Background: ATP-sensitive potassium (KATP) channels translate cellular metabolism (generation of ATP) in an electrical signal.
Results: Mutual repulsion between specific substituted titratable residues in the bundle crossing forces KATP channels to open and changes their apparent ATP sensitivity.
Conclusion: ATP-dependent gating involves conformational changes in the bundle crossing region of KATP channels.
Significance: This reflects an engineered method for control of ion channel activity by a non-natural mechanism.
Keywords: ATP, Biophysics, Cell pH, Membrane Proteins, Potassium Channels, KATP Channel, Bundle Crossing, Gating, Inwardly Rectifying Channel, Slide Helix
Abstract
Numerous inwardly rectifying potassium (Kir) channels possess an aromatic residue in the helix bundle crossing region, forming the narrowest pore constriction in crystal structures. However, the role of the Kir channel bundle crossing as a functional gate remains uncertain. We report a unique phenotype of Kir6.2 channels mutated to encode glutamate at this position (F168E). Despite a prediction of four glutamates in close proximity, Kir6.2(F168E) channels are predominantly closed at physiological pH, whereas alkalization causes rapid and reversible channel activation. These findings suggest that F168E glutamates are uncharged at physiological pH but become deprotonated at alkaline pH, forcing channel opening due to mutual repulsion of nearby negatively charged side chains. The potassium channel pore scaffold likely brings these glutamates close together, causing a significant pKa shift relative to the free side chain (as seen in the KcsA selectivity filter). Alkalization also shifts the apparent ATP sensitivity of the channel, indicating that forced motion of the bundle crossing is coupled to the ATP-binding site and may resemble conformational changes involved in wild-type Kir6.2 gating. The study demonstrates a novel mechanism for engineering extrinsic control of channel gating by pH and shows that conformational changes in the bundle crossing region are involved in ligand-dependent gating of Kir channels.
Introduction
Inwardly rectifying potassium (Kir) channels comprise a transmembrane pore-forming domain conserved within the cation channel superfamily(1). In contrast to their voltage-dependent cousins (Kv, Nav, and Cav channel families), Kir channels do not possess a canonical voltage-sensing domain (2–5). Rather, their ligand sensitivities and gating behaviors are determined by a cytoplasmic ligand-binding domain, and apparent voltage sensitivity in the physiological milieu arises from blockade by intracellular cations (Mg2+ and polyamines) (6). Kir channels exhibit diverse ligand dependence: some are regulated by G-proteins, others by intracellular nucleotides, and still others by intracellular pH, whereas all Kir channels are stimulated by phosphatidylinositol 4,5-bisphosphate (PIP2)2 (7–9). The growing understanding of regulation of Kir channels continues to highlight their role as important integrators of signaling pathways and underscores the importance of understanding Kir gating mechanisms.
Kir6.x channels are the pore-forming subunits of ATP-sensitive potassium (KATP) channels. They are distinguished from other Kir channels by their sensitivity to intracellular nucleotides (ATP and ADP), and the uniquely diverse pharmacology of KATP makes these channels a valuable model for characterizing Kir channel gating (5, 10–13). Many Kir channel types (most notably the G-protein-coupled inwardly rectifying potassium (GIRK) and KATP channels) possess a conserved aromatic residue at the helix bundle crossing (see Fig. 1, A and B), also the locus of the narrowest pore constriction in crystal structures of bacterial Kir homologs (Phe-146 in KirBac1.1 and Tyr-132 in KirBac3.1) (see Fig. 1B) (14). Although crystal structures suggest that this position is important for Kir channel closure, the notion of a functional gate at the Kir channel bundle crossing has been frequently questioned (15–18).
FIGURE 1.
Reverse pH dependence of Kir6.2(F168E) channels. A, structure of the KirBac1.1 channel, with bundle crossing residue Phe-146 highlighted in red. B, alignment of multiple Kir channel M2 segments. The first amino acid corresponds to the putative “glycine hinge” residue. The highlighted position corresponds to the aromatic bundle crossing residue depicted in A. C and D, continuous inside-out patch clamp recordings of WT Kir6.2 and Kir6.2(F168E) (both coexpressed with SUR1 in COSm6 cells), respectively, with internal pH changes as indicated. A small concentration of Ba2+ was included in solutions to assess leak with brief pulses to +50 mV.
We undertook a mutagenic approach to investigate the functional importance of residues in the bundle crossing region and inner cavity of Kir6.2 (19). Through these studies, we have uncovered several mechanisms for extrinsic/non-natural control of Kir6.2 gating. We previously reported a point mutation that introduces voltage-dependent gating into normally voltage-independent Kir6.2 (20). Here, we report the generation of Kir6.2 channels with a very pronounced pH-dependent gating mechanism that arises after introduction of a glutamate residue at the helix bundle crossing position (Phe-168). Our findings indicate that deprotonation of the F168E glutamate drives channel opening, likely due to a mutual repulsion of similar charges in the bundle crossing. Additionally, we demonstrate that conformational changes driven by the engineered pH dependence are closely related to gating motions arising from intrinsic ATP/PIP2-dependent gating. These results demonstrate the importance of conformational motions in the helix bundle crossing of Kir channels and illustrate general principles that underlie a mechanism for extrinsic or “engineered” control of ion channel gating.
EXPERIMENTAL PROCEDURES
KATP Channel Constructs
All mutant channel constructs were generated from a mouse WT Kir6.2 gene expressed in the pcDNA3.1(−) plasmid (Invitrogen). Point mutations were introduced by overlapping PCR-based methods, followed by subcloning of desired fragments into pcDNA3.1(−). Concatenated channel constructs were synthesized using PCR amplification of individual subunits with primers that introduced the desired flanking restriction sites, followed by subcloning into the pcDNA3.1(−) vector and verification by sequencing and digestion. Tetrameric channels were first constructed as two separate dimers to simplify sequencing and then combined into a final tetrameric construct. Channel subunits were subcloned between the XbaI-NotI (“slot 1”), NotI-EcoRI (“slot 2”), EcoRI-BamHI (“slot 3”), and BamHI-HindIII (“slot 4”) restriction sites. Gly6 linkers were included at the C-terminal end of each subunit in the tetramer, followed by two (or three for NotI) amino acids corresponding to the introduced restriction sites. Start codons in slots 2–4 were removed to minimize spurious translation initiation within the tetrameric constructs. A C-terminal GFP tag was also included in the tetrameric channel constructs to track synthesis of full-length tetramers. We found empirically that currents from cells transfected with the tetrameric constructs matured more slowly than those from cells transfected with the monomeric constructs, and the GFP tag assisted significantly in finding strongly expressing cells.
Channel Expression and Electrophysiology
COSm6 cells were maintained in culture in a 5% CO2 incubator at 37 °C in DMEM supplemented with 10% FBS and penicillin/streptomycin. Cells were transfected with channel constructs and hamster SUR1 using Lipofectamine 2000. For non-GFP-tagged constructs, cells were also transfected with a GFP plasmid to allow selection of transfected cells for recording.
Kir6.2ΔC36 constructs were expressed in Xenopus laevis oocytes. Ambion mMESSAGE T7 kits were used to synthesize mRNA from pcDNA3.1(−) constructs encoding either Kir6.2ΔC36 or Kir6.2(F168E)ΔC36, followed by injection (10–20 ng) into Xenopus oocytes 1 day after isolation. Inside-out patches were excised from oocytes 1–3 days after injection.
Data were filtered at 1 kHz, digitized at 5 kHz, and stored directly on a computer hard drive using Clampex software (Axon Inc.). The standard pipette (extracellular) and bath (cytoplasmic) solution used in these experiments had the following composition: 140 mm KCl, 1 mm K-EGTA, 1 mm K-EDTA, and 4 mm K2HPO4. The solution pH was adjusted with KOH or HCl to the desired level. Because the recording solutions are not very strongly buffered, the solution pH was verified each experimental day and verified after the addition of other solutes that can affect solution pH (namely Ba2+ and ATP). Solutions were delivered at room temperature by pressure-driven flow into a chamber designed to prevent mixing of experimental solutions and to allow rapid solution exchange. Identical solutions were used for recordings from both mammalian cell lines and Xenopus oocytes. Chemicals were purchased from Sigma-Aldrich or Fisher.
Western Blot Detection of KATP Channel Surface Expression
COSm6 cells in 6-well plates were transfected with Kir6.2 channel cDNA (600 ng) and FLAG-tagged SUR1 (1.2 μg in the pECE vector). After 3 days of incubation, cell surface proteins were isolated by biotinylation (Pierce cell surface protein isolation kit). Western blotting was carried out with samples of total cell lysates, as well as biotinylated fractions, using 7.5% SDS-PAGE, followed by transfer to nitrocellulose membrane and labeling with mouse anti-FLAG primary antibody (Sigma-Aldrich) and HRP-conjugated goat anti-mouse secondary antibody (ABM, Vancouver, Canada). Blots were visualized by ECL methods (Femto ECL detection kit, Pierce) using a FluorChem SP gel imager (Alpha Innotech).
RESULTS
Mutant Kir6.2 Activated by Intracellular Alkalization
We observed an unexpected phenotype in Kir6.2 channels with a glutamate substitution at Phe-168 (at the helix bundle crossing). A reasonable prediction for this experiment was that close proximity of charged glutamate side chains might force the M2 helices apart and thereby bias the channels toward an open state. Surprisingly, we observed that Kir6.2(F168E) ion channels conducted very little current around pH 7. However, intracellular alkalization (using inside-out patch recordings) caused a very large current increase (Fig. 1D). This activation was rapid and completely reversible, and currents exhibited voltage-dependent block by Ba2+ and spermine (Fig. 1D), demonstrating that the pH-dependent currents are carried by Kir6.2(F168E). A good measure of the pKa of the effect was not possible because the response did not saturate without significant seal breakdown (usually above pH 9). Remarkably, the alkaline pH-dependent activation of Kir6.2(F168E) was completely reversed from the WT Kir6.2 property of inhibition by alkalization (Fig. 1C) (21).
Mechanism for Alkalization-dependent Activation of Kir6.2(F168E)
We speculated that glutamate substitution at position 168 results in a profound pKa shift of the side chain carboxylic acid. When glutamates and/or aspartates are nearby, a pKa shift of the carboxylic acid reduces the energetic penalty arising from close approach of like charges and can be especially dramatic if the side chains are in a hydrophobic environment (22). This often appears as two carboxylates sharing a proton or one behaving as a protonated (uncharged) species, thereby minimizing repulsive coulombic interactions. In studies of ion channels, the best known example of this arrangement appears in the KcsA selectivity filter, where the Glu-71 and Asp-80 side chains are apposed and are presumed to share a proton (Fig. 2C). A similar arrangement is apparent in KirBac1.1 (23) (Fig. 2C). A satisfactory explanation for the pH dependence of F168E channels might be that alkalization promotes deprotonation of the carboxylates and forces a conformational change of the helix bundle crossing due to mutual repulsion (Fig. 2, A and B).
FIGURE 2.
Hypothetical mechanism for pH dependence of Kir6.2(F168E) channels. A and B, carboxylate side chains are forced into close proximity by the channel pore scaffold and may undergo a significant pKa shift. At sufficiently high pH, the carboxylate side chains will be deprotonated, resulting in mutual repulsion that forces motion around the helix bundle crossing. C, close arrangement of carboxylate side chains in the selectivity filters of KcsA and KirBac1.1. This orientation likely results in a substantial pKa shift of carboxylates.
To test whether alkalization-dependent activation of Kir6.2(F168E) requires mutual repulsion of F168E charges, we generated tetrameric constructs with one, two, or three F168E subunits. Significant alkalization-dependent activation was observed for channel constructs with two (FFEE or FEFE, with different orientations of F168E subunits in the tetramer) or three (FEEE) copies of F168E (Fig. 3, A–C and G). However, channels carrying a single F168E (FFFE) did not exhibit alkalization-dependent activation (Fig. 3, D and G). Additionally, 1:1 dimers of WT and Kir6.2(F168E) channels were activated by alkalization (Fig. 3G, 'FE'+'FE'). The degree of alkalization-dependent activation was notably weaker in the FE dimeric construct. We have not investigated this in great detail but suspect that this may be related to a higher intrinsic open probability of this construct (which would limit the extent of activation that could occur). A similar effect is discussed below for Kir6.2(F168E/I296L) mutant channels in Fig. 8.
FIGURE 3.
Subunit stoichiometry of F168E effects on pH-dependent gating. A–D, tandem-linked channel tetramers containing one (FFFE; D), two (FFEE and FEFE; B and C, respectively), or three copies (FEEE; A) of the F168E mutant were characterized for their pH dependence. Channels with more than one F168E subunit exhibited activation at alkaline pH. E and F, representative inside-out patches from Xenopus oocytes expressing Kir6.2(F168E)ΔC36 and WT Kir6.2ΔC36 in the absence of SUR1 exhibit pH responses similar to those of the full-length channel. G, summary data for tetrameric channels with composition indicated by F (for WT subunit) or E (for F168E subunit). Data are also included for a WT Kir6.2 + F168E dimeric channel ('FE'+'FE') (n = 5 for FEEE, FFEE, FEFE, and FFFE tetramers; n = 4 for the FE dimer; n = 10 for WT Kir6.2ΔC36; and n = 11 for Kir6.2(F168E)ΔC36).
FIGURE 8.
Open state stability mutations affect the phenotype of Kir6.2(F168E) channels. Representative inside-out patch records are presented for Kir6.2(F168E/I296L) (A) and Kir6.2(F168E/R176A) (B) channels, with pH jumps as indicated. C, summary data for both mutant channels (n = 4 for I296L and n = 7 for R176A).
Importantly, previous studies of tetrameric Kir6.2 channel constructs have reported a high intrinsic open probability (24, 25). To ensure that the absence of alkalization-dependent activation of FFFE channels did not arise because of an intrinsically high Po (from which little further channel activation could be observed), we also examined the effects of pH after polylysine-mediated rundown. After polylysine treatment resulting in ∼60% current reduction, inhibition upon alkalization persisted, similar to WT Kir6.2 (Fig. 3D, panels i and ii). These findings indicate that at least two copies of the F168E mutation are required for alkalization-dependent activation, consistent with a mechanism of mutual repulsion of F168E.
Alkalization-dependent activation of Kir6.2(F168E) channels does not rely on the presence of a SUR subunit. We expressed WT and Kir6.2(F168E) channels in the absence of SUR by deleting the C-terminal 36 amino acids (26). Similar to full-length channels expressed with SUR1, F168E channels exhibited alkalization-dependent activation, whereas WT Kir6.2 channels were inhibited over a similar pH range (Fig. 3, E–G).
Alkalization-dependent Activation Reflects Increased Channel Open Probability
Patch clamp records resolving single channel openings demonstrate that alkalization promotes opening of Kir6.2(F168E). Fig. 4A depicts a representative membrane patch switched repeatedly between pH 6.5 and 8.5, demonstrating increased channel activity at alkaline pH. Expanded segments of the recording (Fig. 4B, panels i–iii) highlight that there are no apparent differences in single channel current amplitude, but channel Po is pH-dependent. Also, single channel conductance was ∼80 picosiemens at −50 mV, comparable with reports for WT Kir6.2 (20, 24, 27). These findings indicate that alkalization of F168E channels increases current by promoting channel opening rather than increasing microscopic conductance.
FIGURE 4.
Microscopic effects of pH-dependent activation of Kir6.2(F168E) channels. A, continuous record at −50 mV of a tetrameric (FEEE) construct (three copies of Kir6.2(F168E) + one copy of WT Kir6.2), with pH jumps between 6.5 and 8.5 as indicated. Downward deflections at pH changes are solution exchange artifacts and were minimized during figure preparation. The FEEE tetramer was used because it exhibited similar pH dependence as F168E channels but took considerably longer to express currents and was very amenable to recording single channel currents. B, expanded view of channel openings in regions i, ii, and iii indicated in A. Significant changes in single channel current magnitude were not observed, but openings were far more frequent at alkaline pH. Similar observations were made in four patches.
Position Dependence of Alkalization-dependent Activation
Alkalization-dependent activation conferred by the F168E mutation appears to be position-specific, as glutamate substitutions at other pore-lining positions did not exhibit similar pH-dependent activation (Fig. 5). There are also several titratable side chains in the vicinity of F168E (His-70, Lys-170, and His-175), raising the possibility of pH-dependent salt bridges between side chains (Fig. 6A). We ruled this out by systematically neutralizing His-70, Lys-170, and His-175 on a Kir6.2(F168E) background. Each double mutant channel exhibited alkalization-dependent activation comparable with Kir6.2(F168E) (Fig. 6, B–E). Importantly, His-175 has been identified as a determinant of intrinsic pH sensitivity in Kir6.2 (28). Persistence of alkalization-dependent activation in Kir6.2(F168E/H175A) double mutant channels indicates that the pH dependence of the F168E mutation does not arise from a modulation of the intrinsic pH sensor of the channel. Overall, these data indicate that the F168E mutation is sufficient for alkalization-dependent activation and that pH changes likely trigger a conformational change in the vicinity of the F168E substitution.
FIGURE 5.
Position-specific requirements for alkalization-dependent activation of Kir6.2. Glutamate mutants at inner cavity-lining positions were tested for pH dependence. Representative currents at −50 mV with pH jumps as indicated are presented for each pore-lining mutant channel. Only Kir6.2(F168E) channels (boldface arrow, lower right) exhibited activation at alkaline pH.
FIGURE 6.
Neutralization of titratable residues near F168E. A, molecular model of the bundle crossing region of Kir6.2. B–E, double mutants Kir6.2(H70A/F168E) (n = 7), Kir6.2(F168E/K170A) (n = 7), and Kir6.2(F168E/H175A) (n = 5) were examined for pH dependence. All double mutants exhibited alkalization-dependent activation comparable with Kir6.2(F168E) channels.
Surface Expression of Alkalization-dependent Channel Mutant
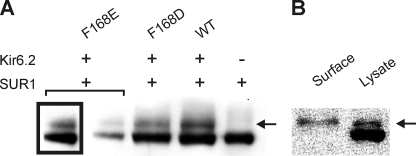
We also determined the cell surface expression level of Kir6.2(F168E) by assessing the presence of a mature glycosylated form of SUR1 (indicated by arrows in Fig. 7). Interestingly, we were unable to detect functional currents from Kir6.2(F168D) channels despite the similarity of aspartate and glutamate side chains, so cell surface expression of F168D channels was also examined, along with WT Kir6.2. For all channel + FLAG-SUR1 combinations examined, two FLAG-SUR1 bands were detected by Western blotting (Fig. 7A). F168E channels consistently generated smaller protein levels relative to WT or Kir6.2(F168D) channels and required longer exposures to convincingly resolve bands (Fig. 7A, boxed). FLAG-SUR1 migration on SDS-PAGE is interpreted with the lower band corresponding to a core-glycosylated immature form and the upper band corresponding to a mature glycosylated form expressed at the cell surface (29, 30). We confirmed the validity of this interpretation by demonstrating that FLAG-SUR1 expression in the absence of Kir6.2 failed to generate the mature glycosylated form (Fig. 7A) and that cell surface biotinylation isolated only the upper band (Fig. 7B). These findings demonstrate that Kir6.2(F168E) and Kir6.2(F168D) channels are trafficked with efficiency similar to WT Kir6.2. However, Kir6.2(F168D) channels are not sufficient to generate functional currents over the pH range tested in our experiments.
FIGURE 7.
Surface expression of Kir6.2 bundle crossing mutants. A, Western blots for FLAG-SUR1 in cells transfected with FLAG-SUR1 + WT Kir6.2, Kir6.2(F168E), or Kir6.2(F168D) or in the absence of any Kir6.2 subunit. Two bands were typically observed in cells transfected with FLAG-SUR1 together with a channel construct. Only the lower molecular weight band was apparent when FLAG-SUR1 was transfected alone. A longer exposure is also presented for Kir6.2(F168E) + SUR1 (boxed), which typically exhibited lower intensity bands relative to other transfections. The arrow indicates the higher molecular weight band corresponding to mature glycosylated FLAG-SUR1. B, Western blot for FLAG-SUR1 in cells transfected with WT Kir6.2 + FLAG-SUR1. Purification of surface-biotinylated protein resulted in a single immunoreactive band corresponding to the mature glycosylated form of FLAG-SUR1 (indicated by the arrow), whereas the entire cell lysate generated both the core and mature glycosylated forms.
Alkalization-dependent Activation Is Influenced by Channel Open Probability
We tested whether the pH-influenced activity of Kir6.2(F168E) is related to the intrinsic channel gating mechanism. First, we introduced the F168E mutation together with a gain-of-function Kir6.2 mutation (I296L) that acts by increasing channel open probability (31, 32). The Kir6.2(F168E/I296L) double mutant exhibited large currents at acidic pH and was weakly potentiated upon alkalization (Fig. 8, A and C). In many patches, pH-activated currents from Kir6.2(F168E/I296L) appeared to saturate at alkaline pH (Fig. 8A). These data suggest that the Kir6.2(F168E/I296L) channels exhibit a high open probability at low pH. In a complementary experiment, we used the R176A mutation (which disrupts PIP2 sensitivity) to generate channels with low open probability (Fig. 8B) (33). In Kir6.2(F168E/R176A) double mutant channels, very little current activation was observed until the most extreme alkaline pH, consistent with a greater stimulus required to force these channels open. These findings further support that alkalization of Kir6.2(F168E) channels modulates the native gating mechanism.
Coupling of the Bundle Crossing Gate and ATP-binding Site
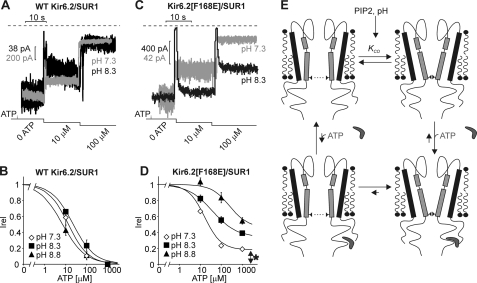
To test stringently whether pH-dependent activation of F168E channels resembles channel gating, we examined whether pH affects ligand sensitivity. To preface this experiment, note that the ATP-binding site is allosterically coupled to the channel gate, and therefore, manipulations (e.g. mutations, changes in lipid conditions) that change the stability of open versus closed states also alter the apparent Kir6.2 channel ATP sensitivity (Fig. 9E) (7, 8, 34). For instance, as membrane PIP2 content (and consequently, channel Po) is increased, ATP inhibition of Kir6.2 grows progressively weaker. If motions triggered by alkalization affect opening and closing transitions at the same gate that is operated by the native ATP regulatory mechanism, then pH-induced changes in the closed-open equilibrium of Kir6.2(F168E) channels may translate into changes in apparent ATP sensitivity, similar to the effects of increased membrane PIP2. We tested this possibility by measuring ATP sensitivity at various internal pH values (Fig. 9, A–D). In WT Kir6.2 channels, internal pH had little effect on ATP inhibition (Fig. 9, A and B), ruling out a nonspecific effect of pH on ATP binding. In contrast, Kir6.2(F168E) channels exhibited significantly weakened ATP inhibition at alkaline pH (Fig. 9, C and D). Thus, Kir6.2(F168E) channels are reasonably well inhibited by ATP but with a sensitivity that is modified by internal pH. This indicates that pH-dependent conformational changes in Kir6.2(F168E) channels are likely coupled to the ATP-binding site and resemble gating motions in WT Kir6.2 channels.
FIGURE 9.
Alkalization-dependent activation alters ATP inhibition of Kir6.2(F168E) channels. A, continuous records at −50 mV for a patch expressing WT Kir6.2 + SUR1, with ATP concentration jumps as indicated. Raw current records were extracted from the same patch and normalized to the peak current in 0 ATP at either pH 7.3 (gray) or 8.3 (black). B, summary of ATP dose response for WT Kir6.2 at pH 7.3 (n = 8), 8.3 (n = 4), and 8.8 (n = 4). Irel is the ratio of current in ATP to the control current level (0 ATP) at a given pH. C and D, identical experiments and summarized data as in A and B for Kir6.2(F168E) channels (n = 10 for pH 7.3 and n = 5 for pH 8.3 and 8.8). Dose-response curves were fit with the equation Irel = (1 − C)/(1 + ([ATP]/Kd)h) + C. E, schematic model illustrating state preference for ATP binding and pH-dependent changes in open state stability.
Another interesting feature of these data is that ATP inhibition of Kir6.2(F168E) did not eliminate macroscopic currents, resulting in a plateau of conductance (asterisk in Fig. 9D, pH 7.3). We have confirmed that the plateau persists at very high ATP concentrations and that currents can be inhibited by spermine (Fig. 10), indicating that the plateau current is carried by Kir6.2(F168E) channels. However, the mechanistic basis for this plateau is unclear. One intriguing possibility is that the glutamate substitution results in a “leaky” closed state, in which channels are in a quasi-closed ATP-bound conformation, but the pore is not completely occluded due to ablation of the Phe-168 aromatic residue. Such partial closings were not apparent in single channel records, but it is noteworthy that the plateau current was best resolved (Fig. 10) at pH 7.3 (where Po is quite low). Therefore, the relative magnitude of the plateau to control (0 ATP) current would not represent the true conductance ratio of a leaky closed state to a fully open state because all closed channels could contribute to the plateau conductance, whereas at pH 7.3, only a small fraction of channels would be likely to open in 0 ATP.
FIGURE 10.
ATP inhibition does not cause complete closure of Kir6.2(F168E) channels. A, ATP inhibition of Kir6.2(F168E) channels was measured at pH 7.3, resulting in a clear plateau conductance that persisted even at 5 mm ATP, with considerably reduced current fluctuations relative to the 0 ATP condition. The presence of a plateau conductance was validated by blocking currents completely with a pulse from −50 to +50 mV in the presence of spermine. B, currents of similar magnitude through WT Kir6.2 channels exhibited virtually complete inhibition by ATP.
DISCUSSION
Targeted pH-dependent Conformational Changes
Most Kir channel crystal structures have captured a presumed closed state with a narrow constriction in the bundle crossing region (14, 35). KirBac3.1 is the only Kir channel with structural data that may represent multiple channel states: a cryo-EM study modeled substantial rearrangements of the inner M2 helices during channel opening (comparable with the differences between the KcsA and MthK structures) (36), whereas a more recent report of multiple crystal structures of KirBac3.1 implies far more subtle rearrangements around the helix bundle crossing and favors a model in which the selectivity filter regulates channel conduction (18). These disparate reports mirror earlier uncertainties arising from functional characterization of ligand-dependent “gated access” of cysteine-reactive probes. Specifically, several studies have reported no gating-dependent changes in accessibility of the Kir channel inner cavity (15, 17), whereas other reports have described the kinetic details for cysteine modification by methanethiosulfonate reagents and demonstrated that gated access can indeed be observed at sufficiently low channel Po (37, 38).
Relationship to WT Kir6.2 Gating
We cannot claim to have resolved these lingering uncertainties completely, although we would argue that the peculiar Kir6.2(F168E) phenotype sheds light on mechanisms of Kir channel gating and coupling to ligand-binding sites. We have demonstrated that it is possible to force channel opening with extrinsic stimuli that induce a conformational change around the helix bundle crossing. This finding does not provide direct evidence that the helix bundle crossing forms a functional gate in Kir6.2 but certainly indicates that conformational changes in this region can promote channel opening and are functionally coupled to the intrinsic ATP-binding site. Also, this induced gating motion likely resembles ATP-dependent conformational changes because forced channel opening changes the ATP sensitivity of the channel. Thus, conformational rearrangements of the bundle crossing are, at the very least, an important step in transducing ligand binding to the channel gate.
Intriguingly, the presence of a hydrophilic glutamate at position 168 also prevented complete channel inhibition even at very high ATP concentrations. This resulted in a discrete plateau conductance with little fluctuation noise (Fig. 10), indicating a possible important role for hydrophobic residues for tight closure of the bundle crossing gate. An earlier study characterizing ATP inhibition in a variety of Kir6.2(F168X) mutants suggested the importance of large hydrophobic side chains in this position to achieve strong ATP sensitivity, although a plateau conductance was not explicitly reported (39). Our findings differ from this report because the ATP sensitivity of Kir6.2(F168E) is only modestly altered relative to that of WT Kir6.2 (Fig. 9, B and D) unless alkaline pH is imposed.
We emphasize that a rightward shift of ATP sensitivity (as reported in Ref. 39) does not necessarily indicate that channel closure is prevented, just that the closed state is destabilized relative to the open state. However, a plateau conductance (especially in the absence of any significant shift in ATP sensitivity) likely indicates that ATP-bound “closed” channels retain some permeability. Given the conservation of hydrophobic residues at the bundle crossing (Fig. 1B), it may be surprising that the presence of a hydrophilic glutamate is not more disruptive. For instance, single channel records from Kir6.2(F168E) exhibit well defined closures (as well as we are able to resolve) (Fig. 4), and macroscopic currents can be reduced considerably by ATP (Fig. 9) or internal acidification (Figs. 1 and 10). Together, these observations suggest that any conductance arising from “quasi-closed” F168E channels must be quite small and barely distinguishable from the closed state in single channel records.
Mechanism of Engineered pH Dependence
This study demonstrates a simple but novel mechanism to control channel gating rapidly and reversibly by introducing a protonatable side chain at a position that is important for channel opening and closing. A likely mechanism underlying this phenotype is pH-dependent mutual repulsion of glutamate side chains at F168E driving movement of the M2 helices and opening the channel pore. It should be noted that there are likely many factors influencing the engineered (non-native) pH-dependent gating. For example, despite carrying a similar carboxylic acid side chain, aspartate is not a suitable surrogate to observe the pH-dependent gating mechanism. No functional currents were detected from Kir6.2(F168D) channels, which could be due to poor tolerance of mutations in this functionally important region or possibly because aspartate undergoes a pKa shift that pushes the pH dependence beyond a practical range for patch clamp experiments. Additionally, other potentially interesting protonatable side chains (His and Lys) have not provided useful data, and this may be because the protonated state that would lead to channel opening by mutual repulsion would also carry a ring of positive charge around the mouth of the inner cavity. Prior experiments involving introduction of positive charges (by cysteine modification) in the bundle crossing region resulted in dramatic reduction of macroscopic conductance (40, 41), likely by introducing a significant electrostatic barrier for ion permeation.
Hydrophobic Bundle Crossing of Kir Channels
In most Kir channels, one or more pore-lining residues in the narrow bundle crossing are occupied by large hydrophobic side chains, and this has been highlighted as potentially important for channel closure by the formation of a hydrophobic seal (14, 42). It is noteworthy that this arrangement differs from other common models of gating (Kv channels and KcsA) where the bundle crossing comprises side chains with much smaller volumes (e.g. valines in Kv channels) (43, 44). In eukaryotic Kir channels strongly regulated by cytoplasmic ligand binding (i.e. KATP and GIRK pore-forming subunits), a phenylalanine is conserved in the final turn of the M2 helix (Fig. 1B). There appears to be less selective pressure for a bundle crossing aromatic in Kir channels that physiologically are predominantly open and regulated by polyamine block (Kir2 subfamily) or pH (Kir1 and Kir4 subfamilies). This dichotomy may reflect a stringent physiological requirement for tight channel closure in the ligand-gated subgroup, although we suspect that this may relate to the conservation of a gating mechanism that requires transduction from a cytoplasmic ligand-binding site. The role of aromatic side chains in the Kir channel bundle crossing will be clarified with further exploration of the ligand-dependent gating mechanisms of Kir channels.
Acknowledgments
We thank Show-Ling Shyng (Oregon Health & Science University, Portland, OR) for providing the FLAG-SUR1 clone and offering advice on detection of SUR1 cell surface expression. H. T. K. is grateful to Colin G. Nichols and Thomas Baukrowitz for assistance during the early exploratory phases of this project.
Note Added in Proof
After acceptance of this article, a crystal structure of Kir2.2 in complex with PIP2 appeared in press (Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2, epub August 28, doi 10:1038/nature10370), demonstrating conformational changes in the helix bundle crossing region that are likely associated with Kir channel opening.
This work was supported by a Canadian Institutes of Health Research operating grant and New Investigator Awards from the Heart and Stroke Foundation of Canada and the Michael Smith Foundation for Health Research (to H. T. K.).
- PIP2
- phosphatidylinositol 4,5-bisphosphate.
REFERENCES
- 1. Kubo Y., Adelman J. P., Clapham D. E., Jan L. Y., Karschin A., Kurachi Y., Lazdunski M., Nichols C. G., Seino S., Vandenberg C. A. (2005) Pharmacol. Rev. 57, 509–526 [DOI] [PubMed] [Google Scholar]
- 2. Kubo Y., Reuveny E., Slesinger P. A., Jan Y. N., Jan L. Y. (1993) Nature 364, 802–806 [DOI] [PubMed] [Google Scholar]
- 3. Kubo Y., Baldwin T. J., Jan Y. N., Jan L. Y. (1993) Nature 362, 127–133 [DOI] [PubMed] [Google Scholar]
- 4. Ho K., Nichols C. G., Lederer W. J., Lytton J., Vassilev P. M., Kanazirska M. V., Hebert S. C. (1993) Nature 362, 31–38 [DOI] [PubMed] [Google Scholar]
- 5. Inagaki N., Gonoi T., Clement J. P., 4th, Namba N., Inazawa J., Gonzalez G., Aguilar-Bryan L., Seino S., Bryan J. (1995) Science 270, 1166–1170 [DOI] [PubMed] [Google Scholar]
- 6. Lopatin A. N., Makhina E. N., Nichols C. G. (1994) Nature 372, 366–369 [DOI] [PubMed] [Google Scholar]
- 7. Shyng S. L., Nichols C. G. (1998) Science 282, 1138–1141 [DOI] [PubMed] [Google Scholar]
- 8. Baukrowitz T., Schulte U., Oliver D., Herlitze S., Krauter T., Tucker S. J., Ruppersberg J. P., Fakler B. (1998) Science 282, 1141–1144 [DOI] [PubMed] [Google Scholar]
- 9. Rohács T., Chen J., Prestwich G. D., Logothetis D. E. (1999) J. Biol. Chem. 274, 36065–36072 [DOI] [PubMed] [Google Scholar]
- 10. Aguilar-Bryan L., Nichols C. G., Wechsler S. W., Clement J. P., 4th, Boyd A. E., 3rd, González G., Herrera-Sosa H., Nguy K., Bryan J., Nelson D. A. (1995) Science 268, 423–426 [DOI] [PubMed] [Google Scholar]
- 11. Inagaki N., Gonoi T., Clement J. P., Wang C. Z., Aguilar-Bryan L., Bryan J., Seino S. (1996) Neuron 16, 1011–1017 [DOI] [PubMed] [Google Scholar]
- 12. Shyng S., Nichols C. G. (1997) J. Gen. Physiol. 110, 655–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clement J. P., 4th, Kunjilwar K., Gonzalez G., Schwanstecher M., Panten U., Aguilar-Bryan L., Bryan J. (1997) Neuron 18, 827–838 [DOI] [PubMed] [Google Scholar]
- 14. Kuo A., Gulbis J. M., Antcliff J. F., Rahman T., Lowe E. D., Zimmer J., Cuthbertson J., Ashcroft F. M., Ezaki T., Doyle D. A. (2003) Science 300, 1922–1926 [DOI] [PubMed] [Google Scholar]
- 15. Proks P., Antcliff J. F., Ashcroft F. M. (2003) EMBO Rep. 4, 70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Claydon T. W., Makary S. Y., Dibb K. M., Boyett M. R. (2003) J. Biol. Chem. 278, 50654–50663 [DOI] [PubMed] [Google Scholar]
- 17. Xiao J., Zhen X. G., Yang J. (2003) Nat. Neurosci. 6, 811–818 [DOI] [PubMed] [Google Scholar]
- 18. Clarke O. B., Caputo A. T., Hill A. P., Vandenberg J. I., Smith B. J., Gulbis J. M. (2010) Cell 141, 1018–1029 [DOI] [PubMed] [Google Scholar]
- 19. Kurata H. T., Phillips L. R., Rose T., Loussouarn G., Herlitze S., Fritzenschaft H., Enkvetchakul D., Nichols C. G., Baukrowitz T. (2004) J. Gen. Physiol. 124, 541–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kurata H. T., Rapedius M., Kleinman M. J., Baukrowitz T., Nichols C. G. (2010) PLoS Biol. 8, e1000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu H., Wu J., Cui N., Abdulkadir L., Wang R., Mao J., Giwa L. R., Chanchevalap S., Jiang C. (2001) J. Biol. Chem. 276, 38690–38696 [DOI] [PubMed] [Google Scholar]
- 22. Lindman S., Linse S., Mulder F. A., André I. (2007) Biophys. J. 92, 257–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berneche S., Roux B. (2002) Biophys. J. 82, 772–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Craig T. J., Ashcroft F. M., Proks P. (2008) J. Gen. Physiol. 132, 131–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shyng S., Ferrigni T., Nichols C. G. (1997) J. Gen. Physiol. 110, 141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tucker S. J., Gribble F. M., Zhao C., Trapp S., Ashcroft F. M. (1997) Nature 387, 179–183 [DOI] [PubMed] [Google Scholar]
- 27. Lorenz E., Alekseev A. E., Krapivinsky G. B., Carrasco A. J., Clapham D. E., Terzic A. (1998) Mol. Cell. Biol. 18, 1652–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang R., Su J., Zhang X., Shi Y., Cui N., Onyebuchi V. A., Jiang C. (2006) J. Membr. Biol. 213, 155–164 [DOI] [PubMed] [Google Scholar]
- 29. Zerangue N., Schwappach B., Jan Y. N., Jan L. Y. (1999) Neuron 22, 537–548 [DOI] [PubMed] [Google Scholar]
- 30. Yan F. F., Casey J., Shyng S. L. (2006) J. Biol. Chem. 281, 33403–33413 [DOI] [PubMed] [Google Scholar]
- 31. Koster J. C., Remedi M. S., Dao C., Nichols C. G. (2005) Diabetes 54, 2645–2654 [DOI] [PubMed] [Google Scholar]
- 32. Proks P., Girard C., Haider S., Gloyn A. L., Hattersley A. T., Sansom M. S., Ashcroft F. M. (2005) EMBO Rep. 6, 470–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shyng S. L., Cukras C. A., Harwood J., Nichols C. G. (2000) J. Gen. Physiol. 116, 599–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Enkvetchakul D., Loussouarn G., Makhina E., Shyng S. L., Nichols C. G. (2000) Biophys. J. 78, 2334–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nishida M., Cadene M., Chait B. T., MacKinnon R. (2007) EMBO J. 26, 4005–4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuo A., Domene C., Johnson L. N., Doyle D. A., Vénien-Bryan C. (2005) Structure 13, 1463–1472 [DOI] [PubMed] [Google Scholar]
- 37. Phillips L. R., Nichols C. G. (2003) J. Gen. Physiol. 122, 795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Phillips L. R., Enkvetchakul D., Nichols C. G. (2003) Neuron 37, 953–962 [DOI] [PubMed] [Google Scholar]
- 39. Rojas A., Wu J., Wang R., Jiang C. (2007) Biochim. Biophys. Acta 1768, 39–51 [DOI] [PubMed] [Google Scholar]
- 40. Kurata H. T., Marton L. J., Nichols C. G. (2006) J. Gen. Physiol. 127, 467–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Loussouarn G., Makhina E. N., Rose T., Nichols C. G. (2000) J. Biol. Chem. 275, 1137–1144 [DOI] [PubMed] [Google Scholar]
- 42. Tao X., Avalos J. L., Chen J., MacKinnon R. (2009) Science 326, 1668–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Long S. B., Campbell E. B., MacKinnon R. (2005) Science 309, 897–903 [DOI] [PubMed] [Google Scholar]
- 44. Doyle D. A., Morais Cabral J., Pfuetzner R. A., Kuo A., Gulbis J. M., Cohen S. L., Chait B. T., MacKinnon R. (1998) Science 280, 69–77 [DOI] [PubMed] [Google Scholar]