Abstract
Neurally adjusted ventilatory assist (NAVA) delivers airway pressure (Paw) in proportion to the electrical activity of the diaphragm (EAdi) using an adjustable proportionality constant (NAVA level, cm⋅H 2O/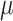 V). During systematic increases in the NAVA level, feedback-controlled down-regulation of the EAdi results in a characteristic two-phased response in Paw and tidal volume (Vt). The transition from the 1st to the 2nd response phase allows identification of adequate unloading of the respiratory muscles with NAVA (NAVAAL). We aimed to develop and validate a mathematical algorithm to identify NAVAAL. Paw, Vt, and EAdi were recorded while systematically increasing the NAVA level in 19 adult patients. In a multistep approach, inspiratory Paw peaks were first identified by dividing the EAdi into inspiratory portions using Gaussian mixture modeling. Two polynomials were then fitted onto the curves of both Paw peaks and Vt. The beginning of the Paw and Vt plateaus, and thus NAVA AL, was identified at the minimum of squared polynomial derivative and polynomial fitting errors. A graphical user interface was developed in the Matlab computing environment. Median NAVAAL visually estimated by 18 independent physicians was 2.7 (range 0.4 to 5.8) cm⋅H 2O/
V). During systematic increases in the NAVA level, feedback-controlled down-regulation of the EAdi results in a characteristic two-phased response in Paw and tidal volume (Vt). The transition from the 1st to the 2nd response phase allows identification of adequate unloading of the respiratory muscles with NAVA (NAVAAL). We aimed to develop and validate a mathematical algorithm to identify NAVAAL. Paw, Vt, and EAdi were recorded while systematically increasing the NAVA level in 19 adult patients. In a multistep approach, inspiratory Paw peaks were first identified by dividing the EAdi into inspiratory portions using Gaussian mixture modeling. Two polynomials were then fitted onto the curves of both Paw peaks and Vt. The beginning of the Paw and Vt plateaus, and thus NAVA AL, was identified at the minimum of squared polynomial derivative and polynomial fitting errors. A graphical user interface was developed in the Matlab computing environment. Median NAVAAL visually estimated by 18 independent physicians was 2.7 (range 0.4 to 5.8) cm⋅H 2O/ V and identified by our model was 2.6 (range 0.6 to 5.0) cm⋅H 2O/
V and identified by our model was 2.6 (range 0.6 to 5.0) cm⋅H 2O/ V. NAVAAL identified by our model was below the range of visually estimated NAVAAL in two instances and was above in one instance. We conclude that our model identifies NAVAAL in most instances with acceptable accuracy for application in clinical routine and research.
V. NAVAAL identified by our model was below the range of visually estimated NAVAAL in two instances and was above in one instance. We conclude that our model identifies NAVAAL in most instances with acceptable accuracy for application in clinical routine and research.
Keywords: Diaphragm electrical activity, neurally adjusted ventilatory assist, patient-ventilator interaction
I. Introduction
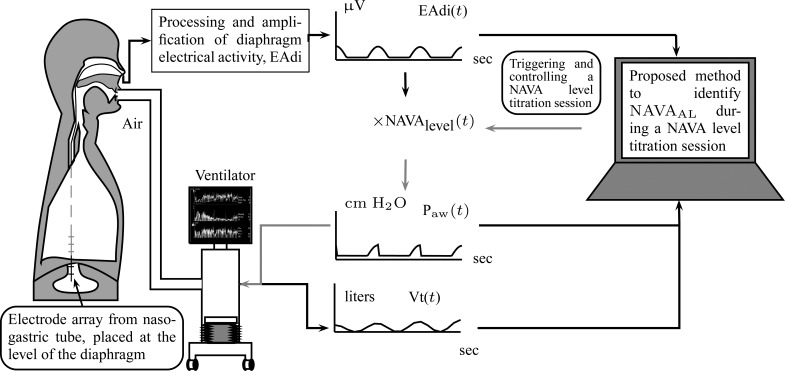
Neurally adjusted ventilatory assist (NAVA) is a new mode of mechanical ventilation that delivers airway pressure (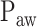 ) in linear proportion to the electrical activity of the diaphragm (EAdi), a signal arising from the diaphragm's neural activation during spontaneous breathing (Fig. 1) [1]. The NAVA level refers to an adjustable proportionality constant that determines the amount of
) in linear proportion to the electrical activity of the diaphragm (EAdi), a signal arising from the diaphragm's neural activation during spontaneous breathing (Fig. 1) [1]. The NAVA level refers to an adjustable proportionality constant that determines the amount of  delivered per unit of EAdi. Thus,
delivered per unit of EAdi. Thus, 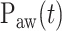 [cm ⋅H2O] = EAdi(
[cm ⋅H2O] = EAdi(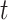 ) [
) [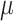 V] ⋅
V] ⋅ 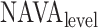 (
( ) [cm ⋅H2O/
) [cm ⋅H2O/ 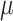 V]. EAdi is a validated measure of global respiratory drive that is controlled via lung-protective feedback mechanisms, which integrate information from pulmonary and extra-pulmonary mechanoreceptors, from blood gases, and from voluntary input [2]–[5]. If the assist delivered with NAVA exceeds the subject's respiratory demand, EAdi is reflexively down regulated, resulting in less assist for the same NAVA level and vice versa [6]–[11].
V]. EAdi is a validated measure of global respiratory drive that is controlled via lung-protective feedback mechanisms, which integrate information from pulmonary and extra-pulmonary mechanoreceptors, from blood gases, and from voluntary input [2]–[5]. If the assist delivered with NAVA exceeds the subject's respiratory demand, EAdi is reflexively down regulated, resulting in less assist for the same NAVA level and vice versa [6]–[11].
Fig. 1.
Principles of neurally adjusted ventilatory assist (NAVA) [1]. The diaphragm electrical activity (EAdi) derived from electrodes on a naso-gastric feeding tube is first amplified and processed. The EAdi signal is then multiplied by an adjustable gain factor (NAVA level) and used to control the pressure generator of a mechanical ventilator. Thus, NAVA delivers pressure to the airways (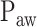 ) in direct synchrony and linear proportionality to the patient's neural inspiratory drive as reflected by the EAdi (
) in direct synchrony and linear proportionality to the patient's neural inspiratory drive as reflected by the EAdi ( = EAdi(
= EAdi( ) ⋅
) ⋅ 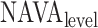 (
( ). Vt = tidal volume.
). Vt = tidal volume.  = NAVA level that provides adequate unloading of respiratory muscles.
= NAVA level that provides adequate unloading of respiratory muscles.
Several experimental and clinical studies with NAVA demonstrated that during ramp increases in the NAVA level, transpulmonary pressure and tidal volume (Vt) initially increase (1st response) before being limited due to feedback-controlled down-regulation of EAdi (2nd response) [6],[7],[8],[9]–[11]. Hence, the breathing pattern response to systematic increases in the NAVA level is directed towards prevention of lung overdistension [6], [7], [8], [9], [10], [12]. Interestingly, in rabbits loaded with various inspiratory resistors, the transition from the 1st to the 2nd response phase occurred when the animals' inspiratory effort was reduced to levels similar to those observed during spontaneous breathing (i.e., when breathing without assist and without additional load) [10]. Thus the transition from the 1st to the 2nd response phase presumably reflects the transition from an initial insufficient ventilatory assist to an adequate level of respiratory muscle unloading ( ). Therefore, reliable identification of
). Therefore, reliable identification of 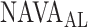 during a NAVA level titration procedure is of potential clinical relevance, since it may help to individualize the support level during NAVA.
during a NAVA level titration procedure is of potential clinical relevance, since it may help to individualize the support level during NAVA.
We hypothesized that identification of  can be modeled. In Section II, we aimed to develop a mathematical algorithm that would objectively identify the transition from the 1st to the 2nd response phase based on
can be modeled. In Section II, we aimed to develop a mathematical algorithm that would objectively identify the transition from the 1st to the 2nd response phase based on 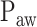 and Vt responses during NAVA level titration procedures that were performed in a previously reported clinical study on 19 critically ill adults [11]. In Section III,
and Vt responses during NAVA level titration procedures that were performed in a previously reported clinical study on 19 critically ill adults [11]. In Section III,  as identified by the algorithm was compared to
as identified by the algorithm was compared to  as visually estimated by 18 independent observers [11]. A discussion of the method is outlined in Section IV, and conclusions are drawn in Section V.
as visually estimated by 18 independent observers [11]. A discussion of the method is outlined in Section IV, and conclusions are drawn in Section V.
II. Development of an Algorithm to Calculate 
Identification of  is based on the analysis of EAdi,
is based on the analysis of EAdi, 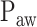 , and Vt recordings while systematically increasing the NAVA level. The principles of such a NAVA level titration procedure have been described elsewhere [6], [7], [9]–[11]. Briefly, first the NAVA level was reduced to a minimum of 0 cm⋅H2O/
, and Vt recordings while systematically increasing the NAVA level. The principles of such a NAVA level titration procedure have been described elsewhere [6], [7], [9]–[11]. Briefly, first the NAVA level was reduced to a minimum of 0 cm⋅H2O/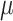 V. When sufficient EAdi was detectable (i.e., at least twice the EAdi trigger threshold), the NAVA level was increased by 0.1 cm ⋅H2O/
V. When sufficient EAdi was detectable (i.e., at least twice the EAdi trigger threshold), the NAVA level was increased by 0.1 cm ⋅H2O/ 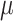 V every 20 sec while continuously monitoring and recording the EAdi, Paw, and Vt signals (NAVA tracker, Maquet, Solna, Sweden) in NT1 format. The NT1 files were converted into Matlab format for further processing. In the study by Passath et al. [11], the data of one patient were recorded with different software and were, therefore, not included in the experimental part of the present work. A characteristic example of such a titration session is depicted in Fig. 2.
V every 20 sec while continuously monitoring and recording the EAdi, Paw, and Vt signals (NAVA tracker, Maquet, Solna, Sweden) in NT1 format. The NT1 files were converted into Matlab format for further processing. In the study by Passath et al. [11], the data of one patient were recorded with different software and were, therefore, not included in the experimental part of the present work. A characteristic example of such a titration session is depicted in Fig. 2.
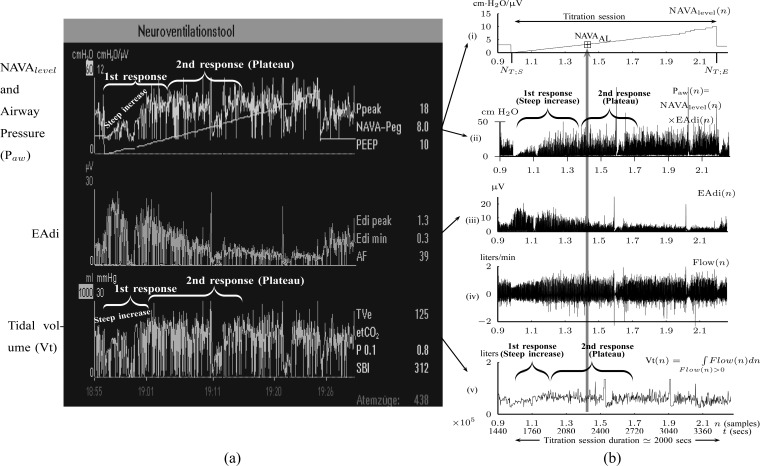
Fig. 2.
Example of a NAVA level titration session as used for estimating  (a) visually or (b) with the proposed algorithm.
(a) visually or (b) with the proposed algorithm. 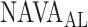 refers to the adequate NAVA level early after the transition from the initial steep increase in
refers to the adequate NAVA level early after the transition from the initial steep increase in  and Vt(
and Vt( ), referred to as 1st response, to the less steep increase or plateau in
), referred to as 1st response, to the less steep increase or plateau in  and Vt(
and Vt(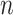 ), referred to as 2nd response [6]–[11]. Flow(
), referred to as 2nd response [6]–[11]. Flow( ) is the air flow. In (a), the Vt(
) is the air flow. In (a), the Vt( ) is estimated on a breath-by-breath basis. If there is false triggering of the ventilator (e.g., based on an EAdi artifact) a minimal Vt (normally a few milliliters) is delivered. Since there is no minimal threshold for Vt, the ventilator displays whatever Vt(
) is estimated on a breath-by-breath basis. If there is false triggering of the ventilator (e.g., based on an EAdi artifact) a minimal Vt (normally a few milliliters) is delivered. Since there is no minimal threshold for Vt, the ventilator displays whatever Vt( ) is delivered in the graph. In (b), the Vt(
) is delivered in the graph. In (b), the Vt(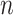 ) is calculated as the integral of Flow(
) is calculated as the integral of Flow( ) per inspiration as it is described in Section II-B (Step 4A).
) per inspiration as it is described in Section II-B (Step 4A).
A. Visual Estimation of 
A visual method for estimating  was described and validated recently [6], [7], [9]–[11]. Briefly, by observing time plots of
was described and validated recently [6], [7], [9]–[11]. Briefly, by observing time plots of 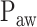 and Vt on the ventilator monitor or on printouts (Fig. 2),
and Vt on the ventilator monitor or on printouts (Fig. 2),  was determined as the NAVA level early after the transition from an initial steep increase in
was determined as the NAVA level early after the transition from an initial steep increase in 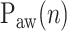 and Vt(
and Vt(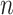 ) (1st response) to a less steep increase or even a plateau in both parameters (2nd response). For validation of the visual method, an arbitrarily chosen number of 17 independent physicians blinded to the
) (1st response) to a less steep increase or even a plateau in both parameters (2nd response). For validation of the visual method, an arbitrarily chosen number of 17 independent physicians blinded to the  selected during the study were instructed post-hoc identify a NAVA level immediately following the transition from a steep to a less steep increase in
selected during the study were instructed post-hoc identify a NAVA level immediately following the transition from a steep to a less steep increase in  and Vt on screen prints of the original trend graphs. The
and Vt on screen prints of the original trend graphs. The 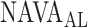 as estimated during the clinical study and post-hoc by the 17 independent physicians was reported previously [11] and used for comparison to
as estimated during the clinical study and post-hoc by the 17 independent physicians was reported previously [11] and used for comparison to 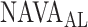 , as identified by the algorithm developed in the present study.
, as identified by the algorithm developed in the present study.
B. Algorithm-Based Calculation of 
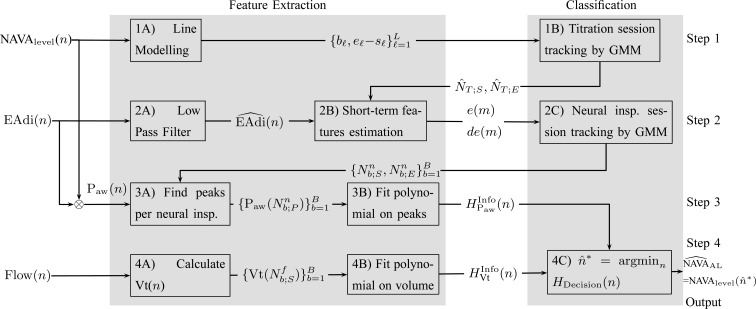
The method to mathematically identify 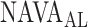 is divided into four steps. The procedure is outlined in Fig. 3. The first step is the identification of the titration session from
is divided into four steps. The procedure is outlined in Fig. 3. The first step is the identification of the titration session from 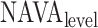 (
(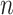 ) represented by nodes 1(A) and 1(B). The second step is the tracking of inspiration sessions from
) represented by nodes 1(A) and 1(B). The second step is the tracking of inspiration sessions from 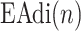 represented by nodes 2(A), 2(B), and 2(C). The third step consists of identifying the peaks in the
represented by nodes 2(A), 2(B), and 2(C). The third step consists of identifying the peaks in the 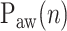 per inspiration and of fitting a polynomial function to the
per inspiration and of fitting a polynomial function to the 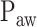 peaks, as shown in nodes 3(A) and 3(B), respectively. The fourth step consists of calculating Vt(
peaks, as shown in nodes 3(A) and 3(B), respectively. The fourth step consists of calculating Vt( ) from Flow(
) from Flow( ), and fitting a polynomial function to the Vt, as shown in nodes 4(A) and 4(B). The derivation of
), and fitting a polynomial function to the Vt, as shown in nodes 4(A) and 4(B). The derivation of  based on polynomials can be found in node 4(C). The sampling rate of all signals used was
based on polynomials can be found in node 4(C). The sampling rate of all signals used was 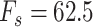 Hz. All steps are described in greater detail below.
Hz. All steps are described in greater detail below.
Fig. 3.
Outline of the algorithm to identify  based on the signals
based on the signals  (
(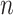 ) for the NAVA level,
) for the NAVA level,  for electrical activity of the diaphragm, and Vt
for electrical activity of the diaphragm, and Vt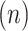 for tidal volume that was derived from the inspiratory flow.
for tidal volume that was derived from the inspiratory flow.
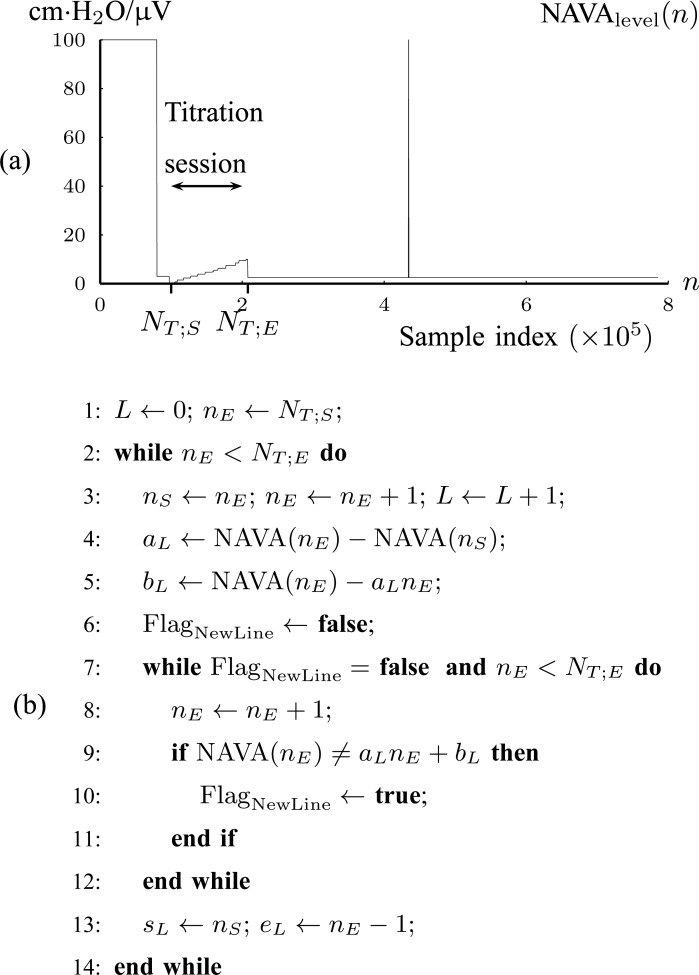
1. Step 1. Identification of the titration session based on changes in the 
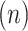
1A) Let  and
and 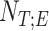 denote the samples where titration session starts and ends, respectively. We wish to identify
denote the samples where titration session starts and ends, respectively. We wish to identify  and
and 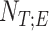 .
.  (
(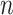 ) is modeled with
) is modeled with 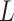 straight line segments as
straight line segments as 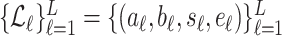 where
where
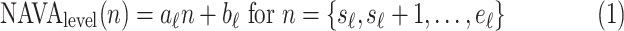 |
with 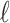 being the index of the line segment
being the index of the line segment  ,
,  the first-order line coefficient,
the first-order line coefficient, 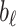 the zero-order coefficient,
the zero-order coefficient,  the starting sample, and
the starting sample, and  the ending sample of the
the ending sample of the  th line segment. It should be noted that there is no noise in
th line segment. It should be noted that there is no noise in 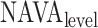 (
(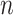 ). The line segments are found by fitting a sequence of lines to
). The line segments are found by fitting a sequence of lines to  n) as follows. The first line is fitted to
n) as follows. The first line is fitted to  (
(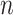 ) for
) for  to
to 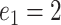 .
. 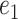 is updated by
is updated by 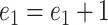 as long as
as long as
 |
If (2) is violated, a new line begins, estimated from the next two samples. The benefit of this transformation of 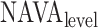 (
( ) into lines is that a great compression of signal data is accomplished. The algorithm is summarized in Fig. 4(b).
) into lines is that a great compression of signal data is accomplished. The algorithm is summarized in Fig. 4(b).
Fig. 4.
(a) Tracking of the NAVA level titration session in Patient 1 (Step 1). (b) Algorithm for modeling { (
( with lines
with lines  (Step 1A).
(Step 1A).
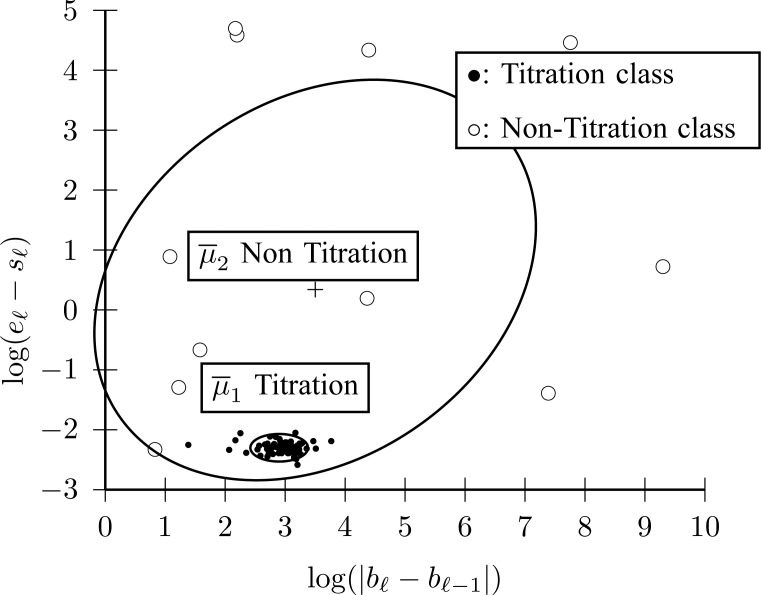
1B) Let 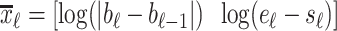 be the 2-D vector that will be used for classifying
be the 2-D vector that will be used for classifying  into
into  (Titration class) or into
(Titration class) or into  (Nontitration class). The first feature of
(Nontitration class). The first feature of  is the difference of offset level between the previous and current line segments, which, according to the inspection of Fig. 4(a), should be an almost constant number for
is the difference of offset level between the previous and current line segments, which, according to the inspection of Fig. 4(a), should be an almost constant number for  . The second feature of
. The second feature of  is the length of each line, which should also be a statistically constant number for
is the length of each line, which should also be a statistically constant number for  . A Gaussian Mixture Modelling (GMM) algorithm is used that searches for a component with a small determinant in
. A Gaussian Mixture Modelling (GMM) algorithm is used that searches for a component with a small determinant in 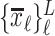 space where the number of components is limited to 2. The algorithm used for GMM was found in a previous investigation and is publicly available [13], [14]. Let
space where the number of components is limited to 2. The algorithm used for GMM was found in a previous investigation and is publicly available [13], [14]. Let  denote a Gaussian component, with
denote a Gaussian component, with  and
and  being its mean vector and its covariance matrix, respectively. Thus,
being its mean vector and its covariance matrix, respectively. Thus,  and
and 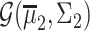 are found, where
are found, where 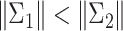 , with
, with  being the determinant of a matrix inside the delimiters. The titration tracking procedure of the signal of Fig. 4(a) is depicted in Fig. 5. A prediction
being the determinant of a matrix inside the delimiters. The titration tracking procedure of the signal of Fig. 4(a) is depicted in Fig. 5. A prediction 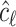 for each line is given according to the Bayes classifier
for each line is given according to the Bayes classifier
 |
where the probability density function (pdf) for each class is given by 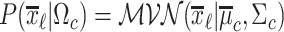 , with
, with  being the multivariate normal pdf. Let
being the multivariate normal pdf. Let  and
and  be the estimated sample index where titration starts and ends, respectively. Then
be the estimated sample index where titration starts and ends, respectively. Then 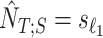 and
and 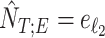 , where
, where
 |
The estimated  interval is depicted in Fig. 6. The benefit of this step is that the titration session is tracked without the need of a trigger input from the ventilation machine.
interval is depicted in Fig. 6. The benefit of this step is that the titration session is tracked without the need of a trigger input from the ventilation machine.
Fig. 5.
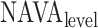 (
( ) titration session tracking by 2 Gaussian components for Fig. 4. The component with small dispersion corresponds to Titration class (Step 1B).
) titration session tracking by 2 Gaussian components for Fig. 4. The component with small dispersion corresponds to Titration class (Step 1B).
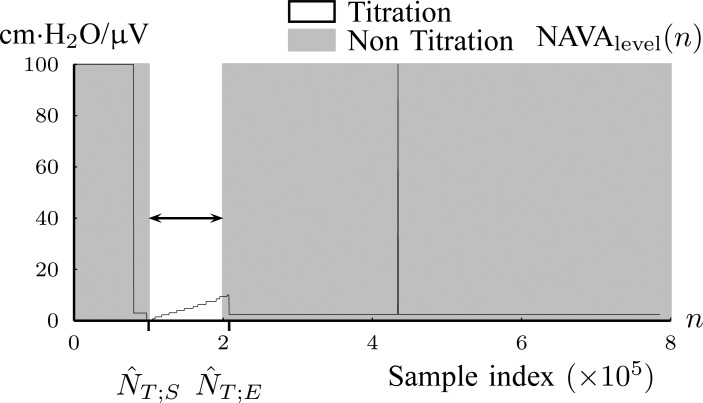
Fig. 6.
The result of titration tracking procedure of Fig. 5. The lines that belong to  are assigned to the Titration class (Step 1B).
are assigned to the Titration class (Step 1B).
2. Step 2. Tracking of neural inspiration sessions
The electrical activity of the diaphragm, denoted as 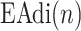 for
for  is used to track neural inspiration sessions. This is accomplished by employing the GMM clustering algorithm that searches for three Gaussian components in 2-D feature space. The first feature is the logarithm of the short-term energy, estimated as follows.
is used to track neural inspiration sessions. This is accomplished by employing the GMM clustering algorithm that searches for three Gaussian components in 2-D feature space. The first feature is the logarithm of the short-term energy, estimated as follows.
2A) A moving average (low pass filter, LPF) of order 40 is applied to  to eliminate frequency components above 4 Hz that are not related to breathing, i.e.,
to eliminate frequency components above 4 Hz that are not related to breathing, i.e.,
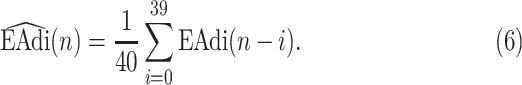 |
The 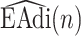 for Patient 1 is shown in Fig. 7, where only 6 breaths out of 350 are shown for visualization reasons. The LPF does not introduce negative values of
for Patient 1 is shown in Fig. 7, where only 6 breaths out of 350 are shown for visualization reasons. The LPF does not introduce negative values of  that cause problems when the logarithm operator is applied in the following step.
that cause problems when the logarithm operator is applied in the following step.
Fig. 7.
Tracking of neural inspiration sessions using 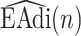 signal (Step 2C).
signal (Step 2C).
2B) Next, short-term energy is estimated. That is, 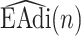 is split into frames
is split into frames  , where
, where  is an orthogonal window of length
is an orthogonal window of length 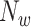 ending at sample
ending at sample  . In our investigation
. In our investigation 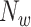 equals 15, and
equals 15, and 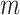 starts from 15 samples, which correspond to 240 msec. m is updated by m:=m+15. Patients in intensive care typically have breath cycles of approximately 1 to 4 sec duration. Overlapping is avoided because each sample should be assigned to one class. The first feature is the logarithm of energy for the
starts from 15 samples, which correspond to 240 msec. m is updated by m:=m+15. Patients in intensive care typically have breath cycles of approximately 1 to 4 sec duration. Overlapping is avoided because each sample should be assigned to one class. The first feature is the logarithm of energy for the 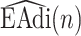 frame ending at
frame ending at 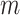
 |
where  . The second feature is the derivative of the first feature, given by
. The second feature is the derivative of the first feature, given by 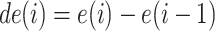 . The energy and the energy derivative are chosen because the
. The energy and the energy derivative are chosen because the  curve should be divided into valleys (expirations) and mountains (inspirations). It was found experimentally that the logarithm operator transforms the distribution of energy from exponential to normal. In this manner, the GMM clustering algorithm can be applied to the feature distribution as described next.
curve should be divided into valleys (expirations) and mountains (inspirations). It was found experimentally that the logarithm operator transforms the distribution of energy from exponential to normal. In this manner, the GMM clustering algorithm can be applied to the feature distribution as described next.
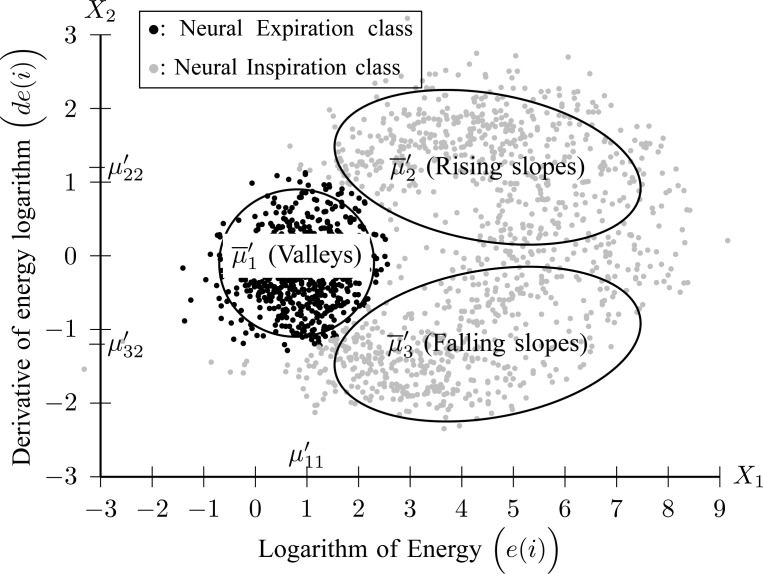
2C) GMM is applied to feature space 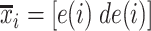 where three Gaussian components are searched for. The clustering result for Patient 1 is depicted in Fig. 8.
where three Gaussian components are searched for. The clustering result for Patient 1 is depicted in Fig. 8.
Fig. 8.
Clustering of 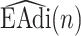 frames to Neural Inspiration and Expiration classes (Step 2C).
frames to Neural Inspiration and Expiration classes (Step 2C).
Each component 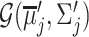 is described by its center (
is described by its center ( ) and its covariance matrix (
) and its covariance matrix (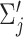 ), for
), for 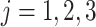 . The component with the center of lowest energy
. The component with the center of lowest energy  corresponds to Neural Expiration class, denoted as
corresponds to Neural Expiration class, denoted as  . The Neural Inspiration class, denoted as
. The Neural Inspiration class, denoted as 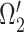 , consists of two Gaussian components. The component with a center signified by maximum derivative of energy
, consists of two Gaussian components. The component with a center signified by maximum derivative of energy 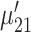 corresponds to rising slopes, and the component signified by minimum derivative of energy
corresponds to rising slopes, and the component signified by minimum derivative of energy  stands for falling slopes of
stands for falling slopes of 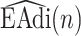 . The Bayes classifier is again employed in order to assign each frame to Inspiration or Expiration class. Let
. The Bayes classifier is again employed in order to assign each frame to Inspiration or Expiration class. Let  be a frame with measurements
be a frame with measurements  and label
and label  . The predicted label of
. The predicted label of 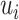 is given by
is given by  , with
, with 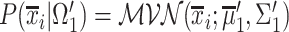 and
and 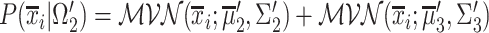 .
.
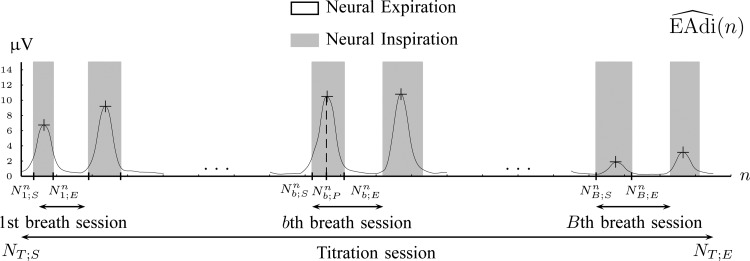
A neural inspiration session is constituted by a sequence of frames that belong to the Neural Inspiration class (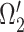 ). The results of this step are shown in Fig. 7. Let
). The results of this step are shown in Fig. 7. Let  be the breath index, where
be the breath index, where 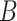 is the total number of breaths. The beginning and the end of the
is the total number of breaths. The beginning and the end of the  th neural inspiration session are denoted as
th neural inspiration session are denoted as 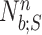 and
and  , respectively.
, respectively.
3A) Neural inspiration peaks estimation: Let  be the airway pressure signal. The neural inspiration peaks indices are found by
be the airway pressure signal. The neural inspiration peaks indices are found by
 |
for  . The airway pressure at neural inspiration peaks is the signal
. The airway pressure at neural inspiration peaks is the signal  .
.
3B) Polynomial fit to airway pressure peaks: The polynomial
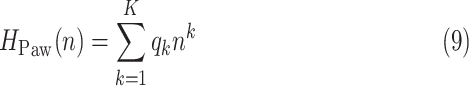 |
of order 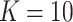 , with
, with 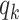 being the polynomial coefficients, is fitted onto
being the polynomial coefficients, is fitted onto 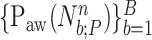 with the reweighted least-squares method [15]. By finding the
with the reweighted least-squares method [15]. By finding the 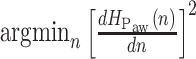 one is able to derive the time index of plateau of airway pressure peaks. The order of the polynomial is chosen empirically, so that it is a trade-off between tracking the underlying number of curve peaks and capturing the trivial sudden peaks. However, this is not the only information needed for choosing the optimum time index. Also, the signal formed by the sequence of polynomial fit error values
one is able to derive the time index of plateau of airway pressure peaks. The order of the polynomial is chosen empirically, so that it is a trade-off between tracking the underlying number of curve peaks and capturing the trivial sudden peaks. However, this is not the only information needed for choosing the optimum time index. Also, the signal formed by the sequence of polynomial fit error values
 |
for 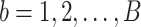 is taken into consideration.
is taken into consideration.  peaks may present great variance around the fitted polynomial, a fact denoting the patient's inability to synchronize his breath with the ventilation machine. So, another polynomial of order
peaks may present great variance around the fitted polynomial, a fact denoting the patient's inability to synchronize his breath with the ventilation machine. So, another polynomial of order  is fitted onto
is fitted onto 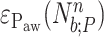 , i.e.,
, i.e.,
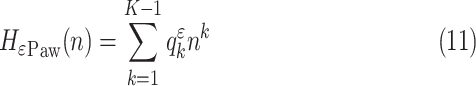 |
with 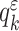 being its coefficients. The polynomial of
being its coefficients. The polynomial of 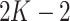 order
order
 |
includes both information about airway pressure peaks plateau and small variance, where the latter indicates that the plateau is stable.
4A) Tidal volume estimation: The 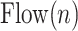 signal for Patient 1 is depicted in Fig. 9.
signal for Patient 1 is depicted in Fig. 9.
Fig. 9.
The air flow signal, 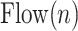 , is divided into inspirations and expirations by zero crossing indices (Step 4A).
, is divided into inspirations and expirations by zero crossing indices (Step 4A).
Let the tidal volume 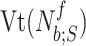 be the air inhaled during
be the air inhaled during  th flow inspiration, where
th flow inspiration, where 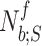 and
and  are the starting and ending index of
are the starting and ending index of  th airflow inspiration. A flow inspiration session is defined as the time during which air flow is positive. So, a flow inspiration session is found by applying the zero crossings method on
th airflow inspiration. A flow inspiration session is defined as the time during which air flow is positive. So, a flow inspiration session is found by applying the zero crossings method on 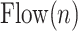 . Then, the tidal volume is found by integrating the inspiration flow for each
. Then, the tidal volume is found by integrating the inspiration flow for each  inspiration
inspiration
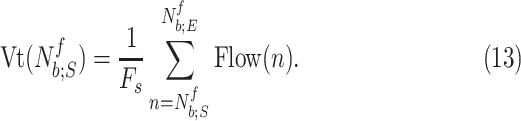 |
4B) Polynomial fit to tidal volume: The polynomial
 |
is fitted onto 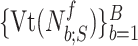 , where
, where  are the polynomial coefficients, in a similar manner as in Step 3B. The sequence of fit errors, i.e.,
are the polynomial coefficients, in a similar manner as in Step 3B. The sequence of fit errors, i.e.,
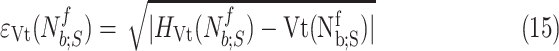 |
for  is also exploited. The polynomial
is also exploited. The polynomial
 |
is fitted onto (15), where 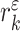 are the polynomial coefficients. So, the information about the tidal volume plateau and its variance is given by
are the polynomial coefficients. So, the information about the tidal volume plateau and its variance is given by
 |
4C) Estimation of plateau:
 equals a certain
equals a certain 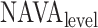 (
(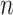 ) when signals
) when signals  and
and 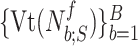 reach a plateau and simultaneously present small variance around the fitted polynomial. Let
reach a plateau and simultaneously present small variance around the fitted polynomial. Let  be the time index when the plateau occurs and small variance is observed. An estimate of
be the time index when the plateau occurs and small variance is observed. An estimate of  , denoted as
, denoted as 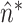 is found when both (12) and (17) are minimized. A function that includes information about the time index where polynomial derivatives and fitting errors are minimized is
is found when both (12) and (17) are minimized. A function that includes information about the time index where polynomial derivatives and fitting errors are minimized is
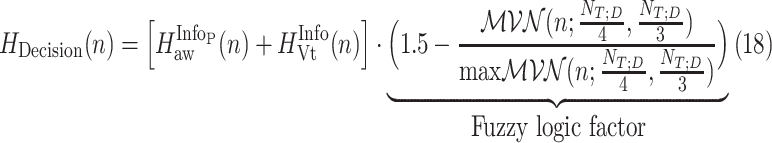 |
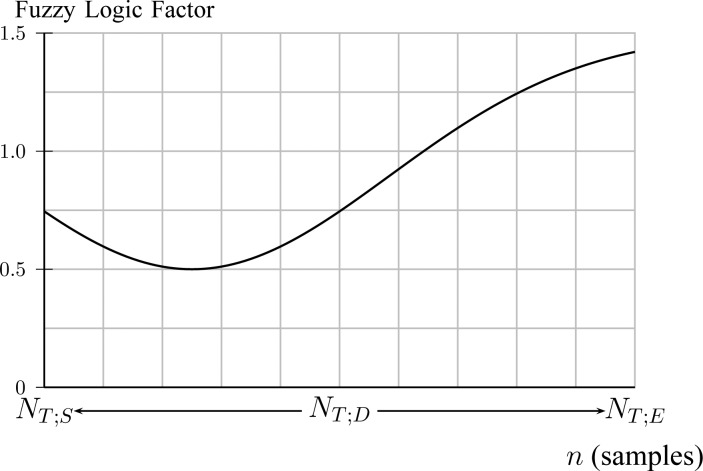
where the fuzzy logic factor is plotted in Fig. 10.
Fig. 10.
A fuzzy logic factor used for exploiting  bias to 0.25 of total duration of titration session (Step 4C).
bias to 0.25 of total duration of titration session (Step 4C).
The fuzzy logic factor is biased toward the first quarter of titration session duration. It will be shown in experiments that physicians are highly biased at  = 2.5. Since NAVA is increasing from 0 to 10 linearly through time, this corresponds to a bias in time toward
= 2.5. Since NAVA is increasing from 0 to 10 linearly through time, this corresponds to a bias in time toward  . The optimum time index is then given by
. The optimum time index is then given by
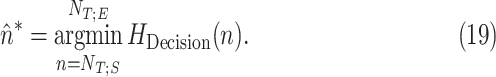 |
Finally, we define 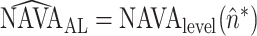 . As an example, in Fig. 11, the curves resulting from (9), (14), and (18) are plotted for Patient 1.
. As an example, in Fig. 11, the curves resulting from (9), (14), and (18) are plotted for Patient 1.
Fig. 11.
Time index of plateau,  , is found when
, is found when  is minimized, as described in Steps 3 and 4.
is minimized, as described in Steps 3 and 4.
The signals 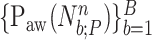 and
and 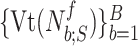 are also plotted in order to demonstrate the polynomial fitting. It is inferred that
are also plotted in order to demonstrate the polynomial fitting. It is inferred that  is minimized at
is minimized at 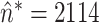 , which is close to
, which is close to  which was given by the clinician. The
which was given by the clinician. The 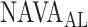 is 2.5, whereas the algorithm found
is 2.5, whereas the algorithm found 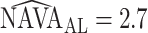 .
.
III. Experiments
For all titration sessions performed in the 19 patients, 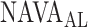 calculated by our algorithm was compared to
calculated by our algorithm was compared to 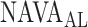 as visually estimated by the investigators when performing the clinical study (i.e., by author LB) and by an arbitrarily chosen number of 17 independent physician observers posthoc using printouts of the signal trajectories [Fig. 2(a)] [11]. Median
as visually estimated by the investigators when performing the clinical study (i.e., by author LB) and by an arbitrarily chosen number of 17 independent physician observers posthoc using printouts of the signal trajectories [Fig. 2(a)] [11]. Median 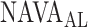 , as estimated by the 18 physicians, was 2.5 cm⋅H2O/
, as estimated by the 18 physicians, was 2.5 cm⋅H2O/ V with a range from 0.4 to 5.8 cm ⋅H2O/
V with a range from 0.4 to 5.8 cm ⋅H2O/ 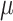 V. In the study by Passath et al. [11], the number of steps necessary to reach
V. In the study by Passath et al. [11], the number of steps necessary to reach 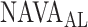 and the highest NAVA level used differed among patients. The highest NAVA level used in the 19 patients included in the present work was (median [range]) 4.9 (1.9–7.4) cm⋅H2O/
and the highest NAVA level used differed among patients. The highest NAVA level used in the 19 patients included in the present work was (median [range]) 4.9 (1.9–7.4) cm⋅H2O/ V and the time to reach this level was 978 (377–1478) sec. The time to reach
V and the time to reach this level was 978 (377–1478) sec. The time to reach  was 498 (198–997) sec.
was 498 (198–997) sec.
Median 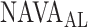 identified by the algorithm was 2.6 cm⋅H2O/
identified by the algorithm was 2.6 cm⋅H2O/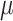 V with a range from 0.6 to 5.0 cm ⋅H2O/
V with a range from 0.6 to 5.0 cm ⋅H2O/  V. In most cases,
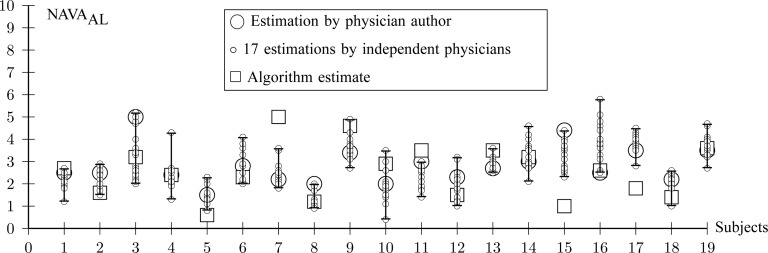
V. In most cases,  identified by the algorithm was within the range of
identified by the algorithm was within the range of  estimated by the physicians (Fig. 12). In Patient 7, the
estimated by the physicians (Fig. 12). In Patient 7, the 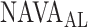 identified by the algorithm was higher, and in Patients 15 and 17 it was lower than the
identified by the algorithm was higher, and in Patients 15 and 17 it was lower than the 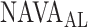 estimated by the physicians. In order to calculate the correlation between
estimated by the physicians. In order to calculate the correlation between 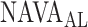 , as identified by the observers with the results of our algorithm, we computed the multiple correlation coefficient (MCC) [16]. MCC ranges from 0 (no correlation) to 1 (linearly dependent). In our case, MCC indicates the correlation between the matrix of
, as identified by the observers with the results of our algorithm, we computed the multiple correlation coefficient (MCC) [16]. MCC ranges from 0 (no correlation) to 1 (linearly dependent). In our case, MCC indicates the correlation between the matrix of 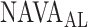 estimates for all observers across all patients with the algorithm result. Furthermore, the Pearson concordance coefficient is used to estimate the concordance between a single observer and the algorithm [11]. The confidence limits are estimated at 95% level of significance. The MCC between
estimates for all observers across all patients with the algorithm result. Furthermore, the Pearson concordance coefficient is used to estimate the concordance between a single observer and the algorithm [11]. The confidence limits are estimated at 95% level of significance. The MCC between 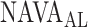 as identified by the algorithm and as estimated by the 18 physicians is 0.54±0.06. The Pearson concordance coefficients between the
as identified by the algorithm and as estimated by the 18 physicians is 0.54±0.06. The Pearson concordance coefficients between the  as identified by each observer and the algorithm are presented in Table I. In the last row, the concordance between median
as identified by each observer and the algorithm are presented in Table I. In the last row, the concordance between median  for all observers and the algorithm is computed. It can be seen that the concordance of the
for all observers and the algorithm is computed. It can be seen that the concordance of the  between each observer and the algorithm is always positive. The lower limit of the concordance coefficient is slightly negative, with a median value of −0.13. The upper confidence limit median is 0.69.
between each observer and the algorithm is always positive. The lower limit of the concordance coefficient is slightly negative, with a median value of −0.13. The upper confidence limit median is 0.69.
Fig. 12.
Comparison between 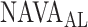 independently estimated by one of the authors (L.B., a physician) and by 17 independent physicians based on visual inspection of the airway pressure (
independently estimated by one of the authors (L.B., a physician) and by 17 independent physicians based on visual inspection of the airway pressure (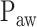 ) and tidal volume (Vt) response to systematic increases in the NAVA level (circles) and
) and tidal volume (Vt) response to systematic increases in the NAVA level (circles) and  identified by the algorithm described in this paper (squares).
identified by the algorithm described in this paper (squares).
Table I. Pearson Concordance Coefficient of  Estimates between Physician Observers and Algorithm.
Estimates between Physician Observers and Algorithm.
| Observer | Coefficient | Lower Limit | Upper limit |
|---|---|---|---|
| 1 (author L.B.) | 0.21 | −0.27 | 0.61 |
| 2 | 0.25 | −0.23 | 0.63 |
| 3 | 0.41 | −0.06 | 0.73 |
| 4 | 0.37 | −0.10 | 0.71 |
| 5 | 0.40 | −0.06 | 0.72 |
| 6 | 0.52 | 0.09 | 0.79 |
| 7 | 0.41 | −0.05 | 0.73 |
| 8 | 0.20 | −0.28 | 0.60 |
| MI 9 | 0.36 | −0.11 | 0.70 |
| 10 | 0.28 | −0.20 | 0.65 |
| 11 | 0.24 | −0.24 | 0.62 |
| 12 | 0.48 | 0.03 | 0.77 |
| 13 | 0.33 | −0.15 | 0.68 |
| 14 | 0.20 | −0.28 | 0.60 |
| 15 | 0.41 | −0.06 | 0.73 |
| 16 | 0.23 | −0.25 | 0.62 |
| 17 | 0.14 | −0.33 | 0.56 |
| 18 | 0.43 | −0.03 | 0.74 |
| Median observer | 0.34 | −0.13 | 0.69 |
A graphic user interface (GUI) for the algorithm is presented in Fig. 13. The GUI includes most of the figures presented in Section II-B. The final result is compared to the ground truth, i.e., the 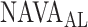 estimated visually, and displayed as bands in the uppermost panel of Fig. 13.
estimated visually, and displayed as bands in the uppermost panel of Fig. 13.
Fig. 13.
The graphic interface provides a synopsis of the signal processing steps described in Figs. 2, 5, 8, and 11, and allows for real time assessment of how the algorithm identifies 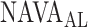 . Ground truth
. Ground truth  denotes the visually estimated adequate NAVA level.
denotes the visually estimated adequate NAVA level.
IV. Discussion
We developed a multistep algorithm and a user interface to identify adequate assist ( ) based on analysis of the Vt,
) based on analysis of the Vt, 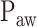 , and EAdi responses during a systematic increase in the NAVA level. The algorithm revealed results that were comparable to the previously used visual method for estimating
, and EAdi responses during a systematic increase in the NAVA level. The algorithm revealed results that were comparable to the previously used visual method for estimating 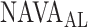 .
.
Delivering mechanical ventilatory assist during spontaneous breathing aims at unloading the respiratory muscles from excessive work of breathing while preventing both fatigue and disuse atrophy of respiratory muscles. However, determining an assist level that adequately meets the patient's needs is not straightforward. Both too high and too low assist may cause harm. While respiratory muscle fatigue may result from insufficiently unloading the patient from his work of breathing [17], disuse atrophy may follow prolonged delivery of assist in excess of the patient's needs [18]–[20].
Thus, defining an adequate level of respiratory muscle unloading based on the patient's individual response to changes in the assist level is of clinical relevance but requires reliable measurement of the respiratory drive. The recent introduction of a technology to monitor EAdi, a validated measure of respiratory drive [2]–[5], provides the opportunity to integrate the patient's response in the process of identifying an adequate level of assist. NAVA is unique in that it directly translates changes in the respiratory drive into changes of the ventilatory pattern. Since with NAVA the ventilator receives the same control signal as the diaphragm, it conceptually acts as an additional external respiratory muscle pump that is directly controlled by the patient's respiratory drive. Thus, NAVA provides the patient with far-reaching control over the ventilatory pattern and with the ability to limit the assist once the inspiratory efforts occur at a level that corresponds to nonloaded conditions, i.e., at a satisfactory, and hence adequate, assist level with NAVA ( ) [6], [7], [9]–[11].
) [6], [7], [9]–[11].
In the present study, we demonstrate that 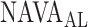 can be identified using a multistep polynomial fitting model based on analyzing the Vt, Paw, and EAdi responses during systematic increases in the NAVA level. The
can be identified using a multistep polynomial fitting model based on analyzing the Vt, Paw, and EAdi responses during systematic increases in the NAVA level. The 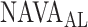 identified by the algorithm was in agreement with the
identified by the algorithm was in agreement with the 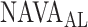 estimated visually for most patients. We previously demonstrated not only good reproducibility among physicians for visual estimation of
estimated visually for most patients. We previously demonstrated not only good reproducibility among physicians for visual estimation of  [10], [11] but also stable cardio-pulmonary function without evidence of respiratory failure or distress when implementing
[10], [11] but also stable cardio-pulmonary function without evidence of respiratory failure or distress when implementing  for various time spans [6], [7], [9]–[11].
for various time spans [6], [7], [9]–[11].
In 3 out of 19 titration sessions, the 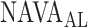 identified by the algorithm was either clearly above or clearly below the range of
identified by the algorithm was either clearly above or clearly below the range of 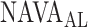 estimated visually. We assume that the discrepancy between the methods in these three patients is most likely due to the fact that the physicians outperformed the current version of the algorithm in recognizing pattern irregularities, as illustrated in Fig. 14. Also, the current version of the ventilator.s graphic interface does not differentiate between real breaths and artifacts when displaying the trend graphs. Therefore the graphs may be difficult to read for users non-experienced with the NAVA level titration procedure. This suggests that, although
estimated visually. We assume that the discrepancy between the methods in these three patients is most likely due to the fact that the physicians outperformed the current version of the algorithm in recognizing pattern irregularities, as illustrated in Fig. 14. Also, the current version of the ventilator.s graphic interface does not differentiate between real breaths and artifacts when displaying the trend graphs. Therefore the graphs may be difficult to read for users non-experienced with the NAVA level titration procedure. This suggests that, although 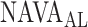 identified by the algorithm was within the range of
identified by the algorithm was within the range of  estimated visually for >80% of the titration sessions, a visual verification is advisable before using
estimated visually for >80% of the titration sessions, a visual verification is advisable before using  identified by the current version of the algorithm. Further refinement and validation of the algorithm is required before it can be safely implemented in clinical practice.
identified by the current version of the algorithm. Further refinement and validation of the algorithm is required before it can be safely implemented in clinical practice.
Fig. 14.
NAVA level titration session in patient 17. In this patient the algorithm identified the transition from a steep increase in peak airway pressure ( ) to a less steep increase or plateau in
) to a less steep increase or plateau in  (i.e., the adequate NAVA level,
(i.e., the adequate NAVA level, 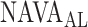 ) clearly below the range of
) clearly below the range of  as visually estimated by the clinicians. The discrepancy is most likely due to a short, transitory interruption of the
as visually estimated by the clinicians. The discrepancy is most likely due to a short, transitory interruption of the 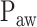 increase during the initial steep increase, i.e., during the 1st response phase (asterisk). We assume that the physicians outperformed the current version of the algorithm in recognizing pattern irregularities.
increase during the initial steep increase, i.e., during the 1st response phase (asterisk). We assume that the physicians outperformed the current version of the algorithm in recognizing pattern irregularities.
Of note, since the transition from the 1st to the 2nd response does not occur acutely, some inter-individual variability and discrepancy between methods used in determining 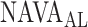 can be expected. Also, as
can be expected. Also, as 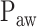 and Vt do not or only minimally change after the transition from the 1st to the 2nd response phase, any NAVA level within the 2nd response phase can be expected to have only minor, if any, effects on breathing pattern.
and Vt do not or only minimally change after the transition from the 1st to the 2nd response phase, any NAVA level within the 2nd response phase can be expected to have only minor, if any, effects on breathing pattern.
The mathematical algorithm developed is based on post processing of the signals obtained. The algorithm not only allows faster identification of  than the visual method but is also independent of observer-related biases and inter-individual variability. However, the algorithm should be modified to identify
than the visual method but is also independent of observer-related biases and inter-individual variability. However, the algorithm should be modified to identify  in real-time, and thus help shorten the time needed for a titration session.
in real-time, and thus help shorten the time needed for a titration session.
V. Conclusion
 can be identified quickly and reliably using our polynomial fitting model based on the analysis of the
can be identified quickly and reliably using our polynomial fitting model based on the analysis of the  , Vt, and EAdi responses to systematic increases in the NAVA level. The correlation between the
, Vt, and EAdi responses to systematic increases in the NAVA level. The correlation between the  identified by the algorithm and the
identified by the algorithm and the  estimated visually suggests that our model has acceptable accuracy for application in clinical routine and research.
estimated visually suggests that our model has acceptable accuracy for application in clinical routine and research.
Biography
Authors' photographs and biographies not available at the time of publication.
Funding Statement
The study was supported by grants from the Swiss National Science Foundation (SNF, 3200B0-113478/1) and from the Stiftung für die Forschung in Anästhesiologie und Intensivmedizin, Bern (18/2006), awarded to L. Brander.
Contributor Information
Dimitrios Ververidis, Email: jimver04@gmail.com.
Mark van Gils, Email: Mark.vanGils@vtt.fi.
Christina Passath, Email: christina.passath@insel.ch.
Jukka Takala, Email: jukka.takala@insel.ch.
Lukas Brander, Email: lukas.brander@bluewin.ch.
References
- [1].Sinderby C., Navalesi P., Beck J., Skrobik Y., Friberg N. C. S., and Lindström S. G. L., “Neural control of mechanical ventilation in respiratory failure,” in Nat. Med., vol. 5, no. 12, pp. 1433–1436, 1999. [DOI] [PubMed] [Google Scholar]
- [2].Sinderby C., Beck J., Spahija J., Weinberg J., and Grassino A., “Voluntary activation of the human diaphragm in health and disease,” in J. Appl. Physiol., vol. 85, no. 6, pp. 2146–2158, 1998. [DOI] [PubMed] [Google Scholar]
- [3].Beck J., Sinderby C., Lindström L., and Grassino A., “Effects of lung volume on diaphragm EMG signal strength during voluntary contractions,” in J. Appl. Physiol., vol. 85, no. 3, pp. 1123–1134, 1998. [DOI] [PubMed] [Google Scholar]
- [4].“ATS/ERS Statement on respiratory muscle testing,” in American Thoracic Society/European Respiratory Society. Std., 2002.
- [5].Jolley C., Luo Y., Steier J., Reilly C., Seymour J., Lunt A., Ward K., Rafferty G., and Moxham J., “Neural respiratory drive in healthy subjects and in COPD,” in Eur. Respir. J., vol. 33, no. 2, p. 289, 2009. [DOI] [PubMed] [Google Scholar]
- [6].Allo J., Beck J., Brander L., Brunet F., Slutsky A., and Sinderby C., “Influence of neurally adjusted ventilatory assist and positive end-expiratory pressure on breathing pattern in rabbits with acute lung injury,” in Crit. Care Med., vol. 34, no. 12, pp. 2997–3004, 2006. [DOI] [PubMed] [Google Scholar]
- [7].Sinderby C., Beck J., Spahija J., de Marchie M., Lacroix J., Navalesi P., and Slutsky A., “Inspiratory muscle unloading by neurally adjusted ventilatory assist during maximal inspiratory efforts in healthy subjects,” in Chest, vol. 131, no. 3, pp. 711–717, 2007. [DOI] [PubMed] [Google Scholar]
- [8].Beck J., Campoccia F., Allo J., Brander L., Brunet F., Slutsky A., and Sinderby C., “Improved synchrony and respiratory unloading by neurally adjusted ventilatory assist (NAVA) in lung-injured rabbits,” in Pediatric Res., vol. 61, no. 3, pp. 289–294, 2007. [DOI] [PubMed] [Google Scholar]
- [9].Brander L., Poi H., Beck J., Brunet F., Hutchison S., Slutsky A., and Sinderby C., “Titration and implementation of neurally adjusted ventilatory assist in critically ill patients,” in Chest, vol. 135, p. 695, 2009. [DOI] [PubMed] [Google Scholar]
- [10].Lecomte F., Brander L., Jalde F., Beck J., Qui H., Elie C., Slutsky A., Brunet F., and Sinderby C., “Physiological response to increasing levels of neurally adjusted ventilatory assist,” in Resp. Phys. Neurob., vol. 166, no. 2, pp. 117–124, 2009. [DOI] [PubMed] [Google Scholar]
- [11].Passath C., Takala J., Tuchscherer D., Jakob S. M., Sinderby C., and Brander L., “Physiological response to changing positive end-expiratory pressure during neurally adjusted ventilatory assist in sedated, critically ill adults,” in Chest., vol. 138, pp. 578–587, 2010. [DOI] [PubMed] [Google Scholar]
- [12].Colombo D., Cammarota G., Bergamaschi V., Lucia M., Corte F., and Navalesi P., “Physiologic response to varying levels of pressure support and neurally adjusted ventilatory assist in patients with acute respiratory failure,” in Intensive Care Med., vol. 34, pp. 2010–2018, 2008. [DOI] [PubMed] [Google Scholar]
- [13].Ververidis D. and Kotropoulos C., “Gaussian mixture modeling by exploiting the Mahalanobis distance,” in IEEE Trans. Signal Process., vol. 56, no. 7B, pp. 2797–2811, Jul. 2008. [Google Scholar]
- [14].Ververidis D., “Open code,” [Online]. Available: mathworks.com/matlabcentral/fileexchange.
- [15].Draper N. and Smith H., Applied Regression Analysis, New York: Wiley, 1998. [Google Scholar]
- [16].Anderson T., Introduction to Multivariate Statist. Analysis, New York: Wiley, 1984. [Google Scholar]
- [17].Jubran A., Mathru M., Dries D., and Tobin M. J., “Continuous recordings of mixed venous oxygen saturation during weaning from mechanical ventilation and the ramifications thereof,” in Am. J. Respir. Crit. Care Med., vol. 158, pp. 1763–1769, 1998. [DOI] [PubMed] [Google Scholar]
- [18].Levine S., Nguyen T., Taylor N., Friscia M., Budak M., Zhu J., Sonnad S., Kaiser L., Rubinstein N., Powers S., and Shrager J., “Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans,” in New England J. Med., vol. 358, no. 13, p. 1327, 2008. [DOI] [PubMed] [Google Scholar]
- [19].Sassoon C. S. H., Zhu E., and Caiozzo V. J., “Assist-control mechanical ventilation attenuates ventilator-induced diaphragmatic dysfunction,” in Am. J. Respir. Crit. Care Med., vol. 170, no. 6, pp. 626–632, 2004. [DOI] [PubMed] [Google Scholar]
- [20].Gayan-Ramirez G., Testelmans D., Maes K., Rácz G., Cadot P., Zádor E., Wuytack F., and Decramer M., “Intermittent spontaneous breathing protects the rat diaphragm from mechanical ventilation effects,” in Crit. Care Med., vol. 33, no. 12, pp. 2804–2809, 2005. [DOI] [PubMed] [Google Scholar]