Abstract
Objective: There are a few studies that describe the oral findings in newborn children in various populations but none conducted for a Turkish population. Hence, this study determined the prevalence of intraoral findings in a group of newborns and examined the correlation among these findings with the mother’s systemic and gestational medical complications, cigarette consumption during pregnancy and consanguinity between the parents. Methods: 2,021 full-term, newborn children were examined. Oral cysts, ankyloglossia, attached upper midline frenum and other medical diagnoses at birth were investigated. Medical information for each child and parent was recorded via standard questionnaire. Obtained data was analysed using the Pearson Chi-Square test (P ≤ 0.05). Results: The most common findings were of oral inclusion cysts situated palatally. Conclusions: There was a statistically significant relationship between the presence of oral inclusion cysts with the congenital diabetes and also insulin treatment and cigarette consumption during pregnancy. Moreover, a significant relationship was found between the presence of oral inclusion cysts and gestational diabetes and with the presence of consanguinity between the parents (P = 0.004).
Key words: Oral cysts, natal teeth, consanguinity, gestational diabetes, cigarette consumption
INTRODUCTION
A variety of anomalies may be found in the oral cavity of the newborn child1. Only a few published national population-based studies such as Swedish, Taiwanese, Mexican and Indian studies1., 2., 3., 4. describe oral findings in newborn children. Some of the generally examined oral findings in these studies are; congenital oral cysts situated either palatally or on the alveolar ridges, ankyloglossia, Fordyce spots, natal/neonatal teeth, the attached upper labial frenum, relationship of the alveolar ridges and commissural lip pits. Medical data on each child were also reported to be collected from the birth records1., 2., 3., 4. including gestational age, height, weight, as well as birth complications and medical diagnoses, information on the mother’s health during pregnancy and ethnic affiliation.
Gingival cysts have also been referred to as alveolar cysts or inclusion cysts in some texts5., 6., 7., 8.. Liu and Huang4 stated that previous studies which investigated the incidence of certain oral abnormalities in newborns indicated a relatively high frequency of palatal cysts and gingival cysts. Palatal and alveolar cysts were described clinically5 as varying in colour from white, to grey, to yellow nodules, varying in size from a pinhead to 3 mm, and in numbers from one to six. Alveolar cysts were further classified according to location as either maxillary or mandibular, and anterior or posterior5. In a study by Donley and Nelson5 the clinical prevalence of palatal and alveolar cysts in premature infants was also compared to full-term infants. According to this study, the frequency of cysts has been reported to be high in newborns, but they are rarely seen after 3 months of age5.
Besides oral inclusion cysts, sometimes the chronology of tooth eruption suffers a more significant alteration in terms of onset, and the first teeth may be present at birth or arise during the first month of life9. Natal teeth were defined as teeth present at birth and neonatal teeth as teeth erupted within the first month of life9., 10., 11., 12., 13., 14., 15., 16., 17., 18., 19., 20., 21., 22.. Morphologically natal and neonatal teeth may be conical or may be of normal size and shape and opaque yellow-brownish in colour9 with poor or absent root formation, nevertheless, few cases might resemble normal primary dentition23., 24.. According to researchers9., 10., 13., the presence of natal teeth is due to several factors related to an unknown cause of disturbed biological chronology and there is no conclusive evidence of a correlation between early eruption and systemic disorders, but natal teeth may be associated with certain syndromes13., 25. and cleft palate9., 16., 23.. Natal teeth are discussed in conjunction with case reports and reviews of the literature9., 10., 12., 16., 26., 27., 28., 29., 30.. The estimated incidence is between 1:700 and 1:3,500 births. Girls are more often affected than boys. The two lower central deciduous incisors23., 24., 25., 26. are most frequently involved with less than 10% of natal teeth being supernumerary13., 23., 24., 25..
Ankyloglossia is a developmental anomaly of the tongue which can be seen in newborns, characterised by a short, thick lingual frenum resulting in limitation of tongue movement which can lead to problems with breast-feeding21. According to da Silva et al.17 newborns present some normal oral characteristics, as well as physiological variations, depending on their development stage. Knowledge of these aspects by dental professionals is fundamental to allow proper counselling to parents. However, no epidemiological survey of oral conditions which limits the study population in newborn Turkish children has been published. Turkey is a county where consanguinity of the parents is widespread (21%)31., 32.. Although the relationship between the mother’s health during pregnancy, gestational age, height, weight, as well as birth complications and medical diagnoses and oral findings in newborn children have been reported, the relationship between the oral findings and consanguinity of the parents in a limited ethnicity, has not been studied so far according to the authors’ knowledge. The objective of this research was to determine the prevalence of intraoral findings in a group of newborn Turkish children within the first 3 days of birth who were born within a specific period of time in a metropolitan area of Ankara, capital of Turkey and to study the specific correlation between the observed findings and consanguinity, besides other medical data including systemic and gestational diseases and cigarette consumption during pregnancy and developmental malformations. The second aim was to study the clinical and structural characteristics, the complications and the management of natal teeth.
METHODS
A transverse population-based study based on oral examinations of 2,021 newborn Turkish children examined in the Neonatal Department of Ankara Etlik Children Hospital during a 2-year period of consecutive months between November 2006 and August 2007 was performed. This institutionally approved study investigated the prevalence of several oral abnormalities in newborns delivered at this hospital coming from low to moderate income families. The Ethics Committee of Ankara University, Ankara, Turkey, reviewed and approved the research protocol and informed consent form.
While full-term (greater than 37 weeks gestation)2., 5., 6. babies were included, babies older than 3 days4., 6., who had systemic diseases requiring intensive care or subjected to several complications during delivery and premature babies were excluded from the study sample by convenience sampling3. Parents were informed about the investigation. Infants were identified by the paediatricians and the oral diagnosis specialist and the written consent of parents or legal guardians (required to be 18 years old or above and sufficiently able to read and sign) was obtained. The examiners conducted the exams concurrently to create inter-examiner reliability as previously described by Donley and Nelson5 and had the consensus for the definition of natal teeth and palatal, gingival cysts of the newborn. Also, prior to the study, 40 newborn children not included in the main study were examined simultaneously by the same examiners in order to standardise diagnostic criteria. The degree of agreement/disagreement between the researchers was assessed using the Wilcoxon matched-pairs signed ranks test (P ≤ 0.05). Photographs of the representative cases were also taken.
No biopsies were performed and only visual examinations were used for the diagnoses. Visual examinations were performed by gently opening the jaws of the neonate and by close observation with the help of a gloved index finger, portable lights, mirrors and disposable tongue blades in the hospital nursery2., 3., 4., 5.. All oral findings were recorded onto Excel data sheets. Medical information for each child regarding date of birth, gestational age, sex, birth weight, and medical diagnosis of the newborn at birth, if any, were obtained and collected for each infant from the neonatal birth record and analysed (Table 1). Data on the mother’s age, information on maternal health during pregnancy and medical complications including drug intake and cigarette consumption during pregnancy, systemic diseases, and in view of the possibility of a genetic disorder implied by the consanguinity of the parents, appearance of consanguinity between the parents were collected from the birth records and were recorded via standard questionnaire specifically designed for this study (Table 2).
Table 1.
Medical information of 2,021 newborns
| Gender | Girls (50%) | Boys (50%) |
| Birth weight | <2500 g (low birth weight) (7%) | ≥2500 g (93%) |
Table 2.
Parents’ medical information
| Mothers | ||||||||
| Age at birth (years) | 16–19 (5%) | 20–29 (71%) | 30–39 (24%) | |||||
| Systemic diseases | Diabetes (3%) | Hypertension (2%) | Allergies (0.05%) | Anaemia (0.5%) | Hypothyroid (1%) | Congenital heart disease (0.25%) | Hepatitis B (0.4%) | Asthma (0.05%) |
| Diseases occurred during pregnancy | Gestational diabetes (9%) | Infection diseases (4%) | Dental Infection (2%) | Pregnancy-induced Anaemia (0.4%) | – | – | – | – |
| Drugs used during pregnancy | Antibiotics (9%) | Vitamins (53%) | Fe (18%) | Ant-hypertension (0.64%) | Insulin (2.5%) | |||
| Cigarette consumption | + (13.5%) | − (86%) | ||||||
| Consanguinity between parents | + (12.5%) | − (87%) | ||||||
| Fathers | ||||||||
| Systemic diseases | Diabetes (0.8%) | Hypertension (1.5%) | Allergies (0.00%) | Anaemia (0.00%) | Hypothyroid (0.00%) | Congenital heart disease (0.5%) | Hepatitis B (0.00%) | Asthma (0.00%) |
The oral mucosa was systematically examined for changes and the location of anomalies was recorded. The following terms were used to diagnose and define the conditions being investigated; oral mucosal cysts; small, firm, isolated or multiple, whitish changes of macula- or papule-like appearance, with a clearly defined border to the surrounding mucosa. The changes were subgrouped according to:
- •
-
•
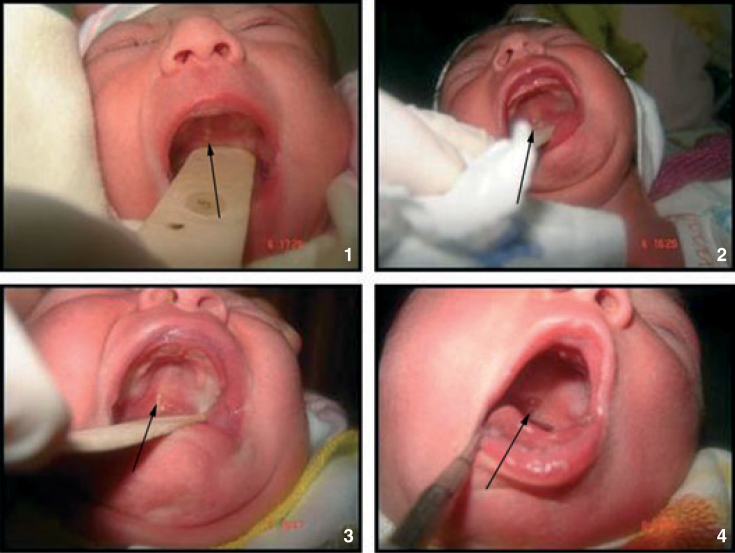
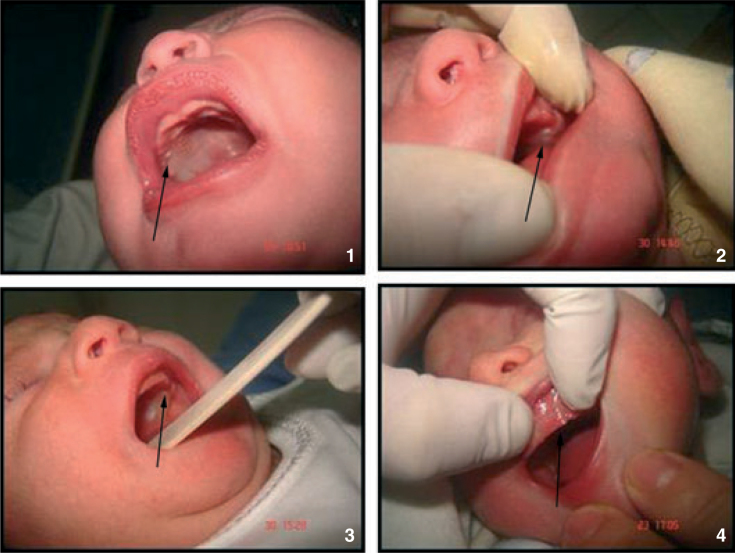
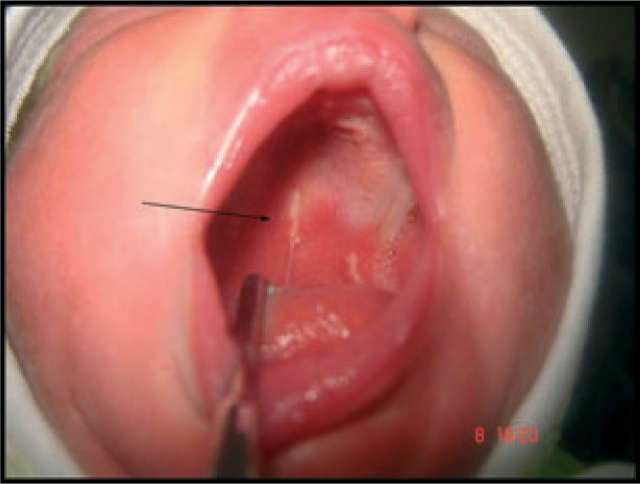
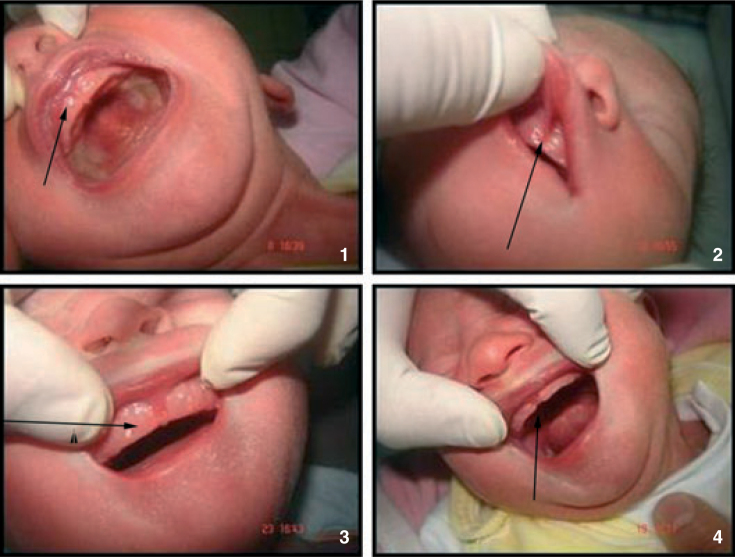
Whether situated palatally or on the alveolar ridges (Figures 1 and 2).
-
•
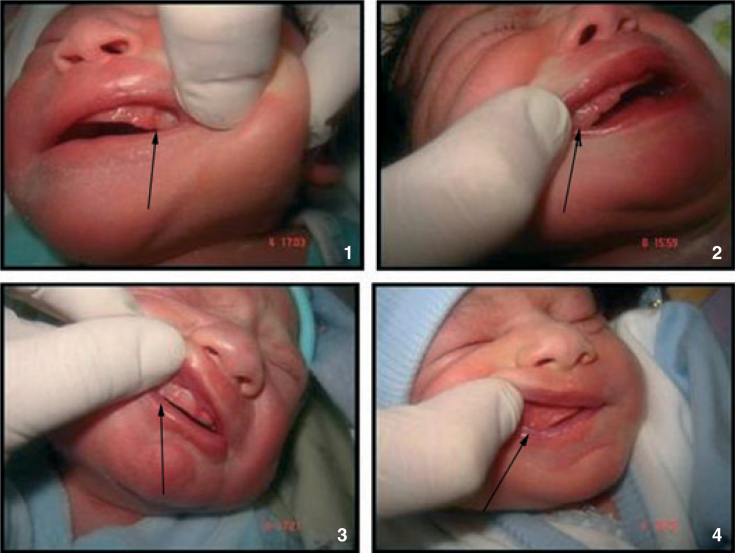
If they were oral gingival cysts (Figure 3).
-
•
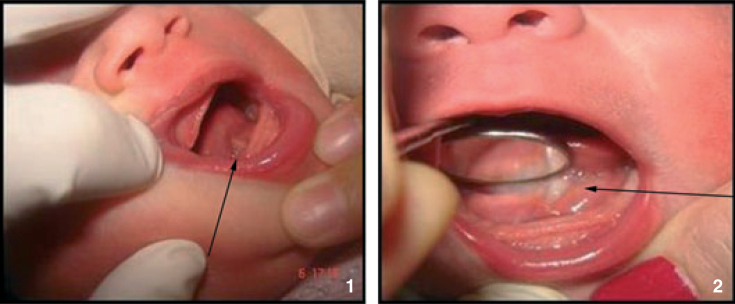
Ankyloglossia; fixation results when the lingual frenum is attached too far forward towards the tongue’s tip preventing protrusion of the tongue2 (Figure 4).
Figure 1.
Oral mucosal cysts situated palatally.
Figure 2.
Oral mucosal cysts situated on the alveolar ridges.
Figure 3.
Oral gingival cysts.
Figure 4.
Ankyloglossia.
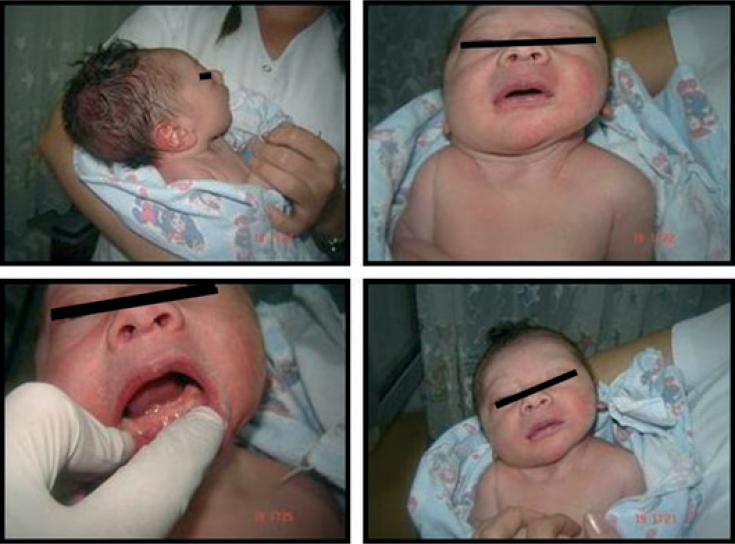
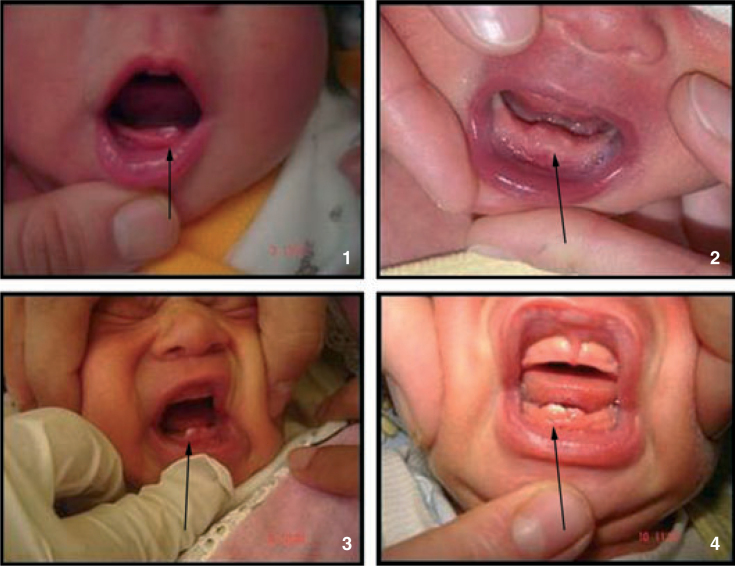
The term median alveolar nodulus was used when the lesions were located along the palatal midline (Figure 5). Attachment of the upper midline frenum (Figure 6), medical diagnosis of the newborn at birth (Figure 7) and natal teeth of the newborn, when the incisal edge of a tooth had penetrated the mucous membrane present at birth (Figure 8) were also recorded.
Figure 5.
Median alveolar nodulus.
Figure 6.
Attachment of the upper midline frenum.
Figure 7.
Sturge-Weber syndrome.
Figure 8.
Natal teeth.
Attachment of the upper midline frenum and data about natal teeth, including the number, colour, shade, mobility, and surrounding gingival tissue were also recorded. After the clinical examination, information about the parents’ role in promoting their baby’s oral health was given to all those who agreed to participate in the study6.
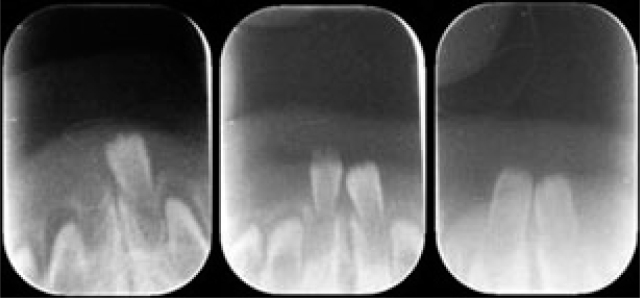
After 10 days, mandibular anterior periapical radiographs were obtained with the parents’ assistance (Figure 9). They confirmed whether the partly erupted teeth were supernumerary teeth or the primary mandibular central incisors and mobility tests were performed in order to decide whether or not to extract these teeth. Necessary precautions were explained to the parents for prevention of dental caries by controlling bacterial plaque and via periodical fluoride application, since in these teeth which erupt prematurely, mineralisation is not complete9.
Figure 9.
Radiographic evaluations of natal teeth.
Teeth diagnosed for extraction had this performed by avoiding unnecessary injury to the gingival tissue after application of topical anaesthetic for safety concerns, with the parents’ consent. A piece of gauze was placed lingual to the tooth in order to obtain pharyngeal protection due to the risk of aspiration during removal. No curettage of the extraction sites was performed18. Because tooth extraction is contraindicated in newborns due to the risk of hemorrhage9, extractions were performed after 10 days dependent on the general health condition of the babies16.
Data were analysed using the Pearson Chi-Square test. Statistical analyses were performed using the SPSS 11.0 program (SPSS Inc, Chicago, IL, USA) for Windows. The value P ≤ 0.05 was considered statistically significant.
RESULTS
The degrees of agreement/disagreement between the researchers were assessed using the Wilcoxon matched-pairs signed ranks test (P ≤ 0.05). The test results indicated that there was no statistically significant difference between the researchers. Ninety-two per cent harmony was detected for the researchers when they examined the sample together.
Accordingly the inter-examiner bias was negligible.
The most common findings, registered in 29% of the children, were of oral mucosal cysts situated palatally (Table 3). The diameter of the cysts varied from 0.1 to 2.0 mm and they were pearl white in colour in all cases. There was no relationship observed between the prevalence of congenital oral inclusion cysts or natal teeth and gender and birth weight (P = 0.32). Furthermore, no relationship was observed between ankyloglossia, median alveolar nodules or attached labial frenum and gender, birth weight and mother’s age (P = 0.17). However, there was a statistically significant relationship between the presence of oral inclusion cysts with the congenital diabetes and also insulin treatment and cigarette consumption during pregnancy. Moreover, a significant relationship was found between the presence of oral inclusion cysts and gestational diabetes and with the presence of consanguinity between the parents (P = 0.004).
Table 3.
Reported percentages of intraoral anomalies
| Sample size | n = 2,021 |
| Babies reported with at least one anomaly | n = 781 (39%) |
| Total anomaly number (there are cases where there is more than one anomaly in a baby) | n = 923 |
| Percentage of oral mucosal cysts situated palatally/number of oral mucosal cysts situated palatally in the total 923 anomalies | 587 (29%)/64% |
| Percentage of oral mucosal cysts situated on the alveolar ridges/number of oral mucosal cysts situated on the alveolar ridges in the total 923 anomalies | 166 (8%)/18% |
| Percentage of oral gingival cysts/number of oral gingival cysts in the total 923 anomalies | 141 (7%)/15% |
| Percentage of ankyloglossia/number of ankyloglossia in the total 923 anomalies | 6 (0.3%)/0.65% |
| Percentage of median alveolar nodulus/number of median alveolar nodulus in the total 923 anomalies | 1 (0.04%)/0.1% |
| Percentage of attachment of the upper midline frenum/number of attachment of the upper midline frenum in the total 923 anomalies | 6 (0.3%)/0.65% |
| Percentage of medical diagnosis/number of medical diagnosis in the total 923 anomalies | 2 (0.1%)/0.2% |
| Percentage of natal teeth/number of natal teeth in the total 923 anomalies | 8 (0.4%)/0.9% |
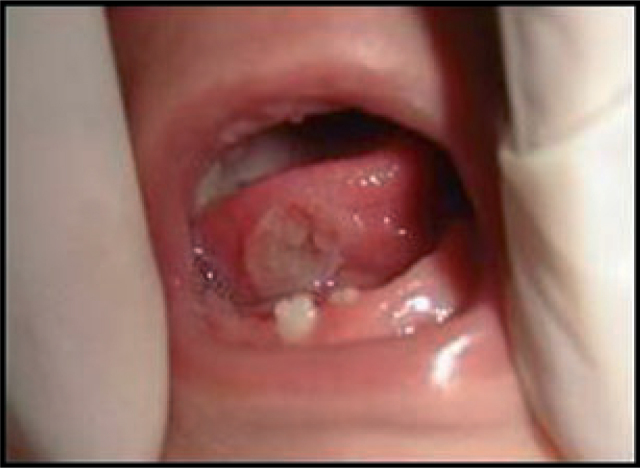
While six of the teeth displayed Grade 1 mobility and did not interfere with feeding, two of them showed Grade 2 mobility, with mild inflammation in the surrounding gingival (Table 4). Because of concerns such as premature loss of a primary tooth as a function of the possible loss of space for the permanent tooth, teeth with minimum mobility were not extracted, but kept under observation. The coronal aspects of two teeth displaying significant mobility, one causing ulceration in the ventral surface of the tongue (Figure 10), had only minimal attachment to the surrounding gingival and were extracted with forceps. The postoperative course was uneventful.
Table 4.
Information regarding natal teeth
| Natal teeth | ||
|---|---|---|
| Position | Mandibular incisor region | 8 |
| Tooth colour | Grey | 8 |
| Surface morphology | Hypoplastic enamel | 8 |
| Mobility | Grade 1 | 6 |
| Grade 2 | 2 | |
| Gingival | Mild inflammation | 8 |
| Treatment | Follow-up | 6 |
| Extraction | 2 | |
Figure 10.
Ulceration caused by the natal teeth.
The medical diagnoses of the neonates at birth revealed one child with Down syndrome and one with Sturge-Weber syndrome (SWS) (Figure 7) and these children’s initial medical diagnoses were confirmed after the 6-month follow-up period.
DISCUSSION
The cysts encountered in this study are microkeratocysts1., 3.. Those located on the alveolar ridges are remnants of the dental lamina and those in the midpalatal region derive from epithelial remnants after fusion of the palatal shelves1. Since it is clinically difficult to differentiate the origin of oral inclusion cysts, recent studies have adopted a classification based on their locations6. Many authors believe the three types (gingival, alveolar and palatal) are actually a single entity which differs as to location and tissue of origin17. Alveolar cysts have also been referred to as gingival cysts or, inclusion cysts in some texts. The clinical description of palatal and alveolar cysts varies in colour from white, to grey, to yellow nodules, in size from a pinhead to 3mm, and in numbers from one to six and filled with keratin5., 21.. According to Ikemura et al.8 it may be suggested that the frequency of visible nodules has a close relation to growth and development in foetal life. Although a variety of terminology for these nodules has been used, these oral cysts can be classified as gingival cyst in the newborn (dental lamina cyst) and median palatal mucosal cyst.
As mentioned by Liu and Huang4, although the mouth opening, the cleanness of the oral cavities, and head position of newborns all displayed wide variation in all newborns, the authors did their best to standardise the examination method. However, as reported by Donley and Nelson5 the mandibular ridge visibility was not as easy to access during the exams since premature infants often experienced oxygen desaturation with tongue manipulation. Mandibular cysts, therefore, might have been missed during the oral examinations.
The majority of children were found to have congenital oral cysts (alveolar, palatally located or gingival). Palatal cysts seemed to be more common than alveolar cysts. These findings are in accordance with previous studies1., 2., 3., 4., 6. and confirm that oral mucosal cysts are the most common finding of newborns regardless of ethnicity.
Freudenberger et al.2 noted that babies born at the same hospital demonstrated a high rate of oral cysts and that many were found in the area of the primary first and second molars rather than in the anterior region. Flinck1 also reported that the palatal cysts were all located along, or close to, the midpalatal raphae of the hard palate; the vast majority being found posteriorly.
Liu and Huang4 found no significant correlation between gender, body weight, gestation age and oral cysts. Donley and Nelson5 reported no significant differences in the prevalence of palatal and alveolar cysts for gender or for race. Although no sexual predilection was noted for the occurrence of oral cysts similar with some studies2., 4., 5., a statistically significant relationship between the appearance of oral inclusion cysts with the congenital diabetes, insulin treatment during pregnancy, cigarette consumption during pregnancy, gestational diabetes and the presence of consanguinity between the parents (P = 0.004) were noted in this study.
Differences between this and reported studies may be attributed to different ethnicities, selected population, sample variations, diagnostic and evaluation criteria, examination protocols studied in the researches and different definitions of the cysts1., 2., 3., 4., 6.. As mentioned by Freudenberger et al.2 further variance among these studies may also be due to differences in postnatal age. Examination of newborns within 3 days of birth may contribute to the higher frequency of palatal and gingival cysts of the newborn4 as the majority of the oral inclusion cysts, especially the palatal, are transient in nature and disappear shortly after birth, after 2–3 or 4–5 months1., 6.. The frequency of inclusion cysts is high in newborns, but they are rarely seen after 3 months of age3., 4., 5.. While Paula et al.6 reported that even after 2 months from the beginning of the clinical examinations the most frequent cyst was palatal, Flinck1 mentioned that the appearance of palatal cysts seems to be restricted to the foetal period, whereas alveolar cysts, derived from the dental lamina, may continue to appear in postnatal life. The reduction in number from the foetal period to the postnatal period of both alveolar and palatal cysts is thought to be due to the cyst wall fusing with the oral epithelium and discharging the cystic keratin5. According to Paula et al.6 it is possible that suction and deglutition may play an important role in the involution observed in palatal cysts, since every time the newborn swallows the tongue puts pressure against both the hard and soft palate. No treatment is required, as these cysts usually disappear during the first 3 months of life21.
Although inclusion cysts are transient lesions, it is important that professionals involved in newborn care are able to promptly identify them in order to avoid unnecessary therapeutic procedures and provide suitable information for the parents, thereby reducing concern4., 6.. Another study2 showed that children born with oral nodules are more likely to be colonised with Streptococcus mutans at an earlier age, thereby giving a possible increased caries risk; they concluded that further studies following children from infancy to early childhood are necessary2. Flinck1 concluded that a prospective, longitudinal study is needed to clarify the appearance and disappearance of these cysts in the oral cavity during the first year of life.
Natal teeth
Natal teeth are reported to be rare in extremely preterm infants23. Donley and Nelson5 reported that the factors associated with prematurity may disrupt the pattern and timing of oral development as evidenced by less prevalence of palatal and alveolar cysts in this group. They reported that the prevalence of palatal and alveolar cysts was observed to rise with increasing gestational age, increasing postnatal age, and increasing birth weight. Because the prevalence of palatal and alveolar cysts in the premature infant was reported to be less than the prevalence of term infants to a statistically significant level5, only full-term infants were included in this study sample.
Ankyloglossia and natal teeth were found to be a little less frequent than in previously reported studies1., 2., 4., although all natal teeth were located in the mandibular anterior area similar to a previous study4. A sexual predilection is controversial because although the frequency of natal teeth was reported with a predilection for females and ankyloglossia with a predilection for males2, no sexual predilection is reported in this study for any of the studied criteria. Similarly Liu and Huang4 reported the frequency of natal teeth of the newborn was not associated with gender, body weight and gestation age.
According to Boras et al.25 the early eruption of a tooth will compromise the normal maturation process of dental enamel with a more likely occurrence of enamel dysplasia. Uzamis et al.14 presented a study in which the surface topography of mandibular incisors was examined using a scanning electron microscope and reported that the enamel exhibited hypoplastic, depressed areas and the incise edge of natal teeth lacked enamel. In addition, root formation of the teeth was not completed, which correlated with findings that teeth may erupt without root formation. In polarised light and microradiographic studies, these teeth showed enamel hypoplasia and dentinal disturbances including the formation of osteodentine and irregular dentine in the cervical portions and interglobular dentine in the coronal region. Enamel thickness of 150 mm was reported with hypomineralisation and size reduction, where for normal primary teeth, the enamel layer reaches 1,000–1,200 mm14. These disturbances can be considered normal when compared with a tooth whose crown formation is completed 3 months postnatally and these ultrastructural studies may be helpful in understanding the mechanism of tooth eruption14., 16..
Premature eruption is a clearly pathological phenomenon with the formation of an incomplete, rootless tooth that would exfoliate within a short period of time9. This structure may result from trauma to the alveolar margin during delivery, with the resulting ulcer acting as a route of infection up to the dental follicle, causing premature loss of the tooth. Hyperactivity of osteoblastic cells within the tooth germ may also be a reason19. Superficial positioning of tooth germs during the developmental period, endocrinal disturbances association with various syndromes, and increased rate of eruption during or after febrile states, inheritance, congenital syphilis and dietary deficiencies can also be various other causes15. The specific aetiology of most cases of natal teeth is unknown although there is a familial trend and some evidence to support an autosomal dominant trait. It has been hypothesised that a more superficial position of the tooth germ might hasten eruption. Given that many of these teeth are smaller than normal, hypoplastic and exfoliate spontaneously within a short time, the possibility of an ectopic superficial placement of the tooth germ seems reasonable9., 21., 25.. There is general agreement in the literature that the aetiology of natal teeth requires further study9.
According to Leung and Robson23 the condition might occur as a familial trait since a positive family history has been reported in 8–62% of cases. Hereditary transmission of an autosomal dominant gene has been suggested and a family in which five siblings were born with natal teeth was reported by Mayhall et al.20 who studied the birth records of Tlinget Indian children. In all instances the prematurely erupted teeth were mandibular central incisors and natal teeth also were noted in two of the maternal grandmothers, one aunt, a cousin, and a great uncle. Thus, these researchers reported a familial tendency of this trait. However, no other family member had a history of natal teeth or oral morbidity at birth in this current study’s patients where natal teeth were observed, similar with the study of Bigeard et al.16
The majority of natal teeth are found in the mandibular incisor region4. The exception is in cleft lip and palate cases, which tend to show natal teeth in the maxillary cleft area3., 4., 16.. Natal teeth are present in 10% of infants with bilateral cleft lip and palate23 and this high prevalence has been attributed to alveolar fissures and to the superficial position of the teeth in this region9.
If the tooth does not interfere with breastfeeding and is otherwise asymptomatic, no treatment is necessary although close monitoring is indicated to ensure that the tooth remains stable 21., 23.. Assessment and observation is much preferred and allows the correctly diagnosed tooth to continue with root development if possible25. Leung and Robson23 reported that root development occurred in the teeth that were not extracted.
The most commonly affected teeth are the lower primary central incisors (85%)21., 23.. The strong predilection for the lower central incisors is consistent with the normal order of eruption of primary deciduous teeth23. Management should aim at preservation of teeth of the normal dentition for aesthetics and maintenance of space for eruption of permanent successors9., 18., 23., 27.. Obviously, one prefers not to take radiographs of newborn infants, however, there are occasions when the natal tooth should be extracted16 and the differential diagnosis can only be made with the help of a radiograph to determine whether the tooth is a supernumerary or normal primary tooth erupting early9., 10., 16., 23., 25.. Supernumerary teeth should always be extracted 9., 23., 25. but the decision to extract a normal mature natal tooth should be made according to scientific knowledge, mobility of the tooth, local or general complications and parental opinion10. For example the tooth may be very loose and in danger of being aspirated or a condition may occur, known as Riga-Fede syndrome, in which the tooth causes an ulcer under the tongue or the mother is having difficulty with breast-feeding the infant16., 18.. From the immunological and haematological point of view the best time for extraction was calculated to be 7–25 days of birth21., 27.. Although for some authors25, aspiration does not seem to be a proven significant clinical concern, two of the reported natal teeth were extracted in this study after a 10-day period because of excessive mobility which is associated with a risk of aspiration, and causing feeding difficulties or soft tissue trauma to the infant. On rare occasions, following spontaneous loss or extraction of these teeth, there may be continued root development (residual neonatal teeth) necessitating further treatment18. According to some authors23 extraction of the tooth should be followed by curettage of the socket to prevent continued development of the cells of the dental papilla. Failure to curette the socket might result in the eruption of odontogenic remnants and necessitate future treatment. For example, Tsubone et al.33 reported a calcified structure in the oral cavity of a young girl, having erupted following the exfoliation of a natal tooth. They termed this structure a residual natal tooth. It was also reported that a natal tooth was extracted by the paediatrician with his fingers and a root fragment remained from which developed a large pulp polyp34. Agostini et al.24 reported a case of an uncommon hamartoma affecting a 4-month-old Caucasian male patient, who previously displayed two natal teeth at the same location. However, Dyment et al.18 suggested that if extraction of natal teeth is required, the practitioner should assess the amount of gingival attachment and decide as to what type of anaesthetic agent, if any, will be required. According to these authors, for extraction in cases where there is minimal gingival attachment it will likely be possible to achieve adequate soft tissue anaesthesia with the application of topical anaesthetic. The authors recommend that no curettage of the extraction site be performed as in most cases the treatment will be adequate and the child will not develop residual natal teeth.
No curettage was applied in this study in order to avoid exposure to injected local anaesthetic and a more stressful surgical procedure, however, the parents were informed of the need for regular follow-up and that in the event of residual tooth formation, a second surgical procedure would be required, as suggested by Dyment et al.18.
There is no conclusive evidence of a correlation between early eruption, developmental anomalies and systemic disorders10, but some investigators3., 4., 16., 23. suggest that natal teeth may be associated with certain syndromes. For example, Koklu and Kurtoglu35 reported the combination of natal teeth and neonatal transient pseudohypoparathyroidism. Mandal et al.36 described the association of natal teeth with congenital hydrocephalus and congenital glaucoma. As the authors mentioned there was no known parental consanguinity although the parents came from a community where consanguinity is common. However, no morphological syndrome was found in patients with natal teeth in the current study; similar to a previous study10. In the Mayhall et al.20 study of Tlinget Indian children no abnormalities were noted in the pregnancy of the mother, the delivery, or the newborn infant other than the presence of teeth similar with this current study.
However, Boras et al.25 gave some examples of association with a number of syndromal conditions, prenatal ingestion of heroin and methadone use, infection or malnutrition, febrile status or hormone imbalances and natal teeth. These authors suggested that teeth present either at birth or erupting within the neonatal period require an accurate and preferably early diagnosis which will allow a decision as to the likely positive benefits of retention of the tooth or early removal. Also the patient should be assessed for the possibility of a systemic or syndromal association and, if indicated, a paediatric consultation should be arranged.
Among the studied anomalies of the newborns, severe conditions were rare, as in other studies1. The medical diagnoses of the neonates at birth revealed one child with Down syndrome and one with Sturge-Weber syndrome (SWS). This syndrome is defined by the association of a facial capillary malformation (port-wine stain), with a vascular malformation of the eye, and/or vascular malformation of the brain (leptomeningeal angioma) 37., 38.. Facial port-wine stains are capillary malformations and SWS is a congenital, severe neurocutaneous syndrome reaching the first branch of trigeminal nerve39. The facial port-wine stain typically involves the forehead and upper eyelid, in a distribution that resembles the area innervated by the first branch (ophthalmic branch) of the trigeminal nerve37. Port-wine stains occur in 3 per 1,000 live births and although the cause remains obscure, recent advances in neuroimaging have provided important insights into the progression of neurologic injury that occurs as a result of impaired blood flow38. Management of patients with SWS is focused on treating associated neurological and ocular abnormalities.37
A statistically significant relationship between the appearance of oral inclusion cysts with the congenital diabetes, insulin treatment during pregnancy, cigarette consumption during pregnancy, gestational diabetes and the presence of consanguinity between the parents (P = 0.004) were noted in this study. The samples of this study were only of Turkish origin, precluding detection of any racial predilections. It is also difficult to compare the results of this study with data from the literature, as there are no published studies available where parents’ consanguinity was studied. This study regarding the oral findings in newborns has been conducted for the first time in Turkey as far as the authors’ are aware. However, it only includes a metropolitan area. In order to be a widespread epidemiologic study, other parts of the country should also be evaluated.
CONCLUSIONS
Reports of common variations observed in the oral cavity of newborns allow proper dental care and counselling to parents17. Longitudinal studies are required to assess any significance of the medical complications including drug and cigarette consumption during pregnancy and consanguinity between the parents in relation to the future development of knowledge about oral findings in neonates.
REFERENCES
- 1.Flinck A, Paludan A, Matsson L, et al. Oral findings in a group of newborn Swedish children. Int J Paediatr Dent. 1994;4:67–73. doi: 10.1111/j.1365-263x.1994.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 2.Freudenberger S, Santos Díaz MA, Bravo JM, et al. Intraoral findings and other developmental conditions in Mexican neonates. J Dent Child (Chic) 2008;75:280–286. [PubMed] [Google Scholar]
- 3.George D, Bhat SS, Hegde SK. Oral findings in newborn children in and around Mangalore, Karnataka State, India. Med Princ Pract. 2008;17:385–389. doi: 10.1159/000141502. [DOI] [PubMed] [Google Scholar]
- 4.Liu MH, Huang WH. Oral abnormalities in Taiwanese newborns. J Dent Child. 2004;71:118–120. [PubMed] [Google Scholar]
- 5.Donley CL, Nelson LP. Comparison of palatal and alveolar cysts of the newborn in premature and full-term infants. Pediatr Dent. 2000;22:321–324. [PubMed] [Google Scholar]
- 6.Paula JD, Dezan CC, Frossard WT, et al. Oral and facial inclusion cysts in newborns. J Clin Pediatr Dent. 2006;31:127–129. doi: 10.17796/jcpd.31.2.rw3h853m3rk242q0. [DOI] [PubMed] [Google Scholar]
- 7.Uauy E, Celis A, Martinez A. Epidemiological study on cysts of the oral mucosa in the newborn. Rev Asoc Odontol Argent. 1980;68:511–513. [PubMed] [Google Scholar]
- 8.Ikemura K, Kakinoki Y, Nishio K, et al. Cysts of the oral mucosa in newborns: a clinical observation. J UOEH. 1983;5:163–168. doi: 10.7888/juoeh.5.163. [DOI] [PubMed] [Google Scholar]
- 9.Cunha RF, Boer FA, Torriani DD, et al. Natal and neonatal teeth: review of the literature. Pediatr Dent. 2001;23:158–162. [PubMed] [Google Scholar]
- 10.El Khatib K, Abouchadi A, Nassih M, et al. Natal teeth: apropos of five cases. Rev Stomatol Chir Maxillofac. 2005;106:325–327. doi: 10.1016/s0035-1768(05)86054-1. [DOI] [PubMed] [Google Scholar]
- 11.Jasmin JR, Jonesco-Benaiche N, Muller-Giamarchi M. Natal and neonatal teeth. Management. Ann Pediatr. 1993;40:640–641. [PubMed] [Google Scholar]
- 12.Seminario AL, Ivancaková R. Natal and neonatal teeth. Acta Medica. 2004;47:229–233. [PubMed] [Google Scholar]
- 13.Adekoya-Sofowora CA. Natal and neonatal teeth: a review. Niger Postgrad Med J. 2008;15:38–41. [PubMed] [Google Scholar]
- 14.Uzamis M, Olmez S, Ozturk H, et al. Clinical and ultrastructural study of natal and neonatal teeth. J Clin Pediatr Dent. 1999;23:173–177. [PubMed] [Google Scholar]
- 15.Kamboj M, Chougule RB. Neonatal tooth-how dangerous can it be? J Clin Pediatr Dent. 2009;34:59–60. doi: 10.17796/jcpd.34.1.27h00760t6271785. [DOI] [PubMed] [Google Scholar]
- 16.Bigeard L, Hemmerle J, Sommermater JI. Clinical and ultrastructural study of the natal tooth: enamel and dentin assessments. ASDC J Dent Child. 1996;63:23–31. [PubMed] [Google Scholar]
- 17.da Silva CM, Ramos MM, Carrara CF, et al. Oral characteristics of newborns. J Dent Child (Chic) 2008;75:4–6. [PubMed] [Google Scholar]
- 18.Dyment H, Anderson R, Humphrey J, et al. Residual neonatal teeth: a case report. J Can Dent Assoc. 2005;71:394–397. [PubMed] [Google Scholar]
- 19.Sarkar S, Sarkar S. Unusual neonatal tooth in maxillary 1st molar region: a case report. J Indian Soc Pedod Prev Dent. 2007;25:41–42. [PubMed] [Google Scholar]
- 20.Mayhall JT. Natal and neonatal teeth among the Tlinget Indians. J Dent Res. 1967;46:748–749. doi: 10.1177/00220345670460042101. [DOI] [PubMed] [Google Scholar]
- 21.American Academy of Pediatric Dentistry Council on Clinical Affairs Guideline on pediatric oral surgery. Pediatr Dent. 2005-2006;27:158–164. [Google Scholar]
- 22.Hegde RJ. Sublingual traumatic ulceration due to neonatal teeth (Riga-Fede disease) J Indian Soc Pedod Prev Dent. 2005;23:51–52. doi: 10.4103/0970-4388.16031. [DOI] [PubMed] [Google Scholar]
- 23.Leung AK, Robson WL. Natal teeth: a review. J Natl Med Assoc. 2006;98:226–228. [PMC free article] [PubMed] [Google Scholar]
- 24.Agostini M, León JE, Kellermann MG, et al. Myxoid calcified hamartoma and natal teeth: a case report. Int J Pediatr Otorhinolaryngol. 2008;72:1879–1883. doi: 10.1016/j.ijporl.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Boras V, Mohamad Z, Savage NW. Supernumerary tooth with associated dentigerous cyst in an infant. A case report and review of differential diagnosis. Aust Dent J. 2007;52:150–153. doi: 10.1111/j.1834-7819.2007.tb00481.x. [DOI] [PubMed] [Google Scholar]
- 26.Groeneveld X, van Damme P. (Neo) natal tooth inperspective. Literature review and report of two cases. Ned Tijdschr Tandheelkd. 1993;100:49–51. [PubMed] [Google Scholar]
- 27.Chawla HS. Management of natal/neonatal/early infancy teeth. J Indian Soc Pedod Prev Dent. 1993;11:33–36. [PubMed] [Google Scholar]
- 28.Primo LG, Alves AC, Pomarico I, et al. Interruption of breast feeding caused by the presence of neonatal teeth. Braz Dent J. 1995;6:137–142. [PubMed] [Google Scholar]
- 29.Gonçalves FA, Birman EG, Sugaya NN, et al. Natal teeth: review of the literature and report of an unusual case. Braz Dent J. 1998;9:53–56. [PubMed] [Google Scholar]
- 30.Barfiwala DR. Natal and neonatal teeth: a review of 50 cases. J Indian Soc Pedod Prev Dent. 1996;14:21–23. [PubMed] [Google Scholar]
- 31.Tunçbílek E, Koc I. Consanguineous marriage in Turkey and its impact on fertility and mortality. Ann Hum Genet. 1994;58:321–329. doi: 10.1111/j.1469-1809.1994.tb00729.x. [DOI] [PubMed] [Google Scholar]
- 32.Denic S, Bener A. Consanguinity decreases risk of breast cancer-cervical cancer unaffected. Br J Cancer. 2001;85:1675–1679. doi: 10.1054/bjoc.2001.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsubone H, Onishi T, Hayashibara T, et al. Clinico-pathological aspects of a residual natal tooth: a case report. J Oral Pathol Med. 2002;31:239–241. doi: 10.1034/j.1600-0714.2002.310408.x. [DOI] [PubMed] [Google Scholar]
- 34.Vergotine RJ, Hodgson B, Lambert L. Pulp polyp associated with a natal tooth: case report. J Clin Pediatr Dent. 2009;34:161–163. doi: 10.17796/jcpd.34.2.96656211337v7517. [DOI] [PubMed] [Google Scholar]
- 35.Koklu E, Kurtoglu S. Natal teeth and neonatal transient pseudohypoparathyroidism in a newborn. J Pediatr Endocrinol Metab. 2007;20:971. doi: 10.1515/jpem.2007.20.9.971. [DOI] [PubMed] [Google Scholar]
- 36.Mandal AK, Hornby SJ, Jones RB. Congenital hydrocephalus associated with congenital glaucoma and natal teeth. Indian J Ophthalmol. 2002;50:322–323. [PubMed] [Google Scholar]
- 37.Baselga E. Sturge-Weber syndrome. Semin Cutan Med Surg. 2004;23:87–98. doi: 10.1016/j.sder.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Comi AM. Update on Sturge-Weber syndrome: diagnosis, treatment, quantitative measures, and controversies. Lymphat Res Biol. 2007;5:257–264. doi: 10.1089/lrb.2007.1016. [DOI] [PubMed] [Google Scholar]
- 39.Maruani A. Sturge-Weber syndrome. Presse Med. 2010;39:482–486. doi: 10.1016/j.lpm.2009.07.030. [DOI] [PubMed] [Google Scholar]