Abstract
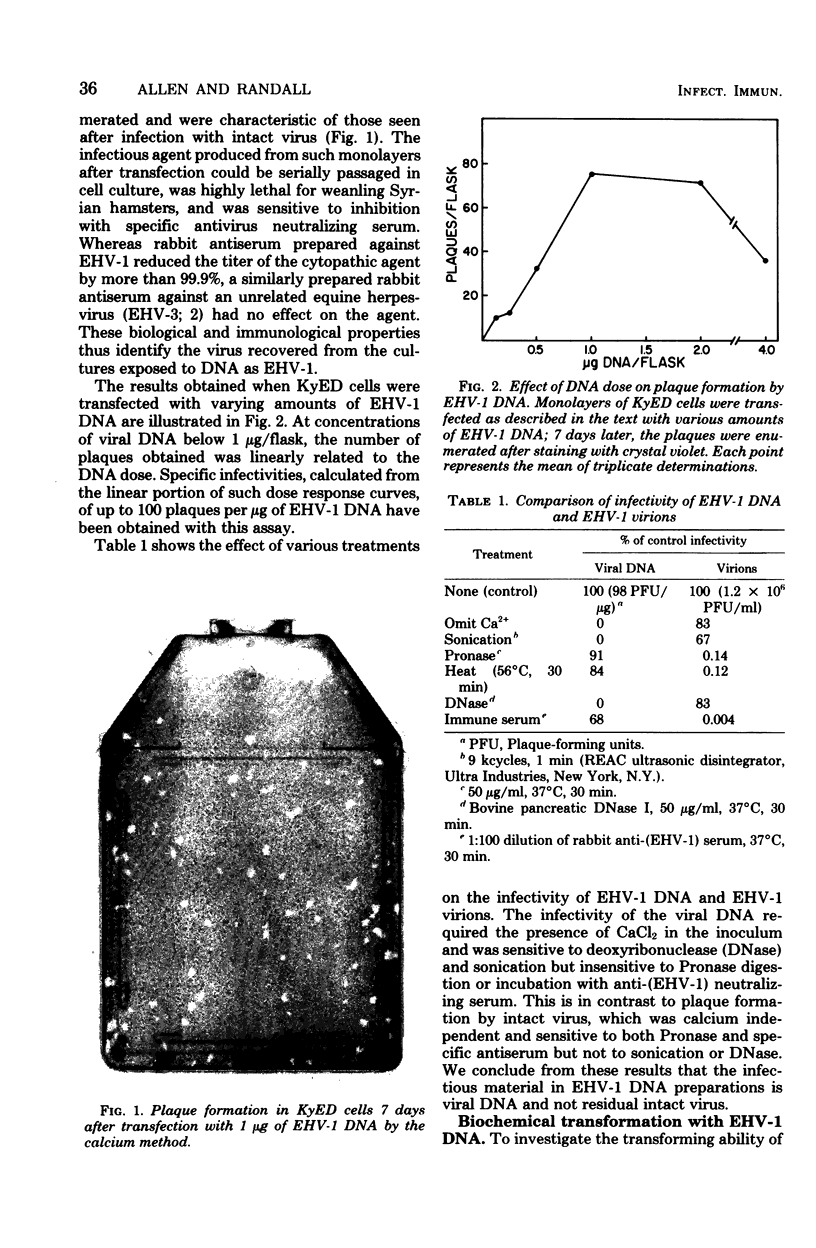
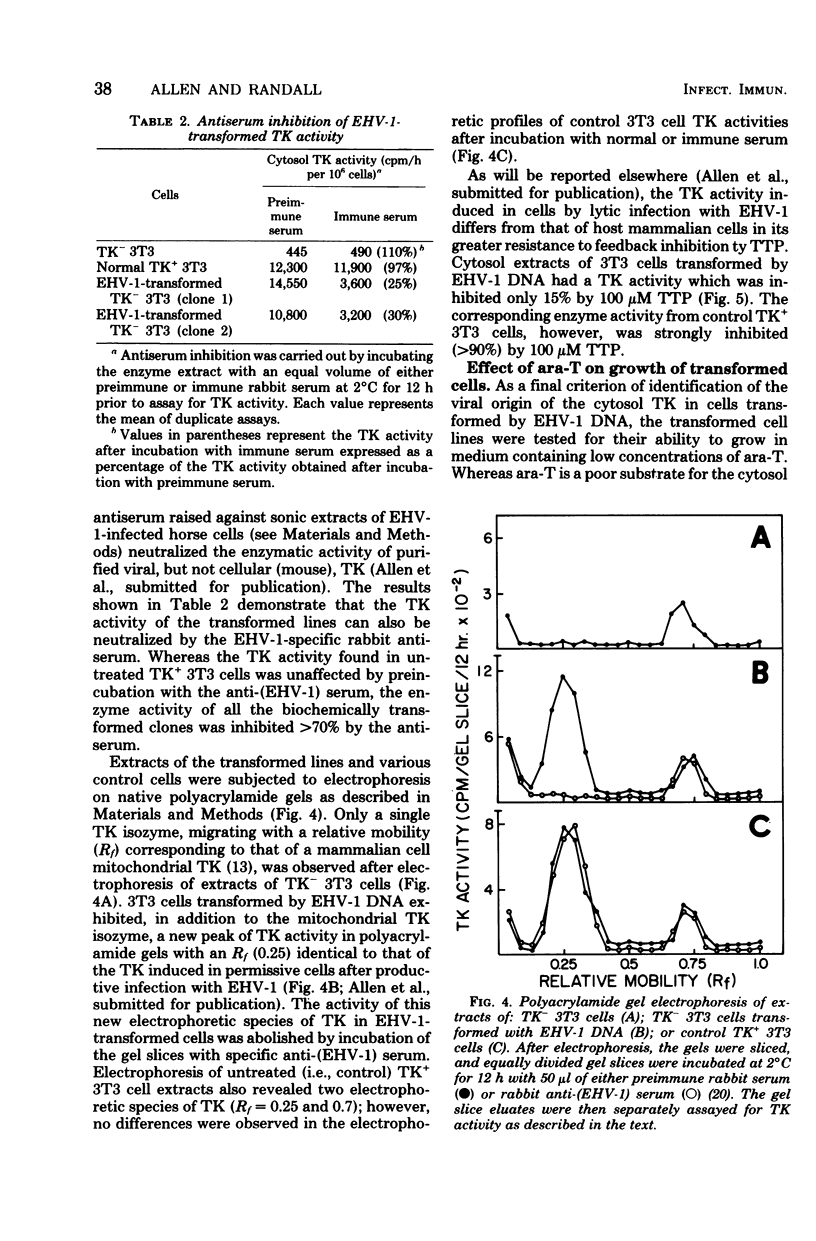
DNA extracted from purified virions of equine herpesvirus type 1 (EHV-1) was examined for its transfectivity and transforming ability. The infectivity of the herpesvirus DNA was demonstrated by addition of calcium phosphate-DNA coprecipitates to monolayers of permissive horse cells, with resultant plaque formation. The efficiency of transfection (50 to 100 plaque-forming units/microgram of DNA) was reduced by treatment of the viral DNA with deoxyribonuclease or sonication but not with Pronase or antivirus neutralizing serum. When nonpermissive mouse 3T3 Cells lacking the enzyme thymidine kinase (TK-) were transfected with intact EHV-1 DNA, clones of cells transformed to the TK+ phenotype were isolated in selective HAT medium (hypoxanthine, aminopterin, thymidine), which prevents growth of the TK- parental phenotype. The efficiency of transformation ranged from one to five transformants per microgram of EHV-1 DNA. The TK activity of the biochemically transformed cells was characterized by biochemical, electrophoretic, and immunological techniques. By these criteria, the TK activity was identical to the EHV-1 TK and different from the host wild-type enzyme. In contrast to the parental TK+ 3T3 cells, the EHV-1-transformed TK+ cells were unable to grow in the presence of arabinosylthymine, a drug selectively phosphorylated by herpesvirus TKs. These results indicate that stable transfer of EHV-1 genes into nonpermissive cells can be achieved with purified viral DNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. P., McGowan J. J., Gentry G. A., Randall C. C. Biochemical transformation of deoxythymidine kinase-deficient mouse cells with UV-irradiated equine herpesvirus type 1. J Virol. 1978 Oct;28(1):361–367. doi: 10.1128/jvi.28.1.361-367.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G. P., O'Callaghan D. J., Randall C. C. Genetic relatedness of equine herpesvirus types 1 and 3. J Virol. 1977 Dec;24(3):761–767. doi: 10.1128/jvi.24.3.761-767.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswell J. F., Allen G. P., Jamieson A. T., Campbell D. E., Gentry G. A. Antiviral activity of arabinosylthymine in herpesviral replication: mechanism of action in vivo and in vitro. Antimicrob Agents Chemother. 1977 Aug;12(2):243–254. doi: 10.1128/aac.12.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetti S., Graham F. L. Transfer of the gene for thymidine kinase to thymidine kinase-deficient human cells by purified herpes simplex viral DNA. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1590–1594. doi: 10.1073/pnas.74.4.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Ostrander M. Deoxythymidine kinase induced in HeLa TK- cells by herpes simplex virus type I and type II. II. Purification and characterization. J Biol Chem. 1976 May 10;251(9):2605–2610. [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W., Ludwig H. Repetitive sequences in complete and defective genomes of Herpesvirus saimiri. J Virol. 1975 Feb;15(2):398–406. doi: 10.1128/jvi.15.2.398-406.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L. Biological activity of tumor virus DNA. Adv Cancer Res. 1977;25:1–51. doi: 10.1016/s0065-230x(08)60631-4. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Veldhuisen G., Wilkie N. M. Infectious herpesvirus DNA. Nat New Biol. 1973 Oct 31;245(148):265–266. doi: 10.1038/newbio245265a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Kemp M. C., Perdue M. L., Rogers H. W., O'Callaghan D. J., Randall C. C. Structural polypeptides of the hamster strain of equine herpes virus type 1: products associated with purification. Virology. 1974 Oct;61(2):361–375. doi: 10.1016/0042-6822(74)90274-8. [DOI] [PubMed] [Google Scholar]
- Kit S., Leung W. C., Trkula D., Jorgensen G. Gel electrophoresis and isoelectric focusing of mitochondrial and viral-induced thymidine kinases. Int J Cancer. 1974 Feb 15;13(2):203–218. doi: 10.1002/ijc.2910130208. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. STUDIES ON THYMIDINE KINASE IN CULTURED MOUSE FIBROBLASTS. Biochim Biophys Acta. 1965 Jan 11;95:14–22. doi: 10.1016/0005-2787(65)90206-6. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Cheng Y. C. Human deoxythymidine kinase. I. Purification and general properties of the cytoplasmic and mitochondrial isozymes derived from blast cells of acute myelocytic leukemia. J Biol Chem. 1976 May 10;251(9):2600–2604. [PubMed] [Google Scholar]
- Maitland N. J., McDougall J. K. Biochemical transformation of mouse cells by fragments of herpes simplex virus DNA. Cell. 1977 May;11(1):233–241. doi: 10.1016/0092-8674(77)90334-8. [DOI] [PubMed] [Google Scholar]
- Matsuya Y., Green H. Somatic cell hybrid between the established human line D98 (presumptive HeLa) and 3T3. Science. 1969 Feb 14;163(3868):697–698. doi: 10.1126/science.163.3868.697. [DOI] [PubMed] [Google Scholar]
- RANDALL C. C., BRACKEN E. C. Studies on hepatitis in hamsters infected with equine abortion virus. I. Sequential development of inclusions and the growth cycle. Am J Pathol. 1957 Jul-Aug;33(4):709–727. [PMC free article] [PubMed] [Google Scholar]
- Sheldrick P., Laithier M., Lando D., Ryhiner M. L. Infectious DNA from herpes simplex virus: infectivity of double-stranded and single-stranded molecules. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3621–3625. doi: 10.1073/pnas.70.12.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walboomers J. M., van Ketel R., Becker-Bloemkolk M. J. A sensitive method for the detection of herpes simplex virus type 2 specific thymidine kinase. Intervirology. 1977;8(3):186–192. doi: 10.1159/000148894. [DOI] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M., Clements J. B., Macnab J. C., Subak-Sharpe J. H. The structure and biological properties of herpes simplex virus DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):657–666. doi: 10.1101/sqb.1974.039.01.079. [DOI] [PubMed] [Google Scholar]




