Abstract
Recruitment of leukocytes circulating in our blood to the sites of infection or tissue damage is the key phenomenon in the acute inflammatory response(s). Among the leukocytes, neutrophils are primarily recruited into the areas of acute inflammation. When neutrophils interact with activated endothelium of the blood vessels, they become migratory and cross the endothelial layer of the blood vessel wall in a process called as leukocyte extravasation. Identifying and understanding the gene regulation of this extravasation phenomenon is one of the key objective of biomedical research aimed at ameliorating or alleviating the symptoms of various diseases, such as rheumatoid arthritis, asthma, anaphylaxis, atherosclerosis, ulcerative colitis etc., that are exacerbated by inappropriate inflammatory stimuli. Here, we decipher and discuss the key genes implicated in the leukocyte transmigration using the acute inflammation model called as the Dextran Sulphate Sodium (DSS) induced Colitis in mice as a classic paradigm.
Keywords: Leukocytes, Neutrophils Endothelium, Transmigration, Extravasation, Acute Inflammation, Colonic Epithelium, DSS Colitis
Background
Arrays of steps are involved in leukocyte extravasation that occurs during the acute inflammatory response(s) [1, 2]. The neutrophils present in the bloodstream first loosely adhere to the endothelium through carbohydratebased ligands to cause sampling of the local micro-environment for signs of inflammation. Activated endothelial cells produce both soluble and surfacebound molecules that trigger tight, integrin-mediated adhesion between the neutrophil and the endothelium. Neutrophils depart the flowing blood stream by first tethering and then rolling on the inflamed endothelial coating of the blood vessels. This occurs primarily in post-capilary and collecting venules in the systemic circulation. Under favourable environment, firmly adherent neutrophils then transmigrate across the endothelium. In vitro studies [3] have shown that the transmigration is a rapid process which is being completed in less than 2 minutes (Figure 1). Each step of neutrophil emigration has been associated with specific cell adhesion molecules (CAMs) and CAM activators and chemo-attractants [4, 5]. The neutrophil-endothelial cell interactions are initiated by interacting sets of CAMs and chemoattractant/activator molecules to form an “adhesion cascade.” The initial phase of inflammation, a transient slowing of neutrophils in postcapillary venules, is mediated by selectins. Subsequently, firm adhesion of neutrophils to the vessel wall occurs via interaction of the CD11/CD18integrins to endothelial ligands such as intercellular adhesion molecule-1 (ICAM-1) [6]. This binding requires activation of CD11/CD18 by exposure of the neutrophil to a variety of activating/chemoattractant molecules, such as platelet activating factor (PAF) or interleukin-8 (IL-8). The transmigration into tissues occurs, a process that requires both a chemotactic stimulus by chemokines and engagement of platelet endothelial cell adhesion molecule-1(PECAM-1) (Figure 2) [5]. Several approaches have been used to probe the role of CAMs in vivo. These include the use of blocking antibodies, chimeric selectin-immunoglobulin proteins, Sialyl Lewis oligosaccharides and peptides, along with the study of humans and animals with genetically determined adhesion deficiencies. These studies demonstrate that CAM blockade can effectively inhibit inflammation [6, 7]. Here, we specifically focus on the acute inflammation induced by Dextran Sulphate Sodium (DSS) using high throughput microarray data obtained from Gene Expression Omnibus (GEO) and discuss how the precise interaction as well as coordination of CAMs and the chemokines facilitate the neutrophil extravasation and transmigration during acute inflammation.
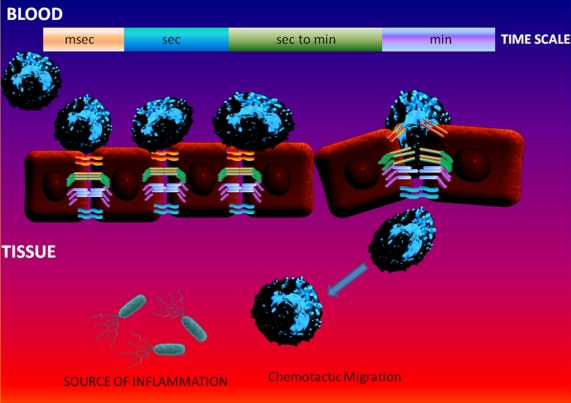
Figure 1.
The Key Cellular Events Implicated in the Neutrophil Extravasation Model. The selectins, chemokines and integrins coordinate in capturing a circulating neutrophil to the vessel wall at the specific sites. This process usually takes within few milliseconds to seconds during the acute inflammatory process(s) triggered by invading microbes such as gram negative bacteria. Further neutrophil exposure to the apical endothelial chemokines causes the chemotaxis and triggers their potential to undergo transendothelial migration within minutes and more importantly without disrupting the integrity of the endothelial barrier surrounding the blood vessels.
Figure 2.
Molecular and Cellular Events in Neutrophil Transmigration during Acute Inflammation. During the‘resting-state’, the junction between endothelial cells lining the blood vessels is closed, and this integrity is mediated by homotypic binding of VE-cadherin, PECAM-1 and JAM molecules. The VE-cadherin is linked to the cytoskeleton via β catenin, and this association is required for its adhesive activity. As it proceeds through the multi-step adhesion cascade, a neutrophil might trigger the dissociation of the VE-cadherin-β-catenin complex by degradation of the β-catenin and redistribution and/or internalization of the VE-cadherin. During transendothelial migration, the homophilic interactions (JAM-JAM and PECAM-1-PECAM-1) between opposing endothelial cells might be disrupted by the neutrophil, which would result in loosening of the junction. Engagement of PECAM-1 might provide a scaffold to ‘walk’ the cell through the junction via haptotactic migration.
Material and Methodology
Meta-Analysis of High-Throughput Genomics Data for deciphering Genes Implicated in the Leukocyte Transmigration in Acute DSS Colitis in Mice
The raw Affymetrix CEL files downloaded from the Gene Expression Omnibus (GSE22307) were normalized with RMA algorithm and subjected to a very stringent Statistical Analysis using One Way Analysis of Variance (ANOVA), followed by Tukey Honestly Significant Difference (HSD) Post-Hoc Test and Multiple Testing Correction (MTC) by applying the Benjamini-Hochberg False Discovery Rate (FDR) (p<0.05) using the latest Genespring GX11 (Agilent, USA). The statistically significant gene list result was filtered based on the standard 2 Fold cut-off compared with the Day 0 expression values. The differentially expressed genes were then classified and clustered based on the Database for Annotation, Visualization, and Integrated Discovery (DAVID) Analysis [8, 9]. Furthermore, the differentially expressed pathways were visualized through KEGG pathway interaction maps [10–12].
Results
The Dextran Sulphate Sodium (DSS) induced acute and chronic colitis models are often performed in experimental animals, especially in the most susceptible strains of mice, such as C57BL/6j to mimic the ulcerative colitis (UC) in humans. Fang et al. (2011) have recently performed a high throughput genomics study by administering 3% Dextran Sulphate Sodium (DSS) in the drinking water to C57BL/6j mice and collected the colonic tissues from individual cohorts at days 0, 2, 4 and 6 for total RNA extraction and detected the differential gene expression patterns using Affymerix GeneChip Mouse Genome 430 2.0 Array [13]. We have obtained the raw CEL files deposited into the Gene Expression Omnibus (GSE22307) to further decipher the key genes implicated in the positive regulation of acute inflammation, leukocyte adhesion, and neutrophil chemotaxis across in acute DSS colitis.
The induction of acute inflammation by the oral administration of DSS causes the expression of various proinflammatory genes in the colonic epithelium. Specific focus into the expression of adhesion molecules as well as the genes implicated in the chemotaxis of leukocytes such as the neutrophils through the Gene Ontology Analysis module in Genespring GX11, have revealed the differential expression of key genes which positively regulate the acute inflammation such as IL-1ß, IL-6, CCL5, CCR5, FcgRI, FcgRIII, FceRIg, and PTGS2, were significantly up-regulated at least 2 fold in the log scale by the acute inflammation triggered by DSS in the colonic tissue (Figure 3A). A plethora of genes positively influencing the leukocyte chemotaxis such as CCL2, CCL7, CCL8, CCL5, IL-1 beta, CXCL1, CXCL2, CXCL3, etc., were significantly up-regulated by the acute inflammation triggered by DSS (Figure 3B). In addition, the adhesion molecules such as ICAM1, VCAM1, ITGAM, ITGAL, and P-Selectin, were up-regulated (at least 2 Fold) (Figure 3C). These adhesion molecules play a key role in the adhesion and transmigration of neutrophils across colonic epithelium as well as vascular endothelium to the site of infection or inflammation. Furthermore, the genes specifically influencing neutrophil chemotaxis such as CXCL1, CXCL2, CXCL3, CCL2, CCL2, C5aR1, ITGAM, ITGB2 etc., were significantly up-regulated by the acute inflammation triggered by DSS (Figure 3D). In addition, the leukocyte activating genes such as CD86, HDAC9, Fc Receptors, Dock2, LCP2 etc. were also up-regulated in this model (Figure 3E). In order to decipher the cellular and molecular processes induced to cause the leukocyte transendothelial migration across the blood vessels, we have performed the DAVID analysis of the differentially expressed genes. Furthermore, we obtained the KEGG pathways for the leukocyte transendothelial migration (Figure 4) as well as Chemokine Signaling (Figure 5) and identified the differentially expressed genes from our gene list in the Canonical Pathway to decipher the key chemokines as well as cytokines implicated in the leukocyte transendothelial migration induced by DSS.
Figure 3.
Genespring GX11 analysis of Affymetrix Gene-Chip Expression Data from the colonic epithelium of 3% DSS-induced Colitis on Day 0, 2, 4, and 6 respectively in C57BL/6j mice. The raw Affymetrix CEL files downloaded from Gene Expression Omnibus (GSE22307) were normalized with RMA algorithm and subjected to One Way ANOVA, Tukey HSD and Bonferroni FDR(p<0.05). The resulting gene list was filtered based on 2 Fold cut-off compared with the Day 0 expression values. The differentially expressed genes were then classified and clustered based on Gene Ontology (GO) Analysis to decipher the differentially expressed genes in (A) Positive Regulation of Acute Inflammation, (B) Chemotaxis, (C) Leukocyte Adhesion, (D) Neutrophil Chemotaxis, and (E) Leukocyte Activation.
Figure 4.
Leukocyte Transendothelial Migration Pathway. KEGG Pathway Analysis of Genes Implicated in the Leukocyte Transendothelial Migration (Red Stars) in the colonic endothelium of 3% DSS-induced Colitis in C57BL/6j mice.
Figure 5.
Chemokine Signaling Pathway. KEGG Pathway Analysis of Genes Implicated in the Chemokine Signaling (Red Stars) in the colonic epithelial tissue of 3% DSS-induced Colitis in C57BL/6j mice.
Discussion
Several classes of adhesion molecules mediate neutrophil attachment to endothelial cells during acute inflammatory state, including selectins, which bind to their carbohydrate-based ligands, and integrins, which in turn bind to CAMs [2]. The initial tethering event is initiated through selectins, and the later development of tight adhesion is proposed to exploit α4β1 (VLA-4)–VCAM (vascular CAM interactions, followed by αL/Mβ2 (LFA-1/Mac-1)–ICAM (intercellular CAM) adhesion [7]. The selectins include a group of three related molecules. L-selectin is constitutively expressed on neutrophils and is shed from the cell surface on cell activation, assumed to occur immediately after rolling begins. P-selectin is found on platelets and is stored in Weibel-Palade bodies where it is rapidly (within few minutes) transported to the cell surface after endothelial cell activation by stimuli such as histamine, thrombin, bradykinin, leukotriene C4 (LTC4), or free radicals. E-selectin (ELAM-1) is expressed solely on endothelial cells where it is synthesized rapidly after cell stimulation by cytokines (TNF-α, IL-1) or endotoxin and then translocated to the luminal surface of the venular endothelium. Each selectin shares a common molecular structure, most notably an NH2-terminal lectin-like domain, which imparts the ability of each selectin to bind to specific carbohydrate ligands [14, 15]. In addition, during the primary phase of acute inflammation, the initial selectin-based adhesive events stimulate sequential activation of downstream adhesion receptors [14, 15]. Involvement of neutrophil L-selectin increases the adhesiveness of β2 integrins for ICAM [14–17]. Further analysis shows that Lselectin cross-linking induces firm adhesion of neutrophils under flow conditions, accompanied by increases in actin polymerization and colocalization of β2 integrins with L-selectin [16]. Engagement of L-selectin induces calcium release, MAP kinases, tyrosine kinases and small G-protein signaling in the neutrophils that may influence β2 integrin adhesiveness [18–20]. It was reported that p38MAPK inhibitors block L-selectin-induced changes to both neutrophil shape and β2- mediated adhesion. Hence, selectins play an important role in initiating the sequential adhesion cascade through both adhesive and signaling events in the neutrophils [21].
For neutrophils, tight adhesion needs activation of the β2-integrin family (CD18), resulting in binding to one of the intercellular adhesion molecules on the surfaces of endothelial cells. The CD11/CD18 integrins, such as Mac-1 and LFA-1, interact with the immunoglobulin superfamily member and ICAM-1. The CD 11b is capable of binding a wide range of ligands on endothelial cells [15, 16, 19, 20]. An important characteristic of the neutrophil integrins is that under baseline conditions they exist in a relatively inactive conformation, rendering the leukocyte non-adhesive. One of the key events of the adhesion cascade is the activation (occasionally accompanied by the inclusion of a protein on the cell surface) and deactivation of these integrins at the proper times and places. Integrin-mediated adhesion is regulated by modulating either the affinity of the integrin for its ligand or by altering the avidity of adhesion through integrin clustering and cell spreading phenomenon. The coordination of adhesion by integrin molecules is mediated by two types of signaling events which are particularly appropriate for transendothelial migration of leukocytes, namely, cross-talk between CAMs and stimulation by chemokines through chemokine receptors on neutrophils [15, 16, 19, 20].
The diapedesis or transmigration of neutrophils across an endothelial monolayer requires a chemotactic gradient [21]. The cytokines, such as TNF-α and IL-1, cause expression of potent chemotactic stimuli such as IL-8, which affect both adherence and transmigration of neutrophils [23]. The plateletendothelial cell adhesion molecule-1 (PECAM-1), a member of the immunoglobulin super family, is expressed at relatively low levels on the surface of leukocytes and platelets but at higher levels (>106molecules per cell) on endothelium. PECAM-1 plays a key role in transmigration of neutrophils across the endothelium [6, 7]. Its localization at the junctions between endothelial cells has suggested a role in transendothelial cell migration. Using an in vitro model, Muller et al. (1993) demonstrated that antibodies against PECAM-1 significantly blocked leukocyte transmigration through TNF-α activated endothelial cell monolayers but did not affect the adhesion of neutrophils [24]. This effect has been confirmed earlier in animal models of neutrophil transmigration [6]. The mechanisms by which PECAM-1 may regulate transendothelial migration are not yet known; however, the possibilities include direct binding of PECAM-I to the leukocyte (perhaps serving as a molecular guide as it passes through the junction), or regulation of adhesion molecules on the surface of the leukocyte (i.e., β2 integrins), thus facilitating transmigration [24]. Available data indicate that cross-linking of PECAM-1 on the surface of leukocytes by antibodies can up-regulate integrinmediated binding [25]. Another process that may play an important role in transendothelial migration is the ability of the endothelial cell monolayer to regulate an opening of its junctions, allowing neutrophil transmigration. Recent work by Huang et al. suggests that transmigrating neutrophils (in response to a chemotactic stimulus) exert an active influence on the endothelial cell monolayer, resulting in increased intracellular endothelial calcium levels and opening of intercellular junctions [26].
Role of Chemokines and Cytokines in Neutrophil Transmigration
Several chemokines regulate the order and timing of integrin adhesions [23], and several chemokines upregulate β2-mediated adhesions of neutrophils [27]. More recently, a role for specific chemokines in distinct steps of neutrophil extravasation has been described [7, 23]. These studies show that the chemokines GROα and fractalkine mediate the initial firm adhesion, whereas the chemokine MCP-1 is required for the later steps of neutrophil spreading and diapedesis [23]. The chemokines play a key role in directing the multi-step model of extravasation by promoting the appropriate sequence of integrin activation/ inactivation and the associated cell behavior. Migration of neutrophils is the result of choreographed changes in both cell morphology and adhesion. Membrane protrusions and the formation of new adhesive contacts at the leading edge of a migrating cell must be coordinated with down-regulation of adhesion and retraction at the rear of the cell [28]. PAF is synthesized by endothelial cells within minutes after stimulation by thrombin, histamine, LTC4, or other agonists, and is expressed on the cell surface where it activates neutrophils and up-regulates CD 11b by binding to a specific cell-surface receptor [29]. Many of the same factors that stimulate PAF expression also cause rapid translocation of P-selectin to the surface of the endothelium. Thus, for at least one set of stimuli both a specific tethering molecule (P-selectin) and a specific activator molecule (PAF) are produced. A similar situation may exist for the neutrophil chemoattractant/activator IL-8, which appears to bind to the luminal surface of activated endothelial cells where it is able to activate neutrophils [29]. Since cytokines, such as TNFα, and other factors (such as endotoxin) cause the synthesis of both IL-8 and E-selectin from endothelial cells [30], it is likely that these mediators may augment neutrophil transmigration by stimulating expression of both the tethering molecule and the activating factor [31]. Important ligands for the CD11/CD18 integrins are ICAM-1, and possibly ICAM-2 [2, 3]. These molecules, members of the immunoglobulin superfamily, are present constitutively on endothelial cells both in vitro and in vivo. CD11a and CDI1b bind to ICAM-1 in different regions of the molecule. Only CD 11a has been shown to be capable of binding to ICAM-2. The cytokines such as TNF-α and IL-1 cause expression of potent chemotactic stimuli such as IL-8 [31, 32], which affect both adherence and transmigration of neutrophils.
Conclusions and Future Directions
Leukocytes such as neutrophils undergo dynamic changes in cell-shape and adhesive properties during transmigration through endothelium of blood vessels in response to inflammatory signals. In this article, we have addressed the dynamics of CAMs and chemokine interactions in mediating this extravasation. Moreover, we discussed the key molecular events which lie beneath these key steps of neutrophil transmigration in acute inflammation induced by DSS in the colon of mice. In future, the evolution of State of the Art cellular and molecular imaging techniques in vitro and in vivo will definitely offer a finer resolution of the spatio-temporal regulation of key CAMs and chemokines to decipher the complete picture of how the activities of individual molecules are orchestrated during neutrophil transmigration in acute inflammatory diseases. This knowledge is pivotal for specifically targeting/designing personalized medicines/therapeutics [33–36] to ameliorate various inflammatory diseases, such as rheumatoid arthritis, asthma, anaphylaxis, atherosclerosis, ulcerative colitis etc., in the near future.
Acknowledgments
Authors would like to thank the “Beacon Biosoft Pvt.Limited” (www.beaconbiosoft.com) for the wonderful graphics on the Molecular and Cellular Mechanism of Neutrophil Extravasation in our article. We would like to extend our sincere thanks and gratitude to Prof. Iain McInnes and Dr. Charles McSharry, Institute of Infection, Immunity and Inflammation, the University of Glasgow, Scotland, UK, for kindly providing us the Genespring GX 11 (Agilent Technologies, USA) and the Dell Precision T7500 Workstation (Dell, UK) respectively.
Footnotes
Citation:Kumar et al, Bioinformation 6(3): 111-114 (2011)
References
- 1.TA Springer, et al. Cell. 1994;76:301. [Google Scholar]
- 2.EJ Brown, et al. Trends Cell Biol. 1997;7:289. doi: 10.1016/S0962-8924(97)01076-3. [DOI] [PubMed] [Google Scholar]
- 3.BN Stein, et al. J Immunol. 2003;171:6097. [Google Scholar]
- 4.PN Pushparaj, et al. J Immunol. 2009;183:1413. doi: 10.4049/jimmunol.0804061. [DOI] [PubMed] [Google Scholar]
- 5.A Yoshizaki, et al. J Immunol. 2010;185:2502. doi: 10.4049/jimmunol.0901778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AA Vaporciyan, et al. Science. 1993;262:1580. doi: 10.1126/science.8248808. [DOI] [PubMed] [Google Scholar]
- 7.AM Albelda, et al. FASEB J. 1994;8:504. [Google Scholar]
- 8.W Huang da, et al. Nat Protoc. 2009;4:44. [Google Scholar]
- 9.W Huang da, et al. Nucleic Acids Res. 2009;37:1. doi: 10.1093/nar/gkn894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.M Kanehisa, et al. Nucleic Acids Res. 2010;38:D355. doi: 10.1093/nar/gkp896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.M Kanehisa, et al. Nucleic Acids Res. 2006;34:D354. doi: 10.1093/nar/gkj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.M Kanehisa, S Goto. Nucleic Acids Res. 2000;28:30. [Google Scholar]
- 13.K Fang, et al. Physiol Genomics. 2011;43:43. [Google Scholar]
- 14.Y van Kooyk, CG Figdor. Curr Opin Cell Biol. 2000;12:542. doi: 10.1016/s0955-0674(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 15.SI Simon, et al. J Immunol. 1995;155:1502. [PubMed] [Google Scholar]
- 16.SI Simon, et al. J Immunol. 1999;163:2891. [PubMed] [Google Scholar]
- 17.AR Burns, et al. J Leukoc Biol. 1999;65:299. doi: 10.1002/jlb.65.3.299. [DOI] [PubMed] [Google Scholar]
- 18.E Crockett-Torabi, et al. J Immunol. 1995;154:2291. [PubMed] [Google Scholar]
- 19.E Crockett-Torabi, JC Fantone. Immunol Res. 1995;14:237. doi: 10.1007/BF02935622. [DOI] [PubMed] [Google Scholar]
- 20.PK Gopalan, et al. J Immunol. 1997;158:367. [PubMed] [Google Scholar]
- 21.JE Smolen, et al. J Biol Chem. 2000;275:15876. doi: 10.1074/jbc.M906232199. [DOI] [PubMed] [Google Scholar]
- 22.NT Luu, et al. J Immunol. 2000;164:5961. [Google Scholar]
- 23.AR Huber, et al. Science. 1991;254:99. [Google Scholar]
- 24.WA Muller, et al. J Exp Med. 1993;178:449. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Y Tanaka, et al. J Exp Med. 1992;176:245. [Google Scholar]
- 26.AJ Huang, et al. J Cell Biol. 1993;120:1371. doi: 10.1083/jcb.120.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.S Goda, et al. J Immunol. 2000;164:4313. [Google Scholar]
- 28.DA Lauffenburger, AF Horwitz. Cell. 1996;84:359. [Google Scholar]
- 29.GA Zimmerman, et al. Immunol Today. 1992;13:93. doi: 10.1016/0167-5699(92)90149-2. [DOI] [PubMed] [Google Scholar]
- 30.A Rot. Immunol Today. 1992;13:291. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- 31.jr Gimbrone MA, et al. Science. 1989;246:1601. [Google Scholar]
- 32.FW Luscinskas, et al. J Immunol. 1989;142:2257. [Google Scholar]
- 33.PN Pushparaj, et al. J Dent Res. 2008;87:992. doi: 10.1177/154405910808701109. [DOI] [PubMed] [Google Scholar]
- 34.PN Pushparaj, et al. Int J Biochem Cell Biol. 2008;40:1817. doi: 10.1016/j.biocel.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 35.J Manikandan, et al. Front Biosci. 2007;12:1344. doi: 10.2741/2152. [DOI] [PubMed] [Google Scholar]
- 36.PN Pushparaj, AJ Melendez. Clin Exp Pharmacol Physiol. 2006;33:504. [Google Scholar]