Abstract
Background and Aims
Self-fertilizing taxa are often found at the range margins of their progenitors, where sub-optimal habitats may select for alternative physiological strategies. The extent to which self-fertilization is favoured directly vs. arising indirectly through correlations with other adaptive life history traits is unclear. Trait responses to selection depend on genetic variation and covariation, as well as phenotypic and genetic responses to altered environmental conditions. We tested predictions of the hypothesis that self-fertilization in Mimulus arises through direct selection on physiological and developmental traits that allow seasonal drought escape.
Methods
Phenotypic selection on mating system and drought escape traits was estimated in field populations of M. guttatus. In addition, trait phenotype and phenotypic selection were compared between experimental wet and dry soil in two greenhouse populations each of M. guttatus and M. nasutus. Finally, genetic variation and covariation for traits were compared between wet and dry soil treatments in a greenhouse population of M. guttatus.
Key Results
Consistent with predictions, selection for early flowering was generally stronger than for mating system traits, and selection for early flowering was stronger in dry soil. Inconsistent with predictions, selection for water-use efficiency was largely absent; selection for large flowers was stronger than for drought escape in the field; and most drought escape and mating system traits were not genetically correlated. A positive genetic correlation between flowering time and flower size, which opposed the adaptive contour, emerged only in wet soil, suggesting that variation in water availability may maintain variation in these traits. Plastic responses to soil moisture treatments supported the idea that taxonomic divergence could have been facilitated by plasticity in flowering time and selfing.
Conclusions
The hypothesis that plant mating systems may evolve indirectly via selection on correlated life history characteristics is plausible and warrants increased attention.
Keywords: Developmental rate, drought escape, floral evolution, flowering time, genetic correlation, heritability, Mimulus, mating system, phenology, phenotypic plasticity, phenotypic selection, water-use efficiency
INTRODUCTION
The evolution of self-fertilization from predominant cross-fertilization is the most common reproductive transition in flowering plants (Stebbins, 1974). Characteristic morphological, life history and physiological changes accompany the transition to predominant self-fertilization. For example, the evolution of selfing is often associated with reductions in flower size and herkogamy (Wyatt, 1988), more rapid floral development (Armbruster et al., 2002; Mazer et al., 2004; Dudley et al., 2007), earlier onset of flowering (Mazer et al., 2004; Martin and Willis, 2007) and, in some plants, a shift toward a ‘drought escape’ physiological strategy in which rapid development, high photosynthetic rate and low water-use efficiency (WUE) minimize exposure to water stress (Kiang and Hamrick, 1978; Mazer et al., 2010; Wu et al., 2010). It is unclear whether these trait changes facilitate the transition to selfing or occur as a consequence of mating system evolution.
Self-fertilization may evolve, for example, because of its direct selective advantage, such as the ability to reproduce when pollinators or mates are scarce, that outweighs any disadvantages, such as inbreeding depression (Lloyd, 1992; Uyenoyama et al., 1993; Goodwillie et al., 2005; Charlesworth, 2006). The advantage of selfing may be more pronounced at range margins where environmental conditions may be sub-optimal, population sizes small and pollinator recruitment difficult (Pujol et al., 2009). Indeed, biogeographical studies have found that selfing species are often found at the periphery of the ranges of their outcrossing progenitors (Solbrig and Rollins, 1977; Kiang and Hamrick, 1978; Schoen, 1982; Wyatt, 1988; Macnair et al., 1989; Moeller, 2006).
Environmental conditions at range margins may also impose selection on physiological or developmental traits that are not directly associated with mating system, but that confer higher fitness in sub-optimal habitats (Kiang and Hamrick, 1978; Macnair et al., 1989; Arntz and Delph, 2001; Angert, 2006). In seasonally dry climates, for example, seeds dispersing into areas that dry earlier in the season will experience strong selection for faster or earlier development (Stanton et al., 2000; McKay et al., 2003; Hall and Willis, 2006; Levin, 2006; Franks et al., 2007). Rapid plant or floral development may permit shorter life spans, which could allow plants to reduce drought exposure. Similarly, plants with more rapidly developing flowers may enjoy reduced evapotranspiration costs associated with maintenance of fresh corolla tissue (e.g. Galen, 1999). Furthermore, rapid floral development may indirectly lead to higher rates of self-fertilization, as faster development can reduce herkogamy or dichogamy (Arroyo, 1973; Fenster et al., 1995; Armbruster et al., 2002; Mazer et al., 2004; Dudley et al., 2007). In this case, selfing may evolve in rapidly drying habitats as an indirect consequence of selection for drought escape because of the advantages of compressed within-flower developmental rates (Mazer et al., 2010).
Spatial or temporal variation in environmental conditions may alter genetic variation and covariation for life history and mating system traits, although the extent to which this constrains or drives trait evolution has received little empirical consideration. Sufficient additive genetic variation is required for mating system and drought adaptation traits to respond to natural selection. On the other hand, traits may evolve indirectly through natural selection if they are correlated with other traits under direct selection (Lande and Arnold, 1983). Environmental fluctuations such as variance in rainfall patterns, however, can alter the strength of selection, the magnitude of additive genetic variation and even the sign of phenotypic correlations among traits. This can lead to shifts in the expectations for evolutionary change over time. For example, Murren et al. (2009) found significant genetic variation for flowering time only under experimentally wet soil conditions. Similarly, Juenger and Bergelson (2000) found that selection on architecture and flowering time of Ipomopsis aggregata plants were significantly correlated, but only when plants experienced herbivore damage. Environmental variation may similarly lead to shifts in genetic correlations, which could mean that constraints on adaptive evolution vary with environmental conditions (Sgrò and Hoffmann, 2004).
Field and greenhouse experiments were conducted to evaluate the hypothesis that mating system divergence between Mimulus guttatus and M. nasutus has been driven by selection on physiological and developmental traits that allow plants to avoid seasonal drought characteristic of their native California habitat. Specifically, the following predictions associated with this hypothesis were tested: (1) if the mating system evolves indirectly through its correlations with drought escape, then physiological or developmental traits should experience stronger direct selection than mating system traits; (2) selection favouring drought escape traits should be stronger in experimentally dry environments; and (3) drought escape traits and mating system traits should be genetically correlated. In addition, the magnitude and direction of phenotypic plasticity in mating system, developmental and physiological traits were examined by comparing plants grown in experimental wet and dry soil treatments. We also tested for the effect of these environments on the expression of genetic variation and genetic correlations among these traits.
MATERIALS AND METHODS
Mimulus
Mimulus guttatus DC (formerly Scrophulariaceae now Phrymaceae, Beardsley et al., 2002) is a herbaceous plant native to western North America that occurs in open, moist environments, such as seeps and springs. Throughout much of its range, it is common in temporary ponds or roadside ditches. In such environments, it grows as a winter annual, germinating with the autumnal rains characteristic of its Mediterranean climate, persisting as a small rosette through the winter, flowering during the spring, and finishing its life with the summer drought. In some environments that remain wet year-round, however, it can spread rhizomatously as a perennial (Dole, 1992). Population sizes fluctuate dramatically (Vickery, 1999) and, as a consequence, founder effects, extinction, recolonization and inbreeding have shaped its history. This is mirrored in its variable mating system, which ranges from about 75 % selfing to complete outcrossing (Ritland and Ritland, 1989; Dudash and Ritland, 1991; Willis, 1993; Ivey and Carr, 2005).
Mimulus guttatus or a similar taxon is considered to be the progenitor of a closely related group of species that vary remarkably in life history, morphology, mating system, physiology and edaphic preference (Vickery, 1978; Ritland and Ritland, 1989; Macnair and Gardner, 1998; Sweigert and Willis, 2003; Beardsley et al., 2004; Wu et al., 2008). Many of these features vary characteristically with mating system. For example, another member of this group, M. nasutus, is highly selfing and, in comparison with M. guttatus, begins flowering earlier, produces smaller flowers with reduced herkogamy, occurs in sites that dry sooner in the summer, completes its life cycle more quickly and is better adapted to drought conditions (Kiang and Hamrick, 1978; Ritland and Ritland, 1989; Martin and Willis, 2007; Wu et al., 2010).
Field study
Phenotypic selection for mating system, developmental and physiological traits was estimated in two annual populations of M. guttatus in Lassen National Forest, Butte County, California, USA. One population (Colby Creek, ‘COL’; 40°7′N, 121°29′W, 1500 m elevation) occurred along the margin of a stream, and the other (Scott's John Meadow, ‘SJM’; 40°06′N, 121°25′W, 1762 m elevation) was in a seasonally wet meadow. Vouchers from each population are housed in the Chico State herbarium (CHSC). On 19 June 2007, when most plants had two pairs of leaves, 100 (COL) and 200 (SJM) seedlings were labelled at regular intervals along transects. Plants were monitored every other day throughout the season, and traits measured as described below. Fruits were collected throughout the season as they matured, and above-ground biomass was collected as plants entered senescence. On 20 August, after the end of the flowering season, above-ground biomass of any remaining plants was collected.
Greenhouse experiments
Two experiments were conducted in the greenhouse. The first examined the effect of water availability on plasticity and selection for mating system, developmental and physiological traits. This study involved two populations each of M. guttatus (Coal Canyon Road, ‘CCR’; 39°36′N, 121°36′W, 155 m elevation; and Dry Creek, ‘DRC’; 39°37′N, 121°37′W, 79 m elevation) and M. nasutus (Cohasset Road, ‘COH’; 39°49′N, 121°50′W, 107 m elevation; and Honeyrun Road, ‘HON’; 39°42′N, 121°46′W, 84 m elevation), all of which occurred in roadside ditches. We did not measure water availability in these populations, but seasonal rainfall totals varied from 512 to 1240 mm in the decade preceding this study (http://www.wrcc.dri.edu/), and soil conditions in which plants occurred varied from inundated pools to apparently quite dry. Based on this apparent variation in access to water, we assumed that phenotypic plasticity in response to water might be observed in these populations. Vouchers from each population are housed in the CHSC. From each population 110 open-pollinated seed families were collected, and approx. 25 seeds from each family were sown onto wet soil in each of two 5 × 5 cm pots. The potting soil was composed of equal parts of peat, sand, pumice and redwood compost. Each set of pots was arranged randomly within trays. Trays were randomly assigned to one of three greenhouse benches, placed under 400 W sodium-vapour lights for a 16 h daylength, and maintained in standing water. After 2 weeks, pots were thinned to leave one randomly chosen seedling in each pot. After thinning, each tray was fertilized once with 800 mL of Peter's 20-20-20 (Scotts, Marysville, Ohio, USA) at 1·5 g L−1.
Twenty-five days after sowing, plants had established rosettes. One set of pots was randomly assigned to receive a wet treatment, in which plants continued to be maintained in standing water for the duration of the experiment. The remaining pots (dry treatment) were provided with 18·3 mL of water every 3–4 d. This amount is equivalent to what would be a seasonal total of 38 cm, which is near the sixth percentile of seasonal rainfall totals at the Chico, California Experiment Station between 1906 and 2006 (http://www.wrcc.dri.edu/). This treatment resembled a consistently dry growing season, and the severity of drought experienced by plants increased later in the experiment because their water requirements increased as plants grew larger, whereas water supplied remained constant following establishment. Water stress was sufficiently great that some plants were wilting between water additions toward the end of the experiment. Volumetric water content (VWC) was monitored throughout the experiment by systematically sampling pots using time domain reflectometry (Hydrosense CD620, Campbell Scientific Inc., Edmonton, Alberta, Canada). Dry treatment plants experienced a mean reduction in VWC of 17 % (4 d after initiation of treatments) to 77 % (after 7 weeks of growth), relative to wet plants. Plants were harvested after 8 weeks, at which point many plants were showing signs of senescence. Thus, plants experienced a form of truncation selection, which their source populations also endure annually at the onset of seasonal drought. With the exception of population DRC, germination and establishment were lower than expected (final sample sizes: CCR, ndry = 30, nwet = 24; DRC, ndry = 104, nwet = 107; COH, ndry = 24, nwet = 17; HON, ndry = 29, nwet = 14).
The second greenhouse experiment examined the effect of water availability on genetic variation and covariation for mating system, developmental and physiological traits in a population of M. guttatus (‘M13W’; 38°33′N, 122°22′W, 305 m elevation). A voucher specimen from this population is housed in the Illinois Natural History Survey herbarium (ILLS). Seeds from 140 open-pollinated plants were collected in the field, and a single plant from each was grown to maturity in the greenhouse. Three plants were randomly assigned without replacement to serve as dams (pollen recipients) to each of 35 randomly chosen sires (pollen donors) in a nested half-sib breeding design. All crosses were performed by hand in a pollinator-free greenhouse. Flowers receiving pollen were emasculated in bud to prevent self-pollination. When performing hand pollinations, the surface of one dehiscing anther from flowers serving as pollen donors was gently pressed into the surface of a receptive stigma on flowers serving as pollen recipients. Seven fruits failed to mature, resulting in a total of 98 available for the experiment. Each matured fruit held several hundred seeds from which approx. 50 were sown onto wet soil in 10 × 10 cm pots. Six emerging seedlings were randomly chosen to be included in the experiment. Due to incomplete germination or failure to establish, 14 seedlings were lost, resulting in a total of 575 plants included in the experiment. Seedlings were transplanted into individual 5 × 5 cm pots, fertilized and otherwise cultured as described above. Each set of seedlings was randomly arranged in trays, and two sets were placed into each of three blocks (greenhouse benches). One of each set was assigned to the wet treatment, and the other to the dry treatment, as described above.
Traits
Traits that are associated with variation in mating system, development rate and physiology were measured. To evaluate potential variation in mating system, variation in flower size and herkogamy was measured. Within Mimulus (Ritland and Ritland, 1989) and other genera (Wyatt, 1988), taxa with larger flowers and increased herkogamy have higher outcrossing rates. Within our study populations, we have not estimated the relationship between herkogamy or flower size and outcrossing rate. Previous work with Mimulus, however, found that herkogamy is correlated with individual plant outcrossing rates (Karron et al., 1997; van Kleunen and Ritland, 2004), and that smaller flowers receive fewer insect visits (Ivey and Carr, 2005), suggesting that small-flowered individuals may rely more on autogamy to maintain fitness (but see van Kleunen and Ritland, 2004). Flower size (FS) was measured as the geometric mean of corolla width measured at the widest point and the length of the corolla tube. Corolla width and length were positively correlated (r > 0·67 in all populations), thus these traits primarily indicated variation in overall flower size. Using the geometric mean reduced dimensionality in our analyses without any meaningful loss of information. Herkogamy was measured as the shortest distance between anthers and stigma (AS). All morphological traits were measured on the first flower to open on the plant.
To characterize variation in developmental rates, we recorded the date the first flower opened on each plant, which represents a consistent and unambiguous stage in the life history of these annual plants. In the field, we recorded the Julian date on which the first flower opened, truncated to the last three integers (JDFF). In the greenhouse, we measured the developmental rate as the number of days between sowing and first flower opening (DFF).
To characterize physiological traits of plants, the first fully expanded peduncular leaf was collected from each plant for elemental analyses. Leaves were analysed for the 13C isotope ratio (δ13C), which can be considered a measure of WUE, integrated over the life of the leaf (Dawson et al., 2002). WUE reflects the ratio of photosynthesis (A) and stomatal conductance (g), and thus could increase by either reducing g (stomatal closure) or increasing A (upregulated photosynthesis). Much of the biochemical machinery necessary for photosynthesis, such as Rubisco, demands nitrogen (N), and positive relationships between A and leaf %N within species are common (Sinclair and Horie, 1989; Reich et al., 1991). Thus, to distinguish the potential roles of A vs. g in WUE variation, leaves were also analysed for %N, which can be interpreted as an indication of potential photosynthetic rate (Wright et al., 2003; Donovan et al., 2007). We were able to collect these measurements only for M. guttatus plants. Leaves for these analyses were collected as each plant began to bolt, but before flowers opened to control for variation in physiological status or allocation associated with developmental stage (Dawson et al., 2002). Leaves were held in an ice chest until returning from the field or greenhouse, when they were dried in a 60 °C oven to a constant mass. Dried leaves were ground to a fine powder by shaking in 2 mL screwtop vials with 3·1 mm diameter methacrylate ball pestles. Sub-samples of the powdered leaf tissue were weighed to the nearest microgram using a Mettler M3 balance (Mettler-Toledo, Inc., Columbus, Ohio, USA), loaded into tin capsules, and analysed by combustion for %N and δ13C on a PDZ Europa ANCA-GSL elemental analyser (Sercon Ltd, Cheshire, UK) at the University of California, Davis Stable Isotope Facility. The data for δ13C are expressed relative to the standard Vienna Pee Dee belemnite (RPDB), where δ13C (‰) = (RS/RPDB – 1) × 1000.
Plant fitness in the field was estimated by counting all seeds matured by plants, which can be considered a measure of lifetime female fitness. Seeds were counted by spreading seeds evenly on white paper, photographing with a digital camera and using imaging software (ImageJ: http://rsbweb.nih.gov/ij/) to count the number of particles. A preliminary experiment found no bias in this method, relative to counting seeds by eye with a click counter, and precision was improved. Plant fitness in the greenhouse was estimated by flower number, which is strongly correlated with seed number in our field sites (0·95 > r > 0·63, C. T. Ivey, unpubl. res.). Finally, all above-ground plant biomass was weighed to the nearest mg, after it was dried to a constant mass at 60 °C.
Analyses
All statistical analyses were performed using SAS (SAS Institute, 2003). For the first greenhouse experiment, trait means between treatments were compared using a mixed-model analysis of variance (ANOVA), in which treatment (wet or dry) was considered a fixed effect while greenhouse bench was included as a random effect. For the second greenhouse experiment, trait means were compared with a mixed-model ANOVA that included treatment as a fixed effect, and greenhouse bench, sire and dam nested within sire as random effects. If residual variances were significantly heterogeneous across treatments, traits were log-transformed for analysis. Means and standard errors in the Results, however, are reported on the back-transformed scale. A significant treatment effect was interpreted as evidence for phenotypic plasticity in response to soil moisture for that trait.
In all experiments, Pearson's product–moment correlations (r) were computed between pairs of traits to examine phenotypic associations among traits.
In the field and first greenhouse study, standardized directional selection differentials (S) for each trait were estimated as the slope of a univariate linear regression of relative fitness on standardized trait values (Lande and Arnold, 1983). Relative fitness was calculated by dividing individual plant fitness by the population mean, and traits were standardized to a mean of 0 and standard deviation of 1. Analyses were performed on individual plants, not family means. Selection differentials provide estimates of total selection on a trait, including both direct and indirect selection (Lande and Arnold, 1983). We also estimated selection gradients (β), which indicate direct selection on a trait after controlling for the effects of other measured traits on fitness. Selection gradients were estimated as the partial regression coefficients from a multiple regression of relative fitness on all measured traits. Model residuals were tested for deviation from a normal distribution using a Shapiro–Wilk (Shapiro and Wilk, 1965) test. In addition, homogeneity of residual variance was tested for by calculating the Spearman's rank correlation between model residuals and relative fitness. If either test indicated a violation of model assumptions, relative fitness was log-transformed. Estimates of selection differentials or gradients from transformed models, however, may be biased (Lande and Arnold, 1983). As a consequence, where it was necessary to transform the data, we present regression coefficients (S and β) from untransformed models, but P-values from transformed models (Mitchell-Olds and Shaw, 1987; Caruso, 2000).
The strength of selection differentials was compared between wet and dry treatments in the greenhouse using a mixed-model analysis of covariance. For these models, plants from both environments for a given population were combined, and relative fitness (flower number) was used as the response. Fixed effects included the standardized trait value (the covariate), treatment (wet or dry) and a treatment × trait interaction term. Greenhouse bench (block) was included as a random effect. A significant interaction term was interpreted as an indication that total selection on the trait differed between environments (Conner, 1989). Model assumptions were tested as described above. Because plants in this experiment were grown in a greenhouse, these estimates of selection may not reflect what plants experience in natural populations, as fitness and other traits routinely differ between greenhouse and field environments. Our primary aim with this analysis was rather to investigate the extent to which variation in water availability might alter patterns of selection when other sources of environmental variation are controlled. In so doing, we follow similar studies (e.g. Dorn et al., 2000; Stanton et al., 2000; Sherrard and Maherali, 2006). As a consequence, although throughout this paper we will refer to the coefficients from these analyses as ‘selection differentials’ or ‘selection gradients’, the full interpretation of these for selection and evolution in natural populations will require further field experimentation.
To estimate genetic variance components and genetic correlations among traits in the second greenhouse experiment, mixed-model restricted maximum likelihood (REML) models as described by Fry (2004; see also Shaw, 1987) were used. Our design permitted estimates of two causal components of genetic variance: additive (VA) and maternal-effect variance (VM); our interpretation of these estimates assumes that variation due to other genetic sources is negligible (Falconer, 1981; Lynch and Walsh, 1998). This analysis also assumes that parents used in the crossing design are fully outbred. Although we do not know whether the parents in this experiment were outbred, a previous study of allozyme frequencies in plants from this population estimated that the population fixation index did not differ significantly from zero (Ivey and Carr, 2005). The magnitude of VA and VM was estimated in univariate REML mixed-models that included sire and dam nested within sire as random effects. We tested the hypotheses that VA = 0 and VM = 0 by constraining in turn each component to 0, re-analysing the model and comparing the difference between the models in the restricted log-likelihood values with a χ2 distribution with d.f. = 1 (Self and Liang, 1987). Because variance cannot be <0, these tests were one-tailed, and the associated P-value was halved, which restricts the range of possible P-values (see Self and Liang, 1987). We estimated the additive genetic correlation (rA) between pairs of traits within each soil moisture environment using the covariance matrix from a similar REML mixed-model that included both traits. We tested the two-tailed hypothesis that additive genetic covariance COVA = 0 using a likelihood ratio test of the full model against one in which the sire covariance between traits was constrained to zero. If this hypothesis was rejected, we tested the one-tailed hypothesis that rA = 1 (or rA = –1, depending on the estimate of the correlation) in which the correlation of sire effects under an ‘unstructured correlations’ (TYPE = UNR) covariance structure was constrained to unity (or –1). If both hypotheses were rejected, genetic correlation estimates are considered 0 < rA < 1 (or 0 > rA > –1, depending on the estimate). More information about this approach is described by Fry (2004). Some of our estimates of rA were >1, which is biologically unreasonable. This was often associated with low, non-significant estimates of VA. In these circumstances, rA estimates were truncated at 1·0. Additive genetic correlations were also estimated across wet and dry environments for each trait. For this analysis, we considered a trait measured on plants growing in different environments to be a distinct trait, and we estimated additive genetic correlations between environments for the trait as described above. The significance of estimates of rA was tested using likelihood ratio tests as described above. Estimates of rA <1 can be interpreted as evidence for significant additive genetic variation in phenotypic plasticity across environments for that trait (Lynch and Walsh, 1998).
RESULTS
Field study
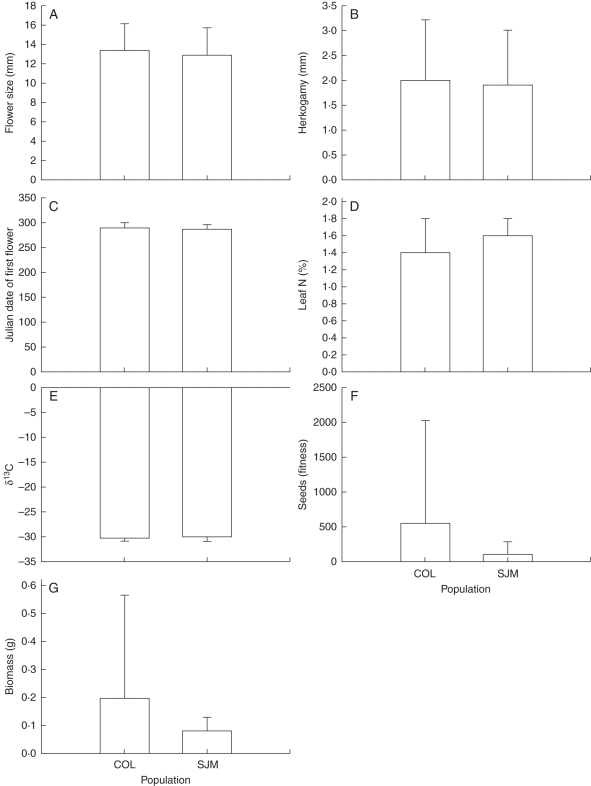
Most (98 % in COL; 92 % in SJM) of the M. guttatus rosettes marked at the beginning of the season were recovered, but few (18 % in COL; 39 % in SJM) of these produced any flowers. As a consequence of the small number of plants available in COL, phenotypic correlations and phenotypic selection were estimated only for SJM plants. The traits measured did not differ substantially between populations, although the variance in seed number and biomass was considerably higher in COL (Fig. 1). Flower size was positively correlated with leaf %N, but otherwise we found no examples of correlations between putative drought escape and mating system traits (Table 1).
Fig. 1.
Means (±s.d.) of flower size (A), anther–stigma separation (B), Julian date of first flower (C), leaf %N content (D), leaf 13C isotope ratio (E), lifetime seed set (F) and above-ground biomass (G) in two field populations of flowering Mimulus guttatus in Lassen National Forest, Butte County, California, USA.
Table 1.
Phenotypic Pearson product–moment correlations between traits measured in a field population (SJM) of Mimulus guttatus in Lassen National Forest, Butte County, California, USA
| AS | JDFF | %N | δ13C | Seeds | Mass | |
|---|---|---|---|---|---|---|
| FS | −0·31* | −0·08 | 0·59* | 0·00 | 0·39** | 0·47*** |
| AS | −0·02 | −0·17 | −0·35 | −0·06 | 0·01 | |
| JDFF | −0·62** | −0·08 | −0·38** | −0·36** | ||
| %N | −0·33 | 0·50* | 0·29 | |||
| δ13C | −0·20 | 0·13 | ||||
| Seeds | 0·28* |
Trait abbreviations are given in the Materials and Methods. *P < 0·05; **P < 0·01; ***P < 0·001.
Selection differentials and selection gradients were significant for several traits in the M. guttatus field population, suggesting evidence for natural selection. Selection differentials through female fitness favoured larger flowers, early flowering and high leaf %N content (Table 2). In addition, selection gradients suggested direct selection favouring larger flowers and early flowering; this model, however, did not include %N and δ13C due to small sample sizes (Table 2). Although the selection gradient for flowering time was statistically significant, it was much weaker than its corresponding selection differential (Table 2), suggesting that much of the strong selection observed for early flowering in the univariate model may have occurred indirectly.
Table 2.
Standardized directional selection differentials (S) and gradients (β) on traits measured in a field population (SJM) of Mimulus guttatus in Lassen National Forest, Butte County, California, USA
| Trait | n | S (s.e.) | β (s.e.) |
|---|---|---|---|
| Flower size | 49 | 0·91 (0·31)*** | 0·98 (0·42)** |
| Anther–stigma separation | 50 | −0·15 (0·35) | 0·17 (0·36) |
| Julian date of first flower (JDFF) | 59 | −0·84 (0·27)** | −0·02 (0·01)* |
| % | 18 | 1·40 (0·61)** | – |
| δ13C | 17 | −0·58 (0·73) | – |
| Biomass | 64 | 0·60 (0·26)† | 0·10 (0·37) |
Fitness was estimated as lifetime seed production. Gradients were not estimated for %N and δ13C due to small sample sizes.
†P < 0·1 *P < 0·05; **P < 0·01; ***P < 0·001.
First greenhouse experiment – plasticity and selection
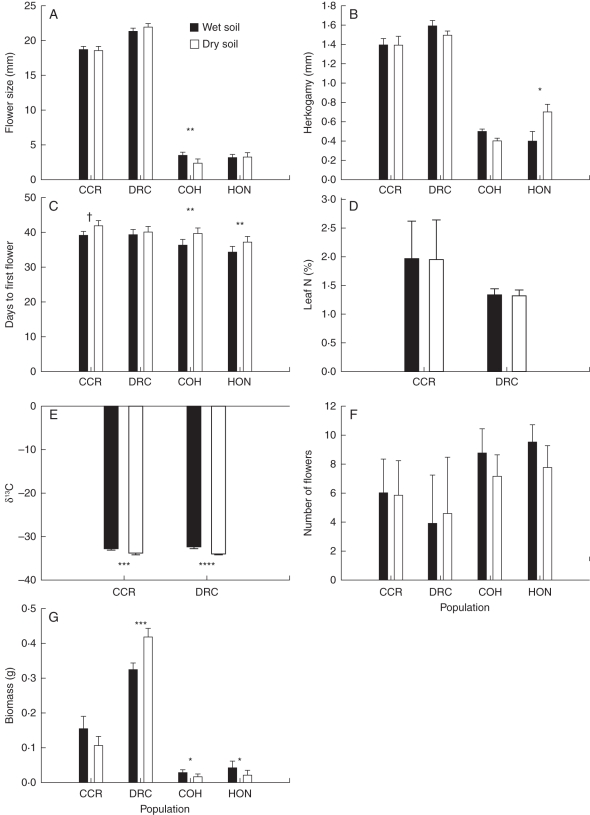
The M. nasutus populations (COH and HON) were overall more phenotypically plastic in response to experimental soil moisture treatment than the M. guttatus populations (Fig. 2; Supplementary Data Table S1, available online). Flower size, for example, was 44 % larger in dry soil than in the wet soil treatment for COH plants, whereas anther–stigma separation was almost half as long in dry soil than in wet soil among HON plants. In the dry soil treatment, plants of both M. nasutus populations flowered about 3 d earlier and surprisingly produced larger (higher biomass) plants (Fig. 2). In contrast, the only trait in the M. guttatus populations (CCR and DRC) that consistently responded plastically to soil moisture was δ13C, which was less negative in dry soil, indicating greater WUE under experimental drought conditions.
Fig. 2.
Least-squares means (±s.e.) of flower size (A), anther–stigma separation (B), days from sowing to first flower (C), leaf %N content (D), leaf 13C isotope ratio (E), number of flowers produced (F) and above-ground biomass (G) in greenhouse populations of Mimulus guttatus (CCR and DRC) and M. nasutus (COH and HON) grown in experimentally dry and wet soil, as indicated. †P < 0·1, *P < 0·05, **P < 0·01, ***P < 0·001, ****P < 0·0001 in mixed-model ANOVA comparisons of trait means between treatments within populations.
Some of the phenotypic correlations among traits for greenhouse plants resembled patterns observed in the field population (Table 3). Leaf %N in M. guttatus plants, for example, was negatively correlated with flowering time and positively correlated with flower number (Table 3), which was similar to what we observed in the field. These relationships, moreover, were maintained in both soil environments (Table 3). Many of the other phenotypic correlations, however, varied between soil moisture treatments. In both M. guttatus populations studied in the greenhouse, for example, biomass and δ13C were positively correlated only in the dry treatment. Similarly, biomass and flower size were strongly positively correlated among M. guttatus plants, but only in the wet treatment. In both M. nasutus populations, on the other hand, flower size and biomass were positively correlated only in the dry treatment (Table 4). Flowering time was negatively correlated with flower number in both environments for both taxa (Table 4).
Table 3.
Pearson product-moment correlations between traits in two greenhouse populations (CCR and DRC) of Mimulus guttatus grown in experimentally wet and dry soil treatments
| FS | AS | DFF | %N | δ13C | Flowers | Biomass | |
|---|---|---|---|---|---|---|---|
| CCR | |||||||
| FS | – | 0·01 | −0·03 | 0·08 | 0·30 | 0·02 | 0·28 |
| AS | 0·78**** | – | 0·06 | −0·20 | 0·30 | 0·02 | 0·33 |
| DFF | 0·05 | 0·04 | – | −0·72*** | 0·08 | −0·73**** | 0·01 |
| %N | −0·46 | −0·41 | −0·83*** | – | −0·27 | 0·48* | −0·40 |
| δ13C | −0·03 | 0·32 | −0·13 | 0·20 | – | 0·06 | 0·64** |
| Flowers | 0·41 | 0·23 | −0·75*** | 0·75*** | 0·36 | – | 0·39* |
| Biomass | 0·81**** | 0·56* | −0·27 | 0·19 | 0·24 | – | – |
| DRC | |||||||
| FS | – | 0·28* | −0·12 | −0·23 | −0·02 | 0·10 | 0·11 |
| AS | 0·08 | – | −0·23* | 0·03 | 0·09 | 0·24* | 0·24 |
| DFF | 0·06 | −0·06 | – | −0·69**** | 0·18 | −0·73**** | 0·03 |
| %N | −0·21 | 0·09 | −0·76**** | – | −0·05 | 0·71**** | 0·05 |
| δ13C | 0·29* | 0·21 | −0·15 | −0·08 | – | 0·01 | 0·43**** |
| Flowers | 0·12 | 0·12 | −0·47**** | 0·47**** | 0·26* | – | 0·03 |
| Biomass | 0·40*** | −0·08 | 0·01 | −0·05 | 0·10 | 0·42**** | – |
Correlations in dry soil are above the diagonal; wet soil below. Trait abbreviations are given in the Materials and Methods.
*P < 0·05, **P < 0·01, ***P < 0·001, ****P < 0·0001.
Table 4.
Pearson product–moment correlations between traits in two greenhouse populations (COH and HON) of Mimulus nasutus grown in experimentally wet and dry soil treatments
| FS | AS | DFF | Flowers | Biomass | |
|---|---|---|---|---|---|
| COH | |||||
| FS | – | −0·21 | −0·38* | 0·50** | 0·75**** |
| AS | −0·18 | – | −0·28 | 0·01 | −0·07 |
| DFF | −0·44 | 0·06 | – | −0·75**** | −0·67**** |
| Flowers | 0·36 | −0·05 | −0·52* | – | 0·89**** |
| Biomass | 0·40 | 0·24 | −0·63** | 0·80*** | – |
| HON | |||||
| FS | – | −0·14 | −0·30 | 0·25 | 0·50** |
| AS | −0·73** | – | −0·10 | 0·21 | 0·09 |
| DFF | −0·16 | −0·19 | – | −0·78**** | −0·73**** |
| Flowers | 0·16 | 0·09 | −0·93**** | – | 0·49** |
| Biomass | 0·39 | −0·06 | −0·86**** | 0·95**** | – |
Correlations in dry soil are above the diagonal; wet soil below. Trait abbreviations are given in the Materials and Methods.
*P < 0·05, **P < 0·01, ***P < 0·001, ****P < 0·0001.
Selection differentials in greenhouse populations of M. guttatus indicated that early flowering and high leaf %N were favoured in both environments (Table 5), which is similar to what was observed in the field. Selection for high biomass was significantly stronger in the wet treatment in both M. guttatus populations, suggesting that allocation to biomass may be adaptive only where water is abundant. In the M. nasutus populations, early flowering and higher biomass were generally favoured in both environments (Table 5).
Table 5.
Standardized directional selection differentials measured in four greenhouse populations of Mimulus grown in experimentally wet and dry soil treatments
| Species | Population | Trait | Dry | Wet | F | P |
|---|---|---|---|---|---|---|
| M. guttatus | CCR | FS | −0·01 (0·14) | 0·41 (0·22)* | 3·08 | 0·08 |
| AS | −0·01 (0·14) | 0·23 (0·24) | 1·70 | 0·2 | ||
| DFF | −0·49 (0·09)**** | −0·73 (0·16)*** | 0·75 | 0·4 | ||
| %N | 0·24 (0·09)* | 1·00 (0·18)*** | 8·80 | 0·006 | ||
| δ13C | 0·08 (0·09) | 0·38 (0·28)* | 2·83 | 0·1 | ||
| Biomass | 0·27 (0·13) | 0·70 (0·15)**** | 4·39 | 0·04 | ||
| DRC | FS | 0·10 (0·12) | 0·09 (0·15) | 0·06 | 0·8 | |
| AS | 0·23 (0·11)* | 0·28 (0·14)* | 0·04 | 0·8 | ||
| DFF | −0·72 (0·08)**** | −0·56 (0·13)**** | 8·81 | 0·004 | ||
| %N | 0·48 (0·08)**** | 0·50 (0·14)** | 0·97 | 0·3 | ||
| δ13C | −0·08 (0·11) | 0·09 (0·14) | 1·28 | 0·3 | ||
| Biomass | 0·20 (0·12) | 0·64 (0·12)**** | 4·75 | 0·03 | ||
| M. nasutus | COH | FS | 0·22 (0·08)** | 0·11 (0·10) | 2·58 | 0·1 |
| AS | −0·02 (0·09) | 0·01 (0·09) | 0·46 | 0·5 | ||
| DFF | −0·33 (0·06)**** | −0·16 (0·10) | 2·93 | 0·09 | ||
| Biomass | 0·41 (0·04)**** | 0·29 (0·07)*** | 2·58 | 0·1 | ||
| HON | FS | 0·15 (0·11) | 0·12 (0·21) | 0·11 | 0·7 | |
| AS | 0·12 (0·11) | −0·02 (0·22) | 0·22 | 0·6 | ||
| DFF | −0·49 (0·07)**** | −0·77 (0·09)**** | 3·57 | 0·07 | ||
| Biomass | 0·28 (0·10)* | 0·67 (0·07)**** | 5·46 | 0·03 |
Trait abbreviations are given in the Materials and Methods. Fitness was estimated as the total number of flowers produced.
*P < 0·05, **P < 0·01, ***P < 0·001, ****P < 0·0001 in a test of the hypothesis that selection differential = 0. F and P present the results of a test of the hypothesis that selection differentials do not differ between environments (trait × treatment interaction term from analysis of covariance).
Selection gradients differed somewhat between the greenhouse populations of M. guttatus, which may have been a consequence of the different sample sizes (see above). In these multivariate models, some evidence was found that early flowering and larger plants were adaptive in CCR (Table 6). Among DRC plants, however, apparent direct selection for early flowering was observed only in the dry treatment, and direct selection for larger plants only in the wet treatment. For M. nasutus plants, relatively uniform selection favouring high biomass was found in both environments. Early flowering was also favoured, weakly, and gradients were significant only in the dry soil treatment (Table 6), again suggesting that rapid development can be more advantageous in drought conditions.
Table 6.
Standardized directional selection gradients measured in four greenhouse populations of Mimulus grown in experimentally wet and dry soil treatments
| Species | Pop. | Trait | Dry | Wet |
|---|---|---|---|---|
| M. guttatus | CCR | FS | −0·08 (0·06) | −0·47 (0·47) |
| AS | −0·11 (0·05) | 0·38 (0·24) | ||
| DFF | −0·44 (0·10)† | −0·47 (0·19)* | ||
| %N | −0·06 (0·09) | 0·15 (0·24) | ||
| δ13C | 0·01 (0·07) | −0·43 (0·19) | ||
| Biomass | 0·27 (0·09)* | 0·73 (0·24)† | ||
| DRC | FS | −0·11 (0·08) | 0·01 (0·10) | |
| AS | 0·03 (0·07) | 0·18 (0·10)† | ||
| DFF | −0·54 (0·11)**** | −0·14 (0·19) | ||
| %N | 0·01 (0·11) | 0·31 (0·18) | ||
| δ13C | −0·11 (0·08) | 0·08 (0·13) | ||
| Biomass | 0·16 (0·09) | 0·68 (0·11)**** | ||
| M. nasutus | COH | FS | −0·15 (0·05)* | −0·01 (0·08) |
| AS | −0·03 (0·04) | −0·10 (0·07) | ||
| DFF | −0·11 (0·05)** | 0·03 (0·09) | ||
| Biomass | 0·45 (0·07)**** | 0·35 (0·09)** | ||
| HON | FS | −0·12 (0·05) | −0·25 (0·06)* | |
| AS | 0·04 (0·05) | −0·13 (0·06) | ||
| DFF | −0·14 (0·07)* | −0·29 (0·10) | ||
| Biomass | 0·53 (0·09)**** | 0·56 (0·09)*** |
Trait abbreviations are given in the Materials and Methods. Fitness was estimated as the total number of flowers produced.
*P < 0·05, **P < 0·01, ***P < 0·001, ****P < 0·0001 in a test of the hypothesis that selection gradient = 0.
Second greenhouse experiment – genetic variation and covariation
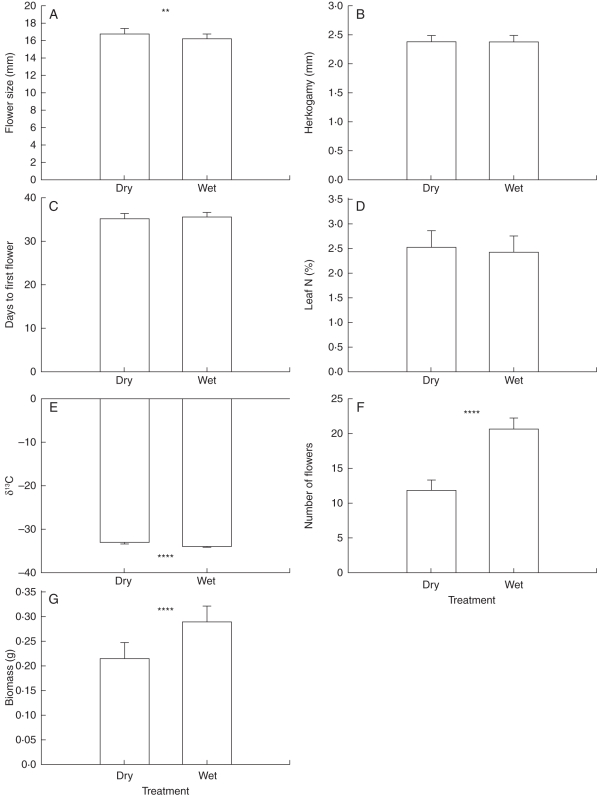
The M. guttatus plants from the nested half-sib experiment revealed more phenotypic plasticity than those from the first greenhouse experiment (Fig. 3; Supplementary Data Table S1, available online). We also had greater statistical power to detect experimental treatment effects in the nested half-sib experiment (n = 574 plants), such as, for example, a 3 % difference in flower size. Nonetheless, some treatment effects were large; for example, the dry treatment resulted in a >40 % reduction in flower number and a 25 % reduction in biomass. In the first greenhouse experiment, biomass in one of the M. guttatus populations (DRC) had a similar response to the dry treatment (23 % reduction), but no such response was observed in the other population, nor was flower number altered by water treatment in either population (Fig. 2).
Fig. 3.
Least-squares means (±s.e.) of flower size (A), anther–stigma separation (B), days from sowing to first flower (C), leaf %N content (D), leaf 13C isotope ratio (E), number of flowers produced (F) and above-ground biomass (G) in a greenhouse population of Mimulus guttatus grown in experimentally dry and wet soil. **P < 0·01, ****P < 0·0001 in mixed-model ANOVA comparisons of trait means between treatments.
Among plants in the nested half-sib experiment, wet soil conditions resulted in greater phenotypic variation for most traits, with the exception of δ13C (WUE), which was more variable under dry conditions (Table 7). For most traits, however, this did not result in differences between treatments in our ability to detect significant contributions of additive or maternal genetic variance. For example, significant additive genetic variation of a similar magnitude was detected for days to first flower, number of flowers and above-ground biomass in both soil moisture treatments. A striking exception to this was observed in flower size; in the dry treatment, flower size was not significantly heritable, whereas in wet soil >70 % of phenotypic variation could be attributed to the effects of additive genes. We found marginally significant heritability for %N in the wet treatment, and a modest, but significant maternal genetic component for δ13C in the dry treatment (Table 7). Otherwise, however, we found little evidence for genetic control of %N or δ13C. Our power to estimate genetic variation and covariation for traits involving leaf %N or δ13C, however, was limited by a small sample size; we were able to measure these phenotypes on 236 (41 %) of the plants in the experiment. Thus, estimates of genetic variation and correlations involving these traits should perhaps be regarded as provisional.
Table 7.
Percentage of phenotypic variance explained by additive and maternal genetic components of variance, along with total phenotypic variance, for traits measured in a greenhouse population of Mimulus guttatus growing in experimentally dry and wet soil treatments
| Dry |
Wet |
P |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trait | Additive | Maternal | VP | Additive | Maternal | VP | rA | rA = 0 | rA = 1 | |
| FS | 15·4 | 18·1† | 6·666 | 70·3**** | 0·0 | 8·528 | 1·00 | 0·003 | 0·5 | |
| AS | 38·1† | 20·5† | 0·880 | 15·0 | 27·8 | 0·885 | 1·00 | 0·04 | 0·1 | |
| DFF | 54·6** | 0·0 | 14·39 | 48·2* | 0·0 | 19·32 | 0·68 | 0·04 | 0·07 | |
| %N | 13·1 | 0·06 | 0·032 | 38·0† | 0·0 | 0·033 | 1·00 | 0·13 | 0·2 | |
| δ13C | 0·0 | 27·8* | 0·627 | 35·0 | 0·0 | 0·254 | 1·00 | 0·4 | 0·5 | |
| Flowers | 46·1** | 0·0 | 25·39 | 54·6** | 0·01 | 134·7 | 0·48 | 0·2 | 0·02 | |
| Biomass | 68·9*** | 0·0 | 0·011 | 62·6** | 0·01 | 0·033 | 0·90 | 0·003 | 0·2 | |
Note that the additive component is also an estimate of narrow-sense heritability (h2) × 100. Also indicated is the additive genetic correlation for each trait across environments and P-values for two log-likelihood ratio tests. The first tested the two-tailed hypothesis that rA = 0; the second tested the one-tailed hypothesis that rA = 1. A cross-environment genetic correlation = 1 suggests a lack of genetic variation for plasticity.
Trait abbreviations are given in the Materials and Methods.
†P < 0·1, *P < 0·05, **P < 0·01, ***P < 0·001, ****P < 0·0001 in log-likelihood ratio tests of the hypothesis that each variance component = 0.
Additive genetic correlations (rA) of traits measured across the two soil water environments indicated that the plasticity observed for biomass (Fig. 3) did not have an additive genetic basis (i.e. rA did not differ significantly from 1: Table 7). In contrast, plasticity for flower number across environments reflected additive genetic differences among plants in response to water (Table 7). This suggests that genotypes that performed well in wet soil are not the same as those that perform well under dry conditions. There was marginally significant evidence for rA <1 for date of first flower, which would indicate that genotypes vary in developmental rate depending on soil water availability.
As observed in the first greenhouse experiment and the field experiment, early flowering M. guttatus plants in the nested full-sib experiment also had high leaf %N and more flowers (Table 8). Similarly, we again found that plants with less negative δ13C (higher WUE) had higher biomass, but only under dry soil conditions. In contrast to the first experiment, however, no difference was found between treatments in the positive correlation between flower size and biomass, although flower size was significantly phenotypically correlated with most traits in both treatments in the nested full-sib experiment (Table 8). In addition, flower size and biomass were positively genetically correlated in both soil environments (Table 8). Few significant additive genetic correlations were detected overall, even among those traits for which we had a robust sample size (i.e. not involving leaf %N or δ13C). Two exceptions, however, are noteworthy. First, flowering time was positively correlated with flower size, but only in the wet treatment. In addition, flowering time was negatively correlated with flower number, but only in the dry treatment (Table 8).
Table 8.
Correlations between traits in a greenhouse population of Mimulus guttatus grown in a nested full-sib breeding design in experimentally wet and dry soil treatments
| FS | AS | DFF | %N | δ13C | Flowers | Biomass | |
|---|---|---|---|---|---|---|---|
| Phenotypic | |||||||
| FS | – | 0·17** | 0·26**** | −0·40**** | 0·29** | −0·04 | 0·50**** |
| AS | 0·26**** | – | −0·03 | 0·03 | 0·01 | 0·10 | 0·23**** |
| DFF | 0·25**** | −0·12* | – | −0·38**** | 0·00 | −0·55**** | −0·04 |
| %N | −0·32*** | −0·07 | −0·26** | – | −0·46**** | 0·34*** | 0·08 |
| δ13C | 0·08 | 0·14 | −0·21* | −0·36**** | – | 0·16 | 0·31*** |
| Flowers | 0·16** | 0·22*** | −0·54**** | 0·07 | 0·25** | – | 0·48**** |
| Biomass | 0·54**** | 0·29**** | −0·07 | −0·07 | 0·13 | 0·69**** | – |
| Genetic | |||||||
| FS | – | 0·57 | −0·44 | –1·00† | 0·00 | 0·39 | 1·00* |
| AS | 0·36 | – | 0·02 | 0·22 | 0·00 | 0·49 | 0·42 |
| DFF | 0·69* | −0·08 | – | −0·67 | 0·00 | −0·59* | −0·06 |
| %N | −0·38 | −0·58 | −0·50 | – | 0·00 | −0·04 | –1·00 |
| δ13C | −0·28 | −0·62 | 0·18 | –1·00 | – | 1·00† | 0·00 |
| Flowers | −0·11 | 0·81 | −0·36 | −0·24 | 0·27 | – | 0·58† |
| Biomass | 0·64* | 0·64 | 0·40 | −0·65 | −0·08 | 0·55 | – |
Correlations in dry soil are above the diagonal; wet soil below. The upper matrix shows Pearson product–moment phenotypic correlations, while the lower matrix shows bivariate additive genetic correlations. Trait abbreviations are given in the Materials and Methods.
P < 0·1, *P < 0·05, **P < 0·01, ***P < 0·001, ****P < 0·0001 in tests of the hypothesis that the correlation = 0. Genetic correlations in bold also differ significantly from 1 or –1 (α = 0·05).
DISCUSSION
Transitions in mating systems are often accompanied by characteristic changes in a suite of morphological, physiological and developmental traits. For example, self-fertilizing taxa often have smaller flowers (Wyatt, 1988), higher photosynthetic rates (Mazer et al., 2010) and more rapid development rates (Fenster et al., 1995; Armbruster et al., 2002) when compared with their outcrossing relatives. These traits, however, can allow plants to minimize exposure to water stress in areas with a seasonal drought (Geber and Dawson, 1990; Galen, 1999; Arntz and Delph, 2001; McKay et al., 2003), and thus they may evolve due to selection by agents not directly affecting the mating system. We examined whether the transition to self-fertilization is directly favoured in Mimulus or whether it might evolve as an indirect consequence of adaptation to drought. Our results provide some support for predictions of the hypothesis that self-fertilization evolves jointly with drought escape traits, and generally suggest that the hypothesis warrants further scrutiny.
Was selection stronger for drought escape than selfing?
Our first prediction, which stated that physiological or developmental traits should experience stronger direct selection than mating system traits, received mixed support. Selection gradients favouring rapid development (early flowering), for example, were stronger than for herkogamy or flower size, for both M. guttatus and M. nasutus in the greenhouse. On the other hand, the selection gradient favouring large flowers was much stronger than that for flowering time in the field study of M. guttatus, even though there was also significant selection favouring early flowering. This may reflect the success of large flowers in attracting pollinators (Ivey and Carr, 2005). Larger flowers are associated with increased outcrossing rates within the genus Mimulus (Ritland and Ritland, 1989), so, if this pattern holds within populations, it could indicate selection for outcrossing in the field. Even though drought escape may be associated with selfing, selection favouring outcrossing is expected if genetic load is substantial and outweighs fitness costs associated with vulnerability to drought.
The role of flower size in the mating system of M. guttatus may be indirect (e.g. van Kleunen and Ritland, 2004). A previous field study, for example, found that experimentally limiting access to pollinators led to a significant change in the selection gradient toward reduced herkogamy, but there was no effect on selection for flower size (Fishman and Willis, 2007). Similarly, a recent study in which the evolution of floral traits in M. guttatus was monitored for five generations following experimental exclusion of pollinator access (i.e. enforcing autogamy) found no phenotypic divergence in flower size in response to pollinator removal (Bodbyl-Roels and Kelly, 2011). Interestingly, flowering time was one of only two traits that diverged between treatments; plants receiving visits from pollinators evolved toward earlier flowering (a phenology more closely coincident with the timing of pollinator release) (Bodbyl-Roels and Kelly, 2011). These results raise uncertainty about the role of flower size in reproductive assurance and mating system. Furthermore, they suggest that flowering time in M. guttatus may be particularly responsive to environmental change, which is similar to the findings from a previous study in Brassica rapa (Franks et al., 2007).
No evidence was found for direct selection on the physiological traits we measured, δ13C ratio and %N in leaves. Low WUE can be advantageous in ephemerally wet environments as a mechanism for avoiding drought exposure (e.g. McKay et al., 2003; Heschel and Riginos, 2005). In our study, however, selection differentials as well as gradients for WUE, as measured by δ13C, were either not significant or weak in greenhouse and field studies. In addition, although strong selection differentials favouring high leaf %N were found in the field and greenhouse, this appeared to be indirect, based on our greenhouse study. Because of the prevalence of Rubisco in plants, leaf N is associated with the potential photosynthetic rate among some plants (Wright et al., 2003; Donovan et al., 2007), and high photosynthetic rates can be adaptive for plants in ephemerally wet environments, allowing them to mature quickly and thereby avoid water stress (Dudley, 1996; Mazer et al., 2010). On the other hand, leaf N may vary because of plant nutrient status independent of photosynthetic potential (Lambers et al., 1998), thus interpreting positive selection for leaf N can be challenging (Donovan et al., 2009). In the field, for example, N availability is likely to be patchy, which could lead to bias in the positive estimates of selection we observed for leaf %N, if availability of N covaried with both fitness and leaf N (e.g. Rausher, 1992). However, we also observed positive selection differentials for leaf %N in greenhouse experiments, where N availability was constant across plants. The similarity in this relationship between both field and greenhouse environments supports the idea that variation in %N is due to constitutive differences among plants in leaf N content, perhaps reflecting allocation to photosynthesis (Sinclair and Horie, 1989).
Positive phenotypic correlations between δ13C and flowering time were not observed, as would be expected if rapid development were associated with low WUE. This combination of traits is considered to reflect a ‘drought escape’ strategy that is adaptive in some plants (Geber and Dawson, 1990; Stanton et al., 2000; McKay et al., 2003; Heschel and Riginos, 2005; Wu et al., 2010; Mazer et al., 2010), although empirical support for this pattern has been mixed (Sherrard and Maherali, 2006; Donovan et al., 2007; Wu et al., 2010). Thus, our results suggest that early flowering in M. guttatus was not mediated by decreased WUE, as might be expected if plants achieved rapid development by using ‘excess’ water early in the spring when it is more abundant (sensu Mazer et al., 2010). Instead, flowering time was negatively correlated with leaf %N availability, which is positively associated with photosynthetic rates in some plants (Arntz et al., 2000; Wright et al., 2001). In all of our experiments, negative phenotypic correlations between leaf %N and flowering time were observed, which supports the idea that variation in leaf %N reflects allocation to growth and development.
Was selection for drought escape stronger in dry soil?
Our second prediction, that selection for drought escape should be stronger in dry environments, was generally supported. The selfing species, M. nasutus, which naturally occurs in more ephemerally wet environments (Kiang and Hamrick, 1978; Martin and Willis, 2007), had stronger selection gradients for rapid development (early flowering) in the dry treatment in our greenhouse experiments. This was also the case for one of the M. guttatus populations we studied (DRC). This mirrors the results of previous field reciprocal transplant studies which found that early flowering in M. guttatus was favoured in interior habitats that dry more quickly (Hall and Willis, 2006; Lowry et al., 2008). Genotypes native to the more mesic costal environment died in the seasonal drought of the interior habitat before they were able to flower. Selection gradients for the other components of drought escape for which we had estimates of selection (WUE measured as δ13C and %N) did not differ significantly across soil moisture treatments.
Were drought escape and mating system traits genetically correlated?
Limited support was found for our third prediction, which was that drought escape and mating system traits should be genetically correlated. A previous study reported a positive genetic correlation between flower size and δ13C for M. guttatus (Kelly et al., 2008), which supports this prediction; however, we found no such relationship in our study. We observed a strong positive genetic correlation between flowering time and flower size among plants grown in the wet soil treatment, which might be construed as support for our third prediction. The direction of this correlation, however, may act to constrain the adaptive evolution of these traits, as our estimates of selection gradients for M. guttatus generally favoured early flowering and large flowers. Our other estimates of genetic correlations provided no evidence for constraints on the adaptive evolution of any other traits, in that few were statistically significant and, of the ones that were, most appeared to follow an adaptive contour. It is noteworthy, therefore, that a genetic correlation between putative drought escape and mating system traits may constrain their evolution, and that this correlation was observed only under the wet soil treatment. Genetic correlations that change with the environment may contribute to the maintenance of genetic variation for traits (Sgrò and Hoffmann, 2004), in this case flower size and flowering time. Thus, in dry years, or dry areas, there may be stronger selection favouring earlier flowering (e.g. population DRC, Table 6), if patterns in the field resemble our experimental greenhouse results. In addition, in the dry treatment, we found a negative genetic correlation between flowering time and flower number, which would reinforce the observed pattern of selection. No other genetic correlations that would constrain the adaptive evolution of flowering time were apparent in the dry treatment (Table 8). In contrast, during wet years or in wet environments, direct selection for early flowering may be more attenuated. Furthermore, no significant genetic correlations between flowering time and flower number were observed in the wet treatment. Thus, under wet conditions, the adaptive evolution of mating system (as reflected in flower size) and developmental rate (as reflected in flowering time) may be limited by this correlation, whereas under dry conditions, these traits may be free to evolve adaptively. In sum, while we found a significant genetic correlation between these two putative drought escape and mating system traits, in support of our prediction, the direction of the correlation does not suggest support for the hypothesis that they evolve jointly, given the selection gradients observed.
Otherwise, we observed sufficient additive genetic variation to expect that most traits experiencing direct selection could evolve adaptively. Narrow-sense heritability (h2) estimates were comparable with reports of h2 for similar traits in previous studies of M. guttatus (Carr and Fenster, 1994; Robertson et al., 1994; van Kleunen and Ritland, 2004; Carr et al., 2006; Ivey et al., 2009). With the exception of flower size, moreover, our estimates of h2 were similar in both dry and wet soil. This contrasts with Murren et al. (2009), who found sire genetic effects for flowering time to vary with experimentally manipulated water availability in two populations of M. guttatus.
Phenotypic plasticity
Overall, M. nasutus was more phenotypically plastic than M. guttatus in response to simulated drought, which is consistent with the idea that genotypes with higher levels of developmental plasticity should be favoured in inbreeding species that occur in temporally fluctuating environments (Bradshaw, 1965). Much of the plasticity we observed in this species could be construed as adaptive, if similar patterns in response to moisture occur in the field. In the dry treatment, for example, M. nasutus flowered earlier and experienced stronger selection for early flowering. Plasticity in flowering time can enhance adaptation to novel, stressful environments (Levin, 2009), especially when accompanied by plastic shifts in traits likely to increase self-fertilization (Levin, 2010), such as the reduced herkogamy we observed in the dry treatment for the HON M. nasutus population. Plastic shifts in flowering time and selfing can reinforce positive assortative mating within the stressful habitat, which may contribute to reproductive isolation (Levin, 2010). Several derived taxa within the M. guttatus species complex, including M. nasutus, occur on edaphically stressful habitats and, in comparison with M. guttatus (which is presumed to resemble the ancestral form), flower earlier and have higher rates of selfing (Macnair and Gardner, 1998; Martin and Willis, 2007). Thus, the plastic responses to soil moisture that were observed within M. nasutus are similar to the among-taxon phenotypic differences between M. guttatus and several of its derivative edaphic specialists. Furthermore, these traits, flowering time and mating system, were found to be the main factors contributing to reproductive isolation between M. guttatus and M. nasutus (Martin and Willis, 2007). Taken together, these observations are consistent with the idea that plasticity in response to a novel edaphic environment (drier soil) may have contributed to divergence between M. guttatus and M. nasutus.
Plasticity in M. guttatus could be adaptive. For example, DRC plants in the wet treatment had greater biomass and experienced strong selection for increased biomass. Interpreting these kinds of plastic responses as adaptive is challenging because the environment affects both phenotype and fitness (Sultan, 2000). In addition, no evidence was found in M. guttatus for additive genetic variation for plasticity among most of the traits that varied phenotypically across water treatments. Nonetheless, shifts in phenotype such as were observed may confer an adaptive advantage even if the plastic response is not likely to evolve (Levin, 2009). Other traits that were measured, most notably flowering time in M. guttatus, experienced apparent direct selection but did not vary with soil moisture treatment. Interestingly, although no significant phenotypic plasticity was observed for flowering time in M. guttatus, this was the only trait (with the exception of flower number, our measure of fitness in the greenhouse) for which we found plasticity to have an additive genetic component, albeit marginally significant (Table 7). Thus, the more plastic genotypes of M. guttatus, were they to disperse into a drier habitat, might be expected to develop (begin flowering) more rapidly, which would probably be an adaptive response. Several previous studies have reported that M. guttatus flowered earlier in drier environments (Galloway, 1995; Hall and Willis, 2006; Murren et al., 2009), but a recent study by Wu et al. (2010) found no soil moisture effect on flowering time for several Mimulus species including M. guttatus. Nonetheless, phenotypic plasticity appears to be an important mechanism allowing M. guttatus to persist in the unpredictable environments in which it occurs. A wide-ranging study comparing the responses of multiple M. guttatus populations to soil moisture and chemistry, for example, concluded that plasticity is more important to the success of local populations than local adaptation (Murren et al., 2006).
Some of the treatment effects we observed may reflect life history trade-offs. For example, in the dry treatment, we observed significant selection gradients favouring rapid development (early flowering) in population DRC and no selection on plant size (biomass), whereas in the wet treatment we observed the reverse pattern. Other studies examining the influence of water availability on selection in M. guttatus have reported similar trade-offs both within (Galloway, 1995) and among (Hall and Willis, 2006; Lowry et al., 2008) populations, although in the latter case this appears to be linked to a chromosomal polymorphism (Lowry and Willis, 2010).
Conclusions
In summary, several of the patterns we observed, for example stronger evidence of direct selection for drought escape in dry environments than in wet environments, were consistent with the hypothesis that selfing may evolve indirectly via selection on drought adaptation. Other patterns, however, such as weak selection for WUE, were not consistent with this hypothesis. We found evidence supporting the idea that temporal or spatial variation in water availability may contribute toward the maintenance of variation in mating system and drought escape traits. Specifically, genetic correlations that may constrain the adaptive evolution of flower size or developmental rate were observed only when plants were grown in wet soil. Patterns in phenotypic plasticity suggest that divergence between taxa could have been facilitated by plasticity in flowering time and self-fertilization in response to changes in soil moisture. Overall, our results suggest that the hypothesis that self-fertilization may evolve in correlation with other life history traits is plausible. The role of environmental variation in the joint evolution of selfing and life history characteristics has received scant attention and warrants additional study.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank B. Bentley, J. P. Bergmann, C. McCarthy and B. Stone for assistance; the US National Forest Service for access to field sites; and C. M. Caruso, M. Ha, J. K. Kelly, S. J. Mazer and an anonymous reviewer for helpful manuscript comments. This work was supported by the Research Foundation of California State University, Chico and a US National Science Foundation Research Opportunity Award supplement to D.E.C. [grant number DEB-0735244].
LITERATURE CITED
- Angert AL. Growth and leaf physiology of monkeyflowers with different altitude ranges. Oecologia. 2006;148:183–194. doi: 10.1007/s00442-006-0361-z. [DOI] [PubMed] [Google Scholar]
- Armbruster WS, Mulder CPH, Baldwin BG, Kalisz S, Wessa B, Nute H. Comparative analysis of late floral development and mating-system evolution in tribe Collinsieae (Scrophulariacae) American Journal of Botany. 2002;89:37–49. doi: 10.3732/ajb.89.1.37. [DOI] [PubMed] [Google Scholar]
- Arntz AM, Delph LF. Pattern and process: evidence for the evolution of photosynthetic traits in natural populations. Oecologia. 2001;127:455–467. doi: 10.1007/s004420100650. [DOI] [PubMed] [Google Scholar]
- Arntz AM, DeLucia EH, Jordan N. Fitness effects of a photosynthetic mutation across contrasting environments. Journal of Evolutionary Biology. 2000;13:792–803. [Google Scholar]
- Arroyo MTK. Chiasma frequency evidence on the evolution of autogamy in Limnanthes floccosa (Limnanthaceae) Evolution. 1973;27:679–688. doi: 10.1111/j.1558-5646.1973.tb00715.x. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Olmstead RG. Redefining Phrymaceae: the placement of Mimulus, tribe Mimuleae, and Phryma. American Journal of Botany. 2002;89:1093–1102. doi: 10.3732/ajb.89.7.1093. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Schoenig SE, Whittall JB, Olmstead RG. Patterns of evolution in western North American Mimulus (Phrymaceae) American Journal of Botany. 2004;91:474–489. doi: 10.3732/ajb.91.3.474. [DOI] [PubMed] [Google Scholar]
- Bodbyl-Roels SA, Kelly JK. Rapid evolution caused by pollinator loss in Mimulus guttatus. Evolution. 2011 doi: 10.1111/j.1558-5646.2011.01326.x. (in press). doi:10.1111/j.1558-5646.2011.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD. Evolutionary significance of pheotypic plasticity in plants. Advances in Genetics. 1965;13:115–155. [Google Scholar]
- Carr DE, Fenster CB. Levels of genetic variation and covariation for Mimulus (Scrophulariaceae) floral traits. Heredity. 1994;72:606–618. [Google Scholar]
- Carr DE, Murphy JF, Eubanks MD. Genetic variation and covariation for resistance and tolerance to Cucumber mosaic virus in Mimulus guttatus (Phrymaceae): a test for costs and restraints. Heredity. 2006;96:29–38. doi: 10.1038/sj.hdy.6800743. [DOI] [PubMed] [Google Scholar]
- Caruso CM. Competition for pollination influences selection on floral traits of Ipomopsis aggregata. Evolution. 2000;54:1546–1557. doi: 10.1111/j.0014-3820.2000.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Charlesworth D. Evolution of plant breeding systems. Current Biology. 2006;16:R726–R735. doi: 10.1016/j.cub.2006.07.068. [DOI] [PubMed] [Google Scholar]
- Conner J. Density-dependent sexual selection in the fungus beetle, Bolitotherus cornutus. Evolution. 1989;43:1378–1386. doi: 10.1111/j.1558-5646.1989.tb02589.x. [DOI] [PubMed] [Google Scholar]
- Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP. Stable isotopes in plant ecology. Annual Review of Ecology and Systematics. 2002;33:507–559. [Google Scholar]
- Dole JA. Reproductive assurance mechansims in three taxa of the Mimulus guttatus complex (Scrophulariaceae) American Journal of Botany. 1992;79:650–659. [Google Scholar]
- Donovan LA, Dudley SA, Rosenthal DM, Ludwig F. Phenotypic selection on leaf water use efficiency and related ecophysiological traits for natural populations of desert sunflowers. Oecologia. 2007;152:13–25. doi: 10.1007/s00442-006-0627-5. [DOI] [PubMed] [Google Scholar]
- Donovan LA, Ludwig F, Rosenthal DM, Rieseberg LH, Dudley SA. Phenotypic selection on leaf ecophysiological traits in Helianthus. New Phytologist. 2009;183:868–879. doi: 10.1111/j.1469-8137.2009.02916.x. [DOI] [PubMed] [Google Scholar]
- Dorn LA, Pyle EH, Schmitt J. Plasticity to light cues and resources in Arabidopsis thaliana: testing for adaptive value and costs. Evolution. 2000;54:1982–1994. doi: 10.1111/j.0014-3820.2000.tb01242.x. [DOI] [PubMed] [Google Scholar]
- Dudash MR, Ritland K. Multiple paternity and self-fertilization in relation to floral age in Mimulus guttatus (Scrophulariaceae) American Journal of Botany. 1991;78:1746–1753. [Google Scholar]
- Dudley LS, Mazer SJ, Galusky P. The joint evolution of mating system, floral traits, and life history in Clarkia (Onagraceae): genetic constraints vs. independent evolution. Journal of Evolutionary Biology. 2007;20:2200–2218. doi: 10.1111/j.1420-9101.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- Dudley SA. Differing selection on plant physiological traits in response to environmental water availability: a test of adaptive hypotheses. Evolution. 1996;50:92–102. doi: 10.1111/j.1558-5646.1996.tb04475.x. [DOI] [PubMed] [Google Scholar]
- Falconer DS. Introduction to quantitative genetics. 2nd edn. London: Longman; 1981. [Google Scholar]
- Fenster CB, Diggle PK, Barrett SCH, Ritland K. The genetics of floral development differentiating two species of Mimulus. Heredity. 1995;74:258–266. [Google Scholar]
- Fishman L, Willis JH. Pollen limitation and natural selection on floral characters in the yellow monkeyflower, Mimulus guttatus. New Phytologist. 2007;177:802–810. doi: 10.1111/j.1469-8137.2007.02265.x. [DOI] [PubMed] [Google Scholar]
- Franks SJ, Sim S, Weis AE. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proceedings of the National Academy of Sciences, USA. 2007;104:1278–1282. doi: 10.1073/pnas.0608379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry JD. Estimation of genetic variances and covariances by restricted maximum likelihood using PROC MIXED. In: Saxton AM, editor. Genetic analysis of complex traits using SAS. Cary, NC: SAS Institute Inc; 2004. pp. 11–34. [Google Scholar]
- Galen C. Are flowers physiological sinks or faucets? Costs and correlates of water use by flowers of Polemonium viscosum. Oecologia. 1999;118:461–470. doi: 10.1007/s004420050749. [DOI] [PubMed] [Google Scholar]
- Galloway LF. Response to natural environmental heterogeneity: maternal effects and selection on life-history characters and plasticities in Mimulus guttatus. Evolution. 1995;49:1095–1107. doi: 10.1111/j.1558-5646.1995.tb04436.x. [DOI] [PubMed] [Google Scholar]
- Geber MA, Dawson TE. Genetic variation and covariation between leaf gas exchange, morphology, and development in Polygonum arenastrum, an annual plant. Oecologia. 1990;85:153–158. doi: 10.1007/BF00319396. [DOI] [PubMed] [Google Scholar]
- Goodwillie C, Kalisz S, Eckert CG. The evolutionary enigma of mixed mating in plants: occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology and Systematics. 2005;36:47–49. [Google Scholar]
- Hall MC, Willis JH. Divergent selection on flowering time contributes to local adaptation in Mimulus guttatus populations. Evolution. 2006;60:2466–2477. [PubMed] [Google Scholar]
- Heschel MS, Riginos C. Mechanisms of selection for drought stress tolerance and avoidance in Impatiens capensis (Balsaminaceae) American Journal of Botany. 2005;92:37–44. doi: 10.3732/ajb.92.1.37. [DOI] [PubMed] [Google Scholar]
- Ivey CT, Carr DE. Effects of herbivory and inbreeding on the pollinators and mating system of Mimulus guttatus (Phrymaceae) American Journal of Botany. 2005;92:1641–1649. doi: 10.3732/ajb.92.10.1641. [DOI] [PubMed] [Google Scholar]
- Ivey CT, Carr DE, Eubanks MD. Genetic variation and constraints on the evolution of defense against spittlebug (Philaenus spumarius) herbivory in Mimulus guttatus. Heredity. 2009;102:303–311. doi: 10.1038/hdy.2008.122. [DOI] [PubMed] [Google Scholar]
- Juenger T, Bergelson J. The evolution of compensation to herbivory in scarlet gilia, Ipomopsis aggregata: herbivore-imposed natural selection and the quantitative genetics of tolerance. Evolution. 2000;54:764–777. doi: 10.1111/j.0014-3820.2000.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Karron JD, Jackson RT, Thumser NN, Schlicht SL. Outcrossing rates of individual Mimulus ringens genets are correlated with anther–stigma separation. Heredity. 1997;79:365–370. [Google Scholar]
- Kelly JK, Holeski LM, Arathi HS. The genetic correlation between flower size and water-use efficiency in monkeyflowers. Evolutionary Ecology Research. 2008;10:147–152. [PMC free article] [PubMed] [Google Scholar]
- Kiang YT, Hamrick JL. Reproductive isolation in the M. guttatus–M. nasutus complex. American Midland Naturalist. 1978;100:269–276. [Google Scholar]
- van Kleunen M, Ritland K. Predicting evolution of floral traits associated with mating system in a natural plant population. Journal of Evolutionary Biology. 2004;17:1389–1399. doi: 10.1111/j.1420-9101.2004.00787.x. [DOI] [PubMed] [Google Scholar]
- Lambers H, Chapin FS, Pons TL. Plant physiological ecology. New York: Springer; 1998. [Google Scholar]
- Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Levin DA. Flowering phenology in relation to adaptive radiation. Systematic Botany. 2006;31:239–246. [Google Scholar]
- Levin DA. Flowering-time plasticity facilitates niche shifts in adjacent populations. New Phytologist. 2009;183:661–666. doi: 10.1111/j.1469-8137.2009.02889.x. [DOI] [PubMed] [Google Scholar]
- Levin DA. Environment-enhanced self-fertilitzation: implications for niche shifts in adjacent populations. Journal of Ecology. 2010;98:1276–1283. [Google Scholar]
- Lloyd D. Self- and cross-fertilization in plants. II. The selection of self-fertilization. International Journal of Plant Sciences. 1992;153:370–380. [Google Scholar]
- Lowry DB, Willis JH. A widespread chromosomal inversion polymorphism contributes to a major life-history transition, local adaptation, and reproductive isolation. PLoS Biology. 2010;8:e1000500. doi: 10.1371/journal.pbio.1000500. doi:10.1371/journal.pbio.1000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry DB, Rockwood RC, Willis JH. Ecological reproductive isolation of coast and inland races of Mimulus guttatus. Evolution. 2008;62:2196–2214. doi: 10.1111/j.1558-5646.2008.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Walsh B. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer; 1998. [Google Scholar]
- Macnair MR, Gardner M. The evolution of edaphic endemics. In: Howard DJ, Berlocher SH, editors. Endless forms – species and speciation. New York: Oxford University Press; 1998. pp. 157–171. [Google Scholar]
- Macnair MR, Macnair VE, Martin BE. Adaptive speciation in Mimulus: an ecological comparison of M. cupriphilus with its presumed progenitor, M. guttatus. New Phytologist. 1989;112:269–279. [Google Scholar]
- Martin NH, Willis JH. Ecological divergence associated with mating system causes nearly complete reproductive isolation between sympatric Mimulus species. Evolution. 2007;61:68–82. doi: 10.1111/j.1558-5646.2007.00006.x. [DOI] [PubMed] [Google Scholar]
- Mazer SJ, Paz H, Bell MD. Life history, floral development, and mating system in Clarkia xantiana (Onagraceae): do foral and whole-plant rates of development evolve independently? American Journal of Botany. 2004;91:2041–2050. doi: 10.3732/ajb.91.12.2041. [DOI] [PubMed] [Google Scholar]
- Mazer SJ, Dudley LS, Hove AA, Emms SK, Verhoeven AS. Physiological performance in Clarkia sister taxa with contrasting mating systems: do early-flowering autogamous taxa avoid water stress relative to their pollinator-dependent counterparts? International Journal of Plant Sciences. 2010;171:1029–1047. [Google Scholar]
- McKay JK, Richards JH, Mitchell-Olds T. Genetics of drought adaptation in Arabidopsis thaliana: I. Pleiotropy contributes to genetic correlations among ecological traits. Molecular Ecology. 2003;12:1137–1151. doi: 10.1046/j.1365-294x.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- Mitchell-Olds T, Shaw RG. Regression analysis of natural selection: statistical inference and biological interpretation. Evolution. 1987;41:1149–1161. doi: 10.1111/j.1558-5646.1987.tb02457.x. [DOI] [PubMed] [Google Scholar]
- Moeller DA. Geographic structure of pollinator communities, reproductive assurance, and the evolution of self-pollination. Ecology. 2006;87:1510–1522. doi: 10.1890/0012-9658(2006)87[1510:gsopcr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Murren CJ, Douglass L, Gibson A, Dudash MR. Individual and combined effects of Ca/Mg ratio and water on trait expression in Mimulus guttatus. Ecology. 2006;87:2591–2602. doi: 10.1890/0012-9658(2006)87[2591:iaceom]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Murren CJ, Chang CC, Dudash MR. Patterns of selection of two North American native and nonnative populations of monkeyflower (Phrymaceae) New Phytologist. 2009;183:691–701. doi: 10.1111/j.1469-8137.2009.02928.x. [DOI] [PubMed] [Google Scholar]
- Pujol B, Zhou S-R, Vilas JS, Pannell JR. Reduced inbreeding depression after species range expansion. Proceedings of the National Academy of Sciences, USA. 2009;106:15379–15383. doi: 10.1073/pnas.0902257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausher MD. The measurement of selection on quantitative traits: biases due to environmental covariances between traits and fitness. Evolution. 1992;46:616–626. doi: 10.1111/j.1558-5646.1992.tb02070.x. [DOI] [PubMed] [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. Leaf age and season influence the relationships between leaf nitrogen, leaf mass per area and photosynthesis in maple and oak trees. Plant, Cell and Environment. 1991;14:251–259. [Google Scholar]
- Ritland C, Ritland K. Variation of sex allocation among eight taxa of the Mimulus guttatus species complex (Scrophulariaceae) American Journal of Botany. 1989;76:1731–1739. [Google Scholar]
- Robertson AW, Diaz A, MacNair MR. The quantitative genetics of floral characters in Mimulus guttatus. Heredity. 1994;72:300–311. [Google Scholar]
- SAS Institute. SAS version 9·1. Cary, NC: SAS Institute, Inc; 2003. [Google Scholar]
- Schoen DJ. The breeding system of Gilia achilleifolia: variation in floral characteristics and outcrossing rate. Evolution. 1982;36:352–360. doi: 10.1111/j.1558-5646.1982.tb05051.x. [DOI] [PubMed] [Google Scholar]
- Self SG, Liang KY. Asymptotic properties of maximum likelihood estimators and likelihood ratio tests under nonstandard conditions. Journal of the American Statistical Association. 1987;82:605–610. [Google Scholar]
- Sgrò CM, Hoffmann AA. Genetic correlations, tradeoffs and environmental variation. Heredity. 2004;93:241–248. doi: 10.1038/sj.hdy.6800532. [DOI] [PubMed] [Google Scholar]
- Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- Shaw RG. Maximum-likelihood approaches applied to quantitative genetics of natural populations. Evolution. 1987;41:812–826. doi: 10.1111/j.1558-5646.1987.tb05855.x. [DOI] [PubMed] [Google Scholar]
- Sherrard ME, Maherali H. The adaptive significance of drought escape in Avena barbata, an annual grass. Evolution. 2006;60:2478–2489. [PubMed] [Google Scholar]
- Sinclair TR, Horie T. Leaf nitrogen, photosynthesis, and crop radiation use efficiency: a review. Crop Science. 1989;29:90–98. [Google Scholar]
- Solbrig OT, Rollins RC. The evolution of autogamy in species of the mustard genus Leavenworthia. Evolution. 1977;31:265–281. doi: 10.1111/j.1558-5646.1977.tb01007.x. [DOI] [PubMed] [Google Scholar]
- Stanton ML, Roy BA, Theide DA. Evolution in stressful environments. I. Phenotypic variability, phenotypic selection, and response to selection in five distinct environmental stresses. Evolution. 2000;54:93–111. doi: 10.1111/j.0014-3820.2000.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Stebbins GL. Flowering plants: evolution above the species level. Cambridge, MA: Belknap; 1974. [Google Scholar]
- Sultan SE. Phenotypic plasticity for plant development, function and life history. Trends in Plant Science. 2000;5:537–542. doi: 10.1016/s1360-1385(00)01797-0. [DOI] [PubMed] [Google Scholar]
- Sweigert AL, Willis JH. Patterns of nucleotide diversity in two species of Mimulus are affected by mating system and asymmetric introgression. Evolution. 2003;57:2490–2506. doi: 10.1111/j.0014-3820.2003.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Uyenoyama MK, Holsinger KE, Waller DM. Ecological and genetic factors directing the evolution of self-fertilization. Oxford Survey of Evolutionary Biology. 1993;9:327–381. [Google Scholar]
- Vickery RK., Jr Case studies in the evolution of species complexes in Mimulus. Evolutionary Biology. 1978;11:405–507. [Google Scholar]
- Vickery RK., Jr Remarkable waxing, waning, and wandering of populations of Mimulus guttatus: an unexpected example of global warming. Great Basin Naturalist. 1999;59:112–126. [Google Scholar]
- Willis JH. Partial self-fertilization and inbreeding depression in two populations of Mimulus guttatus. Heredity. 1993;71:145–154. [Google Scholar]
- Wright IJ, Reich PB, Westoby M. Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Functional Ecology. 2001;15:423–434. [Google Scholar]
- Wright IJ, Reich PB, Westoby M. Least-cost input mixtures of water and nitrogen for photosynthesis. American Naturalist. 2003;161:98–111. doi: 10.1086/344920. [DOI] [PubMed] [Google Scholar]
- Wu CA, Lowry DB, Cooley AM, Wright KM, Lee YW, Willis JH. Mimulus is an emerging model system for the integration of ecological and genomic studies. Heredity. 2008;100:220–230. doi: 10.1038/sj.hdy.6801018. [DOI] [PubMed] [Google Scholar]
- Wu CA, Lowry DB, Nutter LI, Willis JH. Natural variation for drought-response traits in the Mimulus guttatus species complex. Oecologia. 2010;162:23–33. doi: 10.1007/s00442-009-1448-0. [DOI] [PubMed] [Google Scholar]
- Wyatt R. Phylogenetic aspects of the evolution of self-pollination. In: Gottlieb LD, Jain SK, editors. Plant evolutionary biology. New York: Chapman and Hall; 1988. pp. 109–131. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.