Abstract
♦ Background: Accelerated cardiovascular disease (CVD), including peripheral arterial disease (PAD), is very common in patients with end-stage renal disease. Residual renal function (RRF) is a strong predictor of patient survival that is suggested to be linked to the degree of CVD. However, the relationship between PAD and decline in RRF has not previously been measured.
♦ Methods: We studied incident continuous ambulatory peritoneal dialysis patients from Peking University Third Hospital. An ankle brachial index of less than 0.9 was used to diagnose PAD. Residual renal function (RRF) was determined as the mean of 24-hour urea and creatinine clearances (glomerular filtration rate). The Cox proportional hazards model was used to identify factors predicting loss of RRF.
♦ Results: The study included 86 patients (age: 61 ± 14 years; men: 51%), 23 of whom had PAD at baseline. Mean follow-up was 19 months (median: 18 months; range: 6 – 30 months). In univariate analysis, baseline PAD, peritonitis during follow-up, inflammation (C-reactive protein), serum uric acid, Ca×P, and serum phosphate were all significantly associated with a greater-than-50% decrease in RRF during follow-up. In multivariate analysis, only baseline PAD, Ca×P, and peritonitis were independently associated with a decline in RRF.
♦ Conclusions: Our study suggests that PAD may be a clinically important marker of CVD predicting the loss of RRF. It remains to be determined whether interventions aimed at decreasing PAD may also improve renal vascular status and thus slow the rate of RRF decline.
Keywords: Ankle brachial index, end-stage renal disease, cardiovascular disease, inflammation, nutrition, atherosclerosis
Loss of residual renal function (RRF) is an important predictor of morbidity and mortality in patients on maintenance dialysis (1,2). A declining RRF is associated with anemia, inflammation, malnutrition, and ectopic calcification (3,4). Meanwhile, preservation of RRF contributes to increased solute clearance and an improved fluid balance (5). Continuous ambulatory peritoneal dialysis (CAPD) is known to be better than hemodialysis (HD) at maintaining RRF (6), but a decline of RRF also occurs in CAPD. This decline is thought to reflect both progression of the primary kidney disease and increased kidney vascular dysfunction related to chronic kidney disease itself (7). Consistent with that hypothesis, cardiac disease and hypotensive events have both been shown to be associated with a decline in RRF (8–11).
Meanwhile, peripheral arterial disease (PAD), a manifestation of atherosclerosis in the lower extremities, is common in dialysis patients (12,13). Given that PAD is the result of an atherosclerotic process similar to that in cardiovascular disease (CVD) and that PAD is associated with a rise of serum creatinine in the community (14), we hypothesized that PAD may also be a marker of vascular bed degeneration and a predictor of RRF decline. We conducted a prospective observational study to investigate the predictive value of PAD for loss of RRF in incident CAPD patients.
METHODS
PATIENTS
Our prospective study included incident CAPD patients in the outpatient clinic of the Division of Nephrology at Peking University Third Hospital who started dialysis between 1 March 2006 and 1 December 2007. Patients were excluded if they had died within 3 months of starting dialysis (n = 6); if they had transferred to other centers or to HD or transplantation within 3 months (n = 11); and if their baseline urine volume was less than 200 mL daily or their glomerular filtration rate (GFR) was below 2 mL/min (n = 8).
The 86 patients who qualified for the study were followed until 1 June 2009. On the day that GFR was evaluated, 24-hour urine was collected. A significant decline in RRF was defined as a GFR loss of more than 50% from baseline or a daily urinary volume of less than 100 mL. Patients were censored at death or transfer to HD.
The local ethics committee approved the study, and informed consent was obtained from each patient enrolled.
PAD ASSESSMENT
We defined PAD as an ankle brachial index (ABI) below 0.9. We measured ABI as described by Feigelson et al. (15), calculated the value for each leg, and used the lower of the two values for analysis. The ABI measurement was conducted within the first month after the patient started peritoneal dialysis therapy.
Demographic data were collected on the same day that the ABI was measured; the laboratory results used were the most recent before the ABI measurement. Residual renal function was determined as the mean of 24-hour urea and creatinine clearances (GFR). Urine volume was obtained from the first and last evaluations of GFR. Diagnosed coronary artery disease (previous myocardial infarction, angina pectoris, a history of coronary artery bypass grafting or coronary stent implantation), and a history of cerebrovascular events were grouped as CVD. Diabetes status was defined as the use of insulin or oral hypoglycemic agents, or a fasting plasma glucose level higher than 7 mmol/L on at least 2 different days. All patients were followed every 3 months, and dialysis adequacy and incidence of peritonitis were recorded.
STATISTICAL ANALYSIS
The statistical analysis was carried out using SPSS (version 13.0: SPSS, Chicago, IL, USA). Continuous variables are presented as mean ± standard deviation, and categorical variables, as percentages. Serum levels of C-reactive protein (CRP) were logarithmically transformed [ln(CRP)] to approximate linearity. The Student t-test and the chi-square test were used, as appropriate, to identify differences between groups. A two-tailed p value less than 0.05 was regarded as statistically significant. The RRF survival curve was plotted using the Kaplan–Meier method and a log-rank test between patients that lost more than 50% of RRF during follow-up and those that did not. A univariate Cox proportional hazards model was used to identify factors predicting the loss of RRF. The variables that achieved statistical significance (p < 0.05) were then selected into a multivariate Cox proportional hazards model to test for relative independence.
RESULTS
CROSS-SECTIONAL ANALYSIS
Of the 86 incident CAPD patients enrolled in the study (age: 61 ± 14 years; men: 51%), 26 had PAD at baseline. Table 1 shows the primary kidney diseases in the study group. Table 2 presents baseline clinical characteristics grouped by PAD status. Briefly, patients with PAD were older and more likely to have diabetes, a lower serum albumin level, a higher CPR level, and a higher baseline GFR.
TABLE 1.
Primary Causes of Renal Failure in the Study Patients

TABLE 2.
Characteristics of Patients With and Without Peripheral Arterial Disease

During follow-up [for a mean of 19 months (median: 18 months; range: 6 – 30 months)], 26 patients experienced a more-than-50% loss of RRF (14 in the PAD group, 12 in the non-PAD group). The mean RRF decline in all patients was 2 ± 4 mL/min (1.3 ± 2.3 mL/min in non-PAD patients, 3.7 ± 6.2 mL/min in PAD patients). Urine volume declined to 636 ± 442 mL from 957 ± 378 mL in non-PAD patients, and to 433 ± 232 mL from 978 ± 340 mL in PAD patients.
UNIVARIATE AND MULTIVARIATE ANALYSES
The presence of PAD, peritonitis during follow-up, inflammation [ln(CRP)], serum uric acid, serum phosphate, and Ca×P were all significantly associated with a greater-than-50% loss of RRF during follow-up (Table 3). Table 4 shows the independently associated factors that predicted a greater-than-50% decline in RRF. In the Kaplan–Meier analysis, the presence of PAD predicted loss of RRF (Table 4, Figure 1).
TABLE 3.
Factors Significantly Associated with a More-Than-50% Loss of Residual Renal Function in the Study Patients

TABLE 4.
Factors Independently Predicting a More-Than-50% Loss of Glomerular Filtration Rate During Follow-Upa in the Study Patients

Figure 1.
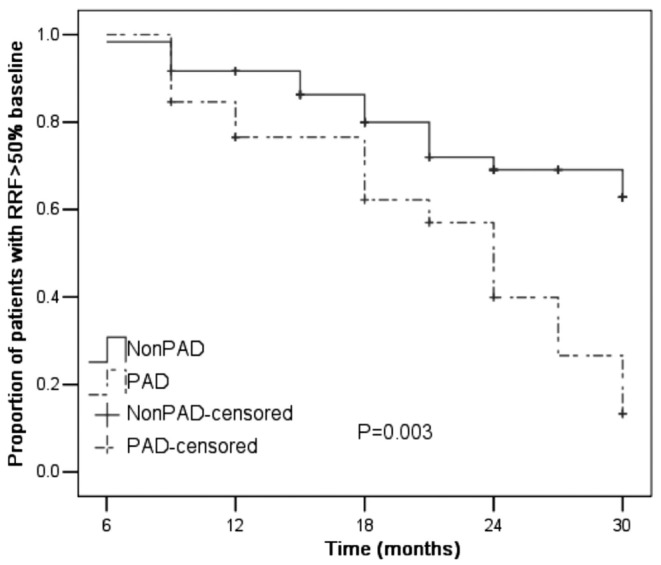
— Kaplan–Meier estimate of preservation of residual renal function (RRF) in patients with and without peripheral arterial disease (PAD).
DISCUSSION
In this study of incident patients starting CAPD, we conclude that the presence of PAD is an independent predictor of a subsequent greater-than-50% loss of RRF. We suggest that these results reflect the fact that PAD is a systemic atherosclerotic process involving the whole vascular bed, and thus also reflects vascular health and disease in the kidney. It is possible that the association between PAD and loss of RRF reflects a shared risk factor profile between end-stage renal disease and atherosclerosis, but the association could not be explained away by controlling for many of the known risk factors for RRF decline.
Renal ischemia, a likely consequence of dysfunction in the renal vasculature, is known to predict loss of renal function (14,16), an observation supported by the fact that volume depletion also independently predicts decline in RRF (17,18).
Atherosclerosis often co-exists in different vascular beds (19). Missouris et al. reported a prevalence of 45% for renal artery stenosis in patients with PAD (20). Given that finding, our study suggests that PAD is a manifestation of atherosclerotic vascular disease in the lower limb vascular bed, likely reflecting a systemic disease also involving the kidneys (14,20–22). Atherosclerosis is also a process of inflammation, which has been found to predict the loss of RRF (1,23). In the present study, ln(CRP) was associated in univariate analysis with a decline in RRF (Table 3). Its loss of significance in the multivariate analysis (Table 4) may reflect the inflammatory process of PAD.
Previous data indicate that the presence of coronary artery disease, another systemic atherosclerotic manifestation, is associated with loss of RRF (9,11,18), supporting our hypothesis. Interestingly, in our study, PAD predicted loss of RRF more powerfully than CVD did, CVD having been excluded from the multivariate model. It should be noted, however, that previous studies (9,18) included congestive heart failure as a manifestation of CVD, something that was not done in the present study. Moreover, in the present study, only 9 patients with CVD had concurrent PAD, suggesting that our CVD definition may be somewhat arbitrary.
In agreement with previous findings (24,25), our data also suggest that peritonitis is an independent risk factor for RRF loss, likely related to the septic vascular damage associated with bacteremia and also potentially to the associated use of nephrotoxic antibiotics (26). Another possible mechanism could be temporary ultrafiltration failure and extracellular fluid volume expansion during peritonitis, leading to elevation of systemic blood pressure, which could have a deleterious effect on remnant nephrons (5).
There is a discrepancy concerning the role of disordered mineral metabolism on RRF. We found that serum phosphorus and Ca×P predict loss of RRF, consistent with previous findings (18,27). However, a recent publication by Noordzij and colleagues (Netherlands Cooperative Study on the Adequacy of Dialysis) failed to demonstrate an effect of disordered mineral metabolism on decline of RRF (28), concluding that the association exists only in pre-dialysis uremic patients in light of the many reports of the accelerative role of mineral disorders on renal function in chronic kidney disease patients (29–32). More studies are needed to clarify this issue.
CONCLUSIONS
Subclinical PAD is a common complication often ignored by nephrologists. The predictive role of PAD for RRF loss, as illustrated in the present study, strongly supports its role as an important marker of generalized CVD risk, providing new impetus for aggressive treatment aimed at minimizing the progression of atherosclerosis.
DISCLOSURES
All of the authors declare that no financial conflicts of interest exist.
REFERENCES
- 1. Chung SH, Heimbürger O, Stenvinkel P, Qureshi AR, Lindholm B. Association between residual renal function, inflammation and patient survival in new peritoneal dialysis patients. Nephrol Dial Transplant 2003; 18:590–7 [DOI] [PubMed] [Google Scholar]
- 2. Vilar E, Wellsted D, Chandna SM, Greenwood RN, Farrington K. Residual renal function improves outcome in incremental haemodialysis despite reduced dialysis dose. Nephrol Dial Transplant 2009; 24:2502–10 [DOI] [PubMed] [Google Scholar]
- 3. Marrón B, Remón C, Pérez–Fontán M, Quirós P, Ortíz A. Benefits of preserving residual renal function in peritoneal dialysis. Kidney Int Suppl 2008; (108):S42–51 [DOI] [PubMed] [Google Scholar]
- 4. Khawar O, Kalantar–Zadeh K, Lo WK, Johnson D, Mehrotra R. Is the declining use of long-term peritoneal dialysis justified by outcome data? Clin J Am Soc Nephrol 2007; 2:1317–28 [DOI] [PubMed] [Google Scholar]
- 5. Singhal MK, Bhaskaran S, Vidgen E, Bargman JM, Vas SI, Oreopoulos DG. Rate of decline of residual renal function in patients on continuous peritoneal dialysis and factors affecting it. Perit Dial Int 2000; 20:429–38 [PubMed] [Google Scholar]
- 6. Ignace S, Fouque D, Arkouche W, Steghens JP, Guebre–Egziabher F. Preserved residual renal function is associated with lower oxidative stress in peritoneal dialysis patients. Nephrol Dial Transplant 2009; 24:1685–9 [DOI] [PubMed] [Google Scholar]
- 7. Fogo AB. The role of angiotensin II and plasminogen activator inhibitor-1 in progressive glomerulosclerosis. Am J Kidney Dis 2000; 35:179–88 [DOI] [PubMed] [Google Scholar]
- 8. Liao CT, Shiao CC, Huang JW, Hung KY, Chuang HF, Chen YM, et al. Predictors of faster decline of residual renal function in Taiwanese peritoneal dialysis patients. Perit Dial Int 2008; 28(Suppl 3):S191–5 [PubMed] [Google Scholar]
- 9. Holley JL, Aslam N, Bernardini J, Fried L, Piraino B. The influence of demographic factors and modality on loss of residual renal function in incident peritoneal dialysis patients. Perit Dial Int 2001; 21:302–5 [PubMed] [Google Scholar]
- 10. Liao CT, Chen YM, Shiao CC, Hu FC, Huang JW, Kao TW, et al. Rate of decline of residual renal function is associated with all-cause mortality and technique failure in patients on long-term peritoneal dialysis. Nephrol Dial Transplant 2009; 24:2909–14 [DOI] [PubMed] [Google Scholar]
- 11. Coronel F, Perez–Flores I, Calvo N, Martinez–Villaescusa M, Cigarran S. Impact of cardiovascular events on residual renal function during the first year of peritoneal dialysis. Perit Dial Int 2007; 27:454–6 [PubMed] [Google Scholar]
- 12. Liu JH, Lin HH, Yang YF, Liu YL, Kuo HL, Wang IK, et al. Subclinical peripheral artery disease in patients undergoing peritoneal dialysis: risk factors and outcome. Perit Dial Int 2009; 29:64–71 [PubMed] [Google Scholar]
- 13. O’Hare AM, Hsu CY, Bacchetti P, Johansen KL. Peripheral vascular disease risk factors among patients undergoing hemodialysis. J Am Soc Nephrol 2002; 13:497–503 [DOI] [PubMed] [Google Scholar]
- 14. O’Hare AM, Rodriguez RA, Bacchetti P. Low ankle-brachial index associated with rise in creatinine level over time: results from the Atherosclerosis Risk in Communities study. Arch Intern Med 2005; 165:1481–5 [DOI] [PubMed] [Google Scholar]
- 15. Feigelson HS, Criqui MH, Fronek A, Langer RD, Molgaard CA. Screening for peripheral arterial disease: the sensitivity, specificity, and predictive value of noninvasive tests in a defined population. Am J Epidemiol 1994; 140:526–34 [DOI] [PubMed] [Google Scholar]
- 16. Shanley PF. The pathology of chronic renal ischemia. Semin Nephrol 1996; 16:21–32 [PubMed] [Google Scholar]
- 17. Jansen MA, Hart AA, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int 2002; 62:1046–53 [DOI] [PubMed] [Google Scholar]
- 18. Moist LM, Port FK, Orzol SM, Young EW, Ostbye T, Wolfe RA, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol 2000; 11:556–64 [DOI] [PubMed] [Google Scholar]
- 19. Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006; 295:180–9 [DOI] [PubMed] [Google Scholar]
- 20. Missouris CG, Buckenham T, Cappuccio FP, MacGregor GA. Renal artery stenosis: a common and important problem in patients with peripheral vascular disease. Am J Med 1994; 96:10–14 [DOI] [PubMed] [Google Scholar]
- 21. Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation 2006; 114:688–99 [DOI] [PubMed] [Google Scholar]
- 22. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Guidelines for the Management of Patients with Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic): executive summary. A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol 2006; 47:1239–312 [DOI] [PubMed] [Google Scholar]
- 23. Chung SH, Heimbürger O, Stenvinkel P, Bergström J, Lindholm B. Association between inflammation and changes in residual renal function and peritoneal transport rate during the first year of dialysis. Nephrol Dial Transplant 2001; 16:2240–5 [DOI] [PubMed] [Google Scholar]
- 24. Breborowicz A, Pawlaczyk K, Polubinska A, Górna K, Wieslander A, Carlsson O, et al. Effect of peritoneal dialysis on renal morphology and function. Nephrol Dial Transplant 2006; 21:3539–44 [DOI] [PubMed] [Google Scholar]
- 25. Shemin D, Maaz D, St Pierre D, Kahn SI, Chazan JA. Effect of aminoglycoside use on residual renal function in peritoneal dialysis patients. Am J Kidney Dis 1999; 34:14–20 [DOI] [PubMed] [Google Scholar]
- 26. Johnson DW, Mudge DW, Sturtevant JM, Hawley CM, Campbell SB, Isbel NM, et al. Predictors of decline of residual renal function in new peritoneal dialysis patients. Perit Dial Int 2003; 23:276–83 [PubMed] [Google Scholar]
- 27. Koizumi T, Murakami K, Nakayama H, Kuwahara T, Ohnishi Y. Role of dietary phosphorus in the progression of renal failure. Biochem Biophys Res Commun 2002; 295:917–21 [DOI] [PubMed] [Google Scholar]
- 28. Noordzij M, Voormolen NM, Boeschoten EW, Dekker FW, Bos WJ, Krediet RT, et al. Disordered mineral metabolism is not a risk factor for loss of residual renal function in dialysis patients. Nephrol Dial Transplant 2009; 24:1580–7 [DOI] [PubMed] [Google Scholar]
- 29. Voormolen N, Noordzij M, Grootendorst DC, Beetz I, Sijpkens YW, van Manen JG, et al. High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant 2007; 22:2909–16 [DOI] [PubMed] [Google Scholar]
- 30. Knight EL, Stampfer MJ, Hankinson SE, Spiegelman D, Curhan GC. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med 2003; 138:460–7 [DOI] [PubMed] [Google Scholar]
- 31. Barsotti G, Giannoni A, Morelli E, Lazzeri M, Vlamis I, Baldi R, et al. The decline of renal function slowed by very low phosphorus intake in chronic renal patients following a low nitrogen diet. Clin Nephrol 1984; 21:54–9 [PubMed] [Google Scholar]
- 32. Kasiske BL, Lakatua JD, Ma JZ, Louis TA. A meta-analysis of the effects of dietary protein restriction on the rate of decline in renal function. Am J Kidney Dis 1998; 31:954–61 [DOI] [PubMed] [Google Scholar]


