Abstract
Polychlorinated biphenyls (PCBs) are persistent worldwide pollutants that are of concern due to their bioaccumulation and health effects. Metabolic oxidation of PCBs results in the formation of hydroxylated metabolites (OHPCBs). Among their biological effects, OHPCBs have been shown to alter the metabolism of endocrine hormones, including inhibition of mammalian cytosolic sulfotransferases (SULTs) that are responsible for the inactivation of thyroid hormones and phenolic steroids (i.e., hSULT1A1, hSULT1B1, and hSULT1E1). OHPCBs also interact with a human hydroxysteroid sulfotransferase that plays a role in the sulfation of endogenous alcohol-containing steroid hormones and bile acids (i.e., hSULT2A1). The objectives of our current study were to examine the effects of a series of OHPCB congeners on the activity of hSULT2A1 and to develop a three-dimensional quantitative structure activity relationship (3D-QSAR) model for OHPCBs as inhibitors of the enzyme. A total of 15 OHPCBs were examined, and the sulfation of 1 μM [3H] dehydroepiandrosterone (DHEA) was utilized as a model reaction catalyzed by the enzyme. All 15 OHPCBs inhibited the sulfation of DHEA, with IC50 values ranging from 0.6 μM to 96 μM, and eight of these OHPCBs were also substrates for the enzyme. Comparative molecular field analysis (CoMFA) provided a predictive 3D-QSAR model with a q2 value of 0.697 and an r2 value of 0.949. The OHPCBs that had the highest potency as inhibitors of DHEA sulfation were those with a 3, 5-dichloro-4-hydroxy substitution pattern on the biphenyl ring system, and these congeners were also substrates for sulfation catalyzed by hSULT2A1.
Introduction
Polychlorinated biphenyls (PCBs) are synthetic chemicals that were produced and sold for decades. Although production of PCBs was banned in the late 1970’s, these compounds are a persistent worldwide problem.1–5 Many of the PCBs with higher numbers of chlorine atoms (e.g., 5 or more) are relatively resistant to biodegradation, accumulate in the food chain, and have significant health effects in humans and other animals.6 Those PCBs with lower numbers of chlorine atoms can be released into the atmosphere from various sources that include, among others, contaminated buildings, water, and soil.1 Significant levels of volatile PCBs have been measured in both indoor and outdoor air.7–12 These airborne PCBs are inhaled and there is evidence of increased blood concentrations of the lower chlorinated PCBs in humans.5 Metabolism of these lower chlorinated PCBs can lead to reactive intermediates that are implicated in carcinogenesis and other toxicities.1,5,13
Mammalian metabolism of PCBs often involves oxidation catalyzed by cytochrome P450 (CYP) enzymes to form hydroxylated PCBs (OHPCBs).6 Further metabolism of OHPCBs may include conjugation reactions such as those catalyzed by cytosolic sulfotransferases (SULTs) and UDP-glucuronosyltransferases (UGTs), with the resulting formation of PCB sulfates and PCB glucuronides, respectively.6,14 While glucuronidation and sulfation might be expected to lead to elimination of the OHPCB, the actual fates of these conjugates are less clear, since some PCB sulfates and glucuronides retain significant lipophilic properties based on the calculated octanol/water partition coefficients.14
The concentrations of OHPCBs have been determined in humans,15–20 and there is evidence for selective retention of OHPCBs in blood relative to the parent PCBs.15 These hydroxylated metabolites have been implicated in some of the adverse health effects observed upon exposure to PCBs. For example, some OHPCBs bind with high affinity to the thyroid hormone transport protein transthyretin,21 and this may contribute to metabolic disruptions in thyroid hormone function in some tissues. The OHPCBs are also potent inhibitors of several cytosolic sulfotransferases (SULTs) that are important in metabolism of endocrine hormones. These SULTs include the family 1 enzymes hSULT1A1 and hSULT1B1 that catalyze sulfation of thyroid hormones.22,23 Inhibition of another family 1 isoform, the estrogen sulfotransferase (hSULT1E1), by OHPCBs can lead to increased levels of estrogens in tissues, thus providing a mechanism for the estrogenic effects seen with these molecules.24
The interactions of OHPCBs with family 2 SULTs, such as hSULT2A1 (previously known as the human hydroxysteroid, or alcohol, sulfotransferase) are, however, not as well understood. The hSULT2A1 plays an important role in the sulfation of alcohol-containing steroids, bile acids and xenobiotics.25–27 Previous studies in our laboratory showed that three OHPCBs interact with hSULT2A1,28 although structure-activity relationships were not available from that work. Therefore, we have now more extensively examined the relationships between the structures of OHPCBs and their interactions with hSULT2A1 as inhibitors and substrates.
Materials and Methods
Chemicals and Reagents
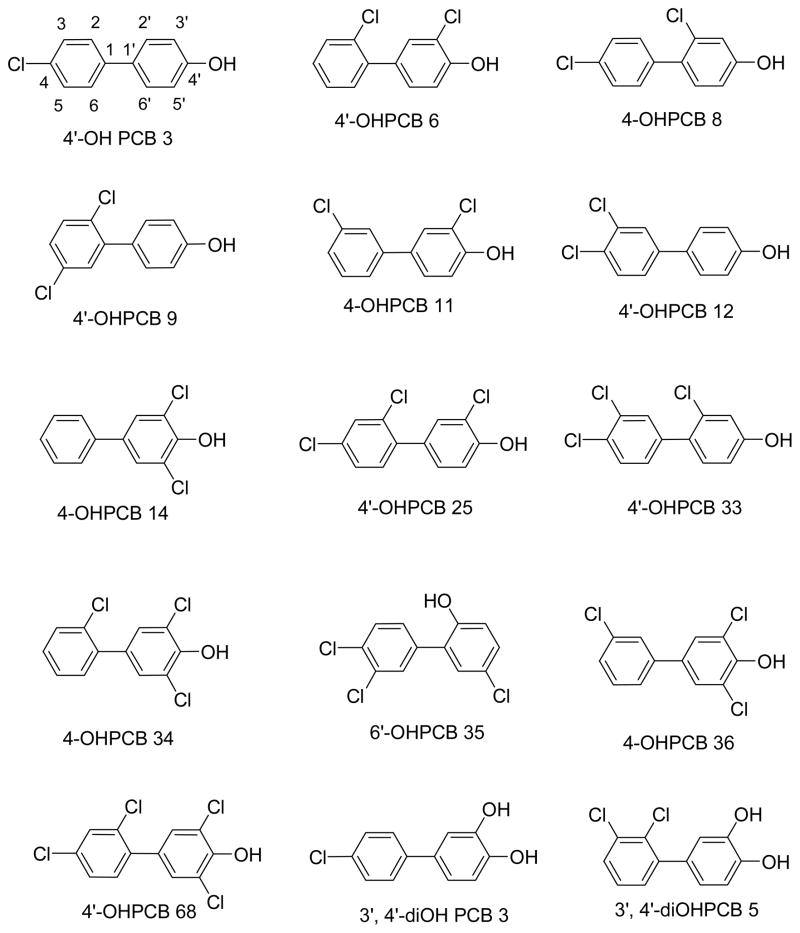
All OHPCBs (structures shown in Figure 1) were synthesized as described previously.29 3′-Phosphoadenosine-5′-phosphosulfate (PAPS) was purchased from Sigma-Aldrich (St. Louis, MO), and it was further purified (> 98% as analyzed by HPLC) using a previously published procedure.30 3H-DHEA (94.5 Ci/mmol) was obtained from Perkin Elmer Life and Analytical Sciences (Boston, MA). Econo-Safe biodegradable scintillation cocktail and Tris-HCl, were purchased from RPI (Mt. Prospect, IL). Hydroxyapatite (Bio-Gel HT) was from Bio-Rad Laboratories (Hercules, CA), and Tween 20 was obtained from J.T. Baker Chemicals (Phillipsburg, NJ). All other chemicals were of the highest purity commercially available.
Figure 1.
Structures of OHPCBs used in this study. The numbering scheme for 4′-OH PCB 3 is indicated.
Expression and Purification of Human SULT2A1
Human sulfotransferase hSULT2A1 was expressed in recombinant Escherichia coli BL21 (DE3) cells using the following minor modification of a previously described procedure.31 After sterile Luria broth (LB) medium was supplemented with filter-sterilized ampicillin to a final concentration of 50 μg/mL, a 3 mL volume of this medium was inoculated with cells and incubated at 28°C for 24 h on a reciprocating shaker at 210 rpm. Aliquots of 100 μL each from this culture were added to each of four culture tubes containing 20 mL of LB medium. Following incubation for 24 h under the above conditions, the contents of each tube were added to four 1 L flasks that each contained 400 mL of sterile LB medium, and the cells were incubated for 1 h at 28°C with shaking at 210 rpm. Cells were then induced by adding 1 mM IPTG (isopropyl-1-thio-D-galactopyranoside) and incubated under the above conditions for 23 h. The resulting cells were harvested by centrifugation at 10,000 × g for 30 min. The cell pellet (19 g, wet weight) was suspended in 20 mL ice-cold buffer containing 0.25M sucrose, 10% (v/v) glycerol, 1 mM phenylmethyl sulfonyl fluoride, 1 μM pepstatin A, 1 mM dithiothreitol, 3.3 μM antipain and 10 mM Tris-HCl at pH 7.5. The cells were disrupted by sonication with a Branson Digital Sonifier (model 450, Branson Ultrasonics Corp., Danbury, CT) and then subjected to centrifugation for 30 min at 12,000 × g. The resulting supernatant fraction was stored at −70°C until purification. Purification of hSULT2A1 was accomplished using the previously described method.32 The purified enzyme was homogeneous as judged by SDS-PAGE with staining by Coomassie Blue. Protein concentrations were determined by the modified Lowry procedure33 with bovine serum albumin as standard. The specific activity of hSULT2A1 during purification steps was determined using the methylene blue paired ion extraction assay.34,35
Inhibition of hSULT2A1 by OHPCBs
The ability of OHPCBs to inhibit the sulfation of DHEA catalyzed by hSULT2A1 was determined using a previously described radiochemical assay method.36 Assays contained 200 μM PAPS, 1 μM [3H]DHEA (final radioactive specific activity of 2 μCi/nmol), varying concentrations of OHPCBs in ethanol (final ethanol concentration of less than 2%, v/v), 0.25 M potassium phosphate at pH 7.0, and 7.5 mM 2-mercaptoethanol in a total volume of 200 μL. After the addition of 0.25 μg of hSULT2A1 to start the reaction, the assay mixture was incubated for 10 min at 37°C. The reaction was terminated by the addition of 0.8 mL 50 mM KOH, unreacted DHEA was extracted with 0.5 mL CHCl3, and radioactivity of the DHEA-sulfate in a 100 μL aliquot of the aqueous layer was determined. The rate of sulfation was expressed as nmoles of DHEA sulfate formed per minute per mg of protein. The means of three determinations at each concentration of inhibitor were fit to a sigmoidal dose-response curve (SigmaPlot v.11; Systat Software, Chicago, IL) to determine IC50 values.
OHPCBs as substrates for hSULT2A1
A previously described HPLC assay35,37 was used to determine the OHPCBs that were substrates for hSULT2A1. Reaction mixtures (total volume of 30 μL) contained 200 μM PAPS, 0.25M potassium phosphate at pH 7.0, 7.5mM 2-mercaptoethanol, OHPCBs dissolved in acetone (final concentration of acetone less than 5%, v/v). Sulfation reactions were initiated by addition of 1 μg of enzyme and incubated at 37°C for 10 min. Reactions were terminated by addition of 30 μL methanol followed by votex-mixing for 30 s. The resulting reaction mixture was kept on ice until analysis of the substrate-dependent formation of the product, adenosine 3′, 5′-diphosphate (PAP) was carried out by HPLC. The limit of detection for this assay method is 0.1 nmol of PAP formed/min/mg of purified recombinant hSULT2A1.31
3D-QSAR Model for Inhibition of hSULT2A1 by OHPCBs
Comparative molecular field analysis (CoMFA)38 was used to generate predictive models for fifteen OHPCBs. Structures of these OHPCBs were constructed in Sybyl 8.0. (Tripos Inc., 2008) and saved in MOL2 format. Gasteiger-Huckel charges were assigned to the compounds, and the gradient convergence criterion set at 0.05 kcal/(Åmol). Alignment was done on a common biphenyl substructure with 4-OHPCB 68 as template. The IC50 values were converted into -log values and served as the dependent variables in the CoMFA analysis. After alignment, each molecule was analyzed in a 3-dimensional cubic lattice with an sp3 carbon atom probe having a net charge of +1 and energy cut-off value of 30 kcal/mol. The CoMFA steric field, calculated according to the Lennard-Jones potential, and the electrostatic field, calculated by the Coulomb potential, were obtained. CoMFA models were derived, and they were evaluated using leave-one-out (LOO) cross validation. Values for q2 (predictive power of the model), the optimum number of components, and r2 (correlation coefficient for fit of the dataset activity to the model) were then obtained. External validation of the method was carried out by removal of three of the compounds and generation of a second model based on the remaining twelve compounds.
Results
OHPCBs as inhibitors of the sulfation of DHEA catalyzed by hSULT2A1
As seen in Table 1, all of the OHPCBs studied (structures in Figure 1) inhibited the hSULT2A1-catalyzed sulfation of DHEA, and there was a greater than 100-fold range in IC50 values. Complete dose-dependent inhibition curves for each OHPCB are provided in the Supporting Information (Figure S1). Among the 15 OHPCBs examined, the two most potent inhibitors of the sulfation of DHEA were OHPCB 34 and 4′-OHPCB 68, with IC50 values of 0.6 and 0.8 μM, respectively. These two compounds possess a 3, 5-dichloro-4-hydroxy substitution pattern, and they also have a chlorine atom ortho to the biphenyl ring junction. Dichlorinated OHPCBs with a chlorine atom ortho to the ring junction (i.e., 4′-OHPCB 9, 4′-OHPCB 6 and 4-OHPCB 8) were generally good inhibitors, with IC50 values of 8 – 10 μM. Furthermore, the dichlorinated OHPCBs with both chlorine atoms in the non-phenolic ring (i.e., 4′-OHPCB 9 and 4′-OHPCB 12) indicated that the position of the ortho chlorine atom at the ring junction in 4′-OHPCB 9 increased the potency of inhibition of hSULT2A1 when compared with the non-ortho analog, 4′-OHPCB 12.
Table 1.
Inhibition of Human SULT2A1 by OHPCBsa
| Compound | IC50 (μM) |
|---|---|
| 4-OHPCB 34 | 0.6 ± 1.2 |
| 4′-OHPCB 68 | 0.8 ± 1.3 |
| 4′-OHPCB 33 | 2.5 ± 1.1 |
| 4′-OHPCB 25 | 4.4 ± 1.3 |
| 4-OHPCB 36 | 4.4 ± 1.2 |
| 4′-OHPCB 9 | 7.5 ± 1.1 |
| 4′-OHPCB 6 | 9.8 ± 1.1 |
| 4-OHPCB 8 | 10 ± 1.2 |
| 4-OHPCB 14 | 13 ± 1.1 |
| 4-OHPCB 11 | 14 ± 1.2 |
| 4′-OHPCB 12 | 16 ± 1.1 |
| 6′-OHPCB 35 | 16 ± 1.1 |
| 3′,4′-diOHPCB 5 | 16 ± 1.1 |
| 4′-OHPCB 3 | 28 ± 1.1 |
| 3′,4′-diOHPCB 3 | 96 ± 1.2 |
The structures of these compounds are shown in Figure 1. IC50 values are the micromolar concentration resulting in 50% inhibition of the sulfation of DHEA catalyzed by hSULT2A1. IC50 values are expressed as means and standard error of the fit of the data in Figure S1 (Supporting Information) based on triplicate determinations of the rate of sulfation at each concentration of inhibitor.
The overall patterns indicating both the importance of an ortho chlorine at the ring junction and the favorable presence of a 3, 5-dichloro-4-hydroxy substitution pattern for inhibition were also observed with the trisubstituted OHPCBs. For example, comparison of 4-OHPCB 34 with 4-OHPCB 36 indicated an approximately 7-fold decrease in IC50 upon changing the chlorine atom in the non-phenolic ring from the meta to ortho position with respect to the ring junction. While the 3′, 4′-diOHPCBs were among the least potent inhibitors, the presence of a chlorine atom ortho to the ring junction in 3′, 4′-diOHPCB 5 did have an effect consistent with the observed differences in IC50 values for other OHPCBs with and without ortho chlorine atoms.
Sulfation of OHPCBs catalyzed by hSULT2A1
Since alternate substrates for an enzyme might be responsible for inhibition of a specific reaction such as the sulfation of DHEA catalyzed by hSULT2A1, we investigated the possibility that some of these OHPCBs might be substrates for sulfation catalyzed by the enzyme. Indeed, eight of the 15 OHPCBs were substrates for hSULT2A1. The initial velocity data for these compounds were fitted to a substrate inhibition equation v=Vmax/(1+ (Km/[S])+[S]/Kis) (Table 2). A concentration of 200 μM PAPS was used due to previous findings that the highest initial velocities at low concentrations of DHEA were achieved with 100 – 300 μM PAPS.36 The values for Km (i.e. the concentration of substrate at half maximal velocity) ranged from 8.1 ± 3.2 μM to 56.8 ± 58.2 μM with 4-OHPCB 34 having the lowest Km value. The values for Vmax (i.e. the maximum reaction velocity) ranged from 8.5 ± 0.5 nmol/min/mg to 57.1 ± 11.4 nmol/min/mg. These data indicated that hSULT2A1 had approximately ten times higher catalytic efficiency (kcat/Km) for OHPCBs with a 3, 5-dichloro-4-hydroxy substitution pattern, i.e. 4-OHPCB 14, 4-OHPCB 34, and 4-OHPCB 36. As observed in the kinetic profiles shown in Figure 2, at higher concentrations of substrate, a decrease in rate is seen due to substrate inhibition. The values calculated for the substrate inhibition constant, Kis, provide a quantitative measure of this effect. As it has been shown previously for DHEA, this substrate inhibition in hSULT2A1 is likely the result of the formation of dead-end complexes (e.g., an enzyme-PAP-substrate complex).36 There are, however, differences in the magnitude of kinetic values for OHPCBs as substrates for hSULT when compared with those for DHEA at the same 200 μM concentration of PAPS. Kinetic values for Vmax, Km, and Kis with DHEA as substrate were 245 ± 40 nmol/min/mg, 0.8 ± 0.3 μM, and 8 ± 3 μM, respectively.36
Table 2.
Kinetic Parameters for the Sulfation of OHPCBs Catalyzed by hSULT2A1
| OHPCB | Vmax (nmol/min/mg) | Km (μM) | Kis (μM) | kcat/Km (min−1mM−1) |
|---|---|---|---|---|
| 4-OHPCB 14 | 44.3 ± 3.9 | 8.5 ± 1.8 | 220 ± 56 | 176 |
| 4-OHPCB 34 | 57.1 ± 11.4 | 8.1 ± 3.2 | 47.4 ± 16.2 | 238 |
| 4-OHPCB 36 | 51.8 ± 16.3 | 13.6 ± 6.7 | 39.4 ± 23.1 | 129 |
| 4-OHPCB 8 | 56.1 ± 42.6 | 56.8 ± 58.2 | 65.4 ± 73.8 | 33 |
| 4′-OHPCB 33 | 21.0 ± 7.4 | 26.2 ± 16.2 | 217 ± 189 | 27 |
| 4′-OHPCB 25a | 8.5 ± 0.5 | 8.6 ± 2.0 | - | 33 |
| 4-OHPCB 11 | 21.4 ± 18.3 | 36.6 ± 43.9 | 51.9 ± 65.7 | 20 |
While 4′-OHPCB 25 and 4′-OHPCB 68 (not shown) were substrates for hSULT2A1, reliable kinetic constants for Kis (both OHPCBs) and for Km and Vmax (4′-OHPCB 68) were not obtained due to low solubility. Values of Km and Vmax for 4′-OHPCB 25 were estimated using the Michaelis-Menten equation. Note: kcat/Km values are based on a subunit Mr of 33,785.
Figure 2.
The ability of purified recombinant hSULT2A1 to catalyze sulfation of OHPCBs. Assays were carried out as described in Materials and Methods. (A) Sulfation of 4-OHPCB 14 (◆), 4-OHPCB 34 (◇), 4-OHPCB 8 (●), 4′-OHPCB 33 (□), and 4-OHPCB 11 (▼). (B, C, and D) Sulfation of 4′OHPCB 68, 4-OHPCB 36, and 4′-OHPCB 25, respectively. Data are fit to the following equation for substrate inhibition: v = Vmax/(1+ (Km/[S])+[S]/Kis). Data points are the means ± standard error of triplicate determinations.
Development of 3D-QSAR models for the interaction of OHPCBs with hSULT2A1
Comparative molecular field analysis (CoMFA) was employed to develop a three dimensional quantitative structure activity relationship for the OHPCB-mediated inhibition of the sulfation of DHEA catalyzed by hSULT2A1. The IC50 values that were obtained from the inhibition studies (Table 1) were converted into pIC50 (-log IC50 (M)) values for the CoMFA analysis. The biphenyl core structure of each OHPCB was aligned with a template molecule (4′-OHPCB 68) in order to get the conformation for correlation analysis and model development. The template molecule, 4′-OHPCB 68, was chosen following comparison to other OHPCBs that might serve as template. This template had the lowest number of components with a reasonable q2 value, and parameters following comparison of other potential template structures are provided in the Supporting Information (Table S1). The superimposition of all the compounds on the template molecule, 4′-OHPCB 68 is shown in Figure 3. The CoMFA model derived using this template had a steric contribution of 38% and an electrostatic contribution of 62% with q2 of 0.697, r2 of 0.949, and a standard error (S.E.) of 0.159. The differences between the actual pIC50 values and the predicted pIC50 values were less than 0.5 (Table 3). As seen in Figure 4, there as a good correlation observed between the experimental and predicted values for pIC50. Also noted in Figure 4 (the most potent inhibitors of DHEA sulfation catalyzed by hSULT2A1 were also substrates for the enzyme).
Figure 3.

Superimposition of all 15 hydroxylated polychlorinated biphenyls (OHPCBs) with 4′-OHPCB 68 as template molecule using a common substructure-based alignment.
Table 3.
Experimental IC50 values compared to Predicted IC50 values in the CoMFA of 15 OHPCBs (Model 1)
| Compound | IC50 (μM) | Actual pIC50 | Predicted pIC50 | Differencea |
|---|---|---|---|---|
| 4′-OHPCB 3 | 28.1 | 4.551 | 4.525 | 0.026 |
| 4′-OHPCB 6 | 9.8 | 5.009 | 5.117 | 0.108 |
| 4-OHPCB 8 | 10.0 | 5.000 | 5.303 | 0.303 |
| 4′-OHPCB 9 | 7.5 | 5.125 | 5.114 | 0.011 |
| 4-OHPCB 11 | 14.0 | 4.854 | 4.843 | 0.011 |
| 4′-OHPCB 12 | 15.8 | 4.801 | 4.763 | 0.039 |
| 4-OHPCB 14 | 13.0 | 4.886 | 5.082 | 0.196 |
| 4′-OHPCB 25 | 4.4 | 5.357 | 5.273 | 0.084 |
| 4′-OHPCB 33 | 2.5 | 5.602 | 5.503 | 0.099 |
| 4-OHPCB 34 | 0.6 | 6.222 | 6.022 | 0.199 |
| 6′-OHPCB 35 | 15.5 | 4.810 | 4.721 | 0.088 |
| 4-OHPCB 36 | 4.4 | 5.357 | 5.268 | 0.088 |
| 4′-OHPCB 68 | 0.8 | 6.097 | 6.153 | 0.056 |
| 3′,4′-diOH PCB 3 | 95.7 | 4.019 | 3.947 | 0.072 |
| 3′,4′-diOH PCB 5 | 15.9 | 4.799 | 4.853 | 0.054 |
Represents the difference between the experimental and the predicted values for pIC50 (i.e., -log IC50).
Figure 4.
Actual versus predicted values of -log IC50 for the set of 15 OHPCBs as inhibitors of the sulfation of DHEA catalyzed by hSULT2A1. Circled data points indicate those OHPCBs that also serve as substrates for sulfation catalyzed by hSULT2A1.
Although the leave-one-out cross validation parameter q2 provides a measure of the predictive power of the 3D-QSAR model, it is also useful to analyze the ability of the procedure to predict IC50 values with more than one compound that is not among the compounds used for developing the CoMFA model. Thus, we removed 4-OHPCB 14, 4-OHPCB 25 and 4-OHPCB 12 from the training set and analyzed an additional CoMFA model developed with the remaining 12 compounds (Figure 5). The IC50 values predicted from this model for 4-OHPCB 12 and 4-OHPCB 25 were similar to the experimental IC50 values obtained (Table 4), and there was a 3.8 fold difference between the predicted and the actual IC50 values for 4-OHPCB 14. The model had a q2 of 0.540, r2 of 0.957, and a S.E of 0.163 at 4 components. The steric contribution and the electrostatic contributions were 29% and 71% respectively. Therefore, the external validation process confirmed that the overall methodology employed for CoMFA model development was predictive for the inhibition of sulfation of DHEA catalyzed by hSULT2A1.
Figure 5.
Actual versus predicted values of –log IC50 for the set of 12 OHPCBs (Model 2).
Table 4.
Predicted and Experimental values of IC50 for 3 OHPCBs using a CoMFA for 12 OHPCBs (Model 2)a
| Test compounds | Predicted (pIC50) | Actual (pIC50) | Predicted IC50 (μM) | Actual IC50 (μM) |
|---|---|---|---|---|
| 4′-OHPCB 12 | 4.73 | 4.80 | 18.6 | 15.8 |
| 4-OHPCB 25 | 5.39 | 5.36 | 4.10 | 4.40 |
| 4-OHPCB 14 | 5.47 | 4.89 | 3.37 | 13.0 |
Three compounds were removed from the training set in Model 1, and a second, related, CoMFA model was derived based on the 12 remaining compounds. Model 2 was then used to predict IC50 values for 4′-OHPCB 12, 4-OHPCB 25 and 4-OHPCB 14.
Discussion
The cytosolic sulfotransferases catalyze sulfation reactions that are involved in the regulation of hormones, detoxication of bile acids and xenobiotics, activation of therapeutic drugs to obtain desired pharmacological effects, and conversion of xenobiotics to more reactive products that can cause cancer and other toxic responses.39–43 As detailed in the Introduction, the potential roles of sulfation in endocrine disruption, carcinogenesis, and other toxicities of the hydroxylated metabolites of PCBs are of increasing interest. We are particularly interested in interaction of the sulfotransferases with those OHPCB metabolites containing lower numbers (e.g., fewer than five) of chlorine atoms, since these may be formed metabolically following exposure to the parent PCBs in contaminated indoor and outdoor air. The studies presented here were specifically focused on the interactions of OHPCBs with hSULT2A1 due to the importance of this family 2 SULT in the metabolism of alcohol-containing steroid hormones and bile acids.
Structural characteristics of OHPCBs, due to the number and substitution pattern of the chlorine atoms, are important determinants of their overall metabolism and toxicity. Since the large number of potential OHPCB congeners precludes a detailed examination of each of the lower chlorinated OHPCBs, it is important to develop models that will be useful in predicting the effect of OHPCBs on hSULT2A1 based on fundamental structural characteristics. Such models will be particularly useful in assessing the potential for individual OHPCB congeners to interfere with the sulfation of hormones and other endogenous substrates for hSULT2A1 as well as determining the potential for interference with metabolism of drugs and other xenobiotics. Preliminary attempts to correlate IC50 values with either the acid-base constants of the OHPCBs or their octanol/water partition coefficients did not provide predictive models (data not shown). As a result, we turned to CoMFA in order to develop a 3D-QSAR model for the interaction of OHPCBs with hSULT2A1. A set of 15 OHPCBs was used to determine their ability to inhibit the catalysis of sulfation of DHEA by hSULT2A1. Those OHPCBs with 3, 5-dichloro-4-hydroxy substitutions proved to be the most potent inhibitors, and they were also substrates for sulfation reactions catalyzed by hSULT2A1. Additionally, the presence of a chlorine atom ortho to the ring junction in the OHPCB enhanced the inhibition of sulfation of DHEA. Whether the OHPCB served as an alternate substrate or solely as an inhibitor, the CoMFA model provided a mechanism for prediction of the ability of a lower chlorinated OHPCB to interfere with the sulfation of a substrate (e.g., DHEA) catalyzed by the hSULT2A1.
The finding that the best inhibitors of the enzyme were also alternative substrates, as well as the seamless correlation in IC50 values between those OHPCBs that were alternate substrates and those that were solely inhibitors of the enzyme, is indicative of a similarity in interactions occurring with the enzyme. Since substrates must bind at the active site for the sulfation reaction to occur, it is reasonable to assume that all of OHPCBs examined bind at the active site of the enzyme. This should not, however, be interpreted to imply that the mode of inhibition is competitive, since there are numerous examples where binding at the active site of an enzyme can display a non-competitive mode of inhibition.44 Indeed, difficulties in directly comparing our IC50 data with kinetic constants for sulfation of the OHPCBs that are substrates imply a more complex behavior than would be expected for simple competitive inhibition. Such complex behavior is common with homodimeric bisubstrate enzymes, where interaction of a substrate/inhibitor might occur with multiple forms of the enzyme that result either from formation of different ternary complexes or from conformational changes in the enzyme subsequent to binding of one substrate molecule. Binding of the first substrate molecule can then affect binding of a second substrate molecule either to a second active site in the homodimer or to an allosteric site. Indeed, differences in ternary enzyme complexes and conformational changes in hSULT2A1 upon binding of DHEA to the homodimer have been reported.36,45–47 Thus, an OHPCB substrate/inhibitor binding to the sulfuryl acceptor site in hSULT2A1 might induce similar conformational changes with kinetic constants that would deviate from those expected for simple competitive inhibition. While we cannot yet verify or rule out such interactions with the OHPCBs, additional studies to more fully determine the nature of these binding interactions of OHPCBs with hSULT2A1 are in progress. Moreover, differences in the formation of dead-end enzyme complexes36 as well as conformational changes36,45,46 have also been implicated in substrate inhibition observed with DHEA. As recently seen in comparisons of DHEA and raloxifene,46 there are mechanistic differences in hSULT2A1-catalysis that are dependent upon the structure of the substrate. Thus interactions with OHPCBs may also involve structure-dependent differences that lead to alterations in modes of binding and substrate inhibition, and further investigation will be required to elucidate these relationships.
Consideration of the in vivo significance of the sulfation of OHPCBs in humans is currently limited to in vitro studies interpreted in the context of serum concentrations of OHPCBs, the presence of specific SULTs in relevant tissues, the substrate specificities of these enzymes, and the concentrations of endogenous substrates for the SULTs. As an example of endogenous substrate for hSULT2A1, DHEA is present at a human serum concentration that varies with several factors, but it is usually within a range of 5–24 nM.48 Androsterone, another substrate for hSULT2A1, has been reported to be present at 2.5 – 5.0 nM in serum.48 Concentrations of OHPCBs in blood have often been reported on the basis of serum or plasma fresh weights, while tissue concentrations of OHPCBs have often been based on lipid weight. This causes difficulties in making direct comparisons. However, we have used previously reported concentrations of 0.082 – 0.328 ng/g49 and 0.117 – 11.6 ng/g20 based on whole blood wet weight in humans to estimate an approximate range of OHPCB concentrations of 0.3 – 40 nM.28 Comparison of these concentrations with Km values for DHEA indicate that both DHEA and OHPCBs are present at concentrations less than their Km values (i.e., the rate of the hSULT2A1-catalyzed reaction is directly dependent upon the concentration of OHPCB or DHEA). Thus, our observation that some OHPCBs inhibit the sulfation of DHEA at concentrations lower than the non-saturating concentration of DHEA used in our assays implies that they may compete effectively for DHEA as substrate for hSULT2A1 in some cells. These results point to a need for future examination of the potential in vivo importance through the use of intact human cell systems as well as development of methods for the extraction and analysis of the sulfated metabolites from human blood.
Although our present study focused on inhibition of the sulfation of DHEA, these interactions and the predictive capacity of CoMFA models would likely hold for other steroid substrates for hSULT2A1 (e.g., pregnenolone and androsterone). This would be particularly important when assessing either the potential for endocrine disruption by this mechanism or alteration in the metabolism of drugs or other xenobiotics through inhibition of hSULT2A1. Additionally, we would expect from our findings that those OHPCBs that are the best substrates for hSULT2A1 would be predicted to be the best inhibitors of the enzyme in its catalysis of the sulfation of DHEA, other steroid substrates, or other xenobiotics. While the model(s) derived in this study proved to be useful in prediction of the ability of OHPCBs to inhibit the sulfation of DHEA catalyzed by hSULT2A1, there are some potential limitations that should be considered. The models did not include highly chlorinated OHPCBs, particularly those that have two or more chlorines ortho to the ring junction). Furthermore, the highly chlorinated OHPCBs may have additional structural interactions with hSULT2A1 that are different from those OHPCBs included in the present work. Although an attempt was made to examine one such higher chlorinated OHPCB (i.e., 4-OHPCB 165) in the context of these studies, this was not possible due to the limited solubility of this compound under the assay conditions.
In summary we have examined the ability of OHPCBs to inhibit the sulfation of DHEA catalyzed by hSULT2A1 and determined those cases where the inhibition was due to the OHPCB serving as an alternate substrate for hSULT2A1. We have also developed a 3D-QSAR model that will aid in predicting the actions of other similar OHPCBs on hSULT2A1. Such models will be important in determining the role of lower chlorinated OHPCBs in disruption of sulfation of endogenous and xenobiotic molecules. As more is learned about the biological activities/toxicities of the PCB-sulfates, these models may also assist in predicting which lower chlorinated PCB congeners would potentially pose a threat to human health through mechanisms involving the formation of PCB-sulfates.
Supplementary Material
Acknowledgments
Funding Sources:
This study was supported by the National Institutes of Health through research grants P42 ES013661 (LWR, MWD, HJL) from the National Institute of Environmental Health Sciences and R01 CA38683 (MWD) from the National Cancer Institute. We also acknowledge programmatic support through the University of Iowa Environmental Health Sciences Research Center (NIEHS/NIH P30 ES05605). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations
- CoMFA
comparative molecular field analysis
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone sulfate
- DE52
Diethylaminoethyl cellulose anion exchanger
- DTT
dithiothreitol
- HPLC
high performance liquid chromatography
- hSULT2A1
human hydroxysteroid sulfotransferase 2A1
- IPTG
isopropyl-1-thio-D-galactopyranoside
- LB
Luria broth
- OHPCB
hydroxylated polychlorinated biphenyl
- 4′-OHPCB 3
4′-hydroxy-4-monochlorobiphenyl
- 4′-OHPCB 6
4′-hydroxy-2, 3′-dichlorobiphenyl
- 4-OHPCB 8
4- hydroxy-2, 4′-dichlorobiphenyl
- 4′-OHPCB 9
4′-hydroxy-2, 5-dichlorobiphenyl
- 4-OHPCB 11
4-hydroxy-3, 3′-dichlorobiphenyl
- 4′-OHPCB 12
4′-hydroxy-3, 4-dichlorobiphenyl
- 4-OHPCB 14
4-hydroxy-3, 5- dichlorobiphenyl
- 4′-OHPCB 25
4′-hydroxy-2, 3′, 4-trichlorobiphenyl
- 4′-OHPCB 33
4′-hydroxy-2′, 3, 4-trichlorobiphenyl
- 4-OHPCB 34
4-hydroxy-2′, 3, 5-trichlorobiphenyl
- 6′-OHPCB 35
6′-hydroxy-3, 3′-4 trichlorobiphenyl
- 4-OHPCB 36
4-hydroxy-3, 3′, 5-trichlorobiphenyl
- 4′-OHPCB 68
4′-hydroxy-2, 3′, 4, 5′-tetrachlorobiphenyl
- 3′, 4′-diOHPCB 3
3′, 4′-dihydroxy-4-monochlorobiphenyl
- 3′, 4′-diOHPCB 5
3′, 4′-dihydroxy-2, 3-dichlorobiphenyl
- PAP
adenosine 3′,5′-diphosphate
- PAPS
3′-phosphoadenosine 5′-phosphosulfate
- PCB
polychlorinated biphenyl
- SULT
cytosolic sulfotransferase
- 3D-QSAR
three-dimensional quantitative structure activity relationship
- Tris-HCl
[tris(hydroxymethyl)aminomethane] hydrochloride
References
- 1.Hansen LG. Stepping backward to improve assessment of PCB congener toxicities. Environ Health Perspect. 1998;106(Suppl 1):171–189. doi: 10.1289/ehp.98106s1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holoubek I, Kocan A, Holoubkova I, Kohoutek J, Falandysz J. Contaminated sites worldwide: The case of the central and eastern european countries. In: Robertson LW, Hansen LG, editors. PCBs: Recent Advances in Environmental Toxicology and Health Effects. University of Kentucky Press; Lexington, KY: 2001. pp. 81–83. [Google Scholar]
- 3.Hornbuckle K, Robertson L. Polychlorinated biphenyls (PCBs): sources, exposures, toxicities. Environ Sci Technol. 2010;44:2749–2751. doi: 10.1021/es100801f. [DOI] [PubMed] [Google Scholar]
- 4.Ludewig G, Esch H, Robertson LW. Polyhalogenierte Bi- und Terphenyle. Chapter 20. Wiley-VCH; Weinheim: 2007. [Google Scholar]
- 5.Safe SH. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol. 1994;24:87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- 6.Safe S. Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs): biochemistry, toxicology, and mechanism of action. Crit Rev Toxicol. 1984;13:319–395. doi: 10.3109/10408448409023762. [DOI] [PubMed] [Google Scholar]
- 7.Currado GM, Harrad S. Comparison of polychlorinated biphenyl concentrations in indoor and outdoor air and the potential significance of inhalation as a human exposure pathway. Environ Sci Technol. 1998;32:3043–3047. [Google Scholar]
- 8.Harrad S, Hazrati S, Ibarra C. Concentrations of polychlorinated biphenyls in indoor air and polybrominated diphenyl ethers in indoor air and dust in Birmingham, United Kingdom: implications for human exposure. Environ Sci Technol. 2006;40:4633–4638. doi: 10.1021/es0609147. [DOI] [PubMed] [Google Scholar]
- 9.Herrick RF, McClean MD, Meeker JD, Baxter LK, Weymouth GA. An unrecognized source of PCB contamination in schools and other buildings. Environ Health Perspect. 2004;112:1051–1053. doi: 10.1289/ehp.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu D, Martinez A, Hornbuckle KC. Discovery of non-aroclor PCB (3,3′-dichlorobiphenyl) in Chicago air. Environ Sci Technol. 2008;42:7873–7877. doi: 10.1021/es801823r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez A, Wang K, Hornbuckle KC. Fate of PCB congeners in an industrial harbor of Lake Michigan. Environ Sci Technol. 2010;44:2803–2808. doi: 10.1021/es902911a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wethington DM, 3rd, Hornbuckle KC. Milwaukee, WI, as a source of atmospheric PCBs to Lake Michigan. Environ Sci Technol. 2005;39:57–63. doi: 10.1021/es048902d. [DOI] [PubMed] [Google Scholar]
- 13.Ludewig G, Lehmann L, Esch H, Robertson LW. Metabolic Activation of PCBs to Carcinogens in Vivo - A Review. Environmental Toxicology and Pharmacology. 2008;25:241–246. doi: 10.1016/j.etap.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James MO. Polychlorinated biphenyls: metabolism and metabolites. In: Robertson LW, Hansen LG, editors. PCBs: Recent advances in environmental toxicology and health effects. The University Press of Kentucky; Lexington: 2001. pp. 35–46. [Google Scholar]
- 15.Bergman A, Klasson-Wehler E, Kuroki H. Selective retention of hydroxylated PCB metabolites in blood. Environ Health Perspect. 1994;102:464–469. doi: 10.1289/ehp.94102464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dirtu AC, Jaspers VL, Cernat R, Neels H, Covaci A. Distribution of PCBs, their hydroxylated metabolites, and other phenolic contaminants in human serum from two European countries. Environ Sci Technol. 2010;44:2876–2883. doi: 10.1021/es902149b. [DOI] [PubMed] [Google Scholar]
- 17.Fängström B, Athanasiadou M, Grandjean P, Weihe P, Bergman Å. Hydroxylated PCB metabolites and PCBs in serum from pregnant Faroese women. Environ Health Perspect. 2002;110:895–899. doi: 10.1289/ehp.110-1240989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabrio T, Piechotowski I, Wallenhorst T, Klett M, Cott L, Friebel P, Link B, Schwenk M. PCB-blood levels in teachers, working in PCB-contaminated schools. Chemosphere. 2000;40:1055–1062. doi: 10.1016/s0045-6535(99)00353-7. [DOI] [PubMed] [Google Scholar]
- 19.Park JS, Bergman A, Linderholm L, Athanasiadou M, Kocan A, Petrik J, Drobna B, Trnovec T, Charles MJ, Hertz-Picciotto I. Placental transfer of polychlorinated biphenyls, their hydroxylated metabolites and pentachlorophenol in pregnant women from eastern Slovakia. Chemosphere. 2008;70:1676–1684. doi: 10.1016/j.chemosphere.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandau CD, Ayotte P, Dewailly E, Duffe J, Norstrom RJ. Analysis of hydroxylated metabolites of PCBs (OH-PCBs) and other chlorinated phenolic compounds in whole blood from Canadian inuit. Environ Health Perspect. 2000;108:611–616. doi: 10.1289/ehp.00108611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lans MC, Klasson-Wehler E, Willemsen M, Meussen E, Safe S, Brouwer A. Structure-dependent, competitive interaction of hydroxy-polychlorobiphenyls, -dibenzo-p-dioxins and -dibenzofurans with human transthyretin. Chem Biol Interact. 1993;88:7–21. doi: 10.1016/0009-2797(93)90081-9. [DOI] [PubMed] [Google Scholar]
- 22.Schuur AG, Brouwer A, Bergman A, Coughtrie MW, Visser TJ. Inhibition of thyroid hormone sulfation by hydroxylated metabolites of polychlorinated biphenyls. Chem Biol Interact. 1998;109:293–297. doi: 10.1016/s0009-2797(97)00140-3. [DOI] [PubMed] [Google Scholar]
- 23.Schuur AG, van Leeuwen-Bol I, Jong WM, Bergman A, Coughtrie MW, Brouwer A, Visser TJ. In vitro inhibition of thyroid hormone sulfation by polychlorobiphenylols: isozyme specificity and inhibition kinetics. Toxicol Sci. 1998;45:188–194. doi: 10.1006/toxs.1998.2504. [DOI] [PubMed] [Google Scholar]
- 24.Kester MH, Bulduk S, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Bergman A, Safe SH, Kuiper GG, Schuur AG, Brouwer A, Visser TJ. Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs. Endocrinology. 2000;141:1897–1900. doi: 10.1210/endo.141.5.7530. [DOI] [PubMed] [Google Scholar]
- 25.Falany CN, Comer KA, Dooley TP, Glatt H. Human dehydroepiandrosterone sulfotransferase. Purification, molecular cloning, and characterization. Ann N Y Acad Sci. 1995;774:59–72. doi: 10.1111/j.1749-6632.1995.tb17372.x. [DOI] [PubMed] [Google Scholar]
- 26.Falany CN, Vazquez ME, Kalb JM. Purification and characterization of human liver dehydroepiandrosterone sulphotransferase. Biochem J. 1989;260:641–646. doi: 10.1042/bj2600641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otterness DM, Wieben ED, Wood TC, Watson RWG, Madden BJ, McCormick DJ, Weinshilboum RM. Human liver dehydroepiandrosterone sulfotransferase: molecular cloning and expression of cDNA. Mol Pharmacol. 1992;41:865–872. [PubMed] [Google Scholar]
- 28.Liu Y, Apak TI, Lehmler HJ, Robertson LW, Duffel MW. Hydroxylated polychlorinated biphenyls are substrates and inhibitors of human hydroxysteroid sulfotransferase SULT2A1. Chem Res Toxicol. 2006;19:1420–1425. doi: 10.1021/tx060160+. [DOI] [PubMed] [Google Scholar]
- 29.Lehmler HJ, Robertson LW. Synthesis of hydroxylated PCB metabolites with the Suzuki coupling. Chemosphere. 2001;45:1119–1127. doi: 10.1016/s0045-6535(01)00052-2. [DOI] [PubMed] [Google Scholar]
- 30.Sekura RD. Adenosine 3′-phosphate 5′-phosphosulfate. Methods Enzymol. 1981;77:413–415. [Google Scholar]
- 31.Sheng JJ, Duffel MW. Enantioselectivity of human hydroxysteroid sulfotransferase ST2A3 with naphthyl-1-ethanols. Drug Metab Dispos. 2003;31:697–700. doi: 10.1124/dmd.31.6.697. [DOI] [PubMed] [Google Scholar]
- 32.Gulcan HO, Liu Y, Duffel MW. Pentachlorophenol and other chlorinated phenols are substrates for human hydroxysteroid sulfotransferase hSULT2A1. Chem Res Toxicol. 2008;21:1503–1508. doi: 10.1021/tx800133d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bensadoun A, Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976;70:241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- 34.Sekura RD, Jakoby WB. Phenol Sulfotransferases. J Biol Chem. 1979;254:5658–5663. [PubMed] [Google Scholar]
- 35.Sheng J, Sharma V, Duffel MW. Current Protocols in Toxicology. John Wiley & Sons, Inc; New York: 2001. Measurement of aryl and alcohol sulfotransferase activity; pp. 4.5.1–4.5.9. [DOI] [PubMed] [Google Scholar]
- 36.Gulcan HO, Duffel MW. Substrate inhibition in human hydroxysteroid sulfotransferase SULT2A1: studies on the formation of catalytically non-productive enzyme complexes. Arch Biochem Biophys. 2011;507:232–240. doi: 10.1016/j.abb.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duffel MW, Binder TP, Rao SI. Assay of purified aryl sulfotransferase suitable for reactions yielding unstable sulfuric acid esters. Anal Biochem. 1989;183:320–324. doi: 10.1016/0003-2697(89)90486-7. [DOI] [PubMed] [Google Scholar]
- 38.Cramer RD, III, Patterson DE, Bunce JD. Comparative molecular field analysis (CoMFA). 1 Effect of shape on binding of steroids to carrier proteins. J Am Chem Soc. 1988;110:5959–5967. doi: 10.1021/ja00226a005. [DOI] [PubMed] [Google Scholar]
- 39.Duffel MW. Sulfotransferases. In: Guengerich FP, McQueen CA, editors. Comprehensive Toxicology, Vol. 4 Biotransformation. Elsevier; Oxford: 2010. pp. 367–384. [Google Scholar]
- 40.Duffel MW, Marshall AD, McPhie P, Sharma V, Jakoby WB. Enzymatic aspects of the phenol (aryl) sulfotransferases. Drug Metab Rev. 2001;33:369–395. doi: 10.1081/dmr-120001394. [DOI] [PubMed] [Google Scholar]
- 41.Gamage N, Barnett A, Hempel N, Duggleby RG, Windmill KF, Martin JL, McManus ME. Human sulfotransferases and their role in chemical metabolism. Toxicol Sci. 2006;90:5–22. doi: 10.1093/toxsci/kfj061. [DOI] [PubMed] [Google Scholar]
- 42.Glatt H, Boeing H, Engelke CE, Ma L, Kuhlow A, Pabel U, Pomplun D, Teubner W, Meinl W. Human cytosolic sulphotransferases: genetics, characteristics, toxicological aspects. Mutat Res. 2001;482:27–40. doi: 10.1016/s0027-5107(01)00207-x. [DOI] [PubMed] [Google Scholar]
- 43.Pacifici GM, Coughtrie MW, editors. Human cytosolic sulfotransferases. CRC Press; Boca Raton, FL: 2005. [Google Scholar]
- 44.Blat Y. Non-competitive inhibition by active site binders. Chem Biol Drug Des. 2010;75:535–540. doi: 10.1111/j.1747-0285.2010.00972.x. [DOI] [PubMed] [Google Scholar]
- 45.Cook IT, Leyh TS, Kadlubar SA, Falany CN. Lack of substrate inhibition in a monomeric form of human cytosolic SULT2A1. Horm Mol Biol Clin Investig. 2010;3:357–366. [PMC free article] [PubMed] [Google Scholar]
- 46.Cook IT, Leyh TS, Kadlubar SA, Falany CN. Structural rearrangement of SULT2A1: effects on dehydroepiandrosterone and raloxifene sulfation. Horm Mol Biol Clin Investig. 2010;1:81–87. doi: 10.1515/HMBCI.2010.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou M, Rehse P, Chang HJ, Luu-The V, Lin SX. Crystallization and preliminary crystallographic results of apo and complex forms of human dehydroepiandrosterone sulfotransferase. Acta Crystallogr D Biol Crystallogr. 2001;57:1630–1633. doi: 10.1107/s0907444901010964. [DOI] [PubMed] [Google Scholar]
- 48.Labrie F, Belanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82:2396–2402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]
- 49.Guvenius DM, Aronsson A, Ekman-Ordeberg G, Bergman A, Noren K. Human prenatal and postnatal exposure to polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorobiphenylols, and pentachlorophenol. Environ Health Perspect. 2003;111:1235–1241. doi: 10.1289/ehp.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.