Abstract
This study investigated the potential use of synovium-derived stem cells (SDSCs) as a cell source for cartilage tissue engineering. Harvested SDSCs from juvenile bovine synovium were expanded in culture in the presence (primed) or absence (unprimed) of growth factors (1 ng/mL transforming growth factor-β1, 10 ng/mL platelet-derived growth factor-ββ, and 5 ng/mL basic fibroblast growth factor-2) and subsequently seeded into clinically relevant agarose hydrogel scaffolds. Constructs seeded with growth factor-primed SDSCs that received an additional transient application of transforming growth factor-β3 for the first 21 days (release) exhibited significantly better mechanical and biochemical properties compared to constructs that received sustained growth factor stimulation over the entire culture period (continuous). In particular, the release group exhibited a Young's modulus (267±96 kPa) approaching native immature bovine cartilage levels, with corresponding glycosaminoglycan content (5.19±1.45%ww) similar to native values, within 7 weeks of culture. These findings suggest that SDSCs may serve as a cell source for cartilage tissue engineering applications.
Introduction
Articular cartilage is the soft tissue lining diarthrodial joints and functions as the weight-bearing cushion of the joint.1,2 The harsh loading environment and the avascular nature of mature cartilage lead to a poor intrinsic healing capacity after injury. As a result, cell-based therapies, including tissue engineering strategies for growing clinically relevant grafts, are being intensively researched.3–8 An autologous cell source would be ideal for growing clinically relevant engineered cartilage; however, using cells from an osteoarthritic or injured tissue to grow engineered cartilage with mechanical and biochemical properties similar to healthy native tissue poses several challenges.9,10 The clinical potential of stem cells has driven efforts toward their optimization for tissue engineering applications.
The synovium is a thin layer of tissue that lines the noncartilaginous surfaces within articular joints. The tissue can be harvested with minimal invasiveness by clinicians during arthroscopy. Synovium-derived stem cells (SDSCs) have the potential for tissue engineering applications aimed at cartilage repair or regeneration.5,11,12 A pure population of SDSCs (type B-synovial fibroblasts) can be isolated through multiple passages or by negative isolation, to remove cells that are positive for CD14 (type A-synovial macrophages).11 The cells can then be expanded in culture for three-dimensional (3D) encapsulation and the expansion media can be optimized to promote chondrogenesis.11,13 Previous studies have induced chondrogenesis of SDSCs in vitro using a growth factor cocktail including basic fibroblast growth factor (bFGF), insulin-like growth factor I, and transforming growth factor-β1 (TGF-β1), with TGF-β1 contributing to chondrogenesis of the SDSCs.11,14 While the SDSCs in these studies showed potential to produce extracellular matrix components similar to chondrocytes (i.e., collagen II and aggrecan), few studies have demonstrated that SDSCs can be used to achieve a tissue-engineered material with mechanical properties similar to native cartilage.11,15
Agarose hydrogels have been used widely in tissue engineering applications for growth of cartilage tissue for clinical implantation.6,16,17 Agarose hydrogels have been used extensively for long-term growth of tissue constructs seeded with chondrocytes harvested from immature bovine or canine articular cartilage.18–20 These studies were able to achieve near native compressive mechanical properties and glycosaminoglycan (GAG) content over 4–6 weeks of in vitro culture. Autologous and juvenile allogeneic chondrocytes are used in clinical strategies for cartilage repair by companies such as Genzyme (Carticel®) and Zimmer/Isto (DeNovo®), respectively.21,22 Agarose hydrogels are currently employed for autologous cartilage repair strategies clinically (CARTIPATCH).17 These 3D scaffolds act to prevent cell and elaborated extracellular matrix leakage from the implantation site; they also stabilize the chondrocyte phenotype and promote a homogeneous distribution of cells within the defect.17 However, clinical application of cartilage tissue engineering may need to rely on other cell sources, due to an insufficient supply of healthy chondrocytes in damaged or osteoarthritic cartilage, donor-site morbidity, and a lack of suitable donor tissues. As such, the clinical potential of stem cells has driven efforts toward optimization of stem cells for tissue engineering applications.
Therefore, this study explored the potential of using SDSCs as a tissue engineering strategy for growing clinically relevant cartilage grafts. The two challenges addressed here were the priming of SDSCs toward a chondrogenic lineage in 2D culture, and the elaboration of construct tissue composition and material properties approaching those of native cartilage. Accordingly, the first objective was to investigate the influence of growth factor priming of SDSCs in 2D culture on the subsequent properties of tissue formed by these cells after seeding in 3D hydrogel scaffolds. The second objective was to determine the impact of transient or continuous exposure of constructs to TGF-β3 growth factor in 3D culture.
Materials and Methods
Synovial tissue was harvested aseptically from two juvenile bovine knee joints (2–4 weeks old), and digested using collagenase type V (Sigma-Aldrich) in serum-containing medium (37°C, 5% CO2). Synoviocytes were isolated, plated at a density of 180 cells/cm2, and cultured with medium containing alpha-minimum essential medium, 10% fetal bovine serum, 100 U/mL penicillin, 100 mg/mL streptomycin, and amphotericin B (Invitrogen Co.).
To obtain a pure population of SDSCs, a negative isolation procedure was performed following the first passage (P1) using CD14 Dynabeads (Invitrogen Dynal).15 Cells were detached by trypsinization for 12 min (Trypsin EDTA; Cellgro Mediatech, Inc.), and suspended in phosphate-buffered saline (PBS) with 0.1% bovine serum albumin and 2 mM EDTA at a concentration of 10×106 cells/mL. The Dynabeads were suspended in PBS with 0.1% bovine serum albumin, and then mixed with the synoviocytes (50 μL of beads per 1×109 cells) for 30 min at 4°C on a rotational shaker. The synovial fibroblasts and the CD14 cells bound to the Dynabeads were separated using the DynaMag-15 magnet (Invitrogen Dynal). The isolated SDSCs were passaged until P3 before expanding for 3D culture.
The expression of surface markers on Dyna-ed synovial cells was studied by flow cytometry. Cells were stained with fluorescein isothiocyanate- or phycoerythrin-conjugated monoclonal antibodies against CD31 (Thermo Scientific), CD34 (Abcam), CD49c (Thermo Scientific), and CD73 (BioLegend). Unstained cells were used as a negative fluorescence control. Cells were incubated in the dark at room temperature for 15 min, after which they were washed and resuspended in 0.5 mL of PBS containing 2 mM EDTA for analysis. Immediately after incubation, cell fluorescence was evaluated using a FACSCalibur flow cytometer (Becton Dickinson). The resulting data were analyzed using FlowJo software (version 7.6.1).
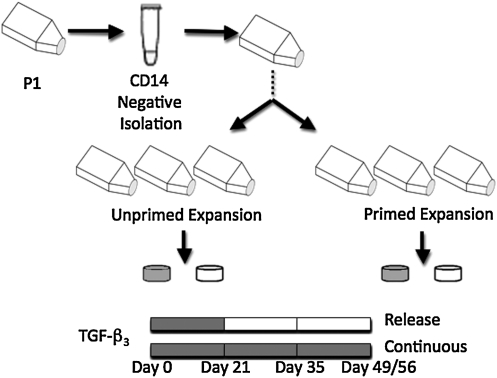
To determine the effect of priming the cells toward a chondrogenic lineage in 2D culture, the culture medium was supplemented with a cocktail of growth factors during P4 expansion (1 ng/mL TGF-β1, 10 ng/mL PDGF-ββ, and 5 ng/mL bFGF-2; primed group; Fig. 1).23 Cells expanded without the cocktail of growth factors served as the control (unprimed group). The synoviocytes were encapsulated in 2% w/v agarose (type VII; Sigma-Aldrich) at a concentration of 60 million cells/mL and discs were cored using a sterile disposable punch (Miltex) to final dimensions of 3 mm diameter and 2.34 mm thickness.
FIG. 1.
Schematic of the study design. Synovium-derived stem cells (SDSCs) were expanded in two-dimensional (2D) culture with (primed) or without (unprimed) growth factors. Once in 3D culture, the culture medium was supplemented with transforming growth factor (TGF)-β3 for the first 21 days (release) or throughout the study (continuous).
Constructs were cultured with serum-free chondrogenic media (Dulbecco's modified Eagle's medium, 1% ITS+ Premix, 50 μg/mL L-proline, 0.1 μM dexamethasone, 10 μg/mL sodium pyruvate, and antibiotics) with ascorbate (50 μg/mL) and TGF-β3 (10 ng/mL) added fresh during each media change. Culture media were changed every other day. The effect of supplementing the culture media with TGF-β3 was evaluated by removing the growth factor from half of the samples at day 21 (release), whereas the other half continued to receive the growth factor for the remainder of the study (continuous; Fig. 1). This study, Study A, was repeated with primed cells that were not isolated with the Dynabeads procedure and were expanded for 3D culture at P2 (Study B). The Dynabeads were not used in the repeat study, since the first study (Study A) observed that less than 5% of the cells were positive for CD14. In addition, the constructs in Study B were cored to final dimensions of 4 mm diameter and 2.34 mm thickness.
Time points for mechanical, biochemical, and histological analyses for Study A were days 0, 21, 35, and 56 (day 49 for Study B). The equilibrium Young's modulus (EY) was determined under unconfined compression at 10% strain. The dynamic modulus (G*) was determined by applying an additional ±1% strain at 0.5 Hz. Biochemistry was performed to determine the collagen and GAG content using the orthohydroxyproline and dimethylmethylene blue assay, respectively.24,25 The DNA content was determined using the PicoGreen kit (Invitrogen Co.). Samples were prepared for histology, and stained with Picrosirius Red for collagen distribution and alcian blue for GAG distribution. Statistics were conducted using a three-way ANOVA with α=0.05, and Tukey's HSD post hoc test, with statistical significance achieved when p≤0.05. The three factors analyzed in the ANOVA were Factor 1=growth factor priming treatment, Factor 2=TGF-β3 application, and Factor 3=time. Factor 1 and Factor 2 each has two levels, whereas Factor 3 has four levels. All data are presented as mean±standard deviation.
Results
Cell characterization and initial 3D culture observations
Flow cytometry analysis indicated that the majority of synoviocytes showed low expression (<5%) of CD31 (endothelial cell marker) and CD34 (hematopoietic cell marker). On the other hand, the expression of CD49c (α3 integrin) was high (>95%) and CD73 (ecto-5′nucleotidase, SH3, SH4) was above 60%.
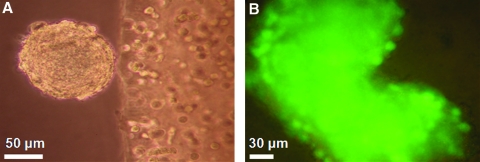
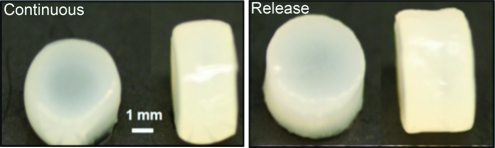
Within the first 21 days of 3D culture, aggregated SDSCs (spherical balls) were observed to emanate from and detach into the culture media. Their presence was generally a precursor of 3D construct growth (Fig. 2A). Cellular viability of the aggregated SDSCs in the culture media, as assessed by the Live/Dead cytotoxicity assay, indicated the presence of living cells throughout the mass (Fig. 2B). Gross morphology of the constructs after 7 weeks in culture revealed an increased volume and tissue opacity of the primed release group as compared to the corresponding continuous group (Fig. 3).
FIG. 2.
(A) Representative image of aggregated SDSCs emanating from the construct on the right half of the image (20× magnification). (B) Viability of the floating cell mass in the culture media as indicated by pervasive green fluorescence of the cells (40× magnification). Color images available online at www.liebertonline.com/tea
FIG. 3.
Representative gross morphology of constructs seeded with growth factor primed synovium cells at day 49 (Study B). The continuous group (left panel) had final average dimensions of φ=4.67±0.23 mm, thickness=2.91±0.11 mm, and the release group (right panel) had final dimensions of φ=4.81±0.19 mm, thickness=2.98±0.11 mm (n=5). Color images available online at www.liebertonline.com/tea
3D culture: Effects of cell priming and TGF-β3 application
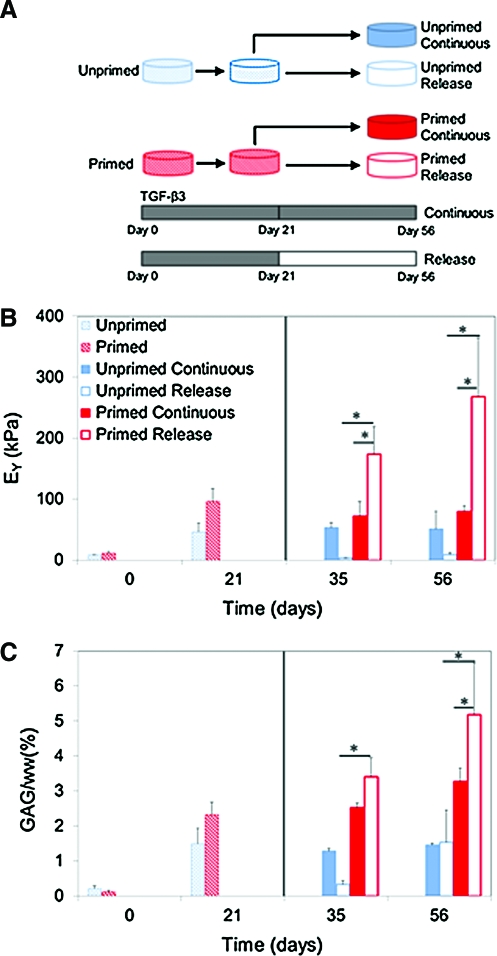
With continuous growth factor administration (from day 0 to day 56), both construct groups (primed or unprimed with growth factor cocktail during 2D expansion) exhibited significant increases in Young's modulus relative to day 0 values (Fig. 4A), reaching as high as 96±21 kPa on day 21 (primed). The primed and unprimed groups exhibited generally similar mechanical properties, except for a transient increase in the primed modulus relative to unprimed constructs observed for day 21. GAG levels of primed constructs were significantly elevated for days 21–56 over corresponding unprimed constructs (p<0.001; Fig. 4B).
FIG. 4.
(A) Schematic of the experimental groups, (B) equilibrium Young's modulus, and (C) glycosaminoglycan (GAG) (%ww) for constructs cultured over 8-week study period. Solid line indicates release at day 21 time point (*p<0.05). Color images available online at www.liebertonline.com/tea
At days 35 and 56, unprimed constructs that received only transient growth factor administration (from day 0 to 21) showed no improvement in mechanical properties relative to day 0, despite a trend of increasing GAG. In contrast, primed constructs exhibited significant temporal increases in Young's modulus upon discontinuation (release) of growth factor (days 35 and 56), with peak values of EY=267±96 kPa and GAG levels of 5.19±1.45%ww attained on day 56. The collagen content in the constructs at day 56 was 0.51±0.03%ww and was similar for release and continuous groups (data not shown). The dynamic modulus followed a similar trend to Young's modulus across all four groups, and reached a maximum of 1.74±0.27 MPa for the primed-release group at day 56. These were the best mechanical properties and biochemical content achieved in the current study.
No significant changes in DNA content were observed over culture time or between the four groups (data not shown). As a measure of cell metabolic activity, GAG was normalized to DNA content, and demonstrated similar trends to the GAG content data for the groups. The primed-release group reached a peak value (for the study) of 137.61±46.17 GAG/DNA on day 56. The corresponding peak collagen content achieved was 14.01±1.68 collagen/DNA (pooled data, n=5) and was similar for the release and continuous groups (data not shown).
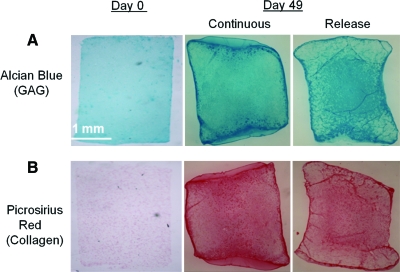
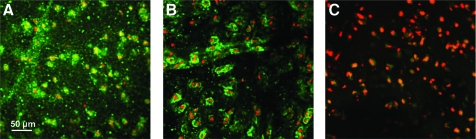
Histological analysis revealed strong GAG and collagen staining in the central region for the release groups at day 49, whereas the continuous groups showed more even distribution throughout (Fig. 5). Immunohistochemical staining indicated the presence of type II and type VI collagen in the primed release groups at day 49 (Fig. 6). At day 56, the mechanical properties of constructs that did not receive any TGF-β3 growth factor over the culture period had not increased from day 0 values (data not shown).
FIG. 5.
Representative GAG and collagen staining at days 0 and 49 (A) alcian blue staining (GAG) and (B) picrosirius red staining (collagen) for constructs seeded with growth factor primed SDSCs. Color images available online at www.liebertonline.com/tea
FIG. 6.
Representative immunohistochemical staining for (A) type II collagen (green), (B) type VI collagen (green), and (C) negative control image (red for cell nuclei) at day 49 (40× magnification). Color images available online at www.liebertonline.com/tea
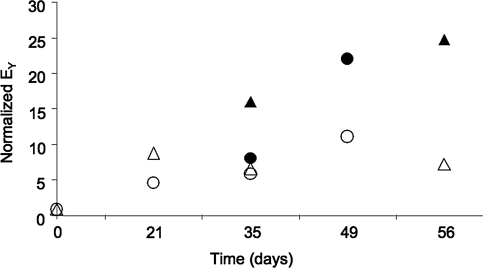
The effect of continued or transient application of TGF-β3 to constructs seeded with growth factor-primed SDSCs was repeated in a second study. In both studies, transient application of TGF-β3 to the constructs led to significantly better tissue properties compared to sustained growth factor application (Fig. 7). While the growth of constructs in the primed-release group was slightly delayed in the second study (day 35), the relative values were comparable between the two studies by day 49.
FIG. 7.
Comparison of the Young's modulus (EY) normalized to day 0 values for the initial study (triangles) and the repeat study (circles). TGF-β3 was added to the culture media either for a transient period of time (release: filled symbols) or continuously throughout the 3D culture (continuous: open symbols). All groups were primed in 2D culture with a growth factor cocktail.
Discussion
This study investigated the potential of SDSCs to grow engineered cartilage. SDSCs have previously been shown to have potential for differentiating down a chondrogenic lineage and are thought to aid in articular cartilage repair in vivo.11 Specifically, we examined the influence of supplementing the cell expansion and tissue culture media with growth factors on the mechanical and biochemical properties of the engineered tissue constructs. Constructs that received application of TGF-β3 for the first 21 days only (release) exhibited significantly higher compressive equilibrium modulus and GAG content compared to constructs that received continuous growth factor stimulation (Fig. 4). This outcome with SDSCs replicates the findings of previous studies that used juvenile chondrocytes or bone marrow-derived mesenchymal stem cells (MSCs), suggesting that SDSCs may be just as effective as these other cell sources.5,26 Within 7 weeks of culture, constructs achieved a Young's modulus approaching native immature bovine cartilage levels (400–1000 kPa; Fig. 4), with corresponding GAG content also similar to native values (approximately 6.0–8.0%ww).5,27 In our previous work, we have reported a strong correlation between Young's modulus and GAG content in chondrocyte-seeded agarose constructs.28 These initial findings are promising in terms of utilizing SDSCs as a source for cartilage tissue engineering applications.
MSCs have been used to grow functional tissue engineered cartilage using bone marrow-derived and SDSCs.15,29–31 Juvenile bovine bone marrow MSCs have been encapsulated in various hydrogels, including agarose and hyaluronan-based hydrogels.29–31 The mechanical properties obtained with primed-SDSC seeded agarose gels reported in this study are several fold greater than the properties achieved by engineered cartilage with bone marrow MSCs.29,31 More recently, SDSCs were seeded in fibrin-PGA scaffolds and cultured in a bioreactor for 4 weeks.15 The mechanical properties achieved with SDSCs seeded in the fibrin-PGA scaffold are comparable to those observed in this study, demonstrating the general utility of SDSCs as a cell source for cartilage repair strategies.17
Growth factor priming in 2D was found to be essential for enhancing the mechanical and biochemical properties of the engineered tissue constructs. A recent study showed that priming SDSCs with a growth factor cocktail including TGF-β1, bFGF-2, and PDGF-ββ significantly enhanced cell proliferation and aided in maintaining a rounded chondrocyte-like morphology.32 Evaluation of the individual growth factors has suggested that bFGF-2 increases the proliferation rate of MSCs and TGF-β1 increases the expression of collagen type II and proteoglycans.33,34 Priming the SDSCs during expansion resulted in a faster doubling rate (i.e., shorter time to reach confluence) and the engineered cartilage mechanical and biochemical properties were better than the properties from constructs seeded with unprimed SDSCs (Fig. 4). Further, the extracellular matrix formed by the constructs seeded with primed SDSCs was positive for collagen types II and VI (Fig. 6). In articular cartilage, type II collagen is the primary collagen constituent, and type VI collagen is thought to help facilitate interactions between collagen type II and the cell surface in the pericellular matrix region.35,36 Therefore, this study supports the notion that using a growth factor cocktail is important for promoting SDSC chondrogenesis and cartilaginous extracellular matrix production.
Several studies comparing the cell-surface properties of MSCs isolated from bone marrow, synovium, periosteum, skeletal muscle, and adipose tissue indicate the superior chondrogenic capacity of synovium-derived cells for tissue engineering applications.37 Flow cytometry of the synoviocytes for common markers showed that the cells were negative for endothelial (CD31) and hematopoietic (CD34) markers and had notable expression of CD73, which is in agreement with previous studies with MSCs.38 CD49c has been described by Grogan et al. as one of the antigens highly expressed in monolayer-expanded chondrocyte populations with high chondrogenic potential when used to form in vitro cartilaginous tissue.39 In our laboratory we have observed that juvenile bovine chondrocytes and SDSCs have a high expression of CD49c. This suggests that MSCs, which can be easily acquired from the synovium, can be cultured to differentiate toward a chondrogenic lineage.
Recently, promising cell sources from tissues adjacent to defect sites are being considered for repair and regeneration purposes.40 The adjacent tissue of a defect site has multiple benefits, including a decrease in the number of surgical sites and the increased potential for functional repair in vivo. For example, synovial cells have been stimulated with TGF-β1, causing the cells to migrate across articular cartilage into partial thickness defect sites for in vivo repair.41 The chondrogenic potential of synovium-derived cells is further demonstrated by the disease condition synovial chondromatosis, in which cartilaginous nodules form within the synovium, further suggesting a high chondrogenic potency of synovium.42 Further, synovial cells and chondrocytes produce similar proteins, including cartilage oligomeric matrix protein (COMP), link proteins, and sulfated GAGs.11 Recent studies are focusing on utilizing an SDSC-derived extracellular matrix to serve as an in vitro 3D microenvironment to promote SDSC proliferation and eventual chondrogenesis. Initial results indicate that this 3D environment coupled with growth factor application and a hypoxia may play a key role in recreating an in vitro tissue-specific microenvironment to improve cartilage repair strategies.18,43
Future studies will focus on incorporating physiologic loading bioreactors to expedite functional cartilage growth. Our group has previously demonstrated that chemical and mechanical stimuli (dynamic deformational loading) can act synergistically to further enhance cartilage tissue properties produced by juvenile bovine chondrocytes.5,44 As collagen levels in this study are low compared to native cartilage, application of controlled enzymatic digestion of engineered constructs will be explored in the future, as this strategy has been demonstrated to significantly increase collagen content of chondrocyte-seeded agarose constructs.20,27 Additionally, studies will be extended to using cells from skeletally mature animals that are more clinically relevant.
The synovium tissue is clinically attractive since it can be harvested with minimal invasiveness by clinicians during arthroscopy and thereby provides a source for autologous cells, without causing complications at the donor site due to its high regenerative capacity.45 However, due to the immunoprivileged nature of the joint that permits the use of allografts in cartilage repair, allogeneic SDSC cells could also be used, similar to the chondrocyte strategies being explored in clinical practice by Zimmer/Isto (DeNovo®).22 In conclusion, the findings of this study show that SDSCs provide a potential, clinically relevant source of cells for regenerative strategies for articular cartilage and that priming these cells with growth factors in 2D culture promotes chondrogenesis.
Authors' Contributions
All authors contributed to the conception and design of the study, collection and analysis of data, and drafting and revising the article, and gave final approval of this submitted work.
Acknowledgments
This work was supported by grants from the National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS) of the U.S. National Institutes of Health (NIH) (AR46568 and AR52871, C.T.H.).
Disclosure Statement
The authors certify that there is no conflict of interest related to the work presented in this article.
References
- 1.Guilak F. Sah R.L. Setton L.A. Physical regulation of cartilage metabolism. In: Mow V.C., editor; Hayes W.C., editor. Basic Orthopaedic Biomechanics. New York: Raven Press; 1997. pp. 197–207. [Google Scholar]
- 2.Mow V.C. Bachrach N.M. Setton L.A. Guilak F. Stress, strain, pressure, and flow fields in articular cartilage and chondrocytes. In: Mow V.C., editor; Guilak F., editor; Hayes W.C., editor. Cell Mechanics and Cellular Engineering. New York: Springer-Verlag; 1994. pp. 345–379. [Google Scholar]
- 3.Ronziere M.C. Perrier E. Mallein-Gerin F. Freyria A.M. Chondrogenic potential of bone marrow- and adipose tissue-derived adult human mesenchymal stem cells. Biomed Mater Eng. 2010;20:145. doi: 10.3233/BME-2010-0626. [DOI] [PubMed] [Google Scholar]
- 4.Moutos F.T. Estes B.T. Guilak F. Multifunctional hybrid three-dimensionally woven scaffolds for cartilage tissue engineering. Macromol Biosci. 2010;10:1355. doi: 10.1002/mabi.201000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lima E.G. Bian L. Ng K.W. Mauck R.L. Byers B.A. Tuan R.S., et al. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15:1025. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang A.H. Stein A. Mauck R.L. Evaluation of the complex transcriptional topography of mesenchymal stem cell chondrogenesis for cartilage tissue engineering. Tissue Eng Part A. 2010;16:2699. doi: 10.1089/ten.tea.2010.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong Z. Xiong H. Long X. Wei L. Li J. Wu Y., et al. Use of synovium-derived stromal cells and chitosan/collagen type I scaffolds for cartilage tissue engineering. Biomed Mater. 2010;5:055005. doi: 10.1088/1748-6041/5/5/055005. [DOI] [PubMed] [Google Scholar]
- 8.Buxton A.N. Bahney C.S. Yoo J. Johnstone B. Temporal exposure to chondrogenic factors modulates human mesenchymal stem cell chondrogenesis in hydrogels. Tissue Eng Part A. 2011;17:371. doi: 10.1089/ten.tea.2009.0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed N. Taylor D.W. Wunder J. Nagy A. Gross A.E. Kandel R.A. Passaged human chondrocytes accumulate extracellular matrix when induced by bovine chondrocytes. J Tissue Eng Regen Med. 2010;4:233. doi: 10.1002/term.235. [DOI] [PubMed] [Google Scholar]
- 10.Gavenis K. Schmidt-Rohlfing B. Mueller-Rath R. Andereya S. Schneider U. In vitro comparison of six different matrix systems for the cultivation of human chondrocytes. In Vitro Cell Dev Biol Anim. 2006;42:159. doi: 10.1290/0511079.1. [DOI] [PubMed] [Google Scholar]
- 11.Pei M. He F. Vunjak-Novakovic G. Synovium-derived stem cell-based chondrogenesis. Differentiation. 2008;76:1044. doi: 10.1111/j.1432-0436.2008.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han H.S. Lee S. Kim J.H. Seong S.C. Lee M.C. Changes in chondrogenic phenotype and gene expression profiles associated with the in vitro expansion of human synovium-derived cells. J Orthop Res. 2010;28:1283. doi: 10.1002/jor.21129. [DOI] [PubMed] [Google Scholar]
- 13.Bilgen B. Orsini E. Aaron R.K. Ciombor D.M. FBS suppresses TGF-beta1-induced chondrogenesis in synoviocyte pellet cultures while dexamethasone and dynamic stimuli are beneficial. J Tissue Eng Regen Med. 2007;1:436. doi: 10.1002/term.56. [DOI] [PubMed] [Google Scholar]
- 14.Fox D.B. Warnock J.J. Stoker A.M. Luther J.K. Cockrell M. Effects of growth factors on equine synovial fibroblasts seeded on synthetic scaffolds for avascular meniscal tissue engineering. Res Vet Sci. 2009;88:326. doi: 10.1016/j.rvsc.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Pei M. He F. Kish V.L. Vunjak-Novakovic G. Engineering of functional cartilage tissue using stem cells from synovial lining: a preliminary study. Clin Orthop Relat Res. 2008;466:1880. doi: 10.1007/s11999-008-0316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kock L.M. Schulz R.M. van Donkelaar C.C. Thummler C.B. Bader A. Ito K. RGD-dependent integrins are mechanotransducers in dynamically compressed tissue-engineered cartilage constructs. J Biomech. 2009;42:2177. doi: 10.1016/j.jbiomech.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 17.Selmi T.A. Verdonk P. Chambat P. Dubrana F. Potel J.F. Barnouin L., et al. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Joint Surg Br. 2008;90:597. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- 18.Ng K.W. Lima E.G. Bian L. O'Conor C.J. Jayabalan P.S. Stoker A.M., et al. Passaged adult chondrocytes can form engineered cartilage with functional mechanical properties: a canine model. Tissue Eng Part A. 2010;16:1041. doi: 10.1089/ten.tea.2009.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mauck R.L. Soltz M.A. Wang C.C. Wong D.D. Chao P.H. Valhmu W.B., et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 20.Ng K.W. Kugler L.E. Doty S.B. Ateshian G.A. Hung C.T. Scaffold degradation elevates the collagen content and dynamic compressive modulus in engineered articular cartilage. Osteoarthritis Cartilage. 2009;17:220. doi: 10.1016/j.joca.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genzyme Announces Positive Results from Carticel(R) Study at Major Sports Medicine Meeting. Genzyme-Carticel press release. 2007. www.medicalnewstoday.com/releases/76976.php www.medicalnewstoday.com/releases/76976.php
- 22.Zimmer Holdings and ISTO Technologies Announce Start of Neocartilage Clinical Trial. Zimmer-Isto Press Release. 2007. www.zimmer.com/z/ctl/op/global/action/1/id/9707/template/CP www.zimmer.com/z/ctl/op/global/action/1/id/9707/template/CP
- 23.Barbero A. Ploegert S. Heberer M. Martin I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003;48:1315. doi: 10.1002/art.10950. [DOI] [PubMed] [Google Scholar]
- 24.Farndale R.W. Sayers C.A. Barrett A.J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 25.Stegemann H. Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 26.Huang A.H. Stein A. Tuan R.S. Mauck R.L. Transient exposure to transforming growth factor beta 3 improves the mechanical properties of mesenchymal stem cell-laden cartilage constructs in a density-dependent manner. Tissue Eng Part A. 2009;15:3461. doi: 10.1089/ten.tea.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bian L. Crivello K.M. Ng K.W. Xu D. Williams D.Y. Ateshian G.A., et al. Influence of temporary chondroitinase ABC-induced glycosaminoglycan suppression on maturation of tissue-engineered cartilage. Tissue Eng Part A. 2009;15:2065. doi: 10.1089/ten.tea.2008.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauck R.L. Seyhan S.L. Ateshian G.A. Hung C.T. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng. 2002;30:1046. doi: 10.1114/1.1512676. [DOI] [PubMed] [Google Scholar]
- 29.Connelly J.T. Wilson C.G. Levenston M.E. Characterization of proteoglycan production and processing by chondrocytes and BMSCs in tissue engineered constructs. Osteoarthritis Cartilage. 2008;16:1092. doi: 10.1016/j.joca.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erickson I.E. Huang A.H. Chung C. Li R.T. Burdick J.A. Mauck R.L. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Eng Part A. 2009;15:1041. doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang A.H. Hung C.T. Mauck R.L. Functional Cartilage Tissue Engineering with Adult Stem Cells: Current Status and Future Direction. Stem Cell and Regenerative Medicine. Oak Park, IL: Bentham Science Publishers; 2010. pp. 49–79. [Google Scholar]
- 32.Francioli S.E. Martin I. Sie C.P. Hagg R. Tommasini R. Candrian C., et al. Growth factors for clinical-scale expansion of human articular chondrocytes: relevance for automated bioreactor systems. Tissue Eng. 2007;13:1227. doi: 10.1089/ten.2006.0342. [DOI] [PubMed] [Google Scholar]
- 33.Solchaga L.A. Penick K. Porter J.D. Goldberg V.M. Caplan A.I. Welter J.F. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203:398. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi A. Regulation of differentiation pathway of skeletal mesenchymal cells in cell lines by transforming growth factor-beta superfamily. Semin Cell Biol. 1995;6:165. doi: 10.1006/scel.1995.0023. [DOI] [PubMed] [Google Scholar]
- 35.Loeser R.F. Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin Geriatr Med. 2010;26:371. doi: 10.1016/j.cger.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keene D.R. Engvall E. Glanville R.W. Ultrastructure of type VI collagen in human skin and cartilage suggests an anchoring function for this filamentous network. J Cell Biol. 1988;107:1995. doi: 10.1083/jcb.107.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakaguchi Y. Sekiya I. Yagishita K. Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 38.Dominici M. Le Blanc K. Mueller I. Slaper-Cortenbach I. Marini F. Krause D., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 39.Grogan S.P. Barbero A. Diaz-Romero J. Cleton-Jansen A.M. Soeder S. Whiteside R., et al. Identification of markers to characterize and sort human articular chondrocytes with enhanced in vitro chondrogenic capacity. Arthritis Rheum. 2007;56:586. doi: 10.1002/art.22408. [DOI] [PubMed] [Google Scholar]
- 40.Fan J. Ren L. Liang R. Gong Y. Cai D. Wang D.A. Chondrogenesis of synovium-derived mesenchymal stem cells in photopolymerizing hydrogel scaffolds. J Biomater Sci Polym Ed. 2010;21:1653. doi: 10.1163/092050609X12531835454314. [DOI] [PubMed] [Google Scholar]
- 41.Hunziker E.B. Rosenberg L.C. Repair of partial-thickness defects in articular cartilage: cell recruitment from the synovial membrane. J Bone Joint Surg Am. 1996;78:721. doi: 10.2106/00004623-199605000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Maurice H. Crone M. Watt I. Synovial chondromatosis. J Bone Joint Surg Br. 1988;70:807. doi: 10.1302/0301-620X.70B5.3192585. [DOI] [PubMed] [Google Scholar]
- 43.He F. Chen X. Pei M. Reconstruction of an in vitro tissue-specific microenvironment to rejuvenate synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A. 2009;15:3809. doi: 10.1089/ten.TEA.2009.0188. [DOI] [PubMed] [Google Scholar]
- 44.Bian L. Fong J.V. Lima E.G. Stoker A.M. Ateshian G.A. Cook J.L., et al. Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Eng Part A. 2010;16:1781. doi: 10.1089/ten.tea.2009.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Theoret C.L. Barber S.M. Moyana T. Townsend H.G. Archer J.F. Repair and function of synovium after arthroscopic synovectomy of the dorsal compartment of the equine antebrachiocarpal joint. Vet Surg. 1996;25:142. doi: 10.1111/j.1532-950x.1996.tb01390.x. [DOI] [PubMed] [Google Scholar]