Abstract
Sodium appetite is an instinct that involves avid specific intention. It is elicited by sodium deficiency, stress-evoked adrenocorticotropic hormone (ACTH), and reproduction. Genome-wide microarrays in sodium-deficient mice or after ACTH infusion showed up-regulation of hypothalamic genes, including dopamine- and cAMP-regulated neuronal phosphoprotein 32 kDa (DARPP-32), dopamine receptors-1 and -2, α-2C- adrenoceptor, and striatally enriched protein tyrosine phosphatase (STEP). Both DARPP-32 and neural plasticity regulator activity-regulated cytoskeleton associated protein (ARC) were up-regulated in lateral hypothalamic orexinergic neurons by sodium deficiency. Administration of dopamine D1 (SCH23390) and D2 receptor (raclopride) antagonists reduced gratification of sodium appetite triggered by sodium deficiency. SCH23390 was specific, having no effect on osmotic-induced water drinking, whereas raclopride also reduced water intake. D1 receptor KO mice had normal sodium appetite, indicating compensatory regulation. Appetite was insensitive to SCH23390, confirming the absence of off-target effects. Bilateral microinjection of SCH23390 (100 nM in 200 nL) into rats’ lateral hypothalamus greatly reduced sodium appetite. Gene set enrichment analysis in hypothalami of mice with sodium appetite showed significant enrichment of gene sets previously linked to addiction (opiates and cocaine). This finding of concerted gene regulation was attenuated on gratification with perplexingly rapid kinetics of only 10 min, anteceding significant absorption of salt from the gut. Salt appetite and hedonic liking of salt taste have evolved over >100 million y (e.g., being present in Metatheria). Drugs causing pleasure and addiction are comparatively recent and likely reflect usurping of evolutionary ancient systems with high survival value by the gratification of contemporary hedonic indulgences. Our findings outline a molecular logic for instinctive behavior encoded by the brain with possible important translational–medical implications.
The mammalian brain embodies many genetically determined neural organizations subserving specific behavioral patterns of high survival value, historically termed instincts. Sodium appetite, an exemplar, is generated by depletion of body salt content, adrenocorticotropic hormone (ACTH) mimicking stress, and reproduction (1). This appetite is specific for sodium salts, paralleling thirst for water. Salt appetite may be gratified very rapidly by drinking saline solution, which is akin to a dehydrated animal satiating thirst (1). A water-depleted Saharan donkey, the camel, and the dog can repair near 10% of loss of body weight in 10 min (2, 3) as can rabbits, goats, and sheep (4). Severely sodium-depleted sheep will drink 2–3 L of 300 mmol NaHCO3 solution in 5 min. Gratification, with complete loss of interest in salt (5), precedes significant absorption from the gut of fluid drunk. This process may take 10–15 min to be evident by commencing plasma concentration change (4, 6). The high survival value arising from evolution of this integrative physiological strategy is that animals in the wild can drink and rapidly exit the source of salt, or water if thirsty, thus evading predation by carnivores.
Salt appetite is conspicuous in herbivores and omnivores, because sodium is very scarce in soil and vegetation in large regions of the planet (7). Carnivores obtain obligatory sodium intake with meat. Furthermore, response to salt taste is present in invertebrates, although salt appetite behavior is moot (1).
The hypothalamus is a major locus of organization of instinctive behaviors (8–10). Furthermore, sodium-deficient mice develop an avid, specific sodium appetite. Like other species, mice will rapidly gratify appetite over 10 min if presented with sodium solution (5, 11). Chronic administration of ACTH (5, 11) also produces a robust sodium appetite. Salt appetite during reproduction reflects the imperious need of sodium for the developing young in utero and during lactation.
We generated sodium appetite in mice by depletion or infusion of ACTH. Hypothalami and remainder of the brain were collected. A third group was offered 0.3 M NaCl to drink after induction of sodium appetite. Avid intake occurred over 10 min, and then, these gratified animals were euthanized. Using genome-wide coverage microarrays, the aim was to determine hypothalamic gene regulatory effects of sodium deficiency vs. control animals. Also, we determined if any gene expression changes evoked by sodium appetite were changed by rapid gratification of the appetite. Did the gestalt of neural inflow through 5th, 7th, 9th, and 10th cranial nerves with rapid drinking of 0.3M NaCl (1, 12, 13) modify any gene regulatory changes evoked by sodium appetite? Additionally, we compared gene expression in hypothalamus with the remainder of the brain.
We also used gene set enrichment analysis (GSEA) (14), a biocomputational method that interrogates regulation of groups of genes related to a particular physiological or pathological process. Gene sets in hypothalami of mice with sodium depletion and sodium appetite were significantly altered relative to sodium-replete controls. We found, unexpectedly, that genes regulated with sodium appetite were enriched for gene sets associated with cocaine and opiate addiction (15), with up-regulation of enrichment by a combination of both methods of sodium appetite induction. No less surprising was the loss of enrichment for addiction gene sets on rapid gratification of sodium appetite. Based on the apparent presence of a specific gene regulatory program in the mammalian hypothalamus, we then focused on the lateral hypothalamus for verification of gene regulation in orexinergic neurons. Finally, moving to specific animal behavior, we were able to substantially reduce sodium appetite by systemic and hypothalamic local pharmacological manipulation.
Results
Genome-wide coverage microarrays (Fig. 1A and Fig. S1A) showed significant regulation of hypothalamic genes, including those genes previously identified as part of monoaminergic neurotransmission and gratification pathways like dopamine- and cAMP-regulated neuronal phosphoprotein 32 kDa (DARPP-32), proenkephalin, dopamine receptors-1 and -2, α-2C adrenoceptor, striatally enriched protein tyrosine phosphatase (STEP), and immediate early gene activity-regulated cytoskeleton associated protein (ARC) (Fig. 1A). Furthermore, by 10 min after gratification by drinking 0.3 M NaCl (Fig. 1A), we noted a perplexingly rapid loss of tight control of gene regulation. This finding means that up-regulated genes became less up-regulated, showing increased variation, and likewise, down-regulated genes became less down-regulated. Quantitative RT-PCR (qRT-PCR) confirmed these results (Fig. 1B).
Fig. 1.
Gene expression study from hypothalamus. (A) Regulated genes either linked to monoaminergic signaling and/or gratification or belonging to the addiction gene sets. The far right column with numerals next to the heat map indicates maximum fold regulation (log2) for the given gene. Note that all shown genes were regulated between at least one Na+ appetite group vs. control, and the regulation is apparently less tight in the gratified state; in some cases, it is reverted (e.g., PENK for furosemide group and PPP1R1B and ADRA2C for ACTH group) (Fig. S1). (B) RNA validation of gene expression by qRT-PCR. Fold regulation was calculated using the ΔΔCt method (44). Note that all fold regulations showed P values < 0.01 for Na+ appetite (blue bars). Red bars represent fold regulation of genes whose expression was stimulated with appetite that reversed after gratification (P < 0.05); white bars represent genes regulated by appetite that do not reverse with gratification (P > 0.05). (C) Coregulation of genes as illustrated by enrichment scores resulting from GSEA for individual Na+ appetite groups and in combination (i.e., common genes regulated by both stimuli); normalized enrichment scores (allowing comparison of enrichment between sets) and nominal P values (statistical significance of the enrichment score for a single gene set) are shown. Left shows Na+ appetite, and Right shows the gratified state. Note the striking loss of normalized enrichment score > 1.5 and nominal P value < 0.05 in gratified animals for both addiction gene sets with significant enrichment when using the combined (furosemide plus ACTH common genes) dataset. (D) Detailed analysis of gene expression in gratified animals showed a loss of significant gene regulation, which is illustrated in Left; it shows the P value of fold regulation vs. control for the top 100 genes. Right shows the proportion of the top 600 genes whose expression is regulated during appetite (blue bars) that revert their initial direction of regulation in response to gratification (red bars) and the number of those genes with a statistically significant difference gratified vs. Na+ appetite (green bars).
To fully take advantage of our microarray results, we next used GSEA (14) to assess whether groups of functionally related genes were coregulated (i.e., enriched), suggesting a concerted regulation (Fig. 1C and Fig. S1B). Coregulations of gene sets that were implicated from previously conducted microarray studies of addiction (15) were shown for each condition, namely sodium deprivation-induced sodium appetite, as well as coregulation induced by ACTH. Moreover, their respective combination greatly increased significance levels. This unexpected result indicates that genes previously associated with addiction are shared between two different stimuli that give rise to the common motivation (salt appetite), whereas other gene sets, which are not shared between stimulatory modes, become diluted on combination. In contrast and as a confirmation of the specificity of the observed results, there was no significantly enriched set found in the remainder of the brain (SI Results). Thus, GSEA greatly increased the power of the microarray analysis method. Importantly, after gratification of sodium appetite, there was no more increase in enrichment on combination of groups, suggesting that gratification disrupts this closely coordinated gene regulatory program in the hypothalamus (Fig. 1C). Overall, this points to critical involvement of the observed gene regulatory program, including individually regulated genes, in generation of sodium appetite. This concept was confirmed by a detailed analysis of gratification-associated gene regulatory changes (Fig. 1D). However, it is likely that other regulated genes will follow a pattern, where return to the normal will be caused by full absorption of sodium drunk and rectification of blood and brain chemistry, which had originally been disturbed by induction of sodium appetite. In relation to the two methods used to produce sodium appetite, differential gene regulatory responses could be documented (Datasets S1, Tables S1–S3). Na+ deficiency-specific gene regulation comprised significantly more genes previously associated with addiction (7/100 vs. 1/100 with ACTH from addiction gene sets). Gene regulation specific for ACTH-evoked Na+ appetite was characterized by synaptic function-associated genes, which were not observed in Na+ deficiency.
We first focused additional analysis on protein expression of DARPP-32 in the hypothalamus using sodium-depleted rats. DARPP-32 was selected because of its known critical relevance in reward pathway dopaminergic signaling (16, 17), and the gene was regulated robustly in both sodium appetite conditions as shown by microarray and confirmed by qPCR (Fig. 1 A and B). In keeping with previous reports (16–18), we found DARPP-32 expression in suprachiasmatic and supraoptic neurons (Fig. S2). This labeling pattern was found in both sodium-depleted and control mice with no clearly appreciable difference. However, in sodium-depleted animals, we detected up-regulation of DARPP-32 in the periventricular nuclei (Fig. S2 A and B) and also in the lateral hypothalamus (Fig. 2). These latter neurons coexpressed orexin (first known as hypocretin) (19), indicating that sodium deprivation leads to DARPP-32 up-regulation in this orexinergic/hypocretin subpopulation, which has key relevance to addiction-related behavior (17, 18) (Fig. 2). We also focused on the immediate-early gene Arc (activity-regulated cytoskeletal-associated protein), which is shown to function as a master regulator of protein-dependent synaptic and thus, neuronal plasticity. Extending our finding of regulated Arc hypothalamic gene expression (Fig. 1A), we detected ARC protein in lateral hypothalamic orexinergic neurons that also expressed DARPP-32 (Fig. 2 A–E). This coexpression (Fig. 2D) suggests a possible neural plasticity mechanism, whereby ARC could function to prime lateral hypothalamic orexinergic neurons for subsequent activation of reward circuitry underlying gratification behavior (20). Interestingly, a previous study reported an increase of dendritic processes in the nucleus accumbens shell in sodium deficiency and also, a sensitization of sodium-depleted animals to amphetamine (21). Morphological alterations seem characteristic of ARC effects. Regulated expression of ARC in lateral hypothalamic orexinergic neurons prompted us to assess enrichment of gene sets associated with neural activity (22). However, we found them to not be enriched in each sodium appetite condition evaluated separately and in combination (Fig. S1B and Table S1). This finding suggests hypothalamic ARC regulation evoked by sodium appetite may be part of a neural plasticity mechanism somewhat specific for this instinct. Whether adaptation to a state of sodium deficiency or ACTH exposure, both evoking sodium appetite, can regulate other immediate early genes, particularly the long-term adaptation and drug addiction related ΔFOS-B, remains to be determined (23, 24).
Fig. 2.
Orexinergic lateral hypothalamic neurons up-regulate DARPP-32 and ARC in sodium-depleted rats. (A Left) Panels of micrographs show orexinergic labeling, colocalization with DARPP-32 immunoreactivity, and colocalization with ARC. Note the increased immunoreactivity of lateral hypothalamic neurons in sodium-depleted animals. (Scale bar: 30 μm.) (Right) An anatomic orientation of the hypothalamus with the target area depicted in A. (B) Representative confocal micrographs showing partial subcellular colocalization of ARC and DARPP-32 in orexin-expressing lateral hypothalamic neurons. (Scale bar: 30 μm.) (C) Morphometry (densitometry) of DARPP-32 and ARC expression in lateral hypothalamic orexinergic neurons. DARPP-32 and ARC are regulated in sodium depletion; differences are statistically significant at levels of P < 0.01 (t test; n = 3 animals per group and n = 17–40 orexin-expressing neurons per animal). (D) Morphometry of colabeling in lateral hypothalamic neurons. The blue bar indicates, based on data from three sodium-deprived rats (n = 19, 32, and 33 lateral hypothalamic neurons positive for orexin), the percentage of colocalization of DARPP-32. The threshold was set at three times the average of nondepleted control animals. The red bar indicates the 100% colocalization of DARPP-32 with ARC in the orexin-DARPP-32–coexpressing neurons.
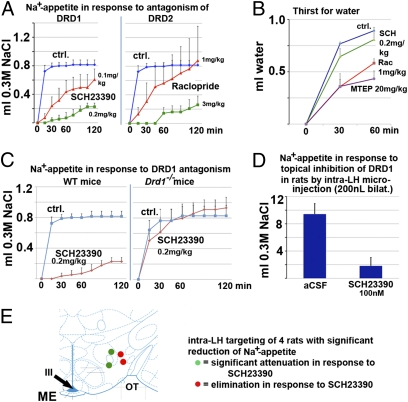
Our experiments have led us to an exciting hypothesis, namely the existence of a hypothalamic gene regulatory program subtending sodium appetite. This mechanism would prime the animal with an intention to ingest salt to experience gratification. Therefore, we decided next to target reward pathways in rats and mice with deprivation-induced sodium appetite to address the question of in vivo relevance of the hypothalamic gene regulatory program for instinctive behavior. We used selective antagonists of dopamine receptors and metabotropic glutamate receptor 5 (mGlu5). This use was in light of their roles in reward and self-administration of drugs of abuse (25, 26) (Fig. 3 and Fig. S3 A and B). We observed a dose-related decrease of gratification behavior on antagonism of both D1(5)-R and D2(3)-R (Fig. 3A). As for the role of mGlu5, known to be present on both D1 and D2 receptor-bearing neuronal populations, this role was substantiated in two rodent species using two different antagonists (Fig. S3 A and B). Thus, D1(5)-R, D2(3)-R, and mGlu5 are functionally relevant in depletion-induced sodium appetite.
Fig. 3.
Modulation of reward pathways in sodium appetite. A shows dose-dependent effects of specific inhibition of D1(5) and D2(3) receptors with specific compounds, SCH23390 and raclopride, on sodium appetite of sodium-depleted mice. Cumulative 0.3 M NaCl was assessed every 15 min. Note the striking effect of both compounds at higher doses and attenuation at lower doses, with raclopride-treated mice showing higher variation. B shows cumulative intake of water in response to osmotic dehydration in mice, the attenuating effects of raclopride (lower dose), mGlu5 antagonist, and MTEP, and the complete lack of effect of SCH23390 (higher dose). C illustrates normal depletion-evoked sodium appetite in Drd1−/− mice; however, there is complete lack of effect of SCH23390 (again, higher dose) in these mice, reiterating the specificity and complete absence of off-target effects of this compound at 0.2 mg/kg. (D) The bar diagram shows averaged sodium appetite for four rats with intrahypothalamic catheters on injection of 200 nL aCSF and the striking reduction when they were infused with 200 nL SCH23390 at 100 nM; P < 0.01 (t test for paired samples) for this dataset (Materials and Methods and Fig. S3). E shows a neuroanatomic schematic, with red circles indicating the location of the injection needle in case of complete elimination of sodium appetite and green circles indicating attenuation (Fig. 2A and Fig. S3B).
To address specificity of the affected instinct, control experiments were conducted to observe water intake after i.p. hypertonic saline injection into mice. Thirst, as a genetically programmed ingestive behavior, parallels salt appetite. In contrast to salt appetite, the D1(5)-R antagonist SCH23390, applied at identical dose, had no effect on osmotically evoked water intake. Antagonism of D2(3)-R as well as mGlu5 caused a moderate reduction of water intake (Fig. 3B). One explanation for these findings is that osmotically stimulated thirst is modulated to a greater degree by D2 receptor pathways (27, 28). Having established a selectivity of D1(5)-R antagonism for sodium appetite, we further investigated this receptor pathway. Drd1−/− mice showed a completely normal depletion-induced sodium appetite, suggesting compensatory up-regulation of alternative pathways. Nevertheless, we were able to record Drd1−/− mice to be completely refractory to the inhibitory effects of SCH23390 at the same dose that could eliminate gratification behavior in WT controls (Fig. 3C). This finding convincingly shows the absence of off-target effects of SCH23390. We next asked the question of local specificity of D1(5)-R signaling in the hypothalamus. For this experiment, rats were microinjected into their lateral hypothalamus (LH) area first with artificial cerebrospinal fluid (aCSF) control solution (200 nL). Only animals that showed robust sodium appetite were subsequently injected with SCH23390 (100 nM in 200 nL injection volume; n = 4). This injection led to a large reduction of sodium appetite at the level of complete elimination in two of four animals (Fig. 3D). Subsequent aCSF injection did not suppress robust sodium appetite (Fig. S3B). Thus, D1(5)-R signaling in the LH area is at least partially responsible for the significant effects on sodium appetite of SCH23390 on systemic application.
Discussion
An instinctive behavior pattern of which salt appetite is an exemplar reflects a genetically hard-wired neural organization naturally selected because of its high survival value. The constitution of the behavior pattern is a trinity—a closely integrated neural system. When strongly aroused, it incorporates an imperious sensation and a compelling specific intention. The subjective element of the trinity of components, the primordial emotion, coalesces all three physiological entities. There is a characteristic specificity. That is, a sexually aroused animal does not have an intention directed to salt intake, and a hungry animal does not have a specific intention to drink water.
The multiple gene changes that we are reporting here likely engender the behavior pattern that is aroused and executed, although our observations do not translate into understanding of a general mechanism. The reversal of a substantial element of the gene changes within 10 min of gratification by drinking 0.3 M NaCl also suggests their involvement in the response to sodium deficiency, and the contemporaneous loss of appetite with gratification suggests that these gene expression changes are implicated in causation of the changed subjective state. It should be noted that it is not determined how rapidly the protein changes evoked by the initial gene expression are reversed by gratification. Additionally, no delineations have been attempted with regard to genes that may control other physiological organizations in sodium deficiency such as control of blood pressure.
In relation to the complexity of the underlying mechanisms, it can be recognized that many factors may be involved in the genesis of sodium appetite. Adrenal steroids and angiotensin are important in the mouse (1). However, with ruminants (e.g., sheep and cattle), sodium concentration in the CSF is a major causal factor, its role probably reflecting that the impact of a sodium-deficient environment falls primarily on the herbivores (1). Thus, differences between species reflect the different selection pressures operative in the evolution of the instinct. Furthermore, a quintet of steroid and peptide hormones is involved in the powerful sodium appetite of pregnancy and lactation. Thus, causation of sodium appetite is complex, and quantitative delineation of the factors contemporaneously operating in the hypothalamus to contrive gene regulatory change in particular species is likely to be as complex as genesis of hunger.
However, the results reported here represent an important first step toward better understanding gene regulatory dynamics in the hypothalamus that subtend gratification (see also ref. 29). Going beyond, we showed here the functional relevance of dopamine and metabotropic glutamate receptor signaling for sodium appetite, and we identified a specific functional role for D1(5) receptors without effect on a related instinct, thirst for water.
Selective antagonism of D1(5) receptors by microinjection into the lateral hypothalamus virtually eliminated sodium appetite, thus reiterating the key role of this location as suggested by our DARPP-32-ARC immunohistochemistry. Our result concurs with a body of previous work showing the role of the mesolimbic dopaminergic system in sodium appetite generated by chronic administration of deoxycorticosterone acetate (30).
Phylogenetically, brain pathways linked to gratification of primal needs have evolved over much more than 100 million years. For example, salt appetite is highly developed in kangaroos. The data reported here suggest that human desire to use drugs such as cocaine and opiates reflects that, in recent human history, drugs of abuse have functionally exploited these phylogenetically ancient gratification mechanisms. This concept was proposed by Koob and Bloom (31, but also see ref. 32), and our data presented here give experimental validation of the idea. This lock into ancient pathways could constitute a partial explanation for the recalcitrance of drug addiction to therapeutic attempts aimed at abstinence. Animals or human addicts with nonsatisfied primal needs show altered responses to drugs of abuse (21, 33).
Beyond our finding of the hypothalamic up-regulation of DARPP-32 after sodium depletion, future studies need to more precisely address the molecular nature of the respective neural circuitry. This need translates into asking which circuits are differentially activated in sodium appetite and possibly, other instinctive needs beyond the established link between lateral hypothalamus orexinergic neurons and nucleus accumbens (34–36). To complement, one of our observations is concordant with a recent advance in addiction biology, namely that orexinergic lateral hypothalamic neurons are involved in addiction behaviors (34, 36). As early as 1964, Wolf (38) showed that bilateral lesions in the lateral hypothalamus greatly reduced the drinking response to sodium deficiency, although he commented that such lesions might damage structures nearby (e.g., zona incerta and median forebrain bundle). Another study reported it as a brain subregion with particularly dynamic gene regulation in cocaine-addicted rats (39).
Our experiments provide a foundation to directly address the question of which aggregate of gene regulatory mechanism underpins each specific instinct and its gratification pathways in the hypothalamus. It is attractive to view the hypothalamic gene regulatory mechanisms characterized here as a scaffold for instinctive behavior (as outlined above), including the compelling intention to specific gratification. Such specific gratification was termed the consummatory act by Lorenz (40). The fact that regulated ensembles of genes are associated with the changes of the subjective state represented by sodium appetite adds a molecular–genetic dimension to the hypothesis that the primordial emotions may have been the phylogenetic origin of consciousness (41, 42).
Materials and Methods
Animals.
All animal studies were conducted with specific approval of the respective animal care and use committees of the Florey Neuroscience Institutes and Duke University.
Sodium Deprivation.
Mice (C57/Bl6; male) were housed individually. After 7 d habituation to low Na food, they then had control measurements taken of body weight, food, water, and 0.3 M NaCl intake over the next 7 d. Then, the 0.3 M NaCl solution was removed, and mice were injected one time per day for 2 d with 1.2 mg furosemide i.p. (Aventis). On the third day, mice were euthanized, and a diencephalic tissue block incorporating all hypothalamus with minimal adjacent tissue (cuts being just rostral to optic chiasm, just caudal to mammillary body, and superiorly through thalamus) was collected into liquid nitrogen. The remainder of the brain served as control. Control mice had a diet with 0.5% NaCl.
Gratification.
With gratification studies, on the third day, 0.3 M NaCl was returned to the mice. These mice drank avidly; ∼10 min later, they were killed, and brain segments were collected.
For pharmacology, mice were treated as above, and then, on the third day, they were offered back the 0.3 M NaCl drinking solution; 0.3 M NaCl intake, water intake, food intake, and body weight were measured every 15 min for the following 1 h, and these measurements were followed by daily measurements (5 d). After this control experiment, the protocol was repeated; however, 10–20 min before the return of the 0.3 M NaCl drinking solution, the mice were injected with 0.1 mL saline s.c. (n = 6 mice), the D2/D3 antagonist raclopride at 1 or 3 mg/kg in 0.1 mL saline s.c. (n = 6 mice in each case), the D1/D5 antagonist SCH23390 at 0.1 or 0.2 mg/kg in 0.1 mL saline s.c. (n = 6 mice in each case), or the mGlu5 antagonist methyl-4-thiazolyl-ethynyl-pyridine (MTEP) at 20 mg/kg i.p. in 0.1 mL (n = 6 mice). Again, intakes were measured every 15 min for the next 2 h, and then, measurements were taken daily.
Drd1a KO mice were used from our own colony (J.D.) (43).
Rats (male Sprague–Dawley rats; mean = 300 g at 12 wk of age) were Na+ deprived with low Na+ food (0.02%, TD90.228; Harlan) and deionized water for 1 wk. On days 3–7 of that week, animals were injected with 5 mg/kg per d furosemide i.p. On day 7, two groups (n = 6 each) were formed and injected with mGlu5 inhibitor, fenobam (i.p., 25 mg/kg in 1 mL), or vehicle 20–30 min before access to 0.3 M NaCl. Rats were permitted to gratify with 0.3 M NaCl, and its intake was measured at 20 min.
ACTH Infusion.
Female C57/B16 mice were offered water, 0.005 M NaCl, and low Na+ food (0.02% Na+). After 1 wk of daily control measurements of body weight, food, water, and 0.005 M NaCl, a miniosmotic pump (Alzet) was implanted s.c. to deliver ACTH (Ciba) at 2.8 μg/d for 12 d. Control 0.005 M NaCl intake was 1.6 mL/d. On the last 4 d of ACTH infusion, the mean intake was 9.3 mL/d. After 12 d of ACTH infusion, mice were euthanized, and hypothalami and the remainder of the brain were removed as above. For the gratification group with ACTH, the same protocol as above was followed, except that 300 mM NaCl solution was available from the beginning of ACTH infusions until euthanasia.
RNA Isolation and Labeling, DNA Microarrays, RNA and Microarray Probe Preparation, and Hybridization.
Total RNA was isolated from the hypothalamus and the remainder of the brain from each mouse using the TR1ZOL-RNA-easy method, and RNA quality was assessed using spectrophotometry and microelectrophoresis; RNA was labeled, and DNA microarray analysis was conducted using the standard procedures as provided in detail in SI Materials and Methods.
GSEA.
GSEA (8) was conducted using the standard procedures that are described in more detail in SI Materials and Methods.
PCR.
Evaluation of mRNA abundance of regulated genes, as suggested by microarray, was performed by qRT-PCR using commercially available primer sets (Openbiosystems), and the SYBR-green and ΔΔCt methods for quantitative assessment (44) were used to perform a relative comparison between sodium appetite and the control group.
Immunohistochemistry.
Standard methods were used (SI Materials and Methods).
Statistical Methods.
Statistical methods for gene arrays and GSEA are described above. Mean and SEM of quantified outcome parameters were compared with their respective controls. Group comparisons were performed using Student unpaired t test or two-factor repeated measures ANOVA followed by Newman–Keuls test for multigroup comparison. Nonparametric statistical testing was performed using the Kruskall–Wallis ANOVA and posthoc Nemenyi test. Minimum significance was set at P < 0.05 for all testing.
Supplementary Material
Acknowledgments
The authors appreciate valuable discussions with Drs. Marc Caron, Nicole Calakos, Sidney Simon, and Sayan Mukherjee and the expert technical assistance of James M. Friedman (all of Duke University). Support for this work was given by The G. Harold and Leila Y. Mathers Foundation, The Robert J. Jr and Helen C. Kleberg Foundation, the Search Foundation, Ms. Diana Gibson, Mr. Robert Albert, Mr. Baillieu Myer, Dr. Mark Nelson, Duke University (to W.B.L.), National Health and Medical Research Council (Australia) Fellowships 454369 (to M.J.M.) and 454303 (to A.J.L.), The Derek Denton Endowment Fund, a Trinity College Undergraduate Fellowship (to S.J.H.), and a Robert H. Ebert Clinical Scholarship of the Klingenstein Fund (to W.B.L.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The microarray data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession nos. GSE29451, GSM729057–GSM729100, and GSM729100).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109199108/-/DCSupplemental.
References
- 1.Denton D. The Hunger for Salt. An Anthropological, Physiological and Medical Analysis. Berlin: Springer; 1983. [Google Scholar]
- 2.Adolph EF, Dill DB. Observations on water metabolism in the desert. Am J Physiol. 1938;123:369–378. [Google Scholar]
- 3.Schmidt-Nielsen K, Schmidt-Nielsen B, Jarnum SA, Houpt TR. Body temperature of the camel and its relation to water economy. Am J Physiol. 1957;188:103–112. doi: 10.1152/ajplegacy.1956.188.1.103. [DOI] [PubMed] [Google Scholar]
- 4.Bott E, Denton DA, Weller S. Water drinking and sheep with an oesophageal fistula. J Physiol. 1965;176:323–336. doi: 10.1113/jphysiol.1965.sp007553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denton D, McBurnie M, Ong F, Osborne P, Tarjan E. Na deficiency and other physiological influences on voluntary Na intake of BALB/c mice. Am J Physiol. 1988;255:R1025–R1034. doi: 10.1152/ajpregu.1988.255.6.R1025. [DOI] [PubMed] [Google Scholar]
- 6.Thrasher TN, Nistal-Herrera JF, Keil LC, Ramsay DJ. Satiety and inhibition of vasopressin secretion after drinking in dehydrated dogs. Am J Physiol. 1981;240:E394–E401. doi: 10.1152/ajpendo.1981.240.4.E394. [DOI] [PubMed] [Google Scholar]
- 7.Blair-West JR, et al. Physiological, morphological and behavioural adaptation to a sodium deficient environment by wild native Australian and introduced species of animals. Nature. 1968;217:922–928. doi: 10.1038/217922a0. [DOI] [PubMed] [Google Scholar]
- 8.Denton DA, McKinley MJ, Weisinger RS. Hypothalamic integration of body fluid regulation. Proc Natl Acad Sci USA. 1996;93:7397–7404. doi: 10.1073/pnas.93.14.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hess WR. Diencephalon. The Biology of Mind. Chicago: University of Chicago Press; 1964. [Google Scholar]
- 10.Johnson AK, Thunhorst RL. The neuroendocrinology of thirst and salt appetite: Visceral sensory signals and mechanisms of central integration. Front Neuroendocrinol. 1997;18:292–353. doi: 10.1006/frne.1997.0153. [DOI] [PubMed] [Google Scholar]
- 11.Denton DA, et al. Effect of adrenocorticotrophic hormone on sodium appetite in mice. Am J Physiol. 1999;277:R1033–R1040. doi: 10.1152/ajpregu.1999.277.4.R1033. [DOI] [PubMed] [Google Scholar]
- 12.Richter C. Salt Appetite of Mammals: Its Dependence on Instinct and Metabolism. Paris: Masson et Cie; 1956. pp. 577–633. [Google Scholar]
- 13.Curtis KS, Stricker EM. Enhanced fluid intake by rats after capsaicin treatment. Am J Physiol. 1997;272:R704–R709. doi: 10.1152/ajpregu.1997.272.2.R704. [DOI] [PubMed] [Google Scholar]
- 14.Subramanian A, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li CY, Mao X, Wei L. Genes and (common) pathways underlying drug addiction. PLoS Comput Biol. 2008;4:e2. doi: 10.1371/journal.pcbi.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stipanovich A, et al. A phosphatase cascade by which rewarding stimuli control nucleosomal response. Nature. 2008;453:879–884. doi: 10.1038/nature06994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svenningsson P, et al. Diverse psychotomimetics act through a common signaling pathway. Science. 2003;302:1412–1415. doi: 10.1126/science.1089681. [DOI] [PubMed] [Google Scholar]
- 18.Ouimet CC, Miller PE, Hemmings HC, Jr, Walaas SI, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. J Neurosci. 1984;4:111–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Lecea L, et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fumagalli F, et al. Single session of cocaine intravenous self-administration shapes goal-oriented behaviours and up-regulates Arc mRNA levels in rat medial prefrontal cortex. Int J Neuropsychopharmacol. 2009;12:423–429. doi: 10.1017/S1461145708009681. [DOI] [PubMed] [Google Scholar]
- 21.Roitman MF, Na E, Anderson G, Jones TA, Bernstein IL. Induction of a salt appetite alters dendritic morphology in nucleus accumbens and sensitizes rats to amphetamine. J Neurosci. 2002;22:RC225 (1–5). doi: 10.1523/JNEUROSCI.22-11-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang G, et al. Identification of activity-dependent gene expression profiles reveals specific subsets of genes induced by different routes of Ca(2+) entry in cultured rat cortical neurons. J Cell Physiol. 2007;212:126–136. doi: 10.1002/jcp.21008. [DOI] [PubMed] [Google Scholar]
- 23.Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- 24.Kelz MB, et al. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- 25.Bird MK, Lawrence AJ. The promiscuous mGlu5 receptor—a range of partners for therapeutic possibilities? Trends Pharmacol Sci. 2009;30:617–623. doi: 10.1016/j.tips.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Caine SB, et al. Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. J Neurosci. 2007;27:13140–13150. doi: 10.1523/JNEUROSCI.2284-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shigemoto R, et al. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- 28.Testa CM, Standaert DG, Landwehrmeyer GB, Penney JB, Jr, Young AB. Differential expression of mGluR5 metabotropic glutamate receptor mRNA by rat striatal neurons. J Comp Neurol. 1995;354:241–252. doi: 10.1002/cne.903540207. [DOI] [PubMed] [Google Scholar]
- 29.Hindmarch C, Yao S, Beighton G, Paton J, Murphy D. A comprehensive description of the transcriptome of the hypothalamoneurohypophyseal system in euhydrated and dehydrated rats. Proc Natl Acad Sci USA. 2006;103:1609–1614. doi: 10.1073/pnas.0507450103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas LR, Pompei P, McEwen BS. Correlates of deoxycorticosterone-induced salt appetite behavior and basal ganglia neurochemistry. Ann N Y Acad Sci. 1999;897:423–428. doi: 10.1111/j.1749-6632.1999.tb07912.x. [DOI] [PubMed] [Google Scholar]
- 31.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 32.Bejerot N. Addiction as an artificially induced instinct. A theory. Nord Med. 1971;85:20–27. [PubMed] [Google Scholar]
- 33.Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76:353–364. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- 34.DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73:759–768. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- 35.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 36.Hoebel BG. Hypothalamic self-stimulation and stimulation escape in relation to feeding and mating. Fed Proc. 1979;38:2454–2461. [PubMed] [Google Scholar]
- 37.Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolf G. Effect of dorsolateral hypothalamic lesions on sodium appetite elicited by desoxycorticosterone and by acute hyponatremia. J Comp Physiol Psychol. 1964;58:396–402. doi: 10.1037/h0048232. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed SH, et al. Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc Natl Acad Sci USA. 2005;102:11533–11538. doi: 10.1073/pnas.0504438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorenz KZ. The comparative method in studying innate behaviour patterns. In: Danelli JF, Brown R, editors. Physiological Mechanisms in Animal Behaviour. Symposium of the Society of Experimental Biology IV. Cambridge, UK: Cambridge University Press; 1950. pp. 221–268. [Google Scholar]
- 41.Denton DA, McKinley MJ, Farrell M, Egan GF. The role of primordial emotions in the evolutionary origin of consciousness. Conscious Cogn. 2009;18:500–514. doi: 10.1016/j.concog.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Denton DA. The Primordial Emotions: The Dawning of Consciousness. New York: Oxford University Press; 2006. [Google Scholar]
- 43.Smith DR, et al. Behavioural assessment of mice lacking D1A dopamine receptors. Neuroscience. 1998;86:135–146. doi: 10.1016/s0306-4522(97)00608-8. [DOI] [PubMed] [Google Scholar]
- 44.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.