Abstract
Far-eastern spotted fever is an emerging disease caused by Rickettsia heilongjiangensis, a tick-borne obligate intracellular bacterium. In this study, R. heilongjiangensis was used to infect BALB/c mice by inoculation of retro-orbital venous plexus to imitate a blood infection caused by tick biting. We found that R. heilongjiangensis rapidly entered the circulation for systemic dissemination and the pathogen existed in liver, spleen, lungs, and brain of the mice at least 9 days post-infection (p.i.). Severe pathological lesions were observed in liver, lungs, and brain at Day 6 p.i. In addition, the elevated levels of inflammatory cytokines, including interferon-γ, tumor necrosis factor, and CC chemokine, were detected in the infected organs at Day 3 p.i. Our results reveal that R. heilongjiangensis may cause an infection in BALB/c mice and the pathological lesions in the infected mice are associated with host inflammatory response induced by R. heilongjiangensis.
Introduction
Rickettsiae are obligate intracellular bacteria requiring the environment of the host cells for replication. The genus Rickettsia has been classified into three groups: one that includes Rickettsia bellii; the typhus group, which includes Rickettsia prowazekii, the agent of the louse-borne epidemic typhus, and Rickettsia typhi, the agent of the flea-borne murine typhus; the spotted fever group (SFG), which includes more than 20 recognized species that are associated mainly with ticks, but also with fleas and mites.1
Before 1980, North Asian tick fever caused by Rickettsia sibirica was recognized as the only spotted fever rickettsiosis in Russia and China. In 1992, a rickettsial strain isolated from the blood of a patient with febrile exanthematous illness in Japan was identified as a novel species, Rickettsia japonica, and the rickettsiosis was named as Japanese spotted fever.2 Rickettsia heilongjiangensis was first isolated from Dermacentor sylvarum ticks in the northeastern area of China in 1983 and was classified within the R. japonica subgroup of SFG rickettsiae.3 Rickettsia heilongjiangensis was demonstrated to be pathogenic for humans based on serologic findings and pathogen identification.4–8 The disease caused by Rickettsia heilongjiangensis has been named as far-eastern spotted fever (FESF) and the disease has been diagnosed in patients in Northeastern China,4 Siberia and Far East of Russia,5,7,8 and Honshu island of Japan,6 suggesting that FESF is an important emerging infectious disease in these areas.
A 17.46% infection rate of R. heilongjiangensis was found among the residents in nine counties of northeastern China in a seroepidemic investigation, and several strains of R. heilongjiangensis were isolated from blood samples of patients in these counties.4 Rickettsia heilongjiangensis was also identified in the skin biopsy samples of patients and isolated from humans in Russia.5,7,8 In addition, R. heilongjiangensis have been isolated and identified from different ticks, including D. sylvarum, Haemaphysalis concinna, and Haemaphysalis japonica douglasi, in the disease-endemic areas.4–9 Screening testing of Haemaphysalis ticks inhabiting Russian Far Eastern regions showed that up to 28.13% of H. concinnae and 4.48% of H. japonica douglasii ticks harbor R. heilongjiangensis, suggesting that H. concinnae may serve as the main vector for the transmission of R. heilongjiangensis in this area.9 Most of the FESF cases have been reported in the summer in the disease-endemic areas,4–6 which coincides with the peak activity of ticks.
To reveal its pathogenic characteristics and pathogenesis of FESF, R. heilongjiangensis was used to infect BALB/c mice, and then the rickettsial burden and pathologic lesions as well as immune and inflammatory responses in the mice were assessed in this study.
Materials and Methods
Growth and purification of R. heilongjiangensis.
Rickettsia heilongjiangensis (054 type strain), isolated from D. sylvarum in Heilongjiang province of China, was grown in Vero cells in Dulbecco's minimal essential medium supplemented with 4% heat-inactivated fetal bovine serum and 2 mM L-glutamine for 7–9 days at 33°C in a 5% CO2.10 The infected cells were harvested and disrupted by vortexing with glass beads, and the host cell debris was removed by centrifugation at 500 × g for 10 min.11 Supernatants containing rickettsiae were centrifuged at 10,400 × g for 30 min.12 Rickettsial pellet was suspended in K36 buffer,13 and then layered on 20–45% linear Renografin gradient for centrifugation at 141,000 × g for 90 min with rotor SW28 (Beckman, Fullerton, CA).12 The light band of the rickettsial layers was collected and subjected to a second round of Renografin gradient centrifugation. The rickettsiae were collected and washed with phosphate buffered saline 3 times and then suspended in culture media. The rickettsiae were determined by a quantitative polymerase chain reaction (qPCR) (see below). The viable organisms were ~95% of the collected organisms that were measured using a bacterial viability kit (LIVE/DEAD BacLightBacterial Viability Kits, Invitrogen, Carlsbad, CA). The absence of other bacteria and fungi was verified by spreading diverse agar plates. The absence of mycoplasma was checked using a mycoplasma test kit (EZ-PCR Mycoplasma Test Kit, Biological Industries, Kibbutz Beit-Haemek, Israel). One part of the purified organisms was treated at 60°C for 30 min and the heat-inactivated organisms were used as controls of the viable organisms.
R.heilongjiangensis infection of mice.
Female BALB/c mice (5 weeks of age) were purchased from the Laboratory Animal Center of Beijing in China. The animal usage was approved by the Beijing Administrative Committee for Laboratory Animals and the animal care met the standard of the committee. Mice were divided into three groups (25 mice for group 1 and 2, 5 mice for group 3). Mice in group 1 were inoculated with 105 viable R. heilongjiangensis organisms and mice in group 2 were inoculated with an equal number of heat-inactivated organisms by injection of retro-orbital venous plexus (intravenous route).14,15 Mice inoculated with phosphate buffered saline were used as a negative control group (group 3). At Days 1, 3, 6, and 9 post-infection (p.i.), 5 mice in group 1 and group 2 were killed and their blood was collected in EDTA tubes and their liver, spleen, lung, and brain tissues were aseptically excised, respectively. Mice in group 3 were killed and their blood and tissue samples were collected at Day 9 p.i. Each tissue sample was divided into two parts, one was stored at −80°C for qPCR analysis of rickettsial load and the other was fixed in 10% neutral formalin for histopathological analysis. Five mice in group 1 and group 2 were bled by tail-cutting at Days 7, 14, and 28 p.i., respectively. Five blood samples/day were mixed and the separated serum sample was used to evaluate the antibody levels against R. heilongjiangensis.
Histopathological analysis.
The formalin-fixed tissue samples were embedded with paraffin and the sections of paraffin-embedded tissues were stained with hematoxylin-eosin to assess the pathological changes in the infected mouse tissues under light microscopy.
Detection of R. heilongjiangensis in blood and tissues.
DNA was extracted from each mouse blood (100 μL) or organ tissue (10 mg) using a DNeasy Blood &Tissue kit (Qiagen, GmbH, Germany). The total DNA of each sample was eluted from the column with 100 μL of elution buffer. The rickettsial DNA copies were measured using qPCR targeting a 98 bp fragment of ompB gene with primers RompB3944 (5′-ATCTGAAGCGGGAGCAATACC-3′) and RompB4041 (5′-CATCAGTATAGAAAGGTTTTGCCCATA-3′) and a TaqMan-MGB probe (FAMTACATTATCAACAGCCTCGTCAP). Reactions were performed in an ABI7300 system (Applied Biosystems, Carlsbad, CA). Each qPCR run included a standard curve of 10-fold serial dilutions of a known concentration of R. heilongjiangensis ompB-recombined plasmid that was previously developed in our laboratory (unpublished data).
Determination of cytokines in tissues after expression of cytokines.
For RNA extraction of tissues, 25 mg of each tissue sample was treated with RNeasy Midi kit (Qiagen) and the extracted RNA was treated with RNase-free DNase (Qiagen) to remove DNA. Ten nanogram (ng) of each DNA-free RNA sample was used to produce complementary DNA (cDNA) with the oligo (dT) primer and M-MLV reverse transcriptase (TaKaRa, Dalian, China) in qPCR analysis. Briefly, amplification was conducted in a 20 μL volume containing 10 μL of Syber Green PCR Master mixture, 5 μL of cDNA template, 1 μL (10 pmol) of each primer, and 3 μL of H2O. The primers used to amplify genes encoding interferon-gamma (IFN-γ), tumor necrosis factor (TNF), CC chemokine (regulated on activation, normal T cell expressed and secreted, RANTES), and β-actin gene (control) were synthesized according to the sequences described previously.14 The fold change (FC) in expression of the target gene relative to the β-actin gene was calculated as follows: FC = 2−ΔΔCt where ΔΔCt = (CtTarget – CtActin)sample – (CtTarget – CtActin)reference.14 The Ct values were defined as the cycle numbers at which the fluorescence signals were detected,16 and sample values represented by tissues from viable organism-infected mice and the reference values as tissues from mice inoculated with heat-inactivated organisms.
Antibody determination.
The Renografin gradient purified R. heilongjiangensis was applied by pen point to 12-well microscope slides and the slides were fixed with methanol after drying. The antigen-coated slides were used to detect the antibodies to R. heilongjiangensis in sera by indirect immunofluorescence assay (IFA). The serum samples from mice inoculated with viable organisms or heat-inactivated organisms of R. heilongjiangensis were diluted from 1:25 to 3,200 for analysis of specific IgG titers by IFA according to our previous description.17
Statistical analysis.
The analysis for each group was performed on at least five samples. Results were expressed as median with 25% and 75% distribution, and minimum and maximum values for qPCR.14 Cytokine and antibody levels were expressed as mean ± SD. Quantitative data were compared with the Mann-Whitney U test. Differences are considered significant when P < 0.05. Statistical analyses were performed using GraphPad Prism version 5.00 for Windows.
Results
R.heilongjiangensis infection of BALB/c mice.
BALB/c mice infected with 105 viable R. heilongjiangensis organisms by the intravenous route showed decreased activity and ruffled fur from Days 2 to 5 p.i. and they returned to normal physical appearance and activity after 6 days p.i. However, BALB/c mice inoculated with 105 heat-inactivated organisms survived without any signs of illness during the course of the experiment. Rickettsemias of BALB/c mice infected with R. heilongjiangensis were determined by qPCR. The rickettsial DNA copies were detectable in whole blood from mice infected with viable organisms at Day 1, significantly (P < 0.05) increased at Day 3 p.i.; the highest levels were determined at Day 6, significantly (P < 0.05) decreased at Day 9 p.i. (Figure 1A). The rickettsial load in tissues from mice infected with viable organisms was also analyzed by qPCR. The rickettsial DNA copies were detectable in liver (Figure 1B), spleen (Figure 1C), lungs (Figure 1D), and brain (Figure 1E) from mice infected with R. heilongjiangensis at Day 1 p.i., respectively, and the highest levels were determined at Day 6 p.i.; the DNA copies in these tissues were significantly (P < 0.05) increased between Days 1 and 3 p.i. and significantly (P < 0.05) decreased between Days 6 and 9 p.i.
Figure 1.
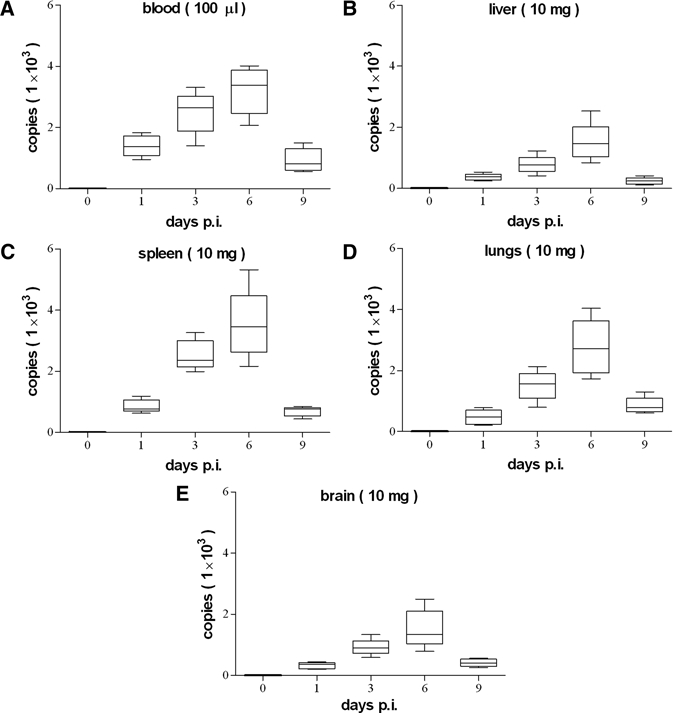
Rickettsial loads in blood and organs of R. heilongjiangensis-infected mice.
Lesions caused by R. heilongjiangensis.
Pathological lesions were observed in liver, lungs, and brain of the R. heilongjiangensis-infected mice at Day 6 p.i. and the lesions in these organs were much less severe at Day 9 p.i. In liver tissues, inflammatory infiltrates consisting of mononuclear cells and polymorphonuclear leukocytes were focused on the portal area of the liver and slight swelling of liver cells was observed (Figure 2A and B). For lung lesions, interstitial pneumonia was characterized by numerous inflammatory infiltrates of mononuclear cells and polymorphonuclear leukocytes and alveolar interstitial thickening (Figure 2C and D). Microhemorrhages were found in brain parenchyma (Figure 2E and F). In contrast to live R. heilongjiangensis, heat-inactivated organisms did not induce any hepatic, pulmonary, and cerebral lesions in mice.
Figure 2.

Pathological lesions in R. heilongjiangensis-infected mice. The tissue samples of the organs were obtained from the R. heilongjiangensis-infected mice at Day 6 p.i. Perivascular infiltration of inflammatory cells and swelling of liver cells were observed in the liver of mice infected with viable R. heilongjiangensis (A and B, original magnifications ×400). Lung lesion consisting of perivascular inflammatory infiltration and alveolar interstitial thickening are observed (C and D, original magnifications ×200). Microhemorrhage was found in the brain (E and F, original magnifications ×100).
Immune response to R. heilongjiangensis.
The humoral immunity was assessed in R. heilongjiangensis-infected mice. Antibodies (IgGs) to R. heilongjiangensis were measured in mouse sera by IFA. The specific antibodies were detected in sera from mice infected with viable organisms at Day 7 and progressively increased until Day 21 p.i. (Figure 3). The specific antibodies were also detected in sera from mice inoculated with heat-inactivated organisms, but the antibody levels were significantly (P < 0.05) lower than that of viable organism-infected mice.
Figure 3.

Specific antibody responses in the mice after R. heilongjiangensis infection.
IFN-γ, TNF, and RANTES transcripts in organs of R. heilongjiangensis-infected mice were assessed by qPCR. Their transcripts were significantly increased in liver, spleen, lungs, and brain from viable organism-infected mice at Day 3 p.i. and decreased at Day 6 (Figure 4A, C, E, and G). Though cytokine transcripts were also observed in these organs of mice inoculated with heat-inactivated organisms, the levels were significantly (P < 0.05) lower than that of viable organism-infected mice at Day 3, respectively (Figure 4B, D, F, and H).
Figure 4.

Messenger RNA (mRNA) expression of interferon-gamma (IFN-γ), tumor necrosis factor (TNF), and RANTES in tissues of mice infected with R. heilongjiangensis. V, depicts that mice were inoculated with viable organisms; I, depicts that mice were inoculated with inactivated organisms.
Discussion
Mediannikov and colleagues5 investigated FESF in the Far East area of Russia and described the characteristics of the disease. Most of the patients naturally infected by R. heilongjiangensis had fever, chills, headache, dizziness, myalgias, arthralgias, and anorexia after an incubation period of 4–7 days, but no specific symptoms appeared during the first several days.5 Leukocytosis was found in certain patients at admission, suggesting a bacterial infection, and later most of the patients appeared with a macular or maculopapular rash and certain patients had a primary lesion (eschar) at the site of tick attachment and lymphadenopathy regional to the eschar.5 Almost half of the patients had hepatomegaly accompanied with an increased alanine aminotransferase and/or aspartate aminotransferase activity, indicating that these patients had a liver lesion caused by R. heilongjiangensis infection.5
Tick-born SFG rickettsiae are transmitted through the bite of an infected tick. After rickettsial infection in humans, rickettsemia develops with disseminated infection of the vascular endothelium, culminating in meningoencephalitis and interstitial pneumonitis that lead to increased vascular permeability, noncardiogenic pulmonary edema, and even hypotensive shock.18 In this study, BALB/c mice were infected with R. heilongjiangensis by injection of retro-orbital venous plexus. In this murine model, rickettsiae passed the cutaneous immune system by the intravenous inoculation and rapidly disseminated by a systemic endothelial vascular infection. Our results show that rickettsiae were detectable in blood at Day 1 p.i. and the amount of rickettsiae in blood was significantly increased at Day 3 p.i., suggesting a severe rickettsemia had developed in the mice caused by rickettsial survival and growth in vascular endothelial cells. In addition, rickettsiae were loaded in the liver, spleen, lungs, and brain from Days 1 to 9 after R. heilongjiangensis infection. The highest level of rickettsial load was observed in these organs at Day 6 p.i. This result demonstrated that the rickettsiae were rapidly disseminated to multiple organs of the mice, including lungs and brain, the most important organs relating to severe pathologic injury and death of human rickettsioses.19 The SFG rickettsiae were identified in vascular endothelial cells in multiple foci in the brain and lung tissues of the patient in a fatal human SFG rickettsiosis.20
In Rickettsia australis-infected BALB/c murine model, rickettsiosis was developed with progressively severe vasculitis, interstitial pneumonia, and multifocal hepatic necrosis.21 In this study, R. heilongjiangensis-infected BALB/c mice also exhibited most of the features of human spotted fever, including hematogenous dissemination, multifocal inflammatory lesions, and inflammatory infiltrates. Noticeably, the lungs of BALB/c mice were a primary site of the infection and interstitial pneumonitis and cerebral hemorrhages were observed in the infected mice, suggesting that R. heilongjiangensis may cause severe rickettsiosis.
In natural SFG rickettsial infection, rickettsiae are initially inoculated by arthropod saliva into blood lakes in the skin generated by the vector during feeding, and the fate and severity of the infection may depend on the path of infection at the primary phase of infection. The first path, from the dermis into capillaries, may lead to rapid systemic spread by infection of the vascular endothelium, whereas the second path, from the dermis into lymphatics of the subcutis, with initial infection of the lymphatic endothelium, may lead to infection of dendritic cells, efficient antigen presentation in lymph nodes and earlier containment of the infection by the adaptive immune response.22 Under both scenarios, there is a component of systemic endothelial vascular infection that induces the innate immune response. In this study, we also found that R. heilongjiangensis infection of BALB/c mice was accompanied by the development of a humoral response. The specific antibodies were detected in sera from mice infected with viable organisms at Day 7 and progressively increased until Day 21 p.i. The specific antibodies appeared concomitantly with suppression of rickettsial infection.
To explore the roles of cytokine in R. heilongjiangensis infection, the messenger RNA (mRNA) expression of IFN-γ and TNF was assessed in this study. As a result, IFN-γ and TNF mRNA were highly expressed in the R. heilongjiangensis-infected organs at Day 3 p.i. Although proinflammatory factors IFN-γ and TNF are known to protect hosts from intracellular microorganisms,23 inflammation is by far the most common cause of tissue injury. The severe tissue lesions of the infected organs observed at Day 6 might be induced by the high-expressed IFN-γ and TNF at Day 3 p.i., although their expression declined at Day 6. Additionally, the high-level RANTES transcript was found in liver and spleen at Day 3, particularly in the spleen at Day 6 p.i. (final observed point). The presence of RANTES transcript in the organs suggests that RANTES is associated with the elimination of R. heilongjiangensis in bodies because RANTES is involved in granuloma formation and protective Th1 responses.24
Cytokines were hypothesized to play roles both in the pathogenesis of vasculopathic rickettsioses and in the immunity against rickettsiae.14,25,26 The significant decline in the mRNA expression of IFN-γ and TNF appeared concomitantly with a marked increase in rickettsial load in the infected organs between Days 3 and 6 p.i., which suggests that the host defenses were suppressed so that rickettsiae grew rapidly during this infection course. Although the expression of IFN-γ and TNF was declined in the Rickettsia-infected organs except for the spleen at Day 6 p.i., the rickettsial load in the infected organs was significantly decreased between Days 6 and 9, indicating that the specific antibodies and other immune factors that were not measured in this study play important roles in the rickettsial elimination. The hypothetical roles of the various cytokines in infection caused by R. heilongjiangensis will be defined by future experiments.
Conclusions
In this study, by intravenous inoculation, R. heilongjiangensis established disseminated intracellular infection in BALB/c mice and caused pathological lesions and inflammatory cytokine expression in major organs similar to what is observed in human spotted fever. Our results reveal that R. heilongjiangensis may cause an infection in BALB/c mice and the pathological lesions in the infected organs are associated with host inflammatory response induced by R. heilongjiangensis.
Footnotes
Financial support: This research was supported by a grant (2010CB530200/2010CB530205) from the National Basic Research Program of China and a grant (2008ZX10004-002) from National Science and Technology Major Project (Infectious Disease Control and Prevention) of China.
Authors' addresses: Changsong Duan, Yanfen Meng, Xile Wang, Xiaolu Xiong, and Bohai Wen, Beijing Institute of Microbiology and Epidemiology, Beijing, China, E-mails: xysage@163.com, myf810110@sohu.com, wangxile80@163.com, xiongxlbob@hotmail.com, and bohaiwen@sohu.com.
Reprint requests: Bohai Wen, Beijing Institute of Microbiology and Epidemiology, 20 Dong-Da-Jie St., Fengtai, Beijing 100071, China, E-mail: bohaiwen@sohu.com.
References
- 1.Raoult D, Berbis P, Roux V, Xu W, Maurin M. A new tick-transmitted disease due to Rickettsia slovaca. Lancet. 1997;350:112–113. doi: 10.1016/S0140-6736(05)61814-4. [DOI] [PubMed] [Google Scholar]
- 2.Uchida T, Uchiyama T, Kumano K, Walker DH. Rickettsia japonica sp. nov., the etiological agent of spotted fever group rickettsiosis in Japan. Int J Syst Bacteriol. 1992;42:303–305. doi: 10.1099/00207713-42-2-303. [DOI] [PubMed] [Google Scholar]
- 3.Fournier PE, Dumler JS, Greub G, Zhang J, Wu Y, Raoult D. Gene sequence-based criteria for identification of new Rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J Clin Microbiol. 2003;41:5456–5465. doi: 10.1128/JCM.41.12.5456-5465.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu YM, Zhang ZQ, Wang HJ, Yang Q, Feng L, Wang JW. Investigation on the epidemiology of Far-East tick-borne spotted fever in the northeastern area of China. Zhonghua Liu Xing Bing Xue Za Zhi. 2008;29:1173–1175. [PubMed] [Google Scholar]
- 5.Mediannikov OY, Sidelnikov Y, Ivanov L, Mokretsova E, Fournier PE, Tarasevich I, Raoult D. Acute tick-borne rickettsiosis caused by Rickettsia heilongjiangensis in Russian Far East. Emerg Infect Dis. 2004;10:810–817. doi: 10.3201/eid1005.030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ando S, Kurosawa M, Sakata A, Fujita H, Sakai K, Sekine M, Katsumi M, Saitou W, Yano Y, Takada N, Takano A, Kawabata H, Hanaoka N, Watanabe H, Kurane I, Kishimoto T. Human Rickettsia heilongjiangensis infection, Japan. Emerg Infect Dis. 2010;16:1306–1308. doi: 10.3201/eid1608.100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shpynov SN, Fournier PE, Rudakov NV, Samoilenko IE, Reshetinokova TA, Yastrebov VK, Schaiman MS, Tarasevich IV, Raoult D. Molecular identification of a collection of spotted fever group rickettsiae obtained from patients and ticks from Russia. Am J Trop Med Hyg. 2006;74:440–443. [PubMed] [Google Scholar]
- 8.Mediannikov O, Makarova V, Tarasevich I, Sidelnikov Y, Raoult D. Isolation of Rickettsia heilongjiangensis strains from humans and ticks and its multispacer typing. CMI. 2009;15((Suppl 2)):288–289. doi: 10.1111/j.1469-0691.2008.02239.x. [DOI] [PubMed] [Google Scholar]
- 9.Mediannikov O, Sidelnikov Y, Ivanov L, Fournier PE, Tarasevich I, Raoult D. Far eastern tick-borne rickettsiosis: identification of two new cases and tick vector. Ann N Y Acad Sci. 2006;1078:80–88. doi: 10.1196/annals.1374.010. [DOI] [PubMed] [Google Scholar]
- 10.Ammerman N, Beier-Sexton M, Azad A. Laboratory maintenance of Rickettsia rickettsii. Curr Protoc Microbiol. 2008 doi: 10.1002/9780471729259.mc03a05s11. Chapter 3: Unit 3A.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson BA, Wisseman CL, Jr, Waddell A, Silverman DJ. Some characteristics of heavy and light bands of Rickettsia prowazekii on renografin gradients. Infect Immun. 1981;34:596–604. doi: 10.1128/iai.34.2.596-604.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss E, Coolbaugh J, Williams J. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975;30:456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss E, Rees HB, Hayes JR. Metabolic activity of purified suspensions of Rickettsia rickettsii. Nature. 1967;213:1020–1022. [Google Scholar]
- 14.Bechah Y, Capo C, Grau GE, Raoult D, Mege JL. A murine model of infection with Rickettsia prowazekii: implications for pathogenesis of epidemic typhus. Microbes Infect. 2007;9:898–906. doi: 10.1016/j.micinf.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Steel C, Stephens A, Hahto S, Singletary S, Ciavarra R. Comparison of the lateral tail vein and the retro-orbital venous sinus as routes of intravenous drug delivery in a transgenic mouse model. Lab Anim (NY) 2008;37:26–32. doi: 10.1038/laban0108-26. [DOI] [PubMed] [Google Scholar]
- 16.Desnues B, Raoult D, Mege JL. IL-16 is critical for Tropheryma whipplei replication in Whipple's disease. J Immunol. 2005;175:4575–4582. doi: 10.4049/jimmunol.175.7.4575. [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Niu D, Wen B, Chen M, Qiu L, Zhang J. Protective immunity against Q fever induced with a recombinant P1 antigen fused with HspB of Coxiella burnetii. Ann N Y Acad Sci. 2005;1063:130–142. doi: 10.1196/annals.1355.021. [DOI] [PubMed] [Google Scholar]
- 18.Walker DH, Valbuena GA, Olano JP. Pathogenic mechanisms of diseases caused by Rickettsia. Ann N Y Acad Sci. 2003;990:1–11. doi: 10.1111/j.1749-6632.2003.tb07331.x. [DOI] [PubMed] [Google Scholar]
- 19.Walker DH, Herrero-Herrero JI, Ruiz-Beltran R, Bullon-Sopelana A, Ramos-Hidalgo A. The pathology of fatal Mediterranean spotted fever. Am J Clin Pathol. 1987;87:669–672. doi: 10.1093/ajcp/87.5.669. [DOI] [PubMed] [Google Scholar]
- 20.Zavala-Castro JE, Zavala-Velazquez JE, Walker DH, Ruiz Arcila EE, Laviada-Molina H, Olano JP, Ruiz-Sosa JA, Small MA, Dzul-Rosado KR. Fatal human infection with Rickettsia rickettsii, Yucatan, Mexico. Emerg Infect Dis. 2006;12:672–674. doi: 10.3201/eid1204.051282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng HM, Wen J, Walker DH. Rickettsia australis infection: a murine model of a highly invasive vasculopathic rickettsiosis. Am J Pathol. 1993;142:1471–1482. [PMC free article] [PubMed] [Google Scholar]
- 22.Valbuena G, Feng HM, Walker DH. Mechanisms of immunity against rickettsiae. New perspectives and opportunities offered by unusual intracellular parasites. Microbes Infect. 2002;4:625–633. doi: 10.1016/s1286-4579(02)01581-2. [DOI] [PubMed] [Google Scholar]
- 23.Kasanen IH, Inhila KJ, Nevalainen JI, Vaisanen SB, Mertanen AM, Mering SM, Nevalainen TO. A novel dietary restriction method for group-housed rats: weight gain and clinical chemistry characterization. Lab Anim. 2009;43:138–148. doi: 10.1258/la.2008.008023. [DOI] [PubMed] [Google Scholar]
- 24.Chensue SW, Warmington KS, Allenspach EJ, Lu B, Gerard C, Kunkel SL, Lukacs NW. Differential expression and cross-regulatory function of RANTES during mycobacterial (type 1) and schistosomal (type 2) antigen-elicited granulomatous inflammation. J Immunol. 1999;163:165–173. [PubMed] [Google Scholar]
- 25.Walker DH. Biology of Rickettsial Diseases. Boca Raton, FL: CRC Press; 1988. p. 11538. (Pathology and pathogenesis of the vasculotropic rickettsioses). [Google Scholar]
- 26.Manor E, Sarov I. Inhibition of Rickettsia conorii growth by recombinant tumor necrosis factor alpha: enhancement of inhibition by gamma interferon. Infect Immun. 1990;58:1886–1890. doi: 10.1128/iai.58.6.1886-1890.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


