Abstract
Recent reports suggest that a growing number of human immunodeficiency virus (HIV)-infected persons show signs of persistent cognitive impairment even in the context of combination antiretroviral therapies (cART). The basis for this finding remains poorly understood as there are only a limited number of studies examining the relationship between CNS injury, measures of disease severity, and cognitive function in the setting of stable disease. This study examined the effects of HIV infection on cerebral white matter using quantitative morphometry of the midsagittal corpus callosum (CC) in 216 chronically infected participants from the multisite HIV Neuroimaging Consortium study currently receiving cART and 139 controls. All participants underwent MRI assessment, and HIV-infected subjects also underwent measures of cognitive function and disease severity. The midsagittal slice of the CC was quantified using two semi-automated procedures. Group comparisons were accomplished using ANOVA, and the relationship between CC morphometry and clinical covariates (current CD4, nadir CD4, plasma and CSF HIV RNA, duration of HIV infection, age, and ADC stage) was assessed using linear regression models. HIV-infected patients showed significant reductions in both the area and linear widths for several regions of the CC. Significant relationships were found with ADC stage and nadir CD4 cell count, but no other clinical variables. Despite effective treatment, significant and possibly irreversible structural loss of the white matter persists in the setting of chronic HIV disease. A history of advanced immune suppression is a strong predictor of this complication and suggests that antiretroviral intervention at earlier stages of infection may be warranted.
Keywords: HIV, Corpus callosum, Nadir CD4, White matter, CART
Introduction
Prior to the advent of combination antiretroviral therapies (cART), both neurocognitive impairment (i.e., dementia (Antinori et al. 2007; Levy and Bredesen 1988; Navia et al. 1986; Navia and Price 1987)) and structural neuroimaging abnormalities including white matter volume reduction (Aylward et al. 1995; Di Sclafani et al. 1997; Jernigan et al. 2005; Stout et al. 1998), white matter signal abnormalities (Archibald et al. 2004), caudate atrophy (Paul et al. 2002; Stout et al. 1998), and cortical thinning (Thompson et al. 2005) were frequently observed in late stage human immunodeficiency virus (HIV) infection. Since the advent of cART, there has been a dramatic reduction in the incidence of dementia (Ances and Ellis 2007; McArthur et al. 2004) though the number of patients experiencing mild to moderate cognitive dysfunction may be on the rise (McArthur et al. 2003; McCutchan et al. 2007; Sacktor et al. 2002). Findings such as these have led researchers to postulate that the central nervous system (CNS) acts as a protected reservoir for HIV infection in the setting of cART (Anthony and Bell 2008; Hult et al. 2008), and despite adequate improvements in systemic immune function and viral suppression, the brain remains vulnerable to HIV-associated injury.
To date, there have been a limited number of studies directly examining the effects of HIV on the CNS in the context of cART. Early studies by the HIV Neuroimaging Consortium (HIVNC) and others using magnetic resonance spectroscopy (MRS) have demonstrated regional metabolite changes indicating increased inflammation and neuronal injury, especially in the frontal white matter and subcortical nuclei (Chang et al. 2004; Sacktor et al. 2005; Yiannoutsos et al. 2004; Harezlak et al. 2011). Several studies have also successfully used diffusion tensor imaging (DTI) to demonstrate general, regional, and tract specific reduction of fractional anisotropy in white matter areas of HIV patients (Chen et al. 2009; Pfefferbaum et al. 2007, 2009; Ragin et al. 2004; Tate et al. 2010; Wu et al. 2006). Combined, these findings suggest that HIV infection is deleterious to brain white matter, though there have been very few studies directly examining white matter atrophy, particularly in the context of stable HIV disease and cART.
To address this gap in the literature, the present study examined the structural morphometry of the midsagittal corpus callosum (CC; a large prominent white matter structure) among HIV-infected patients on cART enrolled in the HIVNC, a large multi-center prospective study designed to assess the effects of chronic HIV disease and treatment on CNS function. We also examined the relationship between CC morphometry and various host and disease-related factors to identify relationships between these measures.
Methods
Participants
Structural MRI and clinical data from 216 HIV-infected participants (181 M; 35 F) enrolled in the multisite HIVNC study were examined. Potential subjects were recruited by nurse or physician referral from outpatient HIV/AIDS clinics affiliated with the Consortium. Each potential participant underwent a screening interview administered by trained nurses to identify eligibility. All eligible study patients were informed to the nature and scope of the study and were required to provide signed consent. All recruitment and data management procedures were performed in accordance with IRB-approved protocols.
Inclusion criteria were as follows: Patients were required to have a nadir CD4 count <200 cells/mm3, on stable antiretroviral regimen with any FDA-approved antiretroviral therapy (i.e., nucleoside, non-nucleoside, and/or protease inhibitors) for at least 12 consecutive weeks prior to study screening, hemoglobin >9.0 gm/dl, serum creatinine ≤3× upper limits of normal (ULN), and AST (SGOT), ALT (SGPT), and alkaline phosphatase ≤3× ULN. Exclusion criteria included severe premorbid or comorbid psychiatric disorders (schizophrenia, bipolar disorder, and active depression), confounding neurologic disorders (chronic seizures, stroke, head trauma resulting in loss of consciousness >30 min, multiple sclerosis), brain infection (e.g., fungal meningitis, toxoplasmosis, progressive multifocal leukoencephalopathy, or neoplasms), active alcohol and drug abuse or related medical complications within 6 months of study, and diabetes mellitus with a fasting glucose >140 mg/dl.
In a post hoc attempt to enrich the sample, we also examined all the available structural MRI data of 139 control participants (67 M; 72 F) from a previously published study (Bigler et al. 1996, 1999) for a limited number of statistical procedures. Exclusion criteria for the control participants were as follows: diagnostic history of severe psychiatric disorders (schizophrenia, bipolar, or major depression), history of neurologic disorder (epilepsy, stroke, head injury with loss of consciousness >5 min), and/or alcohol or drug abuse history. Additional demographic data for HIV and control participants are displayed in Table 1.
Table 1.
Descriptive statistics for major demographic variables
| ADC Stage
|
Total HIV | Controls | ||
|---|---|---|---|---|
| 0 and 0.5 | ≥1 | |||
| N | 181 | 35 | 216 | 139 |
| Gender (% male) | 82 | 89 | 88 | 48 |
| Age (years) | 46.7±8.3a | 52.2±8.5 | 47.6±8.6 | 37.0±13.6 |
| Ethnicity (% Caucasian) | 69 | 74 | 69 | 85 |
| Education level (% high school or less) | 39 | 31 | 38 | 23 |
| % IV drug use | 20 | 40 | 23 | Not available |
| Duration of infection (years) | 11.5±6.6 | 15.0±5.7 | 12.0±6.6 | Not applicable |
| CD4 count (log10 cells/ml) | 2.42±0.36 | 2.46±0.34 | 2.43±0.35 | Not applicable |
| Nadir CD4 (log10 cells/ml) | 1.44±0.58 | 1.46±0.61 | 1.44±0.58 | Not applicable |
| % Undetectable plasma RNA viral load | 75 | 86 | 76 | Not applicable |
| % known on ART at baseline (uninterrupted) | 92 | 94 | 93 | Not applicable |
The numbers after ± are standard deviations
Clinical measures
CD4 cell count and HIV RNA plasma concentrations were obtained from each of the HIV-infected patients within 4 weeks of MRI examination using standardized methods (i.e., Roche Amplicor techniques). Each patient’s nadir CD4 count (lowest lymphocyte count) was also acquired from the patient’s medical history. Additionally, we quantified duration of HIV infection by subtracting the diagnosis date from the date of the image acquisition. This resulted in a length of disease variable quantified in years and months. For a subset of HIV-infected patients (N=86), cerebral spinal fluid (CSF) RNA concentrations were also available and examined.
Neurocognitive status was assessed using the AIDS Dementia Complex (ADC) scale as previously described (Price et al. 1988). Patients were assessed on both clinical and neuropsychological tests and rated as having no impairment, stage 0; subclinical impairment, 0.5; mild impairment, stage 1; moderate, stage 2; or severe, stage 3. Neuropsychological impairment was defined as performance of at least 1.0 standard deviation below normative values on two or more neuropsychological tests or at least 2.0 standard deviations below normative values on one or more tests that included measures of attention, executive function, working memory, learning/memory, and psycho-motor speed. Subsequent to the inception of this study, Antinori and colleagues published a new classification in which ADC stage 0.5 (neuropsychological impairment without functional disturbances) would correspond to asymptomatic neurocognitive impairment, ADC stage 1 would correspond to mild neurocognitive disorder, and ADC stage 2 or greater would correspond to HIV-associated dementia (Antinori et al. 2007). For the purpose of group comparison, we combined patients with ADC stages 0 and 0.5 into an asymptomatic group (N=181) and patients with ADC stages ≥1 into a symptomatic group (N= 35). Of the 86 patients with CSF RNA measures, 24 participants had detectable CSF RNA (~28%), and 17 were classified as having ADC stage ≥1 (~20%).
Neuroimaging
Imaging data used in the current analyses were available from seven sites from across the USA, all of which used GE Signa 1.5-T scanners (GE Medical Systems, Milwaukee, WI, USA) with similar operating systems and standard GE quadrature head coils. The consistent utilization of prescribed imaging parameters and the quality of the imaging data were assessed using automated header checking programs and manual inspection (e.g., for artifact, complete anatomical coverage, etc.). The whole head T1-weighted spoiled gradient-echo volumetric (repetition time/echo time (TR/TE)=16/600; field of view (FOV)=20 cm; slice thickness=1.3 mm; matrix size=256×128, with 1 NEX) images were analyzed in this study.
For the group comparison analyses, we quantified the CC utilizing the same methods in a cohort of healthy controls from a large imaging study with compatible acquisition parameters (Bigler et al. 1999). Data for this comparison cohort were obtained using a GE 1.5-T scanner with similar resolution in the sagittal plane utilizing the following scan parameters: TR/TE=15/500, FOV=24 cm, slice thickness= 2 mm, and matrix size=256×256, with 1 NEX.
Morphometric analyses
For each HIV-infected and control participant, the midsagittal slice of the CC was selected and manually traced by several trained raters, who were unaware of subject’s diagnosis, using the publicly available software 3D Slicer (SPL, Brigham and Women’s Hospital, Boston, MA, USA). The midsagittal slice was identified using the falx cerebri and cerebral aqueduct as anatomical landmarks, as well as 3D Slicer’s view of the horizontal plane for visual confirmation in accordance with established protocols (Denenberg et al. 1991). Methods of CC delineation are visually represented and further explained in Fig. 1. Intra- and inter-rater reliability for manual tracing was periodically examined throughout the study and found to be greater than or equal to 0.99 (intraclass correlation coefficient) suggesting highly reproducible results.
Fig. 1.
Illustration of quantification method. The left side panels show the modified Witelson protocol where the CC is divided into five functionally related areas. The right side of the figure shows the linear widths measurement method developed by our lab to quantify the width of the CC at 100 equidistant points along the length of the CC. First step for each method (see A) is to manually trace the midsagittal CC. In step B, the CC is divided into different area based on a standard division of the linear length. In step C, the CC is labeled and quantified. In step D, the manual tracing is subjected to a smoothing algorithm and the median curve of the CC is produced (black line). In step E, the median curve is divided into 100 equal points where the line is then bisected to produce a linear width at that point
Once manually traced, the label map was then subjected to two automated procedures designed to divide the CC into five standardized areas and quantify the linear width along the craniocaudal extent of the CC. The CC was first divided into five standardized areas using a modified Witeleson protocol (Kochunov et al. 2005; Witelson 1989). This protocol uses standardized divisions of the linear length of the CC after rotating the CC such that the long axis of the CC is parallel to the horizon as illustrated in Fig. 1. The actual areas in square millimeters for each segment of the CC were calculated by summing the number of pixels contained within the defined sub-regions and multiplying by pixel size.
In addition, the width of the CC was measured at 100 equidistant points along the length of the CC. This approach yields a much more detailed morphometric profile, which is particularly important in the context of diseases with subtle CNS effects. For this study, we used a conformal mapping technique developed by our laboratory (Center for Neurological Imaging, Brigham and Women’s Hospital) and implemented in Matlab® (Sampat et al. 2009; Sander et al. 2008). Briefly, this method consisted of two steps. First, the pixelated boundary from the manual tracing is smoothed. Second, the complex geometric plane of the smoothed tracing is conformally mapped to a more simple geometric polygon where the widths can be measured at 100 equidistant points along the CC midline (for a more detailed description of the method, the reader is referred to Sampat et al. 2009; also see Fig. 1).
Head size correction
Semi-automated segmentation was used to derive total intracranial volume (TICV) for each participant (Bigler et al. 1999; Dale et al. 1999). TICV was used to correct the CC area and linear widths using the following equation so that direct comparison of the measures could be made between individuals with largely varying head sizes (e.g., males and females):
Statistics
Differences in CC morphometry (areas and linear widths) measures between healthy HIV negative controls and HIV-infected patients were tested for differences using ANCOVA controlling for age. The relationship between the CC areas and clinical covariates for the HIV-infected patients was examined using linear regression models with current CD4, nadir CD4, plasma RNA (a binary variable representing detectable or undetectable RNA levels), duration of HIV infection, age, and ADC stage as candidate predictors. Variable selection for the regression models was performed using the Akaike information criterion (AIC) to choose only the important predictive factors of the CC areas. This method is preferred here to obtain a parsimonious model of the relationship between the candidate covariates (Cohen et al. 2010a) and the outcomes. In the CC linear width analyses, all candidate predictors were used in the linear regression models, and a smoothing method adjusting for multiple comparisons was utilized to obtain both the regression coefficients and their associated confidence bands (Laird et al. 1992). In a subsample of patients (N=86), the relationship between both the area and linear width analyses and CSF RNA was also examined. All statistical analyses were conducted in R-2.9.2 (R Development Core Team 2011).
Results
Demographic variable comparisons
There were significant differences between the three experimental groups (control, asymptomatic, and symptomatic) for age (p=0.0008) and between asymptomatic and symptomatic patients for duration of illness (p=0.002) with the symptomatic patients having longer average duration of illness. The asymptomatic and symptomatic groups were statistically similar for measures of disease severity and immunological function as measured by CD4 cell count (nadir and baseline), detectable plasma RNA, and CSF RNA (Table 1). To control for the effects of age and duration of illness, statistical analyses for group comparisons were made with these variables as covariates.
CC area group comparisons
Symptomatic HIV-positive subjects (ADC stage ≥1) showed reductions in all five CC areas when compared with healthy controls. No significant differences were found in any the CC areas between asymptomatic subjects (ADC stage 0) and subjects with subclinical impairment (ADC stage 0.5). Therefore, these groups were combined into an asymptomatic group for further analyses. This asymptomatic group differed from control participants for all the area measures except the isthmus (p=0.20), while the differences for genu were only marginally significant (p=0.05). Furthermore, the genu (p= 0.04) and the isthmus (p=0.03) were significantly smaller in the symptomatic group compared with the asymptomatic group (Table 2).
Table 2.
The area difference and actual p value are shown for comparison between the three groups separately adjusted for age and gender (significant comparisons are bolded)
| Area measure | NA vs controls | ADC vs controls | ADC vs NA |
|---|---|---|---|
| Genu | −11.34 (0.048) | −28.35 (0.002) | −18.58 (0.042) |
| Anterior midbody | −4.78 (0.015) | −9.52 (0.002) | −5.01 (0.090) |
| Posterior midbody | −4.57 (0.013) | −8.05 (0.006) | −3.62 (0.170) |
| Isthmus | −2.45 (0.204) | −8.36 (0.006) | −5.93 (0.025) |
| Splenium | −10.50 (0.009) | −18.09 (0.004) | −8.03 (0.182) |
CC linear width group comparisons
Regardless of linear width position examined, both HIV-infected groups displayed significantly smaller linear widths on average compared with control participants (Fig. 2). The symptomatic group demonstrated significant reductions in width in the genu (line segments 6–22) as well as marginally significant differences in the anterior midbody (line segments 25–33) compared with the asymptomatic group. In contrast, significant differences between the two HIV-infected groups were not found in the line segments corresponding to the posterior midbody, isthmus, or splenium. Combined these findings show that the linear widths for the asymptomatic group generally fell between those measured for the other two groups, consistent with a dose effect (Fig. 2).
Fig. 2.
Comparison of the line widths between the groups. a The actual beta (black line; x-axis) and the 95% confidence interval (blue dashed line) when comparing the ADC patients and controls. b The actual beta (black line) and the 95% confidence interval (blue dashed line) when comparing the NA patients and controls. c The actual beta (black line) and the 95% confidence interval (blue dashed line) when comparing the ADC and NA patients. d The actual linear width differences along the whole length of the CC for each of the groups (controls = black line, NA = red dashed line, and ADC = green dashed line)
Determinants of area measures
Multivariable linear regression models with variable selection done by AIC were used to determine the predictive association between each area measure and six clinical measures of interest. ADC stage was modestly associated with the genu (p=0.047) and the isthmus (p=0.027) while nadir CD4 cell count was associated with the posterior midbody (p=0.026). In the subsample of patients with detectable CSF RNA, no significant associations were found between the candidate predictors and the CC areas.
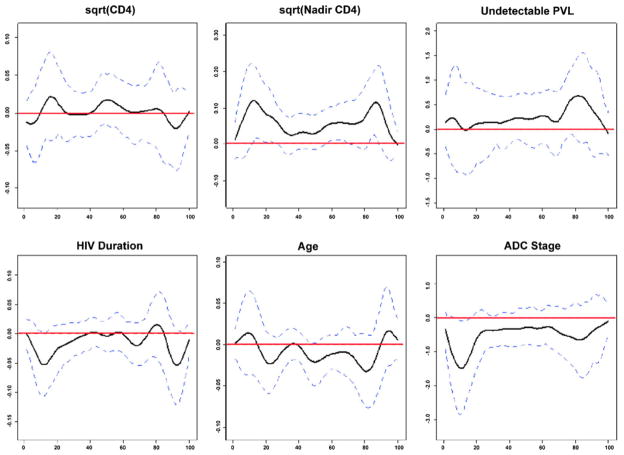
Determinants of linear width
Plots of the beta coefficients and confidence intervals for each regression result are shown in Fig. 3. From these plots, it is clear that the regression results were not significant for current CD4, presence of plasma RNA, HIV duration, and age regardless of linear width examined. In contrast, a lower CD4 cell count was significantly associated with shorter line segments corresponding to the genu (line segments 12–19), isthmus (line segments 59–66), and splenium (line segments 84–88). In addition, segments corresponding to the genu of the corpus callosum (line segments 7–18) were significantly shorter in patients with higher ADC stage. Again, in the subsample of patients with CSF RNA measurements, none of the considered predictors was significantly associated with the linear widths examined (plot not shown).
Fig. 3.
Figure illustrating the beta values (black line; x-axis) and the 95% confidence interval (blue dashed lines) for each multivariable regression analysis
Discussion
This study examined structural abnormalities in the mid-sagittal CC in a large cohort of HIV-infected patients with history of chronic disease on stable combination therapies. The results demonstrate several important findings, the most significant of which is a reduction in area and width in HIV-infected patients compared with controls. Specifically, both asymptomatic and symptomatic HIV-infected groups showed significant atrophy relative to controls regardless of area or linear width examined. In addition, differences were found between the HIV-infected groups in the genu (area and linear widths), anterior midbody (linear widths), and isthmus (area) with symptomatic subjects demonstrating the greatest degree of atrophy. These results suggest that despite treatment, there is significant and quantifiable CNS injury associated with HIV infection in the setting of chronic yet treated infection regardless of symptom presentation. Further, the strong association with nadir CD4 count suggests that this complication may evolve during the early stages of HIV infection in response to progressive immune suppression and may be irreversible despite subsequent exposure to effective antiretroviral therapies.
These findings are based on a large sample (N=216) of chronically infected subjects with a history of advanced disease (mean nadir CD4=27.5 cells/mm3) but who have since shown significant immune reconstitution in response to treatment (mean baseline CD4=269.1 cells/mm3; see Table 1). To our knowledge, this is the largest study of the CC in treated HIV subjects and extends findings from previous studies demonstrating significant reductions in total CC area between controls and more clinically advanced HIV-infected subjects or in subjects with additional comorbid risk factors such as drug and alcohol abuse (Cardenas et al. 2009; Chiang et al. 2007; Pfefferbaum et al. 2006; Thompson et al. 2005, 2006; Wohlschlaeger et al. 2009). A novel feature to our study was the utilization of 100 equidistant linear widths in addition to five regional divisions as previously described (Thompson et al. 2006). Results of area analyses clearly demonstrate significant regional thinning across a wide range of neurologically symptomatic and asymptomatic subjects with greater decreases occurring in subjects with more cognitive impairment. Group differences were further illustrated in the analysis of the linear widths, which demonstrated a similar dose response to the area measures, particularly in the anterior segments.
Although the basis for these findings is not entirely understood, these measures likely reflect underlying structural changes in the CC. One recent pathological study (Wohlschlaeger et al. 2009) found a significant decrease in CC area (~8%) in HIV-infected patients compared with controls that was associated with a global reduction in the absolute number of nerve fibers (~12%), with the most atrophy and reduction of nerve fibers occurring in the frontal and occipital regions of the CC (~28% in the anterior midbody and ~13% in the splenium). Moreover, the authors demonstrated alterations in the shape and caliber of axons that are also reflected in gross morphologic measures. In addition, there is an emerging body of literature based on DTI that demonstrates white matter tract changes in associated with HIV infection (Pfefferbaum et al. 2002, 2007, 2009; Ragin et al. 2004, 2005; Sullivan and Pfefferbaum 2003; Tate et al. 2010; Wu et al. 2006). Alterations in the diffusion signal and tractography have been observed in several areas of the brain, including the CC suggesting disorganization of the micro- and macro-structure of the white matter (Chiang et al. 2007). Thus, quantification of the midsagittal CC may provide an effective and sensitive approach to monitoring pathological changes in the white matter in HIV-infected patients, especially given the fact that the measurement error for the CC was less than 1% as shown in this study.
The current study shows several areas of atrophy across the length of the CC and suggests that in the era of cART many areas of the brain may be vulnerable to the effects of HIV, particularly in those subjects who have experienced severe immunological suppression. This is consistent with recent studies that have shown greater involvement of cortical regions, including reductions in volumes of the frontal and parietal region (Cohen et al. 2010b). The significant relationship between ADC stage and CC thinning, particularly in anterior third or genu of the CC, suggests that increased frontal involvement may contribute to worsening symptomology. More recently, we have shown that metabolic injury of the basal ganglia and frontal white matter as measured by MRS was modestly predictive of ADC, consistent with previous immunopathological studies (Rostasy et al. 1999). Whether the addition of CC measurements to these indices would improve diagnostic accuracy and thereby provide a useful diagnostic biomarker for ADC will need to be determined in future studies.
There have been few studies describing host or viral factors that may contribute to varying patterns of HIV-associated brain injury in the context of chronic disease and treatment. One important aim of this study therefore was to examine whether factors such as CD4 cell count and/or plasma HIV RNA levels exerted an effect on CC morphology. We also included age and duration of infection as covariates to test the hypothesis that aging and chronicity of infection may also contribute to thinning of the CC. To our surprise, other than the expected associations with ADC stage, a significant predictive relationship was only found for nadir CD4+ cell count while no significant effect was found for age, duration of HIV infection, plasma, or CSF HIV RNA levels. This finding stands in contrast to study by Thompson et al. (2006) who showed significant associations with the baseline CD4 value. However, the sample size was substantially smaller in that study (N=30), and the nadir CD4 count was not included in the analysis.
To our knowledge, this is the first study of a large cohort of HIV-infected subjects to demonstrate a significant relationship between nadir CD4 and in vivo morphologic measures of the white matter. It also extends recent reports that demonstrated a predictive association between nadir CD4 cell count and cognitive impairment (Cysique et al. 2010; Munoz-Moreno et al. 2008; Robertson et al. 2007; Valcour et al. 2006) as well as reduced cortical volumes in a smaller sample from the HIVNC cohort (Cohen et al. 2010b). The results of this study suggest that despite reconstitution of the immune system in response to cART, the extent of immune compromise experienced before the start of treatment may be an important factor leading to significant white matter atrophy. This finding also raises a larger question about when to initiate therapy in patients who have yet to experience an AIDS defining event. Accumulating evidence suggests that early initiation of treatment (CD4 cell count ≥350 cells/mm3) is associated with improved outcomes (Emery et al. 2008). Further, data from the When to Start Consortium (18 cohort studies, ~45,000 patients) demonstrated that patients who deferred treatment until CD4 cell count fell between 251 and 350 cells/mm3 were at increased risk for the development of AIDS and experienced higher mortality rates compared with those initiating therapy earlier (351 to 450 cells/mm3; Sterne et al. 2009). Even in the context of treatment, HIV-infected patients with lower nadir CD4 cell count do not experience the same degree of immune reconstitution when compared with patients with higher nadir CD4 cell counts (D’Amico et al. 2005). The results from the current study provide further support for early intervention although additional studies will be needed to determine the extent to which such treatment prevents or ameliorates CNS injury.
It is interesting to note that although there were significant group differences in the CC areas and linear widths, several asymptomatic subjects demonstrated moderate to marked atrophy qualitatively similar to that observed in the overall symptomatic group (Fig. 4). Distinguishing clinical features, which would suggest why so much atrophy would occur in some of the asymptomatic subjects but not others, are not apparent from the current analysis although, as mentioned above, a history of advanced immunosuppression as reflected by a low nadir CD4 count may be one contributing factor. Importantly, these observations suggest that subgroups of HIV-infected patients on treatment are experiencing significant atrophy despite the absence of cognitive symptoms in the setting of relatively stable disease. Future prospective studies are needed to understand the dynamic relationships between regional changes in the CC and the onset of cognitive impairment in treated patients.
Fig. 4.

Figure illustrating readily observable qualitative difference across controls and patients with similar demographic and clinical variables. ADC ADC staging, Nadir Nadir CD4 cell count, PVL plasma viral load (all patients were less than 75), CCD4 current CD4 cell count
There are potentially several limitations to this study. One is the use of archival control data collected as part of another study. Although significant attempts were made to choose a control dataset that had similar acquisition parameters, significant demographic or other features could have limited the comparisons of these groups. Age and education were two areas shown to be different but notably the HIV-infected groups, including asymptomatic individuals, showed a higher percentage with greater than a high school education (53–72%) compared with controls (44%), suggesting higher education did not confer a protective effect. In addition, several statistical measures were undertaken to limit the effects of such demographic variables. Second, the cross-sectional design of the study limits the predictive assessment of the effects of various clinical, cognitive, and morphometric relationships that will need to be addressed in future prospective studies.
It is well established that the CC represents a major communication pathway between the hemispheres that is responsible for the functional integration of complex motor, cognitive, and behavioral tasks. As such, quantification of the CC may provide a robust approach to assess the functional integrity of the cerebral hemispheres among HIV-infected patients. We employed a novel method of CC measurement that allows for greater sensitivity when examining the relationship between clinical measures and morphology and potentially in detecting longitudinal changes. Further, the linear width measurement provided additional sensitivity as this approach identified additional segments (genu and splenium) that were not detected in the area analysis. We also show that in the context of a large multi-center study, it is possible to acquire reliable CC measurements. In fact, intra- and inter-rater reliability measures (Cronbach’s alpha >0.99) demonstrated a high degree of agreement. Combined these findings suggest that these methods may complement each other to provide a useful and robust in vivo approach to examine the progression of subtle and focal changes in the white matter of HIV-infected patients and their effects on cognitive function in the setting of chronic disease and treatment.
Acknowledgments
We gratefully acknowledge the use of imaging data from the following HIV MRS Consortium sites: University of California San Diego, University of California Los Angeles (UCLA), Harbor UCLA Medical Center, Stanford University, Rochester Medical Center, University of Colorado Science Center, and University of Pittsburgh. We also gratefully acknowledge the use of imaging data of typically aging individuals from the laboratory of Erin D. Bigler. We also acknowledge the volunteer assistance of Stella Barth, Molly Siegel, Bessie Liu, Kelsey Han, and Alissa D’Gama. This publication was supported by the following: National Institutes of Health grants R01NS 35524, T32DA13911, RO1AI38855, RO1MH60565, RO1MH65857, RO1DA15045, RO1NS38841, and 1UO1MH083500; National Institute of Mental Health grant K23-MH073416 to DFT; and the AIDS Clinical Trials Group (NIAID).
Contributor Information
David F. Tate, Departments of Radiology and Psychiatry, Center for Neurological Imaging, The Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA. Department of Neurology, Alzheimer’s Disease Center, Boston University Medical Center, Boston, MA, USA. Department of Neurology, Henry M. Jackson Foundation, Defense & Veterans Brain Injury Center, San Antonio Military Medical Center, San Antonio, TX 78324, USA
Mehul Sampat, Departments of Radiology and Psychiatry, Center for Neurological Imaging, The Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Jaroslaw Harezlak, Indiana University School of Medicine, Indianapolis, IN, USA.
Mark Fiecas, Department of Biostatistics, Brown University, Providence, RI, USA.
Joseph Hogan, Email: dftatephd@mac.com, Department of Biostatistics, Brown University, Providence, RI, USA.
Jeffrey Dewey, Departments of Radiology and Psychiatry, Center for Neurological Imaging, The Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Daniel McCaffrey, Departments of Radiology and Psychiatry, Center for Neurological Imaging, The Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Daniel Branson, Departments of Radiology and Psychiatry, Center for Neurological Imaging, The Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Troy Russell, Departments of Radiology and Psychiatry, Center for Neurological Imaging, The Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Jared Conley, Departments of Radiology and Psychiatry, Center for Neurological Imaging, The Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Michael Taylor, University of California, San Diego, San Diego, CA, USA.
Giavoni Schifitto, University of Rochester Medical Center, Rochester, NY, USA.
J. Zhong, University of Rochester Medical Center, Rochester, NY, USA
Eric S. Daar, University of California Los Angeles/Harbor, Torrance, CA, USA
Jeffrey Alger, University of California Los Angeles, Los Angeles, CA, USA.
Mark Brown, University of Colorado, Denver, CO, USA.
Elyse Singer, University of California Los Angeles, Los Angeles, CA, USA.
T. Campbell, University of Colorado, Denver, CO, USA
D. McMahon, University of Pittsburgh, Pittsburgh, PA, USA
Y. Tso, Stanford University, Palo Alto, CA, USA
Janetta Matesan, Indiana University School of Medicine, Indianapolis, IN, USA.
Scott Letendre, University of California, San Diego, San Diego, CA, USA.
S. Paulose, Stanford University, Palo Alto, CA, USA
Michelle Gaugh, University of Rochester Medical Center, Rochester, NY, USA.
C. Tripoli, University of Pittsburgh, Pittsburgh, PA, USA
Constantine Yiannoutsos, Indiana University School of Medicine, Indianapolis, IN, USA.
Erin D. Bigler, Departments of Psychology and Neuroscience, Brigham Young University, Provo, UT, USA
Ronald A. Cohen, Brown Medical School, The Miriam Hospital, Providence, RI, USA
Charles R. G. Guttmann, Departments of Radiology and Psychiatry, Center for Neurological Imaging, The Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Bradford Navia, Email: bradford.navia@tufts.org, Departments of Neurology and Psychiatry, Tufts-New England Medical Center, Boston, MA, USA. Department of Public Health, Jaharis Family Center for Biomedical Research, Tufts Medical School, Boston, MA 02111, USA.
References
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Bell JE. The neuropathology of HIV/AIDS. Int Rev Psychiatry. 2008;20:15–24. doi: 10.1080/09540260701862037. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald SL, Masliah E, Fennema-Notestine C, Marcotte TD, Ellis RJ, McCutchan JA, Heaton RK, Grant I, Mallory M, Miller A, Jernigan TL. Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Arch Neurol. 2004;61:369–376. doi: 10.1001/archneur.61.3.369. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Brettschneider PD, McArthur JC, Harris GJ, Schlaepfer TE, Henderer JD, Barta PE, Tien AY, Pearlson GD. Magnetic resonance imaging measurement of gray matter volume reductions in HIV dementia. Am J Psychiatry. 1995;152:987–994. doi: 10.1176/ajp.152.7.987. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Blatter DD, Johnson SC, Anderson CV, Russo AA, Gale SD, Ryser DK, MacNamara SE, Bailey BJ. Traumatic brain injury, alcohol and quantitative neuroimaging: preliminary findings. Brain Inj. 1996;10:197–206. doi: 10.1080/026990596124511. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Johnson SC, Blatter DD. Head trauma and intellectual status: relation to quantitative magnetic resonance imaging findings. Appl Neuropsychol. 1999;6:217–225. doi: 10.1207/s15324826an0604_4. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Meyerhoff DJ, Studholme C, Kornak J, Rothlind J, Lampiris H, Neuhaus J, Grant RM, Chao LL, Truran D, Weiner MW. Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretro-viral therapy. J Neurovirol. 2009;15:324–333. doi: 10.1080/13550280902973960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Lee PL, Yiannoutsos CT, Ernst T, Marra CM, Richards T, Kolson D, Schifitto G, Jarvik JG, Miller EN, Lenkinski R, Gonzalez G, Navia BA. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004;23:1336–1347. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- Chen Y, An H, Zhu H, Stone T, Smith JK, Hall C, Bullitt E, Shen D, Lin W. White matter abnormalities revealed by diffusion tensor imaging in non-demented and demented HIV+ patients. Neuroimage. 2009;47:1154–1162. doi: 10.1016/j.neuroimage.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Dutton RA, Hayashi KM, Lopez OL, Aizenstein HJ, Toga AW, Becker JT, Thompson PM. 3D pattern of brain atrophy in HIV/AIDS visualized using tensor-based morphometry. Neuroimage. 2007;34:44–60. doi: 10.1016/j.neuroimage.2006.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Harezlak J, Gongvatana A, Buchthal S, Schifitto G, Clark U, Paul R, Taylor M, Thompson P, Tate D, Alger J, Brown M, Zhong J, Campbell T, Singer E, Daar E, McMahon D, Tso Y, Yiannoutsos CT, Navia B HIV Neuroimaging Consortium. Cerebral metabolite abnormalities in human immunodeficiency virus are associated with cortical and subcortical volumes. J Neurovirol. 2010a;16(6):435–444. doi: 10.3109/13550284.2010.520817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, Paul R, Taylor M, Thompson P, Alger J, Brown M, Zhong J, Campbell T, Singer E, Daar E, McMahon D, Tso Y, Yiannoutsos CT, Navia B. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol. 2010b;16:25–32. doi: 10.3109/13550280903552420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Letendre SL, Ake C, Jin H, Franklin DR, Gupta S, Shi C, Yu X, Wu Z, Abramson IS, Grant I, Heaton RK. Incidence and nature of cognitive decline over 1 year among HIV-infected former plasma donors in China. AIDS. 2010;24:983–990. doi: 10.1097/QAD.0b013e32833336c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico R, Yang Y, Mildvan D, Evans SR, Schnizlein-Bick CT, Hafner R, Webb N, Basar M, Zackin R, Jacobson MA. Lower CD4+ T lymphocyte nadirs may indicate limited immune reconstitution in HIV-1 infected individuals on potent antiretroviral therapy: analysis of immunophenotypic marker results of AACTG 5067. J Clin Immunol. 2005;25:106–115. doi: 10.1007/s10875-005-2816-0. [DOI] [PubMed] [Google Scholar]
- Dale A, Fischl B, Sereno M. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Cowell PE, Fitch RH, Kertesz A, Kenner GH. Corpus callosum: multiple parameter measurements in rodents and humans. Physiol Behav. 1991;49:433–437. doi: 10.1016/0031-9384(91)90261-l. [DOI] [PubMed] [Google Scholar]
- Di Sclafani V, Mackay RD, Meyerhoff DJ, Norman D, Weiner MW, Fein G. Brain atrophy in HIV infection is more strongly associated with CDC clinical stage than with cognitive impairment. J Int Neuropsychol Soc. 1997;3:276–287. [PMC free article] [PubMed] [Google Scholar]
- Emery S, Neuhaus JA, Phillips AN, Babiker A, Cohen CJ, Gatell JM, Girard PM, Grund B, Law M, Losso MH, Palfreeman A, Wood R. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–1144. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Alger J, Singer E, Campbell T, Yiannoutsos C, Cohen R, Navia B the HIV Neuroimaging Consortium. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011;25(5):625–633. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hult B, Chana G, Masliah E, Everall I. Neurobiology of HIV. Int Rev Psychiatry. 2008;20:3–13. doi: 10.1080/09540260701862086. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Lancaster J, Hardies J, Thompson PM, Woods RP, Cody JD, Hale DE, Laird A, Fox PT. Mapping structural differences of the corpus callosum in individuals with 18q deletions using targetless regional spatial normalization. Hum Brain Mapp. 2005;24:325–331. doi: 10.1002/hbm.20090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird N, Donnelly C, Ware J. Longitudinal studies with continuous response. Stat Meth Med Res. 1992;1:3–25. doi: 10.1177/096228029200100302. [DOI] [PubMed] [Google Scholar]
- Levy R, Bredesen D. Central nervous system dysfunction in acquired immunodeficiency syndrome. JAIDS J Acquir Immune Defic Syndr. 1988;1:41. [PubMed] [Google Scholar]
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- McArthur JC, McDermott MP, McClernon D, St Hillaire C, Conant K, Marder K, Schifitto G, Selnes OA, Sacktor N, Stern Y, Albert SM, Kieburtz K, deMarcaida JA, Cohen B, Epstein LG. Attenuated central nervous system infection in advanced HIV/AIDS with combination antiretroviral therapy. Arch Neurol. 2004;61:1687–1696. doi: 10.1001/archneur.61.11.1687. [DOI] [PubMed] [Google Scholar]
- McCutchan JA, Wu JW, Robertson K, Koletar SL, Ellis RJ, Cohn S, Taylor M, Woods S, Heaton R, Currier J, Williams PL. HIV suppression by HAART preserves cognitive function in advanced, immune-reconstituted AIDS patients. AIDS. 2007;21:1109–1117. doi: 10.1097/QAD.0b013e3280ef6acd. [DOI] [PubMed] [Google Scholar]
- Munoz-Moreno JA, Fumaz CR, Ferrer MJ, Prats A, Negredo E, Garolera M, Perez-Alvarez N, Molto J, Gomez G, Clotet B. Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res Hum Retroviruses. 2008;24:1301–1307. doi: 10.1089/aid.2007.0310. [DOI] [PubMed] [Google Scholar]
- Navia B, Price R. The acquired immunodeficiency syndrome dementia complex as the presenting or sole manifestation of human immunodeficiency virus infection. Arch Neurol. 1987;44:65. doi: 10.1001/archneur.1987.00520130051017. [DOI] [PubMed] [Google Scholar]
- Navia B, Cho E, Petito C, Price R. The AIDS dementia complex: II neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Paul R, Cohen R, Navia B, Tashima K. Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neurosci Biobehav Rev. 2002;26:353–359. doi: 10.1016/s0149-7634(02)00006-4. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Sullivan EV. Alcoholism and AIDS: magnetic resonance imaging approaches for detecting interactive neuropathology. Alcohol Clin Exp Res. 2002;26:1031–1046. doi: 10.1097/01.ALC.0000021146.01778.55. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Adalsteinsson E, Kemper CA, Deresinski S, Sullivan EV. Contribution of alcoholism to brain dysmorphology in HIV infection: effects on the ventricles and corpus callosum. Neuroimage. 2006;33:239–251. doi: 10.1016/j.neuroimage.2006.05.052. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV. Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain. 2007;130:48–64. doi: 10.1093/brain/awl242. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Kemper CA, Deresinski S, Sullivan EV. Frontostriatal fiber bundle compromise in HIV infection without dementia. AIDS. 2009;23:1977–1985. doi: 10.1097/QAD.0b013e32832e77fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RW, Brew B, Sidtis J, Rosenblum M, Scheck AC, Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. R Development Core Team; Vienna: 2011. http://www.R-project.org. [Google Scholar]
- Ragin AB, Storey P, Cohen BA, Epstein LG, Edelman RR. Whole brain diffusion tensor imaging in HIV-associated cognitive impairment. AJNR Am J Neuroradiol. 2004;25:195–200. [PMC free article] [PubMed] [Google Scholar]
- Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, Epstein LG. Diffusion tensor imaging of subcortical brain injury in patients infected with human immunodeficiency virus. J Neurovirol. 2005;11:292–298. doi: 10.1080/13550280590953799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- Rostasy K, Monti L, Yiannoutsos C, Kneissl M, Bell J, Kemper TL, Hedreen JC, Navia BA. Human immunodeficiency virus infection, inducible nitric oxide synthase expression, and micro-glial activation: pathogenetic relationship to the acquired immunodeficiency syndrome dementia complex. Ann Neurol. 1999;46:207–216. [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Skolasky RL, Ernst T, Mao X, Selnes O, Pomper MG, Chang L, Zhong K, Shungu DC, Marder K, Shibata D, Schifitto G, Bobo L, Barker PB. A multicenter study of two magnetic resonance spectroscopy techniques in individuals with HIV dementia. J Magn Reson Imaging. 2005;21:325–333. doi: 10.1002/jmri.20272. [DOI] [PubMed] [Google Scholar]
- Sampat MP, Jesrani P, Meier DS, Guttmann CR. Quantification of callosal widths using conformal mapping: application to multiple sclerosis. International Society for Magnetic Resonance in Medicine; Hawaii: 2009. [Google Scholar]
- Sander C, Sampat MP, Berger A, Meier DS, Guttmann CR. Functional correlates of corpus callosum thickness in multiple sclerosis. International Society for Magnetic Resonance in Medicine; Toronto: 2008. [Google Scholar]
- Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, Harris R, Funk MJ, Geskus RB, Gill J, Dabis F, Miro JM, Justice AC, Ledergerber B, Fatkenheuer G, Hogg RS, Monforte AD, Saag M, Smith C, Staszewski S, Egger M, Cole SR. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JC, Ellis RJ, Jernigan TL, Archibald SL, Abramson I, Wolfson T, McCutchan JA, Wallace MR, Atkinson JH, Grant I. Progressive cerebral volume loss in human immunodeficiency virus infection: a longitudinal volumetric magnetic resonance imaging study. HIV Neurobehavioral Research Center Group. Arch Neurol. 1998;55:161–168. doi: 10.1001/archneur.55.2.161. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging in normal aging and neuropsychiatric disorders. Eur J Radiol. 2003;45:244–255. doi: 10.1016/s0720-048x(02)00313-3. [DOI] [PubMed] [Google Scholar]
- Tate DF, Conley J, Paul RH, Coop K, Zhang S, Zhou W, Laidlaw DH, Taylor LE, Flanigan T, Navia B, Cohen R, Tashima K. Quantitative diffusion tensor imaging tractography metrics are associated with cognitive performance among HIV-infected patients. Brain Imaging Behav. 2010;4:68–79. doi: 10.1007/s11682-009-9086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci USA. 2005;102:15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Lu A, Lee SE, Lee JY, Lopez OL, Aizenstein HJ, Toga AW, Becker JT. 3D mapping of ventricular and corpus callosum abnormalities in HIV/AIDS. Neuroimage. 2006;31:12–23. doi: 10.1016/j.neuroimage.2005.11.043. [DOI] [PubMed] [Google Scholar]
- Valcour V, Yee P, Williams AE, Shiramizu B, Watters M, Selnes O, Paul R, Shikuma C, Sacktor N. Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in human immunodeficiency virus type 1 infection—The Hawaii Aging with HIV Cohort. J Neurovirol. 2006;12:387–391. doi: 10.1080/13550280600915339. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112(Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Wohlschlaeger J, Wenger E, Mehraein P, Weis S. White matter changes in HIV-1 infected brains: a combined gross anatomical and ultrastructural morphometric investigation of the corpus callosum. Clin Neurol Neurosurg. 2009;111:422–429. doi: 10.1016/j.clineuro.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Wu Y, Storey P, Cohen BA, Epstein LG, Edelman RR, Ragin AB. Diffusion alterations in corpus callosum of patients with HIV. AJNR Am J Neuroradiol. 2006;27:656–660. [PMC free article] [PubMed] [Google Scholar]
- Yiannoutsos CT, Ernst T, Chang L, Lee PL, Richards T, Marra CM, Meyerhoff DJ, Jarvik JG, Kolson D, Schifitto G, Ellis RJ, Swindells S, Simpson DM, Miller EN, Gonzalez RG, Navia BA. Regional patterns of brain metabolites in AIDS dementia complex. Neuroimage. 2004;23:928–93. doi: 10.1016/j.neuroimage.2004.07.033. [DOI] [PubMed] [Google Scholar]