Abstract
Plasmodium falciparum malaria is a major cause of mortality and severe morbidity. Its virulence is related to the parasite's ability to evade host immunity through clonal antigenic variation and tissue-specific adhesion of infected erythrocytes (IEs). The P. falciparum erythrocyte membrane protein 1 (PfEMP1) family is central to both. Here, we present evidence of a P. falciparum evasion mechanism not previously documented: the masking of PfEMP1-specific IgG epitopes by nonspecific IgM. Nonspecific IgM binding to erythrocytes infected by parasites expressing the PfEMP1 protein VAR2CSA (involved in placental malaria pathogenesis and protective immunity) blocked subsequent specific binding of human monoclonal IgG to the Duffy binding-like (DBL) domains DBL3X and DBL5ε of this PfEMP1 variant. Strikingly, a VAR2CSA-specific monoclonal antibody that binds outside these domains and can inhibit IE adhesion to the specific VAR2CSA receptor chondroitin sulfate A was unaffected. Nonspecific IgM binding protected the parasites from FcγR-dependent phagocytosis of VAR2CSA+ IEs, but it did not affect IE adhesion to chondroitin sulfate A or lead to C1q deposition on IEs. Taken together, our results indicate that the VAR2CSA affinity for nonspecific IgM has evolved to allow placenta-sequestering P. falciparum to evade acquired protective immunity without compromising VAR2CSA function or increasing IE susceptibility to complement-mediated lysis. Furthermore, functionally important PfEMP1 epitopes not prone to IgM masking are likely to be particularly important targets of acquired protective immunity to P. falciparum malaria.
Keywords: evolutionary selection, immunoevasion, sequestration, pregnancy
The protozoan parasite Plasmodium falciparum is causing the most virulent form of malaria in humans and is the parasite responsible for most severe malaria cases and malaria-related deaths. In 2009, there were about 225 million clinical cases and about 800,000 malaria deaths. The high virulence of P. falciparum is related to the characteristic accumulation of late-stage infected erythrocytes (IEs) in various tissues, which interferes with splenic clearance of IEs (and thus, leads to development of high parasitemias) and can lead to life-threatening inflammation and circulatory disturbances (1, 2).
The tissue-specific accumulation (sequestration) of IEs is mediated, at least in part, by members of the P. falciparum erythrocyte membrane protein 1 (PfEMP1) family of clonally variant proteins that the parasites put on the surface of the erythrocytes that they infect (3). Different PfEMP1 proteins serve as ligands that can interact with different host vascular receptors. PfEMP1-specific IgG is a central component of protective immunity acquired in response to infection by P. falciparum parasites (4). However, such naturally acquired protection takes years to develop because of the substantial interclonal (polymorphic) and intraclonal variability of PfEMP1 proteins; also, the parasites express different PfEMP1 proteins in a mutually exclusive manner and can switch expression among the different variants (5, 6).
Severe P. falciparum malaria complications, which are concentrated among patients without substantial acquired immunity (7), are associated with infection by parasites that express particular types of PfEMP1 with structural and functional similarities (8, 9). Thus, parasites obtained from pediatric patients with cerebral malaria and severe anemia often express PfEMP1 variants that can form rosettes of uninfected erythrocytes around a central IE (10), and parasites causing placental malaria in pregnant women uniformly express a particular PfEMP1 protein (VAR2CSA) that mediates adhesion to chondroitin sulfate A (CSA) in the intervillous space (11). Noticeably, rosette-forming PfEMP1 proteins (12) and CSA-adhering PfEMP1 proteins (13, 14) share the ability to bind nonspecific IgM, although CSA-adhering IEs are not prone to rosette formation (15, 16). Although the molecular details of the interaction between rosette-forming PfEMP1 proteins and nonspecific IgM are known in considerable detail (17), the biological significance of the nonspecific IgM binding to PfEMP1 in general, and to VAR2CSA in particular, is essentially unknown (18). Here, we present evidence that nonspecific IgM binding to VAR2CSA represents a hitherto unknown immunoevasive mechanism that allows the parasites to shield a functionally important protein from specific IgG-dependent immune attack without compromising its function or rendering IEs susceptible to destruction by complement-mediated lysis.
Results and Discussion
IEs that sequester in the placenta characteristically adhere to CSA (19), express the PfEMP1 protein VAR2CSA on their surface (11), and bind IgM nonspecifically (13, 20). The biological significance of the high VAR2CSA affinity for CSA is well-established, whereas the role of nonspecific IgM binding to VAR2CSA is essentially unknown, although it has been proposed to augment placental IE sequestration (20).
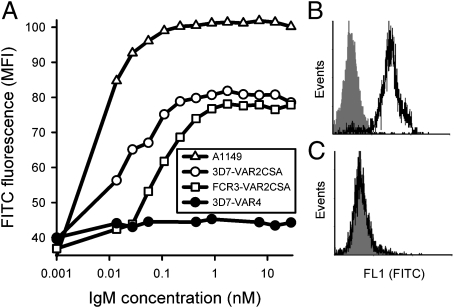
In accordance with the earlier reports, we observed marked nonspecific IgM labeling of erythrocytes infected by 3D7 and FCR3 parasites grown in serum-free medium and selected in vitro to express VAR2CSA on the IE surface (3D7-VAR2CSA+ and FCR3-VAR2CSA+, respectively) (Fig. 1 A and B). Erythrocytes infected by a primary parasite isolate obtained from the peripheral blood of a pregnant Ghanaian woman (A1149) were similarly labeled by nonspecific IgM (Fig. 1A). The large majority of unselected A1149-IEs expressed VAR2CSA and adhered to CSA (Fig. S1), indicating that the predominant in vivo sequestration focus of this isolate was the placenta, which is in accordance with previous findings regarding parasites isolated from the peripheral blood of pregnant women living in areas of stable transmission of P. falciparum parasites (21). In contrast, erythrocytes infected by 3D7 parasites selected in vitro to express the CSA-nonadhering PfEMP1 protein PFD1235w (3D7-VAR4+) (22) did not bind nonspecific IgM (Fig. 1 A and C). The concentration of nonspecific IgM required for saturating IgM labeling of VAR2CSA+ IEs varied ∼10-fold among the different isolates (Fig. 1A).
Fig. 1.
Nonspecific IgM binding to the surface of P. falciparum-infected erythrocytes. (A) Relationship between concentration of IgM and nonspecific IgM binding to field isolate A1149 (△), 3D7-VAR2CSA+ (○), FCR3-VAR2CSA+ (□), and 3D7-VAR4+ (●) late-stage IEs (identified by ethidium bromide nuclear labeling). Representative flow cytometry histograms showing nonspecific IgM binding (at 10 nM concentration) to the surface of VAR2CSA+ (B) and VAR4+ late-stage IEs (C). Background labeling is shown in gray. Data typical of at least three experiments are shown.
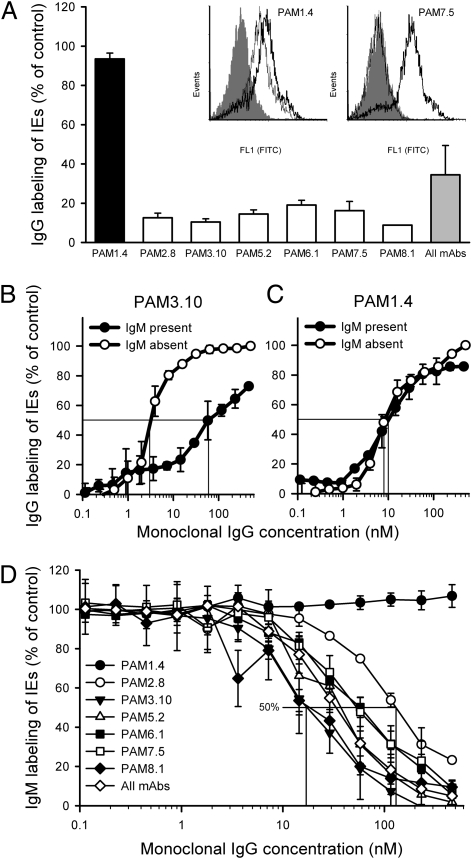
When FCR3-VAR2CSA+ and 3D7-VAR2CSA+ IEs grown in serum-free medium were preincubated with a physiologically plausible concentration (10 nM) of IgM, subsequent labeling of the IEs by six of seven VAR2CSA-specific human monoclonal IgG antibodies was abrogated (Fig. 2A and Fig. S2). All of the IgG antibodies that were inhibited by IgM preincubation are specific for either the DBL3X or DBL5ε domain of VAR2CSA (23). The unaffected antibody (PAM1.4) recognizes neither of these domains and instead, seems to recognize a conformational and possibly discontinuous epitope in VAR2CSA (23, 24). Preincubation of VAR4+ IEs with IgM had no effect on subsequent labeling with the VAR4-specific human monoclonal antibody AB01 (Fig. S3). With the exception of the PAM1.4 antibody, VAR2CSA-specific monoclonal IgG labeling of VAR2CSA+ IEs from cultures maintained in serum-containing medium (and thus, exposed to nonspecific IgM during culture) was much lower than labeling of parallel cultures from serum-free medium (Fig. S4). The labeling pattern, thus, completely resembled that of parasites grown in serum-free medium and preincubated with nonspecific IgM before VAR2CSA-specific monoclonal IgG labeling (Fig. 2A and Fig. S2). We conclude that physiologically realistic levels of nonspecific IgM can markedly interfere with antigen-specific IgG recognition of VAR2CSA+ IEs.
Fig. 2.
Relationships between nonspecific IgM binding and VAR2CSA-specific IgG binding to VAR2CSA+-infected erythrocytes. (A) The effect of preincubation of FCR3-VAR2CSA+ IEs with nonspecific IgM (10 nM) on subsequent labeling by VAR2CSA-specific monoclonal IgG antibodies (150 nM) that either are (white bars) or are not (black bar) specific for the DBL3X or DBL5ε domains of VAR2CSA. IgG labeling is expressed as the percentage (mean and SD) of IgG labeling without IgM preincubation (control). A pool of all of the IgG antibodies (all mAbs) at a final concentration of 150 nM is also shown (gray bar). Inset shows representative flow cytometry histograms of binding of PAM1.4 and PAM7.5 to VAR2CSA+ IEs without (heavy line) or after (thin line) preincubation with nonspecific IgM (background labeling is shown in gray). Labeling of 3D7-VAR2CSA+ IEs by (B) PAM3.10 (150 nM) or (C) PAM1.4 (150 nM) in the presence of 10 nM IgM (●; IgM and IgG added simultaneously) or in the absence of IgM (○). IgG labeling is expressed as the percentage of IgG labeling at 150 nM in the absence of IgM (control). Drop lines corresponding to the IgG concentration required to obtain half-maximal IgG labeling in the absence and presence of IgM are indicated (Table 1). (D) Nonspecific IgM labeling of FCR3-VAR2CSA+ IEs in the presence of various concentrations of VAR2CSA-specific monoclonal IgG antibodies (IgM and IgG added simultaneously). IgM labeling is expressed as the percentage of IgM labeling in the absence of IgG (control). Drop lines corresponding to the PAM2.8 and PAM3.10 concentrations required to obtain half-maximal IgM labeling are indicated (Table 1). Figure data (means and SD) are typical (A) or complete (B–D) from at least three experiments.
The above deduction was strengthened by our observation that 5- to 30-fold more VAR2CSA-specific monoclonal IgG was required to obtain half-maximal labeling of VAR2CSA+ IEs in the presence than in the absence of IgM (10 nM) when IgG and IgM were added simultaneously (Fig. 2B and Table 1). Again, PAM1.4 was the exception, because labeling of both FCR3-VAR2CSA+ and 3D7-VAR2CSA+ IEs with this monoclonal antibody was essentially unaffected by the presence of IgM (Fig. 2C and Table 1). Furthermore, these results indicate that the interaction between nonspecific IgM and VAR2CSA is of higher affinity than previously supposed and is comparable with that between specific IgG and VARCSA (24). In support of this latter inference, we found that high concentrations of specific monoclonal IgG were required to markedly impact nonspecific IgM binding to VAR2CSA (Fig. 2D and Table 1). As expected from the above findings, PAM1.4 did not have any effect on nonspecific IgM binding (Fig. 2D and Table 1).
Table 1.
Competitive binding of nonspecific IgM and VAR2CSA-specific IgG to VAR2CSA+ P. falciparum-infected erythrocytes
| IgG (nM) required for 50% IgG labeling with and without nonspecific IgM (10 nM)* |
IgG (nM) required for 50% reduction of IgM binding† |
|||||
| FCR3-VAR2CSA+ IEs |
3D7-VAR2CSA+ IEs |
|||||
| −IgM | +IgM | −IgM | +IgM | FCR3-VAR2CSA+ IEs | 3D7-VAR2CSA+ IEs | |
| PAM1.4 | 25 | 50 | 8 | 10 | >>500 | >>500 |
| PAM2.8 | 7 | 35 | 3 | 50 | 130 | >500 |
| PAM3.10 | 2 | 10 | 3 | 60 | 20 | >>500 |
| PAM5.2 | 1 | 15 | 4 | 100 | 40 | >500 |
| PAM6.1 | 10 | 50 | 1 | 30 | 60 | 50 |
| PAM7.5 | 2 | 15 | 5 | 100 | 60 | >>500 |
| PAM8.1 | 1 | 8 | n.a.‡ | n.a.‡ | 20 | n.a.‡ |
| All | 5 | 15 | 4 | 30 | 40 | 210 |
Data are based on more than three independent flow cytometry experiments with similar results.
*Approximate IgG concentration (nM) required for 50% saturating IgG labeling in the absence and presence of nonspecific IgM, respectively. Fig. 1 B and C (drop lines) has illustrations.
†Illustrations (drop lines) are in Fig. 2D.
‡3D7-VAR2CSA does not contain the epitope recognized by PAM8.1 (23), and no IE labeling was seen in the experiments conducted here.
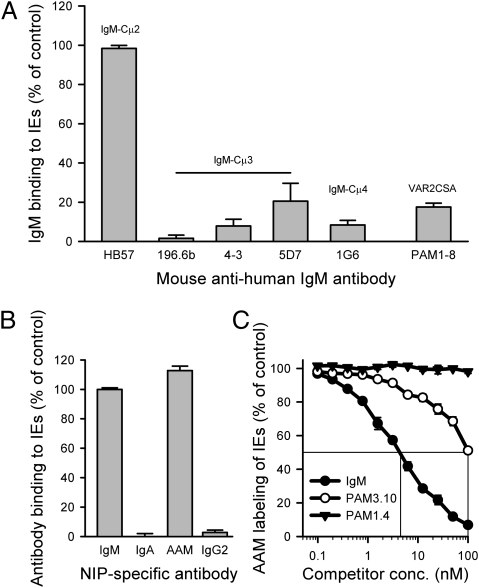
Nonspecific IgM binds to DBL4β in the PfEMP1 protein TM284var1 through residues in Cμ4 located in the armpit between two adjacent IgM monomers within the pentameric structure (17, 18). It seems that this binding causes IgM to straddle TM284var1 in a manner that might stabilize the formation of rosettes around TM284var1+ IEs (17, 25). Previous data indicate that the VAR2CSA binding site of IgM Fc is very similar to its TM284var1 binding site (17). We confirmed this similarity in the present study, because antibodies with specificity for Cμ4 or the adjacent Cμ3 domain effectively inhibited nonspecific IgM binding to VAR2CSA+ IEs, whereas a Cμ2-specific antibody was ineffective (Fig. 3A). The Cμ4 specificity of the interaction with VAR2CSA+ IEs was additionally underpinned by experiments with different antibody classes, showing that only pentameric antibodies containing a human Cμ4 domain (IgM and the domain-switched chimeric AAM antibody, where the Cα3 domain of IgA was replaced by Cμ4 of IgM) could bind VAR2CSA+ IEs (Fig. 3B). Finally, nonspecific IgM as well as the VAR2CSA-specific PAM3.10 antibody (recognizing an epitope affected by IgM masking) (Fig. 2 A and B and Figs. S2 and S4) competed efficiently with the AAM antibody for binding to VAR2CSA+ IEs in a dose-dependent manner, whereas the PAM1.4 antibody (recognizing an epitope not masked) (Fig. 2 A and C and Figs. S2 and S4) did not compete efficiently (Fig. 3C). We conclude that nonspecific, pentameric IgM binds to VAR2CSA through the Cμ4 domain of IgM.
Fig. 3.
IgM domain specificity of nonspecific IgM binding to VAR2CSA. (A) Binding of nonspecific IgM (10 nM) to FCR3-VAR2CSA+ IEs after preincubation of the IgM with various mouse monoclonal antibodies (100 nM) with specificity for the Cμ2 (HB57), Cμ3 (196.6b, 4–3, and 5D7), or Cμ4 (1G6) domain of IgM. Inhibition of IgM binding by preincubation of IEs with a pool of all seven human monoclonal VAR2CSA-specific IgG antibodies (PAM1-8; final concentration is 150 nM) is shown for comparison. (B) Binding to VAR2CSA+ IEs of recombinant chimeric 3-iodo-4-hydroxy-5-nitrophenacetyl (NIP)-specific human IgM, IgA, domain-swapped IgA in which the Cα3 domain has been replaced by Cμ4 from human IgM (AAM), and IgG2. Binding of the recombinant human antibodies to IEs was detected with a phycoerythrin (PE)-conjugated antibody specific for the mouse λ-light chain shared by all these antibodies [described previously by Ghumra et al. (17)]. Only IgM or the domain-swapped IgA containing Cμ4 bound to the IEs. (C) Dose-dependent competition for binding to VAR2CSA+ IEs between the AAM antibody (10 nM) on the one hand and varying concentrations of nonspecific IgM and monoclonal VAR2CSA-specific IgG antibodies PAM1.4 and PAM3.10 on the other hand. Data (means and SD) typical of three independent experiments are shown.
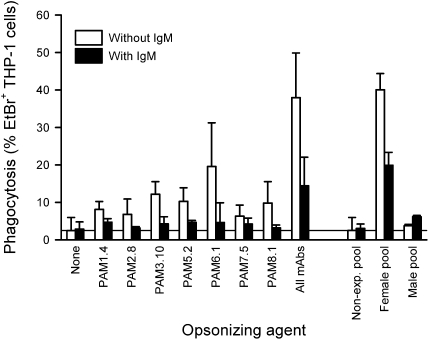
Interference with FcγR-dependent phagocytosis of IEs would seem a plausible selective mechanism driving nonspecific IgM masking of IgG epitopes in VAR2CSA. We have previously shown that all of the VAR2CSA-specific monoclonal IgG1 antibodies used here can opsonize VAR2CSA+ IEs (24), and therefore, we examined whether nonspecific IgM binding could protect VAR2CSA+ IEs from destruction by phagocytosis. Indeed, incubation of FCR3-VAR2CSA+ IEs (Fig. 4) and A1149-IEs (Fig. S5A) with nonspecific IgM before opsonization by the VAR2CSA-specific monoclonal antibodies markedly reduced subsequent IE ingestion by the phagocytic cell line THP-1. IE opsonization by plasma from P. falciparum-exposed multigravid women was similarly affected by nonspecific IgM, whereas plasma from nonexposed controls and P. falciparum-exposed men, as expected, did not opsonize the VAR2CSA+ IEs (Fig. 4 and Fig. S5). Phagocytosis of specific IgG-opsonized VAR2CSA+ IEs maintained in serum-containing medium was much lower than phagocytosis of IEs maintained in serum-free medium, which would be expected from the presence of nonspecific IgM in serum (Fig. S5B). We conclude that Cμ4-dependent binding of IgM to VAR2CSA+ IEs provides parasites with a survival advantage by reducing FcγR-dependent phagocytosis.
Fig. 4.
Phagocytosis of antibody-opsonized VAR2CSA+ IEs. FcγR-dependent THP-1 phagocytosis of monoclonal VAR2CSA-specific IgG- or plasma-opsonized FCR3-VAR2CSA+ IEs without (white bars) or with (black bars) preincubation of IEs with nonspecific IgM (10 nM). Plasma pools were prepared from adult donors without P. falciparum exposure (nonexp. pool), P. falciparum-exposed men (male pool), and previously pregnant P. falciparum-exposed women (female pool). Summary (means and SDs) of three independent experiments is shown.
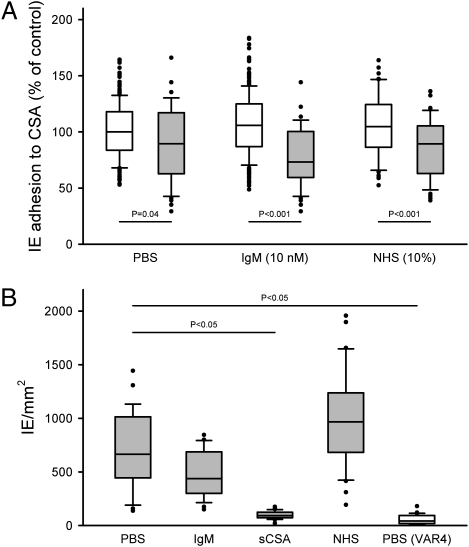
The adhesion of IEs to CSA is considered an important survival strategy of placenta-sequestering P. falciparum parasites (11, 19), and levels of IgG inhibiting IE binding to CSA are associated with protective immunity to placental malaria (26, 27). Nonspecific IgM masking might prevent binding of adhesion-inhibitory IgG, but nonspecific IgM binding near the CSA adhesive domain of VAR2CSA might likewise reduce the ability of VAR2CSA+ IEs to attach to CSA. We found that preincubation of VAR2CSA+ IEs with nonspecific IgM [10 nM or nonimmune serum (10%)] did not reduce their subsequent ability to bind to CSA under conditions of physiologically realistic flow (Fig. 5A). In contrast, a pool of VAR2CSA-specific IgG significantly reduced adhesion whether nonspecific IgM was present or not (Mann–Whitney test, P < 0.04 in all cases) (Fig. 5A). To substantiate the in vivo relevance of these findings, we tested whether nonspecific IgM binding to VAR2CSA+ IEs affected their adhesion to cryosections of placental tissue in a static adhesion assay (28). VAR2CSA+ IEs bound to the sections in high numbers, and this adhesion was completely inhibited by soluble CSA (Fig. 5B). Preincubation of VAR2CSA+ IEs with nonspecific IgM or nonimmune serum did not significantly change their adhesiveness to placental cryosections (Fig. 5B), although adhesion was somewhat higher in the presence of serum, which is in line with a previous report (29). Parasites expressing the nonplacental PfEMP1 variant VAR4 (which does not mediate binding to CSA) did not adhere to the placental tissue sections (Fig. 5B). These results, therefore, fully support the data obtained in the flow-based adhesion inhibition assay (Fig. 5A). We conclude that nonspecific IgM binding to VAR2CSA+ IEs is unlikely to affect parasite survival by interfering with IE adhesion to CSA, possibly because the CSA binding region of VAR2CSA is kept free of nonspecific IgM that might obstruct the interaction between VAR2CSA and CSA.
Fig. 5.
CSA-specific adhesion of VAR2CSA+ IEs. (A) Adhesion of VAR2CSA+ IEs to CSA under flow after preincubation of IEs in PBS, nonspecific IgM (10 nM), or nonimmune human serum (10%) in the absence (open boxes) or presence (shaded boxes) of a pool of VAR2CSA-specific monoclonal IgG antibodies (final concentration of 150 nM). (B) Adhesion of VAR2CSA+ IEs (shaded boxes) or VAR4+ IEs (open box) to placental cryosections after preincubation of IEs in PBS, nonspecific IgM (10 nM), soluble CSA (7.5 μg/mL), or nonimmune human serum (10%). The box plots show median (central line), central 50% (box), central 80% (whiskers), and outlying data points. The statistical significance levels of intergroup differences are indicated. All data from a minimum of three independent experiments, normalized to assay mean adhesion without IgM (control), are included.
The high-affinity CSA binding region of VAR2CSA was recently mapped to the N-terminal DBL2X-CIDRPAM domains (30), whereas binding of nonspecific IgM to VAR2CSA has variously been reported to involve DBL2X, DBL6X, and particularly, DBL5ε (13) or only DBL6ε (14). Our observation (Fig. 5 and Fig. S1) that nonspecific IgM binding to VAR2CSA did not markedly affect adhesion of VAR2CSA+ IEs to CSA argues against DBL2X as a major IgM binding domain. Conversely, the insensitivity of PAM1.4 to masking by nonspecific IgM (Fig. 2 and Fig. S2) and the remarkable ability of this monoclonal antibody to block adhesion of placental IEs to CSA ex vivo (24) indicate that the PAM1.4 epitope is located near the high-affinity CSA binding region in the native VAR2CSA molecule. Furthermore, the susceptibility of all our DBL3X- and DBL5ε-specific monoclonal IgG antibodies to nonspecific IgM masking (Fig. 2 and Fig. S2) and their inability to inhibit IE adhesion to CSA (24) indicate that DBL3X is positioned near DBL5ε in the correctly folded VAR2CSA molecule and that neither domain is directly involved in VAR2CSA-mediated adhesion to CSA. It is noteworthy in this context that VAR2CSA-specific immunity acquired in response to placental P. falciparum infection seems highly focused on these two domains (23). Taken together, the present evidence suggests that nonspecific binding of IgM to VAR2CSA has evolved to shield epitopes that are immunogenic but not critical to molecular function (adhesion to CSA) from recognition by specific IgG, whereas functionally important epitopes are kept clear of this masking. If so, epitopes in VAR2CSA that are both targeted by adhesion-inhibitory IgG and accessible to such IgG despite nonspecific IgM binding (such as the epitope recognized by PAM1.4) would seem to be of particular interest in the quest to develop VAR2CSA-based vaccines against placental malaria (31).
A pentameric structure for IgM, a potent C1q-dependent activator of the complement system, is required for nonspecific IgM binding to PfEMP1 proteins through Cμ4 (17). C1q binding to the Fc part of antibodies seems dependent on conformational changes induced by antigen engagement, but it is unclear whether interaction through Fab is required or whether Fab-independent immobilization is sufficient (32). Therefore, we considered whether the apparent survival advantage afforded by nonspecific IgM binding to VAR2CSA would be counterbalanced by an increased susceptibility to complement-mediated lysis. If so, this finding might explain the paucity of nonspecific IgM binding among PfEMP1 variants in general. However, in experiments with plasma C1q, we did not find evidence of C1q deposition on VAR2CSA+ IEs, and nonspecific IgM (or VAR2CSA-specific monoclonal IgG) bound to the surface of IEs did not lead to C1q deposition (Fig. S6). This finding indicates that either Cμ4-mediated binding of IgM to VAR2CSA does not induce the conformation change in IgM required for C1q deposition or the C1q binding sites in IgM are inaccessible when the pentameric molecule is bound to IEs through Cμ4. In any case, nonspecific IgM masking of IEs does not seem to involve a fitness cost to the parasites in terms of increased susceptibility to complement-mediated lysis.
In summary, we provide evidence that the ability of VAR2CSA to bind IgM through its Cμ4 domain is immunoevasive in character. It seems to confer a selective advantage on the parasite by decreasing IE susceptibility to FcγR-dependent phagocytosis without reducing IE adhesion to CSA or rendering IEs susceptible to complement-mediated lysis. Consequently, identification of PAM1.4-like epitopes that are resistant to nonspecific IgM masking (in this study) is relatively conserved among different P. falciparum clones (24), and identification of PAM1.4-like epitopes that are capable of inhibiting IE adhesion to CSA (24) would seem an obvious priority in current efforts to develop vaccines against placental malaria based on VAR2CSA (31). Furthermore, the detailed topology of the interaction of IgM-Cμ4 and VAR2CSA deserves attention, because it might provide additional clues relevant to vaccine development. Finally, it should be investigated whether the immunoevasive character of the interaction between nonspecific IgM and VAR2CSA also applies to the interaction between nonspecific IgM and rosetting PfEMP1 proteins, which are also mediated through IgM-Cμ4 (17), or whether that interaction serves other purposes (for example, by facilitating or stabilizing rosette formation) (17, 18, 25). We are currently pursuing all these leads.
Materials and Methods
Antibodies and Immune Plasma.
Nonspecific human IgM was obtained from Sigma-Aldrich. The isolation and characterization of the seven VAR2CSA-specific human monoclonal IgG1 antibodies used here (PAM1.4, PAM2.8, PAM3.10, PAM5.2, PAM6.1, PAM7.5, and PAM8.1) have been described in detail elsewhere (23, 24). PAM2.8, PAM6.1, and PAM8.1 recognize epitopes in the DBL3X domain, whereas PAM3.10, PAM5.2, and PAM7.5 recognize epitopes in DBL5ε (23). PAM1.4 recognizes a conformational, possibly discontinuous, epitope in VAR2CSA (24). The human monoclonal IgG1 antibody AB01 with specificity for PFD1235w-DBL4γ was obtained in a manner similar to the VAR2CSA-specific antibodies (23). We also used a panel of recombinant chimeric 3-iodo-4-hydroxy-5-nitrophenacetyl (NIP)-specific antibodies used in a previous study to identify the binding site on human IgM for PfEMP1 (17). The purified mouse anti-human IgM-Fc–specific antibodies (196.6b, 1G6, 4–3, 5D7, and HB57) used here have been previously described (17, 33). The chimeric domain-swapped IgA in which the Cα3 domain is replaced by Cμ4 from human IgM (AAM) mainly exists as the pentameric structure required for interaction with PfEMP1, as previously described (17, 34). Finally, human plasma pools from nonexposed adults and P. falciparum-exposed men and multigravid women collected as part of a previous study (23) were used as control reagents in some experiments.
P. falciparum Parasites.
We used the long-term, in vitro-propagated P. falciparum parasites 3D7 and FCR3 grown in 0 Rh+ erythrocytes with or without human serum (10%) as described (23). Parasites were selected for expression of VAR2CSA on the IE surface by VAR2CSA-specific monoclonal antibodies or repeated panning on the human choriocarcinoma cell line BeWo as described (35, 36). 3D7 parasites were selected for IE surface expression of PFD1235w (VAR4) in a similar manner using specific anti-sera (37). Finally, we included field isolate A1149, obtained from the peripheral blood of a Ghanaian pregnant woman.
Antibody Reactivity with the Surface of P. falciparum-Infected Erythrocytes.
Labeling of the surface of intact and unfixed late-stage IEs by human IgG and IgM was tested essentially as described previously (23). In brief, erythrocytes infected by late-stage parasites were enriched by exposure of parasite cultures to a strong magnetic field, labeled by ethidium bromide (10 mg/mL) and monoclonal IgG and/or nonspecific IgM (30 min a 4 °C), and followed by either FITC-conjugated goat anti-human IgG (http://www.beckmancoulter.com) or FITC-conjugated goat anti-human IgM (http://www.sigmaaldrich.com; 30 min at 4 °C). In experiments with chimeric domain swap antibodies, we used PE-conjugated goat anti-mouse λ-chain–specific IgG (http://www.southernbiotech.com) and IEs not labeled by ethidium bromide. Antibody surface labeling of IEs was quantified by flow cytometry using a Beckman Coulter FC500 instrument (http://www.beckmancoulter.com) followed by analysis of collected data files using WinList software (http://www.vsh.com).
IE Adhesion to CSA.
Binding of VAR2CSA+ IEs to CSA under flow was measured essentially as described before (24). Vena8 channels (www.cellixltd.com) were coated overnight at 4 °C in a humid chamber with 20 μg/mL decorin (www.sigmaaldrich.com) in PBS. The channels were blocked with 2% BSA for 1 h at room temperature. IEs were prepared and adjusted to 2% parasitemia and 1% hematocrit in culture media and incubated (37 °C for 30 min) in PBS with nonspecific IgM (10 nM) or soluble CSA (50 μg/mL). IE suspensions (40 μL) were loaded into the channels using the VenaFlux Platform (www.cellixltd.com) and a shear stress of 0.075 Pa; 10 digital 20-frame movie sequences were recorded through a Leica microscope (200× magnification) at 10 random positions along the channel 5–8 min after inoculation of the sample. The number of bound IEs at each position was determined using Image-Pro Analyzer 7.0 software (www.mediacy.com).
Binding of IEs to placental cryosections was measured as described before (28). In brief, 1-cm3 cubes of fresh tissue from the maternal face of the placenta were frozen in liquid nitrogen, cut into 5-μm sections, and incubated with VAR2CSA+ or VAR4+ IEs preincubated in PBS, nonspecific IgM, soluble CSA, or nonimmune serum. After removal of nonadhering IEs, the tissue sections were stained with Giemsa. For each parasite/treatment combination, IEs adhering to the intervillous compartment of the placental sections were counted on >25-photo micrographs (400× magnification) of random fields by an operator not knowing the parasite/treatment combination.
Antibody Opsonization for Phagocytosis.
We measured the opsonizing capacity of the human monoclonal antibodies essentially as described (24). In brief, magnet-enriched, late-stage IEs (2 × 108/mL) were labeled by ethidium bromide (10 mg/mL; 10 min at room temperature), washed two times in RPMI medium supplemented with 2% FCS, and exposed to either nonspecific IgM (10 nM) or PBS (10 min) followed by VAR2CSA-specific monoclonal IgG (30 min at room temperature). After two additional washes, the opsonized IEs were incubated at a 20:1 ratio with the human monocytic leukemia line THP-1 (TIB-202; http://www.lgcstandards-atcc.org) on a rocking table (30 min at 37 °C). IEs that had not been ingested by the THP-1 cells were lysed (15 mM NH4Cl, 10 mM NaHCO3, 1 mM EDTA; 3 min with shaking for 30 s). The THP-1 cells were then washed two times in ice-cold PBS and supplemented with 2% FCS, and the percentage of ethidium bromide-positive cells was measured by flow cytometry as above.
C1q Deposition on Infected Erythrocytes.
Binding of complement component C1q IEs was measured as described previously (17). In brief, purified late-stage IEs were incubated in PBS (30 min at 4 °C) with or without either IgM or IgG (300 μg/mL), washed three times, incubated (30 min at 4 °C) in Veronal buffer (http://www.oxoid.com) supplemented with either purified human C1q (10 μg/mL; http://www.sigma-aldrich.com) or nonimmune plasma (10%) as the C1q source, washed three times, and labeled (30 min at 4 °C) with FITC-conjugated rabbit anti-human C1q (http://www.abcam.com), anti-human IgG, or anti-human IgM. After two washes in Veronal buffer, the labeled IEs were analyzed by flow cytometry as above.
Supplementary Material
Acknowledgments
Katrine Vegener and Maiken Visti are thanked for excellent technical assistance. Louise Joergensen is thanked for the P. falciparum 3D7 parasites expressing VAR4 (PFD1235w). Patricia Mongini is thanked for IgM-specific monoclonal antibodies. Casper Hempel is thanked for assistance with the placental cryosections. The research leading to these results was funded by Danish Medical Research Council Grant 271-07-0301, Danish Consultative Research Committee for Development Research (FFU) Grant 104.Dan.8 l/306, European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement 242095, Rigshospitalet Grant 9615.05330, and the University of Copenhagen Program of Excellence in membrane topology and quaternary structure of key membrane proteins involved in P. falciparum malaria pathogenesis and immunity. Work in the R.J.P. laboratory was supported by Wellcome Trust Grant 082915/B/07/Z.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103708108/-/DCSupplemental.
References
- 1.Maitland K, Marsh K. Pathophysiology of severe malaria in children. Acta Trop. 2004;90:131–140. doi: 10.1016/j.actatropica.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: Pathogenesis and immunity. Lancet Infect Dis. 2007;7:105–117. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- 3.Su XZ, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 4.Hviid L. Naturally acquired immunity to Plasmodium falciparum malaria in Africa. Acta Trop. 2005;95:270–275. doi: 10.1016/j.actatropica.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Smith JD, et al. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scherf A, et al. Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. EMBO J. 1998;17:5418–5426. doi: 10.1093/emboj/17.18.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bull PC, et al. Plasmodium falciparum-infected erythrocytes: Agglutination by diverse Kenyan plasma is associated with severe disease and young host age. J Infect Dis. 2000;182:252–259. doi: 10.1086/315652. [DOI] [PubMed] [Google Scholar]
- 8.Kraemer SM, Smith JD. Evidence for the importance of genetic structuring to the structural and functional specialization of the Plasmodium falciparum var gene family. Mol Microbiol. 2003;50:1527–1538. doi: 10.1046/j.1365-2958.2003.03814.x. [DOI] [PubMed] [Google Scholar]
- 9.Lavstsen T, Salanti A, Jensen AT, Arnot DE, Theander TG. Sub-grouping of Plasmodium falciparum 3D7 var genes based on sequence analysis of coding and non-coding regions. Malar J. 2003;2:27. doi: 10.1186/1475-2875-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson J, et al. Human cerebral malaria: Association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990;336:1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- 11.Salanti A, et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med. 2004;200:1197–1203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowe JA, Shafi J, Kai OK, Marsh K, Raza A. Nonimmune IgM, but not IgG binds to the surface of Plasmodium falciparum-infected erythrocytes and correlates with rosetting and severe malaria. Am J Trop Med Hyg. 2002;66:692–699. doi: 10.4269/ajtmh.2002.66.692. [DOI] [PubMed] [Google Scholar]
- 13.Rasti N, et al. Nonimmune immunoglobulin binding and multiple adhesion characterize Plasmodium falciparum-infected erythrocytes of placental origin. Proc Natl Acad Sci USA. 2006;103:13795–13800. doi: 10.1073/pnas.0601519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semblat JP, Raza A, Kyes SA, Rowe JA. Identification of Plasmodium falciparum var1CSA and var2CSA domains that bind IgM natural antibodies. Mol Biochem Parasitol. 2006;146:192–197. doi: 10.1016/j.molbiopara.2005.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maubert B, Fievet N, Tami G, Boudin C, Deloron P. Plasmodium falciparum-isolates from Cameroonian pregnant women do not rosette. Parasite. 1998;5:281–283. doi: 10.1051/parasite/1998053281. [DOI] [PubMed] [Google Scholar]
- 16.Rogerson SJ, Beeson JG, Mhango CG, Dzinjalamala FK, Molyneux ME. Plasmodium falciparum rosette formation is uncommon in isolates from pregnant women. Infect Immun. 2000;68:391–393. doi: 10.1128/iai.68.1.391-393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghumra A, et al. Identification of residues in the Cμ4 domain of polymeric IgM essential for interaction with Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) J Immunol. 2008;181:1988–2000. doi: 10.4049/jimmunol.181.3.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czajkowsky DM, et al. IgM, Fc μ Rs, and malarial immune evasion. J Immunol. 2010;184:4597–4603. doi: 10.4049/jimmunol.1000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 20.Creasey AM, Staalsoe T, Raza A, Arnot DE, Rowe JA. Nonspecific immunoglobulin M binding and chondroitin sulfate A binding are linked phenotypes of Plasmodium falciparum isolates implicated in malaria during pregnancy. Infect Immun. 2003;71:4767–4771. doi: 10.1128/IAI.71.8.4767-4771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ofori MF, et al. Expression of variant surface antigens by Plasmodium falciparum parasites in the peripheral blood of clinically immune pregnant women indicates ongoing placental infection. Infect Immun. 2003;71:1584–1586. doi: 10.1128/IAI.71.3.1584-1586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen ATR, et al. Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes. J Exp Med. 2004;199:1179–1190. doi: 10.1084/jem.20040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barfod L, et al. Human pregnancy-associated malaria-specific B cells target polymorphic, conformational epitopes in VAR2CSA. Mol Microbiol. 2007;63:335–347. doi: 10.1111/j.1365-2958.2006.05503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barfod L, et al. Chondroitin sulfate A-adhering Plasmodium falciparum-infected erythrocytes express functionally important antibody epitopes shared by multiple variants. J Immunol. 2010;185:7553–7561. doi: 10.4049/jimmunol.1002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somner EA, Black J, Pasvol G. Multiple human serum components act as bridging molecules in rosette formation by Plasmodium falciparum-infected erythrocytes. Blood. 2000;95:674–682. [PubMed] [Google Scholar]
- 26.Duffy PE, Fried M. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect Immun. 2003;71:6620–6623. doi: 10.1128/IAI.71.11.6620-6623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staalsoe T, et al. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet. 2004;363:283–289. doi: 10.1016/S0140-6736(03)15386-X. [DOI] [PubMed] [Google Scholar]
- 28.Rasti N, Moll K. Placental malaria. In: Moll K, Ljungström I, Perlmann H, Scherf A, Wahlgren M, editors. Methods in Malaria Research. Manassas, VA: MR4/ATCC; 2008. pp. 56–58. [Google Scholar]
- 29.Flick K, et al. Role of nonimmune IgG bound to PfEMP1 in placental malaria. Science. 2001;293:2098–2100. doi: 10.1126/science.1062891. [DOI] [PubMed] [Google Scholar]
- 30.Dahlbäck M, et al. The chondroitin sulfate A-binding site of the VAR2CSA protein involves multiple N-terminal domains. J Biol Chem. 2011;286:15908–15917. doi: 10.1074/jbc.M110.191510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hviid L. The role of Plasmodium falciparum variant surface antigens in protective immunity and vaccine development. Hum Vaccin. 2010;6:84–89. doi: 10.4161/hv.6.1.9602. [DOI] [PubMed] [Google Scholar]
- 32.Czajkowsky DM, Shao Z. The human IgM pentamer is a mushroom-shaped molecule with a flexural bias. Proc Natl Acad Sci USA. 2009;106:14960–14965. doi: 10.1073/pnas.0903805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudich SM, Winchester R, Mongini PK. Human B cell activation. Evidence for diverse signals provided by various monoclonal anti-IgM antibodies. J Exp Med. 1985;162:1236–1255. doi: 10.1084/jem.162.4.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braathen R, Sorensen V, Brandtzaeg P, Sandlie I, Johansen FE. The carboxyl-terminal domains of IgA and IgM direct isotype-specific polymerization and interaction with the polymeric immunoglobulin receptor. J Biol Chem. 2002;277:42755–42762. doi: 10.1074/jbc.M205502200. [DOI] [PubMed] [Google Scholar]
- 35.Haase RN, et al. Plasmodium falciparum parasites expressing pregnancy-specific variant surface antigens adhere strongly to the choriocarcinoma cell line BeWo. Infect Immun. 2006;74:3035–3038. doi: 10.1128/IAI.74.5.3035-3038.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soerli J, et al. Human monoclonal IgG selection of Plasmodium falciparum for the expression of placental malaria-specific variant surface antigens. Parasite Immunol. 2009;31:341–346. doi: 10.1111/j.1365-3024.2009.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joergensen L, et al. Surface co-expression of two different PfEMP1 antigens on single plasmodium falciparum-infected erythrocytes facilitates binding to ICAM1 and PECAM1. PLoS Pathog. 2010;6:e1001083. doi: 10.1371/journal.ppat.1001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.