Abstract
The critical closing pressure (Pcrit) is the airway pressure at which the airway collapses and reflects the anatomical contribution to the genesis of obstructive sleep apnea. Pcrit is usually determined during non-rapid eye movement sleep at night, but has been determined under midazolam sedation during the day in the absence of sleep stage monitoring. Indeed, little is known about the effects of midazolam on sleep architecture. Moreover, deeper sedation with midazolam can decrease upper airway muscle activity and increase collapsibility compared with natural sleep. Pcrit under sedation has not been systematically compared with the usual method performed during natural sleep. Therefore, this study aimed to test the hypothesis that Pcrit following low doses of midazolam during the day would be comparable to Pcrit measured during natural sleep in the same patient. Fifteen men (age 54 ± 10 yr, body mass index 30 ± 4 kg/m2) with obstructive sleep apnea underwent a baseline standard overnight polysomnogram (apnea-hypopnea index 38 ± 22 events/h, range: 8–66 events/h), and Pcrit was determined during natural sleep and following midazolam. Sleep induction was obtained with low doses of midazolam (2.4 mg, range 2.0–4.4 mg), and sleep architecture was comparable to natural sleep. Natural sleep and induced sleep Pcrit were similar (−0.82 ± −3.44 and −0.97 ± 3.21 cmH2O, P = 0.663) and closely associated (intraclass correlation coefficient = 0.92; 95% confidence interval, 0.78–0.97, P < 0.001). Natural and midazolam-induced Pcrit correlated with obstructive sleep apnea severity, indicating that both Pcrit measures provided meaningful physiological information. Pcrit determined during the day with sleep induction is similar to natural overnight sleep and is a valid alternative approach in which to determine Pcrit.
Keywords: obstructive sleep apnea, sleep induction, midazolam
obstructive sleep apnea (OSA) is characterized by recurrent partial or complete upper airway collapse during sleep (8). OSA is a common major health care problem associated with multiple daytime symptoms and increased morbidity and mortality due to cardiovascular disease (6, 25). The reasons for upper airway collapse during sleep in patients with OSA are multiple and not completely understood. Upper airway collapse depends on anatomical characteristics, ventilatory control instability during sleep, and neuromuscular control of upper airway caliber (8). The anatomical characteristics of the upper airway play a pivotal role in the genesis of OSA and are dependent on the interaction between bony and soft tissue structures of the upper airway (37).
A Starling resistor model that consists of a collapsible tube (pharynx) interposed by two rigid segments (nose and larynx) was developed to help explain the pressure and flow relationships in the upper airway (31). The critical closing pressure (Pcrit) can be determined using this model and is defined as the pressure at which the pharyngeal airway collapses. Pcrit measurement requires the simultaneous recording of nasal pressure and flow in response to transient pressure reductions from a level of continuous positive airway pressure (CPAP) sufficient to eliminate flow limitation. Pcrit assesses the mechanical properties of the upper airway and its surrounding tissues, and it is primarily thought to reflect anatomical components, although neuromuscular compensation mechanisms may also contribute to Pcrit (40). Pcrit is extensively used to study OSA pathogenesis, provides insight into the spectrum of the disease, and may be useful in defining which patients are likely to respond to certain interventions (11, 34). However, Pcrit determination has several technical challenges due to the necessity of overnight investigators trained in online recognition of sleep staging and airflow patterns. Pcrit assessment has also been performed during deeper sedation or general anesthesia (2, 3, 7, 17, 24). However, the physiological meaning of Pcrit assessed during deeper sedation or anesthesia may be different from Pcrit determined during natural sleep (7). Midazolam and propofol have been used to perform pharyngeal endoscopy during the day, with a view toward providing pathophysiological insight and predicting treatment success with approaches such as upper airway surgery (12, 20). However, endoscopy requires higher sedative doses than would otherwise be necessary to induce sleep. Increasing anesthetic depth has been shown to be associated with decreasing airway muscle activity and increasing upper airway collapsibility (15). Thus upper airway mechanics under sedation may mimic rapid eye movement (REM) sleep in which neuromuscular activity is minimal (9). In contrast, sleep induction with minimum doses of midazolam (hypnotic dose rather than anesthetic dose) may preserve upper airway muscle activity to a comparable level observed during natural sleep and represent a window of opportunity for the determination of Pcrit. This method may, therefore, be an alternative approach to assess upper airway collapsibility during the daytime and may be combined with other useful techniques, such as airway endoscopy, which is becoming more readily utilized in clinical sleep medicine and research (4, 12, 20).
Some studies have reported conflicting effects of multiple sedatives given individually or in combination on sleep architecture in patients requiring sedation in the intensive care unit (ICU) (1, 5, 10, 14). However, few investigators have assessed the individual effect of sedation with benzodiazepines on sleep architecture (29, 33). For instance, in a sleep endoscopy study that used diazepam sedation, significant decreases in slow wave and REM were reported (33). Midazolam during continuous and deep sedation in the ICU was reported to suppress slow-wave and REM sleep (29). In contrast, a decrease in stages 1 and 3 and an increase in stage 2 after oral midazolam taken at night was reported (26). Therefore, sleep architecture effects during midazolam-induced sleep may be different than those effects during deeper sedation.
Sleep architecture after small doses of midazolam has not been compared with sleep architecture during natural sleep. Pcrit obtained during the day after midazolam has not been compared systematically to Pcrit obtained during natural sleep. Moreover, Pcrit may be influenced by the sleep stage, reflecting stage-dependent pharyngeal muscle activity. For instance, pharyngeal muscle activity is significantly reduced during REM compared with non-REM (9). Therefore, the purpose of this study was, first, to assess sleep architecture during sleep induced with minimal and carefully titrated doses of midazolam using online polysomnography (PSG) monitoring and compare with natural sleep, and, second, to validate Pcrit determination during the day after sleep induction with midazolam. We hypothesized that, if sleep architecture after sleep induction is similar to natural sleep, the mechanical properties assessed by the passive Pcrit method during induced sleep would be preserved compared with natural sleep. Thus sleep induction would yield an effective alternative technique to assess airway mechanics. To this end, sleep architecture and Pcrit during natural and induced sleep were compared.
METHODS
Subjects.
Male subjects under investigation or with a recent diagnosis of OSA, aged between 18 and 70 yr, were invited to participate. All subjects gave written, informed consent before study entry, which was approved by the Hospital das Clínicas Ethics Committee. All subjects underwent a detailed clinical evaluation that included height, weight, waist, and neck circumference measurements. Subjects with body mass indexes >40 kg/m2, nasal obstruction, or major craniofacial abnormalities were excluded.
Sleep studies.
Three separate sleep studies were performed and described in detail below: an overnight baseline diagnostic study, a natural sleep Pcrit study during the night, and a midazolam-induced sleep Pcrit study during the morning following the baseline overnight sleep study. The diagnostic and natural sleep Pcrit studies were performed 1 wk apart.
Diagnostic PSG.
All participants underwent a baseline standard overnight polysomnogram (Alice 5, Philips Respironics, Murrysville, PA) at the commencement of the study to determine sleep characteristics and quantify apnea severity. Monitoring included electroencephalography, electrooculography, electromyography, oximetry, measurements of airflow (oronasal thermistor and pressure cannula), and measurements of rib cage and abdominal movements during breathing, as previously described (6). Apnea was defined as complete cessation of airflow (thermistor) for at least 10 s. Hypopnea was defined as a >50% reduction in airflow (nasal pressure) for at least 10 s, associated with oxygen desaturation of 3% or arousal. The apnea-hypopnea index (AHI) was calculated as the total number of respiratory events (apneas plus hypopneas) per hour of sleep.
Sleep induction.
Sleep induction was performed in the sleep laboratory, starting at 8:00 AM following the overnight diagnostic PSG. Patients wore a nasal mask, and sleep was monitored using all PSG channels, except for nasal pressure and thermistor. A peripheral vein was cannulated and maintained with a continuous infusion of saline solution. Midazolam (diluted in a saline solution with a concentration of 1 mg/10 ml) was infused slowly when necessary, as follows. Sleep induction started with the infusion of 5 ml of the solution over 5 min, which was repeated as needed, until sleep was detected through online PSG monitoring. Midazolam infusion was restarted for sleep maintenance only if the patient arose and was not able to fall asleep again for 10 min. The procedures were done by a pulmonologist and a registered nurse trained in upper airway management. Intubation equipment, a defibrillator, and a benzodiazepine antagonist (flumazenil) were readily available, but never required.
Pcrit determination.
Before the start of the study, our investigators underwent several weeks of intensive training at Harvard Medical School on the theory and practice of flow limitation, airway mechanics, and Pcrit measurements. The Brazil and Boston investigators maintained communication throughout the study to ensure robust data acquisition, analyses, and synthesis. During Pcrit determinations, all polysomnographic channels used in the diagnostic PSG were recorded, except for nasal pressure and thermistor. Pcrit measurements were performed with patients in the supine position using the same protocol during both induced sleep and natural sleep studies. Subjects were fitted with a nasal mask, attached to a heated pneumotachograph (model 3700A, Hans Rudolf, Kansas City, MO) and a differential pressure transducer (Validyne, Northbridge, CA) for measurement of inspiratory flow. Mask pressure and flow were recorded on a personal computer using an analog-to-digital converter (National Instruments, Austin, TX) and data acquisition software (Labview, National Instruments, Austin, TX). A modified CPAP device (Philips Respironics, Murrysville, PA) that could deliver both positive and negative airway pressure was connected to the mask. After sleep onset, airway pressure was increased to abolish flow limitation. This level was used as the holding pressure for each patient. Once stable stage 2 or 3 sleep was observed at the holding pressure, CPAP was abruptly reduced by 1–2 cmH2O during expiration and was held at this level for five breaths (Fig. 1). The pressure was then returned to the holding pressure for 1 min before being dropped a further 1–2 cmH2O for another five breaths. This process of progressively dropping CPAP continued until obstructive apnea occurred. If arousal occurred during any pressure drop, the CPAP was returned to the holding pressure until stable sleep resumed. The entire procedure of progressively dropping CPAP until obstruction was repeated three to five times in each subject. Data were analyzed using custom-designed software (Matlab, The MathWorks, Natick, MA) to determine the peak inspiratory flow (V̇imax) for breaths 3–5 during the pressure drop. Each of these breaths was assessed for the presence or absence of inspiratory flow limitation similar to previously established techniques (16, 19, 28, 31). Breaths associated with arousal were excluded from the analysis. Nasal pressure for flow-limited breaths was plotted against V̇imax. Pcrit was determined as the zero-flow intercept from the linear regression of V̇imax vs. nasal pressure, as previously described (Fig. 2) (11, 18, 30, 35). Pcrit was determined both during natural sleep during a full overnight PSG and during the day during induced sleep in each subject.
Fig. 1.

Nasal pressure (Pn) and flow of a representative pressure drop for 5 breaths.
Fig. 2.

Flow [peak inspiratory flow (V̇imax)] vs. Pn and linear regression of a representative critical closing pressure (Pcrit) determination.
Statistical analysis.
Fourteen subjects were necessary to obtain a power of 80% and α of 0.05, considering a minimum Pcrit difference of 1 cmH2O and a standard deviation of the mean Pcrit difference between natural and induced sleep of 1.2 cmH2O (the latter determined in a pilot study) using a two-tailed test. Data were expressed as means ± SD or median (interquartile range), where appropriate. Natural sleep and induced sleep Pcrit were compared using a Bland-Altman plot, as well as a paired Student's T-test. The intraclass correlation coefficient was calculated to evaluate the agreement between both Pcrit measurements. Pearson correlation analyses were performed to assess the association of polysomnographic, anthropometric, and Pcrit variables. Sleep characteristics during natural and induced sleep Pcrit studies were compared with a paired T-test or a Wilcoxon matched-pairs signed-ranks test, where appropriate. A multiple linear regression model was used to assess the independent predictors of AHI variability.
RESULTS
Clinical, anthropometric, and polysomnographic variables of the study group are shown in Table 1. Of the 15 patients, 13 were Japanese descendants, and 2 were Caucasians. A sleeplike state was induced in all patients with a median midazolam dose of 1 mg (0.6–1.2 mg). The mean time between the first and second midazolam infusion was 71 ± 41 min, and the total midazolam dose used during the entire procedure was 2.4 mg (2.0–4.4 mg). The hypnograms of one patient obtained during induced sleep and during natural sleep Pcrit determination are shown in Fig. 3, A and B, respectively. Sleep characteristics of the study group during natural and induced sleep Pcrit studies are shown in Table 2. Induced sleep was able to reproduce all sleep stages observed during natural sleep.
Table 1.
Anthropometric, clinical, and sleep characteristics during the baseline diagnostic study
| N | 15 |
|---|---|
| Age, yr | 54 ± 10 (42−69) |
| BMI, kg/m2 | 30 ± 4 (24−39) |
| Waist circumference, cm | 103 ± 10 (91−124) |
| Neck circumference, cm | 42 ± 4 (37−49) |
| Epworth Sleepiness Scale | 9 ± 5 |
| Hypertension, % | 73 |
| Diabetes, % | 27 |
| Dyslipidemia, % | 40 |
| Coronary artery disease, % | 13 |
| Sleep latency, min | 9 ± 3 |
| Sleep efficiency, % | 80 ± 12 |
| Total sleep time, min | 345 ± 87 |
| S1, % | 8 ± 3 |
| S2, % | 59 ± 8 |
| S3, % | 13 ± 7 |
| REM, % | 16 ± 7 |
| AHI, events/h | 38 ± 22 (9−83) |
| AI, events/h | 19 ± 23 (0−73) |
| HI, events/h | 19 ± 11 (9−46) |
| Sat O2 min, % | 78 ± 11 (60−92) |
Values are means ± SD or median (with interquartile range in parentheses); N, no. of subjects. BMI, body mass index; S1–S3, stages 1−3; REM, rapid eye movement; AHI, apnea-hypopnea index; AI, obstructive apnea index; HI, hypopnea index; Sat O2 min, minimum oxygen saturation.
Fig. 3.

A: example of a hypnogram from a Pcrit study performed after sleep induction. The arrows indicate the timing of midazolam intermittent infusions in this patient. B: example of a hypnogram from a Pcrit study performed during natural sleep at nightime (same patient as A). W, wakefulness; REM, rapid eye movement; N1–N3, non-REM stages 1–3.
Table 2.
Sleep architecture of natural and induced sleep Pcrit studies
| Natural Sleep | Induced Sleep | P | |
|---|---|---|---|
| Sleep latency, min | 6 (4−15) | 9 (4−11) | 0.502 |
| Sleep efficiency, % | 78 ± 10 | 78 ± 12 | 0.844 |
| Total sleep time, min | 204 ± 67 | 139 ± 38 | 0.004 |
| S1, % | 11 ± 5 | 21 ± 8 | 0.001 |
| S2, % | 62 ± 13 | 56 ± 10 | 0.112 |
| S3, % | 20 ± 11 | 16 ± 14 | 0.245 |
| REM, % | 9 ± 8 | 8 ± 8 | 0.712 |
| Arousals, no./h | 25 ± 17 | 22 ± 17 | 0.518 |
| No. of series of pressure drops | 5.1 ± 1.1 | 4.4 ± 1.2 | 0.028 |
| No. of pressure drops | 44 ± 11 | 36 ± 9 | 0.004 |
| CPAP holding level, cmH2O | 9 ± 3 | 10 ± 2 | 0.350 |
| Pcrit, cmH2O | −0.82 ± 3.44 | −0.97 ± 3.21 | 0.663 |
Values are means ± SD (with interquartile range in parentheses). CPAP holding level, continuous positive airway pressure required to abolish airflow limitation; Pcrit, critical closing pressure.
Pcrit and the CPAP holding level determined during natural and induced sleep were similar (Table 2). The Bland-Altman plot (Fig. 4) reveals a good level of agreement between measurements (mean difference 0.15 ± 1.32 cmH2O) in the entire range of Pcrit values. Intraclass correlation coefficient was 0.92 (95% confidence interval 0.78–0.97), P < 0.001.
Fig. 4.

Bland-Altman plot comparing natural and induced sleep Pcrit.
Table 3 reports the correlation coefficients between AHI, anthropometric and polysomnographic variables, and Pcrit (natural and induced sleep). Natural and induced sleep Pcrit were correlated with the AHI and minimum oxygen saturation (Figs. 5 and 6, respectively). The correlation coefficients for natural and induced sleep Pcrit vs. the apnea index alone tended to be higher compared with the Pcrit vs. AHI correlation coefficients (Table 3). Multiple linear regression analysis revealed induced sleep Pcrit, waist circumference, and age as independent variables associated with AHI (Table 4).
Table 3.
Pearson correlation coefficients of the relationships between AHI, natural sleep, and sleep-induced Pcrit and anthropometric and polysomnographic variables
| AHI, events/h |
Natural Sleep Pcrit, cmH2O |
Induced Sleep Pcrit, cmH2O |
||||
|---|---|---|---|---|---|---|
| R | P | R | P | r | P | |
| Age, yr | 0.481 | 0.069 | −0.126 | 0.654 | −0.052 | 0.853 |
| BMI, kg/m2 | 0.420 | 0.119 | 0.469 | 0.078 | 0.329 | 0.231 |
| Waist circumference, cm | 0.420 | 0.010 | 0.470 | 0.077 | 0.361 | 0.187 |
| Neck circumference, cm | 0.499 | 0.058 | 0.501 | 0.057 | 0.426 | 0.114 |
| AHI, events/h | 0.592 | 0.020 | 0.576 | 0.025 | ||
| AI, events/h | 0.881 | <0.001 | 0.664 | 0.007 | 0.715 | 0.003 |
| HI, events/h | 0.134 | 0.634 | −0.256 | 0.356 | −0.384 | 0.157 |
| Sat O2 min, % | −0.485 | 0.067 | −0.810 | <0.001 | −0.757 | <0.001 |
Values in bold, P < 0.05.
Fig. 5.
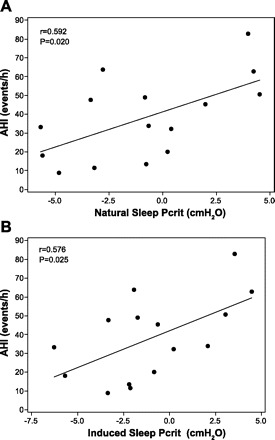
A: relationship between natural sleep Pcrit and apnea-hypopnea index (AHI), according to the predominance of respiratory event. B: relationship between induced sleep Pcrit and AHI, according to the predominance of respiratory event.
Fig. 6.

A: relationship between natural sleep Pcrit and minimum oxygen saturation, according to the predominance of respiratory event. B: relationship between induced sleep Pcrit and minimum oxygen saturation, according to the predominance of respiratory event.
Table 4.
Multiple regression analysis of the variables associated with AHI
| B Coefficient | r2 | P | |
|---|---|---|---|
| Induced sleep Pcrit, cmH2O | 2.95 | 0.332 | 0.015 |
| Waist circumference, cm | 1.07 | 0.216 | 0.010 |
| Age, yr | 1.04 | 0.237 | 0.008 |
| Model | 0.785 | 0.001 |
Value in bold, P < 0.05.
DISCUSSION
This study conveys new information regarding Pcrit determination during drug-induced sleep. First, sleep induction using midazolam in small doses induced a state with similar appearance (architecture) to natural sleep with no observed safety concerns. Second, we showed that Pcrit determined after midazolam is comparable to Pcrit determined during natural overnight sleep. Last, both natural and induced sleep Pcrit were highly associated with polysomnographically determined AHI and anthropometric variables.
Previous studies used midazolam sedation to allow Pcrit measurements (2, 3, 24). However, our study has several important methodological differences. These prior studies evaluated only normal subjects, whereas our study evaluated a clinically relevant population with a wide range of OSA severity and Pcrit levels. In addition, the mean doses of midazolam were significantly higher in these previous studies than in the present study (4–10 mg vs. 2.4 mg, respectively). It is possible that the midazolam dose requirements in the present study were relatively low in part due to the diagnosis of OSA, since we reported a lower dose for sleep induction among OSA patients compared with normal subjects (13). In addition, patients slept with nasal CPAP to determine Pcrit, helping to protect the patient's airway, facilitating sleep. However, deeper sedation was induced in the previous Pcrit studies performed under sedation (2, 3, 24). Moreover, in these studies, the pressure drops required to induce airflow limitation were done regardless of the EEG and sleep stage. Sleep architecture and sleep stages were also not reported, raising questions as to the exact effects of higher midazolam doses on sleep architecture. Finally, we are not aware of any study that compared Pcrit during midazolam-induced sleep with Pcrit determined during natural sleep. In a recent study using midazolam sedation at mean total doses of 6.2 mg, active Pcrit (i.e., Pcrit determined after a few minutes of partial flow limitation to allow an opportunity for upper airway dilator muscle recruitment) was lower than “passive” Pcrit (i.e., Pcrit used in the present protocol). This finding is similar to what is observed during natural sleep among normal subjects, but not among OSA patients who have blunted neuromuscular response (32). These results suggest that neuromuscular compensation to airway obstruction is preserved during midazolam sedation among normal subjects (2). However, the effect of midazolam on neuromuscular responses among OSA patients is not known. Morrison et al. (27) showed that the pharyngeal collapse pressure directly observed by endoscopy was similar after diazepam sedation compared with natural sleep. We have shown good agreement between AHI and minimum oxygen saturation obtained during standard overnight PSG and after midazolam sleep induction (13). These results suggest that sleep induction with relatively small doses of diazepam and midazolam does not increase pharyngeal collapsibility and OSA severity. In contrast, Pcrit determined during general anesthesia in normal subjects is significantly higher than when determined during natural sleep or sedation (7). This observation suggests a significant decrease or elimination of neuromuscular mechanisms involved in upper airway patency during general anesthesia. We extended the previous studies by showing comparable Pcrit values obtained during natural and induced sleep using doses of midazolam that were lower than those previously reported (2, 3, 20). Bland-Altman plot revealed good agreement between measurements in the entire range of Pcrit values. These findings were very similar to the variability of the “passive” Pcrit between nights reported by Kirkness et al. (22). The high intraclass correlation coefficient also strengthens the association between both Pcrit measurement approaches. The lack of significant effect of midazolam on Pcrit found in the present study supports the safety of mild sedation frequently used in clinical practice (23). In addition, midazolam sleep induction may be an alternative approach to determine Pcrit during the day for several reasons. First, we were able to yield similar Pcrit values with the induced sleep Pcrit technique to the standard Pcrit technique in a shorter duration and using fewer drops. Second, standard Pcrit determinations involve many challenges that require experienced investigators or well-trained technicians to perform laborious overnight sleep studies who could be more readily available during the day. Third, Pcrit is frequently assessed under several experimental conditions that usually require more sophisticated equipment and additional experimented investigators and, therefore, could be more easily performed during the daytime. The performance of procedures, e.g., bronchoscopy, generally happen during the daytime, and thus the fact that pharyngeal mechanics under light sedation predict sleeping PCrit may be clinically important (e.g., determining previously unrecognized OSA) and could be performed in conjunction with other clinical procedures.
Further stressing the physiological significance of Pcrit determination obtained during induced sleep, we showed strong correlations among Pcrit and AHI, apnea index, and minimum oxygen saturation. These associations were very similar for both natural and induced sleep Pcrit and are in line with previous studies that only evaluated Pcrit during natural sleep (21, 36, 39). Interestingly, the hypopnea index was not associated with both Pcrit measurements. This finding suggests that OSA pathophysiological traits other than anatomy, for example ventilatory control instability, may be more influential in contributing to the hypopnea index, although this assumption remains to be tested. Sleep-induced Pcrit, waist circumference, and age were all independent predictors of AHI in a multiple linear regression model and could explain 79% of the AHI variability. Very similar results were obtained when natural sleep Pcrit was used in the model. These findings support the independent role of pharyngeal collapsibility determined by Pcrit during either natural or midazolam-induced sleep-like state on the genesis of OSA and provide support for the validity of Pcrit determination during induced sleep.
We used a unique approach to induce sleep in the present study that was based on small doses of midazolam and online recognition of sleep onset using standard sleep staging criteria. After sleep onset, we could observe a normal sequence of sleep stages in the majority of the patients without the necessity of continuous infusion of the drug. Indeed, very small doses of midazolam were required to induce sleep (2.4 mg median total dose). Most of the patients (n = 13 or 80%) had both stage 3 and REM after sleep induction and Pcrit was obtained relatively quickly. Midazolam may induce a dose-dependent decrease on EEG high-frequency activity,(38) raising questions on the reliability of the standard staging criteria to assess sleep under deep sedation (1). In addition, the pressure drops required during Pcrit assessments could interfere with sleep architecture. Having these potential limitations in mind, our patients presented a higher percentage of stage 1 sleep, but overall a similar sleep stage distribution to the studies performed during natural sleep at night. Oto et al. (29) recently described small quantities of slow-wave and REM sleep among patients with intermittent sedation with midazolam in the ICU with total suppression of slow-wave and REM sleep during deep and continuous sedation. In a study of sleep endoscopy that used relatively high doses of diazepam during the procedure (mean = 10.3 mg), the authors only found short periods of stage 3 and REM in 34% of the 50 patients studied (33). These results suggest a dose-dependent effect of benzodiazepines on sleep architecture and are in line with our observations of relatively preserved sleep architecture after sleep induction with small doses of midazolam.
Our study has several limitations. First, sleep induction was performed in a controlled setting by physicians expert in sedation. Second, the use of standard sleep staging criteria may not be precise after midazolam sedation. However, we used low doses of midazolam minimizing EEG artifacts previously reported during deep sedation with midazolam sedation in the ICU (1, 38). Nonetheless, future studies that perform detailed EEG analysis in the absence of upper airway collapsibility measures are required to further investigate the role of midazolam on sleep architecture. Third, the number of patients studied is relatively small. On the other hand, the strong correlations observed points to the consistency of our results. Fourth, we studied only men, and only a few had mild sleep apnea. Thus our conclusions are limited to the population studied and likely differ in healthy individuals without sleep apnea. Last, we studied predominantly Japanese descendents, making these results not necessarily applicable to other races. Conversely, this approach allowed us to reduce the variability introduced by different ethnicities. Further work regarding the generalizability of our results would be useful.
In conclusion, we showed that, in a controlled setting, sleep architecture after low doses of midazolam is similar to natural sleep. Pcrit determined during the day after induced sleep is comparable to the standard Pcrit protocol determined during natural sleep. Thus this novel approach appears to be a valid alternative to determine upper airway collapsibility in patients with OSA.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
P.R.G., D.J.E., A.M., and G.L.-F. conception and design of research; P.R.G., M.G.G., N.J.D., and H.T.M. performed experiments; P.R.G., D.J.E., A.M., and G.L.-F. analyzed data; P.R.G., D.J.E., A.M., and G.L.-F. interpreted results of experiments; P.R.G. prepared figures; P.R.G. and D.J.E. drafted manuscript; P.R.G., D.J.E., M.G.G., N.J.D., H.T.M., A.M., and G.L.-F. edited and revised manuscript; P.R.G., D.J.E., M.G.G., N.J.D., H.T.M., A.M., and G.L.-F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Marcelo Cirelli for the assistance in the development of a Pcrit data analysis software.
REFERENCES
- 1. Ambrogio C, Koebnick J, Quan S, Ranieri M, Parthasarathy S. Assessment of sleep in ventilator-supported critically III patients. Sleep 31: 1559–1568, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ayuse T, Hoshino Y, Kurata S, Schneider H, Kirkness J, Patil S, Schwartz A, Oi K. The effect of gender on compensatory neuromuscular response to upper airway obstruction in normal subjects under midazolam general anesthesia. Anesth Analg 109: 1209–1218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ayuse T, Inazawa T, Kurata S, Okayasu I, Sakamoto E, Oi K, Schneider H, Schwartz AR. Mouth-opening increases upper-airway collapsibility without changing resistance during midazolam sedation. J Dent Res 83: 718–722, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Chan AS, Lee RW, Srinivasan VK, Darendeliler MA, Grunstein RR, Cistulli PA. Nasopharyngoscopic evaluation of oral appliance therapy for obstructive sleep apnoea. Eur Respir J 35: 836–842, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ. Sleep in critically ill patients requiring mechanical ventilation. Chest 117: 809–818, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Drager L, Bortolotto L, Figueiredo A, Krieger E, Lorenzi-Filho G. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med 176: 706–712, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Eastwood PR, Szollosi I, Platt PR, Hillman DR. Comparison of upper airway collapse during general anaesthesia and sleep. Lancet 359: 1207–1209, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Eckert D, Malhotra A, Jordan A. Mechanisms of apnea. Prog Cardiovasc Dis 51: 313–323, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eckert D, Malhotra A, Lo Y, White D, Jordan A. The influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest 135: 957–964, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med 163: 451–457, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis 143: 1300–1303, 1991 [DOI] [PubMed] [Google Scholar]
- 12. Gregorio M, Jacomelli M, Figueiredo A, Cahali M, Pedreira W, Jr, Lorenzi-Filho G. Evaluation of airway obstruction by nasopharyngoscopy: comparison of the Muller maneuver vs. induced sleep. Braz J Otorhinolaryngol 73: 618–622, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gregorio M, Jacomelli M, Inoue D, Genta PR, de Figueiredo A, Lorenzi-Filho G. Comparison of full versus short induced-sleep polysomnography for the diagnosis of sleep apnea. Laryngoscope 121: 1098–1103, 2011 [DOI] [PubMed] [Google Scholar]
- 14. Hardin KA, Seyal M, Stewart T, Bonekat HW. Sleep in critically ill chemically paralyzed patients requiring mechanical ventilation. Chest 129: 1468–1477, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Hillman D, Walsh J, Maddison K, Platt P, Kirkness J, Noffsinger W, Eastwood P. Evolution of changes in upper airway collapsibility during slow induction of anesthesia with propofol. Anesthesiology 111: 63–71, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Hosselet JJ, Norman RG, Ayappa I, Rapoport DM. Detection of flow limitation with a nasal cannula/pressure transducer system. Am J Respir Crit Care Med 157: 1461–1467, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Isono S, Tanaka A, Nishino T. Lateral position decreases collapsibility of the passive pharynx in patients with obstructive sleep apnea. Anesthesiology 97: 780–785, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Jordan A, Wellman A, Edwards J, Schory K, Dover L, MacDonald M, Patel S, Fogel R, Malhotra A, White D. Respiratory control stability and upper airway collapsibility in men and women with obstructive sleep apnea. J Appl Physiol 99: 2020–2027, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jordan A, Wellman A, Fogel R, Pierce R, Malhotra A, Edwards J, Schory K, White D. Pharyngeal critical closing pressure measurement without respiratory effort: a validation study. Am J Respir Crit Care Med 167: A600, 2003 [Google Scholar]
- 20. Kezirian E, White D, Malhotra A, Ma W, McCulloch C, Goldberg A. Interrater reliability of drug-induced sleep endoscopy. Arch Otolaryngol Head Neck Surg 136: 393–397, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Kirkness J, Schwartz A, Schneider H, Punjabi N, Maly J, Laffan A, McGinley B, Magnuson T, Schweitzer M, Smith P, Patil S. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol 104: 1618–1624, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kirkness JP, Peterson LA, Squier SB, McGinley BM, Schneider H, Meyer A, Schwartz AR, Smith PL, Patil SP. Performance characteristics of upper airway critical collapsing pressure measurements during sleep. Sleep 34: 459–467, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lettieri C, Shah A, Holley A, Kelly W, Chang A, Roop S; CPAP Promotion and Prognosis-The Army Sleep Apnea Program Trial Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann Intern Med 151: 696–702, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Litman RS, Hayes JL, Basco MG, Schwartz AR, Bailey PL, Ward DS. Use of dynamic negative airway pressure (DNAP) to assess sedative-induced upper airway obstruction. Anesthesiology 96: 342–345, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Marin J, Carrizo S, Vicente E, Agusti A. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365: 1046–1053, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Monti J, Debellis J, Gratadoux E, Alterwain P, Altier H, D'Angelo L. Sleep laboratory study of the effects of midazolam in insomniac patients. Eur J Clin Pharmacol 21: 479–484, 1982 [DOI] [PubMed] [Google Scholar]
- 27. Morrison DL, Launois SH, Isono S, Feroah TR, Whitelaw WA, Remmers JE. Pharyngeal narrowing and closing pressures in patients with obstructive sleep apnea. Am Rev Respir Dis 148: 606–611, 1993 [DOI] [PubMed] [Google Scholar]
- 28. Norman RG, Ahmed MM, Walsleben JA, Rapoport DM. Detection of respiratory events during NPSG: nasal cannula/pressure sensor versus thermistor. Sleep 20: 1175–1184, 1997 [PubMed] [Google Scholar]
- 29. Oto J, Yamamoto K, Koike S, Imanaka H, Nishimura M. Effect of daily sedative interruption on sleep stages of mechanically ventilated patients receiving midazolam by infusion. Anaesth Intensive Care 39: 392–400, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Owens R, Malhotra A, Eckert D, White D, Jordan A. The influence of end-expiratory lung volume on measurements of pharyngeal collapsibility. J Appl Physiol 108: 445–451, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patil S, Punjabi N, Schneider H, O'Donnell C, Smith P, Schwartz A. A simplified method for measuring critical pressures during sleep in the clinical setting. Am J Respir Crit Care Med 170: 86–93, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Patil S, Schneider H, Marx J, Gladmon E, Schwartz A, Smith P. Neuromechanical control of upper airway patency during sleep. J Appl Physiol 102: 547–556, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Sadaoka T, Kakitsuba N, Fujiwara Y, Kanai R, Takahashi H. The value of sleep nasendoscopy in the evaluation of patients with suspected sleep-related breathing disorders. Clin Otolaryngol Allied Sci 21: 485–489, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Schwartz AR, Gold AR, Schubert N, Stryzak A, Wise RA, Permutt S, Smith PL. Effect of weight-loss on upper airway collapsibility in obstructive sleep-apnea. Am Rev Respir Dis 144: 494–498, 1991 [DOI] [PubMed] [Google Scholar]
- 35. Schwartz AR, O'Donnell CP, Baron J, Schubert N, Alam D, Samadi SD, Smith PL. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am J Respir Crit Care Med 157: 1051–1057, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Sforza E, Petiau C, Weiss T, Thibault A, Krieger J. Pharyngeal critical pressure in patients with obstructive sleep apnea syndrome–clinical implications. Am J Respir Crit Care Med 159: 149–157, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Tsuiki S, Isono S, Ishikawa T, Yamashiro Y, Tatsumi K, Nishino T. Anatomical balance of the upper airway and obstructive sleep apnea. Anesthesiology 108: 1009–1015, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Veselis RA, Reinsel R, Marino P, Sommer S, Carlon GC. The effects of midazolam on the eeg during sedation of critically ill patients. Anaesthesia 48: 463–470, 1993 [DOI] [PubMed] [Google Scholar]
- 39. Wellman A, Jordan A, Malhotra A, Fogel R, Katz E, Schory K, Edwards J, White D. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med 170: 1225–1232, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med 168: 645–658, 2003 [DOI] [PubMed] [Google Scholar]


