Abstract
The interpretation of quantitative phosphoproteomics studies is complicated because each differential phosphorylation event integrates both changes in protein expression and phosphorylation. Here we investigated this phenomenon by performing parallel comparisons of protein expression and phosphorylation in S. cerevisiae. In each of two experiments comparing yeast mutants bearing deletions in FUS3 or STE7 with their wild-type counterparts, we quantified over 4100 proteins, including all members of the yeast mating pathway. We also identified 12,499 unique phosphorylation sites in this work. We demonstrate the critical importance of controlling the protein-level false-discovery rate and provide a novel method to assess the accuracy of protein false-discovery rate estimates. For the first time, 96% of nonredundant phosphopeptide ratios could be calibrated by protein levels, allowing truly differential phosphorylation to be distinguished from altered protein expression. This revealed a starkly different view, with 25% of seemingly differential phosphopeptides now attributed to changes in protein expression. Combined protein expression and phosphorylation surveys uncovered both independent and concerted changes in protein expression and phosphorylation, while highlighting the partially redundant role of a second MAPK (Kss1) in the mating pathway.
Protein phosphorylation networks are master regulators within eukaryotic cells. Reversible phosphorylation of serine, threonine, and tyrosine residues regulate protein localization, activity, or interactions with other macromolecules. Together, these phosphorylation events form intracellular signaling networks that govern virtually all cellular functions, including growth and proliferation, metabolism, gene expression, and responses to environmental cues. Considerable effort has been devoted to characterizing protein phosphorylation using many genetic and biochemical approaches. Recently, methods for high-throughput mapping of protein phosphorylation sites have emerged, founded on advances in sample preparation, mass spectrometry, and informatics for proteomics applications. These methods are providing an unparalleled view of the protein phosphorylation landscape, with individual studies characterizing over 10,000 phosphorylation sites upon thousands of proteins in biological systems ranging from yeast (1) to mammalian cell lines (2–4) and intact organs (5, 6).
When combined with any of several stable isotope labeling techniques, mass spectrometry methods are capable of simultaneous identification and relative quantification of phosphorylation events, enabling quantitative comparison of phosphorylation profiles within multiple cell states (7–13). Though such studies have provided insight into phosphorylation dynamics, the quantitative data require careful interpretation because differential phosphopeptide abundances reflect not only changes in phosphorylation status, but also modified expression of substrate proteins (2, 14–16) (supplemental Fig. S1). This limitation has been long recognized, and theoretically, has a straightforward solution: simultaneously quantifying protein expression and phosphorylation would allow true differential phosphorylation to be distinguished from altered protein expression. However, several technical challenges have made this solution difficult to apply globally. First, because of their relatively low abundance and unfavorable chemical properties, phosphopeptides must be enriched prior to mass spectrometric analysis; thus, a single liquid chromatography-MS (LC-MS)1 analysis can target global protein expression or phosphorylation, but not both. Second, enormous dynamic range often prevents joint quantitation of expression and phosphorylation for many proteins in parallel experiments targeted to each property. One benefit of phosphopeptide enrichment has been identification of low-abundance phosphoproteins that would otherwise be undetectable, even with extensive fractionation. A significant portion of every phosphoproteome profile is invisible without enrichment, precluding independent measurement of protein expression and preventing validation of true differential phosphorylation. Finally, acquiring and assembling proteomics datasets encompassing both global protein expression and phosphorylation are major challenges.
Though these technical challenges have made routine normalization to account for protein expression within quantitative phosphorylation studies difficult, technological improvements are beginning to make this elusive goal a reality. The recent introduction of a hybrid dual-cell linear ion trap-Orbitrap mass spectrometer has offered improved speed and sensitivity for proteomic analyses (17, 18). Coupled with improved sample handling and data analysis, these advances offer the potential for increasingly sensitive and efficient proteomic and phosphoproteomic analyses. As demonstrated by recent proteomic characterization of haploid and diploid yeast (19), due to its small, well-annotated proteome and its extensive resources detailing protein expression levels (20) and protein localizations (21), Saccharomyces cerevisiae is well matched to current technical capabilities and is an ideal model to evaluate the feasibility and importance of accounting for differential protein expression during quantitative phosphoproteomic analyses.
To investigate the influence of protein expression on quantitative phosphoproteome profiling, we examined the effects of deletion of two members of the mitogen-activated protein kinase (MAPK) pathway, which is highly conserved from yeast to humans (22, 23) and in yeast mediates response to mating pheromone (24, 25), ultimately preparing the cell for mating by activating transcription, altering cell morphology, and arresting the cell in G1 phase (26, 27). Within this pathway, Fus3 phosphorylates the Ste12 transcription factor, causing it to bind to pheromone response elements within upstream activating sequences of its target genes. Ste12 also regulates genes involved in invasive growth in haploids and pseudohyphal growth in diploids through a second partially redundant MAPK, Kss1 (26–28). Along with sharing a transcription factor, the mating and filamentous growth pathways also share many kinases involved in the mating response (28). The developmental specificity distinguishing these pathways depends on the catalytic and noncatalytic activities of Fus3 and Kss1 (29). Surprisingly, though they occur side-by-side within the same linear signaling pathway, ablation of each kinase's activity through gene deletion has strikingly different effects. Although deletion of FUS3 produces only a slight mating defect, presumably through Kss1 substitution, deletion of STE7 causes sterility (29).
In this study, we demonstrate the feasibility and importance of accounting for protein expression changes to allow proper interpretation of quantitative phosphoproteomics data. In addition to quantitatively characterizing yeast protein phosphorylation with exceptional depth, we achieved comprehensive analyses of protein expression utilizing new instrumentation and optimized separations, allowing, for the first time, the ubiquitous calibration of protein phosphorylation changes to account for altered protein expression. Deconvoluting these changes in protein expression and phosphorylation profoundly affected subsequent interpretation of observed phosphoproteome dynamics. Applying this strategy to investigate the roles of Fus3 and Ste7 within the yeast MAPK pathway showed both independent and concerted changes in protein expression and phosphorylation and highlighted the partially redundant role of a second MAPK (Kss1) in the mating pathway.
MATERIALS AND METHODS
Yeast Strains, Metabolic Labeling Conditions
All three yeast strains were derived from the same background which contained a lys1Δ (strain BY4742 MAT α, derived from S288c). Wild-type cells were grown in media containing unlabeled lysine (light), while deletion strains (ste7Δ and fus3Δ) were grown in media with lysine enriched in stable isotopes to give ∼8 Da increase in mass (heavy, 13C6, 15N2-lysine; Cambridge Isotopes). After ∼18 h, cell populations had undergone >10 doublings until they reached log-phase (OD600 = 1.0) with quantitative incorporation of heavy lysine.
Cell Lysis and Protein Extraction and Digestion
Light and heavy stable isotope labeling with amino acid in cell culture (SILAC)-labeled yeast cells were harvested by centrifugation, and resuspended at 4 °C in a buffer containing 50 mm Tris pH 8.2, 8 m urea, 75 mm NaCl, 50 mm NaF, 50 mm β-glycerophosphate, 1 mm sodium orthovanadate, 10 mm sodium pyrophosphate and one protease inhibitor mixture tablet (complete mini, EDTA-free; Roche) per 10 ml. Cells were lysed using the MiniBeadbeater (Biospec, Bartlesville, OK) for microcentrifuge tubes at maximum speed, four cycles of 60 s each, with 2 min pauses between cycles to avoid overheating of the lysates. After centrifugation, lysates were transferred to new tubes. The protein concentration in the lysate was determined by bicinchoninic acid (BCA) protein assay (Pierce) and proteins were subjected to disulfide reduction with 5 mm dithiotreitol (56 °C, 25 min) and alkylation with 15 mm iodoacetamide (room temperature, 30 min in the dark). Excess iodoacetamide was quenched with 5 mm dithiotreitol (room temperature, 15 min in the dark). The denatured protein extract was then diluted and digested with 5 ng/μl lys-C (Wako) in 25 mm Tris pH 8.8, 1.5 m urea, at 37 °C for 15 h.
Western Blotting for Kss1
Lysates of wild type, kss1Δ, ste7Δ, and fus3Δ of yeast MAT a and MAT α were resolved using 4 to 12% SDS/PAGE, transferred onto Protran membranes (Whatman, Keene, NH), blocked with 3% milk in Tris-buffered saline/Tween 20 (TBST), incubated with a 1:1000 dilution of primary Kss1 antibody (Santa Cruz, Santa Cruz, CA) at 4 °C overnight, washed, and incubated with a 1:5000 dilution of secondary antibody (horseradish peroxidase-conjugated) in 3% milk in TBST for 1 h at room temperature. Bands were visualized with enhanced chemiluminescence solutions.
Peptide Separation and Phosphopeptide Enrichment
Peptide mixtures were acidified by addition of 10% trifluoroacetic acid to a final concentration of 0.4%, clarified by centrifugation and desalted using tC18 SepPak cartridge (Waters, Milford, MA). For protein expression analysis, we performed the ste7Δ versus wt separation first using hydrophilic interaction chromatography (HILIC) into 30 fractions using a 4.6 × 250 mm TSKgel Amide-80 5 μm particle column (Tosoh Biosciences, Japan) with a 50-min gradient of 5–28% H2O in acetonitrile with 0.125% formic acid. After analysis, we noticed that several fractions could be combined to reduce the number of samples. Thus, the fus3Δ versus wt experiment included only 20 HILIC fractions, but yielded a similar number of protein identifications. For phosphorylation experiments, peptides were separated into 12 fractions by strong cation chromatography (SCX) as described (30).
LC-Tandem MS (MS/MS) Analyses
Dried peptides and phosphopeptides were resuspended in the solvent of 5% acetonitrile and 4% formic acid, respectively, and 2 μl were loaded onto a microcapillary column packed with C18 beads (Magic C18AQ, 5 μm,200 Å, 125 μm × 16 cm) using a Famos autosampler (LC Packings, San Francisco, CA). Peptides were separated by reversed-phase chromatography using an Agilent 1100 binary pump with a 70-min (protein expression) or 90-min (phosphopeptide) gradient of 5–30% acetonitrile (in 0.125% formic acid) and detected in a hybrid dual-cell quadrupole linear ion trap-Orbitrap mass spectrometer (LTQ Orbitrap Velos, ThermoFisher, with a software of Xcalibur 2.0.7 SPI) using a data-dependent Top20 method (31). For each cycle, one full MS scan (resolution: 60,000) in the Orbitrap at a 106 AGC target was followed by up to 20 MS/MS experiments in the LTQ for the most intense ions. Selected ions were excluded from further analysis for 30 s. Ions with charge 1+ or unassigned were also rejected. Maximum ion accumulation times were 1000 ms for each full MS scan and 150 ms for MS/MS scans.
Database Searches and Data Filtering
Following the acquisition of mass spectrometry data, RAW files were converted into mzXML format. Individual precursors selected for MS/MS fragmentation were checked for incorrect monoisotopic peak assignments while refining precursor ion mass measurements (6). All MS/MS spectra were then searched using the Sequest algorithm (version 28) (32). Spectra were matched against a database encompassing sequences of all proteins in the yeast open reading frame (ORFs) database (S288C 2010) downloaded from the Saccharomyces Genome Database. Each protein sequence was listed in both forward and reversed orientations to facilitate estimation of peptide and protein identification false discovery rates (FDRs). Data from protein expression experiments were searched using the following parameters: 10 ppm precursor mass tolerance; 1.0 Da product ion mass tolerance; up to two missed cleavages; variable modifications: oxidation of methionine (+15.9949); fixed modifications: carbamidomethylation of cysteine (+57.0214). Phosphopeptide samples were searched using same parameters, though variable phosphorylation of serines, threonines, and tyrosines (+79.9663) were permitted as well.
The target-decoy method was employed to distinguish correct and incorrect identifications and thus control peptide and protein level FDRs (33). Data for surveying protein expression and phosphorylation were processed separately throughout. Linear discriminant analysis (LDA) was employed to develop a classifier that would distinguish correct and incorrect peptide identifications using numerous parameters such as Xcorr, ΔCn, precursor mass error, and charge state (6). Separate linear discriminant models were trained for each LC-MS analysis using forward and reversed peptide sequences to provide positive and negative training data. This approach is similar to other methods in the literature that employed different features or alternative classifiers (34–36). After scoring, matches to peptide sequences less than six amino acids in length were removed and peptide spectral matches were arranged in descending order by discriminant score and filtered to a 1% FDR based on the number of decoy sequences in the remaining data set. As previously discussed in the literature, because phosphorylated and nonphosphorylated peptides have different score distributions, the dataset was restricted to peptides bearing phosphorylation modifications when determining FDRs for phosphopeptide-enriched samples (37).
When large proteomics data sets such as the phosphorylation and nonphosphorylation surveys described here are assembled, the protein-level FDR often explodes and reaches excessive levels, despite keeping the peptide-level FDR at a constant 1% (see Fig. 3B). Therefore, we applied an additional protein-level filter to each data set to reduce the protein-level FDRs for proteins and phosphoproteins. After associating all peptides with their corresponding proteins, proteins were scored based on their multiplied peptide LDA probabilities. The sorted list was filtered based on reversed protein hits to maximally contain only 1% FP. By strictly controlling the error at the combined data set level, either individual data set (e.g. fus3Δ versus wild type protein expression or ste7Δ versus wild type for phosphorylation) will be even lower.
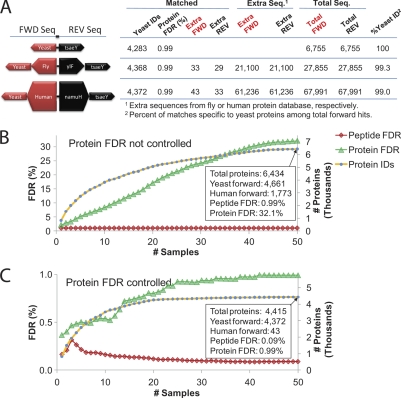
Fig. 3.
Validation of the accuracy of the identification rate (4283 proteins) and false discovery rate (FDR) in the two experiments. A, Three different yeast databases were used to search the MS/MS from the combined 50 samples from both experiments. Each yeast database also contained increasing amounts of known bogus sequences (e.g. randomized fly genome, randomized human genome) followed by a reversed complement of everything. More than 4300 yeast proteins were still identified even in the presence of up to 10× additional (incorrect) sequence. The protein FDR calculated based on the number of accepted reversed protein sequences was < 1%. The number of forward and reverse proteins accepted from fly and human was similar and less than 1% of identified yeast proteins, indicating that >4300 proteins were identified, and the FDR estimate is accurate. B, Combining 50 LC-MS/MS runs requires controlling both peptide- and protein-level FDRs. Cumulative plot for peptide and protein FDRs from 50 LC-MS/MS analyses using the combined yeast-human database in A. The peptide FDR was set to 1% for each fraction, but the protein FDR was uncontrolled. Final protein FDR climbed to 32.1% and 1773 human sequences (incorrect matches) were accepted. C, Same as in B, but all accepted proteins (6434) were now scored and then filtered to 1% FDR based on reversed protein sequences. The cumulative plot shows a greatly reduced peptide FDR and protein FDR that approaches 1%. Only 43 human sequences (incorrect) were accepted under these conditions.
Validation of Protein Detection Rate and FDR Using Extended Databases
To get a second assessment of the FDR of our data sets, all 50 LC-MS/MS protein identification analyses were searched as described above against three different databases (see Fig. 3A) including yeast (6755 sequences), yeast+fly (Flybase Drosophila melanogaster revision 5.23 27,855 sequences), and yeast+human (International Protein Index database, version 3.60 67,991 sequences). To avoid any issues with matching contaminant proteins, the fly and human databases were randomized, i.e. amino acid residues were shuffled randomly using a randomize function in PHP for every protein in the fly and human database. For each database a reversed compliment was created and appended to the end of the forward version. Searches against each database were processed identically, the only difference being the size of each database and the amount of known bogus sequence included. Searches were always filtered initially to a 1% FDR at the peptide level. In addition, protein-level filtering was either applied at the level of the entire data set or not considered such that only 1% false positive protein identifications remained based on the TDA. Matches to sequences known to contain bogus information (e.g. fly- or human-derived proteins) in either orientation (forward or reversed) were counted. A validation of the FDR at the protein level can be assessed by considering the number of fly- or human-derived protein sequences accepted in the final data set. In each case the target-decoy database approach (TDA) and the extended databases method both reported FDRs at the protein level of less than 1%, yet still identified more than 4200 yeast proteins.
Phosphorylation Site Localization and Peptide Quantification
To assign phosphorylation site localizations and measure the assignment confidence, we applied a probabilistic algorithm (37) that considers all phospho-forms of a peptide and uses the presence or absence of experimental fragment ions unique to each to create an ambiguity score (Ascore). The Ascore indicates the likelihood that the best site match is correct when compared with the next best match. We considered sites with Ascore ≥13 (p ≤ 0.05) to be confidently localized. The phosphoproteins identified (1910) is less than those (1958) listed in the supplemental Table S8 (phosphopeptides identified), because we applied an additional criterion for single peptide matches requiring the peptide score to be greater than 10.
Peptide quantification was performed using the Vista algorithm (38). We required a signal-to-noise (S/N) value >5 for both heavy and light species for quantification. For peptides found exclusively as singlets (only heavy or only light peak present), we reported the peak S/N ratio or its inverse, as a proxy for relative abundance measurement. For such peptides, we required a S/N value >10 for the observed species. In addition, if the S/N value of one member of a pair was less than 5, the partner was required to be greater than 10. Finally, to avoid quantifying FPs, any identification from a singlet peak was required to pass a 10-fold more stringent identification threshold (Q-value < 0.001; precision > 99.9%). Raw abundance ratios from each experiment were normalized based on the median distribution ratio. An example of protein identification and relative quantification is shown in supplemental Fig. 2.
Motif Analysis
Phosphopeptides were categorized based on surrounding sequence into acidic, basic, proline-directed, or “other” classes using a decision tree as described (39). For the fold enrichment plot in Fig. 5C, the minimal consensus motif for MAPK (Ser/Thr-Pro) was used. The background frequency of this motif for all centered S and T residues in the yeast proteome was compared with the frequency in each data set. The background fold enrichment was ∼4. Subsequently, the fold enrichments for experiment-specific subsets of up- and down-regulated (or unregulated) sites were calculated.
Fig. 5.
Protein normalization profoundly affects the detection of regulated phosphorylation events. A, Summary of regulated phosphorylation events (>3 S.D.) before and after including protein calibration. Arrows show the direction of regulation. For example, 34% of down-regulated sites in a ste7Δ versus wt experiment became unregulated considering protein changes, and seven new sites achieved significance. B, Examples of the effect of protein normalization. C, Fold enrichment over background of the minimal MAPK consensus motif (Ser/Thr-Pro) for up- and down-regulated sites. The fraction (percent) of sites in each category containing this motif is shown above as a pie graph. No enrichment among regulated sites in the frequency of phosphorylated [ST]P was seen in the fus3Δ versus wt experiment presumably due to compensation by a redundant MAPK, Kss1. Ste7Δ downregulated sites were significantly more enriched for the MAPK motif compared with the entire data set, suggesting MAPK targets are now present. D, Phosphoproteins show more protein-protein interactions on average than non-phosphoproteins.
Data Dissemination
LC-MS/MS raw data files are available for all 50 protein expression LC-MS/MS runs and all 24 phosphorylation analyses at the Tranche website ((https://proteomecommons.org/. Hashes are in the following)
FUS3 deletion phosphorylation data (including 12 raw files)
Eeb7B+prv0TTwMlII762qp/OYab65a/WnXMfrP9EErbfrmgoME22bXHLeTjsry1m+Jhf/zuRKEeR+9+DzC0KisS7aXUAAAAAAAALFQ==
FUS3 deletion protein data (including 20 raw files)
4IsldwgArk0+lflVQqnUCihheQHN8TMvJ80c0yZP28U5ean0BSfS251ma50M9jBgPd+ZfiARSzcua2cS1sez2zTdR6AAAAAAAAAOoA==
STE7 deletion phosphorylation data (including 12 raw files)
fMkp/834/DtnPHeSKF8b1LGYdZOS5qkqFkHNXViXcIftkdfKHeJssNcfdvCeUlKOHT26SuAMYVOMUCnjb4NwQhG4e/EAAAAAAAAKrw==
STE7 deletion protein data (including 30 raw files)
FgabfJJPA+Yjmo/srpwcV+/2IXjcU/yq7/si03nSwSbEzKqXUT6Sjub08fjKuVgHowdmfxksrsxg4RKS13obZ+t5auEAAAAAAAATfg==
Four supplementary tables as Excel files are available (supplemental Table S3–S8) containing proteins identified (supplemental Tables S3), proteins analyzed in both fus3Δ versus wt and ste7Δ versus wt experiments (supplemental Table S4), phosphorylation sites identified (supplemental Table S5), phosphopeptides analyzed in both experiments (supplemental Table S6), total peptides identified (supplemental Table S7) and total phosphopeptides identified (supplemental Table S8), respectively.
RESULTS
Comprehensive Protein Expression Changes for Two Yeast Mutants
To evaluate the effect of protein-level normalization on phosphorylation changes, we first examined our ability to provide comprehensive analyses of the S. cerevisiae proteome from minimally separated lysates. We chose to compare yeast mutants for two kinases in the MAPK mating pathway (Figs. 1 & 2). Metabolic labeling with stable isotopes to quantitatively encode yeast proteomes was applied such that protein expression comparisons for fus3Δ versus wild type and ste7Δ versus wild type could be performed. Lysates from each experiment were mixed equally and digested with endoproteinase lys-C. The peptide mixtures were separated by HILIC with fraction collection. The ste7Δ versus wild type experiment was performed first and 30 HILIC fractions were analyzed by LC-MS/MS using a dual cell quadrupole linear ion trap (dcQLT) - Orbitrap mass spectrometer, which offers improvements in both sensitivity and speed over older orbitrap platforms (17, 18). Based on these results, we determined that 20 fractions could provide the same level of proteome depth such that only 20 fractions were analyzed for the fus3Δ versus wild type experiment. In total, 50 fractions were examined by LC-MS/MS techniques. Data were searched using the Sequest algorithm using a target-decoy database approach to control and assess false discovery rates (FDRs). The final data set (4283 proteins) contained more than 4100 protein identifications from each experiment with an FDR at the protein level estimated to be <1% (Fig. 2B).
Fig. 1.
Experimental workflow. Wild-type yeast was grown in light SILAC labeling media containing normal isotopic abundance of lysine. Two yeast mutants (fus3Δ, and ste7Δ) were grown in media with lysine labeled with heavy isotopes of N and C (∼8 Da increase per lysine). Cultures were harvested at OD600 = 1.0 and then mixed such that fus3Δ:wt and ste7Δ:wt experiment were created. Each experiment was proteolyzed with lys-C. For protein analysis the workflow involved separation by HILIC with fraction collection. For phosphorylation analysis, a tandem affinity enrichment approach was used separating phosphopeptides first by SCX chromatography and then enriching them by IMAC. LC-MS/MS analysis of subsequent fractions was performed using an LTQ Orbitrap Velos system.
Fig. 2.
Collection of comprehensive yeast proteome data sets comparing wt yeast with two mating-pathway mutants (fus3Δ, ste7Δ). A, MAPK cascade controlling the mating pathway and the two mutants to be compared by metabolic labeling and LC-MS/MS techniques. B, Overview of the two data sets acquired in this study. C, D, Measured protein expression changes for fus3Δ:wt and ste7Δ:wt, respectively. Ratios represent protein-level fold changes and are plotted against the summed peptide signal-to-noise values from the MS. Proteins with known pheromone sensitivity or mating pathway function (yellow), and transposable elements (green) are highlighted. E, Validation of Kss1 protein level changes in fus3Δ cells. Kss1 levels were assessed by Western blotting in cells from both mating types and various MAPK pathway mutants. F, MS-based peptide ratios for one peptide (left) and all detected Kss1 peptides (right; error bars are ± one S.D.). Extracted ion chromatograms (±5 ppm) for light (wt) and heavy (fus3Δ) forms for the doubly charged Kss1-derived peptide sequence shown.
Although the log-transformed protein SILAC ratios (Figs. 2C, 2D) were tightly centered around zero (no change) (Standard deviations of log-transformed protein ratio are 0.32 and 0.38, respectively, for ste7 and fus3 protein dataset), FUS3 deletion resulted in the up-regulation of several proteins in the mating pathway, most notably Kss1 (4.7-fold) which was validated by Western blotting (Fig. 2E). In contrast, STE7 deletion (Fig. 2D) did not up-regulate Kss1 (Fig. 2F); rather, it caused down-regulation of several known members of the yeast mating pathway, including Ste3 (10.0-fold), Far1 (5.3-fold), Gpa1 (4.5-fold), and Sst2 (12.5-fold).
Validation of the Accuracy of Protein FDR for Proteome-scale Data Sets
A proven method for both controlling and estimating false positives within a data set is the target-decoy database approach (TDA) (33). A protein sequence database is combined with an equal amount of bogus sequences by reversing each protein. If all assumptions are met, the concatenated database is twice as large, but incorrect matches now choose equally between the forward and reversed proteins. Decoy (reversed protein) matches remaining after filtering provide an estimate of the overall number of FP assignments in the data set. Our filtering approach differentiated true from false positives via linear discriminant analysis (LDA) using several different parameters including Xcorr, ΔCn, precursor mass error, and charge state (6). Though consistent parameters were used throughout, specialized linear discriminant models were trained separately for each LC-MS analysis using peptide matches from forward and reversed protein sequences as positive and negative training data. This approach is conceptually similar to other approaches that have been published previously employing distinct machine learning techniques and relying upon different sets of features (34–36). Following scoring using each run's specialized linear discriminant function, peptide spectral matches within each run were sorted in descending order by their discriminant score and filtered to a 1% FDR as revealed by the number of decoy sequences in the filtered data set.
As proteomics data sets grow larger by combining data from several experiments samples, the protein FDR can increase substantially, despite the fact that the peptide-level FDR remains at 1%. Thus, additional filtering is required at the protein level to ensure that both peptide and protein-level FDRs are controlled. To control protein-level FDRs in this study, peptides from all fractions were combined and assembled into proteins. Each protein was scored based on multiplying all its LDA peptide probabilities. Proteins were then sorted based on this score, thus ranking the proteins in order of decreasing confidence. Proteins were then drawn from this list and added to the final dataset until at most one out of every hundred proteins in the final dataset corresponded to a reversed sequence. All remaining proteins were discarded. After filtering at the protein level, the FDR among those remaining peptides was now substantially lower (∼0.1%).
Final data sets contained more than 4,100 identified proteins (Fig. 2B, Tables S1 and S3) at a protein level FDR of less than 1%. In support of the accuracy of our FDR determinations, we introduced additional bogus sequence information by appending a randomized fly or human proteome to the yeast database (Fig. 3A). Matches to these sequences in either the forward or reversed orientations are also incorrect by definition. We found that 1) more than 4200 proteins were still identified using any of the three databases even with 10× more confounding sequence; 2) matches to forward or reversed fly or human sequences were, as predicted, not significantly different (p = 0.35, 0.15, respectively; cumulative binomial probability) demonstrating that incorrect matches chose equally, and 3) the FDR estimates based solely on the fly and human database searches were also less than 1%. This multispecies database approach provides an excellent (and orthogonal) method to verify protein FDR estimates. We found that >99% of all passing identifications were derived from yeast sequences, estimating the FDR to be <1%. We conclude that these data sets do indeed contain evidence of expression for more than 4200 yeast ORFs.
Comprehensive Phosphorylation Expression Changes for Two Yeast Mutants
Having in hand two comprehensive protein-level data sets, we next collected the corresponding and complementary phosphorylation ones. The workflow for phosphorylation analysis is shown in Fig. 1. Starting with 5 mg of each isotope-labeled lysate and using tandem affinity enrichment of phosphopeptides (30, 40), we analyzed 12 fractions (24 for both experiments) by LC-MS/MS using the same hybrid dual cell quadrupole linear ion trap - orbitrap MS. The numbers of phosphopeptides and total peptides detected in each fraction for the fus3Δ:wt comparison are displayed in Fig. 4A. Using new instrumentation and optimized separations, thousands of phosphorylation sites were detected, while requiring no more instrument time than was devoted to the original surveys of protein expression. For example, within a single 90-min LC-MS analysis, we were able to identify more than 3,200 unique phosphorylation sites from over 8700 matched phosphopeptides (Fig. 4A, fraction #3). In each of the two experiments, over 8000 distinct phosphorylation sites were detected. Combined, these two phosphorylation surveys identified at least 12,499 sites from 1910 proteins (Figs. 4B, 4C). To count numbers of identified phosphorylation sites, each localized site was included once. In addition, nonlocalized sites were counted when no localized phosphorylation sites had been assigned within the range of amino acids where the non-localized site was expected to reside. When multiple nonlocalized sites were found to occupy overlapping sequence ranges, they were only counted as a single phosphorylation event. Thus we report a phosphorylation site count that provides our most conservative estimate of the minimum number of phosphorylation events required to explain all observed phosphopeptides.
Fig. 4.
Phosphoproteome data sets comparing wt yeast with two mating-pathway mutants (fus3Δ, ste7Δ). A, Total peptides and phosphopeptides detected in each of twelve SCX fractions in the fus3Δ:wt experiment using the hybrid dual cell linear ion trap-orbitrap MS. B, C, Summary of phosphorylation sites and phosphoproteins identified. D, E, Phosphorylation level analyses. Log2 ratio distributions for unique phosphopeptides analyzed. Note that greater than 3 S.D. changes were considered significant. F, Fraction of unique phosphopeptides in each data set for which protein normalization was performed. G, Fraction of up- and down-regulated (>3 S.D.) phosphopeptides after protein normalization.
These experiments yielded quantitative ratios for 12,907 (fus3Δ:wt), and 10,588 (ste7Δ:wt) unique phosphopeptides, respectively (supplemental Table S6). Most peptides showed little or no change in phosphorylation. However, ∼2% of phosphopeptides were considered significantly up- or down regulated by >3 S.D. (Figs. 4D, 4E, 4G), including sites on several known substrates of Fus3 (Dig1, Dig2, Ste12, Far1, Sst2). We chose three standard deviations as the cutoff for high significance to facilitate comparisons of before and after protein normalization.
To evaluate the effect of protein normalization on measured phosphorylation changes requires a comprehensive protein-level data set. In total, 96.7 and 96.4% of unique phosphopeptides quantified in fus3Δ and ste7Δ strains, respectively, had corresponding protein ratios (Fig. 4F). Encouraged by the high overlap, we calculated the effect of protein-level changes on our phosphorylation data set for ste7Δ (Fig. 5A). Strikingly, a large fraction of apparent phosphorylation changes were in fact because of changes in protein expression. For example, before normalization, 146 phosphopeptides in the ste7Δ versus wt comparison were down-regulated by more than three S.D., but 49 (34%) of these were no longer regulated after accounting for protein changes. Moreover, seven new phosphopeptides were now significantly down-regulated. Including both up- and down-regulated phosphopeptides, an average of one in four regulated events was removed based on protein normalization. Similarly, an additional 13% of regulated phosphopeptides were identified that had previously been classified as unchanging. The effect of protein normalization for some example proteins from the mating pathway is shown in Fig. 5B. Ste12 is the critical transcription factor in this pathway. S261 from Ste12 appears down-regulated by twofold in the ste7Δ strain, but protein abundance also decreases by about 2 fold, indicating that the phosphopeptide change is due to protein expression differences. In another example, a 2.5 fold up-regulation of S59 and S73 from Hsp12 is perfectly explained by protein abundance changes alone.
MAPKs are proline-directed kinases that target a minimal consensus sequence consisting of Ser/Thr-Pro. This important motif was very significantly enriched over background levels in both data sets (Fig. 5C). In addition, we found this motif even more frequently among ste7Δ down-regulated sites (with a concomitant decrease in enrichment for up-regulated sites), suggesting that bona fide substrates of Kss1 and Fus3 were present. A list of these potential substrates is found in supplemental Table S2. In contrast, FUS3 deletion resulted in no enrichment in the frequency of this motif among regulated sites, likely due to compensating Fus3-like activity of Kss1at this motif. In addition, we found that phosphoproteins, on average, had 1.6-fold more (23 versus 14) annotated interactions in SGD than nonphosphoproteins, as shown in Fig 5D, highlighting the central role of phosphorylation on regulatory proteins.
DISCUSSION
A change in the measured abundance of a phosphopeptide integrates differences in both protein expression and site stoichiometry (supplemental Fig. S1). Thus, the functional interpretation of regulated events from quantitative phosphorylation studies is often incomplete or inaccurate without considering protein expression changes. To assess the magnitude of the issue, we evaluated our ability to collect comprehensive protein expression data sets in S. cerevisiae using modern mass spectrometry techniques and optimized separations. We then applied these global protein data sets to judge the impact of protein normalization in large-scale phosphorylation experiments.
We chose two conserved kinases within the same linear pathway and studied their gene deletion effects on comprehensive protein and phosphorylation levels compared with wild type cells. Quantitative phosphorylation analysis generally relies on stable isotope labeling, enrichment of phosphopeptides, and analysis by high performance mass spectrometry. This culminates in the detection of thousands of phosphorylation events with associated abundance ratios between two cell states. Even given our straightforward ability to detect and quantify these differences, the interpretation of phosphopeptide ratios is highly dependent on underlying protein expression level changes. We encountered this phenomenon repeatedly in both the fus3Δ:wt and ste7Δ:wt data sets. On average, 25% of regulated phosphopeptide abundance changes could be attributed to protein expression differences. Equally important, an additional 13% of new events are detected, demonstrating the key importance of protein normalization to discern bona fide changes in protein phosphorylation status.
A vital issue for protein normalization is the collection of a comprehensive protein level data set. The analysis of more than 4000 expressed yeast proteins at 99% certainty has been proposed as an important milestone in quantitative proteomics and is thought to represent all but a few percent of all yeast protein expression (19). Here, using new instrumentation and optimized separations, we have done this twice with careful assessment of false positives such that the entire data set contained only 0.99% FDR at the protein level. The overlap of the proteins identified with those in a recent study (19) was 94% (Supplemental Fig. 3). Pertaining to this study, the major benefit of the depth of sequence analysis was that 96% of all nonredundant phosphopeptide ratios could be normalized by protein changes.
Some proteins that eluded detection in our data sets were not expected to be expressed, including the mating type-specific pheromone receptor (Ste2) and genes suppressed by growth on glucose as a carbon source (Gal1, Gal3). Furthermore, among the 6607 predicted yeast ORFs are 810 that are annotated as “dubious.” These are likely never expressed and can be used as an external verification of our FDR estimation. We identified only two proteins from these dubious ORFs. Assuming this identification was incorrect, a detection rate of 2/810 represents an FDR of 0.2%, confirming that the true protein FDR is well below 1%. Finally, we reassessed our data sets using a much larger database containing yeast proteins plus additional incorrect sequence information in the form of a randomized fly or human proteome (Fig. 3). Guided by the incorrect hits to proteins from the additional sequence sources, we calculated a second estimate of the FDR for our data sets. Using this new metric, we found that >99.0% of passing identifications were derived from yeast sequences, strongly supporting that these data sets do contain evidence for expression of >4200 identified proteins at <1% FDR.
Common signaling components like MAPK cascades respond to a large number of different stimuli, but are still capable of producing specific signals. In S. cerevisiae, the mating pheromone response and filamentous growth pathways share elements of the same MAPK pathway (Ste20, Ste11, Ste7, and Ste12) (41). The transcription factor, Ste12, is the terminal component of these signaling cascades that drives either filamentous and invasive growth or sexual differentiation. Gene deletion of FUS3 resulted in fundamentally different expression and phosphorylation changes than STE7 (Fig. 6) even though they are found within the same linear pathway and ultimately affect gene transcription through Ste12. The major effect with fus3Δ was the up-regulation of a second MAPK, Kss1, and a transcription factor, Tec1, whose activity provides developmental specificity. Tec1 cooperates with Ste12 to drive filamentous growth (41). In great contrast, in ste7Δ, Kss1 and Fus3 protein levels were unchanged. In addition, even though protein levels for Dig1, Dig2, and Ste12 did not change (Dig1 and Dig2 are involved in the regulation of mating-specific genes and the invasive growth pathway), known Fus3 phosphorylation events on these proteins were now down-regulated. Moreover, several Ste12-dependent mating pathway proteins were also down-regulated (Far1, Ste3, Gpa1, and Sst2). Taken together, these studies help explain the specific signaling idiosyncrasies and underlying phenotypes of each mutant. Notably, deletion of FUS3 results in only a mild mating defect, presumably because Kss1 can redundantly function within the mating pathway. However, STE7 deletion is sterile with no remaining mechanism to activate Fus3, Kss1, or their downstream target, Ste12.
Fig. 6.
Protein and phosphorylation changes associated with FUS3 (A) and STE7 (B) gene deletion with no external stimulus in exponentially growing yeast. Protein abundance changes are shown as fold changes over wt levels from green to red. Phosphorylation changes are calibrated by protein levels and are also shown on the same scale. FUS3 deletion results in the up-regulation of Kss1 which can fulfill a partially redundant role. STE7 deletion results in the inability to activate either Fus3 or Kss1 as kinases. Even without pheromone or external stimulation, many mating-pathway-specific phosphorylation events are down-regulated and known Fus3 substrates have downregulated phosphorylation. All proteins were quantified at least once when considering both experiments, but Tec1and Ste7 (shown in gray) were only quantified in one.
Our results support the need for protein normalization to become a standard part of the phosphoproteomic workflow in both yeast and other organisms as well as experimental systems (cell lines, etc). While this is now possible for unicellular organisms such as S. cerevisiae, the depth of coverage of for mammalian proteomes is still far from saturating. For example, we recently acquired protein expression and phosphorylation data sets for nine mouse tissues (6) where we detected between 4500 and 7500 expressed proteins in each tissue. However, 1000 to 2000 additional proteins in every tissue were identified only in phosphorylation studies, suggesting that we are at best only detecting ∼80% of expressed proteins. Although this falls short of the mark, its impact is sufficient that protein normalization should still be included, wherever possible. Looking forward, better methods, software, and instrumentation are still required prior to the realization of comprehensive proteome analysis in mammalian systems. The yeast results point to a large step forward in depth of analysis and dynamic range.
Acknowledgments
We thank all members of the Gygi lab for help, especially Woong Kim for his help on sample preparation, Deepak Kolippakkam, Ramin Rad and Sean Beausoleil for their bioinformatics support. We are grateful to E. Elion for project advice and critical reading.
Footnotes
* This work was supported in part by NIH grants (HG3456 and GM67945) to S. P. G.
 This article contains supplemental Figs. S1 to S3 and Tables S1 to S8.
This article contains supplemental Figs. S1 to S3 and Tables S1 to S8.
1 The abbreviations used are:
- LC-MS
- liquid chromatography MS
- FDR
- False-discovery rate
- HILIC
- Hydrophilic interaction liquid chromatography
- IMAC
- Immobilized metal affinity chromatography
- LDA
- Linear discriminant analysis
- MAPK
- Mitogen-activated protein kinase
- SCX
- Strong cation chromatography
- SGD
- Saccharomyces Genome database
- TBST
- Tris-Buffered Saline Tween-20
- TDA
- Target-decoy database approach.
REFERENCES
- 1. Holt L. J., Tuch B. B., Villén J., Johnson A. D., Gygi S. P., Morgan D. O. (2009) Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science 325, 1682–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Olsen J. V., Vermeulen M., Santamaria A., Kumar C., Miller M. L., Jensen L. J., Gnad F., Cox J., Jensen T. S., Nigg E. A., Brunak S., Mann M. (2010) Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci. Signal. 3, ra3. [DOI] [PubMed] [Google Scholar]
- 3. Choudhary C., Olsen J. V., Brandts C., Cox J., Reddy P. N., Böhmer F. D., Gerke V., Schmidt-Arras D. E., Berdel W. E., Müller-Tidow C., Mann M., Serve H. (2009) Mislocalized activation of oncogenic RTKs switches downstream signaling outcomes. Mol. Cell. 36, 326–339 [DOI] [PubMed] [Google Scholar]
- 4. Dephoure N., Zhou C., Villén J., Beausoleil S. A., Bakalarski C. E., Elledge S. J., Gygi S. P. (2008) A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 105, 10762–10767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wiśniewski J. R., Nagaraj N., Zougman A., Gnad F., Mann M. (2010) Brain Phosphoproteome Obtained by a FASP-Based Method Reveals Plasma Membrane Protein Topology. J. Proteome. Res. 9, 3280–3289 [DOI] [PubMed] [Google Scholar]
- 6. Huttlin E. L., Jedrychowski M. P., Elias J. E., Goswami T., Rad R., Beausolei S. A., Villén J., Haas W., Sowa M. E., Gygi S. P. (2010) A Tissue-Specific Atlas of Mouse Protein Phosphorylation and Expression. Cell 143, 1174–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grimsrud P. A., Swaney D. L., Wenger C. D., Beauchene N. A., Coon J. J. (2010) Phosphoproteomics for the Masses. ACS. Chem. Biol. 5, 105–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yates J. R., Ruse C. I., Nakorchevsky A. (2009) Proteomics by Mass Spectrometry: Approaches, Advances, and Applications. Annu. Rev. Biomed. Eng. 11, 49–79 [DOI] [PubMed] [Google Scholar]
- 9. Macek B., Mann M., Olsen J. V. (2009) Global and Site-Specific Quantitative Phosphoproteomics: Principles and Applications. Annu. Rev. Pharmacol. Toxicol. 49, 199–221 [DOI] [PubMed] [Google Scholar]
- 10. White F. M. (2008) Quantitative phosphoproteomic analysis of signaling network dynamics. Curr. Opin. Biotechnol. 19, 404–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ding S. J., Qian W. J., Smith R. D. (2007) Quantitative proteomic approaches for studying phosphotyrosine signaling. Expert. Rev. Proteomics. 4, 13–23 [DOI] [PubMed] [Google Scholar]
- 12. Witze E. S., Old W. M., Resing K. A., Ahn N. G. (2007) Mapping protein post-translational modifications with mass spectrometry. Nat. Methods. 4, 798–806 [DOI] [PubMed] [Google Scholar]
- 13. Smolka M. B., Albuquerque C. P., Chen S. H., Zhou H. (2007) Proteome-wide identification of in vivo targets of DNA damage checkpoint kinases. Proc. Natl. Acad. Sci. U.S.A. 104, 10364–10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trinidad J. C., Thalhammer A., Specht C. G., Lynn A. J., Baker P. R., Schoepfer R., Burlingame A. L. (2008) Quantitative analysis of synaptic phosphorylation and protein expression. Mol. Cell. Proteomics. 7, 684–696 [DOI] [PubMed] [Google Scholar]
- 15. Liao L., McClatchy D. B., Park S. K., Xu T., Lu B. W., Yates J. R., 3rd (2008) Quantitative Analysis of Brain Nuclear Phosphoproteins Identifies Developmentally Regulated Phosphorylation Events. J. Proteome Res. 7, 4743–4755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cutillas P. R., Geering B., Waterfield M. D., Vanhaesebroeck B. (2005) Quantification of gel-separated proteins and their phosphorylation sites by LC-MS using unlabeled internal standards - Analysis of phosphoprotein dynamics in a B cell lymphoma cell line. Mol. Cell. Proteomics. 4, 1038–1051 [DOI] [PubMed] [Google Scholar]
- 17. Olsen J. V., Schwartz J. C., Griep-Raming J., Nielsen M. L., Damoc E., Denisov E., Lange O., Remes P., Taylor D., Splendore M., Wouters E. R., Senko M., Makarov A., Mann M., Horning S. (2009) A dual pressure linear ion trap orbitrap instrument with very high sequencing speed. Mol. Cell. Proteomics. 8, 2759–2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Second T. P., Blethrow J. D., Schwartz J. C., Merrihew G. E., MacCoss M. J., Swaney D. L., Russell J. D., Coon J. J., Zabrouskov V. (2009) Dual-pressure linear ion trap mass spectrometer improving the analysis of complex protein mixtures. Anal. Chem. 81, 7757–7765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Godoy L. M. F., Olsen J. V., Cox J., Nielsen M. L., Hubner N. C., Fröhlich F., Walther T. C., Mann M. (2008) Comprehensive mass-spectrometry-based proteome quantification of haploid versus diploid yeast. Nature 455, 1251–1260 [DOI] [PubMed] [Google Scholar]
- 20. Ghaemmaghami S., Huh W. K., Bower K., Howson R. W., Belle A., Dephoure N., O'Shea E. K., Weissman J. S. (2003) Global analysis of protein expression in yeast. Nature 425, 737–741 [DOI] [PubMed] [Google Scholar]
- 21. Huh W. K., Falvo J. V., Gerke L. C., Carroll A. S., Howson R. W., Weissman J. S., O'Shea E. K. (2003) Global analysis of protein localization in budding yeast. Nature 425, 686–691 [DOI] [PubMed] [Google Scholar]
- 22. Widmann C., Gibson S., Jarpe M. B., Johnson G. L. (1999) Mitogen-activated protein kinase: Conservation of a three-kinase module from yeast to human. Physiol. Rev. 79, 143–180 [DOI] [PubMed] [Google Scholar]
- 23. Bardwell L. (2006) Mechanisms of MAPK signalling specificity. Biochem. Soc. Trans. 34, 837–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herskowitz I. (1995) MAP kinase pathways in yeat - for mating and more. Cell 80, 187–197 [DOI] [PubMed] [Google Scholar]
- 25. Mansour S. J., Resing K. A., Candi J. M., Hermann A. S., Gloor J. W., Herskind K. R., Wartmann M., Davis R. J., Ahn N. G. (1994) Mitogen-activation protein (MAP) kinase phosphorylation of MAP kinase kinase - determination of phosphorylation sites by mass-spectrometry and site directed mutagenesis. J. Biochem. 116, 304–314 [DOI] [PubMed] [Google Scholar]
- 26. Elion E. A., Brill J. A., Fink G. R. (1991) Fus3 represses cln1 and cln2 and in concert with kss1 promote signal transduction. Proc. Natl. Acad. Sci. U.S.A. 88, 9392–9396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elion E. A., Satterberg B., Kranz J. E. (1993) Fus3 phosphorylates multiple components of the mating signal-transduction cascade - evidence for ste12 and far1. Mol. Biol. Cell 4, 495–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roberts R. L., Fink G. R. (1994) Elements of a single MAP kinase cascade in Saccharomyces-Cerevisiae mediate, 2-developmental programs in the same cell type mating and invasive growth. Genes Dev. 8, 2974–2985 [DOI] [PubMed] [Google Scholar]
- 29. Schwartz M. A., Madhani H. D. (2004) Principles of map kinase signaling specificity in Saccharomyces cerevisiae. Annu. Rev. Genet. 38, 725–748 [DOI] [PubMed] [Google Scholar]
- 30. Villén J., Gygi S. P. (2008) The SCX/IMAC enrichment approach for global phosphorylation analysis by mass spectrometry. Nat. Protoc. 3, 1630–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haas W., Faherty B. K., Gerber S. A., Elias J. E., Beausoleil S. A., Bakalarski C. E., Li X., Villén J., Gygi S. P. (2006) Optimization and use of peptide mass measurement accuracy in shotgun proteomics. Mol. Cell. Proteomics. 5, 1326–1337 [DOI] [PubMed] [Google Scholar]
- 32. Eng J. K., McCormack A. L., Yates J. R. (1994) An approach to correlate tandem mass-spectral data of peptides with amino-acid-sequences in a protein database. J. Am. Soc. Mass. Spectrom. 5, 976–989 [DOI] [PubMed] [Google Scholar]
- 33. Elias J. E., Gygi S. P. (2007) Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods. 4, 207–214 [DOI] [PubMed] [Google Scholar]
- 34. Du X., Callister S. J., Manes N. P., Adkins J. N., Alexandridis R. A., Zeng X., Roh J. H., Smith W. E., Donohue T. J., Kaplan S., Smith R. D., Lipton M. S. (2008) A computational strategy to analyze label-free temporal bottom-up proteomics data. J. Proteome Res. 7, 2595–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Käll L., Canterbury J. D., Weston J., Noble W. S., MacCoss M. J. (2007) Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods. 4, 923–925 [DOI] [PubMed] [Google Scholar]
- 36. Zhang J., Ma J., Dou L., Wu S., Qian X., Xie H., Zhu Y., He F. (2009) Bayesian Nonparametric Model for the Validation of Peptide Identification in Shotgun Proteomics. Mol. Cell. Proteomics. 8, 547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beausoleil S. A., Villén J., Gerber S. A., Rush J., Gygi S. P. (2006) A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat. Biotechnol. 24, 1285–1292 [DOI] [PubMed] [Google Scholar]
- 38. Bakalarski C. E., Elias J. E., Villén J., Haas W., Gerber S. A., Everley P. A., Gygi S. P. (2008) The Impact of peptide abundance and dynamic range on stable-isotope-based quantitative proteomic analyses. J. Proteome Res. 7, 4756–4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Villén J., Beausoleil S. A., Gerber S. A., Gygi S. P. (2007) Large-scale phosphorylation analysis of mouse liver. Proc. Natl. Acad. Sci. U.S.A. 104, 1488–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ficarro S. B., McCleland M. L., Stukenberg P. T., Burke D. J., Ross M. M., Shabanowitz J., Hunt D. F., White F. M. (2002) Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat. Biotechnol. 20, 301–305 [DOI] [PubMed] [Google Scholar]
- 41. Madhani H. D., Fink G. R. (1997) Combinatorial control required for the specificity of yeast MAPK signaling. Science 275, 1314–1317 [DOI] [PubMed] [Google Scholar]