Abstract
Antigen-specific tolerance is a highly desired therapy for immune-mediated diseases. Intravenous infusion of protein/peptide antigens linked to syngeneic splenic leukocytes with ethylene carbodiimide (Ag-SP) has been demonstrated to be a highly efficient method for inducing peripheral, antigen-specific T cell tolerance for treatment of autoimmune disease. However, little is understood about the mechanisms underlying this therapy. Here, we show that apoptotic Ag-SP accumulate in the splenic marginal zone where their uptake by F4/80+ macrophages induces production of IL-10 which upregulates the expression of the immunomodulatory costimulatory molecule PD-L1 which is essential for Ag-SP tolerance induction. Ag-SP infusion also induces Tregs which are dispensable for tolerance induction, but required for long-term tolerance maintenance. Collectively, these results indicate that Ag-SP tolerance recapitulates how tolerance is normally maintained in the hematopoietic compartment and highlight the interplay between the innate and adaptive immune systems in the induction of Ag-SP tolerance. We show for the first time that tolerance results from the synergistic effects of two distinct mechanisms – PD-L1-dependent T cell-intrinsic unresponsiveness and the activation of Tregs. These findings are particularly relevant as this tolerance protocol is currently being tested in a Phase I/IIa clinical trial in new-onset relapsing-remitting MS.
Introduction
Autoimmune diseases, including multiple sclerosis (MS)2 and Type 1 diabetes, rank third as a major cause of morbidity and mortality in humans. Antigen-specific tolerance remains the most highly desired, yet elusive, technique for treating patients suffering from T cell-mediated autoimmune diseases. Strategies for inducing peripheral T cell tolerance including administration of soluble peptide, altered peptide ligands, anti-CD3 antibody, and co-stimulation blockade (1–3) have been largely unsuccessful. The use of hematopoietic stem cell transplantation or Treg immunotherapy has also been hindered, by the inability to obtain sufficient quantities of stem cells and Tregs of sufficient specificity and stability. Another option with significant promise for inducing long-term T cell tolerance has been the intravenous infusion of peptides cross-linked to the surface of splenic leukocytes (Ag-SP) using ethylene carbodiimide (ECDI) (4–6). Ag-SP tolerance has been shown to both prevent and treat Th1/17-mediated autoimmune diseases (3, 6, 7) and allograft rejection (5). This promising tolerance therapy is currently the focus of a Phase I/IIA clinical trial investigating the safety and efficacy of myelin peptide-coupled PBLs in human MS.
The precise mechanism(s) that underlie(s) Ag-SP tolerance remain to be defined; however, ECDI-induced apoptosis appears to be critical (6). Apoptosis, or programmed cell death, is an event that occurs on a regular basis in the body. Unlike necrosis, which triggers pro-inflammatory immune responses, apoptosis is usually associated with little to no proinflammatory immune activation (8, 9). Nonetheless, apoptotic cells are not invisible to the immune system. The cells responsible for their removal, predominantly macrophages, are capable of targeting apoptotic cells through a number of pathways including recognizing proteins expressed by the dying cells themselves as well as detecting serum opsonins that coat apoptotic cells (8, 9). A large proportion of apoptotic debris is removed by marginal zone macrophages expressing scavenger receptors, including LOX and SRB receptors (8, 9). Within the germinal center, CD68+ tingible-body macrophages are important regulators of apoptotic B cell removal (10). Apoptotic debris can trigger IL-10 production (11–13). Notably, it was recently shown that apoptotic cell infusion can induce regulatory B cells which, through their production of IL-10, can reduce the severity collagen-induced arthritis (11). Overall, the data support the importance of apoptotic cell processing in the maintenance of peripheral self-tolerance. Indeed, dysfunction in these clearance pathways is hypothesized to be a major cause of antibody-mediated autoimmune diseases such as SLE (10, 14).
Numerous immune interactions including CTLA-4-dependent T cell anergy as well as PD-L1 mediated T cell negative co-stimulation have been shown to play a role in long term Ag-SP tolerance induction (7, 15, 16). The immediate responses to infusion of Ag-SP that ultimately lead to long term T cell unresponsiveness have not been examined. We have previously shown that ECDI-induced apoptosis is a critical factor in Ag-SP tolerance and that indirect mechanisms involving host antigen presenting cell (APC) processing of Ag-SP, are also required as indicated by the ability of peptide-coupled allogeneic and MHC-deficient donor splenocytes to efficiently induce tolerance (6). Focusing on the events that occur within the first 72 h after i.v. Ag-SP tolerization, we found that Ag-SP rapidly localize to the splenic marginal zones (MZ) and trigger IL-10 production by F4/80+ MZ macrophages. IL-10 production was critical for tolerance induction, and appears to regulate PD-L1 expression in possibly an autocrine fashion. PD-L1 blockade at the time of Ag-SP infusion abrogated tolerance induction, further highlighting the importance of PD-L1 in the regulation. Lastly, Ag-SP infusion was found to induce Tregs which appear to be dispensable for early tolerance induction, but essential for maintenance of long-term tolerance.
Materials and Methods
Mice
All mice were housed under specific pathogen-free conditions in the Northwestern University Center for Comparative Medicine and maintained according to protocols approved by the Northwestern University Institutional Animal Care and Use Committee. Female SJL/J mice, 5–7 weeks old, were purchased from Harlan Laboratories. 5–7 week old wildtype and IL-10-deficient C57BL/6J mice were purchased from The Jackson Laboratory.
Peptides and reagents
Synthetic peptides MOG35–55 (MEVGWYRSPFSRVVHLYRNGK), PLP139–151 (HSLGKWLGHPDKF), and OVA323–339 (ISQAVHAAHAEINEAGR) were purchased from Genemed Synthesis. PLP178–191 (NTWTTCQSIAFPSK) was purchased from Peptides International.
Immunization
Mice were primed with an emulsion containing 1mg/ml peptide and complete Freund’s adjuvant (CFA) containing 2mg/ml mycobacterium tuberculosis H37Ra (Difco). A 100µl volume of emulsion was injected subcutaneously among three sites on the flank of each mouse.
Induction and clinical evaluation of peptide-induced EAE
Peptide-induced and adoptive transfer EAE was induced in both SJL and C57BL/6 mice as previously reported (17, 18). Individual animals were observed daily and clinical scores were assessed in a blinded fashion on a 0–5 scale as follows: 0 = no abnormality; 1 = limp tail or hind limb weakness; 2 = limp tail and hind limb weakness; 3 = hind limb paralysis; 4 = hind limp paralysis and forelimb weakness; and 5 = moribund. The data are reported as the mean daily clinical score. Paralyzed animals were afforded easier access to food and water.
Coupled-cell tolerance
Tolerance was induced by i.v. injection of chemically treated Ag-coupled splenocytes (Ag-SP), as described previously (5, 6). Briefly, spleens were removed from naive female mice, and the RBCs were lysed. The splenocytes were incubated with ECDI (150 mg/3.2 × 108 cells; Calbiochem) and peptide (1 mg/ml) on ice, shaking for 1 h. The coupled cells were washed 3× and filtered through a 70µM cell strainer to remove cell clumps. The Ag-SP were resuspended at 250×106 cells/ml in PBS. Each mouse received 50×106 Ag-SP in 200 µl of PBS given by i.v. injection at the indicated times before disease induction. This dosage represents delivery of a total of 15–20µg of cell-bound peptide per mouse.
Ag-SP labeling with PKH76, PKH26, and CFSE
Spleen cell membranes were stained with PKH76 (green) or with PKH26 (red) (Sigma Aldrich) dye according to the manufacturer’s instructions before ECDI fixation. 5-carboxyflourescein diacetate succinimidyl ester (CFSE) loading was performed as described in the manufacturers instructions (Cayman Chemical Company). Cells were then treated as described for ECDI fixation.
Delayed-type hypersensitivity (DTH) and in vitro proliferation assays
DTH was performed via ear challenge with 10 µg of peptide as previously reported (6). For proliferation assays, draining lymph nodes (LNs) (axillary, brachial, and inguinal) were harvested from naive mice or primed mice at indicated days following disease induction, counted, and cultured in 96-well microtiter plates at a density of 5×105 cells/well in a total volume of 200 µl of HL-1 medium (BioWhittaker; 1% penicillin/streptavidin and 1% glutamine). Cells were cultured at 37°C with medium alone or with different concentrations of peptide Ag for 72 h. During the last 24 h, cultures were pulsed with 1 µCi/well [3H]TdR, and uptake was detected using a Topcount microplate scintillation counter and results are expressed as mean of triplicate cultures.
Immunohistochemistry
Antibodies used for immunohistochemistry on spleen sections included rabbit polyclonal anti-LOX-1 (Abcam), rabbit polyclonal Scavenger receptor B I (Abcam) and II (Abnova), hamster anti-mouse CD11c (Biolegend), rat anti-mouse F4/80 (Biolegend), or rat anti-mouse IL-10 (BD Pharmigen). Polyclonal anti-rabbit, hamster IgG, rat IgG2a or rat IgG1 antibodies were used respectively as controls (Vector Laboratories, Biolegend, BD Pharmigen). Central nervous system tissues were stained with biotin-conjugated Abs anti-mouse CD4 (H129.19) and anti-mouse F4/80 (Caltag Laboratories). For central nervous system histology, mice were anesthetized and perfused with PBS. Spinal cords were removed by dissection, and 2–3 mm lower lumbar spinal cord (approximately L2–L3) blocks were immediately frozen in OCT (Miles Laboratories) in liquid nitrogen. Spleens were removed from mice infused with PKH26-, PKH76- or CFSE-labeled Ag-SP and fixed in paraformaldehyde for 30 min. to 3 hours at 4°C in the dark. Spleens were then frozen in OCT. The blocks were stored at −80°C in plastic bags to prevent dehydration. Six-micrometer thick cross-sections were cut on a Reichert-Jung Cryocut CM1850 cryotome (Leica) mounted on Superfrost Plus electrostatically charged slides (Fisher), air-dried, and stored at −80°C. Slides were stained using the Tyramide Signal Amplification Direct kit (NEN) according to the manufacturer’s instructions. Nonspecific staining was blocked using either anti-CD16/CD32, (FcIII/IIR, 2.4G2; BD Pharmingen) or 10% horse serum as well as avidin/biotin blocking kit (Vector Laboratories) in addition to the blocking reagent provided by the Tyramide Signal Amplification kit (NEN). Sections were then stained with primary and secondary antibody cocktails. Sections were coverslipped with Vectashield mounting medium with 4',6'-diamidino-2-phenylindole (DAPI) (Vector Laboratories). Slides were examined and images were acquired using a Lica DM5000B fluorescent microscope and Advanced SPOT software. At least eight serial sections from each sample per group were analyzed at ×20, ×40 and ×100 magnification.
ELISA, antibody-mediated neutralization and cell inactivation
IL-10 ELISA was performed using a ready set go IL-10 ELISA kit (eBioscience). As previously described (19), spleens from individual mice were snap frozen, defrosted and homogenized with a hand held homogenizer. The resulting homogenate was ultra-centrifuged before IL-10 quantitation. IL-10 neutralization was performed through intraperitoneal injection of 100 µg/mouse anti-IL-10 antibody (E-bioscience) as described for each experiment. CD20 depletion was performed using 250 µg/mouse anti-CD20 antibody (clone 5D2 kindly gifted by Genentech, Inc.). Treg inactivation was performed via injection of 500 µg/mouse anti-CD25 (clone 7D4) as described previously (20).
Treg Isolation
Tregs were isolated as previously described (21).
Flow cytometric analysis
Cells were isolated from the spleen as previously described (17). Briefly, FcR blocking with CD16/32 was performed. Cells were then stained with either a cocktail of antibodies containing CD4 (Becton Dickinson), CD11c (Becton Dickinson), CD8 (Becton Dickinson), B220 (Becton Dickinson), F4/80 (Biolegend) and PD-L1 (BioXcell) or respective isotype controls. Samples were run on a FACS CANTO flow cytometer with FACS DIVA software (Becton Dickinson). PD-L1 expression was determined based on Mean Fluorescent Intensity relative to isotype controls.
PD-L1 neutralization
Mice were treated intraperitoneally with 500 µg of anti-PD-L1 (clone 10F.9G2) or with control Rat IgG2b on day −7 and additionally with 250 µg on days −5, −3, −1, and +1 relative to immunization with PLP139–151/CFA. Anti-PD-L1 and isotype control Rat IgG2b antibodies were purchased from BioXcell Fermentation and Purification Services (West Lebanon, NH).
Statistical analyses
Comparisons of DTH responses, mean clinical disease scores, proliferation, cytokine responses, or MFIs between any two groups of mice were analyzed by a standard two-tailed t test or one-way ANOVA depending on the precise comparisons made. Values of p < 0.05 were considered significant.
Results
Tolerance Induction Requires Intravenous Administration of Ag-SP and is Spleen Dependent
Intravenous (i.v.) administration of peptides cross-linked to the surface of syngeneic splenic leukocytes (Ag-SP) using ethylene carbodiimide (ECDI) safely and efficiently induces antigen-specific immune tolerance and is effective in prevention and treatment of Th1/Th17-mediated autoimmune diseases (3, 6, 15, 22, 23), Th2-mediated asthma and food allergy models (Smarr and Miller, submitted), and promotes potent alloantigen-specific transplant tolerance in the absence of immunosuppressive drugs (5, 24). A phase I/IIA clinical trial is currently underway to examine the safety and efficacy of Ag-coupled peripheral blood leukocyte tolerance to a cocktail of myelin peptides in patients with new-onset MS. As such, further understanding of the mechanism(s) underlying Ag-SP tolerance induction and the boundaries of this therapy are of critical importance. We first investigated the importance of the route of Ag-SP administration (Figure 1A). Using PLP139–151-SP to induce tolerance in PLP139–151/CFA-induced R-EAE, we confirmed that i.v. administration prevented disease induction, but that i.p. administration was ineffective, as mice that received PLP139–151-SP i.p. developed EAE clinical scores that were similar to control mice treated i.v. with Ag-SP coupled with an irrelevant OVA323–339 peptide (sham tolerized). In contrast, s.c. administration of PLP139–151-SP acted synergistically with immunization, in that treated mice displayed significantly higher disease scores than sham-tolerized controls. We hypothesized that the importance of i.v. administration of Ag-SP for tolerance induction was related to the requirement for i.v. delivery of antigen to organs such as the spleen and liver, which have been associated with tolerance induction (25, 26). We i.v. infused Ag-SP labeled with the green fluorescence membrane labeling agent PKH76. As shown in Figure 1B, Ag-SP accumulated in the MZ of the spleen. Because the spleen is a major site for the removal of circulating senescing erythrocytes and apoptotic hematopoietic cells (25, 27), we examined the effects of Ag-SP administration on expression of certain scavenger receptors known to play a role in the removal of apoptotic cellular debris. While scavenger receptors LOX-1, Scavenger receptor type 1 B (SR1B) and CD68 were not affected by the accumulation of Ag-SP in the spleen at the time points examined, SRIIB was up-regulated within 3 hours after Ag-SP infusion (Figure 1B–H).
Figure 1. Role of the spleen and route of administration in Ag-SP tolerance induction.
(A) SJL/J mice were tolerized with 5×107 Sham OVA323–331-SP given i.v. (OVA323-SP i.v.) or with 5×107 PLP139–151-SP given i.v. (PLP139-SP i.v.), s.c (PLP139-SP s.c.) or i.p. (PLP139-SP i.p.). 5d later, the mice were immunized with 50 µg PLP139–151/CFA and monitored for clinical EAE for 20 days post priming. (B–H) Scavenger receptor response to Ag-SP infusion. SJL/J mice were infused with 5×107 PKH76-labeled PLP139-SP i.v. and spleens harvested for immunohistochemical staining 3 hours later. Expression of scavenger receptors LOX-1 (B), SR1B (C) and CD68 (D) were unaffected, but SRIIB was upregulated (F) compared to non-infused mice (E). Some co-localization of SRIIB and PKH76 was observed (white arrowhead in (H)) but not in isotype controls (G). (I & J) The spleen is required for Ag-SP tolerance. Sham splenectomized (Sham Splx) or splenectomized (Splx) SJL mice were tolerized with OVA323-SP or PLP139-SP on d-7, primed s.c. with 50 µg PLP139–151/CFA on d0 and DTH responses to PLP139–151 determined 8 d later (I). Asterisks denote a significant reduction in DTH responses (*p<0.0005). Data are representative of four experiments of five mice per group. (J) PLP139–151 specific proliferative responses from Sham Splx and Splx SJL mice were determined on d10. Asterisks denote a significant reduction in proliferative responses (*p<0.0001). Data examining the route of inoculation and splenectomized mice are representative of 2–3 experiments of five mice per group, with scavenger receptor examination determined from one experiment with 5 mice per group and at least 5 independent spleen sections examined.
To determine the mechanistic role of the spleen in Ag-SP tolerance, we asked if tolerance could be induced in splenectomized mice. Splenectomized SJL/J mice responded via DTH (Figure 1I) and proliferation (Figure 1J) to PLP139–151/CFA immunization similarly to sham-splenectomized control mice; however, splenectomized animals were resistant to tolerance induction with PLP139–151-SP, as measured by both assays (Figure 1I–J). These data show that i.v. administration is critical in Ag-SP tolerance induction, likely due to the direct delivery of apoptotic Ag-SP to immature ‘tolerogenic’ APCs in the splenic MZ.
Ag-SP Locate to the Splenic MZ and are Cleared within 18 Hours
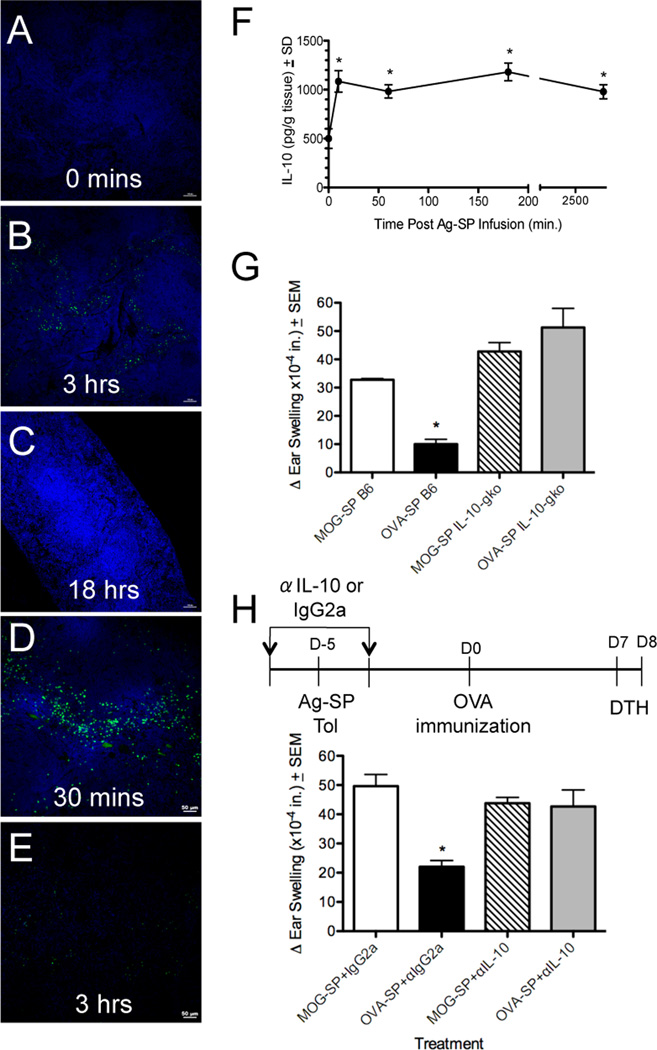
To further elucidate the precise environment of Ag-SP localization within the spleen, we examined the temporal uptake and destruction of PKH-76-labeled OVA323–339-SP (Figure 2A–C). Within 60 min of infusion, PKH-76 labeled OVA323SP were found throughout the spleen, especially within the marginal zone (data not shown). By 3 hours post-infusion, the PKH76 staining appeared to be punctate and fragmented, indicating that the Ag-SP had lost cell membrane integrity (Figure 2B). Using PKH-76 as a marker of membrane debris removal, we found that the Ag-SP were undetectable by 18 hours post-infusion (Figure 2C). This was further supported by experiments using CFSE-labeled Ag-SP (Figure 2D&E). At 30 min post infusion, numerous CFSE labeled OVA323–339-SP can be seen throughout the spleen (Figure 2D); however by 3 hours post-infusion, no evidence of CFSE positive cells remains (Figure 2E). Because CFSE is a cytoplasmic dye, these data suggest that within 3 hours, all infused Ag-SP cells lose their plasma membrane integrity, resulting in CFSE diffusion into the extracellular matrix. In contrast, PKH-76 is a plasma membrane bound dye, which will not dilute or leak during the apoptotic process. Therefore, these results collectively indicate that upon i.v. infusion, the Ag-SP cells rapidly become unstable, lose their membrane integrity within 3 hours of infusion, as supported by our earlier finding that ECDI-fixed cells become rapidly apoptotic (6), and are completely removed by phagocytosis within 18 hours post transfer.
Figure 2. Intravenously infused Ag-SP are rapidly removed from the spleen and trigger IL-10 production.
(A–C) SJL/J mice were tolerized with 5×107 PKH76-labeled OVA323-SP. Groups of 3–5 mice were sacrificed at 0, 3 and 18 h post-infusion. At least 20, 8µM sections were examined from each animal. PKH76-labeled sub-cellular debris present at 3 h (B) post-infusion was completely absent by 18 h (C). (D–E) A separate cohort of at least 4 animals was treated with 5×107 CFSE-labeled OVA323-SP and mice were sacrificed 30 min and 3 h post-infusion. Numerous CFSE-labeled Ag-SP were observed at 30 min (D) but were completely absent by 3 h post infusion (E). IL-10 is secreted in response to Ag-SP infusion. Groups of at least 4 mice were infused with 5×107 OVA323-SP, recipient spleens harvested at 0, 10, 60 and 180 min post-infusion and IL-10 levels in supernatants of individual homogenized spleens (run in triplicate) measured using ELISA. *IL-10 levels significantly higher than baseline (p<0.01). (F). IL-10-deficient mice can not be tolerized with OVA323–339 (G). Wild type (B6) and IL-10-deficient (IL-10gko) C57BL/6 mice were tolerized i.v. with 5×107 syngeneic splenocytes from IL-10gko mice coupled with MOG35–55 (irrelevant peptide control) or OVA323–339 on day −5. On day 0, the mice were immunized with 200 µg OVA323–339/CFA and DTH responses to OVA323–331 ear challenge determined on day 7 (G). IL-10 neutralization prevents Ag-SP tolerance induction (H). Anti-IL-10 or control IgG2a antibody was given 30 min prior and 18 h after MOG35-SP or OVA323-SP infusion on day −5. Animals were immunized with OVA323–339/CFA on day 0, and DTH assessed on day 7. Asterisks denote a significant reduction in DTH responses (*p<0.0005) as compared to MOG35–55-SP controls. Data in all panels are representative of at least 3 experiments of at least 4 mice per group.
IL-10 Secretion is Induced in Response to Ag-SP Infusion and is Critical for Tolerance Induction
The rapid clearance of i.v. administered Ag-SP from the spleen (Figure 2A–E) suggested that the framework for tolerance induction is initiated very early after Ag-SP infusion. Because of the importance of IL-10 in immune regulation (28–31), we investigated the level of IL-10 present in whole-spleen homogenates in response to Ag-SP infusion (Figure 2F). Examination of IL-10 protein revealed that within 10 minutes post-infusion of OVA323–339-SP, IL-10 protein levels increased dramatically. Furthermore, these IL-10 levels remained significantly above the baseline level over the 3 days of testing. To determine the functional role of IL-10 secretion in Ag-SP tolerance induction, we first attempted to tolerize IL-10 deficient animals (IL-10gko), with ECDI coupled splenocytes coupled with OVA323–339-SP. Using DTH as an vivo measure of T cell tolerance induction in OVA323–339/CFA immunized mice, we found that while control mice were successfully tolerized to OVA323–339 showing little to no ear inflammation, IL-10gko mice were not tolerized (Figure 2G). Importantly, we found that donor splenocytes from both wild type (not shown) and IL-10gko (Figure 2G) animals were similarly capable of inducing tolerance in wild type animals, indicating the source of IL-10 was the recipient. These data are a strong indication of the critical nature of IL-10 for the induction of Ag-SP tolerance. However, IL-10gko mice are known to have altered immune regulation, commonly developing autoimmune conditions including colitis (32). Therefore, we administered 100 µg neutralizing IL-10 antibody 30 min prior and 18 hours post OVA323–339-SP infusion in wildtype B6 mice. While immunized mice treated with isotype control antibody displayed a characteristic reduction of DTH responses indicative of tolerance induction, mice receiving anti-IL-10 exhibited ear swelling similar to mice tolerized with the irrelevant MOG35–55-SP peptide (Figure 2H). Collectively, these results confirm that the environment supporting Ag-SP tolerance induction is formed early and is critically dependent on IL-10 production.
B cells are not Required for Ag-SP Tolerance Induction
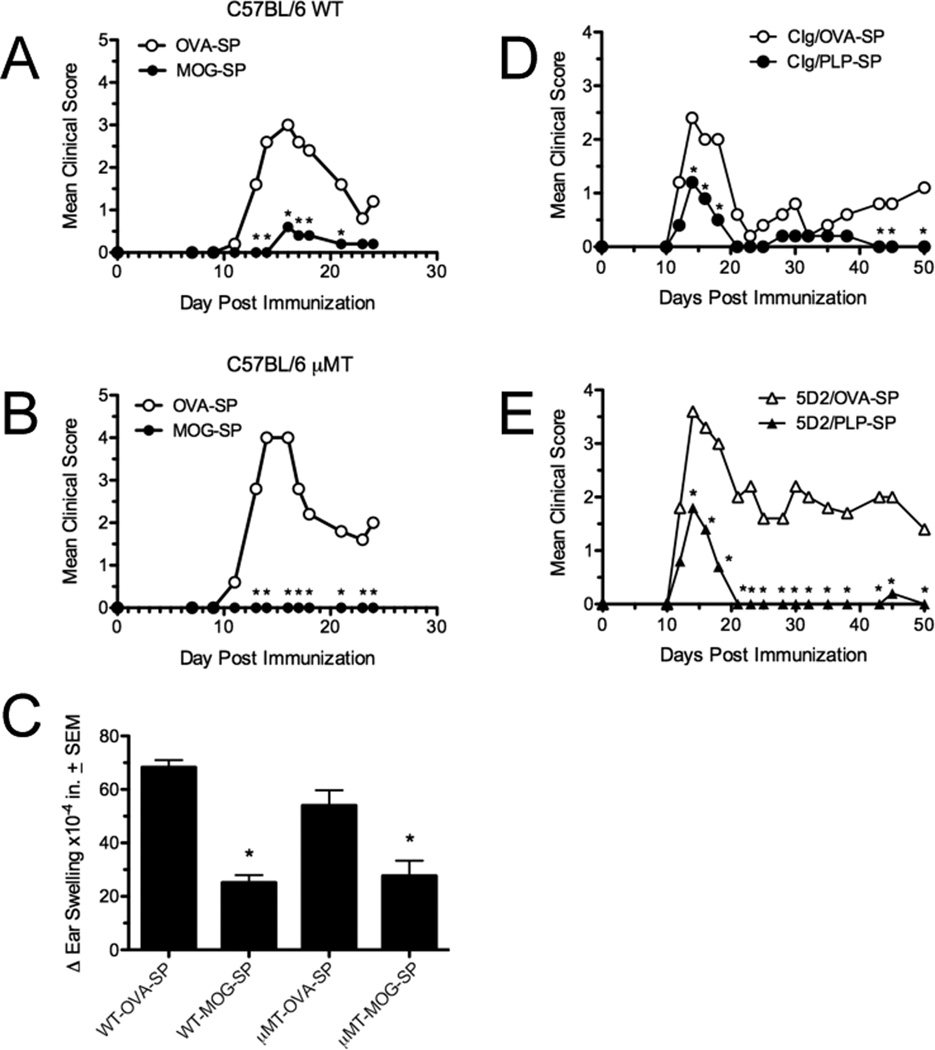
Previously it has been reported that the infusion of apoptotic cells with CFA stimulates IL-10 producing regulatory B cells which can prevent CD4+ T cell activation (11). We investigated the importance of both B cells in Ag-SP tolerance induction. In contrast to this previous study, we found that mice devoid of B cells (µMT mice) can still be tolerized with Ag-SP. Specifically, treatment with MOG35–55-SP was equally capable of preventing MOG35-35/CFA-induced EAE in wildtype (Fig. 3A) and µMT mice (Fig. 3B), and tolerance was similarly reflected in MOG35–55-specific DTH responses (Figure 3C). In addition tolerance could be induced in mice depleted of B cells with anti-CD20 (Fig. 3E). These data discount the importance of B cell-derived IL-10-production in Ag-SP tolerance.
Figure 3. B cells are not required for induction of Ag-SP tolerance.
Wildtype (A) and B cell-deficient (µMT) C57BL/6 mice (B) were tolerized i.v. with 5×107 syngeneic MOG35-SP on day −7, primed with MOG35–551/CFA on day 0, and monitored for clinical EAE disease for 24 days post-priming. Data are representative of 2 experiments of 5 mice per group. On day +25 post-priming, MOG35–55-specific DTH responses were assessed (C). Wildtype SJL/J mice were treated with 250 µg of control Ig (D) or anti-mouse CD20 mAb (clone 5D2) (E) on day −12 followed by i.v. tolerization with 5×107 PLP139-SP on day −7. Anti-CD20 treatment resulted in >95% reduction in B cells in the primary lymphoid organs, peritoneal cavity and the blood within 2 days of antibody injection. On day 0, the mice were primed with PLP139–151/CFA and monitored for disease incidence for 50 days post-priming. Data are representative of 2 experiments of 5 mice per group. Asterisks denote a significant reduction in mean clinical score or DTH responses (*p<0.01).
Regulatory T cells are Critical for Maintenance, but not Induction, of Ag-SP Tolerance
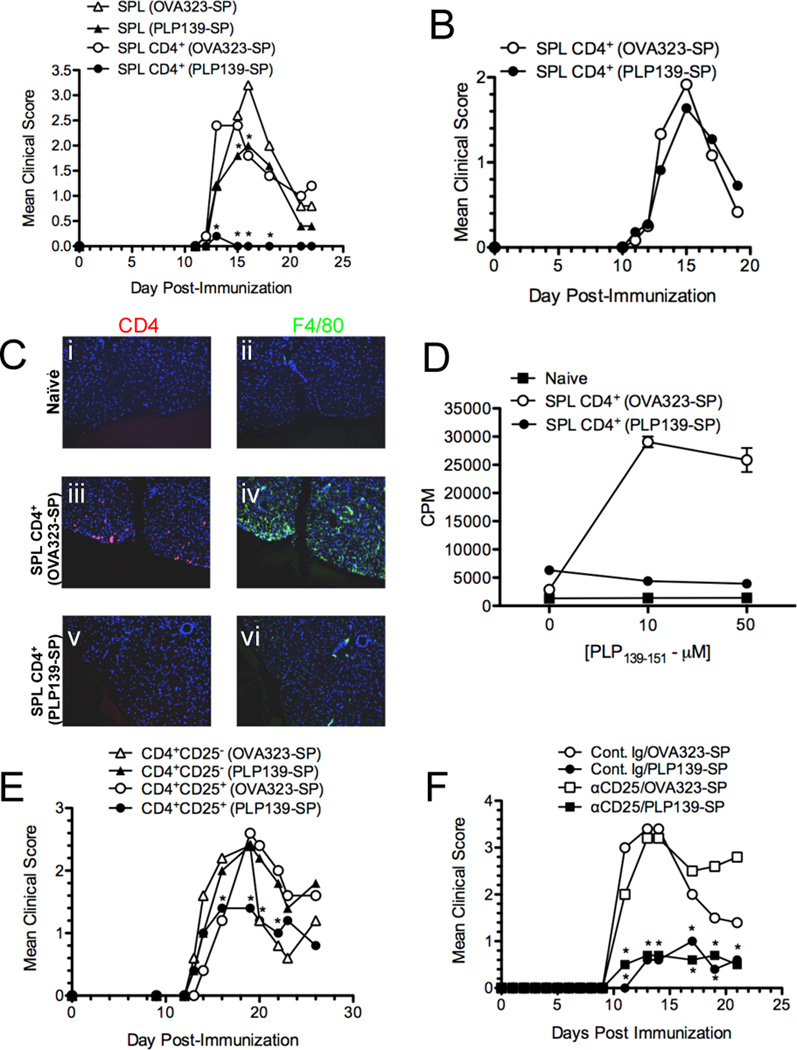
IL-10-producing CD4+CD25+Foxp3+ Tregs have been implicated in immune regulation and tolerance induction in numerous models of inflammation and tolerance (32, 33). The importance of IL-10 in Ag-SP tolerance suggests that Treg may also play a role in the induction of Ag-SP tolerance. To address the role of Tregs, we initially asked if tolerance could be actively transferred from mice treated with Ag-SP. On day −7, SJL mice were tolerized with 5×107 syngeneic splenocytes coupled with either PLP139–151 or OVA323–339. Five days post Ag-SP therapy, bulk splenocytes and purified CD4+ cells were isolated and transferred i.v. into naïve SJL mice, which were then immunized two days later with either PLP139–151/CFA or PLP178-91/CFA and clinical disease was monitored for 24 days. Transfer of bulk splenocytes as well as purified CD4+ T cells significant suppressed clinical EAE compared to animals tolerized to the irrelevant OVA323–339 peptide (Figure 4A). Tolerance transfer was antigen specific, as recipients of T cells from PLP139–151-SP treated mice failed to suppress EAE induced by immunization with PLP178–191 (Figure 4B). Antigen-specific regulation was supported by reductions in CNS inflammation, observed by immunofluorescent staining for CD4+ T cells and F4/80 (microglia/macrophages) on lumbar spinal cord sections (Figure 4Ci–vi), as well as a lack of development of PLP139–151 proliferative responses (Figure 4D) in the animals receiving CD4+ splenocytes from PLP139–151-SP tolerized animals. These data support an important role for CD4+ T cells in disease regulation. To more specifically examine a potential role for Treg cells, we purified CD25+ and CD25− CD4+ splenic T cell populations 5 days post PLP139–151-SP or OVA323–339-SP infusion into, transferring 5×106 of these cells independently into naïve SJL mice, which were then immunized with PLP139-151/CFA and monitored for disease. CD4+CD25+, but not CD4+CD25− cells, transferred from PLP139–151-SP tolerized animals induced significant protection from EAE (Figure 4E). Overall, these data indicate that CD4+CD25+ Tregs are a component of tolerance induced by Ag-SP treatment. However, since we observe rapid IL-10 production almost immediately after Ag-SP infusion, and neutralization of IL-10 at the time of Ag-SP is capable of preventing complete tolerance induction (Figure 2G&H), we next addressed the role of Treg precisely at the time of tolerance induction. Using anti-CD25 antibody to deplete/inactivate Tregs (20), we found that the functional inactivation of Tregs had no measurable effect on tolerance induction, with anti-CD25 treated and isotype control treated Ag-SP tolerized animals both exhibiting significantly reduced clinical disease (Figure 4F).
Figure 4. Tolerance is transferable with CD4+CD25+ T cells.
SJL/J mice were tolerized on day −7 with 5×107 OVA323-SP or PLP139-SP. On day 2, 5×106 bulk splenocytes (SPL) or CD4+ splenocytes (SPL CD4+) from each treatment group were transferred i.v. to naïve recipients which were primed s.c. with 50 µg PLP139–151/CFA (A) or PLP178–191/CFA (B) on day 0 and monitored for clinical disease. Asterisks denote a significant reduction in clinical score in recipients of bulk or CD4+ splenocytes (*p<0.05). Data are representative of 2–3 experiments of 5–8 mice per group. (C) Two mice from the groups receiving splenic CD4+ T cells from OVA323-SP and PLP139-SP primed with PLP139–151/CFA were perfused on day +25. Spinal cords were stained with anti-CD4 (red) or anti-F4/80 (green) mAbs, and counterstained with DAPI (blue). Lumbar regions are shown at 200× magnification. (D) Spleens were harvested from 3 representative mice from each group on day +25 and proliferative responses determined. Data is representative of 2 experiments. (E) SJL/J mice were tolerized on d-7 with OVA323-SP or PLP139-SP as in Panel A. On day −2, 5×106 CD4+CD25− or CD4+CD25+ splenocytes from the tolerized mice were transferred i.v. to naïve recipients which were primed s.c. with 50 µg PLP139–151/CFA and monitored for clinical disease. Asterisks denote a significant reduction in clinical score in recipients of CD4+CD25+ splenocytes (*p<0.05) from PLP139-SP tolerized mice. Data are representative of 2 experiments of 6–8 mice per group. (F) SJL/J mice (5–6 per group) were treated with 500 µg of Cont. Ig or anti-CD25 mAb (clone 7D4) on d-11 and d-9, tolerized with 5×107 OVA323-SP or PLP139-SP on d-7, primed with PLP139–151/CFA on d0, and monitored for clinical signs of disease. Asterisks denote a significant reduction in clinical score of PLP139-SP treated mice (*p<0.01) in both control Ig and anti-CD25 treated mice. Data is representative of 3 separate experiments.
The data suggest that Tregs capable of down-regulating clinical disease are induced by Ag-SP treatment, but that there is a separate non-overlapping tolerance mechanism induced. We hypothesized that while Treg may not be critical for tolerance induction, they may play a role in the long-term maintenance of Ag-SP tolerance. To investigate this possibility, we treated a large cohort of SJL/J mice with either control Ig or anti-CD25 antibody at days −4 and −2 relative to PLP139–151-SP or control OVA323–339-SP infusion (Figure 5A). Separate groups of mice were then immunized with PLP139–151/CFA on days 14, 35, and 63 post Ag-SP treatment. Similarly to data shown above (Figure 4F), we found that that functional inactivation of Tregs had no effect on tolerance induction in animals immunized on either day 14 (Figure 5B) or day 35 (Figure 5C&E) post tolerance induction, as both control Ig and anti-CD25 treated mice tolerized with PLP139–151-SP displayed significantly lower clinical disease and peptide-specific DTH. However, only control Ig-treated, not anti-CD25-treated, mice immunized on day 63 post Ag-SP treatment were protected from disease induction and had significantly downregulated DTH responses (Figure 5 D&F). Overall, the data support two important conclusions. First, Tregs are not required for tolerance induction, and are unlikely to be a significant source of the early IL-10 induced by Ag-SP injection. Second, and more importantly, Tregs appear to play a major role in long-term tolerance maintenance for protection from R-EAE.
Figure 5. Tregs are dispensable for tolerance induction by Ag-SP, but required for long-term tolerance maintenance.
(A) SJL/J mice were treated with 500 µg of Cont. Ig or anti-CD25 mAb (clone 7D4) on days −4 and −2. On day 0, the entire cohort of mice was tolerized with 5×107 OVA323-SP or PLP139-SP. Separate groups of mice were primed with 50 µg PLP139–151/CFA on day +14 (B), day +35 (C), or day +63 (D) post-tolerization and followed for clinical signs of EAE. Data represent the clinical disease pattern of 5–6 mice per group and is representative of 2 separate experiments. (E and F) DTH responses of mice from panels C and D to challenge with PLP139–151 were determined following cessation of clinical disease assessment. Asterisks denote a significant reduction in clinical disease score (*p<0.01) and DTH responses (p<0.05).
Splenic Macrophages Produce IL-10 in Response to Ag-SP in vivo
We next investigated the APC subsets in the spleen involved in tolerance induction. Using PKH26-labeled Ag-SP (red), we examined the association of Ag-SP with DCs (CD11c+; Figure 6A–C) or macrophages (F4/80+; Figure 6D–E) at 8 hours post Ag-SP infusion. Surprisingly, little PKH26-CD11c co-localization was observed in animals that received either non-fixed splenocytes (Figure 6B) or ECDI-fixed splenocytes (Figure 6C). However, fixed splenocytes co-localize at a much higher frequency with cells F4/80 expressing cells (Figure 6F), especially in the MZ.
Figure 6. Macrophage responses to Ag-SP in vivo and in vitro.
In vivo response. Groups of at least 5 C57BL/6 mice were infused with nothing (No Ag-SP A, D & G), 5×107 Non-ECDI fixed PKH26 (red)-labeled splenocytes (PKH-SP (No ECDI) B, E & H), or 5×107 ECDI-fixed PKH26-labeled MOG35–55-SP (PKH-SP C, F & I). 8 hours later the spleens were harvested for immunohistochemistry. Spleen sections (8µM) were stained in green for CD11c (A–C), F4/80 (D–F) and IL-10 (G–I) and counterstained with DAPI (blue, Panels A–F). Similar to the non-fixed splenocyte control (B), little co-localization of Ag-SP with CD11c was observed (C). F4/80 commonly co-localized with PKH-26 in the Ag-SP treated animals (F). No IL-10 staining was observed in the untreated (G) or non-ECDI fixed splenocyte infused animals (H). Strong IL-10 production (indicated by the green stain) was commonly coincident with F4/80+ cells (indicated by the blue stain) (I). In vitro response. The macrophage cell line, J774 (K–M), thioglycollate-elicited (N–P) and non-elicited peritoneal macrophages (Q–S) were cultured on cover slips in 24 well plates and fed 106 OVA323–339-SP labeled with PKH26 (red) overnight. Supernatant was collected for IL-10 analysis and the remaining cover slips were fixed in PFA, counterstained with membrane dye PKH76 (green) and nuclei stained with DAPI (blue). Ag-SP remained PKH26+ after overnight incubation, the cells did not label with DAPI or PKH76 (J). J774 macrophages cultured alone (K) and demonstrating uptake of PKH26+ cell membranes (L), but failure to produce significant IL-10 (M). Thioglycollate-elicited peritoneal macrophages cultured alone (N) and demonstrating significant uptake of both fragments (white arrowhead) and cells (yellow arrowhead) (O), but failure to produce IL-10 (P). Resting peritoneal macrophages were cultured alone (Q) and demonstrating significant uptake of PKH26-labeled Ag-SP (R) and significant production of IL-10 (S). Data represents at least 6 independent wells, conducted in 2–3 separate experiments. Asterisk represents significant increase in the level of IL-10 (p<0.05).
In addition to the co-localization of Ag-SP with F4/80+ macrophages, we also determined the expression profile of IL-10 in serial sections in the same experiments. While little IL-10 was found in the control non-treated or animals receiving non-ECDI fixed PKH-26 labeled splenocytes, IL-10 was strongly expressed in Ag-SP recipients. While a small population of cells was found to express IL-10 in the germinal centers of Ag-SP treated animals (not shown), the most striking number of cells producing IL-10 were F4/80+ and in close proximity to PKH26-expressing Ag-SP (Figure 6I).
Macrophages Produce IL-10 Upon Co-culture with Ag-SP
Attempts to isolate F4/80 macrophages from the spleen failed to produce sufficient yields for functional assessment. As such, IL-10 responses of a macrophage cell line (J774), as well as primary thioglycollate-stimulated and resting peritoneal macrophages, to co-culture with Ag-SP were evaluated. J774 cells phagocytized PKH-26-labeled (red) Ag-SP, but this uptake failed to upregulate production of IL-10 (Figure 6K–M). In an attempt to move to a more physiologically relevant system, we found that thioglycollate-elicited peritoneal macrophages were also capable ingesting Ag-SP, with a significant amount of PKH-26 labeled membrane localized inside the macrophages, but again Ag-SP uptake failed to stimulate IL-10 production (Figure 6N–P). The thioglycollate-stimulated macrophages were rounded up, with multiple nuclei, and exhibited a highly inflammatory phenotype. We have previously shown that LPS injection is capable of preventing Ag-SP tolerance in vivo (7). Since the J774 macrophage line and the thioglycollate-elicited peritoneal macrophages are of a type 1 phenotype, characterized by the production of proinflammatory cytokines, we hypothesized that the normal response to Ag-SP is overcome by the background activation state of these cells. We thus tested non-elicited peritoneal macrophages harvested from multiple mice. We found that these cells exhibited the greatest capacity to ingest Ag-SP as determined by the internalization of PKH26-labeled membrane material and, importantly, that resting macrophages produced significantly upregulated levels of IL-10 upon ingestion of OVA323–339-SP (Figure 6Q–S). The production of IL-10 by these macrophages is consistent with our in vivo immunohistological findings (Figure 6I) as well observations by other investigators examining the response of macrophages to tolerogenic stimuli (33, 34). In conclusion, these results support a scenario in which resting MZ macrophages respond rapidly to Ag-SP and are likely to be the major source of the early IL-10 produced in response to i.v. Ag-SP infusion and to be critical for the induction of tolerance.
IL-10 Regulates PD-L1 Expression on F4/80 Macrophages which is Critical for Ag-SP Tolerance Induction
To this point, our data indicate that the long-term antigen specific tolerance triggered by the infusion of Ag-SP is the result of more than one mechanism, with Tregs primarily required for tolerance maintenance. Numerous previous studies have implicated CD8α+ dendritic cells, as well as different macrophage subpopulations, in tolerance induction secondary to uptake of apoptotic cell debris (34–39). We thus asked if there was a role for one or both of these APC subsets, in Ag-SP uptake, as a sources for IL-10 and/or as major drivers of Ag-SP tolerance.
Further examination of the ratio of APC populations in the spleen 3 hours after Ag-SP infusion revealed that the ratio of the major dendritic cell subsets including CD4+ DCs (CD4+CD11c+CD8α−), CD8α+ DCs (CD8α+CD11c+CD4−) and pDCs (CD8−CD4−CD11cint), remain unchanged, and were further unaffected in mice treated with anti-IL-10 (Figure 7A–B). In contrast, F4/80 expressing macrophages (F4/80+CD11C−CD4−CD8−) significantly increased in relative percentage within 3 hours post Ag-SP infusion (Figure 7B).
Figure 7. Splenic macrophages uptake Ag-SP and express PD-L1 in an IL-10-dependent manner.
Effect of Ag-SP infusion on splenic macrophage ratio. Five groups of SJL/J mice (4–5 mice per group) received IgG2a control antibody, anti-IL-10 alone, OVA323–339-SP + IgG2a antibody, OVA323–339-SP + anti-IL-10 antibody, or no treatment. All antibodies were given 30 min. prior to OVA323-SP infusion. 3h after infusion, animals were sacrificed and splenocytes stained with a cocktail of antibodies as described in the Materials and Methods. (A) Splenic APC populations were enumerated using the gating strategy shown; black population indicates the ungated isotype control for each dot plot. (B) Percentages of CD4+ DCs, CD8α+ DCs, and plasmacytoid DCs (pDC) did not change in any of the treatment groups, but percentages of macrophages increased in an IL-10-dependent fashion. F4/80+ splenic macrophages uptake Ag-SP and express PDL-1. Spleens from CD45.1+ C57BL/6 mice receiving either PBS (C) or 5×107 CD45.2+ PKH-26-labeled, OVA323-SP (D) were harvested 2.5 hours after i.v. administration. Gate R1 represents recipient cells that have taken up donor Ag-SP, while Gate R2 represents intact Ag-Sp. Numbers adjacent to gate represent the percentage of cells within the gate (D). Relative CD45.2 expression on gates R1 (grey line) and R2 (black line) (E). Cells from gate R1 are 85% CD11b+ and 11.6% CD11chigh (F). Cells from gate R3 are 77.5% F4/80int and 11.3% F4/80high. The majority of the cells in gate R3 were CD11cint which is consistent with the phenotype of splenic MZ macrophages (G). >73% of cells from R3 (i.e. those that are of recipient origin, the majority being F4/80+ macrophages) that have engulfed Ag-SP express PD-L1 (H). PD-L1 expression increases in the CD8α+ DC and F4/80+ macrophage populations and expression is reversed by anti-IL-10 in macrophages (I). Data is representative of 2 separate experiments. Asterisks denotes a significant change in APC subset ratio/expression compared to animals treated with IgG2a control antibody (*p<0.05). PD-L1 Expression is Required for Ag-SP Tolerance. PD-L1 blockade prevents Ag-SP tolerance induction. SJL/J mice were treated with anti-PD-L1 or control IgG2a antibody as detailed in the Materials and Methods. Mice were tolerized with OVA323-SP or PLP139-SP on day −7. Animals were immunized with PLP139–151/CFA on day 0, and DTH assessed on day 7. Asterisks denote a significant reduction in DTH responses (*p<0.01) as compared to MOG35–55-SP controls. Results are representative of two separate experiments of at least five mice per group.
To further examine the potential uptake of Ag-SP by macrophages, OVA323-coupled B6 CD45.1 congenic splenocytes were labeled with PKH-26 and injected into CD45.1 mice, which were sacrificed 3 hours after infusion. While no PKH-26 co-localization was observed with recipient CD45.1 in untreated controls (Figure 7C), 3 hours after Ag-SP infusion there was a distinct population of PKH-26+/CD45.1+ cells as well as possibly intact PKH26+CD45.1− donor cells (Gate R1 Figure 7D–E). The PKH-26+CD45.1+ cells were 85% CD11b+F4/80+/CD11c−/lo (Gate R3 Figure 7F), with only 11.6% expressing CD11chi (Figure 7F). Cells from gate R3 were 77.5% F4/80int and 11.3% F4/80high. The majority of the cells in gate R3 were CD11cint which is consistent with the phenotype of splenic MZ macrophages (Figure 7F–G). Significantly, PKH-26 co-localized macrophages also expressed high levels of PD-L1 (Figure 7H). These results show that shortly after i.v. infusion of Ag-SP, macrophages not only change in their relative percentage in the spleen, but they are also the major population taking up the apoptotic Ag-SP debris and expressing PD-L1.
Interestingly, administration of IL-10 neutralizing antibody 30 min prior to Ag-SP infusion completely abrogated the increase in F4/80 expressing macrophages (Figure 7B), suggesting that IL-10 plays may play a role in the overall kinetics of cellular proliferation/migration within the splenic microenvironment. PD1/PD-L1 and IL-10 have been reported to reciprocally regulate each other (40). Therefore, we examined PD-L1 expression on APC populations after Ag-SP infusion. We found that within 3 hours, macrophages displayed the greatest increase in PD-L1 expression (Figure 7I). This was not reflected by increased expression of other molecules such as MHC-II and CD80/86, which remained unchanged on all examined APC populations (data not shown). Interestingly, CD8α+CD11c+CD4− DCs also upregulated PD-L1 within the time frame examined, however unlike the macrophages, neutralization of IL-10 did not significantly alleviate PD-L1 expression on DCs.
Finally to address the functional role of PD-L1 in tolerance induction we infused anti-PD-L1 antibody at the time of PLP131–151-SP infusion and subsequently primed the mice with PLP131–151/CFA. Animals became moribund within 14 days post immunization (data not shown). It has been described previously that administration of anti-PD-L1 antibody early in EAE induction can significantly exacerbate disease (18, 41, 42). Because of the severe disease phenotype observed, we examined tolerance induction in anti-PD-L1 treated animals by DTH at 7 days post-priming. PD-L1 inhibition at the time of Ag-SP infusion completely ablated the induction of tolerance (Figure 7J). Based on the disease observations, it is tempting to speculate that Ag-SP infusion in the presence of anti-PD-L1 may actually prime mice such that later immunization with PLP139–151/CFA results in lethal EAE. We are currently investigating this possibility. Collectively, the data suggest a critical role for IL-10 and build on our and others previous findings regarding the importance of PD-L1 in the induction of Ag-SP tolerance (5, 15, 16).
Discussion
Previously, we and others have shown that Ag-SP infusion is a safe and highly efficient method for the induction of T cell tolerance in models of Th1/Th17 autoimmunity and Th2-mediated allergy. The precise mechanisms driving Ag-SP induced tolerance remain to be completely defined, however it is clear from previous observations that direct Ag-SP-T cell interaction is responsible to some degree for tolerance induction, particularly in in vitro anergy models. This is thought to be the result of TCR stimulation in the absence of secondary co-stimulatory signals (4). However, this mechanism fails to explain the fact that both antigen coupled, ECDI-fixed allogeneic and MHC class I/II-deficient donor cells also effectively induce T tolerance in vivo (6). Here, we show that Ag-SP tolerance recapitulates how tolerance is normally maintained in the hematopoietic compartment and is the result of two parallel, but convergent pathways. First, Ag-SP trigger rapid and sustained IL-10 release from macrophages that appears to modulate PD-L1 expression on these cells which is critical for Ag-SP tolerance induction (5, 7, 15). Second, we show that while not important for Ag-SP tolerance induction, Tregs play a critical role in the long-term maintenance of unresponsiveness.
Tolerance induction with ECDI-coupled-splenocytes was found to critically depend on the route of administration. Intravenous infusion of Ag-SP resulted in complete protection from of PLP131–159 induced EAE, but neither i.p. nor s.c. infusion of Ag-SP prevented disease development. In fact, s.c. infusion of PLP139–151-SP acted synergistically with PLP139–151/CFA immunization, further exacerbating disease instead of tolerizing animals. Relationships between route of administration and tolerance induction have also been described for certain peptides utilized in high zone tolerance induction (43). For example, myelin basic protein (MBP) in human patients induced tolerance only when given via the i.v. route, with intrathecal and s.c. administration having either short lived or no effect on disease (43). The data suggest that the context of antigen delivery, including the cytokine milieu and local cell populations are important in Ag-SP tolerance induction. Currently, it is thought that Ag-SP injection via i.p. or s.c. injection, especially the latter, is uptaken by and/or triggers activation of resident leukocyte populations such as Langerhans cells, dermal dendritic cells and/or γδ T cells. In turn, the production of pro-inflammatory cytokines is likely to negate the upregulation of negative co-stimulatory molecules such as CTLA-4 and PD-L1, which have previously been shown to play important roles in Ag-SP tolerance induction (15, 44). PD-L1 for example has been shown to play a role with Tregs in long-term allo-tolerance after infusion of ECDI-fixed allogeneic splenocytes (15, 42). In addition, the processing and quantity of antigen delivered to secondary lymphoid organs is likely to play a role. Intravenous infusion of Ag-SP results in rapid accumulation of Ag-SP apoptotic debris in the spleen. While both i.p. and s.c. injection of Ag-SP are likely to result in some Ag-SP-associated antigen drainage to the spleen, it is likely that most of the Ag-SP will ultimately be sequestered in local draining lymph nodes. Furthermore, the antigen is likely to be transported by APC from the skin and peritoneal cavity, thereby circumventing the involvement of macrophages in the secondary lymphoid organs.
While apoptotic cells like Ag-SP are thought to be non-immunogenic, they are by no means invisible to the immune system. Work of Barker and colleagues showed that stimulation of macrophages with necrotic, but not apoptotic, cells resulted in triggering of recall responses in OVA-specific T cells (9). Within the spleen, which is critical for Ag-SP tolerance, there are numerous mechanisms through which apoptotic debris is recognized and rapidly removed (9, 45). It appears that these functions are mostly performed by macrophages within the spleen, which encompass a variety of subtypes depending on their anatomical localization and receptor expression. Within the germinal centers exist the tingible body macrophages, identified by the expression of the scavenger receptor CD68 (10). These macrophages have been shown to play a role in B cell tolerance through their uptake of MFGE8-coated apoptotic debris (10). While B cell tolerance is compromised in MFGE8 deficient animals, a role for this pathway in peripheral T cell tolerance has not been described. However, it appears unlikely that this pathway plays a role in Ag-SP-mediated tolerance, as few coupled cells are found within germinal centers or localized with CD68 expressing cells. Another scavenger receptor that has been shown to aid in uptake of apoptotic debris is the oxidized low-density lipoprotein receptor-1 (LOX-1). While this receptor has been shown to mediate DC uptake and response to apoptotic debris (46), we found little co-localization of LOX-1 or CD11c with Ag-SP at the early time points investigated. Similarly, the class B scavenger receptor I (SRBI), which is a phosphatidyl serine recognizing receptor known to play a role in the uptake of apoptotic sperm (47), was also uncommonly found to co-localize with Ag-SP. On the other hand we found that SRBII, an isoform of SRBI (48, 49), co-localized with Ag-SP and was also rapidly upregulated in response to the i.v. infusion of Ag-SP. These findings suggest that there may be a role for SRBII in Ag-SP uptake and T cell tolerance induction. The lack of co-localization with SRBI and LOX-1 requires further investigation. However, it is becoming clear that scavenger receptors may have multiple ligands and functions. SRBI, for example, is known to be expressed on testicular sertoli cells (47), and has also been shown to prevent anemia through its interaction with erythropoietin and high density lipoprotein (50). Finally, there are numerous other scavenger receptors, e.g. MARCO (51) that may also play a role in Ag-SP tolerance induction and are currently under investigation.
In 1997, Voll and colleagues showed that feeding apoptotic cells to peripheral blood-derived macrophages triggered the production of IL-10 (52). IL-10 was originally defined as a T cell cytokine, predominately secreted from Th2 T cells (13). However, it is now known that many cells, including macrophages and regulatory B cells as well as T cells, can produce IL-10 (13). IL-10 plays an important immune regulatory role, preventing inflammatory immune responses and the development of autoimmunity. The infusion of Ag-SP results in rapid and sustained production of IL-10. Importantly, this IL-10 response is critical for the induction of tolerance, as neither IL-10-deficient mice nor mice treated with anti-IL-10 can be tolerized with ECDI-fixed splenocytes. These observations raise the question of whether IL-10 plays a direct role in tolerance induction or whether the IL-10 detected is produced subsequent to tolerance induction, by activated T and B regulatory cells. Indeed, B cell-dependent IL-10 production, in response to apoptotic cells and CFA immunization, has been shown to prevent CD4+ T cell activation. However, we found that animals lacking functional B cells via either genetic deletion (µMT) or following anti-CD20 treatment could still be tolerized to MOG35–55 EAE by MOG35–55-SP. Our data which contrasts with a previous study in which induction of tolerance using apoptotic thymocytes was B cell dependent (11), could be explained by the different methods of induction of apoptosis. In the former study, apoptosis was induced in syngeneic thymocytes using 2-mercaptoethanol (2ME). Furthermore, apoptosis in that study was found only to be 43% at the time of infusion, and it is likely that this mixed apoptotic/non-apoptotic thymocyte population may trigger different responses. Finally, in contrast to specific tolerance induction by Ag-SP, the infusion of apoptotic thymocytes appears to induce a non-specific anti-inflammatory response (11). From our studies, it appears that that B cells are not a critical requirement for Ag-SP tolerance induction, and as such they are not likely to be a major source of IL-10 in response to Ag-SP infusion.
Macrophages have been shown to play a critical role in T cell tolerance induced by antigen administration in the anterior chamber of the eye, in which F4/80 deficiency prevents tolerance induction (53). F4/80+ macrophages in this model mediate the expansion of regulatory T cells. Here we show that PLP139–151 coupled cells induced antigen-specific Tregs. These cells were capable of transferring specific unresponsiveness to naïve recipients. Previously, CD4+CD25+FoxP3+ IL-10 secreting T cells have been implicated in the regulation of colitis and inhibition xenogeneic T cell proliferation (11, 32, 33). The ability to transfer tolerance with CD4+CD25+ T cells strongly suggests that Ag-SP tolerance may in part result from Treg expansion. However, the functional inactivation of Tregs at the time of tolerance induction failed to prevent tolerance induction in mice primed either 14 or 35 days post Ag-SP infusion. Therefore, although Tregs are activated by Ag-SP they appear to be dispensable for induction of short-term tolerance. Instead, we found that Tregs play a role in the maintenance of tolerance as indicated by the failure of mice depleted of Tregs at the time of tolerance induction to regulate disease when primed 63 days after Ag-SP treatment. This finding has significant implications for how tolerance as an immunological phenomenon is viewed. As opposed to one mechanism driving T cell unresponsiveness, tolerance induction after Ag-SP appears to be the result of several parallel mechanisms having specific temporal roles. By day 63 post tolerization, the cell intrinsic regulatory mechanism appears to have waned, with Tregs mediating long-term tolerance. Previous findings from our laboratory, including the fact that treatment with anti-CTLA-4 can reverse established Ag-SP tolerance, further support this hypothesis (7, 44). Another plausible explanation may include regulation of T cells that have recently emigrated from the thymus. Assuming that Ag-SP infusion and the presence of subcutaneous antigen (i.e. the peptide/CFA depot) does not interfere with normal deletion mechanisms, these fresh T cells would be expected to respond to antigen. Further investigation into the mechanisms at the effector T cell and regulatory T cell level are required to address this. Together these mechanisms may combine to regulate T cells that have recently emigrated from the thymus and may have autoantigen specificity.
IL-10 in this model appears to play a major role in the immediate response to Ag-SP and therefore in the short-term tolerance induction phase. Blocking IL-10 with anti-IL-10 antibody within the first 24 hours after Ag-SP abrogates tolerance. Since the data suggest a distinct role for Tregs subsequent to the tolerance induction phase, we propose that this IL-10 is secreted by splenic macrophages. Studies have shown that apoptotic debris do not need to be phagocytized by macrophages to trigger IL-10 production, with cell to cell (i.e., apoptotic cell to macrophage) contact capable of triggering p38 mitogen-activated protein kinase and subsequent IL-10 production (12). This mechanism of macrophage IL-10 production fits with our observations that IL-10 is rapidly produced/secreted prior to the clearance of Ag-SP. We are currently further investigating the potential role of other spleen macrophage subsets to produce IL-10 in response to Ag-SP infusion. Significantly, we show that IL-10 regulates the expression of PD-L1 on splenic F4/80+ macrophages. Previously, we have shown that PD-L1 plays a critical role in the induction of tolerance for protection of allogeneic islet cell transplants (5). In addition, PD-L1 has been shown to regulate interactions between DCs and T cells. Blocking IL-10 with neutralizing antibody prevented Ag-SP mediated PD-L1 upregulation on F4/80+ macrophages, but not on CD8+ DC, suggesting an autocrine pathway in which macrophages regulate the self expression of PD-L1. Exactly how macrophage-expressed PD-L1 induces tolerance induction is currently under examination. Based on observations by Fife and colleagues (15), it is likely that PD-L1 on macrophages acts similar to that on DCs, interacting with PD-1 and triggering enhanced T cell mobility, and thereby preventing T cell swarming around APCs. Furthermore, PD-L1 is known to deliver negative co-stimulatory signals to T cells (54–56) and marginal zone macrophages may also simply activate these pathways directly in autoreactive T cells accounting for the T cell-intrinsic tolerance pathway we have described. It remains to be determined how long the IL-10-mediated PD-L1 regulation is maintained, what are the contributions of other splenic APCs in driving Ag-SP tolerance induction, and how does the APC response induce and/or regulate the Treg-mediated long-term tolerance.
In conclusion, we have shown that Ag-SP tolerance induction recapitulates how tolerance is normally maintained in the hematopoietic compartment, in that marginal zone macrophages are responsible for the daily safe removal of millions of senescing red cells and leukocytes (25, 27), and is the result of multiple mechanisms that appear to play different roles in tolerance induction and maintenance. While Tregs are dispensable at the induction phase of Ag-SP tolerance, they appear to play an essential role in long-term tolerance maintenance. On the other hand, macrophages respond immediately to i.v. infused Ag-SP by rapidly secreting IL-10, which appears to regulate PD-L1 expression on these cells inducing a second Treg-independent, cell-intrinsic tolerogenic signal. Collectively, these findings show for the first time that long-term Ag-SP tolerance induction is the result of multiple temporally distinct innate and adaptive immune mechanisms. Further understanding these interactions should provide significant clues into enhancing tolerance induction in the clinical setting for treatment of immune-mediated diseases.
Acknowledgments
We thank the Northwestern Immunobiology Center Flow Cytometry Core Facility, and all of the Miller lab members for helpful discussions.
Footnotes
This work was funded by National Institutes of Health Grants R01 NS-026543, R01 NS-030871, and a grant from the Myelin Repair Foundation (http://myelinrepair.org/).
Abbreviations used in this paper: Ag, antigen; Ag-SP, antigen-coupled splenocytes; APC, antigen presenting cell; DTH, delayed-type hypersensitivity; EAE, experimental autoimmune encephalomyelitis; ECDI, 1-ethyl-3-(3’-dimethylaminopropyl)-carbodiimide
References
- 1.Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat. Rev. Immunol. 2007;7:622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- 2.Kohm AP, Turley DM, Miller SD. Targeting the TCR: T-cell receptor and peptide-specific tolerance-based strategies for restoring self-tolerance in CNS autoimmune disease. Int. Rev. Immunol. 2005;24:361–392. doi: 10.1080/08830180500371207. [DOI] [PubMed] [Google Scholar]
- 3.Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat. Rev. Immunol. 2007;7:665–677. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J. Exp. Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Getts DR, Xia G, He J, Zhang X, Kaufman DB, Miller SD. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc. Natl. Acad. Sci. USA. 2008;105:14527–14532. doi: 10.1073/pnas.0805204105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turley DM, Miller SD. Peripheral tolerance Induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J. Immunol. 2007;178:2212–2220. doi: 10.4049/jimmunol.178.4.2212. [DOI] [PubMed] [Google Scholar]
- 7.Eagar TN, Turley DM, Padilla J, Karandikar NJ, Tan LJ, Bluestone JA, Miller SD. CTLA-4 regulates expansion and differentiation of Th1 cells following induction of peripheral T cell tolerance. J. Immunol. 2004;172:7442–7450. doi: 10.4049/jimmunol.172.12.7442. [DOI] [PubMed] [Google Scholar]
- 8.Viorritto IC, Nikolov NP, Siegel RM. Autoimmunity versus tolerance: can dying cells tip the balance? Clin. Immunol. 2007;122:125–134. doi: 10.1016/j.clim.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker RN, Erwig L, Pearce WP, Devine A, Rees AJ. Differential effects of necrotic or apoptotic cell uptake on antigen presentation by macrophages. Pathobiol. 1999;67:302–305. doi: 10.1159/000028085. [DOI] [PubMed] [Google Scholar]
- 10.Kranich J, Krautler NJ, Heinen E, Polymenidou M, Bridel C, Schildknecht A, Huber C, Kosco-Vilbois MH, Zinkernagel R, Miele G, Aguzzi A. Follicular dendritic cells control engulfment of apoptotic bodies by secreting Mfge8. J. Exp. Med. 2008;205:1293–1302. doi: 10.1084/jem.20071019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray M, Miles K, Salter D, Gray D, Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc. Natl. Acad. Sci. USA. 2007;104:14080–14085. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung EY, Liu J, Homma Y, Zhang Y, Brendolan A, Saggese M, Han J, Silverstein R, Selleri L, Ma X. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity. 2007;27:952–964. doi: 10.1016/j.immuni.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Kim HJ, Yamamoto S, Kang X, Ma X. Regulation of interleukin-10 gene expression in macrophages engulfing apoptotic cells. J. Interferon Cytokine Res. 2010;30:113–122. doi: 10.1089/jir.2010.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wermeling F, Chen Y, Pikkarainen T, Scheynius A, Winqvist O, Izui S, Ravetch JV, Tryggvason K, Karlsson MC. Class A scavenger receptors regulate tolerance against apoptotic cells, and autoantibodies against these receptors are predictive of systemic lupus. J. Exp. Med. 2007;204:2259–2265. doi: 10.1084/jem.20070600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, Yagita H, Azuma M, Sayegh MH, Bluestone JA. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J. Exp. Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat. Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides 'preferentially' polarize CD4(+) T(H)-17 cells in relapsing EAE. Nat. Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 18.Schreiner B, Bailey SL, Shin T, Chen L, Miller SD. PD-1 ligands expressed on myeloid-derived APC in the CNS regulate T-cell responses in EAE. Eur. J. Immunol. 2008 doi: 10.1002/eji.200838137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Getts DR, Terry RL, Getts MT, Muller M, Rana S, Shrestha B, Radford J, Van Rooijen N, Campbell IL, King NJ. Ly6c+ "inflammatory monocytes" are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J. Exp. Med. 2008;205:2319–2337. doi: 10.1084/jem.20080421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohm AP, McMahon JS, Podojil JR, Smith Begolka W, DeGutes M, Kasprowicz DJ, Ziegler SF, Miller SD. Cutting Edge: Anti-CD25 mAb injection results in the functional inactivation, not depletion of CD4+CD25+ Treg cells. J. Immunol. 2006;176:3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Podojil JR, Luo X, Miller SD. Intrinsic and induced regulation of the age-associated onset of spontaneous experimental autoimmune encephalomyelitis. J. Immunol. 2008;181:4638–4647. doi: 10.4049/jimmunol.181.7.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller SD, Wetzig RP, Claman HN. The induction of cell-mediated immunity and tolerance with protein antigens coupled to syngeneic lymphoid cells. J. Exp. Med. 1979;149:758–773. doi: 10.1084/jem.149.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanderlugt CL, Eagar TN, Neville KL, Nikcevich KM, Bluestone JA, Miller SD. Pathologic role and temporal appearance of newly emerging autoepitopes in relapsing experimental autoimmune encephalomyelitis. J. Immunol. 2000;164:670–678. doi: 10.4049/jimmunol.164.2.670. [DOI] [PubMed] [Google Scholar]
- 24.Martin AJ, McCarthy D, Waltenbaugh C, Goings G, Luo X, Miller SD. Ethylenecarbodiimide-treated splenocytes carrying male CD4 epitopes confer Hya transplant protection by inhibiting CD154 upregulation. J. Immunol. 2010;185:3326–3336. doi: 10.4049/jimmunol.1000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mebius RE, Kraal G. Structure and function of the spleen. Nat. Rev. Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 26.Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J. Autoimmun. 2010;34:1–6. doi: 10.1016/j.jaut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Kraal G. Cells in the marginal zone of the spleen. Int. Rev. Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- 28.Perona-Wright G, Anderton SM, Howie SE, Gray D. IL-10 permits transient activation of dendritic cells to tolerize T cells and protect from central nervous system autoimmune disease. Int. Immunol. 2007;19:1123–1134. doi: 10.1093/intimm/dxm084. [DOI] [PubMed] [Google Scholar]
- 29.Campbell JD, Buckland KF, McMillan SJ, Kearley J, Oldfield WL, Stern LJ, Gronlund H, van Hage M, Reynolds CJ, Boyton RJ, Cobbold SP, Kay AB, Altmann DM, Lloyd CM, Larche M. Peptide immunotherapy in allergic asthma generates IL-10-dependent immunological tolerance associated with linked epitope suppression. J. Exp. Med. 2009;206:1535–1547. doi: 10.1084/jem.20082901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 31.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J. Exp. Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat. Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun L, Yi S, O'Connell PJ. IL-10 is required for human CD4(+)CD25(+) regulatory T cell-mediated suppression of xenogeneic proliferation. Immunol. Cell. Biol. 2010;88:477–485. doi: 10.1038/icb.2009.117. [DOI] [PubMed] [Google Scholar]
- 34.Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, Tanaka M. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J. Clin. Invest. 2007;117:2268–2278. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belz GT, Behrens GM, Smith CM, Miller JF, Jones C, Lejon K, Fathman CG, Mueller SN, Shortman K, Carbone FR, Heath WR. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J. Exp. Med. 2002;196:1099–1104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perruche S, Zhang P, Liu Y, Saas P, Bluestone JA, Chen W. CD3-specific antibody-induced immune tolerance involves transforming growth factor-beta from phagocytes digesting apoptotic T cells. Nat. Med. 2008;14:528–535. doi: 10.1038/nm1749. [DOI] [PubMed] [Google Scholar]
- 37.Qiu CH, Miyake Y, Kaise H, Kitamura H, Ohara O, Tanaka M. Novel subset of CD8{alpha}+ dendritic cells localized in the marginal zone is responsible for tolerance to cell-associated antigens. J. Immunol. 2009;182:4127–4136. doi: 10.4049/jimmunol.0803364. [DOI] [PubMed] [Google Scholar]
- 38.Coquerelle C, Moser M. DC subsets in positive and negative regulation of immunity. Immunol. Rev. 2010;234:317–334. doi: 10.1111/j.0105-2896.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Ciric B, Yang J, Xu H, Fitzgerald DC, Elbehi M, Fonseca-Kelly Z, Yu S, Zhang GX, Rostami A. Intravenous tolerance modulates macrophage classical activation and antigen presentation in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2009;208:54–60. doi: 10.1016/j.jneuroim.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, Hill BJ, Noto A, Ancuta P, Peretz Y, Fonseca SG, Van Grevenynghe J, Boulassel MR, Bruneau J, Shoukry NH, Routy JP, Douek DC, Haddad EK, Sekaly RP. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat. Med. 2010;16:452–459. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carter LL, Leach MW, Azoitei ML, Cui J, Pelker JW, Jussif J, Benoit S, Ireland G, Luxenberg D, Askew GR, Milarski KL, Groves C, Brown T, Carito BA, Percival K, Carreno BM, Collins M, Marusic S. PD-1/PD-L1, but not PD-1/PD-L2, interactions regulate the severity of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2007;182:124–134. doi: 10.1016/j.jneuroim.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc. Natl. Acad. Sci. USA. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warren KG, Catz I, Wucherpfennig KW. Tolerance induction to myelin basic protein by intravenous synthetic peptides containing epitope P85 VVHFFKNIVTP96 in chronic progressive multiple sclerosis. J. Neurol. Sci. 1997;152:31–38. doi: 10.1016/s0022-510x(97)00130-5. [DOI] [PubMed] [Google Scholar]
- 44.Eagar TN, Karandikar NJ, Bluestone J, Miller SD. The role of CTLA-4 in induction and maintenance of peripheral T cell tolerance. Eur. J. Immnol. 2002;32:972–981. doi: 10.1002/1521-4141(200204)32:4<972::AID-IMMU972>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 45.Wermeling F, Karlsson MC, McGaha TL. An anatomical view on macrophages in tolerance. Autoimmun. Rev. 2009;9:49–52. doi: 10.1016/j.autrev.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Parlato S, Romagnoli G, Spadaro F, Canini I, Sirabella P, Borghi P, Ramoni C, Filesi I, Biocca S, Gabriele L, Belardelli F. LOX-1 as a natural IFN-alpha-mediated signal for apoptotic cell uptake and antigen presentation in dendritic cells. Blood. 2010;115:1554–1563. doi: 10.1182/blood-2009-07-234468. [DOI] [PubMed] [Google Scholar]
- 47.Osada Y, Sunatani T, Kim IS, Nakanishi Y, Shiratsuchi A. Signalling pathway involving GULP, MAPK and Rac1 for SR-BI-induced phagocytosis of apoptotic cells. J. Biochem. 2009;145:387–394. doi: 10.1093/jb/mvn176. [DOI] [PubMed] [Google Scholar]
- 48.Webb NR, Connell PM, Graf GA, Smart EJ, de Villiers WJ, de Beer FC, van der Westhuyzen DR. SR-BII, an isoform of the scavenger receptor BI containing an alternate cytoplasmic tail, mediates lipid transfer between high density lipoprotein and cells. J. Biol. Chem. 1998;273:15241–15248. doi: 10.1074/jbc.273.24.15241. [DOI] [PubMed] [Google Scholar]
- 49.Eckhardt ER, Cai L, Sun B, Webb NR, van der Westhuyzen DR. High density lipoprotein uptake by scavenger receptor SR-BII. J. Biol. Chem. 2004;279:14372–14381. doi: 10.1074/jbc.M313793200. [DOI] [PubMed] [Google Scholar]
- 50.Nishiuchi T, Murao K, Imachi H, Yu X, Dobashi H, Haba R, Ishida T. Scavenger receptor class BI mediates the anti-apoptotic effect of erythropoietin. Ann. Med. 2010;42:151–160. doi: 10.3109/07853891003601556. [DOI] [PubMed] [Google Scholar]
- 51.Mukhopadhyay S, Pluddemann A, Gordon S. Macrophage pattern recognition receptors in immunity, homeostasis and self tolerance. Adv. Exp. Med. Biol. 2009;653:1–14. doi: 10.1007/978-1-4419-0901-5_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 53.Lin HH, Faunce DE, Stacey M, Terajewicz A, Nakamura T, Zhang-Hoover J, Kerley M, Mucenski ML, Gordon S, Stein-Streilein J. The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in peripheral tolerance. J. Exp. Med. 2005;201:1615–1625. doi: 10.1084/jem.20042307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding H, Wu X, Wu J, Yagita H, He Y, Zhang J, Ren J, Gao W. Delivering PD-1 inhibitory signal concomitant with blocking ICOS co-stimulation suppresses lupus-like syndrome in autoimmune BXSB mice. Clin. Immunol. 2006;118:258–267. doi: 10.1016/j.clim.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 55.Dong H, Chen X. Immunoregulatory role of B7-H1 in chronicity of inflammatory responses. Cell. Mol. Immunol. 2006;3:179–187. [PMC free article] [PubMed] [Google Scholar]
- 56.Gao W, Demirci G, Strom TB, Li XC. Stimulating PD-1-negative signals concurrent with blocking CD154 co-stimulation induces long-term islet allograft survival. Transplantation. 2003;76:994–999. doi: 10.1097/01.TP.0000085010.39567.FB. [DOI] [PubMed] [Google Scholar]