Abstract
Arbuscular mycorrhizal fungi (AMF) are obligate symbionts with most terrestrial plants. They improve plant nutrition, particularly phosphate acquisition, and thus are able to improve plant growth. In exchange, the fungi obtain photosynthetically fixed carbon. AMF are coenocytic, meaning that many nuclei coexist in a common cytoplasm. Genetic exchange recently has been demonstrated in the AMF Glomus intraradices, allowing nuclei of different Glomus intraradices strains to mix. Such genetic exchange was shown previously to have negative effects on plant growth and to alter fungal colonization. However, no attempt was made to detect whether genetic exchange in AMF can alter plant gene expression and if this effect was time dependent. Here, we show that genetic exchange in AMF also can be beneficial for rice growth, and that symbiosis-specific gene transcription is altered by genetic exchange. Moreover, our results show that genetic exchange can change the dynamics of the colonization of the fungus in the plant. Our results demonstrate that the simple manipulation of the genetics of AMF can have important consequences for their symbiotic effects on plants such as rice, which is considered the most important crop in the world. Exploiting natural AMF genetic variation by generating novel AMF genotypes through genetic exchange is a potentially useful tool in the development of AMF inocula that are more beneficial for crop growth.
INTRODUCTION
Plants and arbuscular mycorrhizal fungi (AMF) probably form the most common symbiosis on earth. These fungi have been shown repeatedly to confer a number of benefits to plants, including increased growth through improved nutrient acquisition, especially phosphate (19). In return, the fungal partner receives photosynthetically fixed carbon (10). Mycorrhizal fungal diversity is also a factor that determines plant diversity and productivity (12, 20, 21). AMF were thought to be asexual, and indeed they can reproduce clonally (11). They also are coenocytic, meaning that nuclei coexist in a common cytoplasm. Some AMF have been shown to be heterokaryotic, containing populations of genetically different nuclei within one fungus (9, 15). The consequences of this heterokaryosis still are poorly understood for either the fungi or the plants with which they form symbioses.
Although it is well understood that AMF diversity is important in ecosystems, little is known about the role of AMF genetic variation in shaping plant growth. Recently, two processes have been shown in the AMF Glomus intraradices that generate genetically novel AMF, namely, genetic exchange and segregation, that give rise to AMF lines (named crossed lines and segregated lines, respectively) that are genetically and phenotypically distinct from their parents (named parental lines) (2, 5). Following segregation, segregated lines were shown to have different effects on rice growth and also induced changes in transcript levels of rice genes that are known to be specifically transcribed during mycorrhizal symbiosis (2). Some crossed lines suppressed the growth of rice compared to that after inoculation with parental lines. More recently, both crossed and segregated AMF lines were shown to exhibit different levels of colonization in rice roots, although this was measured only at one time point, 12 weeks after inoculation (3).
Knowledge about the molecular pathway of arbuscular mycorrhiza (AM) establishment is derived mostly from studies of legumes, but recently AM-specific gene transcription in rice was described by Gutjahr et al. (7). They showed which genes are specifically transcribed during the establishment and development of the symbiosis and, for some genes, where they are transcribed. They characterized 18 genes that are specifically transcribed in the mycorrhizal symbiosis and tested their transcription at 3, 5, 7, and 9 weeks after inoculation. The functions of most of these genes were unknown. Four of them (AM1, AM2, AM3, and AM11) were transcribed very early during the colonization, 3 weeks after inoculation. The other AM-specific genes started to be transcribed at 7 weeks after inoculation (Table 1). The transcription of some of these genes subsequently was shown to be altered by segregation in AMF at one time point, 12 weeks after inoculation (2). Indeed, segregated AMF lines induced different transcription levels of some of these genes compared to levels in rice inoculated with the crossed lines from which they originated.
Table 1.
Genes characterized by Gutjahr et al. (7) and their temporal transcription
| Gene | Transcription |
Description or putative function | ||
|---|---|---|---|---|
| Early (<3 weeks) | Late (>7 weeks) | Systemic | ||
| AM1 | X | Putative classe III peroxidase (Prx53) | ||
| AM3 | X | X | Contains peptidoglycan binding LysM domain 1 | |
| AM10 | X | Similar to putative hypersensitivity-related (Hsr) protein | ||
| AM11 | X | Hypothetical protein with similarity to nucleoid DNA binding protein cnd41 | ||
| AM14 | X | Serine-threonine kinase like | ||
| AM15 | X | Contains peptidoglycan bonding LysM domain 2 | ||
| AM20 | X | Similar to AB-hydrolase associated lipase region | ||
| AM24 | X | Putative cDNA | ||
| AM25 | X | Putative MIP aquaporin, nodulin 26-like | ||
| AM26 | X | Serine-threonine kinase, calcium dependent (EF hand) | ||
| AM29 | X | Similar to Ring-H2 zinc finger protein-like | ||
| PT11 | X | PT11 phosphate transporter | ||
Given that we know genetic exchange in AMF can alter colonization levels in rice and that genetic changes in AMF, through segregation, also can have an effect on the transcription of symbiosis-specific genes, it is pertinent to investigate whether genetic exchange in AMF affects the transcription of symbiosis-specific genes. In particular, we asked whether any observed transcriptional changes in rice are affected by genetic exchange in AMF during the first weeks of the establishment of the symbiosis, whether transcriptional changes are related to colonization levels and plant growth, and whether they are the same genes as those altered by segregation in AMF. Based on previous studies (2, 3), we hypothesized that rice exhibits different levels of symbiosis-specific gene transcription by being inoculated with crossed AMF lines compared to being inoculated with parental AMF lines, and that those differences would change with the passage of time.
We chose two genetically different parental lines of G. intraradices and three AMF lines arising from crosses between the two parental lines to determine the effect of genetic exchange in AMF on mycorrhiza-specific gene transcription in rice. We also measured plant growth and fungal colonization to detect any correlations between phenotype and AM-specific transcription levels. Furthermore, we performed the experiment as a time course, making measurements at 6, 9, and 12 weeks after inoculation with AMF. The time course gave us information about the dynamics of colonization among AMF lines and also the mycorrhiza-specific plant gene transcription over time.
MATERIALS AND METHODS
Origin of the fungal lines.
Glomus intraradices lines C3 and D1 were used in this study. They originate from one agricultural field in Tänikon, Switzerland (14). These lines were isolated from two different plots, each separated by 60 m. These two lines were shown previously to be genetically distinct, to differ in their phenotypic traits, and to differentially affect plant growth (13, 14). In a previous study, isolates C3 and D1 were cocultured together to obtain crossed lines Sb, Sc2, and Sd, which exhibited biparental inheritance (4). Sc2 in the present study corresponds to Sc in the study of Croll et al. (4). Parental and crossed lines were cultivated in standard conditions with root-inducing transferred DNA (Ri T-DNA)-transformed carrot roots prior to inoculation on plants (4). All crossed lines were characterized using amplified fragment length polymorphism (AFLP) by Croll et al. (5). Subsequently, the lines were characterized again after several rounds of subculturing, and the crossed lines displayed the same AFLP patterns (2). In this study, a relatively large amount of root and fungal material is transferred in one subculturing round. This ensures that there is no genetic divergence, as is the case when only one spore is taken to start a new culture. The genotyping of the crossed lines are available in Croll et al. (5) and Angelard (1).
Plant growth conditions, inoculation with AMF, and harvesting.
The substrate for growing plants was composed of 60% sand and 40% loam and was autoclaved twice at 120°C and left open to the air for 2 weeks to oxidize any toxic compounds. The seeds of rice cv. Nipponbar (kindly supplied by Uta Patzowski's group, University of Lausanne) were surface sterilized in 2% NaClO for 10 min, washed repeatedly in sterile water, and placed on filter paper for germination. Eight days after germination, seedlings were put on trays with moist vermiculite and were watered for another 5 days. AMF spores were extracted from 4-month-old cultures using the same method as those of Koch et al. (13). Each pot was filled with 150 ml of soil, and one rice seedling was planted in each pot. Each plant was inoculated with 500 spores of one of the AMF crossed or parental lines. Spores were suspended in water in a tube. The tube was shaken, and then the number of spores per ml was calculated under the binocular microscope. This allowed us to calculate how many ml of suspension would contain 500 spores. Some plants were not inoculated and served as the nonmycorrhizal control. Five AMF lines were used, plus an uninoculated plant treatment with 12 replicates for each AMF treatment and the uninoculated treatment for four sequential harvests, giving a total of 288 pots (6 treatments times 4 harvests times 12 replicates per treatment per harvest). This means that at each harvest, 12 plants of each AMF treatment and 12 uninoculated plants were harvested. Plants were grown in a phytochamber with 60% humidity, 12 h light/12 h dark, and 26°C during the day and 22°C at night. Plants were watered three times a week with 10 ml of tap water. After 3 weeks, 10 ml fertilizer without phosphorus (mixture of 1 g sequestrene [Syngenta] and 0.5 g Hauert-Flovy type A [Hauert] in 1 liter of water) was given twice a week. Twelve plants inoculated with each AMF line and the uninoculated plants were harvested from the phytochamber every 3 weeks for a period of 12 weeks, resulting in four harvest times, at 3, 6, 9, and 12 weeks after inoculation. The shoots of each plant were harvested, dried at 80°C for 2 days, and weighed. The roots were immediately washed and separated into two parts. One-third was stored in 50% ethanol to measure fungal colonization. The other two-thirds were frozen in liquid nitrogen for subsequent RNA extraction and stored at −80°C. No plant died during the experiment. No AMF colonization could be detected in 88 plants that had been inoculated with AMF. Forty-seven of these 88 plants were harvested at 3 weeks and had not yet become colonized. There was no bias regarding the treatment group to which these plants belonged. In subsequent weeks, the number of plants that were not colonized (and that had been inoculated) were 22, 3, and 16 at 6, 9, and 12 weeks, respectively. This left 200 individuals that were used for further analyses.
Measurement of fungal colonization of roots.
Root samples of each plant were stained with trypan blue as described by Munkvold et al. (17). Fungal colonization (i.e., vesicular, arbuscular and hyphal colonization) was measured by following the method of Mcgonigle et al. (16). Fungal colonization, as defined in Mcgonigle et al. (16), corresponds to the percentage of arbuscules plus the percentage of vesicles plus the percentage of hyphae only (without vesicles or arbuscules). Because the amount of roots was too low at 3 weeks after inoculation, we only checked for the presence or absence of the fungi inside roots, but the percentage of root length colonized by AMF was not measured. All of the nonmycorrhizal (NM) plants were checked and found to be AMF free.
RNA extraction and quantitative PCR.
We chose to estimate gene transcription by quantitative PCR, which actually measures the levels of mRNA transcript numbers in a tissue at a given time. RNA extractions were performed directly on frozen roots. Roots of plants harvested at 3 and 6 weeks after inoculation were pooled (among samples of the same AMF treatment and from the same time point) because not enough material was available per plant. Roots were ground with a pestle in liquid nitrogen. We used the RNeasy plant minikit (Qiagen Inc.) to extract RNA by following the manufacturer's recommendations. Precipitation with LiCl also was performed (Ambion). DNase treatment was performed with DNase I grad (Invitrogen). A first PCR on RNA was performed to amplify the housekeeping genes (cyclophilin and ubiquitin), to be sure that there was no DNA contamination, with the following conditions: 94°C for 5 min; 35 cycles of 94°C for 20 s, 58°C for 20 s, and72°C for 50 s; followed by 72°C for 7 min. Reverse transcription with Superscript III enzyme (Invitrogen) with poly(dT) primer was performed by following the instructions of the manufacturer. Each sample was diluted five times. Quantitative PCRs were performed on 12 genes (see Table S1 in the supplemental material for primer sequences) that are known to be upregulated during the first weeks of colonization. Quantitative PCR was performed on all AMF treatments as well as the uninoculated treatment. The uninoculated treatment always showed no amplification compared to that of a water control and was, therefore, removed from any statistical analysis. Quantitative PCR conditions and the mix for each tube were identical to those described by Gutjahr et al. (7). These genes had been found with Affymetrix microarray screening (6) and previously checked by quantitative PCR (7), except for the OsPt11 gene, which was characterized already (18). Power Sybr green was used as the label (Applied Biosystems) for quantitative PCR, and each sample was run in triplicate. Quantitative PCR was performed on a Prism AB7900 thermocycler. Cyclophilin and ubiquitin transcript levels were used to normalize the data. Transcription values were calculated with SDS2.2.1 software (AB) and qBASE 1.3.5 software (8).
Statistical analyses. (i) Plant growth.
To meet the requirements of the statistical tests, Box-Cox transformations were used when needed so that data displayed normal distributions. To test whether the overall growth of NM plants differed from that of AMF-inoculated plants, we ran a two-way analysis of variance (ANOVA) with the six treatments and time as factors. We found a significant effect AMF inoculation on plant growth (F5,173 = 61.21; P = 0.013). The mean weight of mycorrhizal plants was higher during the whole period of the experiment than that of nonmyorrhizal plants, but the differences were not significant. Plants inoculated with crossed line Sd grew significantly larger than nonmycorrhizal plants irrespective of time. The NM treatment was removed, because it could bias the results of the statistical analyses used to identify whether growth differences existed among plants inoculated with different AMF. We performed a crossed two-way ANOVA with harvest time and AMF line as fixed factors.
(ii) Fungal colonization.
The effect of the harvest time and of the AMF treatment on fungal colonization also was tested in a crossed two-way ANOVA with harvest time and AMF line as fixed factors. One-way ANOVAs then were performed for each harvest time separately. When ANOVAs were significant, we carried out a multiple comparison test using a Tukey-Kramer honestly significant difference (HSD) test with an alpha level of 0.05.
(iii) Plant gene transcription.
Analyses of transcription levels were performed for each gene separately. The effects of the harvest time and of the AMF treatment on rice gene transcription were tested in crossed two-way ANOVAs with harvest and AMF treatment as fixed factors. One-way ANOVAs then were performed for each harvest separately, and when ANOVAs were significant we carried out a multiple comparison test using the Tukey-Kramer HSD test with an alpha level of 0.05.
All analyses were performed with the statistical program JMP, version 5.0 (SAS Institute Inc., Cary, NC).
RESULTS
Shoot dry weight and fungal colonization.
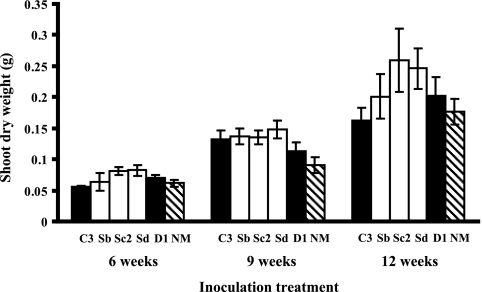
Plant shoot dry weight significantly differed among rice inoculated with different AMF lines, with the lowest values obtained mostly for plants inoculated with the two parental lines and the highest values obtained for plants inoculated with the crossed line Sc2 or Sd (Fig. 1 and Table 2). The significant difference among shoot dry weights of plants inoculated with parental and crossed lines occurred throughout the experiment (Fig. 1 and Table 2). There was no significant interaction between AMF line and harvest time on shoot dry weight (Fig. 1 and Table 2).
Fig. 1.
Plant shoot dry weight (g) of Oryza sativa at 6, 9, and 12 weeks following inoculation. Black bars represent the growth of plants inoculated with parental lines of AMF Glomus intraradices, white bars represent the growth of plants inoculated with crossed lines resulting from a cross between C3 and D1 lines, and hatched bars represent the growth of the uninoculated treatment. Errors bars indicate ± 1 SE. The following numbers of samples of each strain were taken at 6 weeks: NM, 12; C3, 8; D1, 11; Sb, 6; Sc2, 8; and Sd, 5. The following numbers of samples were taken at 9 weeks: NM, 12; C3: 11; D1, 12; Sb, 12; Sc2, 12; and Sd, 10. The following numbers of samples were taken at 12 weeks: NM, 12; C3, 7; D1, 9; Sb, 10; Sc2, 8; and Sd, 8.
Table 2.
Analysis of variance on plant shoot dry weight and fungal colonization
| Source | dfa | SS | F | Pc |
|---|---|---|---|---|
| Plant shoot dry wt | ||||
| Harvest time | 2 | 0.32 | 66.55 | <0.0001*** |
| AMF line | 4 | 0.03 | 2.86 | 0.027* |
| Harvest time × AMF line | 8 | 0.02 | 0.83 | 0.58 |
| Fungal colonization | ||||
| Harvest time | 2 | 5.71 | 94.68 | <0.0001*** |
| AMF line | 4 | 2.53 | 20.96 | <0.0001*** |
| Harvest time × AMF line | 8 | 0.61 | 2.54 | 0.014* |
df, degrees of freedom.
SS, sum of squares.
*, P ≤ 0.05; ***, P ≤ 0.0001.
Genetic exchange significantly altered the AMF colonization levels inside rice roots (Fig. 2 and Table 2). Differences in colonization levels were large. Overall, the crossed lines Sb and Sd exhibited lower colonization levels than the other crossed line (Sc2) and the two parental lines (Fig. 2 and Table 2). Interestingly, the differences in the fungal colonization among AMF lines were not the same with the passage of time (shown by a significant line by harvest time interaction in Table 2). For example, at 6 weeks after inoculation, parental line D1 showed the highest hyphal colonization, while at 9 and 12 weeks after inoculation no differences were found between the two parental lines and the crossed line Sc2 (Fig. 2).
Fig. 2.
Mycorrhizal fungal colonization (percent root length colonized) in the roots of O. sativa at 6, 9, and 12 weeks after inoculation. Black bars represent colonization by parental lines and white bars by crossed lines, originating from crosses between C3 and D1. Error bars indicate ± 1 standard error (SE). Bars topped by different letters within each harvest are significantly different at P ≤ 0.05. The following numbers of samples were taken at 6 weeks: NM, 12; C3, 8; D1, 11; Sb, 6; Sc2, 8; and Sd, 5. The following numbers of samples were taken at 9 weeks: NM, 12, C3, 11; D1, 12; Sb, 12; Sc2, 12; and Sd, 10. The following numbers of samples were taken at 12 weeks: NM, 12, C3, 7; D1, 9; Sb, 10; Sc2, 8; and Sd, 8.
Mycorrhiza-specific gene transcription.
Differences in plant gene transcription among AMF lines was studied for each harvest time separately. Results showed that genetic exchange in AMF had an effect on mycorrhiza-specific plant gene transcription in four genes (Fig. 3 and Table 3). In the other eight genes, no significant differences in transcription were detected. We found significant differences in the transcription of AM1 and AM3 in rice inoculated with different AMF lines 6 weeks following inoculation. Differences in transcription occurred between plants inoculated with parental and crossed lines and between plants inoculated with different crossed lines (Fig. 3a and b). There was also a significant difference in the transcription of AM10 and AM14 at 12 weeks after inoculation between rice inoculated with parental lines and rice inoculated with parental and some crossed lines (Fig. 3c and d and Table 3).
Fig. 3.
Effect of genetic exchange in AMF on transcription of AM1 (a), AM3 (b), AM10 (c), and AM14 (d) genes in the roots of O. sativa. The relative transcription is measured as the relative fluorescence, where the fluorescence values have been standardized relative to the transcription of the housekeeping genes ubiquitin and cyclophilin. Black bars represent the relative transcription of genes when plants were inoculated with parental lines C3 and D1. White bars represent the relative transcription of genes when plants were inoculated with crossed lines Sb, Sc2, and Sd, originating from crosses between C3 and D1 lines. Error bars indicate ± 1 SE. Bars topped by different letters are significantly different at P ≤ 0.05. Plant root samples were pooled to obtain three replicates for each treatment (n = 3).
Table 3.
Analysis of variance on plant gene transcription at 6 and 12 weeks after inoculation for the genes AM1, AM3, AM10, and AM14
| Source and wk after inoculation | dfa | SSb | F | Pc |
|---|---|---|---|---|
| 6 | ||||
| AM1 | 4 | 72.36 | 4.90 | 0.027* |
| AM3 | 4 | 81.25 | 4.00 | 0.045* |
| 12 | ||||
| AM10 | 4 | 4,338,952.1 | 4.78 | 0.024* |
| AM14 | 4 | 674.01 | 3.74 | 0.047* |
df, degrees of freedom.
SS, sum of squares.
*, P ≤ 0.05.
Correlations between gene transcription, fungal colonization, and plant growth.
We found significant positive correlations between gene transcription and fungal colonization at 6 weeks after inoculation for the AM1 (r = 0.90; P = 0.003), AM14 (r = 0.94; P = 0.003), and Pt11 (r = 0.90; P = 0.003) genes. AM11 gene transcription was negatively correlated with shoot dry weight (r = −0.88; P = 0.048) but positively correlated with the arbuscular colonization (r = 0.88; P = 0.049) at 9 weeks after inoculation. Another negative correlation was found between the transcription of the AM25 gene and hyphal colonization (r = −0.889; P = 0.0434). All other genes (except AM29) showed the same trend as that of AM25 at 12 weeks after inoculation, but correlations were not significant. We also found a positive correlation between arbuscular colonization and shoot dry weight (r = 0.399; P = 0.0074) at 12 weeks after inoculation.
DISCUSSION
Here, we showed that genetic exchange in the AMF Glomus intraradices alters rice growth and gene transcription. Gutjahr et al. (7) characterized genes that are transcribed only in rice roots during the establishment of mycorrhizal symbiosis. We used 12 of these genes and measured their transcription at 3, 6, 9, and 12 weeks after inoculation with two genetically different AMF parental lines and three AMF crossed lines arising from genetic exchange between the parental lines. Our results support our hypothesis that genetic exchange in AMF alters the transcription of some of these genes compared to transcription in plants inoculated with parental lines. We also demonstrated that genetic exchange in AMF can alter the speed of AMF colonization and also increase the growth of rice. Finally, we found that some, but not all, of the transcriptional and growth changes were correlated with colonization.
Effect of genetic exchange on rice growth and AMF colonization.
Angelard (1) showed that segregation can improve rice growth by a factor five. She also tested the effect of genetic exchange in AMF on rice growth. She found that genetic exchange in AMF did not improve plant growth. Indeed, she observed a better growth of uninoculated plants and plants inoculated with parental lines than that for plants inoculated with crossed lines. Furthermore, in those experiments rice always grew significantly larger in nonmycorrhizal treatments than in any of the mycorrhizal treatments. We used some of the same crossed lines as those used in a previous study (2). In contrast, we found an overall positive growth effect of the AMF lines on rice with the passage of time, with some crossed lines inducing an even higher plant growth than the parental lines throughout the experiment. We also found that crossed and parental lines did not colonize rice roots in a similar way over time. Angelard et al. (2) also measured the fungal colonization inside rice roots at 12 weeks. They found that the crossed lines Sb and Sc2 exhibited a lower level of colonization in rice than the other AMF lines, while we found that the crossed line Sd exhibited the lowest level of colonization. The environmental conditions were markedly different between the experiments of Angelard (1) and our experiment. This could explain the different results on plant growth and fungal colonization. Our experiment was performed in a phytochamber with a strictly controlled environment, and the experiment of Angelard (1) was performed in the greenhouse, where the environment could have varied greatly during the experiment in terms of light intensity, humidity, and temperature. Furthermore, the pot size in this experiment was much smaller than that used by Angelard (1). The contrasting results obtained from the two experiments showed that the effects of AMF crossed lines on plants likely are dependent upon the environment. It also shows the large potential range of variation in the effects of an AMF, and this should be taken into account for further laboratory or field experiments.
Plant gene expression.
Symbiosis-specific rice genes were found after an extensive study in 2005 (6). Two hundred twenty-four genes were shown to be transcribed only when the mycorrhizal fungus Glomus intraradices was present in the soil. A recent study characterized 18 of these genes (7) and measured their transcription at 3, 5, 7, and 9 weeks after inoculation. Some of them played a role in early transcription (less than 7 weeks after inoculation), and others were characterized as lately transcribed (at least 7 weeks after inoculation). Angelard (1) looked at four of these genes (two early [AM1 and AM3] and two late transcribed genes [AM14 and Pt11]) and showed that segregation in AMF had an effect on rice AM-specific gene transcription. In our study, we used the same four genes plus eight other AM-specific genes (see Table S1 in the supplemental material) identified by Gutjahr et al. (7). They are classified as three early genes and nine late genes. We found a significant effect of AMF lines on the transcription of four genes: AM1, AM3, AM10, and AM14 (Fig. 3 and Table 3). Interestingly, the time at which we found significant effects corresponds to whether the genes were characterized as early or lately transcribed. Only putative functions are known for these genes (see Table S1). AM1 is a putative peroxidase, AM3 contains a peptidoglycan binding LysM domain, AM14 is a serine-threonine kinase-like gene, and AM10 is similar to hypersensitivity-related (Hsr) protein. Angelard (1) found that segregation has significant effects on AM1, AM3, AM14, and Pt11. It is interesting that we also found significant effects for three of these genes. Even if the functions of these genes in symbiosis are not known, we show here that the transcription of those genes is altered by the genetic identity of the fungus following genetic exchange between two genetically distinct AMF.
We showed that symbiosis-specific gene expression was positively correlated with fungal colonization for some of the genes but not all. In our experiments, genetic exchange in AMF had strong effects on colonization rates. It therefore could have been possible that the changes in symbiosis-specific gene expression simply reflect the speed of a given crossed line to colonize rice roots. However, the lack of significant correlation between the transcription of some of the affected genes and colonization levels suggests that the effect on transcription is not so simple.
Conclusions.
In view of the results of our study, further experiments using microarrays or high-throughput sequencing approaches are highly warranted, as they could give a better understanding of how genetic changes in mycorrhizal fungi affect patterns of rice gene transcription over the whole transcriptome. We see this avenue of research of investigating AMF effects on rice as being particularly important given the importance of rice and the current trends in rice cultivation. Most rice cultivation globally occurs in flooded conditions, where AMF are unlikely to easily colonize the plant or provide nutrient benefits. However, there is a strong pressure on rice farmers to adopt a different method of rice cultivation involving much drier conditions, known as the system of rice intensification (SRI). In SRI, rice is not grown in standing water but under conditions that are much more favorable to the development of mycorrhizal symbiosis.
Angelard (1) already showed that segregation in the AMF Glomus intraradices can yield a 5-fold improvement in the growth of rice. Our study shows, for the first time, that genetic exchange in AMF also can have beneficial effects on rice growth. Rice is probably the most important cereal on Earth. It feeds billions of people every day. Improving rice productivity is a priority given the growth of the world population. Consequently, these recent advances in our knowledge of the contribution of AMF genetics to plant productivity could be used for improving crop productivity by manipulating the fungus genetically by using its own natural genetic processes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants 31000AO-105790/1 and 31003A-127371 from the Swiss National Science Foundation.
We thank C. Gutjahr for assistance in the quantification of gene transcripts in rice, U. Paszkowski for providing rice seeds, J. Bonvin for assistance with fungal inoculation, Daniel Croll for providing the crossed lines, and the rest of our group for their useful comments on the manuscript. We also thank the Genomic Technology Facility of the University of Lausanne for assistance with the quantitative PCR.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 22 July 2011.
REFERENCES
- 1. Angelard C. 2010. Effect of genetic alteration in arbuscular mycorrhizal fungi on plant growth and on their response to a change of host. Ph.D. thesis Lausanne University, Lausanne, Switzerland [Google Scholar]
- 2. Angelard C., Colard A., Niculita-Hirzel H., Croll D., Sanders I. R. 2010. Segregation in a mycorrhizal fungus alters rice growth and symbiosis-specific gene transcription. Curr. Biol. 20: 1216–1221 [DOI] [PubMed] [Google Scholar]
- 3. Angelard C., Sanders I. R. 2011. Effect of segregation and genetic exchange on arbuscular mycorrhizal fungi in colonization of roots. New Phytol. 189: 652–657 [DOI] [PubMed] [Google Scholar]
- 4. Becard G., Fortin J. A. 1988. Early events of vesicular arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytol. 108: 211–218 [DOI] [PubMed] [Google Scholar]
- 5. Croll D., et al. 2009. Nonself vegetative fusion and genetic exchange in the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 181: 924–937 [DOI] [PubMed] [Google Scholar]
- 6. Güimil S., et al. 2005. Comparative transcriptomics of rice reveals an ancient pattern of response to microbial colonization. Proc. Natl. Acad. Sci. U. S. A. 102: 8066–8070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gutjahr C., et al. 2008. Arbuscular mycorrhiza-specific signaling in rice transcends the common symbiosis signaling pathway. Plant Cell 20: 2989–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hellemans J., Mortier G., De Paepe A., Speleman F., Vandesompele J. 2007. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8: R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hijri M., Sanders I. R. 2005. Low gene copy number shows that arbuscular mycorrhizal fungi inherit genetically different nuclei. Nature 433: 160–163 [DOI] [PubMed] [Google Scholar]
- 10. Jakobsen I., Rosendahl L. 1990. N2 fixation and root respiration in pea—effects of AM and P. Agric. Ecosyst. Environ. 29: 205–209 [Google Scholar]
- 11. Judson O. P., Normark B. B. 1996. Ancient asexual scandals. Trends Ecol. Evol. 11: A41–A46 [DOI] [PubMed] [Google Scholar]
- 12. Klironomos J. N., McCune J., Hart M., Neville J. 2000. The influence of arbuscular mycorrhizae on the relationship between plant diversity and productivity. Ecol. Lett. 3: 137–141 [Google Scholar]
- 13. Koch A. M., Croll D., Sanders I. R. 2006. Genetic variability in a population of arbuscular mycorrhizal fungi causes variation in plant growth. Ecol. Lett. 9: 103–110 [DOI] [PubMed] [Google Scholar]
- 14. Koch A. M., et al. 2004. High genetic variability and low local diversity in a population of arbuscular mycorrhizal fungi. Proc. Natl. Acad. Sci. U. S. A. 101: 2369–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuhn G., Hijri M., Sanders I. R. 2001. Evidence for the evolution of multiple genomes in arbuscular mycorrhizal fungi. Nature 414: 745–748 [DOI] [PubMed] [Google Scholar]
- 16. Mcgonigle T. P., Miller M. H., Evans D. G., Fairchild G. L., Swan J. A. 1990. A New method which gives an objective-measure of colonization of roots by vesicular arbuscular mycorrhizal fungi. New Phytol. 115: 495–501 [DOI] [PubMed] [Google Scholar]
- 17. Munkvold L., Kjoller R., Vestberg M., Rosendahl S., Jakobsen I. 2004. High functional diversity within species of arbuscular mycorrhizal fungi. New Phytol. 164: 357–364 [DOI] [PubMed] [Google Scholar]
- 18. Paszkowski U., Kroken S., Roux C., Briggs S. P. 2002. Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. U. S. A. 99: 13324–13329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith S. E., Read D. J. 2008. Mycorrhizal symbiosis. Academic Press, London, United Kingdom [Google Scholar]
- 20. van der Heijden M. G. A., Boller T., Wiemken A., Sanders I. R. 1998. Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology 79: 2082–2091 [Google Scholar]
- 21. van der Heijden M. G. A., et al. 1998. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396: 69–72 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.