Abstract
In the few weeks prior to the onset of vision, the retina undergoes a dramatic amount of development. Neurons migrate into position and target appropriate synaptic partners to assemble the circuits that mediate vision. During this period of development, the retina is not silent, but rather exhibits spontaneous correlated activity called retinal waves. The retina assembles and disassembles a series of transient circuits that use distinct mechanisms to generate waves. During the first postnatal week, this transient circuit is comprised of reciprocal cholinergic connections between starburst amacrine cells. A few days before the eyes open, these cholinergic connections are eliminated as glutamatergic circuits involved in processing visual information are formed. Here we discuss the assembly and disassembly of this transient cholinergic network and the role it plays in various aspects of retinal development.
The mammalian retina has long been a model system for study of development of neural circuits in the CNS. The development of the retina requires several steps. First, the 7 cell types that comprise the retina need to be generated in the right proportion. Second, postmitotic cells leave the ventricular zone and migrate to one of three cell layers. Third, they start to form synaptic connections with other retinal neurons. Finally, these groups of synaptically coupled cells evolve into the circuits contained in the adult retina.
Acetylcholine (ACh) signaling plays a key role throughout these developmental stages. Prior to synapse formation, paracrine action of ACh is essential for regulating early developmental events, such as regulation of the cell cycle and the growth of neurites. In addition, cholinergic synapses are among the earliest to mature and thereby constitute the earliest functional circuits in the retina. Here we review the maturation of the retinal cholinergic circuit, describing its role in mediating early spontaneous activity and the development of non-cholinergic circuits.
ACh in the retina is produced by a subtype of amacrine cell termed the starburst amacrine cell (SAC). Of the roughly 30 morphologically distinct amacrine cell types (MacNeil et al., 1999), the starburst amacrine cell is perhaps the best characterized (Famiglietti, 1983; Tauchi & Masland, 1984; Vaney, 1984). So named for its radially symmetric processes, SACs are the only neurons to produce ACh in the adult retina (Hayden et al., 1980). The population of SACs is divided into two sub-populations with somas located in the proximal inner nuclear layer (regular) or in the ganglion cell layer (displaced) (Famiglietti, 1983). In addition to releasing ACh, SACs also release GABA (O'Malley & Masland, 1989) and adenosine (Blazynski, 1989). In the mature retina, ACh release acts on both muscarinic and nicotinic receptors to modulate response properties of many different types of ganglion cells (Masland & Ames, 1976; Masland et al., 1984; Schmidt et al., 1987; Baldridge, 1996; Strang et al., 2005). The SAC is of particular importance in a retinal circuit that detects motion (Fried et al., 2002; reviewed in Taylor & Vaney, 2003), the topic of another review in this series.
In mature retina, ACh released from SACs acts exclusively on other cell types (Baldridge, 1996). However, during development cholinergic signaling occurs between SACs. Long before eye opening, ACh is produced by SACs and functional cholinergic receptors exist on SACs as well as other cells of the developing retina. SACs are spontaneously active and can release ACh onto neighboring SACs and ganglion cells. We refer to this early, transient signaling between SACs as the cholinergic network.
A key developmental function for the cholinergic network is the generation of retinal waves. During an extended period of time prior to visual input, the developing retina exhibits propagating spontaneous activity, termed retinal waves (Meister et al., 1991; Wong et al., 1993; Feller et al., 1996). The cholinergic network appears at birth in mice and mediates the initiation and propagation of waves until the second postnatal week, when waves are mediated by glutamatergic circuits (Bansal et al., 2000; Wong et al., 2000; Zhou & Zhao, 2000).
In this review, we first describe the assembly of the transient cholinergic circuit, which involves early expression of proteins associated with release and action of ACh. Second, we review the process by which SACs orchestrate the wave generating circuit. Third, we describe the transition from cholinergic waves to glutamatergic waves, which requires the disassembly of the cholinergic connections that mediate waves. Last, we review the evidence that SACs and waves play a role in the development of retinal circuits, including dendritic refinement and stratification. (The role of retinal waves in the targeting and refinement of retinofugal synapses has been reviewed elsewhere (Torborg & Feller, 2005; Huberman et al., 2008a; Feldheim & O'Leary, 2010)).
Assembly of the cholinergic network
The proteins associated with cholinergic synapses appear early in development (Figure 1). SACs first begin to express choline-acetyltransferase (ChAT), the enzyme responsible for synthesis of acetylcholine, at embryonic day 17 (E17) in rats (Kim et al., 2000). SAC cell bodies organize into two layers on opposite sides of the nascent inner plexiform layer (IPL) shortly before birth. By postnatal day 3 (P3) two distinct bands are apparent within the IPL, representing regular and displaced populations of SACs (Bansal et al., 2000; Kim et al., 2000). Expression of the vesicular transporter for ACh (VAChT) follows a similar developmental profile, with punctate expression found at birth (Stacy & Wong, 2003). Evidence for presynaptic release machinery, including synaptic vesicle protein 2C (SV2C, (Wang et al., 2003)), synaptophysin (Dhingra et al., 1997), and SNAP-25 (West Greenlee et al., 1998) are present at birth as well.
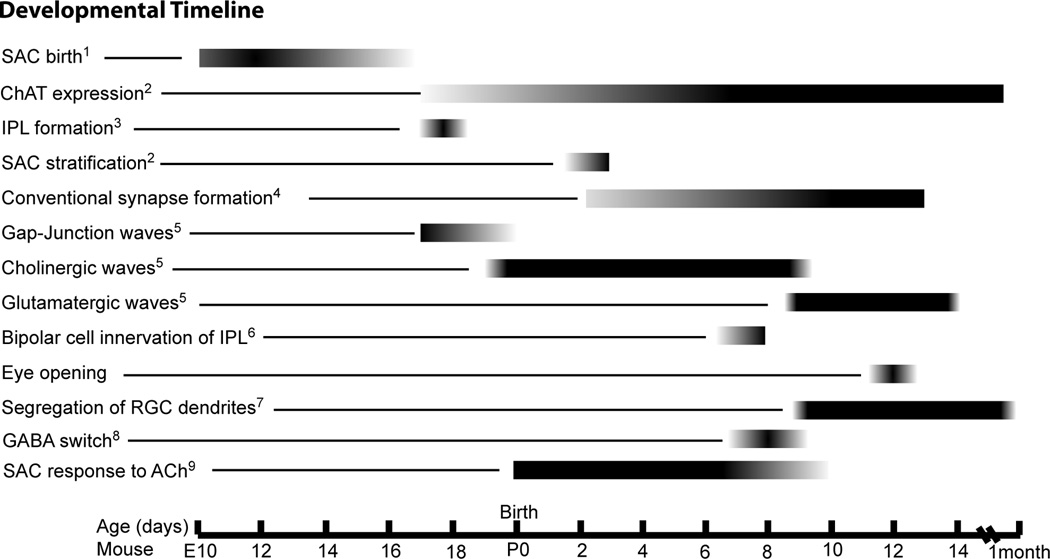
Figure 1. Timeline showing major developmental events in mouse retina.
Starburst cells become postmitotic between E11 and E17. ChAT expression is first seen at E17 and increases during the first week after birth. The IPL first appears at E17 and SAC processes form two discrete bands by P3. Conventional synapses first appear between amacrine and ganglion cells in the IPL at P3. Retinal waves transition through three stages during development: gap-junction, cholinergic, and glutamatergic. Bipolar cells first innervate the IPL at P7. Eyes open around P12. Ganglion cell dendrites stratify into lamina for an extended time beginning around P9 and continuing through the first month. The action of GABA switches from depolarizing to hyperpolarizing around P8. SACs are initially responsive to ACh action on nAChRs, but lose this responsiveness with the corresponding transition to glutamatergic waves.
References:(1) (Voinescu et al., 2009) (2) (Kim et al., 2000) (3) (Hinds & Hinds, 1983) (4) (Fisher, 1979) (5) (Bansal et al., 2000) (6) (Johnson et al., 2003) (7) (Xu & Tian, 2004) (8) (Barkis et al., 2010) (9) (Zheng et al., 2004) (approximate age from rabbit data)
The broad distribution of nicotinic and muscarinic receptors at birth indicates that ACh released from SACs is poised to act on several cell types. mRNA expression of nicotinic acetylcholine receptors (nACHRs) subunits alpha 3–4 and beta 2–4 is present in the embryonic and early postnatal retina (Hoover & Goldman, 1992). Dividing cells in the ventricular zone have M1 muscarinic receptor dependent calcium increases in response to carbachol application, whereas differentiated amacrine and ganglion cells have voltage-gated channel dependent calcium increases in respond to nicotine (Wong, 1995). Among the responsive amacrine cells are SACs themselves, which are depolarized at this early age by ACh acting on nicotinic receptors (Zheng et al., 2004). The presence nAChRs on SACs indicates that they have the potential to form a recurrent excitatory network at this early stage of development.
Despite the abundance of pre- and postsynaptic components for cholinergic signaling, there is little ultrastructural evidence of conventional synapses at this early stage of development. Conventional synapses between amacrine and ganglion cells begin to form after P3 in mice (Fisher, 1979) and ChAT expression precedes synapse formation by at least 2 days in chick retina (Spira et al., 1987). However, despite the lack of structural evidence for synapses, there is physiological evidence for cholinergic synaptic transmission between pairs of SACs at this early age. Paired recordings between SACs from embryonic rabbit showed that SACs can indeed release both ACh and GABA to excite neighboring SACs in a reciprocal fashion (Zheng et al., 2004) (Figure 2D). Postsynaptic nAChR mediated currents are slow, appearing to reflect an asynchronous and/or diffuse release of ACh from the presynaptic SAC. Paired recordings of SACs in early postnatal mice reveal similar slow currents (Ford et al., 2010).
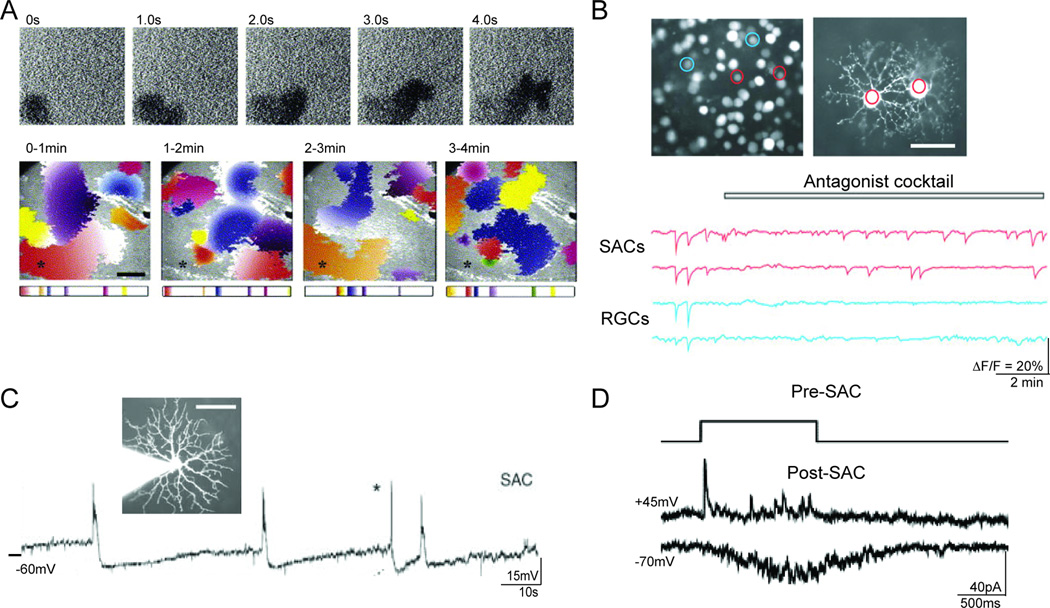
Figure 2. Properties of cholinergic waves are generated by the SAC network.
A. Spatial and temporal properties of waves assessed with calcium imaging.
TOP: Low power calcium imaging of Fura-2AM labeled neonatal ferret retinas show decreases in fluorescence following calcium increases associated with waves.
BOTTOM: Spatial propagation of 30 waves during four consecutive minutes. Each panel shows the propagation of waves that occurred during a one-minute period. Cholinergic retinal waves initiate at random locations and propagate over finite regions. Waves occur at intervals between waves of around one minute for a given region (for example see asterisk). Adapted from (Feller et al., 1996).
B. Cell-autonomous spontaneous depolarization of SACs may initiate retinal waves.
SACs (red regions and traces) from perinatal rabbit retinas loaded with Fura-2AM show spontaneous depolarizations in the presence of antagonists to ionotropic and metabotropic glutamate, GABA, glycine, and ACh receptors, whereas retinal ganglion cells do not (blue regions and traces). Adapted from (Zheng et al., 2006).
C. The inter-wave refractory period may be due to a slow after-hyperpolarization in SACs.
Current clamp recording from a SAC in rabbit retina showing wave evoked and spontaneous (asterisk) depolarizations followed by slow after-hyperpolarizations. Adapted from (Zheng et al., 2006).
D. Waves may propagate via reciprocal connections between SACs.
Voltage clamp recordings from pairs of neighboring SACs show both fast GABAergic postsynaptic currents and slow cholinergic postsynaptic currents. Top: Schematic of voltage command of presynaptic SAC (−70mV to 0mV). Bottom: Evoked PSCs at positive and negative holding potentials to isolate cholinergic and GABAergic currents, respectively. Adapted from (Zheng et al., 2004).
SACs also form GABAergic synapses with other SACs and with subpopulations of ganglion cells. Unlike most GABAergic neurons in the retina, SACs express the GABA synthesizing enzyme GAD-67 rather than the GAD-65 isoform (Brandon & Criswell, 1995). GAD-67 expression is present early in development (Famiglietti & Sundquist, 2010). SACs can release GABA onto neighboring SACs during the first postnatal week in rabbit (Zheng et al., 2004). Direction selective ganglion cells also form GABAergic synapses with SACs well before eye opening (Lee et al., 2010; Wei et al., 2011).
A distinguishing feature of the SAC network is that the somas of SACs form a mosaic -- a term used to describe the regular arrangement of somas with a minimal spacing between them (reviewed in Vaney, 1990; Cook & Chalupa, 2000; Novelli et al., 2005). SAC mosaics arise very early in development. SAC somas are initially positioned randomly with respect to each other as they migrate from the ventricular zone toward the developing ganglion cell layer. By birth in mice, SAC somas have distributed in a mosaic fashion so that there is a minimum distance observed between somas (Galli-Resta et al., 1997; Galli-Resta et al., 2000). The formation of the soma mosaic correlates with the complete coverage of the IPL by SAC processes.
Homotypic interactions between SAC processes are thought to instruct the initial formation of the mosaic (Reese & Galli-Resta, 2002). The developmental window during which these interactions establish the mosaic is finite, as disruption of microtubules (Galli-Resta et al., 2002) or ablation of subsets of SACs by excitotoxicity (Farajian et al., 2004) a few days after birth fails to disrupt the established mosaic. At this point, the mechanisms by which interactions between SAC processes mediate the development of mosaics are unknown.
The role of starburst amacrine cells in generation of retinal waves
The most well studied function of the early cholinergic network in the retina is the generation of retinal waves (Figure 2). Retinal waves consist of bursts of action potentials in retinal ganglion cells that propagate in a wave front across the plane of the retina. Retinal waves occur in many species including turtle (Sernagor et al., 2003), chick (Catsicas et al., 1998; Wong et al., 1998; Sernagor et al., 2000), mice (Mooney et al., 1996; Bansal et al., 2000), rabbit (Zhou, 1998), ferret (Meister et al., 1991; Wong et al., 1993; Feller et al., 1996), and primate (Warland et al., 2006). Across all species, waves share similar characteristic features. Waves initiate at random positions and propagate at speeds ranging from ~100µm/s (mouse, (Singer et al., 2001)) up to 400µm/s (chick, (Sernagor et al., 2000)). Individual waves stop at discrete but shifting borders that reflect recently active regions of retina (Feller et al., 1996). This refractory period following a wave lasts for approximately one minute (Feller et al., 1996; Feller et al., 1997), shortly after which an additional wave can be initiated and spread over the same area (Figure 2A). In addition, this refractory period dictates the frequency with which a local region of the retina experiences a wave.
The circuitry underlying the generation of retinal waves progresses through three stages that reflect the maturation of retinal circuits (Blankenship & Feller, 2010) (Figure 3A). Before formation of the IPL (E17 in mice (Kim et al., 2000), E25 in rabbit (Sharma & Ehinger, 1997)), retinal waves propagate through the developing ganglion cell layer. These early stage waves are blocked by gap-junction antagonists (Syed et al., 2004b). With the initial extension of SAC processes to form the IPL, waves transition from propagation via gap-junctions to propagation via activation of nAChRs (Feller et al., 1996). Finally, a third stage of waves develop several days before the eyes open and co-exist with light responses for a short period of time (Demas et al., 2003). These waves are blocked by ionotropic glutamate receptors (Wong et al., 2000; Zhou & Zhao, 2000; Blankenship et al., 2009).
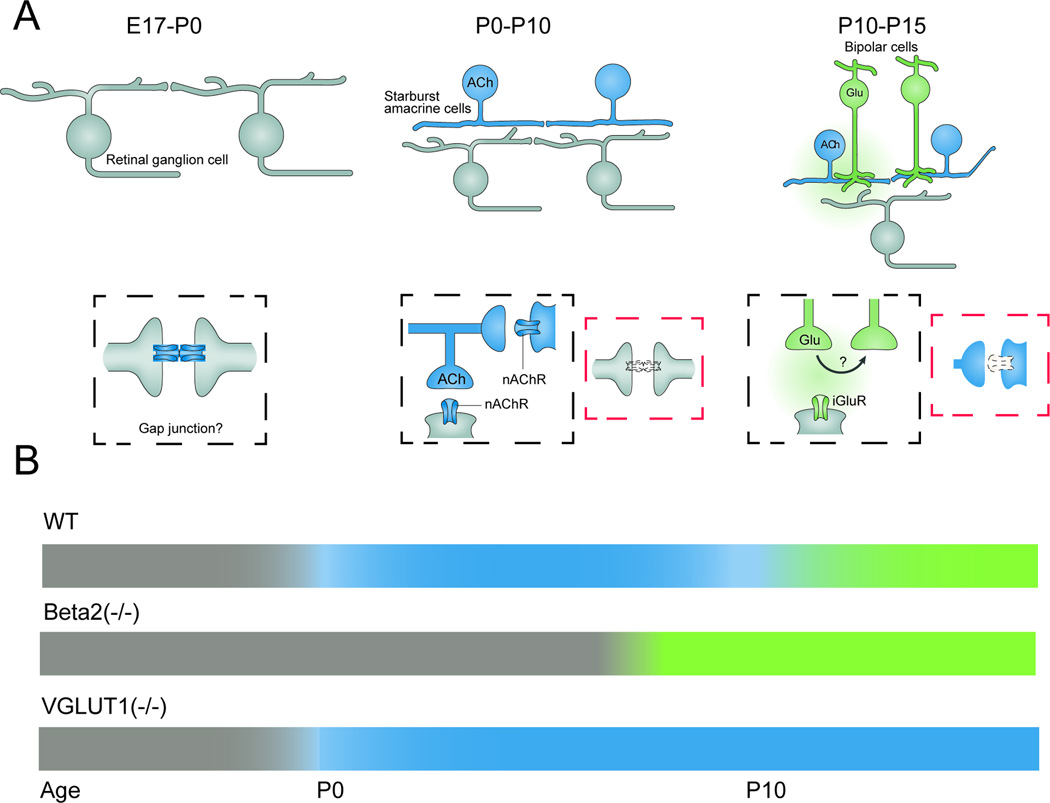
Figure 3. Wave circuits transition through check-points.
A. Schematics of changing circuits that mediate waves. Left: Prior to birth waves are thought to propagate via gap-junctions between ganglion cells. Middle: Postnatal day 1–10, waves are propagated via SAC release of acetylcholine onto other SACs (black box). Acetylcholine also depolarizes ganglion cells. During this period of development, the gap-junction signaling between ganglion cells is reduced (red box).
Right: P10–P15 bipolar cells release glutamate to propagate waves in a mechanism that is thought to involve spillover of glutamate to excite neighboring bipolar cells (black box). Cholinergic signaling between SACs is reduced (red box). Adapted from (Blankenship & Feller, 2010).
B. Summary timeline of how genetic disruption of cholinergic or glutamatergic waves result in an extended action of the previous wave generating circuit
In wild type mice gap-junction mediated waves (gray) are followed by cholinergic waves (blue) starting at P0, then glutamatergic waves (green) at P10. In mice lacking the Beta2 subunit of the nicotinic acetylcholine receptor, gap-junction mediated waves persist until ~P8. In mice lacking vesicular glutamate transporter VGLUT1, cholinergic waves persist through the second postnatal week.
The cholinergic network plays an exclusive role in generating waves during an extended period of development. Neighboring ganglion cells and SACs receive simultaneous slow nAChR and GABA-A mediated currents that give rise to correlated action potential firing (Feller et al., 1996; Zhou, 1998). In perinatal mice, ferrets, and rabbits, waves monitored by calcium imaging are blocked by nAChR antagonists (Feller et al., 1996; Penn et al., 1998; Bansal et al., 2000; Zhou & Zhao, 2000), but not by ionotropic or metabotropic GABA or glutamate receptor antagonists (Zhou & Zhao, 2000). Gap-junction signaling seems to play a role in the generation of these waves in chick (Catsicas et al., 1998), but is involved to a lesser extent in mammalian retina (Bansal et al., 2000; Syed et al., 2004b).
The features of the developing cholinergic network that give rise to waves are now beginning to be understood. The generation of waves requires a source of depolarization for wave initiation, a network of excitatory interactions for propagation and a source of inhibition that limits the spatial extent of waves and dictates the minimum interval between waves. We discuss the properties of the cholinergic network that give rise to these features below.
Initiation
SACs themselves are the likely source of wave initiation. In order for a wave to start, a cell or group of cells must spontaneously excite neighboring cells to initiate the spread of depolarization. Waves initiate at random locations (Feller et al., 1996), suggesting that the cells responsible for the spontaneous excitation that drives wave initiation are present uniformly across the retina. In the presence of neurotransmitter antagonists, SACs from rabbit retinas exhibit spontaneous depolarizations that could be measured with cell attached, whole cell recordings, and calcium imaging (Zheng et al., 2006) (Figure 2B). Spontaneous depolarizations occur fairly regularly with a frequency of about once per 20s in cell attached and current clamp recordings, though spontaneous calcium transients were less regular. These spontaneous depolarizations presumably lead to release of ACh onto neighboring SACs, which may trigger the start of a wave. Indeed, we find that injecting current to depolarize a single SAC can robustly initiate a wave in mouse retina (Ford et al., 2010).
The ion channels that underlie spontaneous depolarizations in SACs are unknown. Activation of TTX sensitive sodium channels, which are present in developing SACs (Zhou & Fain, 1996), are unlikely to be the cause of spontaneous depolarization, as TTX does not block retinal waves (Stellwagen et al., 1999). Spontaneous opening of calcium channels may provide a depolarizing drive. Adult SACs have N-, P/Q-, and R-type calcium channels (Cohen, 2001; Kaneda et al., 2007), but these channels are activated at depolarized potentials. Some L-type voltage gated calcium channels are activated at hyperpolarized potentials (Koschak et al., 2001) and have been implicated in pacemaking neurons (Putzier et al., 2009). Retinal waves are blocked by the L-type channel antagonist nifedipine (Singer et al., 2001), suggesting that these channels may play a role in either the spontaneous depolarization or propagation of waves.
Propagation
Waves propagate through a cholinergic circuit that depends on activation of a specific subtype of nAChRs. Studies of adult rabbit retina have demonstrated strong expression of alpha7 homomeric channels (Dmitrieva et al., 2007) as well as alpha3/beta2 containing heteromeric channels (Keyser et al., 2000) throughout the IPL. Expression of alpha3 and alpha8 containing receptors during the period of cholinergic waves has been shown in chick retina (Hamassaki-Britto et al., 1994). Waves are blocked by α-conotoxin-MII, a toxin that is most specific for alpha3/beta2 containing nAChRs, but not α-bungarotoxin (targeted to homomeric alpha7 nAChRs) or α-conotoxin-AU1B (targeted to alpha3/beta4 containing receptors) (Penn et al., 1998; Bansal et al., 2000). Further evidence for a unique set of receptors mediating waves came from studies using knockout mice -- mice lacking either alpha3 or beta2 subunits lacked cholinergic retinal waves (Bansal et al., 2000; Sun et al., 2008; Stafford et al., 2009). Hence, wave propagation depends upon activation of specific heteromeric alpha3/beta2 nAChRs even though other nAChRs exist in the retina.
In addition to ACh, SACs and ganglion cells receive GABAergic input through GABA-A receptors during waves (Feller et al., 1996; Zhou, 1998). At this developmental age GABA-A mediated currents are depolarizing due to the accumulation of intracellular chloride (Zhang et al., 2006b; Barkis et al., 2010). GABA-A receptor activation is not required for the propagation of waves in rabbit or mice (Syed et al., 2004b; Wang et al., 2007), but does influence the structure of the firing patterns (Wang et al., 2007). Not all studies have reached this conclusion – early waves in both turtle (Sernagor et al., 2003) and ferret (Fischer et al., 1998; but see Stellwagen et al., 1999) depend on GABA-A receptor activation.
A possible explanation for the widespread effects of ACh during waves is that ACh is released by volume transmission – the diffuse release of neurotransmitter in the absence of pre- and postsynaptic specializations (for a comparison of direct vs. volume ACh transmission see (Sarter et al., 2009)). Several lines of evidence support this. First, retinal ganglion cells with dendritic arbors at opposite ends of the IPL are synchronized during waves, despite the fine stratification of SAC processes (Wong & Oakley, 1996). Second, slow synaptic currents recorded between pairs of SACs (Zheng et al., 2004) (Figure 2D) indicate that ACh acts diffusely. Third, cholinergic retinal waves are present prior to the formation of conventional synapses, as identified in electron microscopy studies (Fisher, 1979; Greiner & Weidman, 1981). Finally, in rabbit retina, calcium waves propagate through the ventricular zone that are correlated with waves in the ganglion cells (Syed et al., 2004a). The ventricular zone waves are blocked by muscarinic receptor antagonists as well as nAChR antagonists, implying that ACh released during waves in the ganglion cell layer diffuses tens of microns to the ventricular zone where it activates muscarinic receptors.
Refractory period
The frequency of retinal waves is limited by a refractory period following waves. Over a period of about one minute, waves initiated at random points appear to tile the retina, avoiding regions active recently (Feller et al., 1996)(Figure 2A). Depolarization by focal application of high potassium solution could also cause a refractory period within the affected region. This refractory period is thought to be imparted by a slow after-hyperpolarization (sAHP) following depolarization in SACs (Zheng et al., 2006) (Figure 2C). The sAHP is blocked by cadmium, suggesting that it is activated by calcium entry through voltage-gated calcium channels. Interestingly, the channel underlying the sAHP is inhibited by elevating levels of cAMP, similar to the calcium-activated potassium channel which is thought to underlie sAHPs in various other regions of the brain (Alger & Nicoll, 1980; Lancaster & Adams, 1986; Sah & Isaacson, 1995; Vogalis et al., 2001), the molecular identity of which is unknown. Elevating cAMP increases the frequency of retinal waves (Stellwagen et al., 1999; Hanganu et al., 2006), consistent with the notion that the sAHP limits the frequency of waves.
Recent studies have incorporated the measured features of the cholinergic network into computational models. Modeling studies are of use in identifying the relevant features of a circuit that give rise to the physiological output. For instance, two modeling studies have shown that in order to achieve propagating waves that have finite borders, i.e. do not encompass the entire retina, there must be local variability in the refractory state of the retina (Godfrey & Swindale, 2007; Hennig et al., 2009). This variability is generated by random spontaneous depolarizations of SACs followed by sAHPs that occur in between waves. While spontaneous depolarization of SACs between waves has been observed in rabbit (asterisk Figure 2C) (Zheng et al., 2006), we have not observed these in mouse (Ford et al., 2010). As the details of the SAC network are worked out for different species, there will likely be different, and perhaps disparate, solutions that generate very similar waves.
Role of cholinergic signaling in establishing non-cholinergic retinal circuits
Influence on later progenitor cells
Cholinergic retinal waves occur during a period of rapid retinal development. Progenitor cells in the ventricular zone are dividing and differentiating into each of the 7 types of retinal cells (Figure 1). These newly differentiated cells must then migrate to the proper position within the retina and extend processes to form the neuropil layers. The onset of cholinergic waves correlates with the formation of the IPL, where amacrine and ganglion cells processes stratify into 10 discrete layers (in mice) that process visual information in parallel (Masland, 2001; Roska & Werblin, 2001; Wassle, 2004). Once stratified in these layers, bipolar, amacrine, and ganglion cells must form synapses with their proper partners to form functional circuits that process visual information. Before the onset of vision, these discrete synaptic layers are visible (Kim et al., 2000; Drenhaus et al., 2003; Famiglietti & Sundquist, 2010) and some functional circuits are already established (Elstrott et al., 2008; Wei et al., 2011). To what extent does the cholinergic network shape the outcome of these developmental events?
Rhythmic increases in intracellular calcium play an important role in cell proliferation and differentiation (reviewed in Martins & Pearson, 2008) for retinal neurons born during cholinergic waves. ACh drives increases in intracellular calcium of progenitor cells in the ventricular zone through activation of M1 muscarinic receptors (Wong, 1995), which can alter their progression in the cell cycle (Pearson et al., 2002). Blocking muscarinic receptors in chick eyes leads to an increase in the size of the eye (Pearson et al., 2002). A probable source for ACh acting on muscarinic receptors is from cholinergic waves, which drive correlated calcium waves in the ventricular zone (Syed et al., 2004a). In other parts of the nervous system, elevation of intracellular calcium can also influence neuronal migration (reviewed in Komuro & Kumada, 2005) and neurotransmitter selection (reviewed in Spitzer et al., 2004). It remains to be seen whether calcium increases caused by retinal waves have similar effects.
IPL stratification
The retina is a highly laminar structure. Cell bodies reside in three vertically oriented layers composed of the distinct cell classes. The IPL comprises axons from bipolar cells, processes from amacrine cells, and dendrites of ganglion cells. These processes are separated into the On and Off layers corresponding to the functional responses to light of the corresponding cells. Within On and Off layers, processes are further segregated into at least 10 different strata where distinct neural computations are performed. The structural layering and corresponding functional separation has made the retina an ideal model system for studying synapse specificity – i.e. the process by which pre- and postsynaptic neurons wire to their appropriate partners.
The development of lamina in the IPL occurs in several steps. First, amacrine cells extend processes into the IPL. The processes of different amacrine cells identified by cell-specific antibody staining form discrete layers as soon as they first appear (Famiglietti & Sundquist, 2010). Second, the dendrites of ganglion cells extend into the IPL. While some ganglion cells initially stratify within their appropriate mature lamina, a large portion of ganglion cells stratify diffusely throughout future On and Off layers. Next, On and Off bipolar cell axons extend into the IPL where they stratify into the appropriate layers (Johnson et al., 2003). Finally, ganglion cells restrict their dendrites to their mature lamina (reviewed in (Xu & Tian, 2004)). Below we discuss how SACs could play a role in these developmental steps.
A growing body of literature from zebrafish studies points to a critical role for amacrine cells in patterning the IPL. The processes of amacrine cells have directed growth within their target layers without exuberant growth in other layers (Godinho et al., 2005). While ganglion cells are the first-born cells in the retina, they play a lesser role in formation of layers in the IPL. A genetic model in which ganglion cells are never born have delayed IPL formation, although this is partially corrected and normal lamination of the IPL occurs throughout most of the retina (Kay et al., 2004). Hence, interactions between amacrine cells are sufficient to give rise to the layering within the IPL. Interestingly, bipolar cell axons target correctly to the normal IPL in these mutants, but fail to innervate regions of abnormal IPL (Kay et al., 2004), suggesting that amacrine cells provide the laminar cues for bipolar cell axon targeting. Finally, ganglion cells exhibit directed outgrowth of their processes within established layers of amacrine cell processes (Mumm et al., 2006), implying that amacrine cells can provide lamination cues for ganglion cells. A zebrafish homologue of the SAC has not been conclusively identified (though see (Kay et al., 2004)), so it is unclear what role SACs in particular play in these developmental events.
In mammals, SACs are among the first cells to extend processes within the IPL (Kim et al., 2000) and could potentially serve as a foundation for the layering of subsequent strata within the IPL. Lamination signals, such as members of the immunogblobulin superfamily (Yamagata & Sanes, 2008) or semaphorins (Matsuoka et al., 2011), expressed on SAC processes could instruct the processes of other amacrine, ganglion, and bipolar cells to stratify above, below or between the two cholinergic strata. Despite this early genesis, it is unlikely that SACs provide lamination cues for other amacrine and bipolar cells. Retinal deletion of the transcription factor islet-1 results in a near complete loss of ChAT positive amacrine cells (Elshatory et al., 2007). Despite this loss of differentiated SACs, several markers for other amacrine cell types still form layers within the IPL. Similarly, a genetic model of retinal blastoma lacks several markers of mature SACs but stratification of other amacrine and bipolar cell types are not altered (Chen et al., 2007). Furthermore, SACs ablated by immuno-toxin (Gunhan et al., 2002) or by excitotoxicity with L-glutamate (Reese et al., 2001) at two to three days after birth show no defects in the stratification of bipolar cells, suggesting that SAC processes do not provide an instructive role for stratification of these cell types.
While SACs are unlikely to guide the lamination of other amacrine and bipolar cells, they may instruct the localization of some types of ganglion cell dendrites. Using random dye labeling, a certain class of bi-stratified ganglion cell, mostly likely corresponding to direction selective ganglion cells, was found to co-stratify with the cholinergic processes shortly after birth (Stacy & Wong, 2003), although other mono-stratified ganglion cells never stratified with the cholinergic bands. Recently, genetically labeled mouse lines of functionally identical ganglion cell classes have been developed that allow for consistent characterization across development (Huberman et al., 2008b; Kim et al., 2008; Huberman et al., 2009). The dendrites of genetically identified direction selective ganglion cells co-stratify with SAC processes shortly after birth (Kim 2010, Wei 2011), supporting the hypothesis that SACs provide an instructive role for lamination of certain ganglion cell types.
The dendrites of most retinal ganglion cells initially arborize diffusely within the IPL before restricting their dendrites to distinct lamina (Bodnarenko et al., 1999; Bansal et al., 2000; Sernagor et al., 2001; Xu & Tian, 2004; Coombs et al., 2007; Kim et al., 2010). Some studies have implicated a role for activity in bipolar cells in the segregation of ganglion cell dendrites. Hyperpolarizing ON bipolar cells during the period of glutamatergic retinal waves using the drug APB prevents the segregation into ON and OFF layers (Bodnarenko & Chalupa, 1993; Bodnarenko et al., 1995). Additionally, mice that lack the MHCI receptor CD3zeta have altered glutamatergic retinal waves. The ganglion cells of these mice have reduced dendritic motility and have more diffuse dendrites within the IPL (Xu et al., 2010). However, not all manipulations of activity during development alter dendritic stratification. Preventing synaptic release of glutamate from ON bipolar cells by expression of tetanus toxin fails to prevent the stratification of ganglion cell dendrites, although synapse formation onto ON bipolar cells is reduced (Kerschensteiner et al., 2009).
Is there a role for cholinergic waves in the stratification process? Blocking nAChRs during the period of cholinergic waves reduces the motility of filipodia on the dendrites of ganglion cells (Wong & Wong, 2001), demonstrating that waves can drive structural changes in dendrites. Studies in turtle have demonstrated that blocking cholinergic waves with nAChR antagonists inhibits dendritic growth (Mehta & Sernagor, 2006) and reduces receptive field sizes (Sernagor & Grzywacz, 1996). Additionally, mice lacking the beta2 subunit of the nAChR had a delay in, though did not prevent, the fine stratification of ganglion cell dendrites (Bansal et al., 2000). These findings indicate that cholinergic waves do influence the outgrowth of ganglion cell dendrites but are not the primary factor dictating their final organization.
One intriguing hypothesis for a role of retinal waves is in the development of direction selective circuits. Retinal waves have directional information both in their propagation and in the observation that there is a propagation bias, with more waves traveling toward the nasal direction than the other cardinal axes (Stafford et al., 2009; Elstrott & Feller, 2010). However, direction selective ganglion cells participate equally in all waves irrespective of propagation direction (Elstrott & Feller, 2010) and complete blockade of retinal activity using intraocular muscimol injections did not prevent the development of direction selectivity (Wei et al., 2011). These findings indicate that waves are not likely to provide the instructive signal for the establishment of asymmetric direction selective circuits. However, whether retinal waves play a role in the refinement of DS circuits remains to be determined.
Is there a role for cholinergic waves in the transitions between wave-generating circuits?
Another role of cholinergic waves in development of the retina circuitry may be to terminate early stage gap-junction mediated waves (Figure 3). Knock-out animals that lack nAChR receptor subunits still exhibit wave like activity under certain recording conditions, such as elevated temperature (alpha3(−/−): (Bansal et al., 2000), beta2(−/−) (Sun et al., 2008; Stafford et al., 2009)). This activity is likely mediated by gap-junctions (Sun et al., 2008), as in earlier stage waves. Similarly, a study using a genetic model which eliminates ChAT in a large portion of the retina found normal cholinergic waves in the spared region, but compensatory waves in the region lacking ChAT (Stacy et al., 2005). These studies point to a sequential maturation of the retinal circuitry that relies on checkpoints to make transitions from one stage (gap-junction mediated waves) to the next (cholinergic transmission mediated waves). This sort of checkpoint model of neuronal development (Ben-Ari & Spitzer, 2010) is further evidenced in the disassembly of the cholinergic network to make way for glutamatergic signaling (Blankenship & Feller, 2010).
Disassembly of the cholinergic network
Shortly before eye opening, retinal waves transition from being mediated by ACh to glutamate. Bipolar cell axons invade the IPL and begin to release glutamate at the end of the first week after birth in rodents (Johnson et al., 2003). Shortly thereafter, waves change their pharmacological profile and spatiotemporal properties. Waves are no longer blocked by nAChR antagonists but rather are sensitive to a combination of AMPA and NMDA antagonists (Wong et al., 1998; Zhou & Zhao, 2000; Blankenship et al., 2009). These glutamatergic waves occur as periodic clusters of activity followed by periods of silence (Kerschensteiner & Wong, 2008; Blankenship et al., 2009). Waves exist simultaneously with light responses and disappear a few days after eye opening in a manner that is independent of visual experience (Demas et al., 2003).
With the onset of glutamatergic waves, the SAC network loses the features necessary for propagation of cholinergic waves. First, mature SACs are no longer depolarized by application of nicotine (Baldridge, 1996). This is the result of a rapid decline in nAChR activation that coincides with the start of glutamatergic waves (Zheng et al., 2004). Second, acetylcholinesterase, the enzyme that degrades ACh, is increased during this time window (Hutchins et al., 1995), which would decrease the spread of ACh. Third, the action of GABA becomes hyperpolarizing a few days before the onset of glutamatergic waves (Zhang et al., 2006b; Barkis et al., 2010). Intracellular chloride is reduced as the expression of the potassium-chloride transporter KCC2 is increased in ganglion cells (Zhang et al., 2006a). Mature SAC processes have a graded expression of KCC2 that increases along the length of the process (Gavrikov et al., 2006). When this graded distribution of KCC2 occurs is unknown. Fourth, SACs become less excitable because maturation of processes increases the electronic space constant. The long and skinny processes of SACs likely prevent the spread of depolarization between processes, which would serve to electrically isolate the soma from the processes. A further decrease in the excitability of SACs is imparted by the loss of voltage gated sodium channels (Zhou & Fain, 1996) and an increase in expression of the voltage gated potassium channel Kv3.1 (Ozaita et al., 2004; Zheng et al., 2006).
At this same time, other circuits involving SACs are rapidly maturing. GABAergic connections between SACs are maintained during this period (Zheng et al., 2004) and cholinergic and GABAergic synapses onto direction-selective ganglion cells develop (Lee et al., 2010; Wei et al., 2011). SACs greatly extend their processes as much as 7 fold and develop varicosities at the distal portions that are presumed to be the sites of GABA release (Wong & Collin, 1989).
The nature of cholinergic transmission in the adult retina is not well understood. Many ganglion cells with dendrites distal to the cholinergic strata within the IPL still respond to ACh released from SACs (Schmidt et al., 1987). Paired recordings between SACs and direction selective ganglion cells reveal symmetrical sites of ACh release (Lee et al., 2010), however ultrastructural reconstruction of synaptic contacts onto direction selective ganglion cells failed to find evidence for these cholinergic synapses (Briggman et al., 2011). Furthermore, expression of SNAP-25, a SNARE protein involved in evoked release of neurotransmitter, is transiently highly expressed in SAC processes during the period of cholinergic waves (West Greenlee et al., 1998), suggesting that the presynaptic machinery for neurotransmitter release might change during development.
The onset of glutamatergic waves may play an active role in the disassembly of cholinergic circuits, similar to the transition from gap-junction to cholinergic waves (Blankenship & Feller, 2010) (Figure 3). Mice lacking the vesicular glutamate transporter VGLUT1 lack glutamate release from bipolar cells. These mice still have retinal waves at the period of time when littermate control animals have glutamatergic waves. Interestingly, these later waves in the VGLUT1 knockout mice are unaffected by glutamate receptor antagonists but are blocked by nAChR antagonists (Blankenship et al., 2009). Glutamate signaling from bipolar cells is therefore required to dismantle the cholinergic network.
The mechanisms underlying this transition are not understood. SACs have functional kainate, AMPA, and NMDA receptors shortly after birth (Acosta et al., 2007) suggesting that they can respond to glutamate when it is first released by bipolar cells. In addition to ionotropic receptors, SACs also express the metabotropic glutamate receptor 2 starting shortly after birth in rodents (Koulen et al., 1996). Action of glutamate on either ionotropic or metabotropic receptors on SACs could result in down-regulation of functional nAChRs and perhaps the regulation of other proteins that are necessary for cholinergic waves. The mechanisms involved in this transition will likely be of general importance, as several other developing neural circuits such as the spinal cord, hind brain, cochlea, and hippocampus transition through different stages of spontaneous activity before reaching their mature state (Blankenship & Feller, 2010).
Conclusions
Here we have reviewed the assembly, disassembly and function of cholinergic circuits in retinal development. Starburst amacrine cells release acetylcholine and nicotinic and muscarinic acetylcholine receptors appear early in development to provide signaling that influences events prior to synapse formation. The processes of starburst amacrine cells define the early strata of the developing inner plexiform layer and therefore may play a role in organizing processes of other cells. For a brief period of development, starburst amacrine cells can mutually excite each other. These recurrent excitatory connections provide the substrate for the initiation and propagation of retinal waves. Future work will continue to elucidate how other transient features of developing starburst cells and the nature of their synaptic connections influences the propagation properties of retinal waves.
One outstanding question that remains is how early signaling in the retina influences the formation of retinal circuits. The assembly of this cholinergic circuit is critical for driving the transition from an earlier, gap junction-mediated wave generating circuit. Similarly, maturation of the glutamatergic wave generating circuit drives the disassembly of the cholinergic network. Hence, each circuit is critical for eliminating the circuit before it. In addition, retinal waves drive correlated depolarizations of many synaptic partners (e.g. starburst amacrine cells and direction selective ganglion cells). Whether these early signaling events are critical for the maturation of these functional circuits remains an open question. Answers to these questions require a deeper understanding of the interplay between activity and other cellular mechanisms that underlie the wiring up of functional circuits.
References
- Acosta ML, Chua J, Kalloniatis M. Functional activation of glutamate ionotropic receptors in the developing mouse retina. The Journal of Comparative Neurology. 2007;500:923–941. doi: 10.1002/cne.21225. [DOI] [PubMed] [Google Scholar]
- Alger BE, Nicoll RA. Epileptiform burst afterhyperolarization: calcium-dependent potassium potential in hippocampal CA1 pyramidal cells. Science. 1980;210:1122–1124. doi: 10.1126/science.7444438. [DOI] [PubMed] [Google Scholar]
- Baldridge WH. Optical recordings of the effects of cholinergic ligands on neurons in the ganglion cell layer of mammalian retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1996;16:5060–5072. doi: 10.1523/JNEUROSCI.16-16-05060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20:7672–7681. doi: 10.1523/JNEUROSCI.20-20-07672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkis WB, Ford KJ, Feller MB. Non-cell-autonomous factor induces the transition from excitatory to inhibitory GABA signaling in retina independent of activity. Proc Natl Acad Sci U S A. 2010;107:22302–22307. doi: 10.1073/pnas.1008775108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Spitzer NC. Phenotypic checkpoints regulate neuronal development. Trends in Neurosciences. 2010;33:485–492. doi: 10.1016/j.tins.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nature Reviews. Neuroscience. 2010;11:18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Ford KJ, Johnson J, Seal RP, Edwards RH, Copenhagen DR, Feller MB. Synaptic and extrasynaptic factors governing glutamatergic retinal waves. Neuron. 2009;62:230–241. doi: 10.1016/j.neuron.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazynski C. Displaced cholinergic, GABAergic amacrine cells in the rabbit retina also contain adenosine. Visual Neuroscience. 1989;3:425–431. doi: 10.1017/s0952523800005927. [DOI] [PubMed] [Google Scholar]
- Bodnarenko SR, Chalupa LM. Stratification of ON and OFF ganglion cell dendrites depends on glutamate-mediated afferent activity in the developing retina. Nature. 1993;364:144–146. doi: 10.1038/364144a0. [DOI] [PubMed] [Google Scholar]
- Bodnarenko SR, Jeyarasasingam G, Chalupa LM. Development and regulation of dendritic stratification in retinal ganglion cells by glutamate-mediated afferent activity. J Neurosci. 1995;15:7037–7045. doi: 10.1523/JNEUROSCI.15-11-07037.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnarenko SR, Yeung G, Thomas L, McCarthy M. The development of retinal ganglion cell dendritic stratification in ferrets. Neuroreport. 1999;10:2955–2959. doi: 10.1097/00001756-199909290-00015. [DOI] [PubMed] [Google Scholar]
- Brandon C, Criswell MH. Displaced starburst amacrine cells of the rabbit retina contain the 67-kDa isoform, but not the 65-kDa isoform, of glutamate decarboxylase. Visual Neuroscience. 1995;12:1053–1061. doi: 10.1017/s0952523800006714. [DOI] [PubMed] [Google Scholar]
- Briggman KL, Helmstaedter M, Denk W. Wiring specificity in the direction-selectivity circuit of the retina. Nature. 2011;471:183–188. doi: 10.1038/nature09818. [DOI] [PubMed] [Google Scholar]
- Catsicas M, Bonness V, Becker D, Mobbs P. Spontaneous Ca2+ transients and their transmission in the developing chick retina. Curr Biol. 1998;8:283–286. doi: 10.1016/s0960-9822(98)70110-1. [DOI] [PubMed] [Google Scholar]
- Chen D, Opavsky R, Pacal M, Tanimoto N, Wenzel P, Seeliger MW, Leone G, Bremner R. Rb-mediated neuronal differentiation through cell-cycle-independent regulation of E2f3a. PLoS Biology. 2007;5:e179–e179. doi: 10.1371/journal.pbio.0050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED. Voltage-gated calcium and sodium currents of starburst amacrine cells in the rabbit retina. Visual Neuroscience. 2001;18:799–809. doi: 10.1017/s0952523801185135. [DOI] [PubMed] [Google Scholar]
- Cook JE, Chalupa LM. Retinal mosaics: new insights into an old concept. Trends Neurosci. 2000;23:26–34. doi: 10.1016/s0166-2236(99)01487-3. [DOI] [PubMed] [Google Scholar]
- Coombs JL, Van Der List D, Chalupa LM. Morphological properties of mouse retinal ganglion cells during postnatal development. The Journal of Comparative Neurology. 2007;503:803–814. doi: 10.1002/cne.21429. [DOI] [PubMed] [Google Scholar]
- Demas J, Eglen SJ, Wong ROL. Developmental Loss of Synchronous Spontaneous Activity in the Mouse Retina Is Independent of Visual Experience. The Journal of Neuroscience. 2003;23:2851–2860. doi: 10.1523/JNEUROSCI.23-07-02851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra NK, Ramamohan Y, Raju TR. Developmental expression of synaptophysin, synapsin I and syntaxin in the rat retina. Brain Research. Developmental Brain Research. 1997;102:267–273. doi: 10.1016/s0165-3806(97)00085-0. [DOI] [PubMed] [Google Scholar]
- Dmitrieva NA, Strang CE, Keyser KT. Expression of alpha 7 nicotinic acetylcholine receptors by bipolar, amacrine, and ganglion cells of the rabbit retina. The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society. 2007;55:461–476. doi: 10.1369/jhc.6A7116.2006. [DOI] [PubMed] [Google Scholar]
- Drenhaus U, Morino P, Veh RW. On the development of the stratification of the inner plexiform layer in the chick retina. The Journal of Comparative Neurology. 2003;460:1–12. doi: 10.1002/cne.10602. [DOI] [PubMed] [Google Scholar]
- Elshatory Y, Everhart D, Deng M, Xie X, Barlow RB, Gan L. Islet-1 controls the differentiation of retinal bipolar and cholinergic amacrine cells. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27:12707–12720. doi: 10.1523/JNEUROSCI.3951-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstrott J, Anishchenko A, Greschner M, Sher A, Litke AM, Chichilnisky EJ, Feller MB. Direction selectivity in the retina is established independent of visual experience and cholinergic retinal waves. Neuron. 2008;58:499–506. doi: 10.1016/j.neuron.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstrott J, Feller MB. Direction-selective ganglion cells show symmetric participation in retinal waves during development. J Neurosci. 2010;30:11197–11201. doi: 10.1523/JNEUROSCI.2302-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti [`]Starburst' amacrine cells and cholinergic neurons: mirror-symmetric ON and OFF amacrine cells of rabbit retina. Brain Research. 1983;261:138–144. doi: 10.1016/0006-8993(83)91293-3. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV, Sundquist SJ. Development of excitatory and inhibitory neurotransmitters in transitory cholinergic neurons, starburst amacrine cells, and GABAergic amacrine cells of rabbit retina, with implications for previsual and visual development of retinal ganglion cells. Visual Neuroscience. 2010;27:19–42. doi: 10.1017/S0952523810000052. [DOI] [PubMed] [Google Scholar]
- Farajian R, Raven MA, Cusato K, Reese BE. Cellular positioning and dendritic field size of cholinergic amacrine cells are impervious to early ablation of neighboring cells in the mouse retina. Visual Neuroscience. 2004;21:13–22. doi: 10.1017/s0952523804041021. [DOI] [PubMed] [Google Scholar]
- Feldheim DA, O'Leary DD. Visual map development: bidirectional signaling, bifunctional guidance molecules, and competition. Cold Spring Harb Perspect Biol. 2010;2:a001768. doi: 10.1101/cshperspect.a001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller MB, Butts DA, Aaron HL, Rokhsar DS, Shatz CJ. Dynamic processes shape spatiotemporal properties of retinal waves. Neuron. 1997;19:293–306. doi: 10.1016/s0896-6273(00)80940-x. [DOI] [PubMed] [Google Scholar]
- Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science (New York, N.Y.) 1996;272:1182–1187. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- Fischer KF, Lukasiewicz PD, Wong RO. Age-dependent and cell class-specific modulation of retinal ganglion cell bursting activity by GABA. J Neurosci. 1998;18:3767–3778. doi: 10.1523/JNEUROSCI.18-10-03767.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher LJ. Development of synaptic arrays in the inner plexiform layer of neonatal mouse retina. J Comp Neurol. 1979;187:359–372. doi: 10.1002/cne.901870207. [DOI] [PubMed] [Google Scholar]
- Ford KJ, Felix AL, Feller MB. The role of starburst amacrine cells in initiating retinal waves. City: Society for Neuroscience; (Year) Vol. Program No. 335.6, Neuroscience 2010 Abstracts. [Google Scholar]
- Fried SI, Münch TA, Werblin FS. Mechanisms and circuitry underlying directional selectivity in the retina. Nature. 2002;420:411–414. doi: 10.1038/nature01179. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L, Novelli E, Viegi A. Dynamic microtubule-dependent interactions position homotypic neurones in regular monolayered arrays during retinal development. Development. 2002;129:3803–3814. doi: 10.1242/dev.129.16.3803. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L, Novelli E, Volpini M, Strettoi E. The spatial organization of cholinergic mosaics in the adult mouse retina. The European Journal of Neuroscience. 2000;12:3819–3822. doi: 10.1046/j.1460-9568.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L, Resta G, Tan SS, Reese BE. Mosaics of islet-1-expressing amacrine cells assembled by short-range cellular interactions. J Neurosci. 1997;17:7831–7838. doi: 10.1523/JNEUROSCI.17-20-07831.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrikov KE, Nilson JE, Dmitriev AV, Zucker CL, Mangel SC. Dendritic compartmentalization of chloride cotransporters underlies directional responses of starburst amacrine cells in retina. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18793–18798. doi: 10.1073/pnas.0604551103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey KB, Swindale NV. Retinal Wave Behavior through Activity-Dependent Refractory Periods. PLoS Computational Biology. 2007;3:e245–e245. doi: 10.1371/journal.pcbi.0030245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinho L, Mumm JS, Williams PR, Schroeter EH, Koerber A, Park SW, Leach SD, Wong RO. Targeting of amacrine cell neurites to appropriate synaptic laminae in the developing zebrafish retina. Development. 2005;132:5069–5079. doi: 10.1242/dev.02075. [DOI] [PubMed] [Google Scholar]
- Greiner JV, Weidman TA. Histogenesis of the ferret retina. Experimental Eye Research. 1981;33:315–332. doi: 10.1016/s0014-4835(81)80055-3. [DOI] [PubMed] [Google Scholar]
- Gunhan E, Choudary PV, Landerholm TE, Chalupa LM. Depletion of cholinergic amacrine cells by a novel immunotoxin does not perturb the formation of segregated on and off cone bipolar cell projections. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002;22:2265–2273. doi: 10.1523/JNEUROSCI.22-06-02265.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamassaki-Britto DE, Gardino PF, Hokoc JN, Keyser KT, Karten HJ, Lindstrom JM, Britto LR. Differential development of alpha-bungarotoxin-sensitive and alpha-bungarotoxin-insensitive nicotinic acetylcholine receptors in the chick retina. J Comp Neurol. 1994;347:161–170. doi: 10.1002/cne.903470202. [DOI] [PubMed] [Google Scholar]
- Hanganu IL, Ben-Ari Y, Khazipov R. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. J Neurosci. 2006;26:6728–6736. doi: 10.1523/JNEUROSCI.0752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden SA, Mills JW, Masland RM. Acetylcholine synthesis by displaced amacrine cells. Science (New York, N.Y.) 1980;210:435–437. doi: 10.1126/science.7433984. [DOI] [PubMed] [Google Scholar]
- Hennig MH, Adams C, Willshaw D, Sernagor E. Early-Stage Waves in the Retinal Network Emerge Close to a Critical State Transition between Local and Global Functional Connectivity. J. Neurosci. 2009;29:1077–1086. doi: 10.1523/JNEUROSCI.4880-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds JW, Hinds PL. Development of retinal amacrine cells in the mouse embryo: evidence for two modes of formation. J Comp Neurol. 1983;213:1–23. doi: 10.1002/cne.902130102. [DOI] [PubMed] [Google Scholar]
- Hoover F, Goldman D. Temporally correlated expression of nAChR genes during development of the mammalian retina. Experimental Eye Research. 1992;54:561–571. doi: 10.1016/0014-4835(92)90135-f. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008a;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Manu M, Koch SM, Susman MW, Lutz AB, Ullian EM, Baccus SA, Barres BA. Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron. 2008b;59:425–438. doi: 10.1016/j.neuron.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB, Barres BA. Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron. 2009;62:327–334. doi: 10.1016/j.neuron.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins JB, Bernanke JM, Jefferson VE. Acetylcholinesterase in the developing ferret retina. Experimental Eye Research. 1995;60:113–125. doi: 10.1016/s0014-4835(95)80001-8. [DOI] [PubMed] [Google Scholar]
- Johnson J, Tian N, Caywood MS, Reimer RJ, Edwards RH, Copenhagen DR. Vesicular Neurotransmitter Transporter Expression in Developing Postnatal Rodent Retina: GABA and Glycine Precede Glutamate. The Journal of Neuroscience. 2003;23:518–529. doi: 10.1523/JNEUROSCI.23-02-00518.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M, Ito K, Morishima Y, Shigematsu Y, Shimoda Y. Characterization of voltage-gated ionic channels in cholinergic amacrine cells in the mouse retina. Journal of Neurophysiology. 2007;97:4225–4234. doi: 10.1152/jn.01022.2006. [DOI] [PubMed] [Google Scholar]
- Kay JN, Roeser T, Mumm JS, Godinho L, Mrejeru A, Wong RO, Baier H. Transient requirement for ganglion cells during assembly of retinal synaptic layers. Development. 2004;131:1331–1342. doi: 10.1242/dev.01040. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner D, Morgan JL, Parker ED, Lewis RM, Wong RO. Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature. 2009;460:1016–1020. doi: 10.1038/nature08236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner D, Wong ROL. A precisely timed asynchronous pattern of ON and OFF retinal ganglion cell activity during propagation of retinal waves. Neuron. 2008;58:851–858. doi: 10.1016/j.neuron.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser KT, MacNeil MA, Dmitrieva N, Wang F, Masland RH, Lindstrom JM. Amacrine, ganglion, and displaced amacrine cells in the rabbit retina express nicotinic acetylcholine receptors. Visual Neuroscience. 2000;17:743–752. doi: 10.1017/s095252380017508x. [DOI] [PubMed] [Google Scholar]
- Kim I-J, Zhang Y, Meister M, Sanes JR. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30:1452–1462. doi: 10.1523/JNEUROSCI.4779-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IB, Lee EJ, Kim MK, Park DK, Chun MH. Choline acetyltransferase-immunoreactive neurons in the developing rat retina. The Journal of Comparative Neurology. 2000;427:604–616. doi: 10.1002/1096-9861(20001127)427:4<604::aid-cne8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Yamagata M, Meister M, Sanes JR. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452:478–482. doi: 10.1038/nature06739. [DOI] [PubMed] [Google Scholar]
- Komuro H, Kumada T. Ca2+ transients control CNS neuronal migration. Cell Calcium. 2005;37:387–393. doi: 10.1016/j.ceca.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. alpha 1D (Cav1.3) subunits can form l-type Ca2+ channels activating at negative voltages. The Journal of Biological Chemistry. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- Koulen P, Malitschek B, Kuhn R, Wässle H, Brandstätter JH. Group II and group III metabotropic glutamate receptors in the rat retina: distributions and developmental expression patterns. The European Journal of Neuroscience. 1996;8:2177–2187. doi: 10.1111/j.1460-9568.1996.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Lancaster B, Adams PR. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986;55:1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim K, Zhou ZJ. Role of ACh-GABA cotransmission in detecting image motion and motion direction. Neuron. 2010;68:1159–1172. doi: 10.1016/j.neuron.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The shapes and numbers of amacrine cells: matching of photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. The Journal of Comparative Neurology. 1999;413:305–326. [PubMed] [Google Scholar]
- Martins RAP, Pearson RA. Control of cell proliferation by neurotransmitters in the developing vertebrate retina. Brain Research. 2008;1192:37–60. doi: 10.1016/j.brainres.2007.04.076. [DOI] [PubMed] [Google Scholar]
- Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- Masland RH, Ames A., 3rd Responses to acetylcholine of ganglion cells in an isolated mammalian retina. J Neurophysiol. 1976;39:1220–1235. doi: 10.1152/jn.1976.39.6.1220. [DOI] [PubMed] [Google Scholar]
- Masland RH, Mills JW, Cassidy C. The functions of acetylcholine in the rabbit retina. Proceedings of the Royal Society of London. Series B, Containing Papers of a Biological Character. Royal Society (Great Britain) 1984;223:121–139. doi: 10.1098/rspb.1984.0086. [DOI] [PubMed] [Google Scholar]
- Matsuoka RL, Nguyen-Ba-Charvet KT, Parray A, Badea TC, Chédotal A, Kolodkin AL. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature. 2011;470:259–263. doi: 10.1038/nature09675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta V, Sernagor E. Early neural activity and dendritic growth in turtle retinal ganglion cells. The European Journal of Neuroscience. 2006;24:773–786. doi: 10.1111/j.1460-9568.2006.04933.x. [DOI] [PubMed] [Google Scholar]
- Meister M, Wong RO, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Mooney R, Penn AA, Gallego R, Shatz CJ. Thalamic relay of spontaneous retinal activity prior to vision. Neuron. 1996;17:863–874. doi: 10.1016/s0896-6273(00)80218-4. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Williams PR, Godinho L, Koerber A, Pittman AJ, Roeser T, Chien CB, Baier H, Wong RO. In vivo imaging reveals dendritic targeting of laminated afferents by zebrafish retinal ganglion cells. Neuron. 2006;52:609–621. doi: 10.1016/j.neuron.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli E, Resta V, Galli-Resta L. Mechanisms controlling the formation of retinal mosaics. Prog Brain Res. 2005;147:141–153. doi: 10.1016/S0079-6123(04)47011-3. [DOI] [PubMed] [Google Scholar]
- O'Malley DM, Masland RH. Co-release of acetylcholine and gamma-aminobutyric acid by a retinal neuron. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:3414–3418. doi: 10.1073/pnas.86.9.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaita A, Petit-Jacques J, Völgyi B, Ho CS, Joho RH, Bloomfield SA, Rudy B. A unique role for Kv3 voltage-gated potassium channels in starburst amacrine cell signaling in mouse retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004;24:7335–7343. doi: 10.1523/JNEUROSCI.1275-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R, Catsicas M, Becker D, Mobbs P. Purinergic and muscarinic modulation of the cell cycle and calcium signaling in the chick retinal ventricular zone. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002;22:7569–7579. doi: 10.1523/JNEUROSCI.22-17-07569.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn AA, Riquelme PA, Feller MB, Shatz CJ. Competition in retinogeniculate patterning driven by spontaneous activity. Science (New York, N.Y.) 1998;279:2108–2112. doi: 10.1126/science.279.5359.2108. [DOI] [PubMed] [Google Scholar]
- Putzier I, Kullmann PHM, Horn JP, Levitan ES. Cav1.3 channel voltage dependence, not Ca2+ selectivity, drives pacemaker activity and amplifies bursts in nigral dopamine neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29:15414–15419. doi: 10.1523/JNEUROSCI.4742-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese BE, Galli-Resta L. The role of tangential dispersion in retinal mosaic formation. Prog Retin Eye Res. 2002;21:153–168. doi: 10.1016/s1350-9462(01)00024-6. [DOI] [PubMed] [Google Scholar]
- Reese BE, Raven MA, Giannotti KA, Johnson PT. Development of cholinergic amacrine cell stratification in the ferret retina and the effects of early excitotoxic ablation. Visual Neuroscience. 2001;18:559–570. doi: 10.1017/s0952523801184063. [DOI] [PubMed] [Google Scholar]
- Roska B, Werblin F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature. 2001;410:583–587. doi: 10.1038/35069068. [DOI] [PubMed] [Google Scholar]
- Sah P, Isaacson JS. Channels underlying the slow afterhyperpolarization in hippocampal pyramidal neurons: neurotransmitters modulate the open probability. Neuron. 1995;15:435–435. doi: 10.1016/0896-6273(95)90047-0. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nature Reviews. Neuroscience. 2009;10:383–390. doi: 10.1038/nm2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Humphrey MF, Wässle H. Action and localization of acetylcholine in the cat retina. Journal of Neurophysiology. 1987;58:997–1015. doi: 10.1152/jn.1987.58.5.997. [DOI] [PubMed] [Google Scholar]
- Sernagor E, Eglen SJ, O'Donovan MJ. Differential effects of acetylcholine and glutamate blockade on the spatiotemporal dynamics of retinal waves. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20:RC56–RC56. doi: 10.1523/JNEUROSCI.20-02-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sernagor E, Eglen SJ, Wong RO. Development of retinal ganglion cell structure and function. Progress in Retinal and Eye Research. 2001;20:139–174. doi: 10.1016/s1350-9462(00)00024-0. [DOI] [PubMed] [Google Scholar]
- Sernagor E, Grzywacz NM. Influence of spontaneous activity and visual experience on developing retinal receptive fields. Current Biology: CB. 1996;6:1503–1508. doi: 10.1016/s0960-9822(96)00755-5. [DOI] [PubMed] [Google Scholar]
- Sernagor E, Young C, Eglen SJ. Developmental modulation of retinal wave dynamics: shedding light on the GABA saga. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23:7621–7629. doi: 10.1523/JNEUROSCI.23-20-07621.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma RK, Ehinger B. Mitosis in developing rabbit retina: an immunohistochemical study. Experimental Eye Research. 1997;64:97–106. doi: 10.1006/exer.1996.0189. [DOI] [PubMed] [Google Scholar]
- Singer JH, Mirotznik RR, Feller MB. Potentiation of L-type calcium channels reveals nonsynaptic mechanisms that correlate spontaneous activity in the developing mammalian retina. J Neurosci. 2001;21:8514–8522. doi: 10.1523/JNEUROSCI.21-21-08514.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AW, Millar TJ, Ishimoto I, Epstein ML, Johnson CD, Dahl JL, Morgan IG. Localization of choline acetyltransferase-like immunoreactivity in the embryonic chick retina. J Comp Neurol. 1987;260:526–538. doi: 10.1002/cne.902600406. [DOI] [PubMed] [Google Scholar]
- Spitzer NC, Root CM, Borodinsky LN. Orchestrating neuronal differentiation: patterns of Ca2+ spikes specify transmitter choice. Trends Neurosci. 2004;27:415–421. doi: 10.1016/j.tins.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Stacy RC, Demas J, Burgess RW, Sanes JR, Wong ROL. Disruption and Recovery of Patterned Retinal Activity in the Absence of Acetylcholine. J. Neurosci. 2005;25:9347–9357. doi: 10.1523/JNEUROSCI.1800-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy RC, Wong ROL. Developmental relationship between cholinergic amacrine cell processes and ganglion cell dendrites of the mouse retina. The Journal of Comparative Neurology. 2003;456:154–166. doi: 10.1002/cne.10509. [DOI] [PubMed] [Google Scholar]
- Stafford BK, Sher A, Litke AM, Feldheim DA. Spatial-Temporal Patterns of Retinal Waves Underlying Activity-Dependent Refinement of Retinofugal Projections. Neuron. 2009;64:200–212. doi: 10.1016/j.neuron.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ, Feller MB. Dynamics of Retinal Waves Are Controlled by Cyclic AMP. Neuron. 1999;24:673–685. doi: 10.1016/s0896-6273(00)81121-6. [DOI] [PubMed] [Google Scholar]
- Strang CE, Andison ME, Amthor FR, Keyser KT. Rabbit retinal ganglion cells express functional alpha7 nicotinic acetylcholine receptors. American Journal of Physiology. Cell Physiology. 2005;289:C644–C655. C644–C655. doi: 10.1152/ajpcell.00633.2004. [DOI] [PubMed] [Google Scholar]
- Sun C, Warland DK, Ballesteros JM, van der List D, Chalupa LM. Retinal waves in mice lacking the β2 subunit of the nicotinic acetylcholine receptor. Proceedings of the National Academy of Sciences. 2008;105:13638–13643. doi: 10.1073/pnas.0807178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed MM, Lee S, He S, Zhou ZJ. Spontaneous waves in the ventricular zone of developing mammalian retina. Journal of Neurophysiology. 2004a;91:1999–2009. doi: 10.1152/jn.01129.2003. [DOI] [PubMed] [Google Scholar]
- Syed MM, Lee S, Zheng J, Zhou ZJ. Stage-dependent dynamics and modulation of spontaneous waves in the developing rabbit retina. J Physiol. 2004b;560:533–549. doi: 10.1113/jphysiol.2004.066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchi M, Masland RH. The shape and arrangement of the cholinergic neurons in the rabbit retina. Proc R Soc Lond B Biol Sci. 1984;223:101–119. doi: 10.1098/rspb.1984.0085. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Vaney DI. New directions in retinal research. Trends in Neurosciences. 2003;26:379–385. doi: 10.1016/S0166-2236(03)00167-X. [DOI] [PubMed] [Google Scholar]
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog Neurobiol. 2005;76:213–235. doi: 10.1016/j.pneurobio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Vaney DI. 'Coronate' amacrine cells in the rabbit retina have the 'starburst' dendritic morphology. Proceedings of the Royal Society of London. Series B, Containing Papers of a Biological Character. Royal Society (Great Britain) 1984;220:501–508. doi: 10.1098/rspb.1984.0016. [DOI] [PubMed] [Google Scholar]
- Vaney DI. The mosaic of amacrine cells in the mammalian retina. Oxford, ROYAUME-UNI: Pergamon Press; 1990. [Google Scholar]
- Vogalis F, Furness JB, Kunze WA. Afterhyperpolarization current in myenteric neurons of the guinea pig duodenum. Journal of Neurophysiology. 2001;85:1941–1951. doi: 10.1152/jn.2001.85.5.1941. [DOI] [PubMed] [Google Scholar]
- Voinescu PE, Emanuela P, Kay JN, Sanes JR. Birthdays of retinal amacrine cell subtypes are systematically related to their molecular identity and soma position. The Journal of Comparative Neurology. 2009;517:737–750. doi: 10.1002/cne.22200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-T, Blankenship AG, Anishchenko A, Elstrott J, Fikhman M, Nakanishi S, Feller MB. GABA(A) receptor-mediated signaling alters the structure of spontaneous activity in the developing retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27:9130–9140. doi: 10.1523/JNEUROSCI.1293-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MM, Janz R, Belizaire R, Frishman LJ, Sherry DM. Differential distribution and developmental expression of synaptic vesicle protein 2 isoforms in the mouse retina. The Journal of Comparative Neurology. 2003;460:106–122. doi: 10.1002/cne.10636. [DOI] [PubMed] [Google Scholar]
- Warland DK, Huberman AD, Chalupa LM. Dynamics of spontaneous activity in the fetal macaque retina during development of retinogeniculate pathways. J Neurosci. 2006;26:5190–5197. doi: 10.1523/JNEUROSCI.0328-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Wei W, Hamby AM, Zhou K, Feller MB. Development of asymmetric inhibition underlying direction selectivity in the retina. Nature. 2011;469:402–406. doi: 10.1038/nature09600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West Greenlee MH, Finley SK, Wilson MC, Jacobson CD, Sakaguchi DS. Transient, high levels of SNAP-25 expression in cholinergic amacrine cells during postnatal development of the mammalian retina. The Journal of Comparative Neurology. 1998;394:374–385. doi: 10.1002/(sici)1096-9861(19980511)394:3<374::aid-cne8>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- Wong RO. Cholinergic regulation of [Ca2+]i during cell division and differentiation in the mammalian retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1995;15:2696–2706. doi: 10.1523/JNEUROSCI.15-04-02696.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RO, Collin SP. Dendritic maturation of displaced putative cholinergic amacrine cells in the rabbit retina. The Journal of Comparative Neurology. 1989;287:164–178. doi: 10.1002/cne.902870203. [DOI] [PubMed] [Google Scholar]
- Wong RO, Meister M, Shatz CJ. Transient period of correlated bursting activity during development of the mammalian retina. Neuron. 1993;11:923–938. doi: 10.1016/0896-6273(93)90122-8. [DOI] [PubMed] [Google Scholar]
- Wong RO, Oakley DM. Changing patterns of spontaneous bursting activity of on and off retinal ganglion cells during development. Neuron. 1996;16:1087–1095. doi: 10.1016/s0896-6273(00)80135-x. [DOI] [PubMed] [Google Scholar]
- Wong WT, Myhr KL, Miller ED, Wong RO. Developmental changes in the neurotransmitter regulation of correlated spontaneous retinal activity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20:351–360. doi: 10.1523/JNEUROSCI.20-01-00351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WT, Sanes JR, Wong RO. Developmentally regulated spontaneous activity in the embryonic chick retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1998;18:8839–8852. doi: 10.1523/JNEUROSCI.18-21-08839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WT, Wong RO. Changing specificity of neurotransmitter regulation of rapid dendritic remodeling during synaptogenesis. Nature Neuroscience. 2001;4:351–352. doi: 10.1038/85987. [DOI] [PubMed] [Google Scholar]
- Xu H, Tian N. Pathway-specific maturation, visual deprivation, and development of retinal pathway. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2004;10:337–346. doi: 10.1177/1073858404265254. [DOI] [PubMed] [Google Scholar]
- Xu HP, Chen H, Ding Q, Xie ZH, Chen L, Diao L, Wang P, Gan L, Crair MC, Tian N. The immune protein CD3zeta is required for normal development of neural circuits in the retina. Neuron. 2010;65:503–515. doi: 10.1016/j.neuron.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Fina ME, Vardi N. Regulation of KCC2 and NKCC during development: membrane insertion and differences between cell types. J Comp Neurol. 2006a;499:132–143. doi: 10.1002/cne.21100. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Pathak HR, Coulter DA, Freed MA, Vardi N. Shift of intracellular chloride concentration in ganglion and amacrine cells of developing mouse retina. J Neurophysiol. 2006b;95:2404–2416. doi: 10.1152/jn.00578.2005. [DOI] [PubMed] [Google Scholar]
- Zheng J, Lee S, Zhou ZJ. A transient network of intrinsically bursting starburst cells underlies the generation of retinal waves. Nature Neuroscience. 2006;9:363–371. doi: 10.1038/nn1644. [DOI] [PubMed] [Google Scholar]
- Zheng JJ, Lee S, Zhou ZJ. A developmental switch in the excitability and function of the starburst network in the mammalian retina. Neuron. 2004;44:851–864. doi: 10.1016/j.neuron.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Zhou ZJ. Direct Participation of Starburst Amacrine Cells in Spontaneous Rhythmic Activities in the Developing Mammalian Retina. The Journal of Neuroscience. 1998;18:4155–4165. doi: 10.1523/JNEUROSCI.18-11-04155.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZJ, Fain GL. Starburst amacrine cells change from spiking to nonspiking neurons during retinal development. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8057–8062. doi: 10.1073/pnas.93.15.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZJ, Zhao D. Coordinated transitions in neurotransmitter systems for the initiation and propagation of spontaneous retinal waves. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20:6570–6577. doi: 10.1523/JNEUROSCI.20-17-06570.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]