Abstract
Purpose
Supraclavicular brachial plexus block is considered as one of the most effective anesthetic methods for upper extremity surgeries. Its major drawback, especially in children, is the risk of pneumothorax, vascular puncture, and failure of the procedure due to inaccurate placement of the needle. Ultrasound-guided needle placement may reduce the risk of complications and increase the accuracy of block, particularly in pediatric patients. There are few published experiences about the efficacy and safety of ultrasound-guided supraclavicular block in children and to our knowledge, it seems that there is no published report about its usage in younger children (less than 6 years of age).
Methods
In order to consider the efficacy of ultrasound in younger children, 17 patients aged between 6 months and 6 years were randomly selected. The ultrasound probe was used for proper placement of the needle. After confirmation of the needle location using a nerve locator, the anesthetic agent was injected. The procedure time, establishment time, duration of analgesia, any complications related to the procedure, and the surgeon’s satisfaction were recorded and analyzed.
Results
The procedure time was 10.35 ± 1.22 min, the establishment time was 89.59 ± 18 s, and the duration of analgesia was between 6 and 16 h (mean 9.76 ± 2.57 h). The recovery time was 24.4 ± 6.5 min (range 15–37 min) and the duration of surgeries was 61.3 ± 25.9 min (range 15–110 min). There was no failure of the procedure. Also, there were no complications related to the procedure and the surgeon’s satisfaction during surgery was good or excellent.
Conclusions
This study demonstrates the efficacy and safety of the ultrasound-guided supraclavicular brachial plexus block for orthopedic upper extremity surgeries in patients younger than 6 years of age.
Keywords: Pediatric upper extremity surgery, Ultrasound, Supraclavicular, Brachial plexus block, Pediatric anesthesia, Regional anesthesia
Introduction
Supraclavicular brachial plexus block is a known anesthetic option for upper limb surgery. Long-lasting pain relief and the possibility of earlier hospital discharge are some of the clinical advantages of this option. However, inconsistent block success remains one of the major limitations of brachial plexus blockade and can lead to an unplanned general anesthesia, increase material costs, and prolonged operating room time. Another limitation is the potential for procedure-related complications, such as nerve injury and unintentional vascular puncture. Additionally, children frequently suffer from inadequate management of pain during and especially after painful procedures. Underestimation of the intensity of pain and fear from the risk of complications of opioids may be important factors in this regard. Regional anesthesia may be one of the best solutions for intraoperative and postoperative pediatric pain management; however, due to the lack of proficiency and increased risk of some complications in comparison to adults, peripheral nerve blockade is not the method of choice for most of the anesthesiologists for children, especially in the younger pediatric group. Ultrasound guidance has been introduced in recent years to assist nerve localization and is increasingly being used for performing peripheral nerve blocks. Unlike traditional nerve localization techniques which rely on surface anatomical landmarks, patient report of paresthesia, and/or elicitation of a motor response by electrical nerve stimulation, ultrasound guidance uses sonoanatomic rather than a surface anatomy or neurophysiologic end point for block performance. However, nerve stimulation continues to be used along with ultrasound for additional confirmation of nerve identification [1]. According to the surgeon’s preference and due to the need for a tourniquet at the proximal part of the humerus in all surgeries regardless of the type of surgery, we considered supraclavicular block to be more suitable than more distal ones. This block can be done with only one injection around the plexus and provides sufficient anesthesia at the tourniquet site as well.
There are few published studies related to upper extremity surgery under supraclavicular brachial plexus block in children. The aim of the current study is to demonstrate the efficacy and safety of ultrasound-guided supraclavicular brachial plexus blockade in children younger than 6 years of age.
Materials and methods
After explanation of the risks and benefits of ultrasound-guided supraclavicular brachial plexus block to the parents of 41 American Society of Anesthesiologists Physical Status (ASA ps) I–II patients, and considering our exclusion criteria (patient or parental refusal, obvious or suspicious infection at the injection site), the parents of 17 cases signed a written informed consent and accepted the procedure as the method of anesthesia for their patients. All interviews were done by one physician (HRA) at the Preoperative Anesthesia Clinic and the parents had a minimum of a 24-h period for their decision-making. The patients were between 6 months and 6 years of age and all were scheduled for an elective upper extremity surgery. The data of the patients are presented in Table 1.
Table 1.
Information on the patients
| Procedure | Sex | Age (months) | ASA ps | Procedure time (min) | Establishment time (s) | Duration of analgesia (h) | Recovery time (min) | Duration of surgery (min) |
|---|---|---|---|---|---|---|---|---|
| Syndactyly repair | Fa | 8 | 2 | 10 | 83 | 16 | 19 | 47 |
| Cleft hand repair | F | 6 | 1 | 14 | 94 | 11 | 32 | 95 |
| Pollicization | F | 6 | 1 | 11 | 67 | 8.5 | 16 | 102 |
| Forearm exostosis excision | F | 14 | 2 | 10 | 140 | 10 | 22 | 50 |
| Trigger finger repair | F | 23 | 1 | 10 | 93 | 6.5 | 17 | 27 |
| Forearm exostosis excision | Mb | 48 | 1 | 11 | 81 | 12 | 19 | 53 |
| Pollicization | M | 11 | 2 | 10 | 87 | 10 | 15 | 110 |
| Syndactyly repair | M | 18 | 1 | 12 | 72 | 8.5 | 27 | 65 |
| CRIF of distal radius | F | 8 | 2 | 10 | 78 | 11 | 23 | 50 |
| Cleft hand repair | F | 6 | 2 | 9 | 100 | 12 | 33 | 105 |
| Syndactyly repair | F | 12 | 1 | 10 | 75 | 6 | 28 | 52 |
| Syndactyly repair | F | 19 | 1 | 9 | 80 | 12 | 37 | 61 |
| CRIF of distal radius | M | 51 | 1 | 10 | 90 | 6 | 21 | 47 |
| Syndactyly repair | F | 18 | 1 | 10 | 98 | 6 | 31 | 46 |
| Syndactyly repair | F | 18 | 2 | 11 | 70 | 8.5 | 29 | 55 |
| CRIF of distal radius | F | 69 | 2 | 9 | 110 | 9 | 24 | 53 |
| Fracture of proximal phalanx | M | 70 | 1 | 10 | 105 | 11 | 22 | 25 |
ASA ps American Society of Anesthesiologists Physical Status
CRIF closed reduction and internal fixation
aFemale
bMale
After sedation with intranasal (0.05–0.25 mg/kg) midazolam hydrochloride, the patient was transferred to the block room. A secure intravenous line was introduced and supplemental oxygen (4–6 l/min) through a face mask was applied. Then, a bolus dose of 0.5 mg/kg of Propofol (Propofol 1% MCT/LCT Fresenius, Fresenius Kabi Austria GmbH, Graz, Austria) was injected intravenously. Preparation and draping of the neck and supraclavicular region of the patient was done with povidone iodine solution 10%. Ultrasound-guided supraclavicular block was modified from the technique originally described by Chan et al. [2]. Sonographic view of the brachial plexus was obtained with a 9-MHz, 40-mm linear transducer (PB-L5-9EC-N, SonoAce PICO, Medison, South Korea) of a digital portable ultrasound unit with spectral, color, and wave Doppler (SonoAce PICO, Medison, South Korea). The transducer was oriented transversally in the supraclavicular fossa just above the clavicle. In this view, the brachial plexus is superficial and lateral to the subclavian artery and is visualized as a group of hypoechoic beans (Fig. 1). The location of the subclavian artery is confirmed by a pulse wave Doppler image (Fig. 2).
Fig. 1.
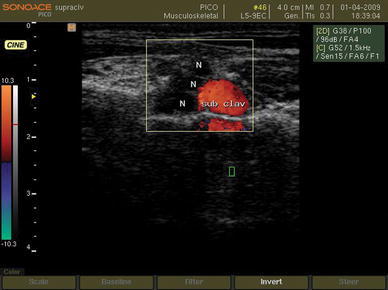
Ultrasound view of the brachial plexus in the supraclavicular region. N brachial plexus, sub clav subclavian artery
Fig. 2.

Subclavian artery (art) and vein (v) in the supraclavicular region are confirmed by Doppler imaging
Supraclavicular block was performed using an insulated 35-mm, 24-gauge needle with a 20° cutting bevel (Polymedic® UPC, te me na SAS, EU) under ultrasonographic guidance. After attachment of the insulated needle to the nerve locator (Polystim II, Polymedic®, te me na SAS, EU), it was introduced through the skin lateral to the probe and in a parallel manner to the ultrasonic beams in order to view the full length of the needle and its pathway through the procedure (Fig. 3).
Fig. 3.
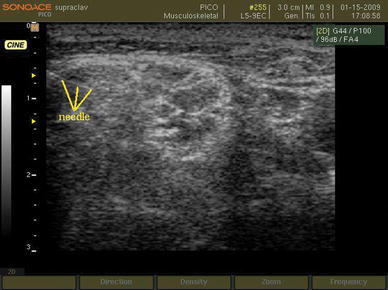
The needle is advanced toward the brachial plexus
We considered motor response to 0.5-mA electrical impulse generated by an electrical nerve locator for the confirmation of adequate proximity of the tip of the needle to the plexus [3–5]. Therefore, the nerve locator was adjusted to 1 Hz, 0.5 mA, and 0.1 ms during the procedure. The needle was advanced toward the brachial plexus and when the tip was visualized adjacent to the hypoechoic beans, it was gently moved to produce a regular muscle twitch. A mixture of lidocaine hydrochloride 0.7% and bupivacaine hydrochloride 0.17% (Bupivacaine 0.5%, Merck Génériques, Lyon, France) was injected at the site after frequent negative aspirations. During injection, spread of the drug around the plexus was visualized through the sonographic view as a hypoechoic area and, if needed, the tip of the needle was transposed to produce a suitable distribution of local anesthetic around the plexus. The injection was stopped when the plexus was surrounded completely by the hypoechoic area (indicating the local anesthetic spread) or a total amount of 5 mg/kg of lidocaine was administered [6].
Throughout the procedure, blood pressure (non-invasive), surface body temperature, oxygen saturation (pulse oximetry), and end tidal CO2 (side stream capnometry) were monitored. The “procedure time” was defined as the time between skin preparation and needle extraction, and was measured in minutes by an anesthetic nurse. The time between the completion of injection and unresponsiveness to painful stimuli on the dependent limb (at the surgical site) was considered as the “establishment time” and was recorded in seconds by an anesthesia resident who was blind to the study. The results are reported as mean ± standard deviation (SD). Responsiveness to the painful stimuli up to 10 min after local anesthetic injection was considered as block failure, which was assessed by the same anesthesia resident. In the case of failure, another method of anesthesia was planned; otherwise, during the surgical procedure, clinical sedation was provided primarily with 30–50 μg/kg/h of propofol infusion and after half an hour, the dose gradually decreased to 20–30 μg/kg/h and continued to the end of surgery.
During surgery, pain was assessed based on the hemodynamic changes (tachycardia, increased blood pressure) after other common causes, such as hypoxia, hypercapnia, hypothermia, and hypovolemia, were ruled out. Providing there was positive response to analgesic administration (decreased heart rate and blood pressure), block failure was regarded and, subsequently, the anesthesia plan was altered again. After the procedure, the infusion of sedative was suspended and the patient was transferred to the recovery room. Our criteria for discharge from the recovery room was according to the existing protocols and includes evaluation of the level of consciousness and awakeness (in children, eye opening, crying, and body movements), vital signs (including pain), and SpO2 without supplemental O2. In the recovery room, the patients were monitored carefully and were also evaluated for pain and delivered to the ward under the supervision of a skilled nurse in companion with the parents. The recovery time was 24.4 ± 6.5 min (range 15–37 min) (Table 1). About 2 h after discharge from the recovery room, oral nutrition was resumed (breast feeding in younger children and fluids in the older patients). Most of the children fall asleep at this stage and the evaluation of motor function necessitates disturbing them (waking them up). This was not acceptable by the parents, so the duration of motor block (muscle relaxation) was inevitably overlooked.
The “duration of analgesia” was considered as the time to the appearance pain considering a behavioral scale based on parental report [7–12]. The first dose of rescue analgesia, hemodynamic changes due to pain, or any complication such as limb irritability or sensitivity due to dysesthesia or partial or complete immobility (paresis/paralysis) were recorded in hours by a blinded orthopedic resident.
Results
Seventeen pediatric patients were enrolled in the study. Their ages were between 6 months and 6 years (mean age 23.8 ± 21.6 months). Five of them (29.4%) were male and 12 (70.6%) were female. Ten patients (58.82%) were in ASA ps I and seven patients (41.18%) were in ASA ps II. The durations of analgesia were between 6 and 16 h (mean 9.76 ± 2.57 h). There was no failure of the blocks before and during the surgical procedures. The surgeon’s satisfaction regarding anesthesia during operation was recorded as excellent in 11 and good in six cases, respectively. The procedure time was between 9 and 14 min (mean 10.35 ± 1.22 min), while the establishment time was between 67 and 140 s (mean 89.59 ± 17.97 s). The mean duration of surgeries was 61.35 ± 26 min (25–110 min) (Table 1). There were no records of any complications relating to the technique during the study. The patients’ data, including surgical procedures, are summarized in Table 1.
Discussion
Among the complications of supraclavicular brachial plexus block, pneumothorax and vascular puncture are the most potentially life-threatening, with pneumothorax being the most common [13, 14]. Because of the anatomic proximity of the cervical pleura, the consequent risk of pneumothorax is higher in pediatric groups [15]. Another main complication of the blind approach is due to the spread of high volumes of local anesthetic [16]. Therefore, for a long time, traditional methods of supraclavicular block of the brachial plexus has not been recommended in pediatric patients [17]. With using real-time ultrasound guidance, the risks of pneumothorax and vascular puncture are dramatically reduced, as the surrounding structures (pleura, subclavian artery, and vein) would be visualized [6]. As a result, the spread of local anesthetic around the target nerve can be assessed and more precisely administered at the correct location [18]. In addition, the block success rate is increased from approximately 85% using the landmark technique to 95% using ultrasound guidance [19, 20]. Ultrasound guidance for regional nerve blocks is rapidly becoming the standard of care in regional anesthesia [21] and, today, ultrasound guidance has been introduced into clinical practice as a possible option to identify peripheral nerves, offering the potential advantage of optimizing the spread of the local anesthetic solution around the nerves under sonographic vision [22].
Since the first study describing the use of ultrasound guidance in pediatric regional anesthesia reported by Marhofer et al. [23], which observed an onset time of 9 min for infracavicular blocks in children with ropivacaine 0.5% in a randomized clinical trial, there have been only a limited number of randomized controlled trials in children comparing ultrasound guidance peripheral nerve block with other techniques [24]. The available evidence among children demonstrates that ultrasound guidance peripheral nerve blocks improve the quality, onset, duration, and success rate of nerve blocks and help to lower the local anesthetic volume needed to perform blocks [23, 25, 26]. To our knowledge, it seems that there is no published report regarding the use of ultrasound-guided supraclavicular brachial plexus block in children younger than 6 years of age. The results of our study are comparable with other studies using the same technique for supraclavicular block in adults and older children. In comparison to other studies related to pediatric regional anesthesia, we used lower concentrations of local anesthetics with acceptable results, which seems to be important regarding the risk of systemic toxicity, especially in the pediatric age group [27]. Also, postoperative analgesia was considerable and satisfactory. In conclusion, ultrasound-guided supraclavicular brachial plexus nerve block is hypothesized be a safe, convenient, and effective anesthesia option for upper extremity surgical procedures in younger children with reasonable procedure, establishment, and analgesia times. Further comparative studies with much bigger groups of patients are required for the careful evaluation of the risks and benefits especially in this age group.
References
- 1.Gray AT. Ultrasound-guided regional anesthesia: current state of the art. Anesthesiology. 2006;104(2):368–373. doi: 10.1097/00000542-200602000-00024. [DOI] [PubMed] [Google Scholar]
- 2.Chan VW, Perlas A, Rawson R, et al. Ultrasound-guided supraclavicular brachial plexus block. Anesth Analg. 2003;97(5):1514–1517. doi: 10.1213/01.ANE.0000062519.61520.14. [DOI] [PubMed] [Google Scholar]
- 3.Bashein G, Haschke RH, Ready LB. Electrical nerve location: numerical and electrophoretic comparison of insulated vs uninsulated needles. Anesth Analg. 1984;63(10):919–924. doi: 10.1213/00000539-198410000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Keschner MT, Michelsen H, Rosenberg AD, et al. Safety and efficacy of the infraclavicular nerve block performed at low current. Pain Pract. 2006;6(2):107–111. doi: 10.1111/j.1533-2500.2006.00071.x. [DOI] [PubMed] [Google Scholar]
- 5.Sinha SK, Abrams JH, Weller RS. Ultrasound-guided interscalene needle placement produces successful anesthesia regardless of motor stimulation above or below 0.5 mA. Anesth Analg. 2007;105(3):848–852. doi: 10.1213/01.ane.0000271912.84440.01. [DOI] [PubMed] [Google Scholar]
- 6.Chan VW, Perlas A, McCartney CJ, et al. Ultrasound guidance improves success rate of axillary brachial plexus block. Can J Anaesth. 2007;54(3):176–182. doi: 10.1007/BF03022637. [DOI] [PubMed] [Google Scholar]
- 7.Li L, Liu X, Herr K. Postoperative pain intensity assessment: a comparison of four scales in Chinese adults. Pain Med. 2007;8(3):223–234. doi: 10.1111/j.1526-4637.2007.00296.x. [DOI] [PubMed] [Google Scholar]
- 8.Beyer JE, McGrath PJ, Berde CB. Discordance between self-report and behavioral pain measures in children aged 3–7 years after surgery. J Pain Symptom Manage. 1990;5(6):350–356. doi: 10.1016/0885-3924(90)90029-J. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson SJ, Kopecky EA, Joshi P, et al. Randomised trial of oral morphine for painful episodes of sickle-cell disease in children. Lancet. 1997;350(9088):1358–1361. doi: 10.1016/S0140-6736(97)08462-6. [DOI] [PubMed] [Google Scholar]
- 10.McGrath PJ, Rosmus C, Canfield C, et al. Behaviours caregivers use to determine pain in non-verbal, cognitively impaired individuals. Dev Med Child Neurol. 1998;40:340–343. [PubMed] [Google Scholar]
- 11.McGrath PJ, McAlpine L. Psychologic perspectives on pediatric pain. J Pediatr. 1993;122(5 Pt 2):S2–S8. doi: 10.1016/S0022-3476(11)80002-8. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell P. Understanding a young child’s pain. Lancet. 1999;354(9191):1708. doi: 10.1016/S0140-6736(05)76696-4. [DOI] [PubMed] [Google Scholar]
- 13.Pande R, Pande M, Bhadani U, et al. Supraclavicular brachial plexus block as a sole anaesthetic technique in children: an analysis of 200 cases. Anaesthesia. 2000;55(8):798–802. doi: 10.1046/j.1365-2044.2000.01330.x. [DOI] [PubMed] [Google Scholar]
- 14.Tobias JD. Brachial plexus anaesthesia in children. Paediatr Anaesth. 2001;11(3):265–275. doi: 10.1046/j.1460-9592.2001.00667.x. [DOI] [PubMed] [Google Scholar]
- 15.Suresh S, Wheeler M. Practical pediatric regional anesthesia. Anesthesiol Clin North America. 2002;20(1):83–113. doi: 10.1016/S0889-8537(03)00056-7. [DOI] [PubMed] [Google Scholar]
- 16.De José María B, Banús E, Navarro Egea M, et al. Ultrasound-guided supraclavicular vs infraclavicular brachial plexus blocks in children. Paediatr Anaesth. 2008;18(9):838–844. doi: 10.1111/j.1460-9592.2008.02644.x. [DOI] [PubMed] [Google Scholar]
- 17.Dalens B. Proximal blocks of the upper extremity: benefit/risk ratio. In: Dalens B, editor. Regional anaesthesia in infants, children and adolescents. Baltimore: Williams & Wilkins; 1995. pp. 275–314. [Google Scholar]
- 18.Riazi S, Carmichael N, Awad I, et al. Effect of local anaesthetic volume (20 vs 5 ml) on the efficacy and respiratory consequences of ultrasound-guided interscalene brachial plexus block. Br J Anaesth. 2008;101(4):549–556. doi: 10.1093/bja/aen229. [DOI] [PubMed] [Google Scholar]
- 19.Kapral S, Krafft P, Eibenberger K, et al. Ultrasound-guided supraclavicular approach for regional anesthesia of the brachial plexus. Anesth Analg. 1994;78(3):507–513. doi: 10.1213/00000539-199403000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Williams SR, Chouinard P, Arcand G, et al. Ultrasound guidance speeds execution and improves the quality of supraclavicular block. Anesth Analg. 2003;97(5):1518–1523. doi: 10.1213/01.ANE.0000086730.09173.CA. [DOI] [PubMed] [Google Scholar]
- 21.Stone MB, Wang R, Price DD. Ultrasound-guided supraclavicular brachial plexus nerve block vs procedural sedation for the treatment of upper extremity emergencies. Am J Emerg Med. 2008;26(6):706–710. doi: 10.1016/j.ajem.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Casati A, Danelli G, Baciarello M, et al. A prospective, randomized comparison between ultrasound and nerve stimulation guidance for multiple injection axillary brachial plexus block. Anesthesiology. 2007;106(5):992–996. doi: 10.1097/01.anes.0000265159.55179.e1. [DOI] [PubMed] [Google Scholar]
- 23.Marhofer P, Sitzwohl C, Greher M, et al. Ultrasound guidance for infraclavicular brachial plexus anaesthesia in children. Anaesthesia. 2004;59(7):642–646. doi: 10.1111/j.1365-2044.2004.03669.x. [DOI] [PubMed] [Google Scholar]
- 24.Rubin K, Sullivan D, Sadhasivam S. Are peripheral and neuraxial blocks with ultrasound guidance more effective and safe in children? Paediatr Anaesth. 2009;19(2):92–96. doi: 10.1111/j.1460-9592.2008.02918.x. [DOI] [PubMed] [Google Scholar]
- 25.Oberndorfer U, Marhofer P, Bösenberg A, et al. Ultrasonographic guidance for sciatic and femoral nerve blocks in children. Br J Anaesth. 2007;98(6):797–801. doi: 10.1093/bja/aem092. [DOI] [PubMed] [Google Scholar]
- 26.Willschke H, Marhofer P, Bösenberg A, et al. Ultrasonography for ilioinguinal/iliohypogastric nerve blocks in children. Br J Anaesth. 2005;95(2):226–230. doi: 10.1093/bja/aei157. [DOI] [PubMed] [Google Scholar]
- 27.Marhofer P, Chan VW. Ultrasound-guided regional anesthesia: current concepts and future trends. Anesth Analg. 2007;104(5):1265–1269. doi: 10.1213/01.ane.0000260614.32794.7b. [DOI] [PubMed] [Google Scholar]


