Abstract
Purpose
To determine the risk of progression to advanced age-related macular degeneration (AMD) conferred by reticular pseudodrusen (RPD), an imaging presentation of reticular macular disease (RMD), in high-risk fellow eyes of subjects with AMD and unilateral choroidal neovascularization (CNV) in a large prospective study.
Design
Cohort study.
Participants
271 subjects with AMD; 94 with RPD and 177 without RPD.
Methods
We studied images from a cohort of 271 subjects with AMD in the NAT 2 Study, a 3-year prospective study of subjects with unilateral CNV and large soft drusen in the fellow eye. The fellow eye, at high risk for developing advanced AMD, was the study eye. There were 5 visits per subject. Imaging at each visit consisted of color, red free, and blue light photography and fluorescein angiography. We analyzed the images for the presence of RPD, following disease progression throughout the 3-year study.
Main Outcome Measures
The development of advanced AMD (CNV or geographic atrophy).
Results
For the 271 subjects who completed the full 3-year study, there was a significantly higher rate of advanced AMD (56% or 53/94) in fellow eyes with RPD at any visit compared to eyes without RPD (32% or 56/177; p = 7.7E-05, χ2 test; relative risk (RR) 1.8; 95% confidence interval (CI) 1.4–2.4). The chance of developing advanced AMD in the fellow eye in females with RPD (66%) was more than double compared to females without RPD (30%, p = 5.1E-06, RR 2.2, 95% CI 1.6–3.1).
Conclusion
To our knowledge, this is the first comprehensive prospective study of reticular macular disease (RMD), a distinct clinical phenotype of AMD which includes RPD. It provides strong confirmation that RMD, a disease entity with stereotypical presentations across imaging modalities, is associated with high risk of progression to advanced AMD, perhaps on an inflammatory or vascular basis. RMD deserves wider recognition and consideration by clinicians caring for patients with AMD.
INTRODUCTION
Reticular pseudodrusen (RPD), described in the Wisconsin Grading System as yellowish, interlacing networks most frequently occurring along the arcades,1 were first identified in blue light photography.2 Subsequent studies have attempted to elucidate the natural course and imaging appearance of this disease entity. RPD historically have been associated with neovascular age-related macular degeneration (AMD),3–5 although a more recent epidemiological study linked their appearance to the development of late-stage AMD without preferential progression to neovascular versus atrophic AMD.6 Patients with RPD have also been shown to have higher mortality rates, even after controlling for factors such as cardiovascular, oncologic, or neurologic diseases.6
The advent of newer imaging modalities has led to the development of a unified description of reticular macular disease (RMD), defined by the presence of RPD in color fundus photography and/or a distinct reticular pattern in scanning laser ophthalmoscope (SLO) photography.7 In addition to an implication of the choriocapillaris in the etiology of RMD,1, 7–8 spectral domain optical coherence tomography (SD-OCT) has recently associated RPD with hyperreflective signals from above the retinal pigment epithelium (RPE).9–12 Hence, multiple retinal layers may be involved.
After SD-OCT findings and review of pathological specimens, one research group has suggested use of the term “subretinal drusenoid deposits” instead of “reticular pseudodrusen.”9–11 However, they compared their SD-OCT findings to previously published histopathologic findings in three unrelated eyes showing subretinal deposits, none of which had a clinical diagnosis of RPD.9 Arnold et al. performed the first histopathologic correlation in an eye with a clinical diagnosis of RPD, which showed significant loss of the small vessels of the middle choroidal layer and increased spacing between the large choroidal veins, leading them to postulate that choroidal vascular disease leads to the development of RPD.3 Recently, Sarks et al. reported pathology from an eye clinically diagnosed with RPD.13 They found that RPD may correlate with material found in the subretinal space, yet they also stated that subretinal deposits are occasionally found in eyes with AMD but without a clinical diagnosis of RPD and therefore kept the term “reticular pseudodrusen.” Until the matter is settled definitively, we prefer to use the more general term “reticular macular disease” (“RMD”) to refer to the disease process and the term “reticular pseudodrusen” (“RPD”) to refer to its specific presentation in color, red free, and blue light photography.
RMD is not uncommon, exhibiting an overall prevalence of 0.7% in the general population and an incidence that increases with age paralleling the incidence of AMD, from 0.4% in patients 43 to 54 years of age to 6.6% in patients 75 to 86 years of age.6 Given the correlation of RMD with poorer survival rates and outcomes in AMD,2, 6 a better understanding of the pathophysiology and natural history of RMD could aid in understanding the AMD process and perhaps other medical problems as well. Our study, a large prospective study of high-risk eyes with RMD, followed 271 subjects, 94 of whom were identified as having RMD on the basis of color, red free, and blue light images, over the course of 3 years. We found a greater incidence of progression to choroidal neovascularization (CNV) and/or geographic atrophy (GA) among subjects with RMD compared to subjects without RMD. We also found that reticular patterns tended to disappear after development of CNV, as has been previously noted.5, 7, 13
METHODS
Subjects
We used data from the NAT 2 Study, sponsored b y Bausch & Lomb, Inc. (Montpellier, France), which had a 3-year follow-up duration for each subject. The research adhered to the tenets of the Declaration of Helsinki and was approved by the Medical Ethics Committee of L’Hôpital Intercommunal de Créteil (Créteil, France). The NAT 2 Study was a prospective, single-center, double-blind, randomized, parallel, placebo-controlled, comparative study. The dataset consisted of subjects 55 to 85 years of age (at the time of the baseline examination) who presented with unilateral CNV and lesions of age-related maculopathy (confluent and/or diffuse soft drusen with or without pigment changes and/or RPD) in the fellow eye. Subjects, therefore, were at high risk for developing CNV in the fellow eye, which was the study eye. The NAT 2 Study compared treatment with a proprietary drug (docosahexaenoic acid (DHA), an omega-3 fatty acid oral supplementation) versus a placebo (olive oil) for the prevention of advanced AMD. The primary end point of the NAT 2 Study occurred when the subject developed CNV in the study eye. We do not have results regarding the possible effects of the proprietary drug, and the results were not available when the images were analyzed.
There were 5 visits per subject: a screening visit (baseline) and 4 follow-up visits (at 6 months and at the end of years 1, 2, and 3). Imaging consisted of color, red free, and blue light photography (at each visit) and fluorescein angiography (FA) (at the baseline examination and then yearly). Images for each subject were initially reviewed by trained graders (MAS, NP), and gradings were subsequently verified by senior graders (RTS, EHS) to confirm the presence of RPD and the classification of patients into either early-stage (grades 2A, 2B, and 3) or late-stage (grades 4A, 4B, and 4C) AMD, according to the international classification system.14 FA allowed detection of CNV and specification of CNV type (classic or occult). In cases where CNV was suspected in the study eye, indocyanine green angiography (ICGA) was performed to confirm the presence of CNV or another disease process. We concluded that GA was present if there were one or more distinct areas (greater than or equal to 500 µm) of RPE loss showing depigmentation of the RPE and prominent choroidal vessels in fundus photography. We included extrafoveal and foveal GA within the central 6000-µm-diameter circle.
Image Acquisition
We acquired color fundus, red free, and blue light photography and fluorescein angiograms digitally on the Topcon TRC-50IA Retinal Fundus Camera (Topcon, Tokyo, Japan). ICGA images of selected subjects were obtained using the Heidelberg Retina Angiograph (HRA)/HRA2 Confocal SLO (Heidelberg Engineering, Inc., Dossenheim, Germany).
Definitions for Multimodal Image Analysis of RPD
RPD, identified in color, red free, and blue light fundus photographs as yellow or light interlacing networks, range from 125 to 250 µm in width (Fig 1).3 Lesions appear in relatively low contrast and occur in regular patterns and well-defined domains generally in the superior arcades.7 FA images were available for all subjects but were examined for supplemental information only and were not used to define RPD, as lesions which appear similar to RPD in FA images are common to other disease processes. We analyzed all images in Adobe Photoshop CS3 (Adobe Systems, Inc., San Jose, CA). For color fundus and red free photographs, we used the standardized autocontrast tool to verify the presence of RPD.7 This technique can be seen in Fig 2. Because monochromatic blue light photographs reportedly improve the visualization of RPD in fundus photography,3, 4, 9–11 we examined the color photographs in the blue channel, as well as the blue light photographs, to distinguish soft drusen from RPD.
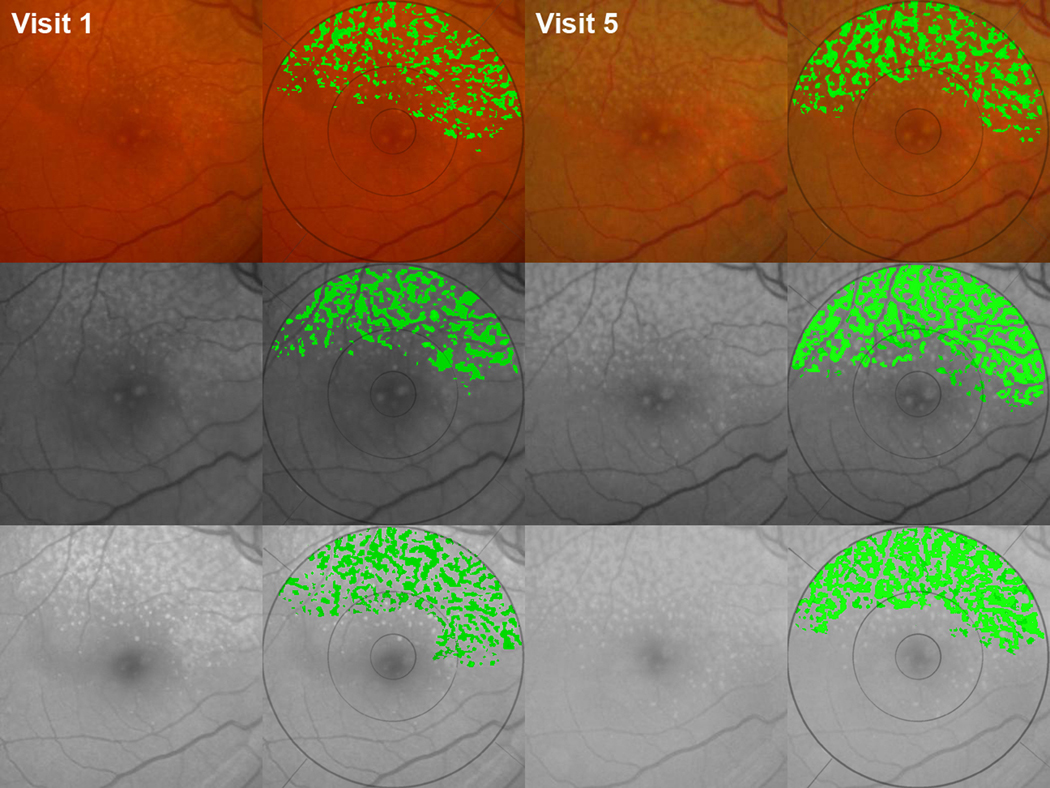
Figure 1. Identifying and tracking progression of reticular pseudodrusen (RPD).
RPD are yellow or light interlacing networks ranging from 125 to 250 µm in width in color, red free, and blue light fundus photos;3 lesions are relatively low contrast and occur in regular patterns with well-defined domains.7 This study eye showed a reticular pattern in all 3 imaging modalities: contrast-enhanced color (top row), red free (middle row), and blue light (bottom row) photos, all of which were precisely registered to each other and cropped.15 The fellow eye (not shown) had choroidal neovascularization. (Column 1) Initial images, visit 1. (Column 2) Our user-interactive drusen segmentation tool applied in the 6000-µm region.16 The reticular pattern is displayed in green. (Columns 3, 4) Follow-up images after 3 years document the progression of RPD without conversion to advanced age-related macular degeneration (AMD).
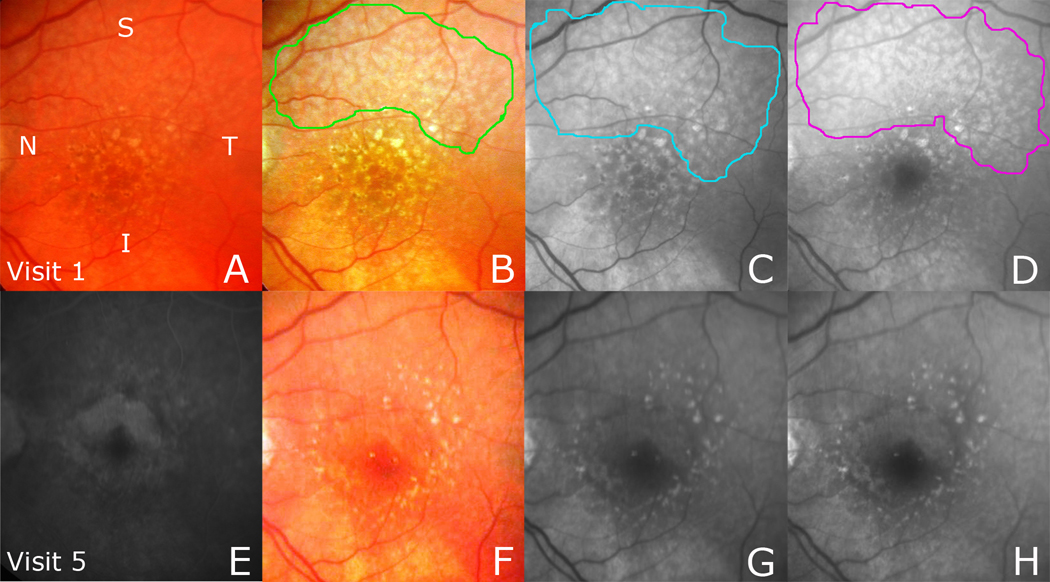
Figure 2. Reticular pseudodrusen (RPD) fading with development of choroidal neovascularization (CNV).
(Top row) A. Original color photo with RPD inapparent. B. The contrast-enhanced color fundus (CF) photo shows a reticular pattern in 2 quadrants, outlined in green, with noticeable large soft drusen and pigment. The red free (RF) photo (C) and the blue light photo (D), registered to the CF photo, show a reticular pattern in 3 quadrants, outlined in blue and magenta, respectively. (Bottom row) Follow-up images were obtained from visit 5 (after 3 years), during which time occult CNV was detected on fluorescein angiography (FA). E. The FA image showing CNV. The reticular patterns in the enhanced CF photo (F), RF photo (G), and blue light photo (H) are faint, even in the areas not involved by exudation.
Statistical Analysis
We wanted to determine whether subjects with RPD in our dataset developed advanced AMD at a higher rate than those who did not have RPD. We separated late-stage AMD into its two subtypes, GA and CNV. Data were analyzed using the χ2 test for categorical variables in univariate analysis to determine the association of RMD with progression to late-stage AMD. Our χ2 test and relative risk calculations were performed using the statistical program R (R Foundation for Statistical Computing, Vienna, France). We used SPSS (SPSS, Inc., Chicago, IL) to perform the Mann–Whitney non-parametric test to compare the statistical distribution of parameters such as age and gender.
RESULTS
The 3-year NAT 2 Study yielded complete data for 271 subjects out of a total of 300. The 29 subjects who did not complete the study were lost to follow up, with one recorded death among them (female, age 86); these subjects were demographically similar to those who completed the full study (72.4%, or 21/29, female with an average age of 75 +/− 5.8 years). Among the subjects who completed the study, there was a significantly higher rate of progression to late-stage AMD in eyes with RPD at any visit (56% or 53/94) compared to eyes without RPD (32% or 56/177; p = 7.7E-05, χ2 test; relative risk (RR) 1.8; 95% confidence interval (CI) 1.4–2.4). We also separately calculated the incidence of the two types of late-stage AMD (GA and CNV) and found a significant rate of subsequent CNV in eyes with RPD (45% or 42/94) compared to eyes without RPD (27% or 47/177, p = 0.002, RR 1.7, 95% CI 1.2–2.4). Significantly more subjects with RPD (20% or 19/94) progressed to GA compared to subjects without RPD (10% or 18/177, p = 0.02, RR 2.0, 95% CI 1.1–3.6). Among subjects with both types of late-stage AMD, there was no significant difference in progression between subjects with RPD (9% or 8/94) and subjects without RPD (5% or 9/177, p = 0.27, RR 1.7, 95% CI 0.7–4.2).
Serial imaging showed faded to absent reticular findings in all eyes that developed CNV (Fig 2). We examined the types of CNV present in subjects with and without RPD. Among the subjects with CNV and RPD, 79% (33/42) had occult CNV, and 21% (9/42) had classic CNV. There were similar findings among the subjects with CNV but without RPD, with 74% (35/47) occult and 26% (12/47) classic. Of the subjects with RPD who developed GA, almost all had the multilobular form, with lobules coalescing to central GA in the midst of the reticular pattern (Fig 3). In contrast to the eyes that developed CNV, the reticular pattern did not fade in the eyes that developed GA. We found no significant difference in the development rates of CNV and GA between subjects with RPD and those without RPD. The CNV development rate was 1.5 years from baseline for subjects with RPD and 1.6 years for subjects without RPD (p = 0.65). The GA development rate was 0.94 years for subjects with RPD and 1.08 years for subjects without RPD (p = 0.53). These rates can be found in Table 1.
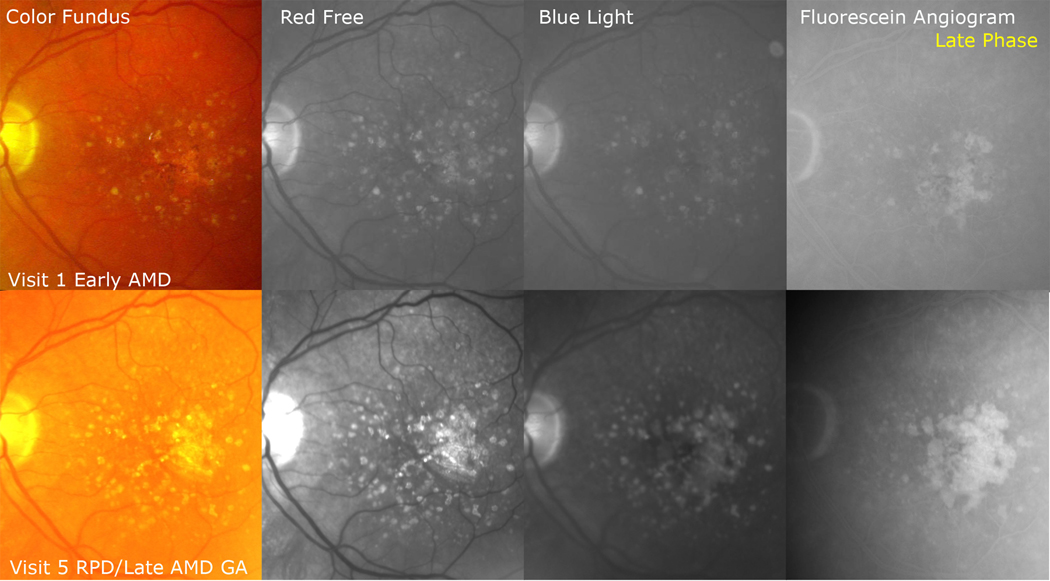
Figure 3. Reticular pseudodrusen with development of geographic atrophy (GA).
(Top row) This study eye initially showed large soft drusen and pigment (early age-related macular degeneration [AMD]) in the color photo and a faint reticular pattern superiorly in the red free and blue light images. The fluorescein angiogram (FA) showed late staining of coalescent soft drusen. (Bottom row) By visit 5, the reticular pattern was more prominent in the enhanced color, red free, and blue light images and surrounded multiple lobules of GA coalescing centrally. The FA shows sharply defined, scalloped lesions with baring of large choroidal vessels characteristic of GA, and the multilobular character of the GA lesions is apparent.
Table 1.
Statistical analysis of the NAT 2 Study comparing subjects with reticular macular disease (RMD) versus subjects without RMD.
| Characteristics | All Subjects | Subjects Without RMD |
Subjects With RMD |
P Value (χ2 test) |
RR (95% CI) |
|---|---|---|---|---|---|
| Sample Size | 271 | 177 | 94 | ||
| Age, Yrs, Mean (SD) | 74.1 (6.7) | 73.8 (6.9) | 74.4 (6.5) | 0.53 | |
| Women, % | 63.1 (171/271) | 60.5 (107/177) | 68.1 (64/94) | 0.19 | |
| Late AMD Progression, % | 40.2 (109/271) | 31.6 (56/177) | 56.4 (53/94) | 7.70E-05 | 1.8 (1.4, 2.4) |
| Progression to CNV, % | 32.8 (89/271) | 26.6 (47/177) | 44.7 (42/94) | 0.002 | 1.7 (1.2, 2.4) |
| Progression to GA, % | 13.6 (37/271) | 10.2 (18/177) | 20.2 (19/94) | 0.02 | 2.0 (1.1, 3.6) |
| Progression to Both CNV & GA, % | 6.3 (17/271) | 5.1 (9/177) | 8.5 (8/94) | 0.27 | 1.7 (0.7, 4.2) |
| Late AMD Progression, Females, % | 43.3 (74/171) | 29.9 (32/107) | 65.6 (42/64) | 5.10E-06 | 2.2 (1.6, 3.1) |
| Late AMD Progression, Males, % | 35.0 (35/100) | 34.3 (24/70) | 36.7 (11/30) | 0.82 | 1.1 (0.6, 2.0) |
| CNV Development Rate, Yrs | 1.55 | 1.60 | 1.50 | 0.65 | |
| GA Development Rate, Yrs | 1.04 | 1.08 | 0.94 | 0.53 | |
| AGE 55–65 | All Subjects |
Subjects Without RMD |
Subjects With RMD |
P Value |
RR (95% CI) |
| Sample Size | 32 | 23 | 9 | ||
| Age, Yrs, Mean (SD) | 60.7 (3.2) | 60.7 (3.4) | 60.9 (2.8) | 0.87 | |
| Women, % | 65.6 (21/32) | 78.2 (18/23) | 33.3 (3/9) | 0.05 | |
| Late AMD Progression, % | 34.3 (11/32) | 30.4 (7/23) | 44.4 (4/9) | 0.45 | 1.5 (0.6, 3.8) |
| Late AMD Progression, Females, % | 20.8 (5/24) | 22.2 (4/18) | 16.7 (1/6) | 0.77 | 0.8 (0.1, 5.5) |
| Late AMD Progression, Males, % | 50.0 (4/8) | 40.0 (2/5) | 66.7 (2/3) | 0.47 | 1.7 (0.4, 6.4) |
| CNV Development Rate, Yrs | 1.31 | 1.25 | 1.50 | 0.73 | |
| GA Development Rate, Yrs | 1.58 | 1.17 | 2.00 | 0.64 | |
| AGE 65–75 | All Subjects |
Subjects Without RMD |
Subjects With RMD |
P Value |
RR (95% CI) |
| Sample Size | 109 | 72 | 37 | ||
| Age, Yrs, Mean (SD) | 71.3 (2.8) | 71.3 (2.8) | 71.2 (2.8) | 0.99 | |
| Women, % | 62.4 (68/109) | 61.1 (44/72) | 64.9 (24/37) | 0.60 | |
| Late AMD Progression, % | 36.7 (40/109) | 30.6 (22/72) | 48.6 (18/37) | 0.06 | 1.6 (1.0, 2.6) |
| Late AMD Progression, Females, % | 38.2 (26/68) | 27.2 (12/44) | 58.3 (14/24) | 0.01 | 2.1 (1.2, 3.9) |
| Late AMD Progression, Males, % | 34.1 (14/41) | 35.7 (10/28) | 30.8 (4/13) | 0.76 | 0.9 (0.3, 2.2) |
| CNV Development Rate, Yrs | 1.51 | 1.66 | 1.35 | 0.37 | |
| GA Development Rate, Yrs | 0.95 | 1.07 | 0.75 | 0.76 | |
| AGE 75–85 | All Subjects |
Subjects Without RMD |
Subjects With RMD |
P Value |
RR (95% CI) |
| Sample Size | 130 | 82 | 48 | ||
| Age, Yrs, Mean (SD) | 79.5 (2.7) | 79.6 (2.7) | 79.4 (2.7) | 0.57 | |
| Women, % | 63.1 (82/130) | 54.9 (45/82) | 77.1 (37/48) | 0.01 | |
| Late AMD Progression, % | 44.6 (58/130) | 32.9 (27/82) | 64.6 (31/48) | 0.0004 | 2.0 (1.4, 2.9) |
| Late AMD Progression, Females, % | 52.4 (43/82) | 35.6 (16/45) | 73 (27/37) | 0.0007 | 2.1 (1.3, 3.2) |
| Late AMD Progression, Males, % | 29.2 (14/48) | 27.0 (10/37) | 36.4 (4/11) | 0.55 | 1.4 (0.5, 3.5) |
| CNV Development Rate, Yrs | 1.62 | 1.68 | 1.61 | 0.89 | |
| GA Development Rate, Yrs | 0.93 | 0.75 | 1.13 | 0.40 | |
Abbreviations: RMD, reticular macular disease; RR, relative risk; CI, confidence interval; yrs, years; SD, standard deviation; AMD, age-related macular degeneration; CNV, choroidal neovascularization; GA, geographic atrophy.
We reviewed the non-study eye for the presence of RPD. Of the subjects with RPD and no progression to advanced AMD in the study eye, 29.3% (12/41) displayed a reticular pattern in the non-study eye. Of the subjects with RPD and eventual signs of advanced AMD in the study eye, 43.4% (23/53) exhibited a reticular pattern in the non-study eye. All reticular patterns in non-study eyes faded over the course of the 3-year study. Subjects without RPD in the non-study eye showed no reticular pattern throughout the 5 visits.
When reviewing gender patterns, we found that 63% (171/271) of all subjects, 68% (64/94) of subjects with RPD, and 61% (107/171) of subjects without RPD were female. We found that, among females, eyes with RPD had more than double the chance of developing advanced AMD (66%) compared to eyes without RPD (30%, p = 5.1E-06, RR 2.2, 95% CI 1.6–3.1). Among males, there was no significant difference in progression to advanced AMD between eyes with RPD and eyes without RPD.
To observe any significance regarding age, we separated our cohort into 3 categories by decades: 55–65, 65–75, and 75–85 years of age. In the early age group (ages 55–65) we found no significant difference in the rate of progression to late AMD between the subjects with RPD and those without RPD. However, in the middle category (ages 65–75), we noted a trend to a higher rate of progression to late-stage AMD among subjects with RPD (49%) compared to subjects without RPD (31%, p = 0.06, RR 1.6, 95% CI 1.0–2.6). This trend increased to statistical significance in the oldest age group (ages 75–85), which exhibited an even higher rate of progression to late-stage AMD for subjects with RPD (65%) versus subjects without RPD (33%, p = 0.0004, RR 2.0, 95% CI 1.4–2.9).
Considering gender and age jointly showed that a significantly higher percentage of eyes of female subjects with RPD progressed to late-stage AMD (58%) compared to eyes of female subjects without RPD (27%, p = 0.01, RR 2.1, 95% CI 1.2–3.9) in the middle age group, as well as the older age group (72% vs. 36%, p = 0.001, RR 2.4, 95% CI 1.3–4.3). In the older age group, we found that 77% (37/48) of the subjects with RPD were women, while only 55% (45/82) of the subjects without RPD were women. All of these results can be found in Table 1.
DISCUSSION
RMD, a sub-phenotype of AMD which includes RPD, has been shown to be associated with advanced AMD.3–7 This study, to our knowledge, is the first prospective study that examines high-risk eyes with early AMD and demonstrates significantly increased progression to both types of advanced AMD (CNV and GA) among such subjects with RMD. We found that 56% of eyes with RPD progressed to late-stage AMD: 45% to CNV, 20% to GA, and 9% to both. Only 32% of eyes without RPD pr ogressed to late-stage AMD: 27% to CNV, 10% to GA, and 5% to both. Previously, a study drawn from the data of the Age-Related Eye Diseases Study (AREDS) reported the clinical course of RPD in eyes with large soft drusen with and without pigment changes over 5 years of follow-up photographs, finding that 50% of eyes progressed to late-stage AMD: 29% to CNV, 36% to GA, and 15% to both (Armstrong JR, et al. IOVS 2005; 46:ARVO E-Abstract 220). These rates were not significantly different from those of similar subjects without RPD, although the rate of transformation to GA was higher. In the NAT 2 Study, we found a significant difference between eyes with RPD and eyes without RPD. The different results recorded in these studies may be attributable to the fact that our study tracked a group at higher risk, comprised of subjects who had already developed unilateral CNV, as opposed to the AREDS subjects, who had not yet developed advanced AMD in either eye. Thus, in our high-risk group, the adverse effect of RMD may have been more evident. Other reported rates of RMD were comparable between our present study and other previous studies. AREDS found the overall incidence of RPD to be 7%, while we previously reported an 8% incidence of RPD for non-advanced AMD patients.5 Several previous studies have documented the increased prevalence of RPD in females. Arnold et al. found that 87% of patients with RPD were women,3 while Klein et al. found the prevalence of RPD to be approximately 2.5 times greater in women than in men.6 In our Columbia Macular Genetics Study, the gender distribution of our group of subjects with RMD was 79% female and 21% male.7 In the 75–85 years of age category of the NAT 2 Study, we found that 77% of subjects with RPD were women, while only 55% of subjects without RPD were women. The current study not only confirms the increased incidence of RPD among women with AMD, but also demonstrates that females with RPD have roughly double the chance of progressing to the advanced stages of AMD compared to females without RPD.
An ongoing hypothesis about RMD suggests that it is an inflammatory disorder driving advanced AMD.5, 6 Accordingly, the higher incidence of RMD in females versus males in our study may reflect the greater susceptibility of females to autoimmune inflammatory disease.17 However, the etiology of RMD may be vascular, as the choriocapillaris has been implicated on ICG imaging.7 Future study for improved understanding of RMD etiology is warranted.
A second hypothesis concerns RMD’s association with increased mortality, suggesting that it is a manifestation of some severe systemic disease. If this disease were to present earlier in life and affect men more severely than women, e.g., if it were vascular, then affected men might have increased mortality before reaching the age of advanced AMD. Thus, the fact that, among AMD patients, more women than men present with RMD might simply reflect this increased earlier mortality of men rather than a true susceptibility to RMD for women.
A limitation of this study was the lack of SLO images to discern the presence of RMD. Previously, we demonstrated the characteristic appearances of RPD in color fundus, red free, indocyanine green, infrared, and autofluorescence images, noting improved identification and visualization of RMD lesions when using multiple imaging modalities.7 In the NAT 2 Study, however, we were limited to color, red free, and blue light photography. As a result, in the absence of infrared and autofluorescence imaging, some subjects with RPD may have been misclassified. Further, we observed the fading of RPD in all modalities as CNV developed, a general phenomenon noted in our previous study, which suggests another reason the frequency of these lesions may have been underestimated.7 Sarks et al.13 believe that the fading of RPD after CNV develops may be related to a wound healing process involving the migration of the RPE toward the area of injury.18, 19
This study was further limited by the unknown differential effect of DHA on the subjects within the NAT 2 Study, particularly as DHA is thought to delay the outcome of CNV in patients presenting with early stages of AMD.20, 21 Because the groups with RMD and without RMD were relatively large, and because DHA and placebo were assigned randomly, one would expect the distribution of DHA-treated subjects between these groups to have been roughly equal and therefore not likely to affect our results. However, this will not be certain until the final results of the NAT 2 study are published.
The strengths of our study were the large cohort size, the prospective design, and the multiple examination points at which to identify RMD and/or the progression of AMD.
In conclusion, we have demonstrated that RMD confers an increased risk of progression to advanced AMD in fellow eyes of patients with unilateral CNV beyond that of the previously established high-risk phenotype of large soft drusen and pigment. This adds to our expanding understanding of the significance of the RMD phenotype. While several recent and past studies have documented the strong association of RMD with advanced AMD, to our knowledge this is the first prospective study that begins with high-risk eyes with early AMD and demonstrates increased progression to advanced AMD among such subjects with RMD compared to subjects without RMD. Further study of the pathophysiology of RMD is clearly indicated, particularly with respect to possible mechanisms by which RMD could drive advanced AMD. Careful attention to identification of this high-risk phenotype in our patients with all available imaging modalities is recommended.
Acknowledgments
Financial Support: Supported by grants from The New York Community Trust (New York, NY), National Eye Institute (Bethesda, MD) grant R01 EY015520 (Smith), and unrestricted funds from Research to Prevent Blindness (New York, NY). The funding organizations had no role in the design or conduct of this research. Images from the NAT 2 Study were obtained from L’Hôpital Intercommunal de Créteil (Créteil, France), Eric H. Souied, MD, PhD. ISRCTN (numeric system for the unique identification of randomized controlled trials) number for the NAT 2 Study: 98246501.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Dr. Souied has financial interest as a consultant for Bausch & Lomb and Novartis and receives lecture fees from Alcon.
Conflict of Interest: No authors have any conflicting interest to disclose.
REFERENCES
- 1.Klein R, Davis MD, Magli YL, et al. The Wisconsin Age-Related Maculopathy Grading System. Ophthalmology. 1991;98:1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 2.Mimoun G, Soubrane G, Coscas G. Macular drusen [in French] J Fr Ophtalmol. 1990;13:511–530. [PubMed] [Google Scholar]
- 3.Arnold JJ, Sarks SH, Killingsworth MC, Sarks JP. Reticular pseudodrusen: a risk factor in age-related maculopathy. Retina. 1995;15:183–191. [PubMed] [Google Scholar]
- 4.Cohen SY, Dubois L, Tadayoni R, et al. Prevalence of reticular pseudodrusen in age-related macular degeneration with newly diagnosed choroidal neovascularization. Br J Ophthalmol. 2007;91:354–359. doi: 10.1136/bjo.2006.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith RT, Chan JK, Busuoic M, et al. Autofluorescence characteristics of early, atrophic, and high-risk fellow eyes in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47:5495–5504. doi: 10.1167/iovs.05-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein R, Meuer SM, Knudtson MD, et al. The epidemiology of retinal reticular drusen. Am J Ophthalmol. 2008;145:317–326. doi: 10.1016/j.ajo.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith RT, Sohrab MA, Busuioc M, Barile G. Reticular macular disease. Am J Ophthalmol. 2009;148:733–743. doi: 10.1016/j.ajo.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold JJ, Quaranta M, Soubrane G, et al. Indocyanine green angiography of drusen. Am J Ophthalmol. 1997;124:344–356. doi: 10.1016/s0002-9394(14)70826-8. [DOI] [PubMed] [Google Scholar]
- 9.Zweifel SA, Spaide RF, Curcio CA, et al. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010;117:303–312. doi: 10.1016/j.ophtha.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Zweifel SA, Imamura Y, Spaide TC, et al. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology. 2010;117:1775–1781. doi: 10.1016/j.ophtha.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Spaide RF, Curcio CA. Drusen characterization with multimodal imaging. Retina. 2010;30:1441–1454. doi: 10.1097/IAE.0b013e3181ee5ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schimtz-Valckenberg S, Steinberg JS, Fleckenstein M, et al. Combined confocal scanning laser ophthalmoscopy and spectral-domain optical coherence tomography imaging of reticular drusen associated with age-related macular degeneration. Ophthalmology. 2010;117:1169–1176. doi: 10.1016/j.ophtha.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 13.Sarks J, Arnold J, Ho IV, et al. Evolution of reticular pseudodrusen. Br J Ophthalmol. doi: 10.1136/bjo.2010.194977. In press. [DOI] [PubMed] [Google Scholar]
- 14.Bird AC, Bressler NM, Bressler SB, et al. International ARM Epidemiological Study Group. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Opthhalmol. 1995;39:367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Smith R, Tian J, Laine AF. A novel registration method for retinal images based on local features. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:2242–2245. doi: 10.1109/IEMBS.2008.4649642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith RT, Chan JK, Nagasaki T, et al. Automated detection of macular drusen using geometric background leveling and threshold selection. Arch Ophthalmol. 2005;123:200–206. doi: 10.1001/archopht.123.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh SJ, Rau LM. Autoimmune diseases: a leading cause of death among young and middle-aged women in the United States. Am J Public Health. 2000;90:1463–1466. doi: 10.2105/ajph.90.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarks J, Tang K, Killingsworth M, et al. Development of atrophy of the retinal pigment epithelium around disciform scars. Br J Ophthalmol. 2006;90:442–446. doi: 10.1136/bjo.2005.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarbin MA. Progressive RPE atrophy around disciform scars. Br J Ophthalmol. 2006;90:396–397. doi: 10.1136/bjo.2005.086041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Querques B, Benlian P, Chanu B, et al. Nutritional AMD treatment phase I (NAT-1): feasibility of oral DHA supplementation in age-related macular degeneration. Eur J Ophthalmol. 2009;19:100–106. doi: 10.1177/112067210901900115. [DOI] [PubMed] [Google Scholar]
- 21.SanGiovanni JP, Chew EY, Agrón E, et al. Age-Related Eye Disease Study Research Group. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch Ophthalmol. 2008;126:1274–1279. doi: 10.1001/archopht.126.9.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]