Abstract
Purpose
The transcription factor TWIST is an important factor in regulation of the epithelial–mesenchymal transition (EMT), which represents the primary stages during the metastasis of tumors. To identify the role of TWIST in the regulation of metastasis in laryngeal carcinoma Hep-2 cells, we investigated whether the alteration of TWIST has an effect on the Hep-2 cells morphology and whether the alteration of TWIST has an effect on the expression of E-cadherin, N-cadherin as well as the ability of cell motion, migration, and invasion.
Methods
Morphological changes of Hep-2 cells that were transfected a mircoRNA against TWIST vector were observed by the reserved microscope. Reverse transcription-polymerase chain reaction was performed in order to examine the mRNA expression of TWIST, E-cadherin, and N-cadherin. Western blotting was performed to examine the protein expression of TWIST, E-cadherin, and N-cadherin. Cell motion ability was examined by Scratch-wound assay. Transwell™ chamber assays were used to determine cell migration and invasion.
Results
Transfecting a mircoRNA down-regulated TWIST expression at mRNA and protein levels. Down-regulation of TWIST expression induced morphological changes, such as the inversion of the EMT. Moreover, down-regulation of TWIST expression up-regulated E-cadherin and down-regulated N-cadherin expressions at mRNA and protein levels, respectively. Furthermore, we confirmed that down-regulation of TWIST expression decreased the motion, invasion, and migration ability of the Hep-2 cells.
Conclusions
Down-regulation of TWIST expression decreases migration and invasion of laryngeal carcinoma Hep-2 cells by regulation of the E-cadherin, N-cadherin expression.
Keywords: TWIST, Laryngeal carcinoma Hep-2 cells, Metastasis, Epithelial–mesenchymal transition (EMT)
Introduction
Laryngeal carcinoma is one of the most common forms of head and neck squamous cell carcinoma (HNSCC), which causes substantial annual morbidity and mortality (Jemal et al. 2002). Although the approaches for the treatment of laryngeal carcinoma have been improved, overall survival rates have been not significantly improved over the past 30 years, mainly due to the metastasis and recurrence of tumors. Metastasis of tumors is a complex process, and various factors are involved in each processes of metastasis (Eccles and Welch 2007). Epithelial–mesenchymal transition (EMT), one of the important mechanisms that induces invasion and metastasis of tumors, is a process by which epithelial cells lose their polarity and are converted to a mesenchymal phenotype (Lee et al. 2006). The most distinguished characteristic of EMT is the morphologic alteration from epithelial to mesenchymal, which is often accompanied by the dissolution of epithelial tight junctions, loss of cell adhesion, down-regulated expression of some epithelial markers, such as E-cadherin, a-catenin, and β-catenin, but acquired expression of mesenchymal components such as N-cadherin, vimentin, and fibronectin, resulting in loss of cell polarity, cell-basement adhesion, and cell–cell contact (Kang and Massague 2004). In the natural state, epithelial cells exist as tight cell clusters that maintain cell–cell or cell-to-matrix contacts, whereas mesenchymal cells were loosely organized, unpolarized cells with reduced adhesion and promoted migratory tendencies. EMT is a prominent characteristic of the most aggressive metastatic cancer cells (Xue et al. 2004; Huber et al. 2005).
Therefore, identification of the target genes that were related with the metastatic progression of laryngeal carcinoma is important for the survival of patients. Recently, the transcription factor TWIST, which was a key factor responsible for metastasis of breast cancer by promoting EMT in vivo system and a master regulator of gastrulation and mesoderm specification (Yang et al. 2004), was shown to be essential to mediate cancer metastasis (Tokes et al. 2005). For example, elevated TWIST expression was related with tumor invasion and metastasis in esophageal squamous cell carcinomas (Yuen et al. 2007). The role of TWIST in promoting EMT processes has also been reported in other solid cancers, such as prostate and uterine cancer (Kwok et al. 2005; Kyo et al. 2006). The overexpression of TWIST may be the key for tumor metastasis and drug resistance, but the precise mechanism is still unclear. Recently, we have reported that TWIST might play a pivotal role in the paclitaxel-induced apoptosis of human laryngeal carcinoma Hep-2 cells (Yu et al. 2009).
Despite many studies are increasing, the biological activities and mechanisms of metastasis in the laryngeal carcinoma remain undefined. There were few reports concerning the effect of TWIST on the invasion and migration ability of laryngeal carcinoma. The experiment was designed to explore the alteration of TWIST expression and its effect on migration, invasion, and the metastatic correlation factor of E-cadherin, N-cadherin in laryngeal carcinoma cell line Hep-2, as well as to evaluate the significance of TWIST expression on morphologies characteristics.
Materials and methods
Cell and reagents
The human laryngeal carcinoma cell line Hep-2 was obtained from the American Type Culture Collection (ATCC, VA, USA). Primary antibodies (anti-TWIST, anti-E-cadherin, anti-N-cadherin, and anti-actin) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All others agents were purchased from Sigma (St. Louis, MO, USA).
Generation of miR-TWIST transfectants
The miR-TWIST vector was generated using the BLOCK-iT™ PolIImiR RNAi Expression Vector kit with EmGFP (Invitrogen, CA, USA) according to the manufacturer’s instruction. The oligo sequences of the miR-TWIST and negative control were as follows:
oligo-F: 5′-TGCTGCTGCCGGTCTGGCTCTTCCTCGTTTTGGCCACTGACTGACGAGGAAGACAGACCGGCAG-3′,
oligo-R: 5′-CCTGCTGCCGTCTGTCTTCCTCGTCAGTCAGTGGCCAAAACGAGGAAGAGCCAGACCGGCAGC-3′.
oligo-control-F: 5′-TGCTGAAATGTACTGCGCGTGGAGACGTTTTGGCCACTGACTGACGTCTCCACGCAGTACATTT-3′.
oligo-control-R: 5′-CCTGAAATGTACTGCGTGGAGACGTCAGTCAGTGGCCAAAACGTCTCCACGCGCAGTACATTTC-3′.
The sequence targeting the TWIST gene-coding region was annealed and inserted into pcDNA 6.2-GM/EmGFPmiR vector to generate the miRNA interfering expression vector. The resulting vectors were then transfected into the Hep-2 cells.
Cell culture and transfection
Hep-2 cells were cultured in Dulbecco modified eagle’s medium (DMEM) containing 10% fetal calf serum, 100 U/mL penicillin, and 100 mg streptomycin at 37°C in a humidified atmosphere composed of 95% air and 5% CO2. Cell transfection was carried out using Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer’s instructions. Cells were grown to 80–90% confluence without antibiotics. Vectors containing the different constructs (10 μg) were diluted in DMEM (100 μl) and then mixed with the transfection solution for 15 min. After washing, the cells were incubated with the transfection mixture at 37°C for 6 h and were allowed to grow in fresh media. For a transient expression of Hep-2 cells, the transfected cells were incubated at 37°C for 48 h and used for analysis.
Observation of morphologic change
The morphologic changes of the Hep-2 cells were observed using the reserved microscope. The photography was taken using a Leica microscope image system (Leica, Manheim, Germany).
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
The detailed experiment procedures were prepared as previously described (Lee et al. 2006). Total RNA was extracted using TRIzol (Invitrogen, CA, USA) and was further purified with RNeasy purification kit (Qiagen, CA, USA). The reverse transcription reaction was performed using the ExScriptRT reagent kit (TaKaRa, Dalian, China) according to the manufacturer’s instructions. The reverse transcription reaction was performed at 42°C for 15 min and terminated by heating at 95°C for 2 min. PCR was performed following the instruction of TaKaRa TaqTM under the following conditions: pre-degeneration, degeneration, renaturation, and elongation. All experiments were conducted 3 times. The primers were as follows:
TWIST: 5′-GGAGTCCGCAGTCTTACGAG-3′; 5′-TCTGGAGGACCTGGTACAGG-3′
E-cadherin: 5′-AGGCCAAGCAGCAGTACATT-3; 5′-ATTCACATCCAGCACATCCA-3′
N-cadherin: 5′-TGGGAATCCGACGAATGG-3′; 5′-TGCAGATCGGACCGGATAC-3′
β-actin: 5′-CTCCTTAATGTCACGCACGATTT-3′; 5′-GTGGGGCGCCCCAGGCACCA-3′
Protein extraction and western blot analysis
The detailed experiments procedures were prepared as previously described (Yu et al. 2009). Cells were harvested, and total proteins were extracted using RIPA Extraction Reagents (Solarbio, Beijing, China). A total of 60 μg protein was separated by 10% SDS–PAGE gel and subsequently transferred onto PVDF membranes. The membranes were blocked with 5% skimmed milk for 2 h at room temperature, incubated with the primary antibody TWIST (1:200), E-cadherin (1:500), N-cadherin (1:500), and β-actin (1:2,000) overnight at 4°C, and then incubated with a secondary antibody. The antigen–antibody complexes were visualized. Relative OD value ratio was calculated with National Institutes of Health software Image J, by comparing it with β-actin from 3 experiments.
Scratch-wound assay
The Hep-2 cells were plated onto six-well plates at a concentration of 5 × 105 cells per well. Cell monolayers were carefully wounded by scratching with a sterile plastic pipette tip. The cells were washed twice with cooled PBS and incubated for 24 h. For each wound, the photographs were taken at 0, 6, 12, and 24 h in the same fields after treatment.
In vitro migration and invasion assays
Cell invasion assays were performed using Transwell™ chambers (Costar, MA, USA). Briefly, after coating the filter with 80 μg of Matrigel (BD, NY,USA) overnight at 4°C, cells (2 × 105 cells per well) were seeded onto the top chamber of a 24 well in serum-free medium. The bottom chamber was filled with 0.6 ml DMEM with 10% FBS as a chemic attractant. After incubation for 24 h, non-invading cells were carefully removed with a cotton swab. The filters were fixed with 95% alcohol and stained with crystal violet for 15 min. The cells on the upper surface were gently removed with a cotton swab, and the cells on the lower surface of the filters were quantified under a microscope at 100× magnification. All experiments were repeated in three replicates, and experiments were repeated three times. To assess migration, in vitro migration assays were done under the same conditions as the Transwell™ invasion assays, but in non-Matrigel-coated Transwell™ chambers.
Statistical analysis
Data were presented as mean ± standard deviation. One-way analysis of variance and least significance difference were applied to analyze the data. P values lower than 0.05 (two-tailed) were considered significant. Statistical calculations were performed using SPSS software package, version 13.0 (SPSS Inc, Chicago, IL, USA).
Results
Down-regulation of TWIST expression changed morphology of Hep-2 cells
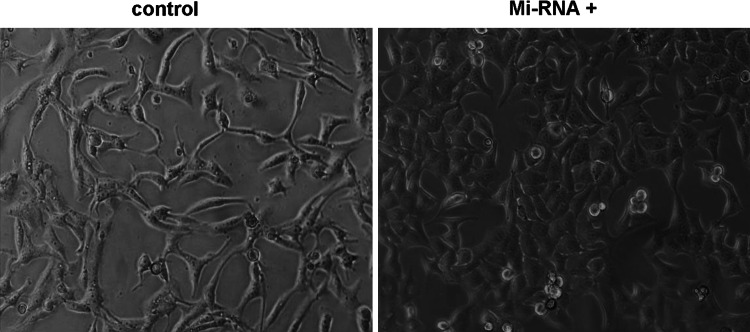
To investigate whether down-regulation of TWIST expression has an effect on morphology of Hep-2, We transfected a mircoRNA against TWIST (miRNA-TWIST) vector and found that down-regulation of TWIST expression in Hep-2 cells resulted in morphologic changes from scattered and fibroblast-like growth to tightly packed colonies (Fig. 1). The phenotype of Hep-2 cells changed from the mesenchymal cell to epithelial cells. The morphology of Hep-2 cells underwent the mesenchymal–epithelial transition (MET). As shown in Fig. 1, the phenomenon indicated that down-regulation of TWIST expression induced morphological changes, such as the inversion of the epithelial–mesenchymal transition (EMT) in laryngeal carcinoma Hep-2 cells.
Fig. 1.
Down-expression of TWIST changed morphology of Hep-2 cells. Morphology of cells in different groups was observed by reserved microscopy (×200 magnification). Morphologic changes from scattered and fibroblast-like growth in control group to tightly packed colonies in miRNA-TWIST vector group. The phenotype of Hep-2 cell changed from the mesenchymal cell to epithelial cells
Down-regulation of TWIST expression inhibited N-cadherin, with up-regulation of E-cadherin at mRNA levels in Hep-2 cells
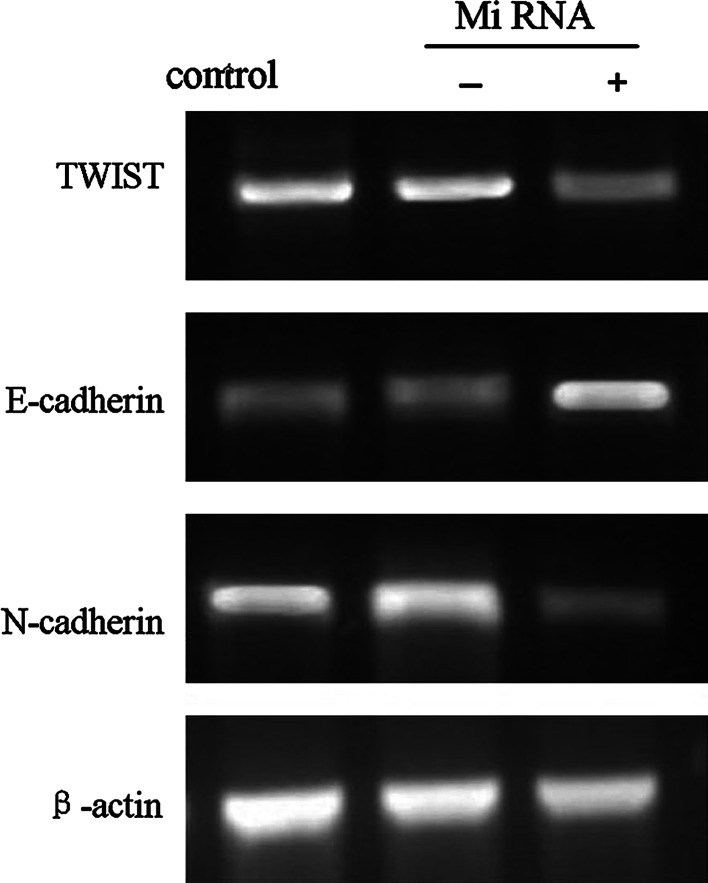
To study the effect of TWIST on the expression of EMT-related molecule in Hep-2 cells, we transfected a miRNA-TWIST vector and assessed the expression of EMT markers at mRNA levels by semi-quantitative RT-PCR. As shown in the Fig. 2, down-regulation of TWIST expression inhibited N-cadherin, with up-regulation of E-cadherin at mRNA levels in Hep-2 cells.
Fig. 2.
The alteration in the expression of TWIST, E-cadherin, and N-cadherin at mRNA level in control group, miRNA-TWIST(−)vector group, and miRNA-TWIST(+)vector group, respectively, was assessed by reverse transcription-polymerase chain reaction analysis
Down-regulation of TWIST expression inhibited N-cadherin, with up-regulation of E-cadherin at protein levels in Hep-2 cells
To further examine whether down-regulation of TWIST had the similar effect, at the protein level, on the expression of E-cadherin, N-cadherin with mRNA level, we found that down-regulation of TWIST inhibited N-cadherin, with up-regulation of E-cadherin at protein levels by western blotting (P < 0.05) (Fig. 3). The alteration in protein agreed with the changes in the mRNA levels, respectively. These results indicated that down-regulation of TWIST inhibited N-cadherin, with up-regulation of E-cadherin, that is, down-regulation of TWIST expression in Hep-2 cells, at least in part, blocked the process of EMT by regulating the E-cadherin, N-cadherin expression.
Fig. 3.
The alteration in expression levels of TWIST, E-cadherin, and N-cadherin protein in different groups by western blotting analysis. aThe alteration in expression of TWIST, E-cadherin, and N-cadherin at protein level in control group, miRNA-TWIST(−)vector group, and miRNA-TWIST(+)vector group, respectively. b–d The optical density value of TWIST (b), E-cadherin (c), and N-cadherin (d) each lane using National Institutes of Health software Image J system was demonstrated in different groups. Each data point in the figure represents the mean ± standard deviation of 3 separate experiments. All experiments were conducted 3 times
Down-regulation of TWIST expression inhibited cells motion ability in Hep-2 cells
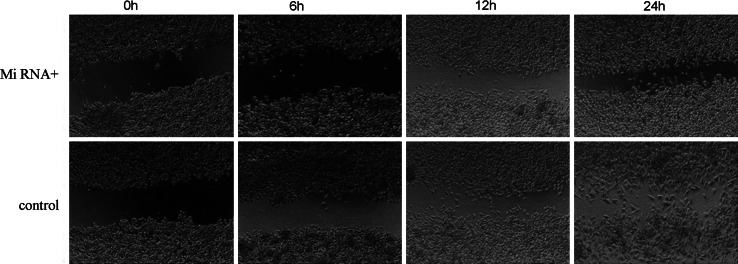
To explore the effect of down-regulation of TWIST expression on the motion ability of Hep-2 cells, the scratch-wound assay was performed. The scratch-wound assay showed that the motion speed of Hep-2 cells that were transfected miRNA for TWIST was more slowly than the controls at the same time point, the former failed to migrate into the center of the wound at 24 h after wounding and the latter was able to move to an almost complete closure (Fig. 4). This suggests that down-regulation of TWIST expression inhibits the cells motion ability in a human laryngeal carcinoma cell line Hep-2.
Fig. 4.
Down-expression of TWIST inhibited cells motion ability in Hep-2 cells at different time points (0, 6, 12, 24 h) was examined by scratch-wound assay (×40 magnification)
Down-regulation of TWIST expression inhibited cell migration and invasion in Hep-2 cells
In order to further assess the function of down-expressed TWIST on cell migration and invasion in Hep-2 cells, we performed the transwell assay. As indicated in Fig. 5, the migrated cell number of control, miRNA-TWIST(−) group, and mRNAi-TWIST(+) group were 144.33 ± 8.62, 127.67 ± 10.60, and 94.67 ± 10.41, respectively (P < 0.05). In vitro invasion assay, we found that the number of invasion cells in miRNA-TWIST(+) group was 34.67 ± 9.02 significantly less than the number of control (89.67 ± 12.01) and miRNA-TWIST(−) group (75.67 ± 10.97) (P < 0.05) (Fig. 5). Taken together, these results clearly showed that down-regulation of TWIST expression inhibited cell migration and invasion in a human laryngeal carcinoma Hep-2 cell line.
Fig. 5.
Down-expression of TWIST inhibited cell migration and invasion in Hep-2 cells in vitro migration and invasion assays. Migration (upper) and invasion (bottom) were examined by the transwell chambers assay in control group, miRNA-TWIST(−)vector group, miRNA-TWIST(+)vector group, respectively (×100 magnification). Each datum point in the figure represented the mean ± standard deviation of 3 separate experiments. All experiments were conducted 3 times
Discussion
Our study found that down-regulation of TWIST expression induced morphological changes, such as the inversion of the epithelial–mesenchymal transition (EMT) in the laryngeal carcinoma Hep-2 cells, and their morphology switched from scattered and fibroblast-like growth to tightly packed colonies. During the EMT, epithelial cells acquire fibroblast-like properties, with changes in cell morphology, construction, adhesion, and the acquisition of invasive properties contribute to tumor progression (Hugo et al. 2007).
It was reported that ectopic TWIST expression in human epithelial cells induced to EMT (Yang et al. 2004). When epithelial cells are undergoing EMT, their morphology switches from an apical-basolateral, polarized epithelial phenotype to a spindle-shaped, fibroblast-like mesenchymal phenotype, which is thought to contribute to the invasion ability of cancer cells (Thiery 2002).
Epithelial cells, in the phenomenon of EMT, changed from well-organized cell–cell adhesion and cell polarity to loss of cell–cell contacts and cell scattering. In addition, it is often associated with loss of epithelial makers, such as E-cadherin and catenins, and gain of mesenchymal markers, such as N-cadherin and vimentin (Yang et al. 2004). Therefore, we hypothesized that alteration of TWIST maybe had a similar effect on the marker of EMT in laryngeal carcinoma cells. Indeed, after suppressing the expression of TWIST,we identified that the expression of the epithelial marker, E-cadherin, was increased, while the expression of mesenchymal markers, N-cadherin, was decreased. The alteration was not only at mRNA levels but also at protein levels. Those results indicated that alterative expression of TWIST, at least in part, had an impact on the process of EMT by regulating the expression of E-cadherin and N-cadherin in laryngeal carcinoma cells.
E-cadherin regulates homophilic cell–cell adhesion and tissue homeostasis in normal epithelia (Christofori and Semb 1999), and it has been presented to suppress invasion in many tumor cell types (Hirohashi 1998; Hsu et al. 2000). Loss of the E-cadherin protein has been involved in increased metastatic potential and poorer prognosis in many of HNSCC (Pyo et al. 2007), while gains of N-cadherin were found to promote metastasis of breast cancer (Hazan et al. 2007). Some reported that N-cadherin-promoted invasive activities can even outweigh E-cadherin tumor suppressive function in human breast cancer cells (Nieman et al. 1999). E-cadherin is necessary for morphogenesis, keeping the epithelial structure and cell survival in the breast cancer cell (Delmas and Larue 2004). Besides the reduction in or absence of E-cadherin expression often is accompanied by a reciprocally increased expression of other proteins, such as N-cadherin during EMT process (Wheelock et al. 2008), it has been found that loss of the adhesion protein is related with acquisition of cell invasiveness and metastatic behavior of the tumors cells. Down-regulation of TWIST expression may lead to suppression of the motion, migration, and invasion ability of Hep-2 cells by regulating the E-cadherin, N-cadherin expression. To test this hypothesis, the scratch-wound assay was performed in vitro. We found that motion speed of laryngeal cancer cells after transfection of miRNA-TWIST was more slowly than the control. At the same time, we found the invasion and migration ability of cells is decreased compared with the control via the in vitro migration and invasion assays. It suggests that alterative expression of E-cadherin and N-cadherin be related with the change of motion, migration, and invasion ability.
In summary, our study implied that down-regulation of TWIST expression conferred morphologic transition of the laryngeal carcinoma cell line Hep-2 from a fibroblastic to epithelial appearance, which was accompanied by a gain of epithelial cell markers, E-cadherin, and loss of the mesenchymal markers, N-cadherin. At the same time, we confirmed that miRNA-mediated knockdown of TWIST inhibited the motion, migration, and invasion ability of Hep-2 cells. These results again strengthened that TWIST was essential in EMT during metastasis and demonstrated that down-regulation of TWIST expression up-regulated E-cadherin and inhibited N-cadherin leading to decrease migration and invasion of the laryngeal carcinoma Hep-2 cell. However, the concrete mechanisms how the TWIST regulates E-cadherin and N-cadherin expression in the laryngeal carcinoma Hep-2 cell remain to be answered in our future work.
Acknowledgments
This work was supported in part Department of Science & Technology of Shandong province (03BS020) and by Shandong Province Outstanding Young Scientist Research Award Fund (bs2009yy018).
Abbreviations
- EMT
Epithelial–mesenchymal transition
- HNSCC
Head and neck squamous cell carcinoma
- DMEM
Dulbecco modified eagle medium
Footnotes
Liang Yu and Hui-zheng Li contributed equally to this work.
Contributor Information
Hai-bo Wang, Email: whbotologic797@163.com.
Wei Xu, FAX: +86-531-85187588, Email: xwhns@yahoo.com.cn.
References
- Christofori G, Semb H (1999) The role of the cell-adhesion molecule Ecadherin as a tumour-suppressor gene. Trends Biochem Sci 24:73–76 [DOI] [PubMed] [Google Scholar]
- Delmas V, Larue L (2004) Cadherins in the mammary gland and the melanocyte lineage. J Soc Biol 198:385–389 [PubMed] [Google Scholar]
- Eccles SA, Welch DR (2007) Metastasis: recent discoveries and novel treatment strategies. Lancet 369:1742–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA (2007) Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol 148:779–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohashi S (1998) Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol 153:333–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu MY, Meier FE, Nesbit M, Hsu JY, Van Belle P, Elder DE, Herlyn M (2000) E-cadherin expression in melanoma cells restores keratinocyte mediated growth control and down-regulates expression of invasion related adhesion receptors. Am J Pathol 156:1515–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber MA, Kraut N, Beug H (2005) Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol 17:548–558 [DOI] [PubMed] [Google Scholar]
- Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, Thompson EW (2007) Epithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progression. J Cell Physiol 213:374–383 [DOI] [PubMed] [Google Scholar]
- Jemal A, Thomas A, Murray T, Thun M (2002) Cancer statistics. CA Cancer J Clin 52:23–47 [DOI] [PubMed] [Google Scholar]
- Kang Y, Massague J (2004) Epithelial-mesenchymal transitions: twist in development and metastasis. Cell 118:277–279 [DOI] [PubMed] [Google Scholar]
- Kwok WK, Ling MT, Lee TW et al (2005) Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res 65:5153–5162 [DOI] [PubMed] [Google Scholar]
- Kyo S, Sakaguchi J, Ohno S et al (2006) High Twist expression is involved in infiltrative endometrial cancer and affects patient survival. Hum Pathol 37:431–438 [DOI] [PubMed] [Google Scholar]
- Lee JM, Dedhar S, Kalluri R, Thompson EW (2006) The epithelial–mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 172:973–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ (1999) N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol 147:631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo SW, Hashimoto M, Kim YS, Kim CH, Lee SH, Johnson KR, Wheelock MJ (2007) Expression of E-cadherin, P-cadherin and N-cadherin in oral squamous cell carcinoma: correlation with the clinicopathologic features and patient outcome. J Craniomaxillofac Surg 35:1–9 [DOI] [PubMed] [Google Scholar]
- Thiery JP (2002) Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2:442–454 [DOI] [PubMed] [Google Scholar]
- Tokes AM, Kulka J, Paku S, Szik A, Paska C, Novak PK, Szilak L, Kiss A, Bogi K, Schaff Z (2005) Claudin-1, -3 and -4 proteins and mRNA expression in benign and malignant breast lesions: a research study. Breast Cancer Res 7:R296–R305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR (2008) Cadherin switching. J Cell Sci 121:727–735 [DOI] [PubMed] [Google Scholar]
- Xue C, Plieth D, Venkov C, Xu C, Neilson EG (2004) The gatekeeper effect of epithelial-mesenchymal transition regulates the frequency of breast cancer metastasis. Cancer Res 63:3386–3394 [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA (2004) Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117:927–939 [DOI] [PubMed] [Google Scholar]
- Yu L, Li HZ, Lu SM, Liu WW, Li JF, Wang HB, Xu W (2009) Alteration in TWIST expression: possible role in paclitaxel induced apoptosis in human laryngeal carcinoma Hep-2 Cell line. Corat Med J 50:536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen HF, Chan YP, Wong ML et al (2007) Up-regulation of TWIST in oesophageal squamous cell carcinoma is associated with neoplastic transformation and distant metastasis. J Clin Pathol 60:510–514 [DOI] [PMC free article] [PubMed] [Google Scholar]