Abstract
In order to investigate the 3D structure of the collagen fibrils in articular cartilage, full thickness canine humeral cartilage was microtomed into perpendicular sections that included both the articular surface and the subchondral bone and approximately 100 successive parallel sections that were each 6 μm thick and from a different cartilage depth. Each section was imaged using polarized light microscopy (PLM) with a 5x objective (2.0μm pixel size), generating two quantitative images (angle and retardation). Selected sections were also imaged using a 40x objective (0.25μm pixel size). At an increased depth from the articular surface, the angle and retardation results in the perpendicular sections showed the well-known 90° change in fibril orientation between the surface and deep cartilage. In contrast, the retardation results of the parallel sections decreased from the articular surface and remained approximately zero through most of the radial zone, while the angle results of the parallel sections only changed about 30°. The territorial matrix morphology surrounding 61 chondrocyte clusters was quantified by its length, aspect ratio, and orientation. The cellular clusters in the surface cartilage were ellipsoidal in both the parallel and perpendicular sections. In the radial zone, the cellular clusters were oriented in vertical columns in the perpendicular sections and as circular groupings in the parallel sections. This orthogonal imaging technique could provide a better understanding of the 3D territorial and interterritorial fibrils in articular cartilage, the disturbance of which could signify the onset of degenerative cartilage diseases such as osteoarthritis.
Keywords: articular cartilage, PLM, birefringence, parallel sections, collagen structure, chondrocyte cluster
Introduction
The extracellular matrix of articular cartilage contains a network of type II collagen fibrils organized into a depth-dependent morphology that includes three consecutive, non-calcified zones: the superficial zone (SZ), the transitional zone (TZ), and the radial zone (RZ). The orientation of the collagen fibrils is approximately parallel with the articular surface in the SZ, random in the TZ, and perpendicular to the articular surface in the RZ [1–3] (Fig. 1a). This depth-dependent organization of the collagen matrix helps maintain the structural integrity of cartilage by withstanding the swelling pressures of negatively charged proteoglycan macromolecules and the deformations of cartilage due to joint loading and motion. Minor disruptions to this fibrillar morphology may be one of the leading causes of pathological diseases, such as osteoarthritis, in cartilage [4–7]. The fibril schematic in Fig. 1a represents the interterritorial matrix that is located far from the proximity of the sparsely distributed cells (chondrocytes). Immediately outside each chondrocyte, there is a narrow region of pericellular matrix, which is further surrounded by a cocoon shaped territorial matrix of fine collagen fibrils [8–10]. If the shape of the cell or cell cluster is not round (spherical), then its long axis normally follows the local orientation of the interterritorial fibrils [10].
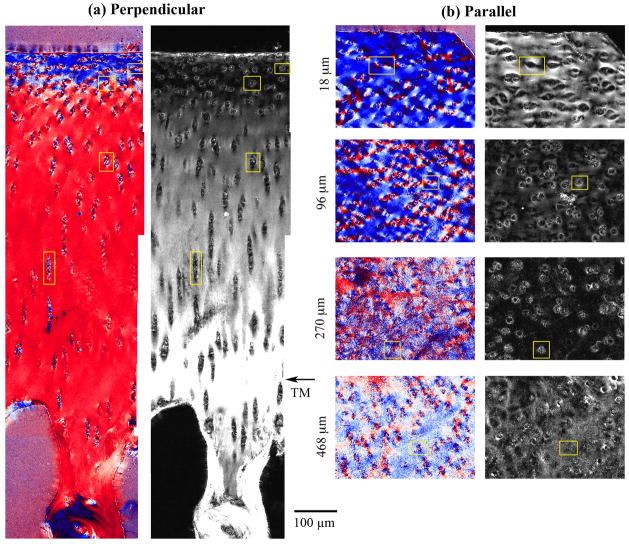
Figure 1.
(a) A schematic representation of the perpendicular (full thickness) and parallel sections from a block of articular cartilage. The short lines illustrate the orientation of collagen fibrils in three histological zones. The horizontal lines on the right face of the block illustrate the parallel sections. (b) The retardation and angle images of a perpendicular section using a 5x objective. Two arrows point to the tidemark line in the cartilage. (c) The retardation and angle images of four parallel sections using a 5x objective at cartilage depths of 18μm (SZ), 96μm (TZ), 270μm (upper RZ) and 468μm (lower RZ). The yellow boxes in the images show the regions where the cartilage was imaged using a 40x objective. All histograms refer to the angle images. The left histogram in (b) is from the first 182μm of the cartilage depth, where the double peaks represent the orientations of the superficial fibrils (centered at 0°) and the radial fibrils (centered at 90°), respectively; the right histogram in (b) is from the remaining deep cartilage, representing only the radial zone. All images are plotted using the same upper and lower limits as shown alongside the scales in (c). (A.S. is articular surface)
Various microscopic imaging techniques have been used to investigate the depth-dependent structure of the collagen network in articular cartilage [11–27]. Among these imaging tools, a computerized form of polarized light microscopy (PLM) has been used [19] in determining quantitative birefringent properties of tissues [18, 27, 28]. Birefringence refers to the differentiated propagations of polarized light when passing through certain materials that have at least two different indices of refraction, resulting in two possible speeds of light transmission in the material [27]. For any pixel, two physical parameters of the specimen can be quantified by this type of 2D imaging technique: angle (in units of degrees) and retardation (in units of nm). The angle value represents the averaged orientations of all collagen fibrils in each image voxel (volume of element in imaging). In articular cartilage, the fibrils should have a nominal 90° difference in the orientations between the superficial zone and the radial zone [19]. The retardation value characterizes the coherence of the fibril organization within individual pixels, i.e., fibril orientations relative to each other in each image voxel. A voxel of uniformly oriented fibrils gives the highest retardation value while randomly oriented fibrils result in the smallest retardation value. It is important to note that in addition to the randomness of the collagen fibrils, the retardation value can also be influenced, in practice, by several other experimental factors, including the fibril diameter, the packing density of the fibrils, and the thickness of the histological section. However, the retardation values from the same tissue section and from identically prepared sections are directly comparable. Previous work shows that the pixel-by-pixel mapping of angle and retardation allows investigations into the disrupted collagen morphology due to early onset lesions [7] and deformation of loaded articular cartilage [26, 29].
Light microscopic imaging of articular cartilage commonly uses thin sections of cartilage that contain the full cartilage thickness from the articular surface to the subchondral bone (termed as the perpendicular sections in this report) [19]. This sectioning and imaging approach allows all structural zones to be imaged together in one experiment. Alternatively, articular cartilage can also be studied using thin sections cut parallel to the articular surface (termed as the parallel sections in this report), i.e., each parallel section is cut from a particular depth in cartilage [30–38]. The goal of this investigation is to map the cartilage anisotropies and cellular morphology by imaging cartilage cut from two orthogonal planes – perpendicular sections and a series of sequentially numbered parallel sections through the entire cartilage depth. We aim to provide new insight into the 3D structural arrangement of collagen fibrils and cellular clusters in articular cartilage.
Materials and Methods
Specimen Preparation
Canine articular cartilage from the central load-bearing region of three healthy and skeletally mature humeral heads was cut into three osteochondral blocks (one block from each joint). The animals had been sacrificed for an unrelated scientific study. The cartilage blocks (~ 1.75mm × 1.75mm × 2mm) were sectioned in two orthogonal directions: perpendicular and parallel to the articular surface (Fig. 1a). Standard paraffin embedding and processing protocols [19] were used in the tissue processing. Briefly, the tissue blocks were fixed in 4% formol-cetylpyridinium chloride, decalcified in 10% EDTA, washed in tap water, and dehydrated in alcohol. The specimens were then infiltrated, embedded in paraffin, and sectioned into 6μm thickness with a microtome (Microm GmbH, Walldorf, Germany). After drying thoroughly, the sections were cleared in three changes of xylene. Each perpendicular section contained the full thickness of cartilage, from the articular surface to the subchondral bone. At least three perpendicular sections were collected from each tissue block. The same cartilage block was then re-embedded in paraffin with a 90° rotation. By microtoming the cartilage block in sections parallel with the articular surface, each parallel section contained cartilage at a particular depth. Approximately 100 parallel sections were obtained from each block and numbered sequentially. One corner of each block was trimmed prior to microtoming to ensure identical orientations among all the parallel sections when imaged.
To minimize the influence of staining chemicals on the birefringent properties of the tissue [39], the sections were not stained. To facilitate the imaging of the same section by both visible light in PLM and infrared light in Fourier-transform infrared imaging (the Fourier-transform infrared imaging results of this project are reported elsewhere [38]), the cartilage sections were placed directly on the MirrIR slides (a specially coated microscope slide from Kevley Technologies, Chesterland, Ohio) without a cover glass. A number of additional cartilage sections from several neighboring blocks were covered with the usual mounting media and cover glass, and were also imaged in PLM as quantitative comparisons [19]. Data obtained from the covered slides were not used in this project.
PLM
The PLM system consisted of a digital imaging attachment (Cambridge Research & Instrumentation Inc, Woburn, MA) mounted on a polarized light microscope (Leica, Wetzlar, Germany). A circular polarizer was used with a liquid crystal compensator, which allowed quantitative calculations of the birefringent properties of the tissue. Based on a series of intensity images1 that each was acquired under a different polarization, this quantitative method calculated two 2D images (angle and retardation) for each cartilage section [19, 40]. Each of the 100 cartilage sections from each block were imaged using a 5x objective (hence resulting in a total magnification of 50 in the microscope system), which has a pixel size of 2.0μm × 2.0μm. Twenty of the sections (at least three consecutive sections in each cartilage zone) were also imaged using a 40x objective (0.25μm × 0.25μm pixel size), which allowed closer inspection of the territorial matrix surrounding the chondrocyte clusters. The pixel dimensions in each objective setting were calibrated using a 10μm stage-micrometer (SWIFT Cat. # MA663, No.10). The parallel sections were monitored with a reference mark on the microscope stage to ensure identical imaging orientation of the cartilage sections. This allowed for direct comparison of angle and retardation values among every section.
Data Analysis
The calculations of the depth-dependent angle and retardation values and their standard deviations from the perpendicular sections used a region of interest (ROI) with a horizontal width of 50μm at each depth (a row of 25 pixels at the 5x objective). Since the 25-pixel average was done at each pixel depth, the pixel resolution along the cartilage depth was still 2.0μm. The angle data were fitted with a hyperbolic tangent function that represented a 90° transition of the collagen fibrils between the SZ and RZ [19]. The retardation data were fitted with a polynomial to illustrate the depth-dependent trends. The calculations of angle and retardation values from the parallel sections used a square ROI of 50μm × 50μm at each depth.
Using a 40x objective, the chondrocytes and their surrounding territorial and interterritorial matrices in each zone of articular cartilage were examined. The shape of each chondrocyte cluster was quantified by its aspect ratio (the ratio of the long axis a by the short axis b), where the ratio can have a minimum value of 1 (representing a circle) or larger values (representing an ellipse). The long axis of each cluster was measured by an angle θ from −90° to 90° to represent the orientation of a chondrocyte cluster, with the articular surface of the specimen as the reference direction.
Results
Each of the three cartilage blocks had at least three full-thickness perpendicular sections and approximately 100 parallel sections, which covered the entire non-calcified cartilage. The full thicknesses of the non-calcified cartilage were measured from the perpendicular sections as 681.0±27.7μm (4 specimens from block 1), 707.0±38.9μm (4 specimens from block 2) and 607.3±93.8μm (3 specimens from block 3). These thicknesses agreed with an earlier measurement of very similar canine humeral cartilage [19]. Since the imaging data obtained from different blocks were highly consistent, all data in this report came from one representative specimen. For the perpendicular sections (Fig. 1b), the image features were highly consistent with the previous studies [19, 40, 41]. For example, the minimum retardation of the cartilage section was averaged at 79.3±12.4μm below the articular surface, marking the most random location of cartilage in terms of its fibril organization. The maximum retardation of the cartilage section was observed around the tidemark (marked in Fig. 1b), which separated the non-calcified and calcified cartilage. Note that the tidemark could be identified in both visible and intensity images [19], but not in the angle and retardation images. This fact suggested that there was little change in the fibril orientation and fibril organization just above and below the tidemark. The angle image of the perpendicular sections showed an approximate 90° change in the fibril orientation between the SZ and RZ cartilage, corresponding to the parallel and perpendicularly oriented fibrils in these two zones, respectively. The 0° of the angle reference was the articular surface, evidenced by the color of the angle images in Fig. 1.
For the parallel sections (Fig. 1c), the first five sections contained partial cartilage sections (e.g., the 18μm slice in Fig. 1c) reflecting either minor non-orthogonality or surface curvature of the cartilage block. The retardation and angle images of all the parallel sections clearly lacked the complex features observed in their perpendicular companions. Instead of the double peaks in the angle histogram of the upper part of the perpendicular sections (the left histogram in Fig. 1b), the angle histograms of the parallel sections consistently exhibited a single peak, reflecting a uniform average orientation of the fibrils. The peak position of the angle histograms shifted away from the 0° reference location with sections of increasing depths but never reached the 90° orientation in the RZ cartilage as in the case of the perpendicular sections.
Note that these quantitative images contained some noise points (i.e., black dots in the retardation images and colorful dots in the angle images), more apparent in the parallel sections than in the perpendicular sections. The noise points inside the cartilage section actually represented the non-birefringent structures of the cartilage in the vicinity of the chondrocyte clusters. Since these structures were not birefringent, they had low retardation (i.e., black dots) or an arbitrary angle (i.e., colorful dots which include blue/white/red colors clustered together).
The depth-dependent profiles from the angle and retardation images of both perpendicular and parallel sections were shown in Fig. 2 (using a 5x objective). Since each perpendicular section contained all histological zones, all data points in the angle and retardation profiles of the perpendicular sections were extracted from the same cartilage section (imaged in a single experiment). In comparison, since each parallel section was located at a particular tissue depth, the images of one parallel section only contributed to one data point in the depth-dependent profiles of the parallel sections. All 100 parallel sections collectively formed the profiles of the angle and retardation in the parallel section results in this report. Since the parallel sections were imaged one section at a time, the parallel profiles contained larger variations, which were evident in Fig. 2. In contrast to the 90° change in the fibril orientation between the SZ and RZ in the perpendicular sections, the change in the fibril orientation in the parallel sections was only about 30° (Fig. 2a). In contrast to the minimum retardation of the perpendicular section occurring distinctly and consistently at a depth of approximately 80μm, the minimum retardation of the parallel sections occurred much deeper in the cartilage and extended through a large region of the cartilage depth (covering a depth from 150 to 400μm). In fact, most of the parallel sections from the RZ cartilage had retardation values close to zero nm (i.e., ~ zero birefringence).
Figure 2.

(a) Average angle profiles when a 5x objective was used. Data points are shown with their respective standard deviations and fitted with a hyperbolic tangent and a polynomial for the perpendicular and parallel sections, respectively. (b) Retardation profiles for the perpendicular and parallel sections processed identically as in (a). Both profiles are fitted using a polynomial to show depth-dependent birefringence.
To examine the angle and retardation of the territorial matrices and the cellular clusters, 20 perpendicular and parallel sections from three histological zones were imaged using a 40x objective (0.25μm pixel size). Fig. 3 shows the images of a two-cell cluster and a four-cell cluster, both extracted from one SZ parallel section. The white/black meshed-shapes in Fig. 3 represented the space that used to be occupied by the cells. Each cellular shape in Fig. 3 was determined by a visual inspection of the visible, angle and retardation images, and likely included the cell and its pericellular matrix. The retardation value was higher than in the background (i.e., interterritorial) cartilage, indicating a higher organization of the territorial matrix (i.e., the “cocoon” structure) in the area surrounding the cells [40]. Since a collagen fibril has neither head nor tail, the angle value in this type of calculation goes from 0° to 180° twice around any circle/ellipse [29, 40], causing the same color to appear on the opposite side of the circle/ellipse in any angle image of the cells in both the SZ and TZ. In the RZ, a diminished “cocoon” was seen in both perpendicular and parallel sections of cartilage. The vector illustration of the angle map, in which each line is the average orientation of two by two pixels, clearly illustrates the “cocoon” feature of the territorial matrix. A total of 61 cells were analyzed, and the measurements are summarized in Table 1. These features of the angle and retardation images were consistent in both the parallel and perpendicular sections, as well as in both uncovered and (additional) covered sections. The dimensions of the cells in this analysis (scaled in Figs. 3 and 5) are consistent with other measurements in literature [42, 43].
Figure 3.
The angle and retardation images of two chondrocyte clusters using a 40x objective. The vector maps show graphically the average orientation of collagen fibrils forming “cocoons” around the cell clusters (meshed areas).
Table 1.
Characteristics of the Cellular Clusters
| Cell Clusters | SZ | TZ | RZ | |||
|---|---|---|---|---|---|---|
| Perpendicular | Parallel | Perpendicular | Parallel | Perpendicular | Parallel | |
| Number | 5 | 10 | 6 | 5 | 6 | 29 |
| Length a (μm) | 11.93±2.57 | 22.13±3.32 | 12.22±1.20 | 13.94±1.09 | 21.35±11.39 | 14.70±3.32 |
| Orientation (°) | 6.4°±5.59° | 6.3°±19.4° | 65.5°±27.4° | 12.4°±56.2° | 90.0°±0.0° | 7.3°±47.4° |
| Aspect Ratio (a/b) | 1.43±0.13 | 1.63±0.27 | 1.26±0.18 | 1.13±0.20 | 2.12±0.99 | 1.01±0.09 |
Figure 5.
The depth-dependent angle (left) and retardation (right) images of the cell clusters using a 40x objective, for the perpendicular sections (a) and parallel sections (b) shown in Fig 4 (the yellow boxes). The yellow rings represent the approximate boundaries of the chondrocyte clusters.
Fig. 4 shows the angle and retardation images of both perpendicular and parallel sections acquired with a 40x objective. Because of a smaller field-of-view at the high magnification, the images of the perpendicular sections shown in Fig. 4a were pieced together from eight separate images using Adobe Photoshop® (San Jose, California). The constant color (i.e., constant quantitative values) between any two neighboring images in Fig. 4a demonstrated the high level of consistency of the PLM instrument and calculation protocol in this work. The images of the perpendicular sections at the high magnification contained identical features when compared to the low magnification images (Fig. 1b). Since each image of the parallel section illustrated the cartilage structure at a particular depth, the largest retardation values occurred in the SZ cartilage, indicating that these SZ sections contained many parallel fibrils in the plane of the cartilage sections. This is consistent with a recent microscopic MRI (μMRI) observation of the SZ cartilage [44]. The retardation values decreased notably from the TZ and remained near zero for most of the RZ cartilage, demonstrating that most fibrils in the RZ cartilage would be cut into mere cross sections in these parallel sections.
Figure 4.
The angle and retardation images using a 40x objective. (a) The angle (left) and retardation (right) images of a full-thickness perpendicular section. (b) The angle (left) and retardation (right) images of four parallel sections outlined in Fig 1c.
The depth-dependent trends of the cellular clusters are shown in Fig. 5, where a yellow ring indicates approximately the boundary of any cluster of chondrocytes. For the SZ and TZ cartilage, each cluster in both perpendicular and parallel sections was clearly surrounded by a well-defined territorial matrix; the angle images show the entire range of orientations twice (2 × 180°) around each cell and the retardation images show higher values in the region adjacent to the cell. In contrast, the chondrocyte cluster in the RZ cartilage had different features. From the perpendicular section, the angle values surrounding each cluster were consistent and nearly uniform; from the parallel sections, the angle values surrounding each cluster were highly inconsistent. The retardation in the RZ cartilage also lacked the higher value feature near the cellular cluster. These features indicated the lack of a well-defined territorial matrix for the cellular clusters in the RZ cartilage when viewed in the parallel plane.
Fig. 6 shows the depth-dependence of the a/b ratio and the orientation of the clusters, which is schematically interpreted in Fig. 7. In the perpendicular sections, the aspect ratio of the clusters decreased from the SZ to TZ then increased in the deep RZ, showing a change in the cluster shape from ellipsoidal in the SZ (Fig. 7a), to spherical in the TZ, and again to ellipsoidal in the RZ (Fig. 7c). When the cluster shapes change, their orientation also changes, with a characteristic 90° difference between the SZ and RZ in the perpendicular sections (Fig. 6b, which is fitted with the same hyperbolic tangent function as in Fig. 2a). For the parallel sections from the SZ and TZ, the aspect ratios of the cell clusters were consistent to these in the corresponding regions of the perpendicular sections (i.e., the xy plane is similar to the yz plane of the cluster in Fig. 7b). However, the aspect ratios of the cell clusters remained approximately unity for most of the deep cartilage (RZ) in the parallel sections. This is consistent with the understanding that most of the cells or cell clusters in the RZ are organized along the fibril bundles (Fig. 7e), thus having an approximately circular cross-section in the parallel sections (Fig. 7d). Since the cells in the RZ remain approximately circular in a cross-section, their orientations were difficult to identify, which caused the spreading of the cell angles for most RZ in Fig. 6b. (Five different chondrocytes were measured at different depths of the parallel sections until the chondrocyte became circular, thus having no identifiable direction.)
Figure 6.
(a) The ratio of the long axis (a) and the short axis (b) of the cell clusters at different depths in the perpendicular and parallel sections. The error bars of the parallel sections are the standard deviation from five clusters taken at each depth. (b) The orientation of the cell clusters at different depths in both perpendicular and parallel sections. Five different clusters of cells were analyzed at each tissue depth. Since the cells appeared circular and therefore have no consistent orientation after a depth of 300 μm in the parallel sections, there were no data points shown.
Figure 7.
The schematics of the cell clusters in SZ (a) and RZ (c) and their 2D sections (b, d, e). a and b refer to the long and short axes of the chondrocyte clusters plotted in Fig. 6.
Discussion
Based on the schematic structure of collagen fibrils in articular cartilage (Fig. 1a), the deep cartilage (RZ) in all perpendicular sections and all parallel sections from the surface portion of the cartilage (SZ) should have collagen fibrils parallel with the 2D plane of the cartilage sections (6μm thick in this work). In contrast, all parallel sections from the deep portion of the cartilage (RZ) should contain mostly the cross sections of the collagen fibrils. Since the fundamental mechanism of polarized light microscopy is birefringence [18, 27, 28], the in-plane fibrils should result in strong birefringence and any cross section of the fibril should result in negligible birefringence.
The depth-dependent structure of the interterritorial fibrils
The depth-dependent structure of collagen fibrils in the schematics of articular cartilage (Fig. 1a) represents the orientation of the interterritorial fibrils that are located far from the proximity of the chondrocytes. Previous PLM works using the perpendicular sections have demonstrated that the interterritorial fibrils of articular cartilage do follow such schematics in a depth-dependent manner [19, 40]. In this project, the same features of the interterritorial matrix in articular cartilage have again been observed in the perpendicular sections (Fig. 2).
In the SZ cartilage, the collagen fibrils in parallel planes were found to have a finite structural anisotropy, established by the motional anisotropy of water molecules in μMRI [44] and the amide anisotropy in Fourier-transform infrared imaging (FTIRI) [36, 38]. These previous assessments of the surface fibril structure by different physical mechanisms are consistent with the birefringent results in this PLM report. For example, the largest retardation in the parallel sections of cartilage actually occurred in the SZ (Fig. 2b). The retardation gradually decreased when one moves towards the deeper cartilage (Fig. 2b), reflected by the decreasing of the long fibril components in the parallel planes. The difference in the retardation values at the same cartilage depth between the perpendicular and parallel sections presented two different views of the same 3D fibril distribution in this portion of the cartilage.
Within the TZ cartilage, the results of the perpendicular sections showed that the collagen fibrils changed their orientation by approximately 90° and the retardation reached approximately zero, indicating a more random orientation of the fibrils while transitioning [19, 41, 45]. In contrast, the fibril angle in the parallel sections did not change by 90° and the minimum of the retardation kept decreasing towards the radial zone cartilage. It was interesting to note that the two retardation profiles did not have their minima coinciding at the same cartilage depth but crossed at a deeper depth of ~ 110μm, probably the most random location in the 3D fibril structure in cartilage. These differences illustrated the difference in the projections of the 3D fibril structure on two orthogonal 2D planes.
Through most of the RZ cartilage, the retardation values fluctuated near zero when viewed from the parallel plane. This represented a near random organization of the fibrils and was consistent with the FTIRI results of the same cartilage sections [38]. Because of this low retardation, any angle measurement was likely to contain large errors, indicated by the large error bars in the angle profiles of parallel sections (Fig. 2a) and the noisy images (Fig. 4b). Any residual value in these measurements could indicate the existence of secondary or tangential fibrils crossing the vertically oriented fibrils [24, 36, 38, 41, 46, 47].
The depth-dependent structure of the territorial fibrils
With a sufficient resolution (e.g., 0.25μm per pixel in this work), PLM could identify another group of collagen fibrils in articular cartilage: the territorial fibrils that form a cocoon shaped structure in the proximity surrounding each chondrocyte or each cluster of chondrocytes [29, 40]. It is clear from this work that the existence of the chondrocytes in articular cartilage altered the smooth distribution of collagen fibrils organized in the interterritorial matrix, shown by the different alterations in the angle and retardation measurements.
The orientation of the territorial matrix followed the orientation of the interterritorial fibrils. The ellipsoidal shape of the chondrocytes was shown to align in a parallel manner with the articular surface in the SZ [10, 45]. This orientation of the cocoon fibrils appeared similar on both orthogonal planes in the SZ cartilage (Fig. 7a and 7b), where the matrix surrounding the chondrocytes could be quantified identically on both perpendicular and parallel sections (Fig. 5). In the TZ cartilage, the orientation of the cocoon fibrils became poorly defined in both angle and retardation (Fig. 6), likely due to the more spherical shape of the cell clusters in this cartilage region [9]. The clusters of chondrocytes in the RZ cartilage were known to be vertically oriented in columns with a diminished territorial matrix using perpendicular sections [14] (Fig. 5), illustrated in Fig. 7.
The area between individual cells in the same cluster was also examined during the course of this project. The angle values in these intercellular spaces were either fluctuating (e.g., Fig. 3a) or consistent (e.g., Fig. 3b). When the angle values fluctuated, their corresponding retardation values were always near zero, indicating a lack of organized fibrils. When the angle values were consistent, the retardation values were finite and similar to the area immediately outside the cells, indicating the fibrils were following the circular contour. A better knowledge of the fine structure of pericellular and territorial matrices would be very useful in understanding the cellular activities and functions in cartilage, but would require imaging of thinner cartilage sections at resolutions greater than those used in this work.
A number of error sources in PLM experiments should be mentioned briefly here. The first source was the averaging effect over the section thickness, since PLM generated 2D images from the 6μm thick tissue sections in this project. Any cell that did not penetrate the entire section thickness would be difficult to identify in the images. This is a limitation in any 2D imaging approach. The true usefulnesses of this PLM technique were the ability to image native tissue (no staining), a relatively inexpensive microscope (polarized light microscope), and the ability to generate two parameter images that characterized the physical properties of the tissue (fibril orientation and fibril organization). The second error source was the artifactual influences in the calculation of the angle and retardation images. Although these quantitative images should, in principle, represent the characteristics of the interterritorial (i.e., the extracellular matrix) cartilage, the cellular and territorial structures in the tissue sections could have introduced some minor variations in the calculations, especially at low magnifications (i.e. the 50x magnification could not identify individual cells or the territorial matrix). Careful investigation in several previous projects had demonstrated that these local variations did not change the overall shapes of the depth-dependent profiles of the angle and retardation images with the appropriate averaging [19]. Finally, the surface roughness of the uncovered tissue sections in our experiments could have also caused some fluctuations in the calculations. Comparisons (data not shown) between the covered sections and the uncovered sections have again shown that these minor fluctuations would not alter the overall shapes of the depth-dependent profiles of the angle and retardation images or the territorial matrix [19].
To the best of our knowledge, this is the first quantitative PLM study of parallel sections of articular cartilage over the full cartilage thickness using a 6μm step-increment. We showed that the birefringence of the parallel sections exhibited some unique features that were very different from the usual perpendicular sections. This 3D orthogonal viewing approach can provide a comprehensive understanding of the territorial and interterritorial fibrils in articular cartilage. A better knowledge of cartilage structure could clearly aid in the early diagnoses of various pathological conditions, such as osteoarthritis, which is known to have local fibril disorganization at the articular surface as the earliest signs of the cartilage degradation.
Acknowledgments
Y Xia is supported by the R01 grants (AR 45172, AR 52353) from the National Institutes of Health. The authors are grateful to Drs. C Les and H Sabbah (Henry Ford Hospital, Detroit) for providing the canine joints, Mr. Ilco Aksovski (Dept of Physics, Oakland University) for an initial exploratory study of parallel sections by PLM, Dr. Nagarajan Ramakrishnan (Current Address: Defence Laboratory, Ratanada Palace, Rajasthan, India) for help with PLM 5x imaging, Prof. Ekrem Cicek (Mehmet Akif Ersoy University, Burdur, Turkey) for exploring PLM cell-cluster imaging, Dr. Matthew Szarko (Current Address: St. George’s, University of London, UK) and Mr. Farid Badar (Dept of Physics, Oakland University) for helpful discussions, Mr. David Kahn (Dept of Physics, Oakland University) and Miss Aimee Xia (University of Michigan, Ann Arbor) for editorial comments.
Footnotes
In this paper, the intensity images refer to the raw optical images taken under various polarizations, which are used to calculate the angle and retardation images. By comparison, a visible image refers to the ordinary optical image taken under no polarization.
References
- 1.Benninghoff A. Form und bau der gelenkknorpel in ihren beziehungen zur funktion. II der aufbau des gelenk-knorpels in semen beziehungen zur funktion. Z Zellforsch U Mikr Anat (Berlin) 1925;2:783–862. [Google Scholar]
- 2.Venn M, Maroudas A. Chemical composition and swelling of normal and osteoarthritic femoral head cartilage. Ann Rheum Dis. 1977;36:121–129. doi: 10.1136/ard.36.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maroudas A, Bayliss MT, Venn M. Further studies on the composition of human femoral head cartilage. Ann Rheum Dis. 1980;39:514–534. doi: 10.1136/ard.39.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton-Wurster N, Todhunter RJ, Lust G. Animal models of osteoarthritis. In: Woessner JFJ, Howell D, editors. Joint cartilage degradation. Basic and clinical aspects. New York: Marcel Dekker, Inc; 1993. pp. 347–384. [Google Scholar]
- 5.Hollander AP, Pidoux I, Reiner A, Rorabeck C, Bourne R, Poole AR. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995;96:2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckwalter JA, Mankin HJ. Articular cartilage. Part II: Degeneration and osteoarthritis, repair, regeneration, and transplantation. J Bone Joint Surgery. 1997;79A:612–632. [PubMed] [Google Scholar]
- 7.Alhadlaq H, Xia Y, Moody JB, Matyas J. Detecting Structural Changes in Early Experimental Osteoarthritis of Tibial Cartilage by Microscopic MRI and Polarized Light Microscopy. Ann Rheum Dis. 2004;63:709–717. doi: 10.1136/ard.2003.011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broom ND. Further insights into the structural principles governing the function of articular cartilage. J Anat. 1984;139:275–294. [PMC free article] [PubMed] [Google Scholar]
- 9.Poole CA. Articular cartilage chondrons: form, function and failure. J Anat. 1997;191:1–13. doi: 10.1046/j.1469-7580.1997.19110001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Youn I, Choi JB, Cao L, Setton LA, Guilak F. Zonal variations in the three-dimensional morphology of the chondron measured in situ using confocal microscopy. Osteoarthritis Cartilage. 2006;14:889–897. doi: 10.1016/j.joca.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Bullough P, Goodfellow J. The significance of the fine structure of articular cartilage. J Bone Joint Surgery. 1968;50B:852–857. [PubMed] [Google Scholar]
- 12.Speer DP, Dahners L. The collagenous architecture of articular cartilage. Correlation of scanning electron microscopy and polarized light microscopy observations. Clin Orthop. 1979;139:267–275. [PubMed] [Google Scholar]
- 13.Yarker YE, Aspden RM, Hukins DW. Birefringence of articular cartilage and the distribution on collagen fibril orientations. Connect Tissue Res. 1983;11:207–213. doi: 10.3109/03008208309004857. [DOI] [PubMed] [Google Scholar]
- 14.Poole CA, Flint MH, Beaumont BW. Morphological and functional interrelationships of articular cartilage matrices. J Anat. 1984;138:113–138. [PMC free article] [PubMed] [Google Scholar]
- 15.Clark JM. The organisation of collagen fibrils in the superficial zones of articular cartilage. J Anat. 1990;171:117–130. [PMC free article] [PubMed] [Google Scholar]
- 16.Xia Y. Relaxation Anisotropy in Cartilage by NMR Microscopy (μMRI) at 14 μm Resolution. Magn Reson Med. 1998;39:941–949. doi: 10.1002/mrm.1910390612. [DOI] [PubMed] [Google Scholar]
- 17.Nieminen MT, Rieppo J, Toyras J, Hakumaki JM, Silvennoinen J, Hyttinen MM, Helminen HJ, Jurvelin JS. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med. 2001;46:487–493. doi: 10.1002/mrm.1218. [DOI] [PubMed] [Google Scholar]
- 18.Arokoski JP, Hyttinen MM, Lapveteläinen T, Takacs P, Kosztaczky B, Modis L, Kovanen V, Helminen HJ. Decreased birefringence of the superficial zone collagen network in the canine knee (stifle) articular cartilage after long distance running training, detected by quantitative polarized light microscopy. Ann Rheum Dis. 1996;55:253–264. doi: 10.1136/ard.55.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia Y, Moody J, Burton-Wurster N, Lust G. Quantitative In Situ Correlation Between Microscopic MRI and Polarized Light Microscopy Studies of Articular Cartilage. Osteoarthritis Cartilage. 2001;9:393–406. doi: 10.1053/joca.2000.0405. [DOI] [PubMed] [Google Scholar]
- 20.Rieppo J, Hallikainen J, Jurvelin JS, Kiviranta I, Helminen HJ, Hyttinen MM. Practical considerations in the use of polarized light microscopy in the analysis of the collagen network in articular cartilage. Microsc Res Tech. 2008;71:279–287. doi: 10.1002/jemt.20551. [DOI] [PubMed] [Google Scholar]
- 21.Gadaleta SJ, Landis WJ, Boskey AL, Mendelsohn R. Polarized FT-IR microscopy of calcified turkey leg tendon. Connect Tissue Res. 1996;34:203–211. doi: 10.3109/03008209609000699. [DOI] [PubMed] [Google Scholar]
- 22.Potter K, Kidder LH, Levin IW, Lewis EN, Spencer RG. Imaging of collagen and proteoglycan in cartilage sections using Fourier transform infrared spectral imaging. Arthritis Rheum. 2001;44:846–855. doi: 10.1002/1529-0131(200104)44:4<846::AID-ANR141>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 23.Camacho NP, West P, Torzilli PA, Mendelsohn R. FTIR microscopic imaging of collagen and proteoglycan in bovine cartilage. Biopolymers. 2001;62:1–8. doi: 10.1002/1097-0282(2001)62:1<1::AID-BIP10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 24.Hughes LC, Archer CW, ap Gwynn I. The ultrastructure of mouse articular cartilage: collagen orientation and implications for tissue functionality. A polarised light and scanning electron microscope study and review. Eur Cell Mater. 2005;9:68–84. doi: 10.22203/ecm.v009a09. [DOI] [PubMed] [Google Scholar]
- 25.Xia Y, Ramakrishnan N, Bidthanapally A. The depth-dependent anisotropy of articular cartilage by Fourier-transform infrared imaging (FTIRI) Osteoarthritis Cartilage. 2007;15:780–788. doi: 10.1016/j.joca.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia Y, Alhadlaq H, Ramakrishnan N, Bidthanapally A, Badar F, Lu M. Molecular and morphological adaptations in compressed articular cartilage by polarized light microscopy and Fourier-transform infrared imaging. J Struct Biol. 2008;164:88–95. doi: 10.1016/j.jsb.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia Y. Averaged and Depth-Dependent Anisotropy of Articular Cartilage by Microscopic Imaging. Semin Arthritis Rheum. 2008;37:317–327. doi: 10.1016/j.semarthrit.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Oldenbourg R, Mei G. New polarized light microscope with precision universal compensator. J Microscopy. 1995;180:140–147. doi: 10.1111/j.1365-2818.1995.tb03669.x. [DOI] [PubMed] [Google Scholar]
- 29.Alhadlaq HA, Xia Y, Hansen FM, Les CM, Lust G. Morphological changes in articular cartilage due to static compression: polarized light microscopy study. Connect Tissue Res. 2007;48:76–84. doi: 10.1080/03008200601130950. [DOI] [PubMed] [Google Scholar]
- 30.Maroudas A, Venn M. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. II Swelling Ann Rheum Dis. 1977;36:399–406. doi: 10.1136/ard.36.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venn MF. Chemical composition of human femoral and head cartilage: influence of topographical position and fibrillation. Ann Rheum Dis. 1979;38:57–62. doi: 10.1136/ard.38.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bayliss MT, Venn M, Maroudas A, Ali SY. Structure of proteoglycans from different layers of human articular cartilage. Biochem J. 1983;209:387–400. doi: 10.1042/bj2090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brocklehurst R, Bayliss MT, Maroudas A, Coysh HL, Freeman MA, Revell PA, Ali SY. The composition of normal and osteoarthritic articular cartilage from human knee joints. With special reference to unicompartmental replacement and osteotomy of the knee. J Bone Joint Surg. 1984;66A:95–106. [PubMed] [Google Scholar]
- 34.Roberts S, Weightman B, Urban J, Chappell D. Mechanical and biochemical properties of human articular cartilage from the femoral head after subcapital fracture. J Bone Joint Surg. 1986;68B:418–422. doi: 10.1302/0301-620X.68B3.3733808. [DOI] [PubMed] [Google Scholar]
- 35.Laasanen MS, Toyras J, Korhonen RK, Rieppo J, Saarakkala S, Nieminen MT, Hirvonen J, Jurvelin JS. Biomechanical properties of knee articular cartilage. Biorheology. 2003;40:133–140. [PubMed] [Google Scholar]
- 36.Ramakrishnan N, Xia Y, Bidthanapally A. Fourier-transform infrared anisotropy in cross and parallel sections of tendon and articular cartilage. J Orthop Surg Res. 2008;3:48. doi: 10.1186/1749-799X-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rolauffs B, Williams JM, Grodzinsky AJ, Kuettner KE, Cole AA. Distinct horizontal patterns in the spatial organization of superficial zone chondrocytes of human joints. J Struct Biol. 2008;162:335–344. doi: 10.1016/j.jsb.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia Y, Mittelstaedt D, Ramakrishnan N, Szarko M, Bidthanapally A. Microscopy Research and Technique. 2010. Depth-Dependent Anisotropies of Amides and Sugar in Perpendicular and Parallel Sections of Articular Cartilage by Fourier Transform Infrared Imaging (FTIRI) in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiraly K, Hyttinen MM, Lapvetelainen T, Elo M, Kiviranta I, Dobai J, Modis L, Helminen HJ, Arokoski JP. Specimen preparation and quantification of collagen birefringence in unstained sections of articular cartilage using image analysis and polarizing light microscopy. Histochem J. 1997;29:317–327. doi: 10.1023/a:1020802631968. [DOI] [PubMed] [Google Scholar]
- 40.Xia Y, Moody J, Alhadlaq H, Hu JN. Imaging the Physical and Morphological Properties of a Multi-Zone Young Articular Cartilage at Microscopic Resolution. J Magn Reson Imaging. 2003;17:365–374. doi: 10.1002/jmri.10269. [DOI] [PubMed] [Google Scholar]
- 41.Xia Y, Moody J, Alhadlaq H, Burton-Wurster N, Lust G. Characteristics of Topographical Heterogeneity of Articular Cartilage over the Joint Surface of a Humeral Head. Osteoarthritis Cartilage. 2002;10:370–380. doi: 10.1053/joca.2002.0523. [DOI] [PubMed] [Google Scholar]
- 42.Hunziker EB, Quinn TM, Hauselmann HJ. Quantitative structural organization of normal adult human articular cartilage. Osteoarthritis Cartilage. 2002;10:564–572. doi: 10.1053/joca.2002.0814. [DOI] [PubMed] [Google Scholar]
- 43.Bush PG, Hall AC. The volume and morphology of chondrocytes within non-degenerate and degenerate human articular cartilage. Osteoarthritis Cartilage. 2003;11:242–251. doi: 10.1016/s1063-4584(02)00369-2. [DOI] [PubMed] [Google Scholar]
- 44.Zheng S, Xia Y. The collagen fibril structure in the superficial zone of articular cartilage by μMRI. Osteoarthritis Cartilage. 2009;17:1519–1528. doi: 10.1016/j.joca.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia Y, Elder K. Quantification of the Graphical Details of Collagen Fibrils in Transmission Electron Micrographs. J Microscopy. 2001;204:3–16. doi: 10.1046/j.1365-2818.2001.00925.x. [DOI] [PubMed] [Google Scholar]
- 46.Broom ND, Marra DL. Ultrastructural evidence for fibril-to-fibril associations in articular cartilage and their functional implication. J Anat. 1986;146:185–200. [PMC free article] [PubMed] [Google Scholar]
- 47.Broom ND, Silyn-Roberts H. The three-dimensional ‘knit’ of collagen fibrils in articular cartilage. Connect Tissue Res. 1989;23:75–88. doi: 10.3109/03008208909005626. [DOI] [PubMed] [Google Scholar]