Abstract
C/EBPβ is an auto-repressed protein that becomes post-translationally activated by Ras-MEK-ERK signalling. C/EBPβ is required for oncogene-induced senescence (OIS) of primary fibroblasts, but also displays pro-oncogenic functions in many tumour cells. Here, we show that C/EBPβ activation by H-RasV12 is suppressed in immortalized/transformed cells, but not in primary cells, by its 3′ untranslated region (3′UTR). 3′UTR sequences inhibited Ras-induced cytostatic activity of C/EBPβ, DNA binding, transactivation, phosphorylation, and homodimerization, without significantly affecting protein expression. The 3′UTR suppressed induction of senescence-associated C/EBPβ target genes, while promoting expression of genes linked to cancers and TGFβ signalling. An AU-rich element (ARE) and its cognate RNA-binding protein, HuR, were required for 3′UTR inhibition. These components also excluded the Cebpb mRNA from a perinuclear cytoplasmic region that contains activated ERK1/2, indicating that the site of C/EBPβ translation controls de-repression by Ras signalling. Notably, 3′UTR inhibition and Cebpb mRNA compartmentalization were absent in primary fibroblasts, allowing Ras-induced C/EBPβ activation and OIS to proceed. Our findings reveal a novel mechanism whereby non-coding mRNA sequences selectively regulate C/EBPβ activity and suppress its anti-oncogenic functions.
Keywords: C/EBPβ, mRNA trafficking, oncogene-induced senescence, Ras signalling, 3’UTR
Introduction
Expression of activated Ras, Raf or other oncoproteins in primary cells often provokes cellular senescence, a permanent state of proliferative arrest that is activated by oncogenic stress (Campisi, 2005; Collado et al, 2007). This cytostatic response, termed oncogene-induced senescence (OIS), functions as an intrinsic tumour suppression mechanism in cells that have sustained critical levels of oncogenic flux (Sarkisian et al, 2007; Prieur and Peeper, 2008). OIS is implemented primarily through induction of the p19Arf-p53 tumour suppressor pathway and CDK inhibitors such as p16Ink4a and p21CIP1 that activate Rb-dependent checkpoints (Adams, 2009). Disruption of these and other anti-oncogenic effectors through mutation or epigenetic silencing leads to senescence bypass and renders cells susceptible to transformation by Ras and other oncogenes (Lowe et al, 2004).
Recent studies have also implicated the transcription factor C/EBPβ and pro-inflammatory mediators such as IL-6, chemokines, and their receptors, comprising a ‘senescence-associated secretory phenotype’ (SASP), in senescence induction triggered by oncogenes or DNA damage (Sebastian et al, 2005; Acosta et al, 2008; Kuilman et al, 2008; Rodier et al, 2009). Genetic experiments demonstrate that C/EBPβ is required for Ras- or BRAF-induced senescence of mouse embryonic fibroblasts (MEFs; Sebastian et al, 2005) and human diploid fibroblasts (Acosta et al, 2008; Kuilman et al, 2008), in part through its ability to activate SASP genes and p15Ink4b. Although C/EBPβ and SASP genes are important regulators of OIS, they differ from classical tumour suppressors in that they are rarely, if ever, inactivated in cancers and also exert pro-oncogenic effects in many transformed cells (Sebastian and Johnson, 2006; Mantovani et al, 2008). Currently, it is unclear how such factors can be critical for establishing senescence while promoting cancer in other contexts.
C/EBPβ is maintained in a latent, low-activity state by several auto-inhibitory elements that suppress its DNA-binding and transactivation functions (Kowenz-Leutz et al, 1994; Williams et al, 1995; Lee et al, 2010a). In response to oncogenic Ras or other stimuli, C/EBPβ becomes de-repressed by signalling through the RAF-MEK-ERK cascade (Nakajima et al, 1993; Kowenz-Leutz et al, 1994; Lee et al, 2010b), in part due to phosphorylation on Thr188 (mouse C/EBPβ) by ERK1/2 that leads to altered binding of mediator complexes (Mo et al, 2004). Oncogenic Ras also stimulates C/EBPβ's anti-proliferative activity and increases the ratio of C/EBPβ homodimers to C/EBPβ:C/EBPγ heterodimers by a mechanism involving phosphorylation on leucine zipper residue Ser273 by p90Rsk kinases (Lee et al, 2010b). These observations, together with the fact that C/EBPγ-deficient MEFs display severe proliferative defects, have led to the notion that the hyperactivated, homodimeric form of C/EBPβ contributes to Ras-induced cell-cycle arrest and senescence in primary cells, whereas β:γ heterodimers are permissive for, or actively promote, mitotic growth (Lee et al, 2010b). However, this model does not explain how transformed cells, particularly those harbouring Ras or BRAF oncogenes, evade the anti-proliferative effects of activated C/EBPβ. In NIH 3T3 cells, endogenous C/EBPβ expression is downregulated by RasV12, providing one possible mechanism (Sebastian and Johnson, 2009). Nevertheless, many transformed cells express relatively high levels of C/EBPβ, suggesting that other means exist to constrain its anti-proliferative activity.
Here, we report the unexpected finding that Ras-induced post-translational activation of C/EBPβ is inhibited by the 3′ untranslated region (3′UTR) of its mRNA, suppressing the cytostatic and pro-senescence functions of C/EBPβ selectively in immortalized and transformed cells. These observations, thus, identify a new function for 3′UTRs and suggest a further basis for senescence bypass in cancer cells.
Results
The Cebpb 3′UTR blocks the Ras-induced cytostatic functions of C/EBPβ
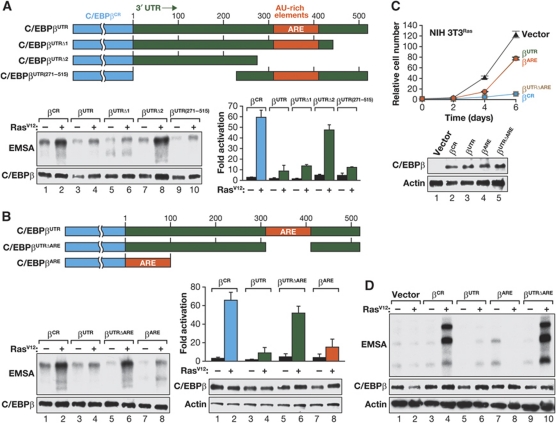
Introduction of oncogenic H-RasV12 in immortalized NIH 3T3 fibroblasts silences C/EBPβ expression through a mechanism requiring absence of the tumour suppressor p19Arf (Sebastian and Johnson, 2009). Ectopic C/EBPβ expression in RasV12-transformed NIH 3T3 (3T3Ras) cells suppresses proliferation and transformation and induces a flat, senescent-like morphology, suggesting that downregulation of endogenous C/EBPβ is involved in neoplastic transformation of these cells. To explore a possible role for Cebpb 3′UTR sequences in C/EBPβ downregulation by RasV12 (e.g., via miRNA-mediated silencing), we used retroviral infection to introduce the C/EBPβ coding region alone (C/EBPβCR) or the coding region plus 3′UTR (C/EBPβUTR) in NIH 3T3 or 3T3Ras cells. The 3′UTR did not significantly affect C/EBPβ protein levels (Figure 1A, bottom panel, lanes 4 and 6), indicating that 3′UTR elements do not confer C/EBPβ silencing in this context. Interestingly, however, the proteins expressed from the two constructs exerted very different effects on cell proliferation. C/EBPβCR inhibited mitotic growth in a Ras-dependent manner, as observed previously (Figure 1A; Supplementary Figure S1A; Sebastian and Johnson, 2009). By contrast, C/EBPβUTR displayed greatly reduced anti-proliferative activity relative to C/EBPβCR despite similar nuclear protein levels and mRNA expression (Figure 1A; Supplementary Figure S1B). Cells expressing C/EBPβUTR also did not acquire the flattened morphology or expression of the senescence marker SA-βGal that were elicited by C/EBPβCR (Figure 1B). Moreover, C/EBPβCR potently inhibited the ability of 3T3Ras cells to form transformed foci and grow as colonies in soft agar, whereas C/EBPβUTR was much less active in suppressing these transformed phenotypes (Supplementary Figure S1C). To ensure that the UTR effect was not an artefact of expressing C/EBPβ from a retroviral transcript, we generated stable 3T3Ras transfectants carrying inducible C/EBPβCR or C/EBPβUTR plasmid vectors. A comparison of two clones expressing equivalent levels of C/EBPβCR and C/EBPβUTR confirmed that the 3′UTR efficiently blocks the anti-proliferative activity of C/EBPβ (Supplementary Figure S1D). Collectively, these observations suggested that the 3′UTR regulates C/EBPβ function rather than its expression.
Figure 1.
Suppression of Ras-induced C/EBPβ activation and its anti-proliferative functions by the 3′UTR. (A) Growth rates of control NIH 3T3 and 3T3Ras cells infected with retroviruses expressing the C/EBPβ coding region alone (C/EBPβCR) or coding region+3′UTR (C/EBPβUTR). Data are the mean values±s.d. from three experiments, each point assayed in triplicate. Lower panel: C/EBPβ western blot of nuclear extracts from the infected cells. (B) Morphology and senescence features of 3T3Ras cells transduced with C/EBPβCR or C/EBPβUTR. Cells expressing C/EBPβCR exhibit decreased proliferation and a flattened cellular morphology (left panels). SA-βGal staining (right) demonstrates senescent-like properties of many cells expressing C/EBPβCR but not C/EBPβUTR. The percentage of SA-βGal-positive cells is shown in the inset. (C) DNA-binding assays (EMSA) of nuclear extracts from cells described in (A). EMSA was performed using a canonical C/EBP site probe. The upper band represents a C/EBPβ homodimer; the intermediate band is composed of β:γ heterodimers and a second unidentified species. Bottom panel: C/EBPβ levels assessed by western blotting. (D) Antibody supershift assays were used to determine the composition of C/EBP EMSA complexes in C/EBPβCR-expressing NIH 3T3 cells. Lanes 1 and 2 show HEK293T cells transfected with C/EBPβCR±RasV12; the upper complex corresponds to C/EBPβ homodimers. The faster-migrating complex present in C/EBPβCR-expressing 3T3Ras cells (lanes 3–5) is a mixture of β:γ heterodimers and a second unidentified DNA-binding species. (E) DNA-binding and transactivation assays of C/EBPβ proteins transiently expressed in 293T cells. Cells were transfected with the indicated vectors and, after serum starvation, nuclear extracts were prepared, normalized for C/EBPβ levels and analysed by EMSA (upper). Transactivation (lower) was analysed using a C/EBP reporter (2xC/EBP-luc). Values represent fold activation over the reporter alone and are the mean values±s.d. of four experiments. C/EBPβ protein levels are shown below the graph.
Electrophoretic mobility shift assay (EMSA) analysis of nuclear extracts from the NIH 3T3 cell lines demonstrated that C/EBPβCR DNA binding was enhanced by RasV12 and homodimeric complexes were increased (Figure 1C and D; Lee et al, 2010b). However, C/EBPβUTR DNA binding was unaffected by Ras and homodimer levels remained low. Ras also stimulated C/EBPβCR-driven transactivation of a reporter gene (2xC/EBP-luc) in transiently transfected NIH 3T3 cells, and this response was blunted by the 3′UTR (see Figure 3E). The 3′UTR effect was not limited to NIH 3T3 cells, as Ras-induced DNA binding and transactivation by C/EBPβUTR was also reduced in transiently transfected human HEK293T (293T) cells (Figure 1E) and in HeLa cells (Supplementary Figure S2A). Thus, the 3′UTR blocks post-translational activation of C/EBPβ in several immortalized/transformed cell lines.
C/EBPβ DNA binding is auto-inhibited by three elements in the N-terminal region of the protein, and mutation of these sequences increases basal DNA-binding activity (Lee et al, 2010a). Appending the 3′UTR to this de-repressed triple mutant did not inhibit its constitutive DNA binding (Supplementary Figure S2B), showing that the 3′UTR specifically blocks signal-induced activation of C/EBPβ and not its binding activity in general. Furthermore, co-expressing a transcript containing the Cebpb 3′UTR alone in trans did not decrease C/EBPβCR DNA binding (Supplementary Figure S2C). Hence, the 3′UTR acts in cis to suppress C/EBPβ post-translational activation.
Since the related C/EBPα protein also has strong cytostatic activity (Johnson, 2005), we investigated whether the Cebpa 3′UTR exerts similar inhibitory effects. Like C/EBPβCR, C/EBPαCR suppressed NIH 3T3 cell proliferation in a Ras-dependent manner, and the Cebpa 3′UTR substantially blocked this anti-proliferative function without affecting C/EBPα expression (Supplementary Figure S3, upper panel). Moreover, fusing the Cebpa 3′UTR to the Cebpb coding region (C/EBPβαUTR) repressed C/EBPβ's ability to inhibit cell proliferation (Supplementary Figure S3, lower panel). Thus, the 3′UTRs of two Cebp genes contain transferable regulatory information that inhibits the Ras-induced cytostatic functions of both proteins.
An AU-rich element and its cognate binding protein, HuR, are required for 3′UTR inhibition
To identify 3′UTR sequences that inhibit C/EBPβ activity, we tested a set of 3′UTR deletion mutants in transfected 293T cells. This analysis showed that a region near the 3′-end of the UTR was required to suppress DNA binding and transactivation (Figure 2A). The inhibitory region spans a sequence previously shown to contain A/U-rich elements (AREs; Gantt et al, 2005; Cherry et al, 2008), regulatory motifs found in many 3′UTRs that control mRNA decay and translation (Barreau et al, 2005). The Cebpb 3′UTR contains a class III ARE composed of several G(U)4−6G repeats and lacks canonical AUUUA elements found in class I and II AREs (Xu et al, 1997). To test its role in C/EBPβ inhibition, we deleted the 110-nt Cebpb ARE region. This mutation (ΔARE) abolished the inhibitory effect of the 3′UTR (Figure 2B). Moreover, the ARE segment alone was nearly as effective as the complete 3′UTR in suppressing C/EBPβ activity. Analysis of the mutant constructs in 3T3Ras cells showed that ΔARE was unable to inhibit C/EBPβ-induced growth arrest, whereas the ARE alone efficiently repressed C/EBPβ (Figure 2C). EMSA confirmed that Ras-induced C/EBPβ DNA binding was also inhibited by the ARE in these cells (Figure 2D). Hence, the ARE region is necessary and sufficient to suppress C/EBPβ activation by oncogenic Ras.
Figure 2.
An AU-rich element (ARE) region is necessary and sufficient for 3′UTR inhibition. (A) Analysis of Cebpb 3′UTR deletion mutants in 293T cells. The constructs depicted in the diagram were tested for C/EBPβ DNA binding (left) and transactivation (right) in transiently transfected cells. Transactivation data are the mean values±s.d. from three experiments. (B) Critical role of the ARE region for Cebpb 3′UTR repression. The constructs shown were analysed as described in (A). Transactivation data are the mean values±s.d. from three experiments. (C) Cytostatic activity of C/EBPβ proteins expressed from WT and 3′UTR mutant constructs in 3T3Ras cells. Data are the mean values±s.d. from two experiments, each point assayed in triplicate. Western blot shows C/EBPβ expression levels in the transduced cells. (D) DNA-binding assays of nuclear extracts from cells described in (C). Extracts from non-transformed NIH 3T3 cells (i.e., without RasV12) infected with the same constructs are included for comparison.
AREs are recognized by several sequence-specific RNA-binding proteins, including members of the Elav1/Hu protein family (Barreau et al, 2005). The ubiquitously expressed HuR protein binds to numerous mRNAs and controls their stability and translation (Abdelmohsen et al, 2008). Because HuR was shown to associate with the Cebpb ARE (Gantt et al, 2005; Cherry et al, 2008), we examined its role in 3′UTR inhibition. shRNA knockdown of HuR in 293T cells (HuRKD) largely abolished 3′UTR inhibition of C/EBPβ DNA binding and transactivation (Figure 3A and C), and inhibition was restored by re-expression of HuR (Figure 3B and C). HuR depletion did not affect activation of Ras effector kinases, as judged by induction of phosphorylated ERK, RSK, and Akt, nor was signalling disrupted by expression of the 3′UTR construct (Supplementary Figure S4). Thus, the inhibitory effects of the 3′UTR are not due to impaired Ras signalling.
Figure 3.
HuR is required for 3′UTR inhibition. (A) 3′UTR inhibition is disrupted in HuR knockdown (HuRKD) 293T cells. Nuclear extracts normalized for C/EBPβ levels were analysed by EMSA. Western blot shows HuR levels in control and HuRKD cells. (B) Re-expression of HuR in HuRKD cells restores 3′UTR inhibition. HuR was co-transfected with the indicated constructs in 293T-HuRKD cells. (C) Transcriptional activity of C/EBPβUTR depends on HuR. Transactivation of the 2 × C/EBP-luc reporter was compared in control and HuRKD cells. Re-expression of HuR restored inhibition by the 3′UTR without significantly affecting C/EBPβCR activity. Data are the mean values±s.d. from two experiments. (D) HuR knockdown in NIH 3T3 cells abolishes 3′UTR inhibition of C/EBPβ cytostatic activity. Control NIH 3T3 cells (upper panel) or 3T3Ras cells (lower panel) were infected with retroviruses expressing shHuR or the empty vector. The cells were subsequently infected with the indicated C/EBPβ constructs and proliferation rates were determined. Data are the mean values±s.d. from two experiments, each point assayed in triplicate. Extracts were analysed for C/EBPβ and HuR expression by western blotting (right). (E) 3′UTR inhibition of C/EBPβ transactivation is impaired in HuRKD NIH 3T3 cells. Transient transactivation assays using the 2 × C/EBP-luc reporter were performed in control and HuRKD NIH 3T3 cells. Data are the mean values±s.d. from two experiments. Ectopic C/EBPβ protein expression could not be assessed due to high levels of endogenous C/EBPβ in these cells.
We also depleted HuR in NIH 3T3 cells to assess its role in 3′UTR-mediated inhibition of C/EBPβ cytostatic activity. HuR silencing alone moderately decreased proliferation of control and Ras-transformed cells (Figure 3D). C/EBPβCR did not appreciably alter the growth of non-transformed 3T3-HuRKD cells but blocked proliferation of 3T3Ras cells, regardless of their HuR status. By contrast, C/EBPβUTR only weakly inhibited the expansion of 3T3Ras cells but displayed potent cytostatic activity in HuRKD-3T3Ras cells (Figure 3D; Supplementary Figure S5A). Moreover, C/EBPβUTR elicited a flattened morphology in HuRKD-3T3Ras cells (Supplementary Figure S5B), but not in 3T3Ras cells (Figure 1B). Hence, HuR is essential for the ability of the 3′UTR to suppress C/EBPβ activation by Ras.
We further investigated whether HuR suppresses endogenous C/EBPβ activity in human tumour cells, since this mechanism could be involved in establishing the transformed phenotype. HuR was ablated in A549 lung tumour cells and proliferation was assessed. HuR silencing decreased cell growth and colony formation (Supplementary Figure S6A), consistent with studies showing that HuR depletion inhibits proliferation of RKO colorectal carcinoma cells (Wang et al, 2000). To determine if this cytostatic response involves C/EBPβ, we depleted C/EBPβ in conjunction with HuR. C/EBPβ knockdown in A549 cells overcame the effect of HuR ablation and increased proliferation and colony formation to levels exceeding even those of control cells. HuR silencing also led to a modest increase in endogenous C/EBPβ DNA binding (Supplementary Figure S6B). Similar results were obtained using MCF7 breast cancer cells and HeLa cells (Supplementary Figure S6C and D). Thus, HuR ablation impairs proliferation of several immortalized human tumour cell lines in a manner that is partially dependent on C/EBPβ, supporting the notion that HuR suppresses C/EBPβ cytostatic activity in tumour cells.
Ras-induced post-translational modifications and homodimerization of C/EBPβ are suppressed by the 3′UTR
We next asked whether Ras-induced modifications on C/EBPβ are regulated by the 3′UTR. Ser64 and Thr188 are phosphorylated by Cdk and ERK1/2 kinases, respectively, in response to oncogenic Ras (Nakajima et al, 1993; Shuman et al, 2004). Analysis by western blotting using phospho-specific antibodies showed that both modifications were induced by Ras in C/EBPβCR, but were nearly undetectable in C/EBPβUTR (Figure 4A). By contrast, C/EBPβUTR underwent phosphorylation on Ser64 and Thr188 in HuR-depleted 293T cells (Figure 4B). Therefore, the 3′UTR blocks inducible phosphorylation on Ser64 and Thr188 in a HuR-dependent manner, consistent with C/EBPβUTR remaining in a low-activity state.
Figure 4.
The 3′UTR inhibits Ras-induced post-translational modifications and dimerization of C/EBPβ. (A) Ras-induced p-Ser64 and p-Thr188 modifications are suppressed by the 3′UTR. C/EBPβCR and C/EBPβUTR were expressed in 293T cells without or with RasV12. Nuclear extracts were analysed by western blotting using the indicated phospho-specific or conventional C/EBPβ antibodies. (B) 3′UTR inhibition of C/EBPβ phosphorylation is disrupted in HuR knockdown cells. The experiment described in (A) was performed using 293T-HuRKD and control cells. (C) 3′UTR inhibition maintains efficient C/EBPβ heterodimerization with C/EBPγ in the presence of Ras signalling. C/EBPβCR and C/EBPβUTR were expressed with RasV12 and increasing amounts of C/EBPγ in 293T cells, and nuclear extracts were analysed by EMSA. (D) C/EBPβCR and C/EBPβUTR transactivation of a luciferase reporter in the presence of RasV12 and increasing amounts of C/EBPγ. Luciferase activity for the C/EBPβ constructs in the absence of C/EBPγ (control) was set to 100%, and the other values represent percent of control. Data are the mean values±s.d. of three experiments.
Ras-induced activation of C/EBPβ is associated with phosphorylation-mediated stabilization of homodimers that are resistant to displacement by C/EBPγ (Lee et al, 2010b). To determine whether the 3′UTR affects C/EBPβ dimerization with C/EBPγ, we expressed C/EBPβ and RasV12 in the presence of increasing amounts of C/EBPγ. EMSA showed that C/EBPβCR heterodimerization with C/EBPγ was relatively inefficient, whereas C/EBPβUTR readily formed heterodimers, especially at the highest dose of C/EBPγ (Figure 4C). Similar results were seen in reporter assays, where increasing amounts of C/EBPγ inhibited C/EBPβUTR transcriptional activity while that of C/EBPβCR was largely unaffected (Figure 4D). These findings indicate that the 3′UTR suppresses formation of transcriptionally active C/EBPβ homodimers, which promote cell-cycle arrest and OIS, and allows preferential heterodimerization with C/EBPγ.
The presence of the 3′UTR alters expression of C/EBPβ target genes
To confirm that C/EBPβCR and C/EBPβUTR possess distinct regulatory activities and to identify target genes that are differentially induced by these two proteins in the presence of oncogenic Ras, we used microarrays to compare the transcriptomes of 3T3Ras, C/EBPβCR-3T3Ras, and C/EBPβUTR-3T3Ras cell lines. Using thresholds of ⩾2-fold change and P⩽0.001, 558 probes (409 genes) displayed significant upregulation by C/EBPβCR relative to control 3T3Ras cells (Figure 5A and B; Supplementary Table S1). By the same criteria, 208 probes (167 genes) were activated by C/EBPβUTR. In all, 124 probes (97 genes) constituted an intersecting set induced by both proteins. Thus, of the genes induced by C/EBPβCR, 76% (312/409) were not significantly activated by C/EBPβUTR. However, even this number underrepresents the inhibitory effect of the 3′UTR, since many of the transcripts upregulated by C/EBPβUTR were activated to a much greater extent by C/EBPβCR (Supplementary Figure S7; Supplementary Table S1). Interestingly, 84 probes (70 genes) were induced by C/EBPβUTR but not by C/EBPβCR, showing that the ‘low-activity’ C/EBPβUTR protein is able to stimulate gene transcription in a manner that is qualitatively distinct from the ‘high activity’ C/EBPβCR form.
Figure 5.
The Cebpb 3′UTR alters C/EBPβ target gene specificity. (A) Heat maps depicting expression levels of genes selectively induced by C/EBPβCR (left), C/EBPβUTR (right), or both proteins (middle). Maps show microarray data from three independently derived cell lines each for 3T3Ras, C/EBPβCR-3T3Ras, and C/EBPβUTR-3T3Ras cells. Darker shading indicates higher expression; dendrograms show hierarchical clustering patterns. (B) Venn diagram showing differential activation of target genes by C/EBPβCR and C/EBPβUTR in 3T3Ras cells. Gene array data were filtered as described in the text to identify significantly upregulated genes. Genes induced significantly by both proteins are depicted in the intersection. (C) qPCR analysis of C/EBPβ-induced SASP genes and other pro-inflammatory mediators. RNA from the indicated NIH 3T3 cell lines was analysed for expression of individual transcripts using quantitative PCR. Data are the mean values±s.d. from two experiments, each assayed in triplicate. (D) Pathway and disease associations of gene signatures selectively activated by C/EBPβCR or C/EBPβUTR in 3T3Ras cells. Genes corresponding to the non-overlapping groups shown in this figure and Supplementary Table S1 were analysed using GeneGo MetaCore software to identify associations with canonical biological pathways or disease states. Pattern matches were ranked by P-value (LOD score) and the top 10 categories are shown.
We also examined expression of individual senescence-associated genes. Several inflammatory SASP genes (IL-6, GROα/Cxcl-1, Cxcl-2, Cxcl-5, and Ccr-1) ranked within the 400 transcripts showing highest fold induction by C/EBPβCR. Quantitative PCR demonstrated that these genes were induced to a much greater extent by C/EBPβCR than by C/EBPβUTR (Figure 5C). For example, Cxcl-1 mRNA was induced 253-fold by C/EBPβCR (relative to 3T3Ras cells) and only 4.3-fold by C/EBPβUTR. Similarly, IL-1α, IL-1β, and S100a9 showed strong preferential activation by C/EBPβCR. In several cases, these transcripts were diminished in 3T3Ras cells compared with control NIH 3T3 cells, possibly due to silencing of endogenous C/EBPβ expression by oncogenic Ras (Sebastian and Johnson, 2009). Other C/EBPβCR-activated genes identified in the arrays that are known to regulate cell-cycle arrest, cell adhesion/migration, or tumour suppression include schlafen2, stefin A3, Btg2, p53 interacting protein 1, Vgll3, and thrombospondin 1 (Thbs1/TSP-1) (Li et al, 2005; Tomasini et al, 2005; Kazerounian et al, 2008; Cody et al, 2009; Katsoulidis et al, 2009; Winkler, 2010). qPCR confirmed that each of these transcripts was significantly increased by C/EBPβCR but was induced much less efficiently by C/EBPβUTR (Supplementary Figure S7B). For example, Stfa3 expression was increased 6600-fold by C/EBPβCR but only 290-fold by C/EBPβUTR, and Vgll3 was induced 130-fold by C/EBPβCR versus 10-fold by C/EBPβUTR. Hence, the impaired activation of several SASP, anti-proliferative, and tumour suppressor genes is consistent with the diminished cytostatic potential of C/EBPβUTR.
We analysed the gene signatures induced by C/EBPβCR or C/EBPβUTR for associations with biological pathways or diseases using GeneGo MetaCore software. The 10 highest ranking associations for each category are shown in Figure 5D. C/EBPβCR-induced genes predominantly corresponded to pathways involved in inflammation and immune function (8/10 categories). By contrast, C/EBPβUTR targets were biased towards developmental pathways (4/10), cytoskeleton remodelling, and TGFβ signalling (4/10). Of note, C/EBPβUTR-activated genes were strongly correlated with neoplastic diseases (7/10 categories), while C/EBPβCR scored highly for cardiovascular diseases, inflammation, and wounds/injuries but not for cancers. The fact that the C/EBPβUTR signature is correlated with TGFβ signalling, epithelial–mesenchymal transition (EMT), and neoplastic diseases raises the intriguing possibility that this form of C/EBPβ has pro-oncogenic/metastatic functions.
The 3′UTR excludes Cebpb mRNA from a perinuclear cytoplasmic domain that contains activated ERK1/2 kinase
One possible mechanism for 3′UTR inhibition could involve localization of 3′UTR-containing transcripts to a cytoplasmic region where the newly translated protein cannot undergo activation. To address this possibility, we analysed the cytoplasmic location of Cebpb mRNAs using an mRNA tagging method in which transcripts are tethered to a nuclear-targeted GFP reporter (GFP-MS2-nls) via the RNA-binding domain of MS2 phage coat protein (Rook et al, 2000). Multiple copies of the MS2 binding site were appended to the CebpbCR and CebpbUTR transcripts and the constructs were analysed in transfected NIH 3T3 cells by confocal microscopy. The GFP reporter alone was exclusively nuclear due to the presence of the nuclear localization signal (Figure 6A). Co-expression of tagged CebpbCR mRNA resulted in detectable cytoplasmic fluorescence, with the GFP signal distributed relatively uniformly throughout the cytoplasm. The CebpbUTR transcript also promoted cytoplasmic localization of the GFP reporter, but in this case GFP was excluded from a region surrounding the nucleus, with fluorescence concentrated in more peripheral areas. Although such perinuclear exclusion was not observed for every GFP-positive cell (79.5±2.4%), the pattern was clearly distinct from that of CebpbCR mRNA, which was nearly always uniformly distributed (93.3±1.8%). Similar results were obtained using HeLa cells (Supplementary Figure S8). Hence, the 3′UTR restricts Cebpb mRNA to a peripheral cytoplasmic domain.
Figure 6.
The 3′UTR excludes Cebpb mRNA from a perinuclear region of the cytoplasm. (A) Localization of CebpbCR and CebpbUTR transcripts in NIH 3T3 cells using a GFP tethering assay. Cells were transiently transfected with the GFP-MS2-nls reporter alone or with tagged CebpbCR or CebpbUTR vectors. GFP-positive cells were imaged by confocal fluorescence microscopy. Nuclei were visualized by DAPI staining. The percentage of cells exhibiting the GFP fluorescence pattern depicted is indicated in parentheses. (B) The ARE is necessary and sufficient to direct Cebpb mRNA to the cytoplasmic periphery. Tagged CebpbUTRΔARE and CebpbARE transcripts were expressed with GFP-MS2-nls in NIH 3T3 cells and imaged by confocal microscopy. (C) HuR is required for perinuclear exclusion of CebpbUTR mRNA. HuRKD NIH 3T3 cells were transfected with tagged CebpbCR or CebpbUTR vectors and the GFP reporter. Control cells expressing the empty knockdown vector were analysed in parallel (Supplementary Figure S9).
Deleting the ARE led to uniform distribution of the CebpbUTR transcript (Figure 6B). Moreover, CebpbARE mRNA was peripherally localized in a pattern similar to CebpbUTR. Compartmentalization of the CebpbUTR transcript required HuR, as it displayed more uniform distribution in HuRKD NIH 3T3 cells compared with its peripheral restriction in control cells (Figure 6C; Supplementary Figure S9). Thus, the presence of Cebpb mRNA in the perinuclear region is strictly correlated with C/EBPβ activation and induction of cell-cycle arrest, while exclusion from this domain is associated with diminished protein activity. To further test the mRNA localization model, we replaced the 3′UTR with three copies of the β-actin 3′UTR ‘zipcode’ element, a sequence that directs β-actin transcripts to the peripheral cytoplasm and leading lamellae of migrating cells (Kislauskis et al, 1994). The Cebpb3 × ZIP transcript was predominantly localized to the periphery in NIH 3T3 cells, substantially mimicking the pattern observed for the Cebpb 3′UTR (Supplementary Figure S10). Significantly, the zipcode sequence also suppressed activation of C/EBPβ DNA binding and transactivation in NIH 3T3 cells and 293T cells (Supplementary Figure S11), although its inhibitory effect was somewhat less than that of the 3′UTR. This quantitative difference may result from less efficient exclusion of the zipcode-containing transcript from the perinuclear region. Nevertheless, our results corroborate the notion that exclusion of Cebpb mRNA from the perinuclear compartment blunts C/EBPβ post-translational activation by Ras signalling.
These data suggest that C/EBPβUTR protein synthesis in the cytoplasmic periphery could restrict its access to critical modifying enzyme(s). C/EBPβ activation by Ras is highly dependent on Raf-MEK-ERK signalling, inducing direct phosphorylation on Thr188 by ERK1/2 and other modifications (Nakajima et al, 1993; Mo et al, 2004; Lee et al, 2010b). Since Thr188 phosphorylation was blocked by the 3′UTR (Figure 4A), we examined the subcellular location of activated ERK1/2 in NIH 3T3 cells expressing a 4-OHT-inducible H-RasV12 protein, ER:RasV12 (Dajee et al, 2002). Western blotting showed significantly increased levels of phospho-ERK 24 and 48 h after induction (Figure 7A). Immunofluorescent staining of the induced cells revealed a strong p-ERK signal that occurs in a punctate pattern confined mainly to the perinuclear region (Supplementary Figure S12). Nuclear p-ERK levels were low or undetectable in these cells, showing that activated ERK is predominantly perinuclear in cells undergoing sustained Ras signalling. Similar perinuclear p-ERK localization was observed in RasV12-expressing (senescent) normal human fibroblasts (Gaumont-Leclerc et al, 2004). Notably, simultaneous imaging of p-ERK and Cebpb mRNA (GFP) revealed a striking overlap between p-ERK and the perinuclear region that is devoid of CebpbUTR mRNA (Figure 7B). These findings provide compelling evidence that C/EBPβ protein synthesis in the perinuclear compartment facilitates coupling with p-ERK (and possibly other C/EBPβ-activating kinases), while 3′UTR-mediated exclusion of the Cebpb transcript prevents functional interactions between nascent C/EBPβ and Ras effector kinase(s).
Figure 7.
Activated ERK1/2 localizes to the perinuclear region of RasV12-expressing NIH 3T3 cells. (A) Phospho-ERK1/2 is induced upon 4-OHT treatment of NIH 3T3 cells stably expressing ER:RasV12. Cells were starved for serum and lysates analysed for p-ERK and total ERK by immunoblotting following 4-OHT stimulation. p-ERK levels in non-transduced, serum-starved NIH 3T3 cells are shown for comparison. (B) Phospho-ERK1/2 is located in the perinuclear region that lacks CebpbUTR mRNA. 3T3ER:RasV12 cells were co-transfected with the GFP-MS2-nls reporter and tagged Cebpb vectors, induced with 4-OHT for 48 h, immunostained for p-ERK (Texas Red), and both markers visualized by confocal fluorescence microscopy.
3′UTR inhibition is absent in primary fibroblasts
C/EBPβ is required for OIS in primary MEFs (Sebastian et al, 2005), and endogenous C/EBPβ DNA-binding activity is stimulated by RasV12 in these cells (Figure 8A; Lee et al, 2010b). These observations suggested that 3′UTR inhibition might be lacking in primary fibroblasts. Consistent with this prediction, both C/EBPβCR and C/EBPβUTR efficiently inhibited proliferation of Ras-expressing Cebpb−/− MEFs and showed comparable transactivation of a reporter gene (Figure 8B and C). IMR90 human diploid fibroblasts also undergo C/EBPβ-dependent senescence in response to oncogenic stress (Kuilman et al, 2008). Accordingly, C/EBPβCR and C/EBPβUTR displayed similar Ras-stimulated DNA-binding and transcriptional activity in these cells (Supplementary Figure S13A and B). Moreover, both transcripts showed uniform cytoplasmic distribution in MEFs and IMR90 cells (Figure 8D; Supplementary Figure S13C). Hence, 3′UTR inhibition is reduced or absent in primary fibroblasts and correlates with an absence of Cebpb mRNA compartmentalization, allowing C/EBPβ to become activated and induce proliferative arrest in primary cells expressing oncogenic Ras.
Figure 8.
C/EBPβ 3′UTR repression is lacking in primary fibroblasts. (A) DNA binding of endogenous C/EBPβ in MEFs is activated by oncogenic Ras. Nuclear extracts from MEFs infected with control or RasV12 retroviruses were analysed for C/EBPβ DNA binding. Western blotting shows similar levels of C/EBPβ in the extracts. (B) Proliferation assays of low passage Cebpb−/− MEFs infected with C/EBPβCR or C/EBPβUTR retroviruses. Data are the mean values±s.d. from two experiments assayed in triplicate. (C) C/EBPβCR and C/EBPβUTR display equivalent RasV12-induced transcriptional activity in MEFs. Cebpb−/− MEFs were transfected with 2xC/EBP-luc and C/EBPβ plasmids, with or without RasV12. Data are the mean values±s.d. from two experiments. (D) The 3′UTR does not regulate Cebpb mRNA localization in MEFs. MEFs were transfected with the GFP-MS2-nls reporter, alone or with tagged CebpbCR or CebpbUTR vectors, and imaged by confocal fluorescence microscopy.
We next asked whether cell-specific control of 3′UTR inhibition involves HuR. As HuR is predominantly nuclear but undergoes nucleocytoplasmic shuttling in a regulated manner (Doller et al, 2008), we investigated whether differential 3′UTR inhibition in MEFs and NIH 3T3 fibroblasts is associated with changes in HuR levels or subcellular partitioning. Cytoplasmic HuR levels were comparable in both control cell populations (Supplementary Figure S14, left panel, lanes 1 and 3). However, oncogenic Ras decreased the cytoplasmic HuR pool in MEFs, whereas it increased cytosolic HuR in NIH 3T3 cells (Supplementary Figure S14, left panel, lanes 2 and 4). Nuclear HuR levels were correspondingly increased or decreased, respectively (Supplementary Figure S14, right panel). Thus, elevated cytoplasmic HuR is observed in immortalized cells under conditions of oncogenic stress. This suggests that increased cytosolic HuR in transformed 3T3Ras cells engages the C/EBPβ 3′UTR repression mechanism. By contrast, primary cells undergoing OIS display reduced levels of cytoplasmic HuR and, concomitantly, lack C/EBPβ 3′UTR inhibition.
Discussion
3′UTRs have well-established roles in controlling mRNA degradation and translation, often through the actions of miRNAs (Conne et al, 2000; Moore, 2005; Bartel, 2009). Here, we report a previously unrecognized function for 3′UTRs in regulating signal-induced activation of the protein product, a process that we term ‘3′UTR regulation of protein activity’ or UPA (Figure 9A). UPA inhibits the cytostatic activities of both C/EBPβ and C/EBPα in RasV12-expressing cells, showing that this mechanism regulates at least two members of the C/EBP family. At present, it is unclear how many other proteins are subject to UPA control. However, since an estimated 5–8% of human genes contain ARE-like sequences (Bakheet et al, 2003), similar mechanisms could theoretically regulate the activities of many proteins.
Figure 9.
Model depicting 3′UTR regulatory functions and the proposed mechanism of C/EBPβ 3′UTR inhibition. (A) The 3′UTR regulation of protein activity (UPA) mechanism identified in this study extends the previously known roles of 3′UTRs, which include control of mRNA decay and regulation of protein translation. (B) UPA is operative in immortalized/transformed cells but not in primary cells. The 3′UTR restricts Cebpb mRNA to the peripheral cytoplasm in tumour cells, in part due to increased levels of cytoplasmic HuR. Other factors are likely to contribute to this HuR effect, as depicted. In primary cells, Cebpb mRNA can enter the perinuclear region, where newly synthesized C/EBPβ is available for activation by p-ERK and possibly other Ras effector kinases. Increased homodimerization of activated C/EBPβ regulates inflammation, cell-cycle arrest, and senescence, whereas in tumour cells the predominantly heterodimeric form of C/EBPβ may contribute to the transformed phenotype.
C/EBPβ activity is constrained by auto-inhibition and heterodimerization with C/EBPγ, and these inhibitory effects are reversed by oncogenic Ras signalling via the MEK/ERK/RSK kinase cascade (Lee et al, 2010a, 2010b). Our results show that UPA quells de-repression of C/EBPβ by oncogenic Ras, providing another barrier to C/EBPβ activation that prevents unscheduled cell-cycle arrest. UPA is restricted to immortalized and transformed cells, raising the possibility that genetic events rendering cells susceptible to transformation by Ras (i.e., loss of tumour suppressors) are involved in activating the C/EBPβ UPA system. This mechanism could link inhibition of C/EBPβ cytostatic activity with oncogenic transformation and may be essential for senescence bypass in tumour cells. Because C/EBPβ also has pro-oncogenic functions and can promote cell proliferation and survival (Sebastian and Johnson, 2006), we suggest that UPA does not inhibit all C/EBPβ activities. Indeed, the C/EBPβUTR-induced gene signature was highly correlated with cancers whereas that of C/EBPβCR tended to be associated with inflammation and non-neoplastic diseases (Figure 5D). Thus, activation of genes associated with cancer, EMT, and TGFβ signalling may underlie the known pro-tumourigenic effects of endogenous C/EBPβ, which is expressed from a 3′UTR-containing transcript.
The Cebpb ARE region is necessary and sufficient for UPA, which also requires the ARE-binding protein, HuR. HuR positively regulates cell proliferation, in part through binding to the 3′UTRs of cyclins A and B and stabilizing the mRNAs encoding these key cell-cycle regulators (Wang et al, 2000). C/EBPβ depletion in several human tumour cell lines partially or completely abrogated the cytostatic response to HuR ablation (Supplementary Figure S6). Thus, C/EBPβ is another important HuR target, and HuR may stimulate cell proliferation at least in part through the UPA mechanism. The connection between HuR and C/EBPβ was also observed in NIH 3T3 cells, where HuR depletion converted C/EBPβUTR into a growth suppressor (Figure 3D). These experiments show that HuR inhibits the cytostatic activity of C/EBPβ, an idea that is further supported by the fact that cytoplasmic HuR levels are decreased in senescent fibroblasts (Wang et al, 2001; Supplementary Figure S14), possibly due to increased energetic stress and elevated AMP-dependent signalling (Wang et al, 2003). The diminished cytoplasmic HuR pool in primary cells may alleviate negative regulation by the 3′UTR and permit C/EBPβ activation.
The UPA mechanism proposed here also relates to the reported association between HuR and cancer. Since elevated HuR levels and/or cytoplasmic abundance have been linked to increased incidence or malignancy of various cancers (Lopez de Silanes et al, 2003; Ido et al, 2008; Mazan-Mamczarz et al, 2008; Yoo et al, 2009), we propose that implementation of C/EBPβ UPA contributes to the progression of premalignant tumours (with senescent features) to fully malignant cancers. Although C/EBPβUTR is resistant to activation in immortalized/transformed cells, the protein is nonetheless expressed and transported to the nucleus. Thus, UPA provides a plausible mechanism for generating the low activity, mostly heterodimeric form of C/EBPβ that may have pro-oncogenic functions in advanced cancers.
Although the full details of UPA remain to be established, our studies strongly implicate the subcytoplasmic location of C/EBPβ synthesis as a critical factor in its post-translational activation by Ras signalling (Figure 9B). In tumour cells, the 3′UTR excludes Cebpb transcripts from a perinuclear domain through a HuR-dependent mechanism. HuR itself does not exhibit the same cytoplasmic distribution and tends to be localized throughout the cytosol in punctate structures (Zou et al, 2006), suggesting that HuR is only one component of a larger complex that assembles on the ARE region to regulate Cebpb mRNA localization. Importantly, the perinuclear region corresponds precisely to the site of active ERK1/2 in cells expressing oncogenic Ras. p-ERK and newly synthesized C/EBPβ are apparently capable of interacting in this compartment, allowing C/EBPβ to undergo activation before nuclear translocation. Since C/EBPβ de-repression involves additional modifications besides ERK1/2 phosphorylation on Thr188, it is likely that other kinases are also enriched in the perinuclear space. This possibility warrants further investigation. In further support of our model, Cebpb mRNA partitioning was not observed in primary cells, consistent with the observation that oncogenic Ras can activate endogenous C/EBPβ and induce senescence in these cells. Thus, UPA may be an important regulatory process that abrogates the senescence program in tumour cells but not in primary cells.
UPA has similarities to, but is distinct from, the 3′UTR zipcode mechanism that localizes β-actin mRNA to the leading edge of migrating fibroblasts (Kislauskis et al, 1994). Zipcode-directed mRNA trafficking restricts β-actin translation to a subcellular domain where high levels of actin are required. In contrast, UPA does not alter the ultimate destination of C/EBPβ (the nucleus) but rather controls its post-translational activation. A study of mRNA localization in Drosophila embryos showed that 71% of the transcripts analysed were distributed in distinct spatial patterns (Lecuyer et al, 2007). Thus, differential mRNA localization may be a common means of controlling protein function and activity. The extent to which 3′UTRs specify this spatial patterning is unknown, but they are likely to have a significant role.
In light of similarities between cancer cells and stem/progenitor cells (Reya et al, 2001), an important physiologic function of UPA may involve constraining the anti-proliferative activity of C/EBP proteins in progenitor or transit amplifying cells during development. C/EBPα is expressed in proliferating haematopoietic progenitors but is also required for subsequent terminal differentiation of granulocytes and functions as a tumour suppressor in myeloid leukaemias (Nerlov, 2004). Therefore, UPA could regulate haematopoiesis and other developmental processes by restricting the anti-proliferative effects of C/EBPα and C/EBPβ to the appropriate lineages and stage of differentiation. Future studies should reveal whether this is an important biological role for C/EBP UPA. In addition, further elucidation of the proteins and pathways responsible for establishing UPA in tumour cells may reveal novel targets for cancer therapy, since disrupting UPA would be predicted to suppress tumour growth and induce senescence.
Materials and methods
Cell lines and reagents
NIH 3T3 cells were obtained from ATCC and cultured in DMEM medium with 10% calf serum. HEK293T cells, WT and Cebpb−/− MEFs (Sebastian et al, 2005), A549 cells, MCF7 cells, HeLa cells, and IMR90 cells were cultured in DMEM with 10% fetal bovine serum. Phoenix ecotropic and amphotropic packaging cells were grown in the same medium. C/EBPβ (C-19), β-actin (C11), HuR (3A2), and ERK1/2 (K23) antibodies were obtained from Santa Cruz Biotechnology. Phospho-C/EBPβ (T188) antibody was obtained from Abcam (pT235) and phospho-S64 antibody has been described previously (Shuman et al, 2004). Phospho-p90RSK (S380), Akt, phospho-ERK1/2 (T202/Y204), and pAkt (S473) antibodies were obtained from Cell Signaling Technology. RSK2 and RSK3 antisera were kindly provided by M Ernst and N Rice.
Plasmids and retroviral constructs
Details of the various expression vectors are presented in the Supplementary data.
Stable transfection and retroviral transduction
Retroviral infection was performed as described (Sebastian et al, 2005). After drug selection, cells were used for proliferation, colony formation, and focus formation assays. Knockdown of C/EBPβ and HuR expression was achieved using pSuperRetro vector-mediated RNAi technology (Oligoengine). The siRNA sequences for C/EBPβ (GATGTTCCTGCGGGGTTGT; Sebastian and Johnson, 2009) and HuR (GAGGCAATTACCAGTTTCA; Lal et al, 2004) were inserted into pSuperRetro; retroviral supernatants were produced using Phoenix packaging cells. Permanent clones of HuRKD HEK293T, A549, and MCF7 cells were selected, and individual colonies were expanded for further analysis. For inducible expression of C/EBPβ, NIH 3T3 cells were transfected with pMTCB6-C/EBPβ (±3′UTR) using CaPO4 precipitation, transfectants were selected with antibiotics, and individual clones were isolated for proliferation assays. C/EBPβ expression was induced with 50 μM ZnSO4.
Proliferation, transformation, and senescence assays
Cell proliferation and transformation were assayed using standard methods (see Supplementary data). For SA-βGal staining, cells were plated in 12-well dishes (12 × 103 cells/well) and stained using a senescence detection kit (EMD Biosciences, USA) according to the manufacturer's instructions. Positive cells were quantified by scoring 100 cells each from two different wells.
Transient transfection and luciferase assays
HEK293T and IMR90 cells were transfected with 200 ng C/EBPβ vector and 100 ng of pcDNA3-H-RasV12 in 60 mm dishes. In all, 700 ng HuR vector was used for HuR re-expression experiments. After transfection, cells were cultured in complete media for 24 h and then serum starved overnight before harvesting. For transactivation assays, 293T cells, NIH 3T3 cells, HeLa cells, IMR90 cells, or WT and Cebpb−/− MEFs were transfected with 100 ng 2xC/EBP-luc reporter and 5 ng C/EBPβ vector with 10 ng RasV12 plasmid. Cells were lysed in 1 × passive lysis buffer (Promega) and luciferase activity was measured using a Monolight 2010 luminometer. Luciferase values were normalized to total protein concentration of the lysate. Data are presented as fold activation of the reporter alone and represent mean values±s.d. of at least three independent experiments.
Protein analysis and EMSA
Nuclear and cytoplasmic extracts were prepared from the retrovirally infected or transiently transfected cells, as described (Sebastian and Johnson, 2009). EMSA was performed by incubating nuclear extracts (normalized for C/EBPβ levels) with 32P-labelled oligonucleotide probe corresponding to a consensus C/EBP binding site, as described (Lee et al, 2010b). In these experiments, β-actin controls were not included because the extracts were equalized for C/EBPβ levels. For supershift assays, extracts were pre-incubated with 200 ng antibodies against C/EBPβ or C/EBPγ (Parkin et al, 2002) for 30 min before the binding reaction. DNA–protein complexes were resolved on 6% polyacrylamide/1 × TBE gels.
mRNA localization
The GFP-MS2-nls reporter system has been described previously (Rook et al, 2000). The pcDNA-Cebpb-Ms2b vector was constructed by cloning the multiple MS2 binding sites from the RSV-lacZ-MS2b vector (Rook et al, 2000) downstream of the Cebpb coding region, without or with the 3′UTR, using BamHI/BglII sites. The CebpbCR-Ms2b vector was modified to Cebpb3 × ZIP-Ms2b by cloning three copies of the 54-nt β-actin zipcode element (5′-ACCGGACTGTTACCAACACCCACACCCCTGTGATGAAACAAAACCCATAAATGC-3′) (Kislauskis et al, 1994) downstream of the MS2 binding sites using BamHI/HindIII restriction sites. NIH 3T3 cells, HeLa cells, IMR90 cells, and WT MEFs were plated at 5 × 104 cells/35 mm dish and transfection was carried out using Polyfect (Qiagen) (for NIH 3T3), Fugene 6 (Roche) (for HeLa), and Transpass D2 and Transpass V (New England Biolabs) (for IMR90 and MEFs, respectively). After 36 h, the cells were fixed with 3.6% formaldehyde for 10 min and stained with 1 μg/ml DAPI (Sigma). Fluorescence images were taken with a Zeiss confocal microscope.
Immunofluorescence
Immunostaining was performed as described (Sebastian et al, 2005) with minor modifications. Cells (5 × 104/35 mm dish) were fixed with 4% paraformaldehyde for 10 min and incubated with phospho-ERK1/2 primary antibody followed by goat anti-rabbit IgG secondary antibody conjugated with Texas red (Santa Cruz Biotechnology sc-2780). Fluorescence images were taken with a Zeiss confocal microscope.
Microarray gene expression profiling
Genome-wide expression analysis was carried out using RNA isolated from the indicated NIH 3T3 cell populations (RNeasy, Qiagen). Each cell population was generated by retroviral transduction, in triplicate, and RNAs from each replicate were used for independent microarray hybridization analysis (LMT/Affymetrix Group, SAIC-Frederick). Labelled cDNAs were hybridized to GeneChip Mouse Genome 430 2.0 arrays (Affymetrix). The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (Edgar et al, 2002) and are accessible through GEO Series accession number GSE30834 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE<http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE30834> 30834<http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE30834>). Detailed statistical methods are described in Supplementary data.
qPCR
RNA isolated from NIH 3T3 cell populations was used to determine expression levels of individual genes. cDNA was generated (Reverse Transcription Kit, Qiagen) from total RNA and qPCR was performed using SYBR Green with QuantiTect Primers (Qiagen) and normalized against β-actin or β2-microglobulin. Analysis was performed using an Applied Biosystems 7500 Real Time PCR System and SDS Software (version 1.4). For each gene, a random representative of the triplicate RNA samples used for microarray analysis was selected for qPCR. Relative gene expression values were determined twice (in triplicate) and the data were averaged. Error bars represent standard error of mean expression levels. For primer sequences, see Supplementary data.
Gene function and pathway analysis
The association of gene expression signatures with biological pathways and diseases was performed using MetaCore software (MetaCoreTM, GeneGo, St Joseph, MI). Gene symbols of transcripts specifically upregulated by C/EBPβCR (312 genes) or C/EBPβUTR (70 genes) (i.e., the non-overlapping sets in Supplementary Table S1) were uploaded to GeneGo and the data were compared with the gene signatures for canonical biological pathways or specific diseases. Associations were ranked by P-value (LOD score; logarithm of the odds) and the top 10 matches were selected.
Supplementary Material
Acknowledgments
We thank M Gorospe for HuR vectors, K Kosick for the GFP reporter/mRNA visualization system, F Rauscher for the MT inducible vector, X Wu for assistance with microarrays, K Peifley and S Lockett for confocal microscopy support, J Hao for generating the pWZL-ER:RasV12 construct, and J Wada for preparation of figures. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Author contributions: SKB, RM, CJH, SL, TS, KS, and PFJ designed the experiments. SKB, RM, CJH, SL, TS, and KS performed experiments; TS and RM made the initial observations and generated several of the plasmid constructs. SKB, RM, CJH, SL, TS, KS, and PFJ analysed the data; OAQ and WGA performed statistical analysis of the array data. SKB, WGA, and PFJ wrote the paper and all authors contributed to editing.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abdelmohsen K, Kuwano Y, Kim HH, Gorospe M (2008) Posttranscriptional gene regulation by RNA-binding proteins during oxidative stress: implications for cellular senescence. Biol Chem 389: 243–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, Takatsu Y, Melamed J, d’Adda di Fagagna F, Bernard D, Hernando E, Gil J (2008) Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133: 1006–1018 [DOI] [PubMed] [Google Scholar]
- Adams PD (2009) Healing and hurting: molecular mechanisms, functions, and pathologies of cellular senescence. Mol Cell 36: 2–14 [DOI] [PubMed] [Google Scholar]
- Bakheet T, Williams BR, Khabar KS (2003) ARED 2.0: an update of AU-rich element mRNA database. Nucleic Acids Res 31: 421–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreau C, Paillard L, Osborne HB (2005) AU-rich elements and associated factors: are there unifying principles? Nucleic Acids Res 33: 7138–7150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J (2005) Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120: 513–522 [DOI] [PubMed] [Google Scholar]
- Cherry J, Jones H, Karschner VA, Pekala PH (2008) Post-transcriptional control of CCAAT/enhancer-binding protein beta (C/EBPbeta) expression: formation of a nuclear HuR-C/EBPbeta mRNA complex determines the amount of message reaching the cytosol. J Biol Chem 283: 30812–30820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody NA, Shen Z, Ripeau JS, Provencher DM, Mes-Masson AM, Chevrette M, Tonin PN (2009) Characterization of the 3p12.3-pcen region associated with tumor suppression in a novel ovarian cancer cell line model genetically modified by chromosome 3 fragment transfer. Mol Carcinog 48: 1077–1092 [DOI] [PubMed] [Google Scholar]
- Collado M, Blasco MA, Serrano M (2007) Cellular senescence in cancer and aging. Cell 130: 223–233 [DOI] [PubMed] [Google Scholar]
- Conne B, Stutz A, Vassalli JD (2000) The 3′ untranslated region of messenger RNA: A molecular ‘hotspot’ for pathology? Nat Med 6: 637–641 [DOI] [PubMed] [Google Scholar]
- Dajee M, Tarutani M, Deng H, Cai T, Khavari PA (2002) Epidermal Ras blockade demonstrates spatially localized Ras promotion of proliferation and inhibition of differentiation. Oncogene 21: 1527–1538 [DOI] [PubMed] [Google Scholar]
- Doller A, Pfeilschifter J, Eberhardt W (2008) Signalling pathways regulating nucleo-cytoplasmic shuttling of the mRNA-binding protein HuR. Cell Signal 20: 2165–2173 [DOI] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE (2002) Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30: 207–210 (http://nar.oupjournals.org/cgi/content/full/30/1/207?ijkey=oxMPOWseARs7o&keytype=ref&siteid=nar) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt K, Cherry J, Tenney R, Karschner V, Pekala PH (2005) An early event in adipogenesis, the nuclear selection of the CCAAT enhancer-binding protein. J Biol Chem 280: 24768–24774 [DOI] [PubMed] [Google Scholar]
- Gaumont-Leclerc MF, Mukhopadhyay UK, Goumard S, Ferbeyre G (2004) PEA-15 is inhibited by adenovirus E1A and plays a role in ERK nuclear export and Ras-induced senescence. J Biol Chem 279: 46802–46809 [DOI] [PubMed] [Google Scholar]
- Ido K, Nakagawa T, Sakuma T, Takeuchi H, Sato K, Kubota T (2008) Expression of vascular endothelial growth factor-A and mRNA stability factor HuR in human astrocytic tumors. Neuropathology 28: 604–611 [DOI] [PubMed] [Google Scholar]
- Johnson PF (2005) Molecular stop signs: regulation of cell-cycle arrest by C/EBP transcription factors. J Cell Sci 118(Pt 12): 2545–2555 [DOI] [PubMed] [Google Scholar]
- Katsoulidis E, Carayol N, Woodard J, Konieczna I, Majchrzak-Kita B, Jordan A, Sassano A, Eklund EA, Fish EN, Platanias LC (2009) Role of Schlafen 2 (SLFN2) in the generation of interferon alpha-induced growth inhibitory responses. J Biol Chem 284: 25051–25064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazerounian S, Yee KO, Lawler J (2008) Thrombospondins in cancer. Cell Mol Life Sci 65: 700–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislauskis EH, Zhu X, Singer RH (1994) Sequences responsible for intracellular localization of beta-actin messenger RNA also affect cell phenotype. J Cell Biol 127: 441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowenz-Leutz E, Twamley G, Ansieau S, Leutz A (1994) Novel mechanism of C/EBP β (NF-M) transcriptional control: activation through derepression. Genes Dev 8: 2781–2791 [DOI] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS (2008) Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133: 1019–1031 [DOI] [PubMed] [Google Scholar]
- Lal A, Mazan-Mamczarz K, Kawai T, Yang X, Martindale JL, Gorospe M (2004) Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO J 23: 3092–3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuyer E, Yoshida H, Parthasarathy N, Alm C, Babak T, Cerovina T, Hughes TR, Tomancak P, Krause HM (2007) Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell 131: 174–187 [DOI] [PubMed] [Google Scholar]
- Lee S, Miller M, Shuman JD, Johnson PF (2010a) CCAAT/enhancer-binding protein beta DNA binding is auto-inhibited by multiple elements that also mediate association with p300/CREB-binding protein (CBP). J Biol Chem 285: 21399–21410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Shuman JD, Guszczynski T, Sakchaisri K, Sebastian T, Copeland TD, Miller M, Cohen MS, Taunton J, Smart RC, Xiao Z, Yu L-R, Veenstra TD, Johnson PF (2010b) RSK-mediated phosphorylation in the C/EBP β leucine zipper regulates DNA binding, dimerization, and growth arrest activity. Mol Cell Biol 30: 2621–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Ding F, Zhang L, Liu Z, Wu Y, Luo A, Wu M, Wang M, Zhan Q (2005) Overexpression of stefin A in human esophageal squamous cell carcinoma cells inhibits tumor cell growth, angiogenesis, invasion, and metastasis. Clin Cancer Res 11(24 Pt 1): 8753–8762 [DOI] [PubMed] [Google Scholar]
- Lopez de Silanes I, Fan J, Yang X, Zonderman AB, Potapova O, Pizer ES, Gorospe M (2003) Role of the RNA-binding protein HuR in colon carcinogenesis. Oncogene 22: 7146–7154 [DOI] [PubMed] [Google Scholar]
- Lowe SW, Cepero E, Evan G (2004) Intrinsic tumour suppression. Nature 432 (7015): 307–315 [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454 (7203): 436–444 [DOI] [PubMed] [Google Scholar]
- Mazan-Mamczarz K, Hagner PR, Corl S, Srikantan S, Wood WH, Becker KG, Gorospe M, Keene JD, Levenson AS, Gartenhaus RB (2008) Post-transcriptional gene regulation by HuR promotes a more tumorigenic phenotype. Oncogene 27: 6151–6163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo X, Kowenz-Leutz E, Xu H, Leutz A (2004) Ras induces mediator complex exchange on C/EBP beta. Mol Cell 13: 241–250 [DOI] [PubMed] [Google Scholar]
- Moore MJ (2005) From birth to death: the complex lives of eukaryotic mRNAs. Science 309 (5740): 1514–1518 [DOI] [PubMed] [Google Scholar]
- Nakajima T, Kinoshita S, Sasagawa T, Sasaki K, Naruto M, Kishimoto T, Akira S (1993) Phosphorylation at threonine-235 by a ras-dependent mitogen-activated protein kinase cascade is essential for transcription factor NF-IL6. Proc Natl Acad Sci USA 90: 2207–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerlov C (2004) C/EBPalpha mutations in acute myeloid leukaemias. Nat Rev Cancer 4: 394–400 [DOI] [PubMed] [Google Scholar]
- Parkin SE, Baer M, Copeland TD, Schwartz RC, Johnson PF (2002) Regulation of CCAAT/enhancer-binding protein (C/EBP) activator proteins by heterodimerization with C/EBPgamma (Ig/EBP). J Biol Chem 277: 23563–23572 [DOI] [PubMed] [Google Scholar]
- Prieur A, Peeper DS (2008) Cellular senescence in vivo: a barrier to tumorigenesis. Curr Opin Cell Biol 20: 150–155 [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414 (6859): 105–111 [DOI] [PubMed] [Google Scholar]
- Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J (2009) Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 11: 973–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook MS, Lu M, Kosik KS (2000) CaMKIIalpha 3′ untranslated region-directed mRNA translocation in living neurons: visualization by GFP linkage. J Neurosci 20: 6385–6393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisian CJ, Keister BA, Stairs DB, Boxer RB, Moody SE, Chodosh LA (2007) Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol 9: 493–505 [DOI] [PubMed] [Google Scholar]
- Sebastian T, Johnson PF (2006) Stop and go: anti-proliferative and mitogenic functions of the transcription factor C/EBPbeta. Cell Cycle 5: 953–957 [DOI] [PubMed] [Google Scholar]
- Sebastian T, Johnson PF (2009) RasV12-mediated down-regulation of CCAAT/enhancer binding protein beta in immortalized fib. Cancer Res 69: 2588–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian T, Malik R, Thomas S, Sage J, Johnson PF (2005) C/EBPbeta cooperates with RB:E2F to implement Ras(V12)-induced cellular senescence. EMBO J 24: 3301–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman JD, Sebastian T, Kaldis P, Copeland TD, Zhu S, Smart RC, Johnson PF (2004) Cell cycle-dependent phosphorylation of C/EBPbeta mediates oncogenic cooperativity between C/EBPbeta and H-RasV12. Mol Cell Biol 24: 7380–7391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasini R, Seux M, Nowak J, Bontemps C, Carrier A, Dagorn JC, Pebusque MJ, Iovanna JL, Dusetti NJ (2005) TP53INP1 is a novel p73 target gene that induces cell cycle arrest and cell death by modulating p73 transcriptional activity. Oncogene 24: 8093–8104 [DOI] [PubMed] [Google Scholar]
- Wang W, Caldwell MC, Lin S, Furneaux H, Gorospe M (2000) HuR regulates cyclin A and cyclin B1 mRNA stability during cell proliferation. EMBO J 19: 2340–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Yang X, Cristofalo VJ, Holbrook NJ, Gorospe M (2001) Loss of HuR is linked to reduced expression of proliferative genes during replicative senescence. Mol Cell Biol 21: 5889–5898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Yang X, Lopez de Silanes I, Carling D, Gorospe M (2003) Increased AMP:ATP ratio and AMP-activated protein kinase activity during cellular senescence linked to reduced HuR function. J Biol Chem 278: 27016–27023 [DOI] [PubMed] [Google Scholar]
- Williams SC, Baer M, Dillner AJ, Johnson PF (1995) CRP2 (C/EBPβ) contains a bipartite regulatory domain that controls transcriptional activation, DNA binding and cell specificity. EMBO J 14: 3170–3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler GS (2010) The mammalian anti-proliferative BTG/Tob protein family. J Cell Physiol 222: 66–72 [DOI] [PubMed] [Google Scholar]
- Xu N, Chen CY, Shyu AB (1997) Modulation of the fate of cytoplasmic mRNA by AU-rich elements: key sequence features controlling mRNA deadenylation and decay. Mol Cell Biol 17: 4611–4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo PS, Sullivan CA, Kiang S, Gao W, Uchio EM, Chung GG, Cha CH (2009) Tissue microarray analysis of 560 patients with colorectal adenocarcinoma: high expression of HuR predicts poor survival. Ann Surg Oncol 16: 200–207 [DOI] [PubMed] [Google Scholar]
- Zou T, Mazan-Mamczarz K, Rao JN, Liu L, Marasa BS, Zhang A-H, Xiao L, Pullmann R, Gorospe M, Wang J-Y (2006) Polyamine depletion increases cytoplasmic levels of RNA-binding protein HuR leading to stabilization of nucleophosmin and p53 mRNAs. J Biol Chem 281: 19387–19394 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.