Abstract
Background
Tuberculosis (TB) and AIDS together present a devastating public health challenge. Over three million deaths every year are attributed to these twin epidemics. Annually, ~ 11 million people are co-infected with HIV and Mycobacterium tuberculosis (Mtb). AIDS is thought to alter the spontaneous rate of latent TB reactivation.
Methodology
Macaques are excellent models of both TB and AIDS. Therefore, it is conceivable that they can also be used to model co-infection. Using clinical, pathological and microbiological data we addressed if latent TB infection in rhesus macaques can be reactivated by infection with Simian Immunodeficiency Virus (SIV).
Results
A low-dose aerosol infection of rhesus macaques with Mtb caused latent, asymptomatic TB infection. Infection of macaques exhibiting latent TB with a rhesus-specific strain of SIV significantly reactivated TB.
Conclusions
Rhesus macaques are excellent model of TB/AIDS co-infection and can be used to study the phenomena of TB latency and reactivation.
Keywords: M. tuberculosis, TB/AIDS co-infection, nonhuman primate, macaque, latency, reactivation
INTRODUCTION
TB and AIDS are responsible for the death of over three million people every year (1, 2). One third of the world population is latently infected with Mtb. About 1.7 million people die of TB each year (1). The situation is equally grim on the AIDS front. An estimated 5 million people are infected with HIV every year (3). Currently there are no promising AIDS vaccines. Recently, the candidate AIDS vaccine that had generated the most hope, failed in clinical trials (4). An estimated 11 million people are co-infected yearly with HIV and Mtb (5). The emergence of drug resistant strains has compounded this situation (6-8). In hotbed areas, an estimated 40% of all TB patients are MDR and 10% of all patients are XDR, a majority of which are also HIV+ (9). It is believed that the deficiency of activated CD4+ T cells - which play a key role in the containment of the pulmonary Mtb infection - is responsible for the increased rates of TB in the post-AIDS world (10). However, the molecular and immunological mechanisms of how both pathogens impact each other are not well understood.
Animal models of infectious disease are necessary to understand mechanisms of pathogenesis and to pre-clinically predict the value of candidate vaccines and drugs. Animal models should mimic human disease as closely as possible. Mice, guinea pigs, rabbits and NHPs have all contributed to our understanding of TB (11-16). Mice have been instrumental in enhancing our understanding of TB, e.g. the role of Mtb virulence factors (11), and the role of various components of the immune system in protection from Mtb infection (17-19). Although easy to use and inexpensive, mice do not sufficiently model critical aspects of human TB. All infected mice eventually succumb to infection, as opposed to a small fraction of immunocompetent humans. Mice also fail to develop necrotic lesions, the precursors of the cavities that characterize late-stage human TB (20). There are advantages as well as limitations to both guinea pig (12) and rabbit (13) models. Immunologic reagents for both these models are scarce. NHPs, such as the rhesus (Macaca mulatta) or the cynomolgus (Macaca fascicularis) macaques have been sparingly used for TB research due to issues like handling, space, availability and expense. However, experimentally infected macaques most accurately reflect human TB in clinical and pathological contexts. They develop acute fatal cavitary or chronic disease based upon the challenge dose (14-16, 21-23). Immunological and molecular reagents are readily available for monkeys due to cross reactivity with humans and their extensive use for studying AIDS. Macaques allow the study of aspects of human TB that are difficult to model in other animals, such as pathology, latency and various outcomes.
Upon infection with a very low dose of highly pathogenic Mtb, cynomolgus macaques either progress to advanced disease, show chronic infection or exhibit latent infection (no signs of clinical disease) (15, 21). In this model, depletion of TNF as well as co-infection with SIV can cause reactivation of TB (24, 25). Recently, we have reported that rhesus macaques infected by exposing them to high-doses of Mtb aerosols develop a rapidly fatal pulmonary TB (22, 23). This model has been used to study both the bacterial (22) as well as host mediators (23) involved in TB, using high throughput approaches. The key difference between this study and the previous ones is that we infected NHPs with Mtb via the aerosol mode. Natural infection with Mtb involves exposure to infectious aerosols. Hence, aerosol mediated infection of NHPs represents the most natural and accurate model to study TB. This method also subjects Mtb to environmental and nutritional stresses comparable to those experienced during the infection process. It is conceivable that such stress primes Mtb for successful infection of primate lungs.
Rhesus macaques have been instrumental in our understanding of the pathogenesis of AIDS (26). HIV and SIV are closely related lentiviruses that cause AIDS in humans and macaques, respectively, with remarkably similar disease progression. Using rhesus macaque adapted strains of SIV it is possible to model a rapid loss of CD4+ cells in the periphery as well as lymphoid organs (27), leading to immunodeficiency in a majority of animals.
In spite of the extensive synergy between TB and AIDS that is apparent from clinical and epidemiological data, co-infection of the two pathogens has never been studied in sufficient detail. Only one recent study (25) attempted to study TB/AIDS co-infection in a macaque model. However, the host species used in this experiment was the cynomolgus macaque, which is not a natural host for SIVmac viruses. We therefore designed experiments aimed at studying TB/AIDS co-infection in an aerosol infection based rhesus macaque model.
MATERIALS AND METHODS
Experimental Animals
Sixteen Indian origin adult rhesus macaques (Macaca mulatta) were used for these studies. The animals were obtained from the TNPRC breeding colony. Before beginning the study, the animals were quarantined for 90 days and tested by both tuberculin skin-test (TST) and an NHP Interferon-gamma release assay (PRIMAGAM) (22), to ensure they were free of prior Mtb infection. SIV-infected animals were housed under BSL-2 conditions while Mtb-infected animals were housed under BSL-3 conditions. Blood draws, bronchoalveolar lavage (BAL), bronchial lymph node (BrLN) biopsies and other procedures were performed as previously described (16, 22).
Mtb and SIV Infection
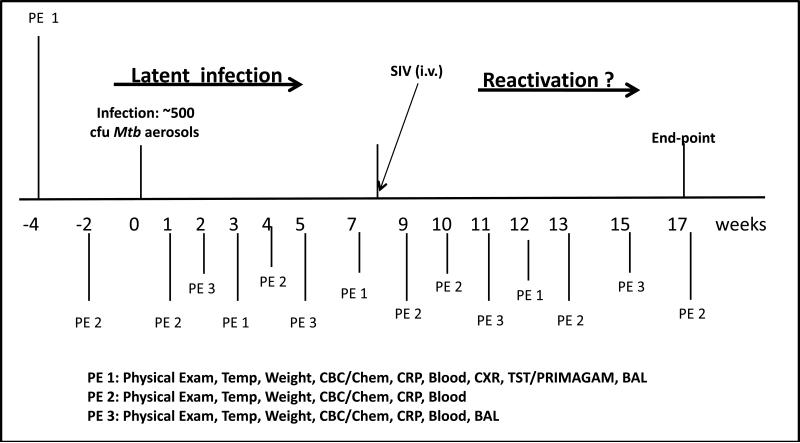
The experimental design of these studies is described in Figure 1. Twelve animals were presented with ~ 500 CFU Mtb CDC1551 via a head-only aerosol method as previously described (22). Mtb infection was confirmed in all animals by conversion to positive TST and PRIMAGAM. Animals were classified as latent if positive for TST or PRIMAGAM test in the absence of signs of clinical TB. For SIV infection, concentrated SIVmac239 stock was diluted in RPMI medium, and 300 TCID50 units of virus injected intravenously. Six animals with latent TB and four Mtb-negative animals were infected with SIV, with the four Mtb-negative animals designated as the SIV-only control group.
Figure 1. The experimental design for Mtb/SIV co-infection in NHPs (A).
All animals were infected with ~ 500 CFU Mtb CDC1551. PE 1, PE 2 and PE 3 correspond to the different collection of clinical activities performed on the two groups of animals at different time-points, including prior to Mtb infection. 300 TCID50 SIVmac239 in PBS buffer was introduced i.v. into six NHPs belonging to group II. The group I animals received placebo (PBS).
Clinical measurements of latent or reactivation TB
Criteria for assessing reactivation included a combination of the following: increased body temperature, prolonged weight or appetite loss, elevated serum C-reactive protein, lung granulomatous immunopathology as observed by thoracic X-rays and by gross and histopathology post-necropsy and the ability to culture Mtb from broncho-alveolar lavages. These clinical measures of TB in rhesus macaques have been described in detail previously (22).
Microbiological measurements of Mtb and SIV from rhesus macaques
Mtb was recovered from macaque blood and BAL samples as well as from homogenized (Biospec Homogenizer, Fisher Scientific, catalog number 11-504-206) tissues (lung, BrLNs, liver, spleen and kidney at necropsy). Tissues were digested using the BBL MycoPrep Mycobacterial System Digestion/Decontamination Kit, (BD Diagnostics, catalog number B4340862), as previously described (22).
SIV-load was determined in plasma by quantitative RT-PCR. Plasma samples were spiked with armored RNA (Asurgen) and centrifuged for 1 hour at 25,000g. Viral RNA was extracted using Proteinase K (2.5 μg/μl; Life Tech), the High Pure Viral RNA kit (Roche Indianapolis, IN) and the RNA Clean and Concentrator kit (ZYMO Research). Viral RNA was reverse-transcribed using MultiScribe™ Reverse Transcriptase (Life Tech). The resulting cDNA was added to TaqMan master mix (Life Tech) along with primers and probe targeting the gag region of SIVmac239 and subjected to 40 cycles of qPCR analyses. Fluorescence signals were detected with an Applied Biosystems 7900HT Sequence Detector. Data were captured and analyzed with Sequence Detector Software (Life Tech). Viral copy numbers were calculated by plotting CT values obtained from samples against a standard curve generated with in vitro-transcribed RNA representing known viral copy numbers (and controlled by addition of known copies of aRNA).
Pathology of TB and TB/AIDS co-infection in rhesus macaques
Methods pertaining to gross and histopathology analysis in the tissues of infected macaques have been described in detail previously (22). Both Mtb and SIV were detected in the lungs of singly and co-infected animals by fluorescently labeled antibodies as described (23, 28).
RESULTS
As shown in Figure 1A, we designed experiments to understand if SIV co-infection could reactivate latent TB infection in rhesus macaques. All animals were exposed to infectious aerosols of Mtb using a head-only exposure system, as previously described (23). The input dose of Mtb was adjusted so that approximately 500 cfu of the low-virulence strain CDC1551 would be presented to and inhaled by the animals. All the animals were examined weekly by a board certified veterinary clinician (Fig 1A). Body temperature and weight, as well as blood chemistry and CBC's were also obtained weekly during the initial phase of Mtb infection, and bimonthly thereafter. Periodically, BAL samples were also obtained from these animals (Fig. 1A). The experiment was designed to allow the identification of animals with latent or asymptomatic TB infection, and those which exhibited signs of reactivated TB following co-infection with SIV. Six animals were only exposed to Mtb aerosols (group I). Of these, one animal (EL04) exhibited signs of active TB from a very early stage post Mtb-infection and was excluded from further analyses. Therefore results are presented for only five NHPs in group I. Six animals were infected intravenously with SIVmac239 at the 9 week post-Mtb infection time-point and designated group II. The five group I animals received a placebo dose of PBS, intravenously. We also had access to a third group (group III) of NHPs that were simultaneously infected with a similar dose of SIVmac239 only.
We used tuberculin skin-test and PRIMAGAM to measure the extent of infection. All twelve animals in group I and II exhibited a positive TST (Table 1) and a positive PRIMAGAM (not shown) between 3-7 weeks post-infection. This conversion confirms that all animals were exposed to Mtb aerosols.
Table 1. TST results.
TST results are tabulated for all 12 animals in groups I and II prior to Mtb infection, and at 4, 8 and 12 weeks post Mtb-infection. Tests were assessed at 24, 48 and 72 hr post-administration. Negative results for each time point are represented by an N while positive results for each time point are represented by a P.
| Group I | Animal | TST result (Pre-infection) | TST result (Week 4) | TST result (Week 9) | TST result (Week 12) |
| NHP 1 | NNN | PPN | NNN | NNN | |
| NHP 2 | NNN | PPP | PPP | NNN | |
| NHP 3 | NNN | N-N | NPP | PPP | |
| NHP 4 | NNN | NPN | NPP | PPP | |
| NHP 5 | NNN | N-N | PPP | PPP | |
| Group II | NHP 1 | NNN | NNN | PPP | NNN |
| NHP 2 | NNN | PPP | PPP | PPP | |
| NHP 3 | NNN | NPN | PPP | PPP | |
| NHP 4 | NNN | PPN | PPP | PPP | |
| NHP 5 | NNN | PPP | PPP | PNN | |
| NHP 6 | NNN | NPP | PPP | NNN |
Clinical correlates of latent Mtb infection and reactivation
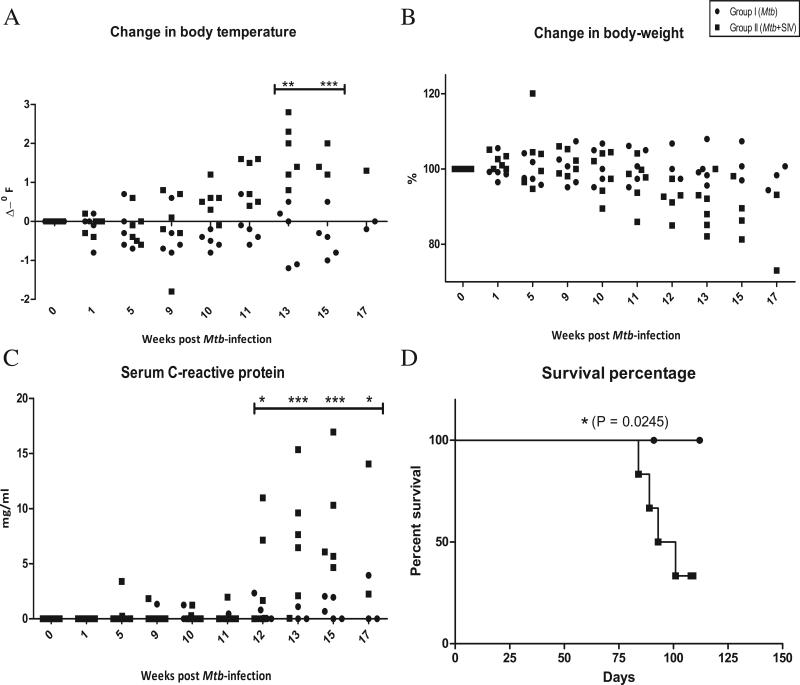
Prior to infection, all animals exhibited normal body temperatures, in the range of 98.5-100°F. We measured the temperature of group I and II animals during the course of Mtb infection, and then following SIV (or placebo) co-infection. These eleven animals exhibited no detectable change in temperature over the first nine weeks post-infection (Fig 2A). Animals in group II, but not group I, exhibited increased body temperatures following SIV co-infection at week 9 (Fig 2A). The differences between group I and II were statistically significant at several time-points.
Figure 2. Clinical correlates of latent TB and reactivation.
We studied change in body temperature, expressed as change in oF (A), change in percent body-weight (B), and serum CRP concentrations, expressed as mg/ml (C). Closed circles represent group I, while closed squares represent group II.
Comparison of the survival percentages for Mtb-infected and Mtb/SIV co-infected NHPs (D). Closed circles represent group I, while closed squares represent group II.
Similarly, none of the eleven animals exhibited any appreciable weight-loss during the first nine weeks post Mtb-infection. However, most animals of group II exhibited weight-loss post SIV-infection (Fig 2B). These differences approached statistical significance with a clear trend of weight-loss in group II NHPs. All animals in this group with the exception of one exhibited notable weight-loss (15-30% of their body weight prior to Mtb-infection), while none of the Mtb-infected animals exhibited significant weight loss (< 10% of their body weight prior to Mtb-infection) (Fig 2B).
We have earlier shown that the serum levels of the systemic inflammatory marker C-reactive protein (CRP) coincide with the advent of active TB. We therefore measured the levels of CRP in the two groups of animals. The serum CRP levels remained close to baseline values prior to SIV infection for most animals. However, the serum CRP levels of all group II animals exhibited a significant increase within 3-7 weeks of SIV-infection (Fig 2C). Serum CRP levels of all group I animals remained significantly lower post-placebo inoculation (Fig 2C).
We also analyzed the extent of lung involvement using chest X rays (CXRs) at different time points during infection. The CXRs were scored by veterinary clinicians on a subjective scale of 0-3, with a score of 0 denoting normal lung, and a score of 3 denoting severe tuberculous pneumonia. During weeks 7-9 post-Mtb infection (prior to co-infection with SIV), all 11 NHPs exhibited CXR scores of 0 or 1, consistent with the diagnosis of latent or asymptomatic TB. However, at week 12 post-Mtb infection (three weeks post-SIV infection), the group II animals exhibited higher CXR scores relative to those in group I (Table 2). These differences were however, not statistically significant.
Table 2. Chest X-rays.
Arbitrary scores from CXRs obtained from all group I and II animals are shown for the following time-points: prior to, 4, 8 and 12 weeks post-Mtb infection. The following subjective scoring system was used: 0 (no pneumonia); 1 (mild pneumonia); 2 (moderate pneumonia); 3 (severe pneumonia).
| Group I | Animal | CXR Score (Pre-infection) | CXR Score (Week 4) | CXR Score (Week 7-9) | CXR Score (Week 12) |
| NHP 1 | 0 | 1 | 1 | 3 | |
| NHP 2 | 0 | 0 | ND | 1 | |
| NHP 3 | 0 | 1 | 1 | 1 | |
| NHP 4 | 0 | 1 | 1 | 1 | |
| NHP 5 | 0 | 0 | 0 | 0 | |
| NHP 6 | 0 | 0 | 0 | 0 | |
| Group II | NHP 1 | 0 | 1 | 1 | 2 |
| NHP 2 | 0 | 0 | 1 | 2 | |
| NHP 3 | 0 | 1 | 1 | 3 | |
| NHP 4 | 0 | 0 | 0 | 1 | |
| NHP 5 | 0 | 0 | 0 | 3 | |
| NHP 6 | 0 | 0 | 0 | 3 |
Infection with a low-dose of a low-virulence Mtb strain caused asymptomatic TB infection in rhesus macaques (Figs 2A-C). Infection was clearly reactivated by SIV co-infection (Figs 2A-C). Reactivation of latent TB significantly reduced the survival of co-infected animals (Fig. 2D). None of the five animals in group I that progressed to asymptomatic infection by week 9 died due to TB related complications during the study. However, of the six animals from group II which were co-infected with SIV, four succumbed to TB related complications. These survival differences were significant (Fig 2D) pointing to the fact that SIV-infection related immunodeficiency reactivates latent TB infection in NHPs.
Microbiological correlates of latent Mtb infection and reactivation
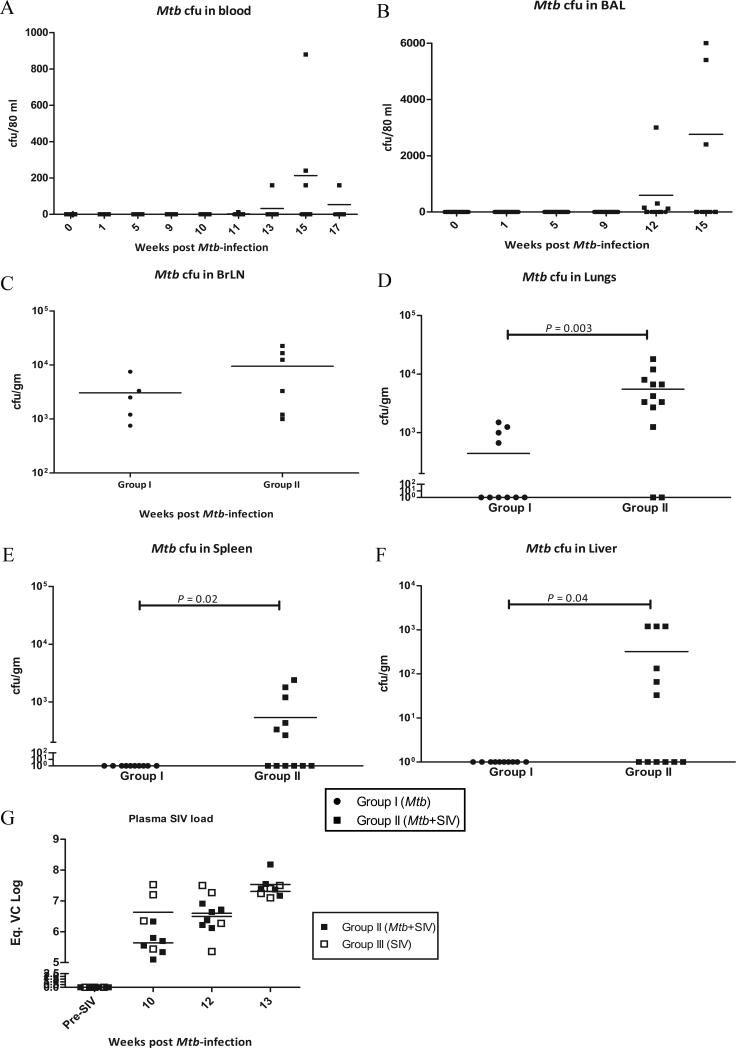
We measured the level of Mtb replication in latent as well as reactivation disease in blood and BAL samples. Post-Mtb infection, but prior to SIV or placebo co-infection, no Mtb CFU's could be detected in the blood of any of the 11 NHPs in group I or II (Fig. 3A). Similarly, BAL samples of all NHPs were negative for Mtb during the post-Mtb infection phase (Fig. 3B). However, post-SIV inoculation, the animals in group II experienced a rapid increase in Mtb replication in lungs, as measured by CFU levels in the BAL (Fig. 3B). Surprisingly, some bacilli could also be detected in the blood of SIV co-infected NHPs (Fig. 3A). This was surprising since the CDC1551 strain is not known to disseminate. Even during high-dose aerosol infection of NHPs with this strain, we have been unable to detect Mtb in blood (Mehra et al, unpublished observations).
Figure 3. Microbiological correlates of latent TB and reactivation.
Mtb CFUs recovered from blood (A) and BAL (B) during different time periods of infection, both expressed as CFU/80 mL. Mtb CFUs at necropsy in BrLN (C), lung (D), spleen (E), liver (F) and kidney (G), expressed as CFU/gram of tissue. Plasma SIV viral copy number as assessed by quantitative RT-PCR (H), prior to and after SIV co-infection. Data is shown for NHPs in group II (closed squares), as well as group III (open squares) for control plasma (prior to SIV infection) and at weeks 10, 12 and 13 post Mtb-infection (i.e. weeks 1, 3 and 4 post-SIV infection) for both groups.
We also measured the bacterial load in BrLNs at different times during infection (Fig. 3C). At necropsy, several randomly chosen regions from both lungs were also used to determine Mtb load (Fig. 3D). Mtb could be detected in BrLNs of all animals and the loads were statistically indistinguishable between the two groups (Fig. 3C). However, a statistically significant higher number of Mtb CFUs could be detected in the lungs of group II animals (Fig. 3D). While the total Mtb burden per gram of tissue was higher in BrLNs than in lungs, the replication of Mtb was significantly increased in lungs by SIV co-infection. Similarly, a higher number of Mtb CFUs could be cultured from the spleen (Fig. 3E), liver (Fig. 3F) and kidneys (not shown) of the group II (SIV co-infected animals) relative to group I (singly infected animals). Taken together, the CFU results indicate that asymptomatic TB was reactivated upon co-infection with SIV.
We also measured the SIV loads present in the plasma of animals in group II in the weeks following inoculation with SIV (Fig 3G). These results were compared to animals in group III, which only received an equivalent dose of SIVmac239. The levels of SIV in the plasma from the two groups of animals were not significantly different, indicating that the course of SIV replication was not influenced by either the presence of a latent TB infection or its reactivation.
Pathology of latent Mtb infection and reactivation
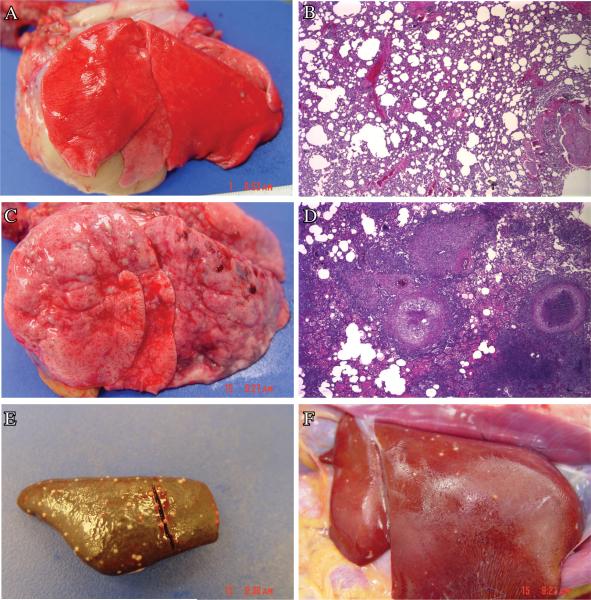
At necropsy, various tissues such as lungs, associated lymphatics, spleen, liver and kidneys were grossly analyzed for the presence of tuberculous lesions in both groups of animals. A few discrete lesions could be observed in the lungs and lymph nodes of group I animals. However, no lesions were present in the extra-thoracic tissues of the NHPs from group I. In contrast, significantly higher lesion counts were visible in the lungs and lymph nodes of the group II animals. These animals also exhibited numerous discrete lesions in spleen, liver and kidney. Representative gross and histopathology lung images for an animal from group I (Fig. 4A and 4B) and group II (Fig. 4C and 4D) are shown. Representative images from the spleen (Fig 4E) and liver of a co-infected (group II) NHP (Fig 4F) are shown. These images clearly show tuberculous lesions in these extra-thoracic tissues, relative to the corresponding, completely normal tissues from the NHPs infected with low-dose Mtb (not shown).
Figure 4. Pathology of latent and reactivation TB.
Representative gross pathology images from the lungs of an NHP from group I (A) and II (B). Representative H&E stained images from the lungs of an NHP from group I (C) and II (D).
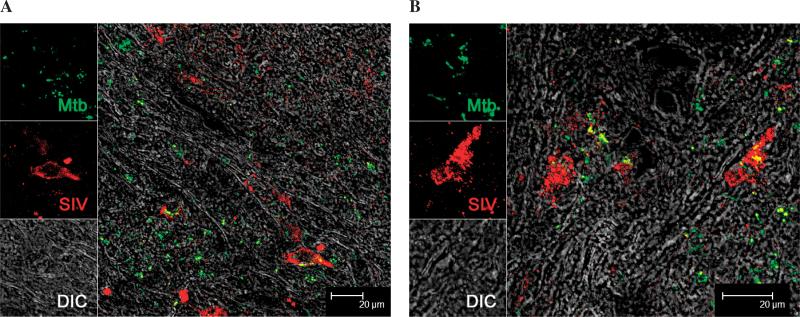
Using confocal microscopy, we studied the presence of SIV in the lung tissues of co-infected animals. We were able to detect the presence of both Mtb and SIV using specific antibodies. Interestingly, SIV along with Mtb appeared to infected macrophages in the lesions obtained from lung as well as lymph nodes. Both in lung (Fig. 5A) and BrLN (Fig. 5B) we were able to detect the co-localization of both Mtb and SIV in the same cells. Additionally, numerous virus-positive cells were located in the vicinity of Mtb-positive cells. This result clearly shows that in-spite of a systemic (intravenous) route of inoculation SIV is able to pathogenize lungs and co-infect the same cells Mtb.
Figure 5. Co-localization of Mtb and SIV in the tissues of co-infected animals.
Multi-label confocal microscopy was used to identify if Mtb and SIV co-localize in the lungs (A) or BrLNs (B) of co-infected animals. A representative image from each tissue is being shown. Specific signal was detected under differential interference contrast (DIC). SIV was detected by using a p28-specific antibody raised in mouse (Microbix Biosystems #AS-59-06) as previously described (28). Mtb was detected by using a polyclonal anti-Mtb antibody raised in rabbit (Abcam #ab905), as previously described (23).
DISCUSSION
It is widely believed that reactivation of latent TB infection in AIDS patients is the single largest reason behind the resurgence of TB as a major cause of human mortality. However, there are only a limited number of studies that have attempted to address this question in a research setting. In part the problem stems from the lack of effective experimental models of co-infection. Mice, the predominant choice of animal system to study Mtb virulence factors as well as the role of host immune mediators in the pathogenesis of the disease, can't be used to model AIDS. NHPs, particularly the Indian rhesus macaque, are an excellent model for studying the pathogenesis of human AIDS (26, 27). Over the last decade, there has been renewed interest in using NHPs to model TB as well (15, 16, 21-25). Both cynomolgus and rhesus macaques have been used to model latent as well as acute TB infections (15, 16). The cynomolgus macaque model has allowed a better understanding of the role played by TNF (24) and regulatory T cells (29) during TB. The rhesus macaque model has allowed the discovery of Mtb genes essential for virulence in primates (22), as well as study host gene-expression (23). Therefore, NHPs appear to be an excellent system for modeling TB/AIDS co-infections. We believe that the model can be used to study the host factors that could lead to reactivation of latent TB infection.
In our studies, we used rhesus macaques as the primate host for latent TB infection. We have already shown that this species can be infected with a high dose of Mtb aerosols (22, 23). We used a highly immunogenic, low-virulence strain of Mtb (CDC1551). We have previously noted that that CDC1551 strain exhibits significantly low virulence as compared to the Mtb Erdman strain (Mehra et al, unpublished observations). In order to generate latent infection with the Erdman strain, as few as 25-50 bacilli may be required. This number of Mtb CFU is difficult to successfully obtain repeatedly when using the aerosol technique. The use of CDC1551 allowed us to perform low-dose aerosol infections with as many as ~ 500 CFU. After a period of time where a large majority of NHPs developed evidence of asymptomatic, latent TB infection, as characterized by positive TST and PRIMAGAM tests, we co-infected one group with SIVmac239, a pathogenic strain of SIV. This is a rhesus macaque adapted strain that is known to generate AIDS related immunodeficiency in this species. The dose used (300TCID50) was in itself nonlethal. This dose was also significantly (about four logs) lower than the dose of SIV (106 TCID50) used by Dietrich et al (25) to generate reactivation of latent TB in cynomolgus macaques and thus represents a more physiologically relevant dose.
Most of the NHPs that were co-infected with SIV significantly reactivated TB infection, as assessed by clinical, pathological and microbiological measures. These included evidence of dissemination of Mtb to extra-thoracic tissues such as spleen, liver and kidney. Clinically, these animals exhibited a higher level of systemic inflammation, as judged by significantly higher serum C-reactive protein values. A statistically significant reduced survival of the co-infected animals, relative to Mtb-infected animals also points to the reactivation of asymptomatic TB infection. In stark contrast, none of the Mtb-only infected animals showed any signs of active TB during the relative time period, as judged by clinical, microbiological and pathological correlates. In the only animal from this initial group of six NHPs which failed to generate latent TB infection, the signs of active TB were apparent from as early as week 3 post-Mtb infection.
Our study suggests that even at a physiologically relevant low-dose, SIV infection rapidly and definitively reactivates latent infection with a low-virulence strain of Mtb in rhesus macaques. These results have far reaching consequences for understanding the immunology of co-infection. The use of a low-dose of SIV to rapidly reactivate latent Mtb infection in rhesus points to the fact that this species is an excellent model for studying the immunological markers of TB/AIDS co-infection. We have already described the system-wide expression of host genes in early and relatively mature TB granuloma lesions (23). In future we would like to study if gene-expression signatures in the granulomatous lesions induced by SIV-mediated reactivation are similar to or different from those induced by active TB. We are also using this model to study the recruitment of and the activation status of different immune cells, such as macrophages, lymphocytes and neutrophils, to the site of pulmonary infection.
Using multi-label confocal microscopy, we have shown that SIV is able to infect lungs and BrLNs of Mtb-infected NHPs, despite its systemic route of inoculation. Further we show co-localization of both pathogens in the lungs as well as BrLNs of the same animals. This is perhaps the first direct illustration of co-infection by Mtb and SIV (or possibly HIV), at the cellular and molecular level. Using this model and the multi-labeling technique, we would in future like to address the hypothesis that SIV infection interferes with the clearance of Mtb infection by modulating anti-tuberculocidal functions of the infected and bystander macrophages.
ACKNOWLEDGEMENTS
This work was funded by the NIH grants RR026006 and AI091457 (both to DK) and supported by NIH grants AI089323, HL10679 (both to DK), AI084793 (to RSV), RR000164 and RR020159; and awards by Louisiana Vaccine Center (to DK), Tulane Research Enhancement Fund (to DK) and Tulane Center for Infectious Diseases (to SM). We acknowledge Dr Preston Marx for providing a stock of SIVmac239; Stephanie Killeen and Satheesh Sivasubramani for Mtb infections and Mary Barnes for PRIMAGAM assay.
Footnotes
The authors declare no conflict of interest in connection with this manuscript. All procedures related to Mtb were approved by the Tulane Institutional Biosafety Committee. All procedures involving animals were reviewed and approved by the TNPRC Institutional Animal Care and Use Committee.
REFERENCES
- 1.Dye C, Scheele C, Dolin P, Pathania V, Raviglione MC. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Prevention and treatment of tuberculosis among patients infected with human immunodeficiency virus: principles of therapy and revised recommendations. Morb Mortal Wkly Rep. 1998;47(RR-20):1–58. [PubMed] [Google Scholar]
- 3.Joint United Nations Programme on AIDS (UNAIDS) WHO . AIDS Epidemic Update Rep. 02.46E 2002. UNAIDS/WHO; Geneva: [Google Scholar]
- 4.Steinbrook R. One Step Forward, Two Steps Back — Will There Ever Be an AIDS Vaccine? New Engl J Med. 2007;357:2653–2655. doi: 10.1056/NEJMp0708117. [DOI] [PubMed] [Google Scholar]
- 5.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–21. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 6.Adegbola RA, Hill P, Baldeh I, Otu J, Sarr R, Sillah J, Lienhardt C, Corrah T, Manneh K, Drobniewski F, McAdam KP. Surveillance of drug-resistant Mycobacterium tuberculosis in The Gambia. Int. J Tuberc. Lung Dis. 2003;7:390–3. [PubMed] [Google Scholar]
- 7.Bifani PJ, Mathema B, Kurepina NE, Kreiswirth BN. Global dissemination of the Mycobacterium tuberculosis W-Beijing family strains. Trends Microbiol. 2002;10:45–52. doi: 10.1016/s0966-842x(01)02277-6. [DOI] [PubMed] [Google Scholar]
- 8.Shah NS, et al. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg. Infect. Dis. 2007;13:380–7. doi: 10.3201/eid1303.061400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh JA, Upshur R, Padayatchi N. XDR-TB in South Africa: No time for denial or complacency. PLoS Med. 2007;4:e50. doi: 10.1371/journal.pmed.0040050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrington M. From HIV to tuberculosis and back again: a tale of activism in 2 pandemics. Clin Infect Dis. 2010;50:S260–266. doi: 10.1086/651500. [DOI] [PubMed] [Google Scholar]
- 11.Orme IM. The mouse as a useful model of tuberculosis. Tuberculosis. 2003;83:112–5. doi: 10.1016/s1472-9792(02)00069-0. [DOI] [PubMed] [Google Scholar]
- 12.McMurray DN. Disease model: pulmonary tuberculosis. Trends Mol Med. 2001;7:135–7. doi: 10.1016/s1471-4914(00)01901-8. [DOI] [PubMed] [Google Scholar]
- 13.Manabe YC, Kesavan AK, Lopez-Molina J, Hatem CL, Brooks M, Fujiwara R, Hochstein K, Pitt ML, Tufariello J, Chan J, McMurray DN, Bishai WR, Dannenberg AM, Jr, Mendez S. The aerosol rabbit model of TB latency, reactivation and immune reconstitution inflammatory syndrome. Tuberculosis. 2008;88:187–96. doi: 10.1016/j.tube.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh GP, Tan EV, dela Cruz EC, Abalos RM, Villahermosa LG, Young LJ, Cellona RV, Nazareno JB, Horwitz MA. The Philippine cynomolgus monkey (Macaca fasicularis) provides a new nonhuman primate model of tuberculosis that resembles human disease. Nat Med. 1996;2:430–6. doi: 10.1038/nm0496-430. [DOI] [PubMed] [Google Scholar]
- 15.Capuano SV, 3rd, Croix DA, Pawar S, Zinovik A, Myers A, Lin PL, Bissel S, Fuhrman C, Klein E, Flynn JL. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect Immun. 2003;71:5831–44. doi: 10.1128/IAI.71.10.5831-5844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gormus BJ, Blanchard JL, Alvarez XH, Didier PJ. Evidence for a rhesus monkey model of asymptomatic tuberculosis. J Med Primatol. 2004;33:134–45. doi: 10.1111/j.1600-0684.2004.00062.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann SH. Protection against tuberculosis: cytokines, T cells, and macrophages. Ann Rheum Dis. 2002;61:ii54–8. doi: 10.1136/ard.61.suppl_2.ii54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grotzkea JE, Lewinsohn DM. Role of CD8+ T lymphocytes in control of Mycobacterium tuberculosis infection. Microbes Infect. 2005;7:776–788. doi: 10.1016/j.micinf.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Boom WH. γδ T cells and Mycobacterium tuberculosis. Microbes Infect. 1999;1:187–195. doi: 10.1016/s1286-4579(99)80033-1. [DOI] [PubMed] [Google Scholar]
- 20.Tsai MC, Chakravarty S, Zhu G, Xu J, Tanaka K, Koch C, Tufariello J, Flynn J, Chan J. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol. 2006;8:218–32. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 21.Lin PL, Pawar S, Myers A, Pegu A, Fuhrman C, Reinhart TA, Capuano SV, Klein E, Flynn JL. Early events in Mycobacterium tuberculosis infection in cynomolgus macaques. Infect Immun. 2006;74:3790–803. doi: 10.1128/IAI.00064-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dutta NK, Mehra S, Didier PJ, Roy CJ, Doyle LA, Alvarez X, Ratterree M, Be NA, Lamichhane G, Jain SK, Lacey MR, Lackner AA, Kaushal D. Genetic requirements for the survival of tubercle bacilli in primates. J Infect Dis. 2010;201:1743–52. doi: 10.1086/652497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehra S, Pahar B, Dutta NK, Conerly CN, Philippi-Falkenstein K, Alvarez X, Kaushal D. Transcriptional reprogramming in nonhuman primate (rhesus macaque) tuberculosis granulomas. PLoS One. 2010;5:e12266. doi: 10.1371/journal.pone.0012266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin PL, Myers A, Smith L, Bigbee C, Bigbee M, Fuhrman C, Grieser H, Chiosea I, Voitenek NN, Capuano SV, Klein E, Flynn JL. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 2010;62:340–50. doi: 10.1002/art.27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diedrich CR, Mattila JT, Klein E, Janssen C, Phuah J, Sturgeon TJ, Montelaro RC, Lin PL, Flynn JL. Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PLoS One. 2010;5:e9611. doi: 10.1371/journal.pone.0009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lackner AA, Veazey RS. Current concepts in AIDS pathogenesis: insights from the SIV/macaque model. Annu Rev Med. 2007;58:461–76. doi: 10.1146/annurev.med.58.082405.094316. [DOI] [PubMed] [Google Scholar]
- 27.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–31. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 28.Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–15. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green AM, Mattila JT, Bigbee CL, Bongers KS, Lin PL, Flynn JL. CD4+ Regulatory T Cells in a Cynomolgus Macaque Model of Mycobacterium tuberculosis Infection. J Infect Dis. 2010;202:533–541. doi: 10.1086/654896. [DOI] [PMC free article] [PubMed] [Google Scholar]