, IL-6, and MCP-1 expression between BMMC from WT and Mcpt6−/− mice (not shown). We then examined whether BMMC from WT and Mcpt6−/− mice behaved differently in inducing cytokine or chemokine expression in T cells, macrophages, monocytes, or neutrophils. Degranulated BMMC supernatants from WT and Mcpt6−/− mice again showed no significant differences in promoting TNF-α, IL-6, and MCP-1 expression in all aforesaid leukocytes (not shown). Yet, EC from WT mice expressed significantly higher levels of the cytokines TNF-α and IL-6, and the chemokines CXCL1/KC, CXCL2/MIP-2 (macrophage inflammatory protein-2), and CXCL5/LIX after stimulation with degranulated BMMC from WT mice than did BMMC from Mcpt6−/− mice (Figure 4A), a finding that agrees with the reduced leukocyte content of Mcpt6−/− AAA lesions (Figure 2B and 2C, and Figure 3B and 3C).
Figure 4.
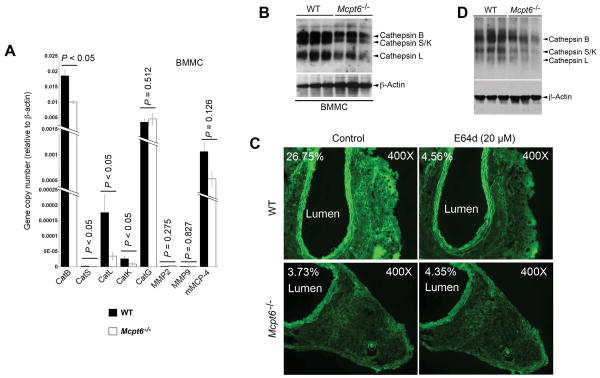
EC cytokine and chemokine expression, monocyte migration, and SMC apoptosis. A. RT-PCR analysis of cytokine and chemokine expression in mouse aortic EC after incubation with degranulated BMMC from WT and Mcpt6−/− mice. Data are mean ± SE of three experiments. B. Peripheral monocyte transmigration assay under different concentrations of SDF-1a. Data are mean ± SE of six experiments. C. RT-PCR determines expression of cathepsins B, S, L, and K in peripheral blood monocytes from WT and Mcpt6−/− mice. Data are mean ± SE of three experiments. P<0.05 was considered statistically significant, Mann-Whitney U test. D. Mouse aortic SMC apoptosis after induction with PDTC with or without BMMC from WT or Mcpt6−/− mice. Representative panels are presented to the left. Green fluorescent cells indicate TUNEL-positive apoptotic cells. P<0.02 was considered statistically significant, Mann-Whitney U test.
To test further whether the absence of mMCP-6 directly affected monocyte transmigration, we assayed transmigration through a collagen-coated Boyden chamber cell using monocytes from WT and Mcpt6−/− mice. Monocytes from Mcpt6−/− mice transmigrated more slowly than those from WT mice under different concentrations of the chemokine SDF-1α (Figure 4B). Reduced transmigration of monocytes from Mcpt6−/− mice suggested altered protease expression. Although monocytes do not express tryptase, these cells are rich sources of cysteinyl cathepsins involved in leukocyte transmigration.28,29 Monocytes from Mcpt6−/− mice expressed significantly less mRNA encoding all tested cathepsins — including cathepsins B, S, L, and K — than those from WT mice (Figure 4C), as determined by RT-PCR.
We have previously shown that MCs induce aortic SMC apoptosis.19 This study revealed reduced AAA media cell apoptosis in Mcpt6−/− mice (Figure 2I), with concurrent increase of lesion SMC content (Figure 2D). Therefore, mMCP-6 may promote SMC apoptosis. We used post-degranulation supernatants of BMMC from WT and Mcpt6−/− mice to test this hypothesis. Live or degranulated WT BMMC enhanced PDTC-induced SMC apoptosis.19 In contrast, the supernatants of degranulated mMCP-6–deficient BMMC showed significantly reduced induction of aortic SMC apoptosis under the same conditions (Figure 4D), supporting the contribution of tryptase mMCP-6 in this process.
Tryptase deficiency affects cysteine protease cathepsin expression and activities
Cysteine protease cathepsins and MMPs contribute to aortic wall remodeling. Mice lacking cysteine proteases, such as cathepsins S, K, or L, resist diet-induced atherosclerosis,28-30 and MMP-9–deficient, MMP-2–deficient, or chymase mMCP-4–deficient mice show reduced experimental AAA formation.31-33 As discussed, MC tryptase participates in MMP and serine protease activation,12,13 processes pertinent to AAA formation. Absence of mMCP-6 may affect the expression and/or activation of these proteases in MCs, thereby providing an additional mechanism underlying reduced AAA formation in Mcpt6−/− mice. BMMC from Mcpt6−/− mice expressed significantly less mRNA encoding cathepsins B, S, L, and K, but did not affect the expression of serine proteases cathepsin G, chymase mMCP-4, MMP-2, and MMP-9 mRNAs, as assessed by RT-PCR (Figure 5A). To test whether reduced cathepsin mRNA levels corresponded to altered enzymatic activities, we performed active site labeling for cysteine proteases with biotinylated JPM. BMMC cell lysates from Mcpt6−/− mice showed reduced cathepsin activities, compared with those from WT mice (Figure 5B). AAA lesions from Mcpt6−/− mice also had reduced activities of cysteinyl cathepsins. Using frozen AAA sections and fluorescein-conjugated elastin as substrate, we performed in situ cathepsin zymography in a buffer that was optimized for cysteine protease cathepsin activities (pH 5.5).19 AAA lesions from WT mice 56 days post-perfusion showed elastolytic activity in the adventitia (green fluorescence), sensitive to the non-selective cathepsin inhibitor E64d (20 μM, Sigma). In contrast, the adventitia in AAA sections from Mcpt6−/− mice 56 days post-perfusion contained much less elastolytic activity (green fluorescence), and E64d showed negligible effect on this activity (Figure 5C) — a finding consistent with reduced cathepsin activities in AAA lesions from Mcpt6−/− mice. Although in situ cathepsin zymography experimental and photograph shuttering conditions were the same between AAA lesions from WT and Mcpt6−/− mice, media green fluorescence was brighter in WT mice than in Mcpt6−/− mice (Figure 5C), likely from increased media inflammatory cells in WT AAA lesions (Figure 1D). To confirm increased cathepsin activities in AAA lesions from WT mice than in Mcpt6−/− mice, we performed cysteinyl cathepsin active site labeling with JPM using AAA tissue extracts. AAA tissue lysates from Mcpt6−/− mice had reduced cathepsin activities compared with those from WT mice 56 days post-perfusion (Figure 5D), consistent with reduced medial elastin degradation in AAA lesions from Mcpt6−/− mice than in those from WT mice at this time point (Supplemental Figure 1A).
Figure 5.
Cysteine protease cathepsin expression and activity in MCs and AAA lesions. A. RT-PCR to assess the expression of common MC proteases in BMMC from WT and Mcpt6−/− mice. Data are mean ± SE of three experiments. P<0.05 was considered statistically significant, Mann-Whitney U test. B. Cysteine protease cathepsin active site JPM-labeling with cell lysate from WT and Mcpt6−/− BMMC. C. WT and Mcpt6−/− mouse frozen AAA cross section in situ elastase activity zymograph in the presence or absence of cathepsin inhibitor E64d. Lumen and percentage of fluorescence intensity are indicated. Images were obtained with the same magnification and shutter speed, and all data are from the 56-day time point experiments. D. Cysteine protease cathepsin active site JPM-labeling with aortic tissue lysates from WT and Mcpt6−/− mice from the 56-day time point experiments. In panels B and D, active cathepsins B, S, K, and L are indicated with arrowheads. ß-actin blot was used to ensure equal protein loading.
Discussion
The release of undefined granular content from activated MCs contributes to arterial remodeling.19,34 The granules in mouse and human MCs contain substantial amounts of different types of neutral proteases. In particular, the chymase and tryptase families of MC-restricted serine proteases participate in pathological events, such as atherosclerosis,35 that pertain to AAA pathogenesis. We have previously shown that the chymase mMCP-4 contributes to AAA by affecting lesion leukocyte infiltration, apoptosis, elastin degradation, and angiogenesis.32 The absence of mMCP-4 (but not its chromosome 14C3 family member mMCP-5) protected mice from elastase perfusion-induced AAA. We now show that the chromosome 17A3.3 tryptase family member mMCP-6 also contributes critically to experimental AAA formation in mice, and biomarker studies also implicate this protease in human AAA.
Identifying novel biomarkers to predict arterial expansion, not only for the aorta but also for the coronary, cerebral, and peripheral arteries, could prove useful as an investigative and a clinical tool. This study showed that human serum tryptase levels correlated significantly but weakly with the aneurysmal expansion rate (P=0.005, R=0.29) (Figure 1A), suggesting that serum tryptase is probably not useful as a stand-alone biomarker in clinical decision-making. Larger and broader clinical studies in the future, however, may yield different conclusions. Statistical significances and high Exp(B) values before and after adjusting for all available risk factors in predicting AAA expansion and subsequent aortic surgery and mortality in this small patient population follow-up study (Tables 2 and 3), and the independence from traditional AAA risk factors make this protease a compelling and attractive biomarker candidate. The present data suggest that both tryptase and chymase participate in mouse AAA development. Our prior study demonstrated that serum chymase levels also correlated with AAA expansion rate,32 but not with the need for surgical repair or with death (not shown), which suggests there are mechanistic differences between the two types of MC proteases. For example, BMMC from Mcpt4−/− mice showed impaired activity in promoting microvessel growth in an aortic ring assay, and AAA lesions from Mcpt4−/− mice had reduced CD31+ microvessels compared with those in WT mouse AAA lesions.32 Although tryptase may participate in angiogenesis,27 we did not find an effect of this protease in promoting microvessel growth in the aortic ring assay (data not shown), and we did not detect significant differences in CD31-positive microvessel numbers in AAA lesions from WT mice and Mcpt6−/− mice (Supplemental Figure 1D). MC tryptase therefore may not influence angiogenesis in this AAA preparation.
This study suggests that tryptase regulates leukocyte recruitment to lesions in experimental AAA. AAA lesions from Mcpt6−/− mice had fewer macrophages and T cells (Figure 2B and 2C). Reconstitution of BMMC from Mcpt6−/− mice did not restore lesion macrophages (Figure 3B) or T cells (Figure 3C). Although other mechanisms may participate, our data suggest that tryptase mMCP-6 stimulates vascular EC expression of chemokines CXCL1/KC, CXCL2/MIP-2, and CXCL5/LIX. This mechanism may contribute to the observed reduction in lesion leukocyte content. Yet, reduced MCP-1 expression in Mcpt6−/− mouse AAA lesions (Figure 2F) remained unexplained. Use of BMMC from Mcpt6−/− mice did not show any effect of this tryptase isoform on MCP-1 expression in any tested cell types, including EC, T cells, macrophages, monocytes, and neutrophils (not shown). Therefore, tryptase may exert indirect effects on lesion MCP-1 expression, including a reduced ability of Mcpt6−/− BMMC to induce EC expression of KC, MIP-2, and LIX (Figure 4A), impaired cathepsin expression, and transmigration of monocytes from Mcpt6−/− mice (Figure 4B and 4C), all which may result in fewer macrophages in AAA lesions and thus account for lower levels of MCP-1.
We have long been interested in why the absence of one protease affects the expression or activities of the others. All tested cathepsins showed lower expression in BMMC from Mcpt6−/− mice than in those from WT mice (Figure 5A and 5B). Although not tested in this study, tryptase is known to induce mitogen-activated protein kinase (MAPK) activation in human eosinophils. Phosphorylation of extracellular signal-regulated kinase-1 and 2 (ERK1/2), MAPK p38, and Jun N-terminal kinase-1 and -2 (JNK1/2) occur within 3 minutes after incubation with 50 ng/mL of recombinant human skin tryptase.36 Conditional activation of ERK can induce the expression of CatB and CatL in 3T3 and K1735 cells.37 The receptor activator of NF-κB ligand (RANKL)-induced CatK expression during osteoclastogenesis depends on p38 MAPK.38 The N-terminal telopeptide of collagen type II enhances expression of cathepsins B, K, and L in articular chondrocytes, and also associates with the activation of p38 MAPK.39 Tryptase may control cathepsin expression in BMMC (Figure 5A) and in other inflammatory cells and vascular cells by regulating MAPK activation. This study tested whether reduced cathepsins in tryptase-deficient MCs resulted from decreased chymase expression. Although BMMC from Mcpt6−/− mice expressed less mMCP-4 than did those from WT mice, this difference did not reach statistical significance. Cathepsin activities fell not only in MCs, but also in AAA lesions from Mcpt6−/− mice. Reduced cathepsin expression and activity may result in part from fewer leukocytes in AAA lesions; leukocytes are a rich source of proteases and inflammatory stimuli that are required for vascular cell protease expression.40,41 The low MHC class II levels in Mcpt6−/− mouse lesions supports this possibility (Figure 2E). Reduced cathepsin expression and activity in Mcpt6−/− mice BMMC and aortic tissue extracts (Figure 5) did not explain fully the aortic wall elastin fragmentation. Increased T-cell content at 7 days post-elastase perfusion and increased macrophage content after 14 days in WT mice relative to Mcpt6−/− mice did not affect elastin degradation grades. Indeed, Mcpt6−/− mice showed more elastin degradation than did WT mice at the 7-day time point (Supplemental Figure 1A). We currently lack an explanation for this finding, although absence of tryptase may lead to compensatory increase of other elastases at this time point, and this speculation merits further investigation. This study also demonstrated that the absence of mMCP-6 affects monocyte cathepsin expression (Figure 4C). This finding is perplexing, as mMCP-6 is a MC-specific protease and should not affect monocyte gene expression. These monocytes, isolated from peripheral blood, may have encountered a different environment. In humans, blood tryptase level (∼100 ng/ml) is about 10-fold that of chymase (∼10 ng/ml),32 20-fold that of cathepsin S (∼5 ng/ml),42 and >20-fold that of cathepsin L (<5 ng/ml).43 A high blood tryptase level may affect blood cells, such as monocyte gene expression. Indeed, tryptase promotes human EC MCP-1 and IL8 expression.44 This study demonstrated that tryptase stimulated mouse EC expressions of IL6, KC, MIP-2, and LIX (Figure 4A). Tryptase in blood, therefore, may have more complex functions than previously appreciated.
Reduced apoptosis and enhanced SMC content in AAA lesions from Mcpt6−/− mice at the 7-day time point suggested that tryptase could promote SMC apoptosis (Figure 2D, Supplemental Figure 1B/1C). Study of cultured SMC affirmed this hypothesis (Figure 4D). At later time points (14 days and 56 days post-perfusion), however, tryptase expression did not affectAAA lesion SMC loss or apoptosis. Our current data do not explain these discrepant changes. As discussed, tryptase induces cysteinyl cathepsin expression via the MAPK pathway.36-39 These cathepsins induce cell apoptosis by cleaving the anti-apoptotic protein Bcl-2 member Bid and creating a pro-apoptotic signal for mitochondrial cytochrome C release.45 Observations in several cathepsin mutant mouse cells support this hypothesis,46,47 but tryptase inhibits Fas-induced fibroblast apoptosis in a concentration-dependent manner, likely via the Rho kinase pathway.48 One mechanism may predominant over the other at different stages of AAA progression; this speculation requires experimental confirmation.
This study provided evidence from human AAA patients and mice with experimental AAA supporting the direct participation of MC tryptase in AAA pathogenesis. Although more mechanisms remain undiscovered, tryptase regulates EC cytokine and chemokine expression, leukocyte migration, SMC apoptosis, and AAA-pertinent cysteinyl cathepsin expression in lesions. All of these functions may contribute to AAA formation. Significant correlation of serum tryptase levels with subsequent surgery and overall mortality indicate that serum tryptase could serve as a biomarker of AAA expansion, and that pharmacological inhibition of tryptase activity might benefit AAA patients.
Supplementary Material
Novelty and Significance.
What is known?
Mast cells contribute to the pathogenesis of both atherosclerosis and abdominal aortic aneurysm (AAA) by releasing inflammatory mediators to affect neighboring inflammatory and vascular cells.
Both chymase and tryptase are unique mast cell-derived serine proteases.
Mast cell chymase plays an important role in AAA formation, and the absence of chymase protects mice from AAA in an experimental model.
What new information does this article contribute?
Serum mast cell tryptase levels are significantly higher in patients with AAA and correlate with subsequent need for surgery and overall mortality.
Absence of tryptase protects mice from aortic elastase perfusion-induced experimental AAA.
Mast cell tryptase contributes to AAA formation by regulating inflammatory cell infiltration, smooth muscle cell (SMC) apoptosis, cysteinyl cathepsin expression, and vasculature remodeling.
Studies from human samples and experimental animals demonstrate that mast cells participate directly in cardiovascular diseases. Using mast cell-deficient mice, several studies have shown that mast cells contribute to both atherosclerosis and AAA by releasing granular mediators to induce vascular cell protease expression, and consequently promoting vasculature remodeling. Tryptase constitutes one of the most abundant human mast cell granular proteins and is important in mast cell activation, inflammatory cell recruitment, SMC proliferation, protease activation, and angiogenesis. These properties are consistent with participation in AAA formation. This study demonstrates that serum tryptase may serve as a novel biomarker for AAAs. Increased serum tryptase concentrations in AAA patients correlated significantly with subsequent surgical repair and overall mortality in a follow-up study. In elastase perfusion-induced experimental AAA, absence of tryptase (mMCP-6) protected mice from AAA progression. Mechanistic studies demonstrated that tryptase contributes to AAA formation by regulating inflammatory cell (macrophage and T cell) recruitment, SMC apoptosis, cysteinyl cathepsin expression, and aortic wall elastin degradation. Although larger AAA population follow-up studies are required to confirm our observations, measurements of serum tryptase level may assist future clinical decision making in treating patients with AAA, and these protease inhibitors may be useful in treating AAA patients.
Acknowledgments
The authors thank Mrs. Eugenia Shvartz for technical assistance and Ms. Sara Karwacki for editorial assistance.
Sources of Funding: This study was supported by NIH grants HL60942, HL81090, HL88547 (GPS); HL56985 (PL); HL36110 (RLS); European FP7 grant no. 200647: Fighting Aneurysmal Disease (JSL); and an EIA award (0840118N) from AHA (GPS).
Non-standard Abbreviations and Acronyms
- AAA
abdominal aortic aneurysm
- AP
anteroposterior
- BMI
body-mass index
- BMMC
bone marrow–derived mast cell
- EC
endothelial cell
- MC
mast cell
- MCP-1
monocyte chemotactic protein-1
- mMCP
mouse mast cell protease
- MMP
matrix metalloproteinase
- PDTC
pyrrolidine dithiocarbamate
- SDF-1α
stromal cell derived factor-1α
Footnotes
Disclosures: None.
References
- 1.Miller JS, Moxley G, Schwartz LB. Cloning and characterization of a second complementary DNA for human tryptase. J Clin Invest. 1990;86:864–870. doi: 10.1172/JCI114786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz LB, Sakai K, Bradford TR, Ren S, Zweiman B, Worobec AS, Metcalfe DD. The alpha form of human tryptase is the predominant type present in blood at baseline in normal subjects and is elevated in those with systemic mastocytosis. J Clin Invest. 1995;96:2702–2710. doi: 10.1172/JCI118337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds DS, Gurley DS, Austen KF, Serafin WE. Cloning of the cDNA and gene of mouse mast cell protease-6. Transcription by progenitor mast cells and mast cells of the connective tissue subclass. J Biol Chem. 1991;266:3847–3853. [PubMed] [Google Scholar]
- 4.Cairns JA, Walls AF. Mast cell tryptase is a mitogen for epithelial cells. Stimulation of IL-8 production and intercellular adhesion molecule-1 expression. J Immunol. 1996;156:275–283. [PubMed] [Google Scholar]
- 5.He S, Peng Q, Walls AF. Potent induction of a neutrophil and eosinophil-rich infiltrate in vivo by human mast cell tryptase: selective enhancement of eosinophil recruitment by histamine. J Immunol. 1997;159:6216–6225. [PubMed] [Google Scholar]
- 6.Jung KS, Cairns JA, Church MK, Shute JK, Walls AF. Human mast cell tryptase can induce eosinophil chemotaxis and secretion. Clin Exp Allergy. 1994;24:988A. [Google Scholar]
- 7.Shin K, Watts GF, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, Lee DM. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J Immunol. 2008;180:4885–4891. doi: 10.4049/jimmunol.180.7.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C, De Sanctis GT, O'Brien PJ, Mizgerd JP, Friend DS, Drazen JM, Brass LF, Stevens RL. Evaluation of the substrate specificity of human mast cell tryptase beta I and demonstration of its importance in bacterial infections of the lung. J Biol Chem. 2001;276:26276–26284. doi: 10.1074/jbc.M102356200. [DOI] [PubMed] [Google Scholar]
- 9.Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, Adachi R. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 10.Shin K, Watts GF, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, Lee DM. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J Immunol. 2008;180:4885–4891. doi: 10.4049/jimmunol.180.7.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Compton SJ, Cairns JA, Holgate ST, Walls AF. The role of mast cell tryptase in regulating endothelial cell proliferation, cytokine release, and adhesion molecule expression: tryptase induces expression of mRNA for IL-1 beta and IL-8 and stimulates the selective release of IL-8 from human umbilical vein endothelial cells. J Immunol. 1998;161:1939–1946. [PubMed] [Google Scholar]
- 12.Gruber BL, Marchese MJ, Suzuki K, Schwartz LB, Okada Y, Nagase H, Ramamurthy NS. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J Clin Invest. 1989;84:1657–1662. doi: 10.1172/JCI114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stack MS, Johnson DA. Human mast cell tryptase activates single-chain urinary-type plasminogen activator (pro-urokinase) J Biol Chem. 1994;269:9416–9419. [PubMed] [Google Scholar]
- 14.Carrell TW, Burnand KG, Wells GM, Clements JM, Smith A. Stromelysin-1 (matrix metalloproteinase-3) and tissue inhibitor of metalloproteinase-3 are overexpressed in the wall of abdominal aortic aneurysms. Circulation. 2002;105:477–482. doi: 10.1161/hc0402.102621. [DOI] [PubMed] [Google Scholar]
- 15.Deng GG, Martin-McNulty B, Sukovich DA, Freay A, Halks-Miller M, Thinnes T, Loskutoff DJ, Carmeliet P, Dole WP, Wang YX. Urokinase-type plasminogen activator plays a critical role in angiotensin II-induced abdominal aortic aneurysm. Circ Res. 2003;92:510–517. doi: 10.1161/01.RES.0000061571.49375.E1. [DOI] [PubMed] [Google Scholar]
- 16.Mäyränpää MI, Trosien JA, Fontaine V, Folkesson M, Kazi M, Eriksson P, Swedenborg J, Hedin U. Mast cells associate with neovessels in the media and adventitia of abdominal aortic aneurysms. J Vasc Surg. 2009;50:388–395. doi: 10.1016/j.jvs.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 17.Tsuruda T, Kato J, Hatakeyama K, Kojima K, Yano M, Yano Y, Nakamura K, Nakamura-Uchiyama F, Matsushima Y, Imamura T, Onitsuka T, Asada Y, Nawa Y, Eto T, Kitamura K. Adventitial mast cells contribute to pathogenesis in the progression of abdominal aortic aneurysm. Circ Res. 2008;102:1368–1377. doi: 10.1161/CIRCRESAHA.108.173682. [DOI] [PubMed] [Google Scholar]
- 18.Valent P, Baghestanian M, Bankl HC, Sillaber C, Sperr WR, Wojta J, Binder BR, Lechner K. New aspects in thrombosis research: possible role of mast cells as profibrinolytic and antithrombotic cells. Thromb Haemost. 2002;87:786–790. [PubMed] [Google Scholar]
- 19.Sun J, Sukhova GK, Yang M, Wolters PJ, MacFarlane LA, Libby P, Sun C, Zhang Y, Liu J, Ennis TL, Knispel R, Xiong W, Thompson RW, Baxter BT, Shi GP. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest. 2007;117:3359–3368. doi: 10.1172/JCI31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He S, Gaça MD, Walls AF. A role for tryptase in the activation of human mast cells: modulation of histamine release by tryptase and inhibitors of tryptase. J Pharmacol Exp Ther. 1998;286:289–297. [PubMed] [Google Scholar]
- 21.He S, Walls AF. Human mast cell tryptase: a stimulus of microvascular leakage and mast cell activation. Eur J Pharmacol. 1997;328:89–97. doi: 10.1016/s0014-2999(97)83033-6. [DOI] [PubMed] [Google Scholar]
- 22.Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ. 2005;330:750. doi: 10.1136/bmj.38369.620162.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindholt JS. Aneurysmal wall calcification predicts natural history of small abdominal aortic aneurysms. Atherosclerosis. 2008;197:673–678. doi: 10.1016/j.atherosclerosis.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Ghildyal N, Friend DS, Stevens RL, Austen KF, Huang C, Penrose JF, Sali A, Gurish MF. Fate of two mast cell tryptases in V3 mastocytosis and normal BALB/c mice undergoing passive systemic anaphylaxis: prolonged retention of exocytosed mMCP-6 in connective tissues, and rapid accumulation of enzymatically active mMCP-7 in the blood. J Exp Med. 1996;184:1061–1073. doi: 10.1084/jem.184.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghorpade A, Baxter BT. Biochemistry and molecular regulation of matrix macromolecules in abdominal aortic aneurysms. Ann N Y Acad Sci. 1996;800:138–150. doi: 10.1111/j.1749-6632.1996.tb33305.x. [DOI] [PubMed] [Google Scholar]
- 26.Henderson EL, Geng YJ, Sukhova GK, Whittemore AD, Knox J, Libby P. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation. 1999;99:96–104. doi: 10.1161/01.cir.99.1.96. [DOI] [PubMed] [Google Scholar]
- 27.Blair RJ, Meng H, Marchese MJ, Ren S, Schwartz LB, Tonnesen MG, Gruber BL. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J Clin Invest. 1997;99:2691–2700. doi: 10.1172/JCI119458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitamoto S, Sukhova GK, Sun J, Yang M, Libby P, Love V, Duramad P, Sun C, Zhang Y, Yang X, Peters C, Shi GP. Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation. 2007;115:2065–2075. doi: 10.1161/CIRCULATIONAHA.107.688523. [DOI] [PubMed] [Google Scholar]
- 29.Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, Kodama T, Tsimikas S, Witztum JL, Lu ML, Sakara Y, Chin MT, Libby P, Shi GP. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;111:897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lutgens E, Lutgens SP, Faber BC, Heeneman S, Gijbels MM, de Winther MP, Frederik P, van der Made I, Daugherty A, Sijbers AM, Fisher A, Long CJ, Saftig P, Black D, Daemen MJ, Cleutjens KB. Disruption of the cathepsin K gene reduces atherosclerosis progression and induces plaque fibrosis but accelerates macrophage foam cell formation. Circulation. 2006;113:98–107. doi: 10.1161/CIRCULATIONAHA.105.561449. [DOI] [PubMed] [Google Scholar]
- 31.Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun J, Zhang J, Lindholt JS, Sukhova GK, Liu J, He A, Abrink M, Pejler G, Stevens RL, Thompson RW, Ennis TL, Gurish MF, Libby P, Shi GP. Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation. 2009;120:973–982. doi: 10.1161/CIRCULATIONAHA.109.849679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, MacFarlane LA, Mallen-St Clair J, Shi GP. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 35.Kaartinen M, Penttila A, Kovanen PT. Mast cells of two types differing in neutral protease composition in the human aortic intima. Demonstration of tryptase- and tryptase/chymase-containing mast cells in normal intimas, fatty streaks, and the shoulder region of atheromas. Arterioscler Thromb Vasc Biol. 1994;14:966–972. doi: 10.1161/01.atv.14.6.966. [DOI] [PubMed] [Google Scholar]
- 36.Temkin V, Kantor B, Weg V, Hartman ML, Levi-Schaffer F. Tryptase activates the mitogen-activated protein kinase/activator protein-1 pathway in human peripheral blood eosinophils, causing cytokine production and release. J Immunol. 2002;169:2662–2669. doi: 10.4049/jimmunol.169.5.2662. [DOI] [PubMed] [Google Scholar]
- 37.Silletti S, Yebra M, Perez B, Cirulli V, McMahon M, Montgomery AM. Extracellular signal-regulated kinase (ERK)-dependent gene expression contributes to L1 cell adhesion molecule-dependent motility and invasion. J Biol Chem. 2004;279:28880–28888. doi: 10.1074/jbc.M404075200. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto M, Kogawa M, Wada S, Takayanagi H, Tsujimoto M, Katayama S, Hisatake K, Nogi Y. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J Biol Chem. 2004;279:45969–45979. doi: 10.1074/jbc.M408795200. [DOI] [PubMed] [Google Scholar]
- 39.Ruettger A, Schueler S, Mollenhauer JA, Wiederanders B. Cathepsins B, K, and L are regulated by a defined collagen type II peptide via activation of classical protein kinase C and p38 MAP kinase in articular chondrocytes. J Biol Chem. 2008;283:1043–1051. doi: 10.1074/jbc.M704915200. [DOI] [PubMed] [Google Scholar]
- 40.Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998;102:576–583. doi: 10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi GP, Dolganov GM. Comprehensive transcriptome of proteases and protease inhibitors in vascular cells. Stroke. 2006;37:537–541. doi: 10.1161/01.STR.0000198816.62266.e9. [DOI] [PubMed] [Google Scholar]
- 42.Taleb S, Lacasa D, Bastard JP, Poitou C, Cancello R, Pelloux V, Viguerie N, Benis A, Zucker JD, Bouillot JL, Coussieu C, Basdevant A, Langin D, Clement K. Cathepsin S, a novel biomarker of adiposity: relevance to atherogenesis. FASEB J. 2005;19:1540–1542. doi: 10.1096/fj.05-3673fje. [DOI] [PubMed] [Google Scholar]
- 43.Yang M, Zhang Y, Pan J, Sun J, Liu J, Libby P, Sukhova GK, Doria A, Katunuma N, Peroni OD, Guerre-Millo M, Kahn BB, Clement K, Shi GP. Cathepsin L activity controls adipogenesis and glucose tolerance. Nat Cell Biol. 2007;9:970–977. doi: 10.1038/ncb1623. [DOI] [PubMed] [Google Scholar]
- 44.Kinoshita M, Okada M, Hara M, Furukawa Y, Matsumori A. Mast cell tryptase in mast cell granules enhances MCP-1 and interleukin-8 production in human endothelial cells. Arterioscler Thromb Vasc Biol. 2005;25:1858–1863. doi: 10.1161/01.ATV.0000174797.71708.97. [DOI] [PubMed] [Google Scholar]
- 45.Stoka V, Turk B, Schendel SL, Kim TH, Cirman T, Snipas SJ, Ellerby LM, Bredesen D, Freeze H, Abrahamson M, Bromme D, Krajewski S, Reed JC, Yin XM, Turk V, Salvesen GS. Lysosomal protease pathways to apoptosis. Cleavage of bid, not pro-caspases, is the most likely route. J Biol Chem. 2001;276:3149–3157. doi: 10.1074/jbc.M008944200. [DOI] [PubMed] [Google Scholar]
- 46.Pennacchio LA, Bouley DM, Higgins KM, Scott MP, Noebels JL, Myers RM. Progressive ataxia, myoclonic epilepsy and cerebellar apoptosis in cystatin B-deficient mice. Nat Genet. 1998;20:251–258. doi: 10.1038/3059. [DOI] [PubMed] [Google Scholar]
- 47.Chen W, Yang S, Abe Y, Li M, Wang Y, Shao J, Li E, Li YP. Novel pycnodysostosis mouse model uncovers cathepsin K function as a potential regulator of osteoclast apoptosis and senescence. Hum Mol Genet. 2007;16:410–423. doi: 10.1093/hmg/ddl474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawamukai N, Yukawa S, Saito K, Nakayamada S, Kambayashi T, Tanaka Y. Mast cell-derived tryptase inhibits apoptosis of human rheumatoid synovial fibroblasts via rho-mediated signaling. Arthritis Rheum. 2010;62:952–959. doi: 10.1002/art.27331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.