Abstract
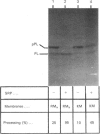
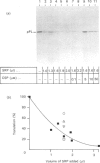
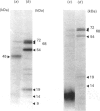
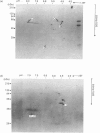
The mechanisms whereby isolated or synthetic signal peptides inhibit processing of newly synthesized prolactin in microsome-supplemented lysates from reticulocytes and wheat-germ were investigated. At a concentration of 5 microM, a consensus signal peptide reverses the elongation arrest imposed by the signal-recognition particle (SRP), and at higher concentrations in addition inhibits elongation of both secretory and non-secretory proteins. A photoreactive form of a synthetic signal peptide cross-links under u.v. illumination to the 54 kDa and 68 kDa subunits of SRP, whereas the major cross-linked protein produced after photoreaction of rough microsomes is of 45 kDa. As SRP-mediated elongation arrest is unlikely to be essential for translocation, it is suggested that signal peptides may interact with components other than SRP in the translation system in vitro.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Walter P., Blobel G. Signal recognition protein is required for the integration of acetylcholine receptor delta subunit, a transmembrane glycoprotein, into the endoplasmic reticulum membrane. J Cell Biol. 1982 May;93(2):501–506. doi: 10.1083/jcb.93.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austen B. M., Hermon-Taylor J., Kaderbhai M. A., Ridd D. H. Design and synthesis of a consensus signal sequence that inhibits protein translocation into rough microsomal vesicles. Biochem J. 1984 Nov 15;224(1):317–325. doi: 10.1042/bj2240317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassüner R., Wobus U., Rapoport T. A. Signal recognition particle triggers the translocation of storage globulin polypeptides from field beans (Vicia faba L.) across mammalian endoplasmic reticulum membrane. FEBS Lett. 1984 Jan 30;166(2):314–320. doi: 10.1016/0014-5793(84)80103-9. [DOI] [PubMed] [Google Scholar]
- Bernstein H. D., Poritz M. A., Strub K., Hoben P. J., Brenner S., Walter P. Model for signal sequence recognition from amino-acid sequence of 54K subunit of signal recognition particle. Nature. 1989 Aug 10;340(6233):482–486. doi: 10.1038/340482a0. [DOI] [PubMed] [Google Scholar]
- Duong L. T., Caulfield M. P., Rosenblatt M. Synthetic signal peptide and analogs display different activities in mammalian and plant in vitro secretion systems. J Biol Chem. 1987 May 5;262(13):6328–6333. [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Cell-free translation of messenger RNA in a wheat germ system. Methods Enzymol. 1983;96:38–50. doi: 10.1016/s0076-6879(83)96007-x. [DOI] [PubMed] [Google Scholar]
- Gilmore R., Blobel G., Walter P. Protein translocation across the endoplasmic reticulum. I. Detection in the microsomal membrane of a receptor for the signal recognition particle. J Cell Biol. 1982 Nov;95(2 Pt 1):463–469. doi: 10.1083/jcb.95.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Kaderbhai M. A., Austen B. M. Dog pancreatic microsomal-membrane polypeptides analysed by two-dimensional gel electrophoresis. Biochem J. 1984 Jan 1;217(1):145–157. doi: 10.1042/bj2170145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg U. C., Walter P., Johnson A. E. Photocrosslinking of the signal sequence of nascent preprolactin to the 54-kilodalton polypeptide of the signal recognition particle. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8604–8608. doi: 10.1073/pnas.83.22.8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majzoub J. A., Rosenblatt M., Fennick B., Maunus R., Kronenberg H. M., Potts J. T., Jr, Habener J. F. Synthetic pre-proparathyroid hormone leader sequence inhibits cell-free processing of placental, parathyroid, and pituitary prehormones. J Biol Chem. 1980 Dec 10;255(23):11478–11483. [PubMed] [Google Scholar]
- Meyer D. I. Signal recognition particle (SRP) does not mediate a translational arrest of nascent secretory proteins in mammalian cell-free systems. EMBO J. 1985 Aug;4(8):2031–2033. doi: 10.1002/j.1460-2075.1985.tb03888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A., Kaderbhai M. A., Austen B. M. Identification of signal sequence binding proteins integrated into the rough endoplasmic reticulum membrane. Biochem J. 1987 Mar 15;242(3):767–777. doi: 10.1042/bj2420767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A., Meredith C., Austen B. M. Isolation and properties of the signal region from ovalbumin. FEBS Lett. 1986 Jul 28;203(2):243–246. doi: 10.1016/0014-5793(86)80751-7. [DOI] [PubMed] [Google Scholar]
- Sanz P., Meyer D. I. Signal recognition particle (SRP) stabilizes the translocation-competent conformation of pre-secretory proteins. EMBO J. 1988 Nov;7(11):3553–3557. doi: 10.1002/j.1460-2075.1988.tb03232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenstedt G., Zimmermann R. Import of frog prepropeptide GLa into microsomes requires ATP but does not involve docking protein or ribosomes. EMBO J. 1987 Mar;6(3):699–703. doi: 10.1002/j.1460-2075.1987.tb04810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel V., Walter P. Each of the activities of signal recognition particle (SRP) is contained within a distinct domain: analysis of biochemical mutants of SRP. Cell. 1988 Jan 15;52(1):39–49. doi: 10.1016/0092-8674(88)90529-6. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7112–7116. doi: 10.1073/pnas.77.12.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum. II. Signal recognition protein (SRP) mediates the selective binding to microsomal membranes of in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):551–556. doi: 10.1083/jcb.91.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C., Wickner W., Zimmermann R. M13 procoat and a pre-immunoglobulin share processing specificity but use different membrane receptor mechanisms. Proc Natl Acad Sci U S A. 1983 May;80(10):2809–2813. doi: 10.1073/pnas.80.10.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin S. L., Walter P. Ribosome pausing and stacking during translation of a eukaryotic mRNA. EMBO J. 1988 Nov;7(11):3559–3569. doi: 10.1002/j.1460-2075.1988.tb03233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R., Mollay C. Import of honeybee prepromelittin into the endoplasmic reticulum. Requirements for membrane insertion, processing, and sequestration. J Biol Chem. 1986 Sep 25;261(27):12889–12895. [PubMed] [Google Scholar]