Abstract
Tissue injury during a critical period of early life can facilitate spontaneous glutamatergic transmission within developing pain circuits in the superficial dorsal horn (SDH) of the spinal cord. However, the extent to which neonatal tissue damage strengthens nociceptive synaptic input to specific subpopulations of SDH neurons, as well as the mechanisms underlying this distinct form of synaptic plasticity, remains unclear. Here we use in vitro whole-cell patch clamp recordings from rodent spinal cord slices to demonstrate that neonatal surgical injury selectively potentiates high-threshold primary afferent input to immature lamina II neurons. In addition, the increase in the frequency of miniature excitatory postsynaptic currents (mEPSCs) after hindpaw incision was prevented by neonatal capsaicin treatment, suggesting that early tissue injury enhances glutamate release from nociceptive synapses. This occurs in a widespread manner within the developing SDH, as incision elevated mEPSC frequency in both GABAergic and presumed glutamatergic lamina II neurons of Gad-GFP transgenic mice. The administration of exogenous nerve growth factor (NGF) into the rat hindpaw mimicked the effects of early tissue damage on excitatory synaptic function, while blocking trkA receptors in vivo abolished the changes in both spontaneous and primary afferent-evoked glutamatergic transmission following incision. These findings illustrate that neonatal tissue damage can alter the gain of developing pain pathways by activating NGF-dependent signaling cascades which modify synaptic efficacy at the first site of nociceptive processing within the CNS.
1. Introduction
Infants treated in neonatal intensive care units routinely experience tissue damage as a consequence of essential surgical and procedural interventions [3,50,52], which can evoke modality-specific cortical responses [51] and pain hypersensitivity [5,19,53]. However, the clinical management of pain in this population remains inadequate [1]. The design of new, evidence-based approaches to relieving pediatric pain is hampered by an incomplete understanding of how immature nociceptive circuits in the central nervous system (CNS) respond to tissue damage at a cellular and molecular level.
The experience-dependent maturation of the nociceptive withdrawal reflex in humans [4] and rodents [23,56] illustrates that pain networks in the CNS remain highly plastic during the neonatal period. It is also clear that the functional organization of the spinal superficial dorsal horn (SDH), which represents the first site of synaptic integration within the pain pathway, undergoes marked changes during early life [20]. For example, nociceptive primary afferent projections to the rat SDH progressively strengthen during the first ten postnatal days [6,18]. This raises the possibility that tissue damage incurred during this sensitive period can enhance nociceptive synaptic input to the developing SDH, either by modulating the excitability of the primary afferents [28] to promote activity-dependent synaptogenesis [26], or by increasing the availability of neurotrophic factors known to influence the growth of sensory fibers within the spinal cord [36]. In fact, anatomical studies show that peripheral inflammation in the neonate leads to an expansion in nociceptive projections to the dorsal horn [44,57]. However, it remains unclear if this results in an altered efficacy of nociceptive synapses within the SDH.
Early tissue damage does evoke an activity-dependent facilitation of spontaneous glutamatergic transmission within the developing SDH [30,31]. While the available evidence points to an elevated glutamate release under pathological conditions, the potential source of this glutamate remains unknown, as immature lamina II neurons receive synaptic inputs from both low-threshold and high-threshold primary afferents [38,40] as well as local excitatory interneurons within the SDH. In addition, since spinal projection neurons are absent from lamina II, the functional implications of the above changes in glutamatergic signaling for the overall output of the spinal pain network will depend on whether the enhanced glutamatergic drive occurs onto GABAergic and/or excitatory interneurons within the region [22].
The present results suggest that neonatal surgical injury increases the efficacy of synapses formed by high-threshold primary afferents within the immature SDH and thus strengthens nociceptive input onto both GABAergic and glutamatergic neurons in lamina II. The data also suggest that NGF-trkA interactions promote synaptic plasticity within spinal glutamatergic circuits under pathological conditions.
2. Materials and methods
All experiments adhered to animal welfare guidelines established by the University of Cincinnati Institutional Animal Care and Use Committee and the Committee for Research and Ethical Issues of the IASP.
2.1. Hindpaw surgical incision
Sprague-Dawley rat pups (postnatal days 2–3) or Gad-GFP mice (FVB-Tg(GadGFP)4570Swn; Jackson Labs; postnatal days 3–56) were anesthetized with isoflurane (2–3%) and a small incision made through the skin and underlying muscle of the plantar hindpaw as described previously [8]. The skin was immediately closed with 5-0 (adult mice), 6-0 (rats) or 7-0 (P3 mice) suture (Ethicon; Cornelia, GA).
2.2. Neonatal capsaicin treatment
At postnatal days (P)0 and P1, rats were anesthetized with isoflurane and capsaicin (50 mg/kg; Sigma, St. Louis, MO) or vehicle solution (80% saline, 10% EtOH and 10% Tween-20) was administered i.p.
2.3. Administration of nerve growth factor (NGF) to hindpaw
Rat pups were anesthetized with isoflurane and NGF (20 ng/μl; 2.5S from mouse; Fisher Scientific, Florence, KY) was injected subcutaneously into the plantar surface of the hindpaw (at 0.5 μl/g body weight) once daily for 2 days (i.e. at P3 and P4 or P17 and P18). Equivalent injections of vehicle solution (0.4% BSA in saline) were used as a control. Patch clamp recordings were obtained 1–2 days after the last injection was administered.
2.4. Administration of trkA inhibitor in vivo
VMD902 is a small organic molecule which selectively inhibits NGF-evoked activation of trkA receptor tyrosine kinase in cell-based functional assays with a potency in the low nanomolar range and suppresses mechanical allodynia and hyperalgesia in rodent models of nociceptive and neuropathic pain (VM Discovery, Inc., Fremont, CA). To examine the role of trkA signaling in injury-evoked synaptic plasticity within the SDH, VMD902 was injected i.p. at 100 mg/kg approximately 1 hour prior to the hindpaw incision (at P2) and again 24 hours later. Pups receiving equivalent injections of the vehicle solution (0.5% CMC-Na / 0.1% Tween-80 in dH2O) were used as a control group.
2.5. Preparation of spinal cord slices
Rats (P3–20) or Gad-GFP mice (P4–58) were deeply anesthetized with sodium pentobarbital (30 mg/kg) and then perfused with ice-cold dissection solution consisting of (in mM): 250 sucrose, 2.5 KCl, 25 NaHCO3, 1.0 NaH2PO4, 6 MgCl2, 0.5 CaCl2, and 25 glucose continuously bubbled with 95% O2 / 5% CO2. The lumbar spinal cord was isolated and immersed in low-melting-point agarose (3% in above solution; Invitrogen, Carlsbad, CA) and parasagittal slices (350–400 μm) were cut from the ipsilateral side using a Vibroslice tissue slicer (HA-752; Campden Instruments, Lafayette, IN). The slices were placed in a chamber filled with oxygenated dissection solution for 30 min then allowed to recover in an oxygenated artificial CSF (aCSF) solution containing the following (in mM): 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.0 NaH2PO4, 1.0 MgCl2, 2.0 CaCl2, and 25 glucose for ≥ 1 hour at room temperature.
2.6. Patch clamp recordings
After recovery, slices were transferred to a submersion-type recording chamber (RC-22; Warner Instruments, Hamden, CT) and mounted on the stage of an upright microscope (BX51WI, Olympus, Center Valley, PA) which was equipped with fluorescence to allow for the identification of GFP-expressing neurons. Slices were then perfused at room temperature with oxygenated aCSF at a rate of 1.5–3 ml/min.
Patch electrodes were constructed from thin-walled single-filamented borosilicate glass (1.5 mm outer diameter; World Precision Instruments, Sarasota, FL) using a microelectrode puller. Pipette resistances ranged from 4 to 6 MΩ and seal resistances were >1 GΩ. Patch electrodes were filled with a solution containing the following (in mM): 130 Cs-gluconate, 10 CsCl, 10 HEPES, 11 EGTA, 1.0 CaCl2, and 2.0 MgATP, pH 7.2 (305 mOsm).
Dorsal horn neurons were visualized with infrared-differential interference contrast and patch clamp recordings were obtained as described previously [7] using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA). Miniature postsynaptic currents (mPSCs) were isolated via the bath application of 500 nM TTX, and mEPSCs were recorded at a holding potential of −70 mV. Under these conditions, mEPSCs are abolished by bath application of the selective AMPAR antagonist NBQX [6]. mIPSCs were recorded at a holding potential of 0 mV, thus minimizing the contribution of NMDA and AMPA/kainate receptor-mediated events [61].
In some experiments, EPSCs were electrically evoked via minimal stimulation of the L4/L5 dorsal roots (0–1 mA, 100 μs – 5 ms duration) delivered via a second patch electrode which was connected to a constant-current stimulator (Master-8, Jerusalem, Israel). The threshold to evoke an EPSC was defined as the current intensity (at a duration of 1 ms) which evoked a measurable EPSC in ≥ 50% of the trials. A-fiber mediated EPSCs were classified as monosynaptic based on their ability to follow repetitive stimulation (5 stimuli at 2X threshold delivered at 10 Hz) with a constant latency and absence of failures. High-threshold EPSCs were considered monosynaptic if no failures were observed during 1 Hz stimulation. To investigate whether surgical incision altered the probability of glutamate release at primary afferent synapses in the dorsal horn, pairs of identical stimuli (at 2X threshold at a frequency of 0.10 Hz) were delivered at an inter-stimulus interval of 75 ms, and the paired-pulse ratio (PPR) was calculated as: PPR = Mean EPSC2 / Mean EPSC1. The coefficient of variation (CV) of the evoked EPSC amplitudes was defined as standard deviation / mean.
Membrane voltages were adjusted for liquid junction potentials (approximately –14 mV) calculated using JPCalc software (P. Barry, University of New South Wales, Sydney, Australia; modified for Molecular Devices). Currents were filtered at 4–6 kHz through a –3 dB, four-pole low-pass Bessel filter, digitally sampled at 20 kHz, and stored on a personal computer (ICT, Cincinnati, OH) using a commercially available data acquisition system (Digidata 1440A with pClamp 10.0 software; Molecular Devices).
2.7. Data analysis and statistics
mPSCs were analyzed via visual inspection using Mini Analysis (version 6.0.3; Synaptosoft, Decatur, GA) while evoked EPSCs were analyzed using Clampfit (Molecular Devices) software. The threshold for mPSC detection was set at twice the mean amplitude of the background noise. Nonparametric statistical tests (Mann–Whitney test for two groups; Prism 5.0 software; GraphPad Software, La Jolla, CA) were used in cases in which the distribution of data failed the D’Agostino & Pearson normality test (Prism) or when the number of observations was insufficient (n < 24) to definitively conclude that data were distributed in a Gaussian manner. Due to considerable variability in the baseline measurements of mEPSC frequency between litters (which could reflect differences in a variety of factors such as the precise hour of birth, litter size, and maternal interactions), littermate-matched controls were used for all experiments and statistical analysis was restricted to comparisons made between experimental groups from the same litter. n refers to the number of neurons sampled in a given group unless specified otherwise. Data are expressed as means ± SEM.
3. Results
3.1. Neonatal surgical injury evokes a sex-dependent facilitation of glutamatergic signaling within the immature SDH
We first examined the effects of hindpaw surgical incision at postnatal day (P) 3, which produces acute mechanical hyperalgesia at this age [58], on the properties of spontaneous excitatory and inhibitory synaptic signaling onto rat SDH neurons at 2–3 days post-injury using whole-cell patch clamp recordings from spinal cord slices in vitro (Fig. 1A). Because the sampled neurons were located approximately 50–125 μm from the edge of the dorsal white matter, the majority of these neurons likely resided in lamina II [33].
Fig. 1.

Neonatal hindpaw incision enhances spontaneous excitatory signaling within the female rat SDH. (A) Examples of mEPSCs recorded at a holding potential (Vh) of −70 mV (top) and mIPSCs isolated at a Vh of 0 mV (bottom) from the same lamina II neuron. (B) Surgical injury at postnatal day (P) 3 significantly increased the frequency (left; **p = 0.004; Mann-Whitney test) but not amplitude (right) of mEPSCs across the general population of lamina II cells at P5–6. (C, D) Separation of the data according to the sex of the pup revealed a selective increase in mEPSC frequency within the female SDH (C; left; **p = 0.009; Mann-Whitney test) using this model of tissue damage.
As illustrated in Fig. 1B, hindpaw incision at P3 led to a significant increase in the frequency of miniature excitatory postsynaptic currents (mEPSCs) in SDH neurons at P5–6 in comparison to naïve littermate controls (Naïve: 0.21 ± 0.03 Hz, n = 114; Incision: 0.28 ± 0.03 Hz, n = 123; p = 0.004; Mann-Whitney test) while no significant change was seen in mEPSC amplitude (Naïve: 15.68 ± 0.65 pA; Incision: 16.72 ± 0.64 pA; p = 0.198), which agrees with our previous findings using other models of neonatal tissue damage [30,31]. However, since these earlier studies did not address whether the enhancement in glutamatergic transmission occurs to a similar extent in male and female rats, the present data (Fig. 1B) were also analyzed according to sex. P3 incision significantly elevated mEPSC frequency in female SDH neurons at P5–6 (Naïve: 0.18 ± 0.02 Hz, n = 63; Incision: 0.31 ± 0.05 Hz, n = 53; p = 0.009; Mann-Whitney test; Fig. 1C; left) while no significant change was seen in mEPSC amplitude (Naïve: 15.0 ± 0.84 pA; Incision: 16.82 ± 0.92 pA; p = 0.074; Fig. 1C; right). In contrast, P3 incision failed to alter mEPSC frequency (Naïve: 0.26 ± 0.07 Hz, n = 51; Incision: 0.25 ± 0.03 Hz, n = 70; p = 0.163; Mann-Whitney test) or mEPSC amplitude within lamina II of male pups at the same time point (Fig. 1D). In both males and females, the properties of miniature inhibitory postsynaptic currents (mIPSCs) were not significantly affected by neonatal incision (data not shown), which is also consistent with our earlier studies [30,31].
These data suggest that localized surgical injury during early life facilitates glutamatergic synapses within the developing SDH of female pups. As a result, female rodents were used for the subsequent experiments designed to better identify which components of the SDH network are responsible for the altered balance between synaptic excitation and inhibition in the days following neonatal tissue injury. However, it should be noted that a meaningful exploration of the basis for these sex-dependent differences in synaptic plasticity falls outside the scope of the present study.
3.2. Early tissue damage strengthens nociceptive synaptic input onto developing SDH neurons
To determine if the above increase in glutamatergic synaptic drive after neonatal hindpaw incision (Fig. 1B, C) reflects changes in primary afferent input onto immature SDH neurons, we used a minimal stimulation protocol to activate monosynaptic sensory inputs to lamina II neurons (Fig. 2). The stepwise increase in the evoked EPSC amplitude seen with increasing dorsal root stimulation suggests an all-or-none recruitment of small numbers of primary afferent inputs to the SDH (Fig. 2A, B), thereby avoiding significant contamination by polysynaptic A-fiber pathways onto the sampled neuron. The distribution of EPSC thresholds across a population of recordings suggests that this approach allows for a clear separation between low-threshold (Aβ-fiber) and high-threshold (Aδ- and C-fiber) primary afferent inputs to neonatal lamina II cells (Fig. 2C).
Fig. 2.
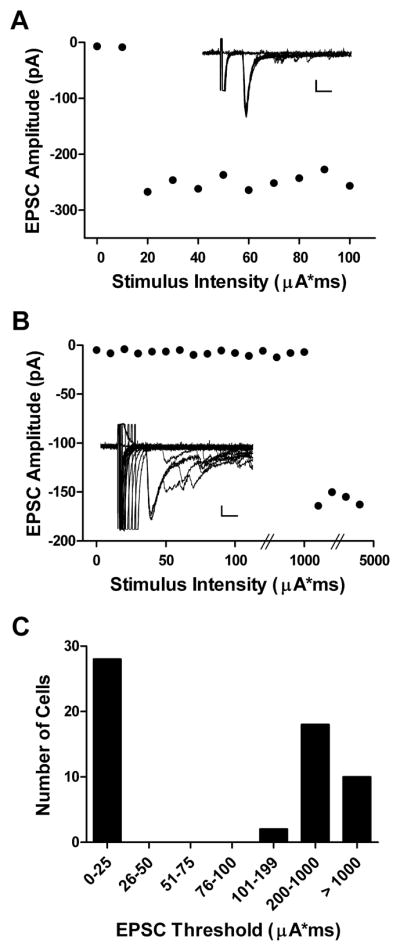
Minimal stimulation of low-threshold vs. high-threshold sensory inputs to developing SDH neurons. (A) Plot of monosynaptic EPSC amplitude vs. intensity of primary afferent stimulation illustrating an example of an EPSC mediated by low-threshold sensory inputs to the immature rat SDH (see inset). Note that increasing the level of dorsal root stimulation to 5X threshold failed to recruit any additional synaptic inputs to this lamina II neuron. Scale bar: 50 pA, 5 ms. (B) A similar step-like increase in EPSC amplitude is seen in a different lamina II cell (inset), but at a ~50-fold higher threshold than seen in A. Scale bar: 25 pA, 5 ms. (C) Histogram showing the distribution of monosynaptic EPSC thresholds across the sampled population of lamina II neurons at P3–4.
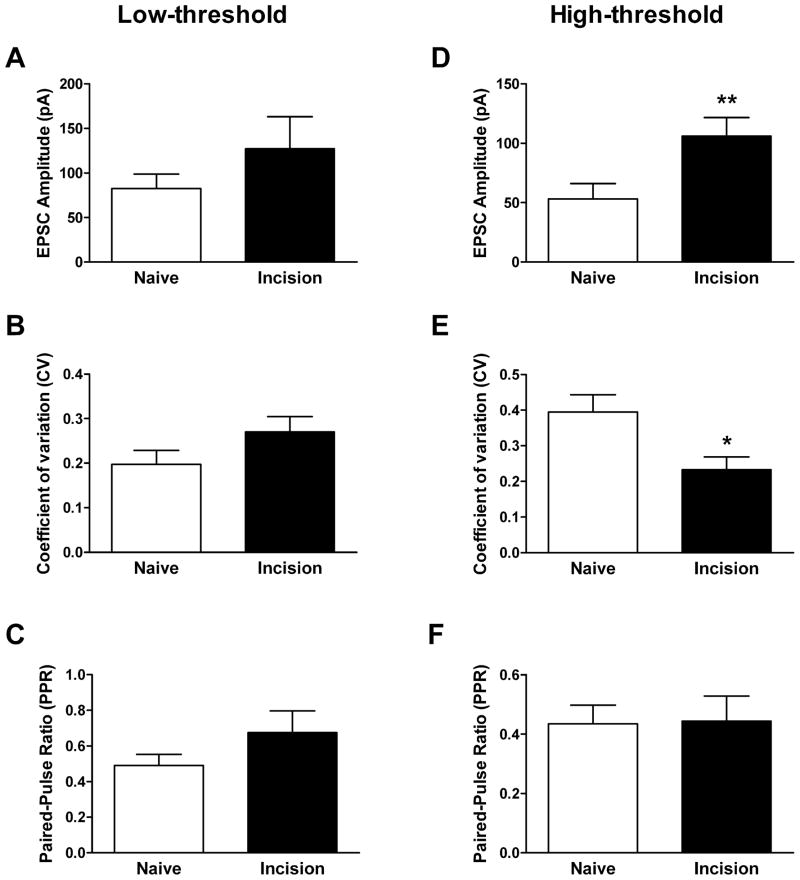
We next characterized the effect of surgical injury at P2 on the properties of low-threshold (LT) and high-threshold (HT) primary afferent inputs to P3–4 SDH neurons. First, neonatal hindpaw incision did not significantly alter the threshold to evoke an EPSC in either the LT or HT groups (data not shown). However, the incision significantly increased the amplitude of HT-mediated EPSCs in lamina II neurons (Naïve: 53.2 ± 13.0 pA, n = 16; Incision: 106.1 ± 15.7 pA, n = 14; p = 0.005; Mann-Whitney test; Fig. 3D), while no change was observed in the amplitude of LT-mediated inputs to SDH neurons in the same spinal cord slices (Naïve: 82.6 ± 16.2 pA, n = 15; Incision: 127.3 ± 35.6 pA, n = 13; p = 0.678; Fig. 3A). The enhanced mean amplitude of HT-mediated synaptic input after injury was associated with a significant reduction in the variability of the EPSC amplitudes as measured by the coefficient of variation (CV of Naïve: 0.40 ± .05; CV of Incision: 0.23 ± 0.04; p = 0.015; Mann-Whitney test; Fig. 3E). Meanwhile, the response to paired high-threshold stimulation in the same neurons was similar in the naïve and incision groups (PPR of Naïve: 0.43 ± 0.06; PPR of Incision: 0.44 ± 0.08; p = 0.917; Fig. 3F). We observed no effect of the incision on either the coefficient of variation (Fig. 3B) or paired-pulse ratio (Fig. 3C) of EPSCs evoked by stimulation of low-threshold primary afferent inputs to the immature SDH. These results clearly suggest that the elevation in spontaneous glutamatergic signaling under pathological conditions (Fig. 1C) is accompanied by a potentiation of high-threshold sensory input to lamina II.
Fig. 3.
Early tissue damage selectively increases the amplitude of high-threshold (HT) primary afferent inputs to lamina II neurons. (A – C) Surgical incision of the rat hindpaw at P2 failed to significantly alter the amplitude (A) and coefficient of variation (B) of EPSCs mediated by low-threshold sensory afferents, as well as the response to paired stimulation (C). (D) P2 incision significantly increased the amplitude of monosynaptic EPSCs evoked by high-threshold stimulation of the attached dorsal root in the same spinal cord slices (**p = 0.005; Mann-Whitney test). (E) Neonatal injury also decreased the CV of HT-mediated EPSCs in the same lamina II neurons (*p = 0.015; Mann-Whitney test). (F) The paired-pulse ratio of HT-mediated EPSCs was unaffected by the hindpaw incision.
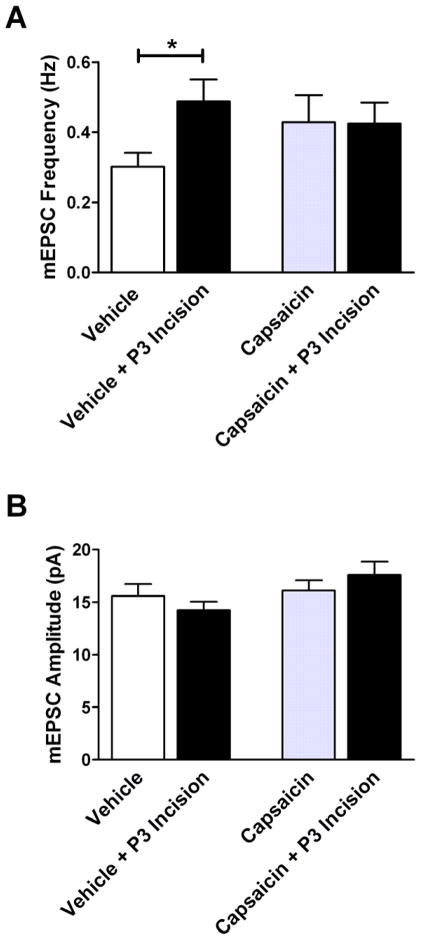
To further test the hypothesis that early incision facilitates nociceptive synaptic input to the developing SDH, we administered systemic capsaicin (CAP) at birth and determined whether this prevented the subsequent effects of P3 surgical injury on excitatory synapses within lamina II at P5–6. Fig. 4 demonstrates that tissue injury fails to modulate glutamatergic signaling in the immature rat SDH in the absence of normal input from TRPV1-expressing sensory neurons, as mEPSC frequency was unaffected by hindpaw incision in pups pre-treated with capsaicin (CAP only: 0.43 ± 0.08 Hz, n = 36; CAP + Incision: 0.42 ± 0.06 Hz, n = 35; p = 0.508; Mann-Whitney test; Fig. 4A, right), while a robust increase in mEPSC frequency was observed following incision in pups receiving vehicle injections during the first days of life (Vehicle: 0.30 ± 0.04 Hz, n = 36; Vehicle + Incision: 0.49 ± 0.06 Hz; n = 36; p = 0.012; Fig. 4A, left). Neonatal incision failed to significantly affect mEPSC amplitude in both the vehicle and capsaicin groups (Fig. 4B). In sum, these data suggest that capsaicin-sensitive primary afferents are key contributors to a distinct form of SDH synaptic plasticity which is exclusively evoked by tissue injury during early life.
Fig. 4.
TRPV1-expressing primary afferents are involved in the facilitation of spontaneous glutamatergic signaling within the rat SDH following neonatal incision. (A) While mEPSC frequency in lamina II neurons is significantly elevated following P3 hindpaw incision in pups receiving vehicle injections from birth (left; *p = 0.012; Mann-Whitney test), no such increase is seen in pups pre-treated with systemic capsaicin at P0–1 (right). (B) Incision at P3 failed to change mEPSC amplitude in both the vehicle (left) and capsaicin-treated (right) pups.
3.3. Neonatal incision facilitates excitatory input onto both GABAergic and presumed glutamatergic neurons of the mouse SDH
To determine which functional subtypes of SDH neurons receive enhanced glutamatergic input following early tissue damage, we administered hindpaw surgical incisions at P3 in transgenic mice which selectively express eGFP in GABAergic interneurons of the dorsal horn via the gad1 promoter [11,15] and characterized the properties of spontaneous synaptic transmission onto both Gad-GFP (Fig. 5A, B) and adjacent non-GFP neurons within lamina II at P4–5. Since eGFP labels ~80% of GABAergic neurons in the neonatal SDH [16] and glycinergic cells likely represent a subset of this GABAergic population [54], the vast majority (80–90%) of sampled non-GFP cells will correspond to glutamatergic neurons [48].
Fig. 5.
Early surgical injury strengthens glutamatergic drive onto both inhibitory and presumed excitatory interneurons within lamina II of the developing spinal cord. (A, B) Examples of sagittal spinal cord sections at P4–5 illustrating the distribution of Gad-GFP neurons throughout the L4–L5 dorsal horn. Scale bars: A, 250 μm; B, 50 μm. (C, D) Hindpaw incision at P3 increased mEPSC frequency (left; *p < 0.05; Mann-Whitney test) in both GABAergic (C) and presumed glutamatergic (D) lamina II neurons without altering mEPSC amplitude (right).
As shown in Fig. 5C–D, hindpaw incision at P3 significantly elevated mEPSC frequency in both GFP (Naïve: 0.08 ± 0.02 Hz, n = 29; Incision: 0.21 ± 0.05 Hz, n = 30; p = 0.014; Mann-Whitney test) and non-GFP neurons (Naïve: 0.14 ± 0.04 Hz, n = 28; Incision: 0.31 ± 0.08 Hz, n = 32; p = 0.042) at P4–5. In both subtypes of lamina II cells, neither the mEPSC amplitude (Fig. 5) nor the properties of miniature inhibitory currents (data not shown) were significantly altered by hindpaw injury. To confirm that rats and mice have a qualitatively similar developmental profile in terms of the response of SDH synapses to tissue injury, hindpaw incision was also administered in Gad-GFP mice at either P17 or during adulthood (P42–56) and the mEPSC properties were analyzed at 1–2 days post-injury. As previously seen in the rat [30,31], tissue damage administered during or after the third postnatal week failed to facilitate glutamatergic signaling across the general population of lamina II neurons in the mouse (data not shown).
3.4. NGF-trkA signaling regulates glutamatergic transmission within the developing SDH
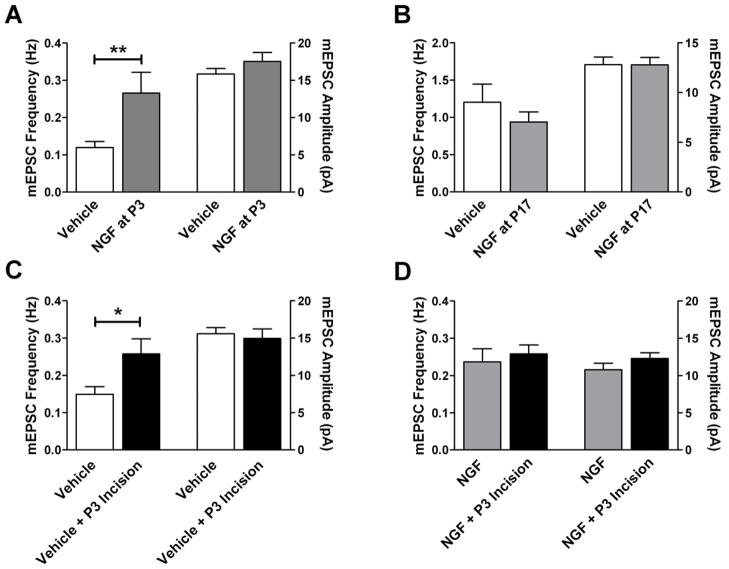
Neonatal tissue damage evokes a robust increase in NGF expression within the skin [10] and elevated NGF levels in the periphery result in the expansion of developing primary afferent inputs to the SDH [36]. This raises the possibility that NGF-related signaling at the injury site is important for the strengthening of glutamatergic synapses within the immature SDH under pathological conditions. First, to determine if exogenous NGF alone can reproduce the age-dependent effects of incision on excitatory SDH synapses, NGF was injected subcutaneously into the rat hindpaw at P3–4 and mEPSC properties were characterized in lamina II neurons 1–2 days later. Fig. 6A shows that the peripheral administration of NGF in the neonate evokes similar effects on SDH glutamatergic synapses as was observed following hindpaw incision (Fig. 1B, 1C), as NGF significantly increased mEPSC frequency compared to littermates receiving equivalent vehicle injections (Vehicle: 0.12 ± 0.02 Hz, n = 45; NGF: 0.27 ± 0.06 Hz, n = 45; p = 0.001; Mann-Whitney test) while mEPSC amplitude was unaffected (Vehicle: 15.8 ± 0.8 pA; NGF: 17.5 ± 1.22 pA; p = 0.44; Mann-Whitney test). In contrast, NGF administration at P17–18 failed to modulate mEPSC frequency or amplitude in the SDH at P19–20 (Fig. 6B), which is consistent with the inability of tissue injury to affect excitatory synapses if administered during the third postnatal week [30,31].
Fig. 6.
Exogenous NGF delivered to the hindpaw is sufficient to evoke an age-dependent facilitation of excitatory signaling in the immature SDH and the effects of surgical injury on glutamatergic function are not observed after NGF pretreatment. (A) Injection of NGF into the hindpaw at P3–4 significantly increased mEPSC frequency in rat lamina II neurons at P5–6 compared to vehicle-treated littermates (left; **p = 0.001; Mann-Whitney test) without altering mEPSC amplitude (right). (B) NGF administration from P17–18 failed to modulate spontaneous glutamatergic transmission compared to vehicle controls at P19–20. (C, D) mEPSC frequency is significantly elevated by P3 hindpaw incision in pups injected with vehicle from P2–4 (C, left; *p = 0.042; Mann-Whitney test), but not in pups treated with NGF during the same period (D, left).
If neonatal surgical injury facilitates excitatory signaling within the SDH by initiating NGF-dependent signaling pathways, then one might predict that the prior administration of exogenous NGF might prevent any subsequent effects of hindpaw incision on glutamatergic function within lamina II. To test this hypothesis, NGF (or vehicle solution) was injected subcutaneously into the rat hindpaw from P2–4 with a subset of these pups also receiving the surgical injury at P3. Patch clamp recordings were obtained at P5–6 in order to characterize spontaneous glutamatergic transmission in the SDH as described above. While mEPSC frequency was significantly elevated by P3 incision in pups treated with vehicle solution (Vehicle only: 0.15 ± 0.02 Hz, n = 29; Vehicle + Incision: 0.26 ± 0.04 Hz, n = 35; p = 0.028; Mann-Whitney test; Fig. 6C, left), this increase failed to occur in pups treated with NGF (NGF only: 0.24 ± 0.04 Hz, n = 35; NGF + Incision: 0.26 ± 0.02 Hz, n = 34; p = 0.18; Fig. 6D, left). As expected, hindpaw incision did not affect mEPSC amplitude in either the vehicle-treated (Fig. 6C, right) or NGF-treated (Fig. 6D, right) groups. The lack of an additive effect of hindpaw incision on mEPSC frequency in the NGF-treated pups suggests that NGF and early tissue damage regulate excitatory synaptic function within the SDH via similar downstream mechanisms.
To determine whether activation of the high-affinity NGF receptor trkA is required for the observed plasticity in spinal glutamatergic circuits under pathological conditions, we also administered neonatal hindpaw incisions in rat pups receiving systemic injections of the trkA inhibitor VMD902, which demonstrates a >200-fold selectivity for trkA over other kinases including trkB and trkC. Blockade of trkA signaling in vivo prevented the facilitation of spontaneous excitatory transmission by early surgical injury, as the average mEPSC frequency in lamina II neurons was similar in the naïve and incised groups at P4–5 (VMD902 only: 0.19 ± 0.02 Hz, n = 48; VMD902 + P2 Incision: 0.18 ± 0.02 Hz, n = 50; p = 0.678; Mann-Whitney test; see Fig. 7B). Meanwhile, as shown in Fig. 7A, the administration of the corresponding vehicle solution did not interfere with the ability of tissue damage to enhance mEPSC frequency in these neurons (Vehicle only: 0.16 ± 0.03 Hz; n = 32; Vehicle + P2 Incision: 0.26 ± 0.03 Hz; n = 32; p = 0.005). In addition, the potentiation of high-threshold primary afferent input to the SDH previously observed after neonatal incision (Fig. 3) failed to occur in pups pre-treated with VMD902 (Fig. 8), as there was no significant difference in EPSC amplitude (VMD902: 59.2 ± 8.2 pA, n = 13; VMD902 + P2 Incision: 62.6 ± 11.3 pA, n = 11; p = 0.95; Mann-Whitney test; Fig. 8A), coefficient of variation (VMD902: 0.38 ± 0.06; VMD902 + P2 Incision: 0.41 ± 0.05; p = 0.73; Fig. 8B) or paired-pulse ratio (VMD902: 0.44 ± 0.07; VMD902 + P2 Incision: 0.48 ± 0.06; p = 0.86; Fig. 8C) between groups. This suggests that similar, trkA-dependent mechanisms underlie the alterations in spontaneous and primary afferent-evoked glutamatergic transmission within the developing SDH following neonatal tissue damage.
Fig. 7.
Disruption of trkA signaling in vivo abolishes the effects of neonatal surgical injury on spontaneous glutamatergic transmission in the developing SDH. (A) Hindpaw incision significantly increased the mEPSC frequency (left), but not amplitude (right), in pups receiving injections of a vehicle solution at P2–3 (**p = 0.005; Mann-Whitney test). (B) This increase failed to occur when the pups were treated with the selective trkA inhibitor VMD902 during the same time period (left; p = 0.678).
Fig. 8.

trkA inhibition prevents the enhancement of high-threshold (HT) primary afferent input to the SDH following early tissue damage. (A – C) There were no significant effects of P2 hindpaw incision on EPSC amplitude (A), coefficient of variation (B) or paired-pulse ratio (C) in pups which were treated with VMD902 prior to the injury.
4. Discussion
The present results collectively demonstrate, for the first time, that neonatal tissue damage leads to a widespread increase in glutamate release at nociceptive primary afferent synapses within the immature rodent SDH. The ability of locally applied NGF to mimic the effects of early surgical injury on SDH synaptic function, along with the results showing that the injury-evoked synaptic plasticity is blocked by trkA antagonists in vivo, suggests that NGF-trkA signaling at the injury site may enhance the flow of noxious information to the developing brain in part by facilitating glutamatergic efficacy within spinal pain circuits.
4.1. Mechanisms underlying the potentiation of nociceptive synaptic input to the SDH after neonatal injury
The conclusion that neonatal injury strengthens nociceptive synaptic input to lamina II is supported by data illustrating that P2 hindpaw incision selectively increases the amplitude of monosynaptic EPSCs evoked by high-threshold (HT) primary afferent stimulation (Fig. 3A, D). The enhanced amplitude of HT-mediated EPSCs was accompanied by a decreased coefficient of variation (CV) but no change in the paired-pulse ratio (Fig. 3E, F). The CV is a commonly used measure of presynaptic function which inversely depends on both the probability of transmitter release (Pr) and the number (n) of transmitter release sites [9]. Meanwhile, the paired-pulse ratio is thought to depend only on Pr [14]. Therefore, these results suggest that the increased strength of HT-mediated inputs onto lamina II cells after tissue damage may reflect an elevation in the number of synapses made by nociceptive primary afferents within the developing SDH, although these data (Fig. 3D–F) could alternatively be explained by an injury-evoked increase in the number of high-threshold fibers that are activated by minimal stimulation of the dorsal root. Nonetheless, an increase in the number of synapses or release sites (n) could also explain why neonatal incision selectively increases the frequency, but not amplitude, of mEPSCs at this time point (Fig. 1B, C). Such a mechanism would be consistent with an expansion of nociceptive projections to the SDH following peripheral inflammation in the neonate [44,57], and suggests that these exuberant terminals make functional synapses under pathological conditions. While electrical stimulation of the immature dorsal root cannot definitively implicate C-fiber neurons in the observed synaptic plasticity [6,43], the central terminals of unmyelinated primary afferents can be identified at the ultra-structural level from the first days of life [41]. Thus, electron microscopy could be used to confirm that neonatal tissue damage increases the number of C-fiber synapses within lamina II during early postnatal development.
The observation that hindpaw incision at P3 fails to alter spontaneous glutamatergic transmission in pups pre-treated with systemic capsaicin (Fig. 4) also suggests that early tissue injury predominantly modulates nociceptive synapses within the developing SDH. While our results point to an important role for TRPV1+ terminals in the injury-evoked synaptic plasticity, we cannot exclude the possibility that the elevated mEPSC frequency after incision actually reflects a heterosynaptic facilitation of TRPV1-negative synaptic inputs following the activation of capsaicin-sensitive afferents in the periphery.
4.2. Early surgical injury enhances glutamatergic function in the SDH by initiating NGF-trkA signaling
Given the electrophysiological evidence pointing to the involvement of small-diameter sensory neurons in the facilitation of glutamatergic signaling in the SDH after neonatal injury (Figs. 3, 4), it is notable that the high-affinity NGF receptor trkA is expressed in ~80% of these cells during early life [37]. NGF is known to be significantly up-regulated in the neonatal skin following wounding [10], which would be expected to sensitize TRPV1-expressing sensory neurons [27,39,49] and evoke a hyperinnervation of the dorsal horn by peptidergic sensory fibers [36]. These findings are consistent with our data showing that the increase in mEPSC frequency produced by surgical injury fails to occur when the incision is preceded by a local delivery of NGF to the hindpaw (Fig. 6), which is unlikely to reflect a ceiling effect since newborn SDH neurons can exhibit mEPSCs at rates of up to 20 Hz when stimulated [6]. While these observations cannot rule out the possibility that NGF and incision act on separate populations of primary afferents in a non-additive manner, it is important to note that the injury-evoked changes in both spontaneous and evoked glutamatergic transmission were abolished by a selective trkA antagonist in vivo (Figs. 7, 8). Collectively, the results suggest that NGF produced in the injured tissue activates trkA receptors to promote the central sprouting of capsaicin-sensitive primary afferents, thus increasing the number of nociceptive synapses within the immature SDH.
4.3. Why is the facilitation of SDH excitatory synapses restricted to injuries occurring during the neonatal period?
While increased expression of NGF at the injury site may drive synaptic plasticity within the developing SDH under pathological conditions, it is unlikely to explain the observed age-dependence of this plasticity [30,31]. Hindpaw incision also robustly elevates NGF expression in both the skin and underlying muscle in the adult [59,60], and NGF is known to promote collateral sprouting of mature nociceptive fibers in the periphery [12,13]. However, elevating peripheral NGF levels during the third postnatal week fails to enhance glutamatergic signaling in lamina II (Fig. 6B). This suggests that postnatal changes within the spinal cord itself may create a progressively less permissive environment for synaptic remodeling within nociceptive circuits following tissue damage.
The disappearance of this form of injury-induced synaptic plasticity at later ages [30] coincides with the onset of myelination in the spinal cord [25]. CNS myelin contains a complement of proteins known to inhibit axonal growth [24], and enhanced collateral sprouting of nociceptive fibers occurs in the myelin-free dorsal horn following dorsal rhizotomy [47]. These studies argue for a potential role of myelin-associated proteins in the termination of the critical period within the SDH. Interestingly, experience-dependent plasticity in the visual cortex persists throughout adulthood in the absence of normal myelin-derived signaling at the Nogo receptor [35].
4.4. Increased glutamatergic drive after neonatal injury does not depend on the functional subtype of SDH neuron
Our results (Fig. 5) suggest that early tissue damage elevates glutamate release onto both excitatory and inhibitory interneurons within the developing SDH. Given the capsaicin sensitivity of the enhanced glutamatergic signaling after injury (Fig. 4), the widespread nature of these synaptic modifications is consistent with earlier findings that all immature lamina II neurons receive direct synaptic input from TRPV1-expressing primary afferents [6]. Sensory processing within lamina II is dominated by glutamatergic interneurons [45] and GABAergic neurons comprise only ~ 30% of the lamina II population in the mature SDH [54]. It should be noted that the neonatal rat SDH contains a higher proportion of GABAergic neurons compared to the adult [34,46]. Nonetheless, it seems likely that excitatory interneurons constitute the majority of the rat lamina II neurons which were randomly sampled in our experiments. As a result, the changes in synaptic efficacy reported here could contribute to a net increase in the excitability and output of the SDH network under pathological conditions. In this regard, it will be important to examine how neonatal tissue damage modulates glutamatergic inputs onto projection neurons in the developing SDH [55].
4.5. Sex differences in the response of the SDH synaptic network to early tissue damage
Given the clinical evidence that women tend to report higher levels of acute postoperative pain [17], it is interesting that early hindpaw incision selectively enhanced glutamatergic signaling within the female rat SDH at 2–3 days post-injury (Fig. 1). Our previous studies used both male and female pups [30,31], and it remains to be determined if such sex-dependent synaptic plasticity occurs across multiple injury models, or whether the male SDH can also exhibit a facilitation of excitatory transmission following more severe or widespread tissue damage. Although the mechanisms underlying these sex differences remain unclear, one possibility is that immature sensory neurons are less sensitive to NGF in males compared to females. During the late embryonic and neonatal period, CNS levels of estrogen are higher in males [2], and continuous estrogen exposure has been shown to decrease trkA levels in both the DRG and brain [21,32].
The long-term effects of neonatal injury on adult pain sensitivity [42] are also exacerbated in female rodents [29], and the developmental window during which hindpaw injury can potentiate glutamatergic function [30] coincides with the critical period during which tissue damage evokes these persistent changes in pain behavior [29,42]. This raises the possibility that the increased efficacy of nociceptive synapses within the SDH following neonatal injury may play a role in triggering more persistent structural and functional alterations within developing central pain networks.
4.6. Conclusions
The present study demonstrates that neonatal tissue damage increases the gain of spinal nociceptive transmission by modifying primary afferent synapses onto developing dorsal horn neurons. Additional work is needed to understand how these specific components of the SDH synaptic network are shaped by repetitive tissue damage, which inevitably occurs during neonatal intensive care treatment. Knowledge of the potential cumulative effects of multiple injuries on developing pain circuits is essential for the design of better analgesic therapies for these infants.
Acknowledgments
This work was supported by the U.S. National Institutes of Health grant NS060858 (MLB) and the University of Cincinnati Millennium Fund. We would also like to thank Dr. Jay Wu of VM Discovery, Inc. for kindly providing the trkA inhibitor VMD902 and Dr. Jun-Ming Zhang for helpful comments regarding the preparation of this manuscript.
Footnotes
The authors declare that they have no conflicts of interest with any of the work presented in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alexander J, Manno M. Underuse of analgesia in very young pediatric patients with isolated painful injuries. Ann Emerg Med. 2003;41:617–622. doi: 10.1067/mem.2003.138. [DOI] [PubMed] [Google Scholar]
- 2.Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- 3.Anand KJ. Pain, plasticity, and premature birth: a prescription for permanent suffering? Nat Med. 2000;6:971–973. doi: 10.1038/79658. [DOI] [PubMed] [Google Scholar]
- 4.Andrews K, Fitzgerald M. The cutaneous withdrawal reflex in human neonates: sensitization, receptive fields, and the effects of contralateral stimulation. Pain. 1994;56:95–101. doi: 10.1016/0304-3959(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 5.Andrews KA, Desai D, Dhillon HK, Wilcox DT, Fitzgerald M. Abdominal sensitivity in the first year of life: comparison of infants with and without prenatally diagnosed unilateral hydronephrosis. Pain. 2002;100:35–46. doi: 10.1016/s0304-3959(02)00288-9. [DOI] [PubMed] [Google Scholar]
- 6.Baccei ML, Bardoni R, Fitzgerald M. Development of nociceptive synaptic inputs to the neonatal rat dorsal horn: glutamate release by capsaicin and menthol. J Physiol. 2003;549:231–242. doi: 10.1113/jphysiol.2003.040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baccei ML, Fitzgerald M. Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. J Neurosci. 2004;24:4749–4757. doi: 10.1523/JNEUROSCI.5211-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 9.Chuhma N, Ohmori H. Postnatal development of phase-locked high-fidelity synaptic transmission in the medial nucleus of the trapezoid body of the rat. J Neurosci. 1998;18:512–520. doi: 10.1523/JNEUROSCI.18-01-00512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Constantinou J, Reynolds ML, Woolf CJ, Safieh-Garabedian B, Fitzgerald M. Nerve growth factor levels in developing rat skin: upregulation following skin wounding. Neuroreport. 1994;5:2281–2284. doi: 10.1097/00001756-199411000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Daniele CA, MacDermott AB. Low–threshold primary afferent drive onto GABAergic interneurons in the superficial dorsal horn of the mouse. J Neurosci. 2009;29:686–695. doi: 10.1523/JNEUROSCI.5120-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diamond J, Coughlin M, Macintyre L, Holmes M, Visheau B. Evidence that endogenous beta nerve growth factor is responsible for the collateral sprouting, but not the regeneration, of nociceptive axons in adult rats. Proc Natl Acad Sci U S A. 1987;84:6596–6600. doi: 10.1073/pnas.84.18.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond J, Holmes M, Coughlin M. Endogenous NGF and nerve impulses regulate the collateral sprouting of sensory axons in the skin of the adult rat. J Neurosci. 1992;12:1454–1466. doi: 10.1523/JNEUROSCI.12-04-01454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- 15.Dougherty KJ, Sawchuk MA, Hochman S. Properties of mouse spinal lamina I GABAergic interneurons. J Neurophysiol. 2005;94:3221–3227. doi: 10.1152/jn.00184.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dougherty KJ, Sawchuk MA, Hochman S. Phenotypic diversity and expression of GABAergic inhibitory interneurons during postnatal development in lumbar spinal cord of glutamic acid decarboxylase 67-green fluorescent protein mice. Neuroscience. 2009;163:909–919. doi: 10.1016/j.neuroscience.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., III Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzgerald M, Gibson S. The postnatal physiological and neurochemical development of peripheral sensory C fibres. Neuroscience. 1984;13:933–944. doi: 10.1016/0306-4522(84)90107-6. [DOI] [PubMed] [Google Scholar]
- 19.Fitzgerald M, Millard C, McIntosh N. Cutaneous hypersensitivity following peripheral tissue damage in newborn infants and its reversal with topical anaesthesia. Pain. 1989;39:31–36. doi: 10.1016/0304-3959(89)90172-3. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 21.Gibbs RB, Wu D, Hersh LB, Pfaff DW. Effects of estrogen replacement on the relative levels of choline acetyltransferase, trkA, and nerve growth factor messenger RNAs in the basal forebrain and hippocampal formation of adult rats. Exp Neurol. 1994;129:70–80. doi: 10.1006/exnr.1994.1148. [DOI] [PubMed] [Google Scholar]
- 22.Graham BA, Brichta AM, Callister RJ. Moving from an averaged to specific view of spinal cord pain processing circuits. J Neurophysiol. 2007;98:1057–1063. doi: 10.1152/jn.00581.2007. [DOI] [PubMed] [Google Scholar]
- 23.Granmo M, Petersson P, Schouenborg J. Action-based body maps in the spinal cord emerge from a transitory floating organization. J Neurosci. 2008;28:5494–5503. doi: 10.1523/JNEUROSCI.0651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu F, Strittmatter SM. Regulating axon growth within the postnatal central nervous system. Semin Perinatol. 2004;28:371–378. doi: 10.1053/j.semperi.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Kapfhammer JP, Schwab ME. Inverse patterns of myelination and GAP-43 expression in the adult CNS: neurite growth inhibitors as regulators of neuronal plasticity? J Comp Neurol. 1994;340:194–206. doi: 10.1002/cne.903400206. [DOI] [PubMed] [Google Scholar]
- 26.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 27.Koltzenburg M, Bennett DL, Shelton DL, McMahon SB. Neutralization of endogenous NGF prevents the sensitization of nociceptors supplying inflamed skin. Eur J Neurosci. 1999;11:1698–1704. doi: 10.1046/j.1460-9568.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- 28.Koltzenburg M, Lewin GR. Receptive properties of embryonic chick sensory neurons innervating skin. J Neurophysiol. 1997;78:2560–2568. doi: 10.1152/jn.1997.78.5.2560. [DOI] [PubMed] [Google Scholar]
- 29.LaPrairie JL, Murphy AZ. Female rats are more vulnerable to the long-term consequences of neonatal inflammatory injury. Pain. 2007;132(Suppl 1):S124–S133. doi: 10.1016/j.pain.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Baccei ML. Excitatory synapses in the rat superficial dorsal horn are strengthened following peripheral inflammation during early postnatal development. Pain. 2009;143:56–64. doi: 10.1016/j.pain.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Walker SM, Fitzgerald M, Baccei ML. Activity-dependent Modulation of Glutamatergic Signaling in the Developing Rat Dorsal Horn by Early Tissue Injury. J Neurophysiol. 2009;102:2208–2219. doi: 10.1152/jn.00520.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liuzzi FJ, Scoville SA, Bufton SM. Long-term estrogen replacement coordinately decreases trkA and beta-PPT mRNA levels in dorsal root ganglion neurons. Exp Neurol. 1999;155:260–267. doi: 10.1006/exnr.1998.6999. [DOI] [PubMed] [Google Scholar]
- 33.Lorenzo LE, Ramien M, St LM, De Koninck Y, Ribeiro-da-Silva A. Postnatal changes in the Rexed lamination and markers of nociceptive afferents in the superficial dorsal horn of the rat. J Comp Neurol. 2008;508:592–604. doi: 10.1002/cne.21691. [DOI] [PubMed] [Google Scholar]
- 34.Ma W, Behar T, Chang L, Barker JL. Transient increase in expression of GAD65 and GAD67 mRNAs during postnatal development of rat spinal cord. J Comp Neurol. 1994;346:151–160. doi: 10.1002/cne.903460111. [DOI] [PubMed] [Google Scholar]
- 35.McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendelson B, Albers KM, Goodness TP, Davis BM. Overexpression of nerve growth factor in epidermis of transgenic mice preserves excess sensory neurons but does not alter the somatotopic organization of cutaneous nerve projections. Neurosci Lett. 1996;211:68–72. doi: 10.1016/0304-3940(96)12641-0. [DOI] [PubMed] [Google Scholar]
- 37.Molliver DC, Snider WD. Nerve growth factor receptor TrkA is down-regulated during postnatal development by a subset of dorsal root ganglion neurons. J Comp Neurol. 1997;381:428–438. doi: 10.1002/(sici)1096-9861(19970519)381:4<428::aid-cne3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Nakatsuka T, Ataka T, Kumamoto E, Tamaki T, Yoshimura M. Alteration in synaptic inputs through C-afferent fibers to substantia gelatinosa neurons of the rat spinal dorsal horn during postnatal development. Neuroscience. 2000;99:549–556. doi: 10.1016/s0306-4522(00)00224-4. [DOI] [PubMed] [Google Scholar]
- 39.Nicholas RS, Winter J, Wren P, Bergmann R, Woolf CJ. Peripheral inflammation increases the capsaicin sensitivity of dorsal root ganglion neurons in a nerve growth factor-dependent manner. Neuroscience. 1999;91:1425–1433. doi: 10.1016/s0306-4522(98)00706-4. [DOI] [PubMed] [Google Scholar]
- 40.Park JS, Nakatsuka T, Nagata K, Higashi H, Yoshimura M. Reorganization of the primary afferent termination in the rat spinal dorsal horn during post-natal development. Brain Res Dev Brain Res. 1999;113:29–36. doi: 10.1016/s0165-3806(98)00186-2. [DOI] [PubMed] [Google Scholar]
- 41.Pignatelli D, Ribeiro-da-Silva A, Coimbra A. Postnatal maturation of primary afferent terminations in the substantia gelatinosa of the rat spinal cord. An electron microscopic study. Brain Research. 1989;491:33–44. doi: 10.1016/0006-8993(89)90085-1. [DOI] [PubMed] [Google Scholar]
- 42.Ren K, Anseloni V, Zou SP, Wade EB, Novikova SI, Ennis M, Traub RJ, Gold MS, Dubner R, Lidow MS. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain. 2004;110:588–596. doi: 10.1016/j.pain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Ringkamp M, Peng YB, Wu G, Hartke TV, Campbell JN, Meyer RA. Capsaicin responses in heat-sensitive and heat-insensitive A-fiber nociceptors. J Neurosci. 2001;21:4460–4468. doi: 10.1523/JNEUROSCI.21-12-04460.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruda MA, Ling QD, Hohmann AG, Peng YB, Tachibana T. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science. 2000;289:628–631. doi: 10.1126/science.289.5479.628. [DOI] [PubMed] [Google Scholar]
- 45.Santos SF, Rebelo S, Derkach VA, Safronov BV. Excitatory interneurons dominate sensory processing in the spinal substantia gelatinosa of rat. J Physiol. 2007;581:241–254. doi: 10.1113/jphysiol.2006.126912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaffner AE, Behar T, Nadi S, Smallwood V, Barker JL. Quantitative analysis of transient GABA expression in embryonic and early postnatal rat spinal cord neurons. Brain Res Dev Brain Res. 1993;72:265–276. doi: 10.1016/0165-3806(93)90192-d. [DOI] [PubMed] [Google Scholar]
- 47.Schwegler G, Schwab ME, Kapfhammer JP. Increased collateral sprouting of primary afferents in the myelin-free spinal cord. J Neurosci. 1995;15:2756–2767. doi: 10.1523/JNEUROSCI.15-04-02756.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shiokawa H, Kaftan EJ, MacDermott AB, Tong CK. NR2 subunits and NMDA receptors on lamina II inhibitory and excitatory interneurons of the mouse dorsal horn. Mol Pain. 2010;6:26. doi: 10.1186/1744-8069-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shu X, Mendell LM. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci Lett. 1999;274:159–162. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- 50.Simons SH, van DM, Anand KS, Roofthooft D, van Lingen RA, Tibboel D. Do we still hurt newborn babies? A prospective study of procedural pain and analgesia in neonates. Arch Pediatr Adolesc Med. 2003;157:1058–1064. doi: 10.1001/archpedi.157.11.1058. [DOI] [PubMed] [Google Scholar]
- 51.Slater R, Cantarella A, Gallella S, Worley A, Boyd S, Meek J, Fitzgerald M. Cortical pain responses in human infants. J Neurosci. 2006;26:3662–3666. doi: 10.1523/JNEUROSCI.0348-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevens B, McGrath P, Gibbins S, Beyene J, Breau L, Camfield C, Finley A, Franck L, Howlett A, McKeever P, O’Brien K, Ohlsson A, Yamada J. Procedural pain in newborns at risk for neurologic impairment. Pain. 2003;105:27–35. doi: 10.1016/s0304-3959(03)00136-2. [DOI] [PubMed] [Google Scholar]
- 53.Taddio A, Shah V, Gilbert-MacLeod C, Katz J. Conditioning and hyperalgesia in newborns exposed to repeated heel lances. JAMA. 2002;288:857–861. doi: 10.1001/jama.288.7.857. [DOI] [PubMed] [Google Scholar]
- 54.Todd AJ, Sullivan AC. Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J Comp Neurol. 1990;296:496–505. doi: 10.1002/cne.902960312. [DOI] [PubMed] [Google Scholar]
- 55.Torsney C, MacDermott AB. Disinhibition Opens the Gate to Pathological Pain Signaling in Superficial Neurokinin 1 Receptor-Expressing Neurons in Rat Spinal Cord. J Neurosci. 2006;26:1833–1843. doi: 10.1523/JNEUROSCI.4584-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waldenstrom A, Thelin J, Thimansson E, Levinsson A, Schouenborg J. evelopmental learning in a pain-related system: evidence for a cross-modality mechanism. J Neurosci. 2003;23:7719–7725. doi: 10.1523/JNEUROSCI.23-20-07719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walker SM, Meredith-Middleton J, Cooke-Yarborough C, Fitzgerald M. Neonatal inflammation and primary afferent terminal plasticity in the rat dorsal horn. Pain. 2003;105:185–195. doi: 10.1016/s0304-3959(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 58.Walker SM, Tochiki KK, Fitzgerald M. Hindpaw incision in early life increases the hyperalgesic response to repeat surgical injury: critical period and dependence on initial afferent activity. Pain. 2009;147:99–106. doi: 10.1016/j.pain.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 59.Wu C, Boustany L, Liang H, Brennan TJ. Nerve growth factor expression after plantar incision in the rat. Anesthesiology. 2007;107:128–135. doi: 10.1097/01.anes.0000267512.08619.bd. [DOI] [PubMed] [Google Scholar]
- 60.Wu C, Erickson MA, Xu J, Wild KD, Brennan TJ. Expression profile of nerve growth factor after muscle incision in the rat. Anesthesiology. 2009;110:140–149. doi: 10.1097/ALN.0b013e318190bc84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshimura M, Nishi S. Blind patch-clamp recordings from substantia gelatinosa neurons in adult rat spinal cord slices: pharmacological properties of synaptic currents. Neuroscience. 1993;53:519–526. doi: 10.1016/0306-4522(93)90216-3. [DOI] [PubMed] [Google Scholar]